Abstract
Background
Unexplained physical symptoms (UPS) are extremely common among primary care attenders, but little is known about their longer-term outcome.
Aim
To investigate the persistence of somatic symptoms at 6 months among a cohort with multiple UPS, and identify prognostic factors associated with worsening symptom scores.
Design and setting
Prospective longitudinal cohort study involving adults attending UK general practice in North and Central London between January and December 2013.
Method
Consecutive adults attending nine general practices were screened to identify those with at least three UPS. Eligible participants completed measures of symptom severity (measured using the Patient Health Questionnaire Somatic Symptom Module [PHQ-15]), physical and mental wellbeing, and past health and social history, and were followed up after 6 months. Multivariable linear regression analysis was conducted to identify prognostic factors associated with the primary outcome: somatic symptom severity.
Results
Overall, 245/294 (83%) provided 6-month outcome data. Of these, 135/245 (55%) reported still having UPS, 103/245 (42%) had symptoms still under investigation, and only 26/245 (11%) reported complete symptom resolution. Being female, higher baseline somatic symptom severity, poorer physical functioning, experience of childhood physical abuse, and perception of poor financial wellbeing were significantly associated with higher somatic symptom severity scores at 6 months.
Conclusion
This study has shown that at 6 months few participants had complete resolution of unexplained somatic symptoms. GPs should be made aware of the likelihood of UPS persisting, and the factors that make this more likely, to inform decision making and care planning. There is a need to develop prognostic tools that can predict the risk of poor outcomes.
Keywords: general practice, primary health care, somatic symptoms, unexplained physical symptoms
INTRODUCTION
Unexplained physical symptoms (UPS) — that is, physical symptoms that lack obvious pathological explanations even after appropriate investigations — are common among primary care attenders.1 They are associated with high morbidity, distress to patients and their families, and costs to the NHS and wider economy.2,3 In the past, outcome studies focused on those meeting psychiatric diagnostic criteria such as the Diagnostic and Statistical Manual for Mental Disorders, and not on more heterogeneous primary care groups with common symptoms such as headaches, back pain, bloating, nausea, and fatigue.4 Depending on the diagnostic criteria and methods of identification, the prevalence of UPS is estimated to vary from 0.8% to 79%.1
Primary care is often the first point of access for health care, and doctors report difficulties with the appropriate management of patients with UPS.5,6 Better early detection and management could reduce the burden on patients and clinicians, and those requiring longer-term input.7 GPs’ decision making and care planning for individuals with UPS could be assisted by the use of prognostic tools to predict the risk of poor outcomes, such as the persistence of symptoms. Identification of factors associated with relevant outcomes is the key component in developing prognostic models.8 However, little is known about outcomes over time and the factors associated with prognostic factors in primary care attenders with UPS.9–12
This UK-based primary care study aimed at 6-month follow-up to investigate outcome in terms of persistence of UPS, and to identify prognostic factors associated with somatic symptom severity, quality of life (QoL), anxiety, depression, and healthcare use.
METHOD
This was a prospective longitudinal cohort study.
Setting and participants
Physical symptoms of unknown cause are referred to as UPS, and this term is used broadly to include those who reported that they had not received a clear diagnosis for their symptoms, even after consultation and investigation, as well as those who were attending for the first time with symptoms bothering them on the Patient Health Questionnaire Somatic Symptom Module (PHQ-15). The authors’ definition of UPS does not imply an underlying psychological cause.
Over an 11-month period (28 January 2013 to approximately 14 December 2013). waiting room attendees aged ≥18 years at nine general practices in North and Central London were invited to complete a screening questionnaire. The authors excluded people not registered at the practice, those planning to move away in the next 6 months, and those unable to understand and complete the questionnaire in English. Those with a medical explanation or diagnoses that fully explained their symptoms, or those with a terminal illness, were also excluded.
How this fits in
Most existing studies of unexplained physical symptoms (UPS) are based on meeting the criteria for severe symptomology and comorbid psychiatric disorders, with few studies conducted among primary care attenders. Existing guidelines are developed from evidence that includes individuals with psychiatric disorders, hypochondriasis, hysteria, or somatoform disorder, and from secondary care populations or community samples, who are likely to have very different illness trajectories and outcomes than patients recruited in primary care. In this study, about half the responders recruited in primary care had persistent unexplained symptoms at 6-month follow-up. Being female, having poor physical wellbeing, more severe symptoms at onset, a past history of physical abuse during childhood, and current stressful circumstances, such as financial difficulties, were associated with higher somatic symptom severity at follow-up.
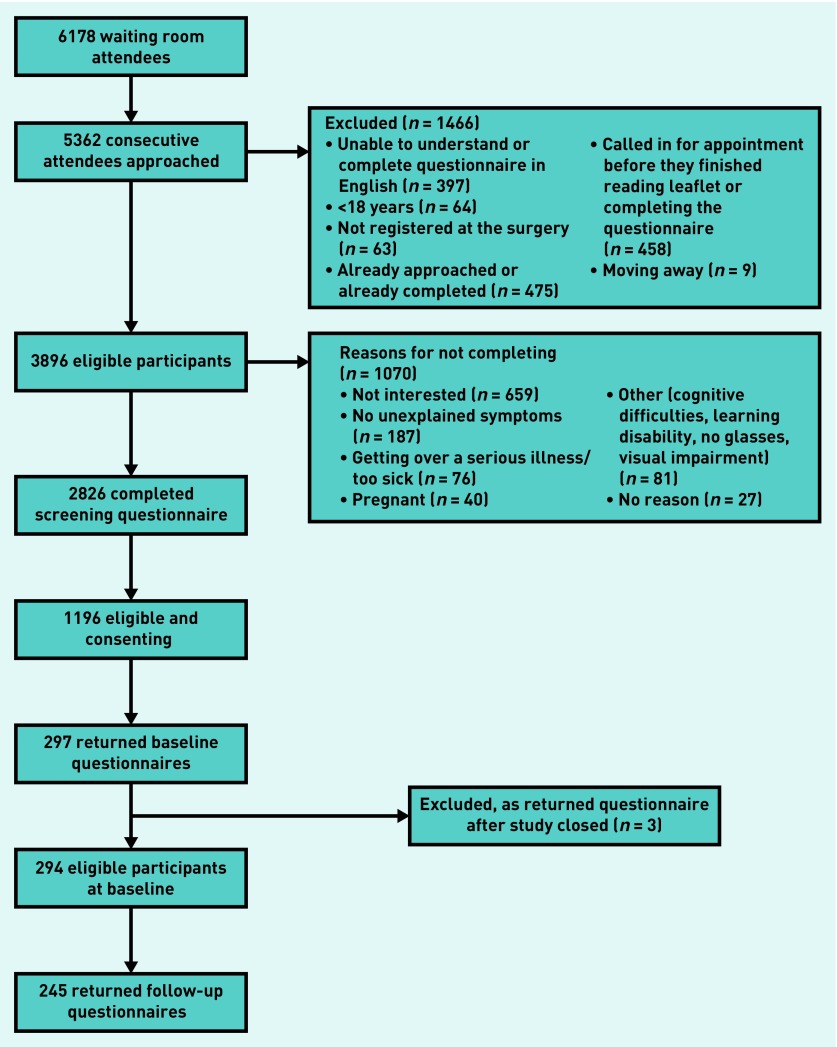
Consecutive attendees were screened and those meeting the eligibility criteria recruited to the main study (Figure 1).
Figure 1.
Screening and recruitment of study cohort.
Phase 1: screening
Participants completed the PHQ-15, a self-administered questionnaire that includes 90% of common presenting somatic symptoms.13,14 The question: ‘During the past 4 weeks, how much have you been bothered by any of the following problems?’ was followed by 15 questions for females and 14 for males about specific body symptoms. A score was calculated based on answers ‘not bothered at all’ (0), ‘bothered a little’ (1), or ‘bothered a lot’ (2).15 Clinical cut-off points are frequently used, with a score of 0–4 considered as minimal severity (at least three symptoms), 5–9 considered to be low severity (at least five symptoms), 10–14 considered to be moderate severity (at least eight symptoms), and ≥14 high severity.15 Those who had previously seen the doctor for their symptoms, as well as those seeing the doctor for the first time, who had at least three symptoms and scored ≥5 on the PHQ-15 were considered eligible, as at this stage their symptoms were considered ‘unexplained’.
Responders were asked to provide any known explanation or diagnosis for their symptoms from their perspective, to determine which were unexplained. These were discussed and a booklet of diagnoses that could potentially explain symptoms was developed, in consultation with practising GPs. The booklet was iteratively updated until no further additions to explanations were identified, which allowed for consistency in inclusion or exclusion. This information was used in the descriptive analysis to categorise responders’ symptoms as unexplained, fully explained by physical diagnoses, or partially explained by a physical diagnosis (for example, diabetes or medicine side effects), psychological explanations (for example, stress, anxiety, or depression), or functional diagnoses (for example, irritable bowel syndrome or chronic fatigue).
Phase 2: main cohort study
Potentially eligible participants were sent a postal questionnaire at baseline, followed by two reminders. Options for completion were post, telephone, or face-to-face. Baseline responders were sent a follow-up questionnaire at 6 months and asked to indicate whether their symptoms had resolved, were still under investigation, had been diagnosed, or were still considered unexplained. Both questionnaires included measures of relevant prognostic factors (Box 1).
Box 1. Potential prognostic factors considered at baseline, scales and measures used for data collection, and outcome variables explored.
| Potential prognostic variables/instruments or questionnaires used | Baseline questionnaire booklet | 6-month follow-up questionnaire bookleta |
|---|---|---|
| Somatic symptom severity (PHQ-15) (primary outcome) | X | X |
| Quality of life (SF-12) | X | X |
| Depression (PHQ-9) | X | X |
| Anxiety (GAD-7) | X | X |
| Panic (PHQ-PD) | X | X |
| Management of symptoms/questions developed for studyb | X | |
| Social functioning (WSAS) | X | |
| Self-efficacy (GSE) | X | |
| Stressful life events (LTE-Q) | X | |
| Childhood experiences/questions developed for the studyb | X | |
|
Sociodemographic information including: Sex, age, ethnicity, marital status, employment status, socioeconomic status/wellbeing, education level, and perceptions of social support/questions developed for the study, ethnic categories informed from the ONS study |
X |
Six-month follow-up questionnaire booklet — only outcome measures collected. GAD-7 = Generalised Anxiety Disorder assessment. GSE = general self-efficacy. PHQ-PD = Patient Health Questionnaire Panic Disorder. LTE-Q = List of Threatening Experiences Questionnaire. ONS = Office for National Statistics. PHQ-15 = Patient Health Questionnaire Somatic Symptom Module. SF-12 = Short Form health questionnaire. WSAS = Work and Social Adjustment Scale. Questionnaires developed for the study were based on existing evidence or questionnaires.
Due to the long and sensitive nature of many questionnaires on trauma and abuse, only two short screening questions were included in the baseline questionnaire booklet. First, a more general question asked whether the participant had experienced any type of trauma while growing up and to clarify what this was, followed by a question about whether they had experienced any type of abuse as a child.
Outcome measurements
The primary outcome was somatic symptom severity score, measured using the PHQ-15.
Data were also collected on secondary outcomes: QoL was measured using the mental and physical health components of the 12-item Medical Outcome Survey Short Form Questionnaire (SF-12);16 depression and generalised anxiety disorder were measured using the Patient Health Questionnaire depression module (PHQ-9)17 and the Generalised Anxiety Disorder assessment (GAD-7).18 The number of primary healthcare contacts in the year before study recruitment and during the study period (either face-to-face or telephone) with doctors, nurses, healthcare assistants, or out-of-hours GP services were obtained from patients’ medical records.
Statistical analysis
Univariable analysis was conducted to determine the association of baseline variables with outcomes using Stata (version 12). This was followed by conceptual group modelling, a method used to reduce the number of variables included in the main modelling.19 Multivariable analyses were conducted using variables that are significantly associated with the outcome variables (P<0.05) and theoretically considered to be measuring similar characteristics among the study population.
For example, a conceptual group consisting of socioeconomic factors — education level, employment status, Index of Multiple Deprivation score, and perception of financial wellbeing — would be placed in the same conceptual group if they were significantly associated with the outcome. This was carried out to avoid potential collinearity in the main modelling process, as well as to ensure the main modelling was not overfitted by including too many explanatory variables for the number of observations. Those variables that remained would then be included in the main multivariable modelling procedure, after which backwards elimination was carried out, starting with the largest P-value and continuing until only variables with P<0.05 remained (and/or were included in the model a priori). All models were adjusted for baseline outcome variable, including baseline PHQ-15. Age and sex were included a priori. Results reported are from mutually adjusted models.
Missing data
Guidance on correcting missing data for each of the scales and measures was used. For the PHQ-15, PHQ-9, and GAD-7 the authors used a conservative approach of assuming the responder was not bothered by the item.20 Missing data were minimal, and accounted for <0.5% of data at each time point.
RESULTS
Baseline characteristics
Of those who had completed the screening questionnaire, 1632/2826 (58%) were potentially eligible for the next stage of the study, while 1196/2826 (42%) also gave their contact details, allowing them to be followed up with the baseline questionnaire. Baseline questionnaires were returned by 294/1196 (25% of those screened), excluding three who sent back questionnaires after the study had closed.
The majority were female (231/294, 79%), and median age was 44 (interquartile range [IQR] 32–57) years. The sample was ethnically diverse, less than half were white British (125/294, 43%), and representative of the practice populations. At baseline, responders were asked about duration of their symptoms; most had experienced symptoms for >1 year. On average, they had moderately severe physical symptom scores and poor physical and mental health functioning, based on the SF-12. One-third of responders fell into the range of clinically significant comorbid depression and anxiety at a cut-off ≥10 on the PHQ-9 and GAD-7 (data not shown). Other clinical characteristics are shown in Table 1.
Table 1.
Baseline clinical characteristics of the study cohort
| Clinical characteristics | Total, n= 294 | Male, n= 63 | Female, n= 231 |
|---|---|---|---|
| Baseline symptom severity (PHQ-15 score), mean (SD) | 11.5 (4.9) | 11.0 (5.0) | 11.7 (4.9) |
|
| |||
| Symptom duration (%) | |||
| <1 year | 63 (21) | 14 (22) | 49 (21) |
| ≥1 year | 212 (72) | 43 (68) | 169 (73) |
| Missing | 19 (6) | 6 (9) | 13 (6) |
|
| |||
| SF-12 score, mean (SD) | |||
| Physical health functioninga | 43.8 (10.6) | 42.9 (10.6) | 44.1 (10.6) |
| Mental health functioning | 39.6 (11.0) | 41.0 (11.1) | 39.2 (11.0) |
|
| |||
| Work and social adjustment score, mean (SD) | 18.7 (11.5) | 19.1 (11.5) | 18.5 (11.5) |
| Missing | 6 | 0 | 6 |
|
| |||
| Self-efficacy score, mean (SD) | 27.4 (7.4) | 27.4 (7.2) | 27.4 (7.4) |
| Missing | 4 | 0 | 4 |
|
| |||
| Anxiety score, mean (SD) | 8.9 (5.8) | 8.2 (5.8) | 9.0 (5.7) |
|
| |||
| Depression score, mean (SD) | 9 (5–14) | 9 (4–14) | 9 (5–14) |
|
| |||
| Panic (PHQ-PD) (%) | |||
| Yes | 62 (21) | 12 (19) | 50 (22) |
| No | 204 (69) | 43 (68) | 161 (70) |
| Missing | 28 (10) | 8 (13) | 20 (9) |
|
| |||
| Stressful life events, median (IQR) | 1 (0–2) | 1 (1–3) | 1 (0–2) |
| Missing | 3 | 1 | 2 |
|
| |||
| Experienced physical illness in the family as a child (%) | |||
| Yes | 94 (32) | 23 (37) | 71 (31) |
| No | 194 (66) | 39 (62) | 155 (67) |
| Missing | 6 (2) | 1 (2) | 5 (2) |
|
| |||
| Experienced mental illness in the family as a child (%) | |||
| Yes | 47 (16) | 5 (8) | 42 (18) |
| No | 242 (82) | 57 (90) | 185 (80) |
| Missing | 5 (2) | 1 (2) | 4 (2) |
|
| |||
| Experienced ≥1 traumatic event as a child (%) | |||
| Yes | 93 (32) | 17 (27) | 76 (33) |
| No | 192 (65) | 43 (68) | 149 (65) |
| Missing | 9 (3) | 3 (5) | 6 (3) |
|
| |||
| Experienced any abuse as a child (%) | |||
| Yes | 77 (26) | 16 (25) | 61 (26) |
| No | 204 (69) | 46 (73) | 158 (68) |
| Missing | 13 (4) | 1 (2) | 12 (5) |
|
| |||
| Type of abuse experienced as a child (%)b | |||
| Physical abuse | |||
| Yes | 31 (11) | 8 (13) | 23 (10) |
| No | 253 (86) | 54 (86) | 199 (86) |
| Missing | 10 (3) | 1 (2) | 9 (4) |
|
| |||
| Sexual abuse | |||
| Yes | 25 (9) | 2 (3) | 23 (10) |
| No | 259 (78) | 60 (95) | 199 (86) |
| Missing | 10 (3) | 1 (2) | 9 (4) |
|
| |||
| Emotional abuse | |||
| Yes | 59 (20) | 13 (22) | 46 (20) |
| No | 225 (77) | 49 (78) | 176 (76) |
| Missing | 10 (3) | 1 (2) | 9 (4) |
Missing data for one male participant.
Possible to tick more than one type of abuse. IQR = interquartile range. PHQ-PD = Patient Health Questionnaire Panic Disorder. PHQ-15 = Patient Health Questionnaire Somatic Symptom Module. SD = standard deviation. SF-12 = Short Form health questionnaire.
Baseline responders had similar characteristics to eligible non-responders who had consented to be contacted after screening (1196/2826, 42%). However, male responders when compared with male non-responders were older (53 years, IQR 36–66 versus 43 years, IQR 30–55), and more males reported symptoms that were partially explained by a diagnosis (49% versus 29%) (Table 1).
Outcomes at 6 months
The follow-up rate was high (245/294, 83%). Responders (n = 245) were slightly older (45 years [IQR 33–58] versus 39 years [IQR 27–49]) than non-responders (n = 49), and had experienced symptoms for longer (36 months [standard deviation {SD} 17–72] versus 24 months [SD 14–58]) and with lower median baseline PHQ-9 scores (8 [IQR 4–14] versus 10 [IQR 6–18]). Otherwise, they were similar in all other respects. At 6-month follow-up, mean scores for all outcome measures were similar to the baseline scores, indicating poor recovery; only 11% (26/245) reported full recovery, 24% (58/245) had received a diagnosis for at least some of their symptoms, 42% (103/245) reported being still under investigation (by GP/specialist), and 55% (135/245) continued to have unexplained symptoms (data not shown). These categories were not mutually exclusive.
Following univariable and conceptual group modelling, 15 variables were included in the modelling to identify prognostic factors associated with symptom severity scores at follow-up. Backward selection was carried out until six variables remained in the final model (Table 2). Being female, higher baseline somatic symptom severity, experience of childhood physical abuse, perception of financial wellbeing as poor, and poorer baseline physical functioning were significantly associated with higher somatic symptom severity scores at 6 months. Six variables were included in the final model. As mentioned in the statistical analysis section, age and sex were included in the model a priori. Although backward selection was conducted until only six variables were left in the model, age was not found to be significantly associated with the primary outcome. Only the five variables that were significantly associated are discussed above. Adjusted somatic symptom severity score at follow-up was, on average, 1.31 (95% confidence interval [CI] = 0.12 to 2.50) points higher among females (Table 2). Depression and anxiety scores were not independently associated with adverse outcome after adjusting for baseline somatic symptom severity.
Table 2.
Summary of baseline predictors which were significantly associated with each of the outcomes in multivariable analysesa
| Baseline variables | Primary outcome | Secondary outcomes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Somatic symptom severity | Physical health functioning | Mental health functioning | Depression | Anxiety | Primary healthcare contact | |||||||
| Coefficient (95% CI) | P-value | Coefficient (95% CI) | P-value | Coefficient (95% CI) | P-value | Coefficient (95% CI) | P-value | Coefficient (95% CI) | P-value | Coefficient (95% CI) | P-value | |
| Female | 1.31 (0.12 to 2.50) | 0.031 | 0.83 (−1.47 to 3.13) | 0.479 | −2.47 (−5.18 to 0.25) | 0.075 | 0.79 (−0.64 to 2.23) | 0.277 | 0.55 (−0.85 to 1.96) | 0.437 | −0.57 (−1.60 to 0.46) | 0.274 |
| Age, years score, mean (SD) | 0.01 (−0.03 to 0.04) | 0.559 | −0.08 (−0.15 to −0.02) | 0.014 | −0.21 (−0.09 to 0.48) | 0.543 | 0.02 (−0.02 to 0.05) | 0.339 | 0.02 (−0.01 to 0.06) | 0.227 | 0.01 (−0.02 to 0.04) | 0.568 |
| Perception of financial wellbeing as doing badly | 1.90 (0.89 to 2.91) | <0.001 | – | – | – | – | – | – | – | – | – | – |
| Somatic symptom score | 0.53 (0.42 to 0.64) | <0.001 | −0.30 (−0.51 to −0.09) | 0.005 | – | – | 0.16 (0.03 to 0.30) | 0.016 | – | – | – | – |
| Mental health functioning score | – | – | – | – | 0.40 (0.27 to 0.54 | 0.001 | – | – | – | – | – | – |
| Physical health functioning score | −0.10 (−0.15 to −0.04) | 0.001 | 0.61 (0.51 to 0.72) | <0.001 | – | – | – | – | – | – | – | – |
| Self-efficacy score | – | – | – | – | – | – | −0.18 (−0.27 to −0.09) | <0.001 | – | – | – | – |
| Anxiety score | – | – | – | – | – | – | – | – | 0.44 (0.27 to 0.61) | <0.001 | – | – |
| Depression score | – | – | – | – | −0.45 (−0.67 to −0.23) | <0.001 | 0.54 (0.42 to 0.65) | <0.001 | 0.20 (0.06 to 0.34) | 0.007 | – | – |
| Experienced physical abuse during childhood | 1.86 (0.27 to 3.45) | 0.022 | – | – | – | – | – | – | – | – | – | – |
| Experienced emotional abuse during childhood | – | – | – | – | – | – | – | – | – | – | 1.28 (0.26 to 2.32) | 0.015 |
| Primary care health service contacts in year before study participation | – | – | −0.18 (−0.31 to −0.04) | 0.011 | – | – | – | – | – | – | 0.40 (0.34 to 0.45) | <0.001 |
Coefficients are also given for female sex and age, which were included in all models a priori.
All final multivariable models for the secondary variables included a fairly narrow range of baseline variables associated with physical outcomes (somatic symptom severity and physical health functioning) and psychological outcomes (mental health functioning, depression, and anxiety), respectively, summarised in Table 2. For all secondary outcomes the baseline measure of the same variable was associated with its follow-up severity, after adjusting for all other variables. Most factors followed an expected association; for example, greater self-efficacy was associated with lower depression scores and better outcome at follow-up. The only factors associated with higher primary healthcare use at follow-up were emotional abuse in childhood and higher healthcare contacts in the year before the study.
DISCUSSION
Summary
In this study, primary care attendees with ≥3 unexplained symptoms had poor quality of life, but only one-third had associated significant symptoms (above diagnostic thresholds) of depression or anxiety. Attenders were likely to continue to be symptomatic at 6-month follow-up; more than half reported UPS at 6 months (55%), close to half (42%) were still under investigation (by GP or specialist), and a few (11%) described themselves as fully recovered. Prognostic factors associated with higher somatic symptom severity at follow-up included being female, higher baseline somatic symptom severity, poorer physical health functioning, perception of poor financial wellbeing, and experience of childhood physical abuse.
Strengths and limitations
To the authors’ knowledge, this is the first UK study of primary care attenders with self-reported UPS recruited on the basis of the severity of their somatic symptoms in which the outcomes of their UPS were explored, as well as the prognostic factors associated with their persistence. Self-reported symptoms are crucial to understanding patients’ needs and help-seeking behaviour. However, as they rely on the patients’ understanding of their diagnosis or explanation and their recall, it is possible that there may be some bias. Nevertheless, the authors believe that the patients’ understanding and perceptions are likely to offer valuable insights on the level of burden, frequency of consultation, and other healthcare use.
Nine general practices with differing levels of deprivation and high ethnic diversity were included, increasing the potential for wider generalisability of the findings to the UK population. The characteristics of responders and non-responders were reasonably similar on key variables, and attrition at follow-up was low (17%), suggesting the findings are likely to be generalisable to a wider population, although potentially less applicable to younger men as these were less well represented among the male responders.
The majority of measures used were validated and reliable, but existing questionnaires were adapted in a few cases. For example, validated questionnaires on childhood abuse are long and potentially too intrusive for a postal questionnaire, so questions were reduced and modified. This may have decreased the sensitivity and specificity of these measures, which may have impacted on the findings. Efforts were taken to ensure that models were adjusted for potential confounding. However, it is possible that there was some impact of unmeasured and unknown confounding variables, such as other comorbidities and current experience of abuse. The study was powered for the primary outcome, somatic symptom severity. Although exploration of the secondary outcomes provides an indication of possible associations, these must be interpreted with caution.
Comparison with existing literature
Comparison with the existing literature is difficult as most existing UPS research has included heterogeneous populations meeting psychiatric diagnostic classifications, such as somatoform disorder, which comprise a very small proportion of those attending primary care.1,21 A review of studies that included populations with both somatoform disorders and hypochondriasis concluded that many unexplained symptoms are transient, and that the majority of patients will improve over time.10 In contrast, this study found that more than half of primary care attenders, who may be anticipated to have potentially less morbidity, reported their symptoms as still unexplained at follow-up. This is in line with comparatively more recent studies which have reported that around half continue to be burdened by their symptoms, albeit these studies include populations meeting the criteria for somatoform disorders11 or bodily distress syndrome (BDS), which is a diagnosis of functional disorders rather than symptoms.12 Only 11% in this present study reported their symptoms as resolved; this is also much lower than rates of resolution reported by Jackson and Passamonti in the US among consecutive primary care attenders at 3-month follow-up, although they did not distinguish between those with explained and unexplained symptoms.9
Although at baseline one-third of the cohort in this study had clinically significant scores for depression and anxiety, neither depression nor anxiety independently predicted persistent somatic symptoms at follow-up. However, a few existing studies based on primary care attenders with psychiatric morbidity have reported persistent somatic symptoms at between 6- and 12-month follow-up.11,22,23
The authors’ finding that greater overall symptom severity at baseline is associated with worse outcome at follow-up is consistent with previous research, though those studies also included populations meeting a variety of different inclusion criteria.9–12,24–26 There is a growing body of literature which suggests that a greater number of symptoms, regardless of whether they are explained or unexplained, contribute to poor outcome.9,13,26 As in this study, a recent review by Tomenson et al in which secondary analysis was conducted on studies from four different sites reported that somatic symptom score was a better predictor of follow-up health status and healthcare use than UPS.26
As in many other studies, the authors found that females were likely to have higher symptom severity compared with males at follow-up, even after adjusting for other variables, including baseline somatic symptoms.26–28 Worse functional disability and poor physical health at baseline are reported to be associated with the persistence of UPS or high somatic symptom scores at 12-month follow-up.11,12 Physical abuse in childhood was associated with an increase in somatic symptom severity at follow-up, suggesting that childhood physical abuse may have a long-term impact on physical health, similar to reports in other studies.29–32
Implications for research and practice
The current study suggests that for many patients in primary care with several bothersome UPS their symptoms may not be transient, and that around half will continue to be affected over time. Baseline symptom severity was found to be a good indicator of how patients are likely to progress over a 6-month period, and can be helpful in considering prognosis by GPs and policymakers, as well as in future research.
A fairly high proportion of the study participants were still undergoing investigations at 6-month follow-up, and there have been concerns that ongoing investigations may perpetuate symptoms, with a number of potential iatrogenic consequences.33 It is vital that individuals are managed appropriately in the long term to reduce the burden on themselves, healthcare resources, and the wider economy. It may be useful to take an approach to health care advocated for other long-term conditions: engaging with the patient, involving them in decisions about their care, and supporting self-management, as well as providing emotional, psychological, and practical support.34 Management strategies used by GPs, both initially and over time, should consider addressing factors such as symptom burden, current physical and mental health, recent stressful life events, and historical factors such as abuse.
A high percentage of patients with UPS in primary care were functionally impaired, with high somatic symptom scores, but only one-third of the cohort had comorbid depression and anxiety. The current findings support assertions that UPS should not be assumed to be of psychological aetiology among heterogeneous primary care attenders,9,35 and accompanying psychological morbidity may not be a key to prognosis.
This study adds value to the area of UPS by providing an evidence base for primary care guidance. Until now such guidance has been based on research in much more heterogeneous populations, with UPS closely aligned to psychiatric morbidity and located in a wide range of settings, including secondary care.36
Funding
Dr Lamahewa was funded for her PhD research by the National Institute of Health Research School for Primary Care, London, UK (NIHR School for Primary Care Research [NSPCR] UKCRN ID [13892]). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Ethical approval
Brent Local Research Ethics Committee 12/LO/1885. Approval received on 7 December 2012.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Haller H, Cramer H, Lauche R, Dobos G. Somatoform disorders and medically unexplained symptoms in primary care: a systematic review and meta-analysis of prevalence. Dtsch Arztebl Int. 2015;112(16):279–287. doi: 10.3238/arztebl.2015.0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zonneveld LN, Vant Spijker A, Passchier J, et al. The effectiveness of training for patients with unexplained physical symptoms: protocol of a cognitive behavioural group training and randomized controlled trial. BMC Public Health. 2009;9(1):251. doi: 10.1186/1471-2458-9-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bermingham SL, Cohen A, Hague J, Parsonage M. The cost of somatisation among the working–age population in England for the year 2008–2009. Ment Health Fam Med. 2010;7(2):71–84. [PMC free article] [PubMed] [Google Scholar]
- 4.American Psychiatric Association . DSM IV: Diagnostic and statistical manual of mental disorders. 4th edition. Washington, DC: American Psychiatric Press Inc.; 1994. [Google Scholar]
- 5.Howman M, Walters K, Rosenthal J, et al. ‘You kind of want to fix it, don’t you?’ Exploring general practice trainees’ experiences of managing patients with medically unexplained symptoms. BMC Med Educ. 2016;16(1):27. doi: 10.1186/s12909-015-0523-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stone L. Managing the consultation with patients with medically unexplained symptoms: a grounded theory study of supervisors and registrars in general practice. BMC Fam Pract. 2014;15(1):192. doi: 10.1186/s12875-014-0192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Royal College of General Practitioners. Royal College of Psychiatrists The management of patients with physical and psychological problems in primary care: a practical guide: Report of a joint working group of the Royal College of General Practitioners and the Royal College of Psychiatrists. 2009. www.rcgp.org.uk/policy/rcgp-policy-areas/management-of-patients-with-physical-and-psychological-problems.aspx (accessed 21 Jan 2019)
- 8.Hemingway H, Croft P, Perel P, et al. Prognosis research strategy (PROGRESS) 1: a framework for researching clinical outcomes. BMJ. 2013;346:e5595. doi: 10.1136/bmj.e5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson JL, Passamonti M. The outcomes among patients presenting in primary care with a physical symptom at 5 years. J Gen Intern Med. 2005;20(11):1032–1037. doi: 10.1111/j.1525-1497.2005.0241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olde Hartman TC, Borghuis MS, Lucassen PL, et al. Medically unexplained symptoms, somatisation disorder and hypochondriasis: course and prognosis. A systematic review. J Psychosom Res. 2009;66(5):363–377. doi: 10.1016/j.jpsychores.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 11.Steinbrecher N, Hiller W. Course and prediction of somatoform disorder and medically unexplained symptoms in primary care. Gen Hosp Psychiatry. 2011;33(4):318–326. doi: 10.1016/j.genhosppsych.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Budtz-Lilly A, Vestergaard M, Fink P, et al. Patient characteristics and frequency of bodily distress syndrome in primary care: a cross-sectional study. Br J Gen Pract. 2015 doi: 10.3399/bjgp15X686545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Creed FH, Davies I, Jackson J, et al. The epidemiology of multiple somatic symptoms. J Psychosom Res. 2012;72(4):311–317. doi: 10.1016/j.jpsychores.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Spitzer RL, Williams JB, Kroenke K, et al. Utility of a new procedure for diagnosing mental disorders in primary care: the PRIME-MD 1000 study. JAMA. 1994;272(22):1749–1756. [PubMed] [Google Scholar]
- 15.Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med. 2002;64(2):258–266. doi: 10.1097/00006842-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Ware JE, Jr, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Kroenke K, Spitzer RL, Williams JB. The PHQ–9. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 19.Marston L, Peacock JL, Calvert SA, et al. Factors affecting vocabulary acquisition at age 2 in children born 23–28 weeks gestation. Arch Dis Child. 2007;49:591–596. doi: 10.1111/j.1469-8749.2007.00591.x. [DOI] [PubMed] [Google Scholar]
- 20.Mallett S, Royston P, Waters R, et al. Reporting performance of prognostic models in cancer: a review. BMC Med. 2010;8(1):21. doi: 10.1186/1741-7015-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kroenke K, Spitzer RL, Williams JB, Löwe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32(4):345–359. doi: 10.1016/j.genhosppsych.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 22.De Gucht V, Fischler B, Heiser W. Personality and affect as determinants of medically unexplained symptoms in primary care: a follow-up study. J Psychiatr Res. 2004;56(3):279–285. doi: 10.1016/S0022-3999(03)00127-2. [DOI] [PubMed] [Google Scholar]
- 23.Gureje O, Simon GE. The natural history of somatization in primary care. Psychosom Med. 1999;29(3):669–676. doi: 10.1017/s0033291799008417. [DOI] [PubMed] [Google Scholar]
- 24.Van der Windt DA, Dunn KM, Spies-Dorgelo MN, et al. Impact of physical symptoms on perceived health in the community. J Psychiatr Res. 2008;64(3):265–274. doi: 10.1016/j.jpsychores.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Hilbert A, Martin A, Zech T, et al. Patients with medically unexplained symptoms and their significant others: illness attributions and behaviors as predictors of patient functioning over time. J Psychiatr Res. 2010;68(3):253–262. doi: 10.1016/j.jpsychores.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 26.Tomenson B, Essau C, Jacobi F, et al. Total somatic symptom score as a predictor of health outcome in somatic symptom disorders. Br J Psychiatry. 2013;203(5):373–380. doi: 10.1192/bjp.bp.112.114405. [DOI] [PubMed] [Google Scholar]
- 27.Kroenke K, Spitzer RL. Gender differences in the reporting of physical and somatoform symptoms. Psychosom Med. 1998;60(2):150–155. doi: 10.1097/00006842-199803000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Barsky AJ, Peekna HM, Borus JF. Somatic symptom reporting in women and men. J Gen Intern Med. 2001;16(4):266–275. doi: 10.1046/j.1525-1497.2001.00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Springer KW. Childhood physical abuse and midlife physical health: testing a multi-pathway life course model. Soc Sci Med. 2009;69(1):138–146. doi: 10.1016/j.socscimed.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barsky AJ, Wool C, Barnett MC, Cleary PD. Histories of childhood trauma in adult hypochondriacal patients. Am J Psychiatry. 1994;151(3):397–401. doi: 10.1176/ajp.151.3.397. [DOI] [PubMed] [Google Scholar]
- 31.Waldinger RJ, Schulz MS, Barsky AJ, Ahern DK. Mapping the road from childhood trauma to adult somatization: the role of attachment. Psychosom Med. 2006;68(1):129–135. doi: 10.1097/01.psy.0000195834.37094.a4. [DOI] [PubMed] [Google Scholar]
- 32.Van Boven K, Lucassen P, van Ravesteijn H, et al. Do unexplained symptoms predict anxiety or depression? Ten-year data from a practice-based research network. Br J Gen Pract. 2011. [DOI] [PMC free article] [PubMed]
- 33.Page LA, Wessely S. Medically unexplained symptoms: exacerbating factors in the doctor–patient encounter. J R Soc Med. 2003;96(5):223–227. doi: 10.1258/jrsm.96.5.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coulter A, Entwistle VA, Eccles A, et al. Personalised care planning for adults with chronic or long-term health conditions. Cochrane Database Syst Rev. 2013;(5):CD010523. doi: 10.1002/14651858.CD010523.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henningsen P, Zimmermann T, Sattel H. Medically unexplained physical symptoms, anxiety, and depression: a meta–analytic review. Psychosom Med. 2003;65(4):528–533. doi: 10.1097/01.psy.0000075977.90337.e7. [DOI] [PubMed] [Google Scholar]
- 36.Chitnis A, Dowrick C, Byng R, et al. Guidance for health professionals on medically unexplained symptoms. London: Royal College of General Practitioners, Royal College of Psychiatrists; 2011. [Google Scholar]