Abstract
Background/Aims
Kefir is a kind of fermented probiotic dairy product. The objective of the present study was to investigate the effects of kefir consumption on the fecal microflora and symptoms of patients with inflammatory bowel disease (IBD).
Materials and Methods
Kefir was serially diluted and inoculated into de Man, Rogosa, and Sharpe agar and incubated at 37°C for 48 to 72 h under anaerobic conditions. This was a single-center, prospective, open-label randomized controlled trial. Forty-five patients with IBD were classified into two groups: 25 for treatment and 20 for control. A 400 mL/day kefir was administered to the patients for 4 weeks day and night. Their stool Lactobacillus, Lactobacillus kefiri, content was quantitated by real-time quantitative polymerase chain reaction before and after consumption. Abdominal pain, bloating, stool frequency, stool consistency, and feeling good scores were recorded in diaries daily by the patients.
Results
A 5×107 CFU/mL count of lactic acid bacteria colony forming units was found in a kefir sample as the total average count. Lactobacillus bacterial load of feces of all subjects in the treatment group was between 104 and 109 CFU/g, and the first and last measurements were statistically significant (p=0.001 in ulcerative colitis and p=0.005 in Crohn’s disease (CD)). The L. kefiri bacterial load in the stool of 17 subjects was measured as between 104 and 106 CFU/g. For patients with CD, there was a significant decrease in erythrocyte sedimentation rate and C-reactive protein, whereas hemoglobin increased, and for the last 2 weeks, bloating scores were significantly reduced (p=0.012), and feeling good scores increased (p=0.032).
Conclusion
According to our data, kefir consumption may modulate gut microbiota, and regular consumption of kefir may improve the patient’s quality of life in the short term.
Keywords: Inflammatory bowel disease, probiotics, kefir, Lactobacillus, Lactobacillus kefiri
INTRODUCTION
Inflammatory bowel disease (IBD), encompassing both ulcerative colitis (UC) and Crohn’s disease (CD), is characterized by a chronic and relapsing inflammation of the gastrointestinal (GI) tract. UC and CD are generally described as chronic IBDs, although they are distinct diseases that differ in both symptoms and inflammation pattern (1).
The term probiotic means “for life”. A viable mono or mixed microorganism culture that can be applied to an animal or a human being positively affects the host by improving the properties of the native microflora (2).
Kefir is a sour, carbonated and fermented milk product. It is a natural probiotic that contains live active cultures of the normal intestinal flora. Most lactic acid bacteria (LAB) in kefir have been considered as probiotic bacteria, such as Lactobacillus kefiri, Lactobacillus casei, Lactobacillus kefiranofaciens, Pediococcus acidilactici, and Lactococcus lactis, and they have potentially imparting health benefits (3–4).
According to the Turkish Food Codex, kefir is a kind of fermented dairy product containing starter cultures or examples of kefir. These cultures use specific forms of L. kefiri, Leuconostoc, Lactococcus, and Acetobacter with lactose fermenting (Kluyveromyces marxianus) and nonfermenting (Saccharomyces unisporus, Saccharomyces cerevisiae, and Saccharomyces exiguous) bacteria. L. kefiri is a heterofermentative bacterium that determines one of the flavor characteristics of kefir drink (5–6).
CD is a serious immunity inflammatory disease that affects any part of the GI system, and the reason is still unknown. None of the treatments can heal the disease completely, but it is possible to keep it under control and enhance the quality of life.
UC is a chronic disease that is located in the mucosa of the large bowel and has recurrence and remission characteristics accompanied by inflammation and ulceration that can occur without any reason.
The human gut microbiota has a community of >100 trillion microbial cells that has been linked with GI conditions, such as IBD. For UC cases in the intestinal flora, Lactobacillus and Bifidobacterium decrease, whereas Bacteroides vulgatus and Fusobacterium increase. In addition, a decrease was reported in Lactobacillus and Bifidobacterium in the CD data (7). The most consistent change is the reduction in Firmicutes (8).
It is thought that in the enteropathogenesis of these illnesses, apart from genetic susceptibility, mucosal immune response disorder and the breakdown of the balance of the intestinal flora play significant roles. Probiotics are becoming increasingly popular. The use of oral probiotic cultures may improve intestinal disorders, such as UC (9).
Lactobacillus is the dominant flora of kefir and has probiotic properties, and L. kefiri is the characteristic microorganism of kefir, so they were selected for the study.
The aim of the present study was to determine the effects of kefir on CD and UC patient’s Lactobacillus flora and their biochemical properties as well as symptoms and quality of life.
MATERIALS AND METHODS
Subjects
The study was performed as an open-label randomized control, single-center, prospective trial. From May 2015 to December 2016, 45 (25 treatment and 20 control group) patients participated in this trial. Three patients left the trial willingly. A total of 45 (25 treatment and 20 control group, 23 male and 22 female) patients completed the study. The trial protocol was assessed and approved by the ethics committee. Written informed consent was obtained from all the participants before the entry into the trial.
Selection of patients
Patients with IBD participated in the study. In the trial, CD Activity Index for CD and Truelove-Witts scoring systems for UC were used for disease assessment scores (10–11). If the score was <450, patients with CD were admitted to the study. If the score was higher, patients with UC were not admitted to the study. Volunteers also had to be >18 years old. Patients with alcohol consumption >20 g/day, allergies or intolerance to milk, antibiotic treatment within the last 1 month, column or bowel operation history up to 3 months before the start of the study, and the presence of active infection within 1 month prior to the start of the study or during the study were excluded from the study. In addition, if a patient requested to leave on his/her own will, or if kefir was not consumed continuously for 2 weeks, the trial protocol was assessed and was not approved.
Treatment of patients
Eligible patients were selected randomly to receive one of the following treatments: 400 mL/day kefir was administered twice a day to the patients for 4 weeks, which contains a total of 2.0×1010 CFU/mL viable Lactobacillus bacteria (treatment group, 25 patients). Treatment was interrupted in case of disease relapse, occurrence of side effects, and poor compliance. Patients were requested to fill out the symptoms diary that has questionnaires of bowel habits. Abdominal pain and bloating were rated on a four-point scale with 0=none, 1=mild, 2=moderate, and 3=severe. Stool consistency results were rated on a daily basis as slurry/watery=0, mash consistency=1, medium watery=2, normal=3, hard feces=4, and very hard or lumpy=5. Feeling good score was rated as very poor=1, worse=2, moderate/normal=3, good=4, and very good=5.
All patients underwent blood analysis (hemoglobin (Hgb), C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR)), and the clinical activity index was calculated before and after the treatment.
The control group did not consume placebo because it was not possible to prepare a control product with a similar flavor, texture, and taste as those of kefir. Ayran and yogurt were similar to kefir, but they also have Lactobacillus and can affect the microbiota results.
Sample collection of feces
For measurement of the initial Lactobacillus quantity of feces, samples were obtained and stored at −20°C. After 4 weeks, patients were asked for a sample, and the stool was stored at −20°C at appropriate conditions for analysis.
Microbiological analysis of kefir
Kefir was serially diluted and inoculated into de Man, Rogosa and Sharpe (Oxoid CM361) agar and incubated at 37°C for 48 to 72 h under anaerobic conditions (anaerobic jars, Anaerocult C Merck) for LAB and Potato Dextrose Agar (Oxoid CM139) at 22°C for 5 days for yeast.
Isolation and identification of kefir Lactobacillus
The colonies obtained in the tests were cultured and purified. Pure bacterial cultures for species identification of Lactobacillus isolates were performed by Vitek® MS MALDI-TOF mass spectrometer (BioMerieux, Marcy I’Etoile, France).
At the same time, the isolates were identified using the API 50 CHL (BioMerieux) test.
PCR analysis of feces
Quantification of Lactobacillus bacteria from human stool samples was performed via real-time quantitative polymerase chain reaction (qPCR) in a culture-independent manner.
Total DNA of the stool was extracted using the Stool DNA Isolation Kit (QIAamp DNA Stool Kit) according to the manufacturer’s instructions. Lactobacillus primers’ specificity and optimization were performed by PCR amplification on a Thermal Cycler T-100 (Bio-Rad, Istanbul, Turkey). The amplified products were analyzed via gel electrophoresis according to size. The amplified region was confirmed as Lactobacillus by the Sanger sequencing method. All of the samples were studied on real-time PCR for both Lactobacillus and L. kefiri quantification separately. The Lactobacillus quantity in the stool samples was analyzed using the Roche LightCycler Nano Software (Bio-Rad) on real-time q-PCR device. For quantification of experiments, a standard curve of positive controls at different known concentrations was used. For positive control, the Lactobacillus gene Lactobacillus rhamnosus strain with the code CECT278ATCC7469 was used. The L. kefiri strain used is ATCC35411 in standard curve experiments. Primers for Lactobacillus spp. were designed according to Wang et al. (2011). L. kefiri primers were designed to be flat/reverse by taking the nucleotide sequences in the National Center for Biotechnology Information as reference (12).
The reaction conditions for PCR amplification were 95°C for 10 min; 40 cycles of 95°C for 1 min, 50°C for 1 min, and 72°C for 1 min; and final elongation at 72°C for 10 min. The quantification protocol used to identify the abundance of fecal L. kefiri was according to Castillo et al. (13).
Statistical analysis of symptom diaries
Statistical analysis of symptom diary data was made using the SPSS 23.0 statistical package program. The Shapiro-Wilk test was used to determine whether the test was normal or not. The Mann-Whitney U test was used for normal distribution data. The Wilcoxon signed-rank test was used to compare dependent samples. The Fisher’s exact, chi-square, and Fisher-Freeman-Halton tests were used to examine the categorical data. For analysis of repeated measures, percent change value (percent change=(last measurement-first measurement)/first measurement) according to the initial measurement was calculated and compared among the groups. Significance level was set at α=0.05.
RESULTS
In IBDs, it is necessary to improve the quality of life. In our study, we aimed to elucidate the effects of kefir, which is a kind of probiotic food, on the intestinal microflora. In addition, we aimed to find kefir’s effects on the quality of life of patients who have CD and UC that have not been investigated in humans previously with IBDs.
We investigated and compared the effects of fermented kefir drink on the changes of feces Lactobacillus flora and L. kefiri of patients with CD and UC and the effects of their biochemical parameters and symptoms. We found that regular kefir usage may improve both the symptoms and the quality of life in the short term in patients with CD and have a positive effect on the biochemical parameters of patients, such as Hgb, ESR, and CPR.
Twenty-five patients as treatment group and twenty patients as control group completed a total of 4 weeks. A 5×107 CFU/mL count of LAB colony forming units was found in a kefir sample as the total average count. A 2.1×104 CFU/mL yeast was found in a kefir sample as the total average count.
Identification of Lactobacillus strains of kefir
Overall, 10 Gram-positive, catalase-negative, rod-shaped isolates were obtained from kefir drink. The LP 1, LP 6, and LP 5b isolates were identified as Lactobacillus pentosus, LB 2 and LB 3 isolates were identified as Lactobacillus brevis, LPL 4 and LPL 5 isolates were identified as Lactobacillus plantarum, LF 7 isolate was identified as Lactobacillus fermentum, LK 9 isolate was identified as L. kefiri, and LL 10 isolate was identified as Lactobacillus lindneri, respectively, using the API 50 CHL test and Vitek® MS MALDI-TOF mass spectrometer. Therefore, we found six different strains of lactobacilli consisting of L. pentosus, L. brevis, L. plantarum, L. fermentum, L. kefiri, and L. lindneri.
Analysis of feces (treatment and control groups of patients) results
The composition of the fecal Lactobacillus microflora of 25 patients was monitored before and after the administration of kefir containing Lactobacillus (daily dose, 2.0×1010 CFU/day). After 1 month of kefir administration, the Lactobacillus amount in the stool of all subjects was between 104 and 109 CFU/g. The L. kefiri bacterial load in the stool of 17 subjects was measured between 104 and 106 CFU/g. The total amount of Lactobacillus in the treatment group of patients with CD was 106–107 CFU/g for all subjects and between 0 and 106 CFU/g for L. kefiri. The amount of Lactobacillus in the control group of patients shown in Table 1 and Figure 1. with CD was found to be between 0 and 107 CFU/g. L. kefiri was found in the range of 0–103 CFU/g. It was not found in 7 out of 10 patients. The amount of Lactobacillus in the treatment group of patients with UC was found to be 104–109 CFU/g for all subjects and 0–105 CFU/g for L. kefiri. The amount of Lactobacillus in the control group of are shown in Table 2 and Figure 2. with UC was found to be 0–105 CFU/g for all subjects. Lactobacillus was found to be 0–106 CFU/g in 18 patients for kefir. L. kefiri was not found in 6 out of 10 patients. Demographic and clinic properties of treatment groups are displayed in Table 3.
Table 1.
Comparison between patients fecal Lactobacillus population and Lactobacillus kefiri count before and after kefir comsumption CD treatment groups and control groups
| No | Microorganisms | Crohn’s Disease Treatment Group (log10) | No | Microorganisms | Crohn’s Disease Control Group (log10) | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| 0.day | 28.day | 0.day | 28.day | ||||
| CDT1 | Lactobacillus | 4.65 | 7.95 | CDC1 | Lactobacillus | 0 | 7.39 |
| Lactobacillus kefiri | 3.41 | 5.95 | Lactobacillus kefiri | 0 | 4.88 | ||
| CDT2 | Lactobacillus | 0 | 6.62 | CDC2 | Lactobacillus | 4.69 | 4.92 |
| Lactobacillus kefiri | 0 | 6.15 | Lactobacillus kefiri | 0 | 0 | ||
| CDT3 | Lactobacillus | 0 | 6.65 | CDC3 | Lactobacillus | 5.47 | 5.63 |
| Lactobacillus kefiri | 0 | 0 | Lactobacillus kefiri | 4.29 | 4.20 | ||
| CDT4 | Lactobacillus | 4.2 | 6.36 | CDC4 | Lactobacillus | 5.37 | 4.2 |
| Lactobacillus kefiri | 0 | 0 | Lactobacillus kefiri | 0 | 0 | ||
| CDT5 | Lactobacillus | 0 | 6.43 | CDC5 | Lactobacillus | 0 | 0 |
| Lactobacillus kefiri | 0 | 4.69 | Lactobacillus kefiri | 0 | 0 | ||
| CDT6 | Lactobacillus | 0 | 6.91 | CDC6 | Lactobacillus | 4.71 | 4.95 |
| Lactobacillus kefiri | 0 | 4.45 | Lactobacillus kefiri | 2.70 | 0 | ||
| CDT7 | Lactobacillus | 4.47 | 6.63 | CDC7 | Lactobacillus | 6.43 | 6 |
| Lactobacillus kefiri | 0 | 4.59 | Lactobacillus kefiri | 0 | 0 | ||
| CDT8 | Lactobacillus | 0 | 6.53 | CDC8 | Lactobacillus | 4.65 | 4.64 |
| Lactobacillus kefiri | 0 | 5.04 | Lactobacillus kefiri | 4.2 | 3.95 | ||
| CDT9 | Lactobacillus | 0 | 6.89 | CDC9 | Lactobacillus | 5.89 | 5.18 |
| Lactobacillus kefiri | 0 | 5.48 | Lactobacillus kefiri | 0 | 0 | ||
| CDT10 | Lactobacillus | 5.99 | 7.82 | CDC10 | Lactobacillus | 5.83 | 5.43 |
| Lactobacillus kefiri | 5.69 | 5.98 | Lactobacillus kefiri | 0 | 0 | ||
Figure 1.
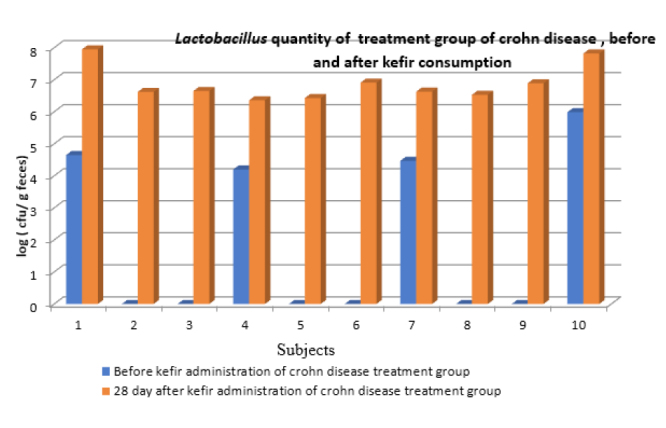
Lactobacillus quantity of treatment group Crohn disease treatment group; before and after kefir consumption
Table 2.
Comparison between patients fecal Lactobacillus population and Lactobacillus kefiri count before and after kefir consumption UC treatment groups and control groups
| No | Microorganisms | Ulcerative Colitis Treatment Group (log10) | No | Microorganisms | Ulcerative Colitis Control Group (log10) | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| 0.day | 28.day | 0.day | 28.day | ||||
| UCT1 | Lactobacillus | 4.93 | 5.54 | UCC1 | Lactobacillus | 5.91 | 0 |
| Lactobacillus kefiri | 0 | 0 | Lactobacillus kefiri | 4.83 | 0 | ||
| UCT2 | Lactobacillus | 0 | 9.17 | UCC2 | Lactobacillus | 3.93 | 4.31 |
| Lactobacillus kefiri | 0 | 5.8 | Lactobacillus kefiri | 0 | 4.4 | ||
| UCT3 | Lactobacillus | 4.65 | 5.59 | UCC3 | Lactobacillus | 3.81 | 6.29 |
| Lactobacillus kefiri | 0 | 0 | Lactobacillus kefiri | 2.95 | 4.4 | ||
| UCT4 | Lactobacillus | 0 | 6.22 | UCC4 | Lactobacillus | 3.85 | 4.6 |
| Lactobacillus kefiri | 0 | 4.55 | Lactobacillus kefiri | 0 | 0 | ||
| UCT5 | Lactobacillus | 4.6 | 5.81 | UCC5 | Lactobacillus | 4.92 | 4.2 |
| Lactobacillus kefiri | 4.07 | 5.04 | Lactobacillus kefiri | 0 | 0 | ||
| UCT6 | Lactobacillus | 0 | 6.11 | UCC6 | Lactobacillus | 4.65 | 4.07 |
| Lactobacillus kefiri | 0 | 5.97 | Lactobacillus kefiri | 2.80 | 3.00 | ||
| UCT7 | Lactobacillus | 0 | 6.46 | UCC7 | Lactobacillus | 5.66 | 5.58 |
| Lactobacillus kefiri | 0 | 0 | Lactobacillus kefiri | 4.82 | 0 | ||
| UCT8 | Lactobacillus | 5.58 | 5.66 | UCC8 | Lactobacillus | 4.07 | 5.81 |
| Lactobacillus kefiri | 0 | 0 | Lactobacillus kefiri | 2.85 | 2.80 | ||
| UCT9 | Lactobacillus | 4.68 | 4.91 | UCC9 | Lactobacillus | 5.47 | 5.15 |
| Lactobacillus kefiri | 4.54 | 4.2 | Lactobacillus kefiri | 0 | 0 | ||
| UCT10 | Lactobacillus | 0 | 4.71 | UCC10 | Lactobacillus | 4.55 | 5.39 |
| Lactobacillus kefiri | 0 | 0 | Lactobacillus kefiri | 0 | 0 | ||
| UCT11 | Lactobacillus | 0 | 5.38 | ||||
| Lactobacillus kefiri | 0 | 5.15 | |||||
| UCT12 | Lactobacillus | 6.43 | 6.65 | ||||
| Lactobacillus kefiri | 0 | 5.78 | |||||
| UCT13 | Lactobacillus | 6.14 | 6.17 | ||||
| Lactobacillus kefiri | 0 | 5.53 | |||||
| UCT14 | Lactobacillus | 0 | 5.18 | ||||
| Lactobacillus kefiri | 0 | 0 | |||||
| UCT15 | Lactobacillus | 0 | 5.83 | ||||
| Lactobacillus kefiri | 0 | 5.15 | |||||
Figure 2.
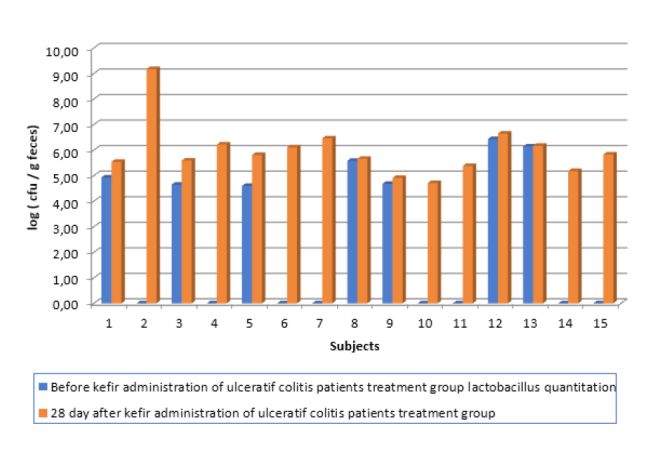
Lactobacillus quantity of treatment group ulcerative colitis disease treatment group; before and after kefir consumption
Table 3.
Demographic and clinic properties of treatment groups
| Ulceratif Crohn’s (n=15) | Colitis Disease (n=10) | p | |
|---|---|---|---|
| Age (median(min-max)) year | 33 (19;68) | 33 (24;65) | 0.643 |
| Gender | |||
| Male | 9 (%60) | 4 (%40) | 0.428 |
| Female | 6 (%40) | 6 (%.60) | |
| Involment place (Localition) | |||
| Colon | 15 (%100) | 1 (%10) | <0.001 |
| Ileum | 0 (%0) | 6 (%60) | |
| Colon+Ileum | 0 (%0) | 3 (%30) | |
| Age of illness (median (min-max)) year | 4 (1;12) | 2 (1;9) | 0.129 |
| Total Kefir consumption (median(min-max)) liter | 11.2 (9.4;11.2) | 11.2 (9.6;11.2) | 0.683 |
| Lactobacillus first measurement (Range) | 0 (0–271.3) | 0 (0–99.1) | 1.000 |
| Lactobacillus last measurement (% change) | 1.87 (0.19–53.4) | 3.4 (0.44–13.7) | 0.914 |
| HGB first measurement | 11.7 (10.6–15.8) | 12.7 (9.3–15.5) | 0.723 |
| HGB last measurement (% change) | 0.05 (−0.09;0.17) | 0.08 (−0.04;0.24) | 0.567 |
| ESR firs measurement | 25 (5;59) | 29 (12;59) | 0.428 |
| ESR last measurement (% change) | −0.15 (−.71;1.18) | −0.20 (−0.53;0.69) | 0.643 |
| CRP first measurement | 0.33 (0.3;6) | 1.1 (0.3;8) | 0.048 |
| CRP last measurement (% change) | −0.06 (−0.94;2.52) | −0.60 (−0.96;0.72) | 0.103 |
Biochemical parameters and symptom diary results
For biochemical parameters, patients with CD showed statistically significant differences in terms of all variables after kefir use. There was a significant decrease in ESR and CRP, whereas patients with CD showed an increase in Hgb after kefir use. The increase in Hgb measurements was found to be higher in patients with CD than in the CD control group (p=0.024 and p=0.029) which is shown in Table 5.
Table 5.
Demographic and clinic properties of treatment and control groups of Crohn’s disease
| Crohn Disease Treatment Group (n=10) | Crohn Disease Control Group (n=10) | p | |
|---|---|---|---|
| Age (median(min-max)) year | 33 (24;65) | 42 (21;66) | 0.529 |
| Gender | |||
| Male | 4 (%40) | 6 (%60) | 0.656 |
| Female | 6 (%.60) | 4 (%40) | |
| Involment Place (Localition) | |||
| Colon | 1 (%10) | 0 (%0) | 0.628 |
| Ileum | 6 (%60) | 10 (%100) | |
| Colon + Ileum | 3 (%30) | 0 (%0) | |
| Age of illness (median (min-max)) year | 2 (1;9) | 2 (1;10) | 0.971 |
| Total Kefir Consumption (median(min-max)) Liter/4 week | 11.2 (9.6;11.2) | - | - |
| Lactobacillus first measurement (Range) | 0 (0;99.1) | 14.26 (0;271.3) | 0.143 |
| Lactobacillus last measurement (% change) | 3.4 (0.44;13.7) | −0.6 (−0.93;0.74) | 0.024 |
| HGB first measurement | 12.7 (9.3;15.5) | 13.2 (10.6;15.9) | 0.481 |
| HGB last measurement (% change) | 0.08 (−0.04;0.24) | −0.01 (−0.13;0.15) | 0.029 |
| ESR first measurement | 29 (12;59) | 20.5(3;89) | 0.353 |
| ESR last measurement (% change) | −0.20 (−0.53;0.69) | −0.09 (−0.77;0.67) | 0.393 |
| CRP first measurement | 1.1 (0.3;8) | 0.40 (0.31;10.80) | 0.481 |
| CRP last measurement (% change) | −0.60 (−0.96;0.72) | −0.18 (−0.97;4.48) | 0.190 |
Demographic and clinic properties of treatment and control groups of ulcerative colitis are shown in Table 4. According to the symptoms diary for patients with CD, the last 2 weeks of bloating was significantly reduced (p=0.012). At the same time, the feeling good score improved in the last 2 weeks, and patients’ conditions improved (p=0.032). The feeling good score was significantly higher in patients with CD as the abdominal pain score was significantly lower in patients with CD in the last 2 weeks than in patients with UC. No statistically significant difference was found between weeks 1 and 2 in patients with UC in terms of abdominal pain, bloating, frequency of stools, defecation consistency, and feeling good. A statistically significant difference was observed between the abdominal pain score (p=0.049) and the feeling good score (p=0.019) in the last 2 weeks when the symptom diary data were compared between patients with CD and UC. According to this, the rate of feeling good was significantly higher in patients with CD as the abdominal pain score was lower in the last 2 weeks than in patients with UC. None of the patients in either of the groups had worsening of disease symptoms. No side effects were observed in all of the subjects.
Table 4.
Demographic and clinic properties of treatment and control groups of ulcerative colitis
| Ulcerative Colitis Treatment Group Group (n=15) | Ulcerative Colitis Control Group Group (n=10) | p | |
|---|---|---|---|
| Age (median(min-max)) year | 33 (19;68) | 43.5 (29;76) | 0.041 |
| Gender | |||
| Male | 9 (%60) | 4 (%40) | 0.428 |
| Female | 6 (%40) | 6 (%60) | |
| Involment Place (Localition) | |||
| Colon | 15 (%100) | 10 (%100) | - |
| Ileum | 0 (%0) | 0 (%0) | |
| Colon + Ileum | 0 (%0) | 0 (%0) | |
| Age of illness (median (min-max)) year | 4 (1;12) | ||
| Total Kefir Consumption (median(min-max)) liter/ 4 week | 11.2 (9.4;11.2) | - | - |
| Lactobacillus first measurement (Range) | 0 (0;271.3) | 4.04 (0.65;81.3) | 0.048 |
| Lactobacillus last measurement (% change) | 1.87 (0.19;53.4) | 0.62 (−1;299.02) | 0.428 |
| HGB first measurement | 11.7 (10.6;15.8) | 12.35 (8.5;15) | 0.531 |
| HGB last measurement (% change) | 0.05 (−0.09;0.17) | 0.02 (−0.06;0.49) | 0.807 |
| ESR first measurement | 25 (5;59) | 25.5 (17;64) | 0.367 |
| ESR last measurement (% change) | −0.15 (−0.71;1.18) | −0.19 (−0.6;0.88) | 1.000 |
| CRP first measurement | 0.33 (0.3;6) | 0.78 (0.31;18.70) | 0.461 |
| CRP last measurement (% change) | −0.06 (−0.94;2.52) | −0.44 (−0.93;5.48) | 0.531 |
According to the results we obtained, it was determined that in some patients using kefir, there was a statistically significant improvement in abdominal pain, bloating, and quality of life when compared with the control group. The feeling good score was significantly higher in patients with CD when the abdominal pain score was significantly lower in patients with CD than in patients with UC in the last 2 weeks. A statistically significant difference was found in terms of bloating and feeling good when the symptom log data of the first 2 weeks and the last 2 weeks of patients with CD were examined.
DISCUSSION
The mean count of lactobacilli in some studies was similar to our study, with 8 log CFU/mL, 7.2 log CFU/mL, and 1.2×107 CFU/mL of lactobacilli, respectively, in kefir (14,15,16). A 5×107 CFU/mL count of LAB colony forming units was found in a kefir sample as the total average count in our study.
In the present study, L. pentosus, L. brevis, L. plantarum, L. fermentum, L. kefiri, and L. lindneri were isolated from kefir. The most common lactobacilli isolated from kefir grains as reported by other studies are: L. brevis, L. kefir, Lactobacillus acidophilus, L. plantarum, L. kefiranofaciens, Lactobacillus kefirgranum, and Lactobacillus parakefiri. The LAB isolated from kefir in our study were the same as the following studies: L. fermentum Witthuhn et al. (16); L. kefiri Bosch et al. (17), Kesmen and Kacmaz (19), and Magalhaes et al. (20); L. plantarum Garrote et al. (18) and Witthuhn et al. (16); and L. brevis Simova et al (21). and Witthuhn et al. (16).
We isolated L. kefiri from kefir. Pintado et al. isolated L. kefiri from Portuguese kefir by using API 50 as the same. Chen et al. also identified L. kefiri from the kefir in Taiwan (22,23).
Our data indicated that the selected LK 9 L. kefiri strains were colonized in the gut of this study of patients. As similarly seen in the study by Toscano et al., after 1 month of L. kefiri LKF01 administration, the Lactobacillus strain was detected in the feces of all subjects participating in our study with a bacterial load of 105–106 CFU/g. According to the same study, L. kefiri showed a strong ability to modulate the gut microbiota composition, leading to a significant reduction of several bacterial genera directly involved in the onset of proinflammatory response and GI diseases (24).
According to Braat et al., there was a decrease in the number of CRP levels of patients with CD consuming L. lactis for 1 week (25). In our study, CRP levels decreased after a 28-day kefir consumption of patients with CD, and it was statistically significant (p=0.015). The number of studies evaluating the immunomodulatory properties of probiotics is increasing. The immunomodulatory properties of kefir may be due to the direct action of the microbicide or may be indirect through different bioactive compounds produced during the fermentation process (25). The immunomodulatory effect of kefir may be attributed to its ability to reduce or repair intestinal permeability of these probiotics. Thus, contact between the antigens in the host and intestinal lumen is reduced, which can reduce the inflammatory response (26). IBD is associated with the intestinal microflora. In humans with IBD, there are a low number of lactobacilli and bifidobacteria and a large number of anaerobic bacteria. Treatment is performed using probiotics to help the patient maintain the remission period (27). In the intestines of individuals with IBD, the numbers of Lactobacillus and Bifidobacterium are lower, and anaerobes are higher. Probiotics do not cure the disease; however, after some time, they may prolong the remission period. This increases the quality of life of patients (25). According to data from our study, a statistically significant difference was observed in abdominal pain score (p=0.049) and feeling good score (p=0.019) for patients who consumed kefir, which contains probiotics. They have positive effects on diseases caused by an imbalance of the intestinal microflora (28).
Some studies show that probiotics have effects on patients with UC and CD (29). According to Tursi et al. (2010), VSL # 3 probiotic mixture reinforcement is safe and can reduce the UC Disease Activity Index (UCDAI) scores in patients affected by mild to moderate UC treated with 5-aminosalicylic acid and/or immunosuppressants. In addition, it improves rectal bleeding and regenerates remission in patients with recurrent UC after 8 weeks of treatment. However, these parameters do not reach statistical significance (30).
The study was performed in a small open-label study in patients with active UC. Compared with 10 patients treated with inactivated bacteria given live L. plantarum 299v, 6 out of 9 patients reached remission (31).
Patients with relapses with mild to moderate UC were treated with 3×250 mg/day probiotic Saccharomyces boulardii for 4 weeks. A 68% remission rate was observed (32).
Patients with UC who were on remission in a placebo-controlled study using fermented pills containing 1×1010 CFU Bifidobacterium breve, Bifidobacterium bifidum, and L. acidophilus were given 100 mL milk for 12 months. At the end of the study period, 73% of patients in the fermented milk group remained in remission, whereas the number was 10% for the placebo group, and a significant difference was detected in clinical remission; however, no difference was found 1 year after colonoscopy (33).
One of the other studies was the one which forty patients with clinical and endoscopic remissions participated in the randomized, placebo-controlled trial. VSL # 3 was infected with 6 g/day for 9 months. Fecal samples showed significantly increased fecal concentration of Lactobacillus, bifidobacteria, and Streptococcus thermophilus after pretreatment and treatment (p<0.01) only in baseline levels in the VSL # 3 treated group (34).
We also found that the amount of Lactobacillus in patients’ feces at the end of 1 month of kefir consumption was between 104 and 109 CFU/g for all subjects. For L. kefiri, it was found to be between 104 and 106 CFU/g in 17 patients, and the change in the amount of Lactobacillus was significant.
In one study related to lactose intolerance, a group of subjects were fed low-fat milk, and another group was fed with kefir. The subjects have lactose intolerance. Lactose intolerance is caused by low β-galactosidase (lactase) activity in the intestine. Diarrhea and pain in the abdomen were observed in the milk group, but these effects were not observed in the kefir group (35). In lactose intolerance, individuals have an osmotic effect by lactose fermentation, which is not digested due to enzyme deficiency, and lactose and methane, hydrogen, and organic acids emerge, which cause discomfort. Dairy products can cause gas and bloating in patients with CD and UC. Nevertheless, since kefir has Lactobacillus that degrades lactose in the intestines, no one complained about lactose intolerance symptoms, such as abdominal pain and gas, in our study (36). Patients with CD and UC who cannot consume dairy products can easily consume kefir, and they do not feel uncomfortable and cannot stay away from calcium source.
In an experiment on 10 patients with IBD, VSL # 3 probiotic mixture was administered to the patients for 2 months, and the stool was analyzed by PCR. As a result, colonization of S. thermophilus, Bifidobacterium infantis Y1, and B. breve Y8 strains was found to be similar to healthy individuals (37).
One study was conducted to directly detect S. thermophilus in human feces, except culture-based techniques or DNA isolation and purification procedures with culture-independent PCR protocol. The persistence of S. thermophilus in the intestines of 10 healthy subjects who were given VSL # 3 or yogurt was investigated. The bacteria sought after 3 days of administration were detected and continued to be found 6 days after treatment suspension.
Manichanh et al. (38) found a significant decrease in the Clostridium family in patients with CD using the DNA microarray-based analysis method, but no significant variation was found in the Bacteroides family.
A 16S rDNA-column library index method was used in the study by Gophna et al. (39) for the analysis of IBD intestinal microbiota. In conclusion, a decrease in the number of Bacteroidetes and Proteobacteria in CD, but a decrease in the Clostridium family, was observed.
The general composition of the intestine is considered most relevant in the etiology and pathogenesis of IBD. However, microbiota analyses are long and labor intensive, and as a result, only cultivable bacterium can detect 20%–30% of microbiota. Owing to complex anaerobic environment requirements, the rest cannot be cultured. Therefore, molecular approaches are widely used for microbiota analysis (40).
In a study investigating whether the fecal microbiome of patients with UC and CD differed from healthy individuals, studies using terminal restriction fragment length polymorphism analysis showed differences. However, the intestinal microbiology of patients with inactive UC is similar to that of healthy individuals. Identification of the intestinal mechanisms of these patients and changes in microbiota structure may contribute to the development of new treatment options for patients with UC and CD (40).
When constantly consumed, the lactobacilli in the kefir settle in the intestines and produce acid components that correct the microflora against the pathogenic bacteria, thus the diseased bacteria can be removed (41).
Although pathogenic bacteria, such as Salmonella and Shigella, have been associated with the presence of kefir starter, these pathogens have not been developed (42). In addition, LAB and yeast present in the microflora have an inhibitory effect on kefir intestinal microorganisms (43). Kefir reduces the time of transit time by allowing feces to be easily thrown away. When antibiotic therapy is applied, it improves the irregular bowel flora (41).
Patients with UC and CD who started to use kefir in our study were seen to have been colonized by kefir probiotics according to the first week and the last 2 weeks when they started to establish a positive balance in the gut. Since the results in the literature are mostly obtained by different symptom evaluation methods, we are unable to make a direct comparison with data from our study.
In our study, the decrease in abdominal pain and bloating scores in the IBD group compared with the control group was similar to Nagendra and Shah (44).
The effect of S. boulardii was also investigated in a study on the effect of CD. Patients who were in remission from CD have been treated with idiopathic remedies. In this treatment, mesalamine was administered to a group of 3×g/day. The other group was S. boulardii for 1 month and 2×1 g/day mesalamine for 6 months. The remission rate in the group administered only mesalamine was 38%. The remission rate for mesalamine and S. boulardii was 94% (32).
In patients with CD, there are experiments with Lactobacillus salivarius UCC118 and Lactobacillus GG as probiotics. The results obtained for these patients are not sufficient, nonetheless promise future work.
In a meta-analysis, probiotics, which failed to prevent remission in CD and prevent clinical and endoscopic recurrence, have been recommended to use probiotic preparations containing a mixture of Lactobacillus, Escherichia coli, or Saccharomyces (45).
A pilot study by Gupta et al. showed that Lactobacillus GG can increase the intestinal barrier function in children with mild to moderate active CD (46).
In a double-blind, randomized, controlled study with Lactobacillus GG, children with CD did not prolong their recurrence time (47).
Saccharomyces boulardii with mesalazine has been found to be effective only in the recurrence control group when administered mesalazine (32).
In the study conducted by Steed et al. in 2010, by reviewing patients with active CD, they were given a symbiotic containing Bifidobacterium longum and as a result found to be effective when compared with the placebo. In the treatment of CD, randomized, controlled trials have proven the effectiveness of probiotics (48).
In our study of the microbial analysis of feces, the kefir treatment group showed significantly higher fecal lactobacilli count than the control group. This has been attributed to their ability to survive at low pH and high bile concentration as in in vitro experiments. These potentially probiotic bacteria colonizing the intestinal mucosa provide a barrier to pathogens through various mechanisms, competition for nutrients, and the production of antimicrobials.
According to Toscane et al. (24), L. kefiri appears to be effective and safe to maintain remission in patients with UC and may be a good treatment option for preventing relapse in this group of patients. L. kefiri LKF01 demonstrated a strong ability in modulating the intestinal microbiota composition, leading to a significant decrease in several bacterial generations at the onset of direct proinflammatory response and GI disorders.
Although the etiology of CD is uncertain, evidence suggests the involvement of intestinal bacteria, and studies have shown that bacterial, fusobacteria, enterococci, E. coli, and fewer bifidobacteria, lactobacilli, eubacteria, Clostridium coccoides, and Clostridium leptum showed higher concentrations in patients with CD. In Faecalibacterium prausnitzii and remission from healthy individuals, populations of fecal bacteria changed (48).
Probiotics can effectively protect UC remission, but little is known about their ability to induce remission. Adult patients with mild to moderate UC were randomized to receive 3.6×1012 CFU VSL # 3 (n=77) twice daily for 12 weeks and placebo (n=70). In the UCDAI, a reduction of 50% was achieved at 6 weeks. UCDAI is a measure of the degree of fecal incidence, rectal bleeding, mucosal appearance, and disease activity of the physician. The percentage of patients with a >50% improvement in the UCDAI score at week 6 was compared with the placebo-treated group (10%; 0.001) in the VSL # 3 given group (25% vs. 32.5%). At week 12, 33 (42.9%) patients receiving VSL # 3 entering remission were compared with 11 (15.7%) placebo patients (p<0.001). In addition, it was observed that the number of patients given VSL # 3 (40%; 51.9%) decreased by 3 points in UCDAI compared with placebo (13%; 18.6%) (p<0.001). The VSL # 3 group showed significantly greater reductions in UCDAI scores and symptoms at 6 and 12 weeks compared with the placebo group (49).
Other studies have confirmed that probiotic bacteria may increase the integrity of tight junctions between intestinal epithelial cells during infections or inflammatory conditions. For this reason, colonization with probiotic bacteria may cause exposure of immune cells to bacterial antigens believed to induce IBD. Experimental colitis showed that the protective effects of probiotic microorganisms (VSL # 3) in a dextran sulfate sodium model were mediated by DNA as recognized by the mucosal Toll-like receptor 9 receptor. This interaction subsequently led to increased endogenous production of bacterial survival beta-defensin and antibacterial peptides. In addition, it has been reported that treatment of VSL # 3 cultured intestinal epithelial cells leads to an increase in transepithelial electrical resistance, a change associated with reduced permeability. In the present study, incubation of intestinal epithelial cells with this probiotic consortium also induced the expression of various mucins, resulting in decreased adhesion of microorganisms and components to the epithelial surface (50). According to our study, probiotics have been evaluated in animal models and in some clinical trials. Oral administration of probiotics with VSL # 3 has been shown to normalize the interleukin 10 barrier function in IBD mice. VSL # 3 is a probiotic cocktail consisting of eight different Gram-positive organisms. Many studies on kefir’s biological activities have revealed that kefir has anti-inflammatory, immunomodulatory, and antimicrobial activities and is a functional food (51). Regular kefir consumption is associated with lactose intolerance and tolerance; antibacterial effect; hypocholesterolemic effect; control of plasma glucose; antihypertensive and anti-inflammatory effects; antioxidant, anticarcinogenic, and anti-allergic activities; and healing effects. Much of the work supporting these findings has been made in vitro or in animal models (52). All studies show that probiotics may play an important role in the management of IBD in the future, despite the fact that current clinical trials do not have statistical power, probably due to limited data. The availability of new techniques to better understand bacterial and host interactions and to better define the microbiota modification in different clinical subclasses may be a key to the success of effective probiotic therapy in patients with IBD (50).
Study disadvantages and limitations
Our study has some limitations. Moreover, the literature on IBD data is insufficient to reach at definite conclusions about the changes in the quality of life. Short-term kefir consumption and changes in the quality of life in our study may not have been revealed by patients. Inadequate number of patients may prevent the statistical significance of the changes.
The small sample size and short time are major weak points of the present study; however, it is very difficult for patients who have UC and CD to consume anything due to their illness. They especially want to know the effect of the symptoms of the diseases before consuming a different food. The lack of study on kefir was also questioned by the patients. One other limitation of our study was that the questionnaires were self-administered by the patients.
One advantage of our study was that we performed both feces analysis and concurrent assessment of bloating, defecation consistency, defecation, and feeling good scores with biochemical parameters at the same time. We also measured the severity of symptoms.
According to data from our study, regular kefir usage may improve both symptoms and quality of life in the short term in patients with IBD. The actual effects of probiotics on intestinal ecology are still to be discussed, as differences in microbial strains have a number of factors to be explored, such as their concentration and formulations.
Kefir has a tart, creamy flavor and apart from having a high nutritional value, it is also known to have a probiotic effect (53). Probiotic bacteria should be produced as an alternative to industrial probiotics through non-transgenic microorganisms isolated from natural food products such as kefir (54).
There are many useful probiotic microorganisms in kefir. It is easy to find and is inexpensive. We investigated the undefined effects of kefir in patients with IBD, Lactobacillus and L. kefiri flora of feces, and biochemical parameters and disease symptoms. Further studies are needed to evaluate the best dose-response effect of kefir, including monitoring patients to assess the persistence of potential beneficial effects in patients with CD and UC following kefir intervention. Unfortunately, countless human research conducted with kefir is often poorly designed. More human studies should be conducted to demonstrate the effect of kefir consumption and reduce the risk of disease. In addition, the actual effects of probiotics affecting intestinal ecology should be investigated, and advanced studies should be conducted on disease-specific food product formulations with customized studies on microbial strains in well-designed randomized clinical trials. The trials should continue on greater patient populations.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Uludağ University School of Medicine Clinical Research Ethics Committee (Decision No: B.30.2.ULU.0.20.70.02-050.99/440, Decision Date: 25.11.2013)
Informed Consent: Written informed consent was obtained from the subjects who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - İ.Y., M.E.D., H.Ö.; Design - İ.Y., M.E.D., H.Ö.; Supervision - İ.Y., M.E.D., H.Ö.; Materials - M.E.D.; Data Collection and/or Processing - İ.Y.; Analysis and/or Interpretation - İ.Y., M.E.D., H.Ö.; Literature Review - İ.Y., M.E.D.; Writing Manuscript - İ.Y.; Critical Review - İ.Y., M.E.D., H.Ö.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Guinane M, Cotter D. Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ. Therap Adv Gastroenterol. 2013;6:295–308. doi: 10.1177/1756283X13482996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schrezenmeir J, de Vrese M. Probiotics, prebiotics, and synbiotics-approaching a definition. Am J Clin Nutr. 2001;73:361–4. doi: 10.1093/ajcn/73.2.361s. [DOI] [PubMed] [Google Scholar]
- 3.Altay F, Karbancıoglu Güler F, Daskaya Dikmen C, Heperkan D. A review on traditional Turkish fermented non-alcoholic beverages: Microbiota, fermentation process and quality characteristics. Int J Food Microbiol. 2013;167:44–56. doi: 10.1016/j.ijfoodmicro.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 4.World Gastroenterology Organisation practice guideline: Probiotics and Prebiotics. 2008. [DOI] [PubMed] [Google Scholar]
- 5.Turkish Food Codex Fermented Dairy Products Directive (No: 2009/25)
- 6.Liu SQ. Practical implications of lactate and pyruvate metabolism by lactic acid bacteria in food and beverage fermentations. Int J Food Microbiol. 2003;83:115–31. doi: 10.1016/S0168-1605(02)00366-5. [DOI] [PubMed] [Google Scholar]
- 7.Rosenstiel P. Stories of love and hate: innate immunity and host-microbe crosstalk in the intestine. Curr Opin Gastroenterol. 2013;29:125–32. doi: 10.1097/MOG.0b013e32835da2c7. [DOI] [PubMed] [Google Scholar]
- 8.Ferrario C, Taverniti V, Milani C, et al. Modulation of fecal Clostridiales bacteria and butyrate by probiotic intervention with Lactobacillus paracasei DG varies among healthy adults. J Nutr. 2014;144:1787–96. doi: 10.3945/jn.114.197723. [DOI] [PubMed] [Google Scholar]
- 9.Plaza-Díaz J, Fernandez-Caballero JÁ, Chueca N, et al. Pyrosequencing analysis reveals changes in intestinal microbiota of healthy adults who received a daily dose of immunomodulatory probiotic strains. Nutrients. 2015;7:3999–4015. doi: 10.3390/nu7063999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baran B, Karaca C. Practical medical management of Crohn’s disease. ISRN Gastroenterol. 2013;2013:1–12. doi: 10.1155/2013/208073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Truelove SC, Witts LJ. Cortisone in ulcerative colitis. Final report on therapeutic trial. Br Med J. 1995;2:1041–8. doi: 10.1136/bmj.2.4947.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang K, Lai HC, Yu CY, et al. Real-Time PCR Analysis of the Intestinal Microbiotas in Peritoneal Dialysis Patient. Appl Environ Microbiol. 2011;78:1107–12. doi: 10.1128/AEM.05605-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castillo M, Martín-Orúe SM, Manzanilla EG, et al. Quantification of total bacteria, Enterobacteria and Lactobacilli populations in pig digesta by real-time PCR. Vet Microbiol. 2006;114:165–70. doi: 10.1016/j.vetmic.2005.11.055. [DOI] [PubMed] [Google Scholar]
- 14.Irigoyen A, Arana I, Castiella M, Torre P, Ibanez FC. Microbiological, physicochemical, and sensory characteristics of kefir during storage. Food Chem. 2005;90:613–20. doi: 10.1016/j.foodchem.2004.04.021. [DOI] [Google Scholar]
- 15.Fontan MCG, Martinez S, Franco I, Carballo J. Microbiological and chemical changes during the manufacture of kefir made from cow’s milk, using a commercial starter culture. Int Dairy J. 2006;16:762–7. doi: 10.1016/j.idairyj.2005.07.004. [DOI] [Google Scholar]
- 16.Witthuhn RC, Schoeman T, Britz TJ. Characterisation of the microbial population at different stages of Kefir production and Kefir grain mass cultivation. Int Dairy J. 2005;15:383–9. doi: 10.1016/j.idairyj.2004.07.016. [DOI] [Google Scholar]
- 17.Bosch A, Golowczyc A, Abraham G, Garrote L, De Antoni L, Yantorno O. Rapid discrimination of lactobacilli isolated from kefir grains by FT-IR spectroscopy. Int J Food Microbiol. 2005;111:280–7. doi: 10.1016/j.ijfoodmicro.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Garrote GL, Abraham AG, De Antoni GL. Chemical and microbiological characterisation of kefir grains. J Dairy Res. 2001;68:639–52. doi: 10.1017/S0022029901005210. [DOI] [PubMed] [Google Scholar]
- 19.Kesmen Z, Kacmaz N. Determination of lactic microflora of kefir grains and kefir beverage by using culture-dependent and culture-independent methods. J Food Sci. 2011;76:276–83. doi: 10.1111/j.1750-3841.2011.02191.x. [DOI] [PubMed] [Google Scholar]
- 20.Magalhaes KT, Pereira GVM, Campos CR, Dragone G, Schwan RF. Brazilian kefir: structure, microbial communities and chemical composition. Braz J Microbiol. 2011;42:693–702. doi: 10.1590/S1517-83822011000200034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simova E, Beshkova D, Angelov A, Hristozova TS, Frengova G, Spasov Z. Lactic acid bacteria and yeasts in kefir grains and kefir made from them. J Ind Microbiol Biotech. 2002;28:1–6. doi: 10.1038/sj/jim/7000186. [DOI] [PubMed] [Google Scholar]
- 22.Pintado E, Da Silva L, Fernandes B, Malcata X, Hogg A. Microbiological and rheological studies on Portuguese kefir grains. Int J Food Sci Tech. 1996;31:15–26. doi: 10.1111/j.1365-2621.1996.16-316.x. [DOI] [Google Scholar]
- 23.Chen HS, Wang SY, Chen MJ. Microbiological study of lactic acid bacteria in kefir grains by culture-dependent and culture-independent methods. Food Microbiol. 2008;25:492–501. doi: 10.1016/j.fm.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Toscano M, De Grandi R, Miniello L, Mattina R, Drago L. Ability of Lactobacillus kefiri LKF01 (DSM32079) to colonize the intestinal environment and modify the gut microbiota composition of healthy individuals. Dig Liver Dis. 2017;49:261–7. doi: 10.1016/j.dld.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 25.Braat H, Rottiers P, Huyghebaert N, et al. IL-10 Producing Lactococcus lactis for the Treatment of Crohn’s Disease. Inflamm Bowel Dis. 2006;12:26–7. doi: 10.1097/00054725-200604002-00063. [DOI] [Google Scholar]
- 26.Santos A, San Mauro M, Sanchez A, Torres JM, Marquina D. The antimicrobial properties of different strains of Lactobacillus spp. isolated from kefir. Syst Appl Microbiol. 2003;26:434–7. doi: 10.1078/072320203322497464. [DOI] [PubMed] [Google Scholar]
- 27.Shah NP. Functional cultures and health benefits. Int Dairy J. 2007;17:1262–77. doi: 10.1016/j.idairyj.2007.01.014. [DOI] [Google Scholar]
- 28.Plaza-Díaz J, Fernandez-Caballero JÁ, Chueca N, et al. Pyrosequencing analysis reveals changes in intestinal microbiota of healthy adults who received a daily dose of immunomodulatory probiotic strains. Nutrients. 2015;7:3999–4015. doi: 10.3390/nu7063999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Özden A. Diğer Fermente Süt Ürünleri. Güncel Gastroenteroloji Dergisi. 2008;12:169–81. [Google Scholar]
- 30.Tursi A, Brandimarte G, Papa A. Treatment of Relapsing Mild-to-Moderate Ulcerative Colitis With the Probiotic VSL#3 as Adjunctive to a Standard Pharmaceutical Treatment: A Double-Blind, Randomized, Placebo-Controlled Study. Am J Gastroenterol. 2010;105:2218–27. doi: 10.1038/ajg.2010.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kordecki HJ, Niedzielin K. May the enrichment of colon microflora with Lactobacillus plantarum improve the results of treatment of irritable bowel syndrome and/or ulcerative colitis? Gut. 2001;49(Suppl III):2880. [Google Scholar]
- 32.Guslandi M, Giollo P, Testoni PA. A pilot trial of Saccharomyces boulardii. Dig Dis Sci. 2000;45:1462–4. doi: 10.1023/A:1005588911207. [DOI] [PubMed] [Google Scholar]
- 33.Ishikawa H, Akedo I, Umesaki, Tanaka R, Imaoka A, Otani R. Randomized controlled trial of the effect of bifidobacteria-fermented milk on ulcerative colitis. J Am Coll Nutr. 2003;22:56–63. doi: 10.1080/07315724.2003.10719276. [DOI] [PubMed] [Google Scholar]
- 34.Gionchetti P, Rizzello F, Venturi A. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:305–9. doi: 10.1053/gast.2000.9370. [DOI] [PubMed] [Google Scholar]
- 35.Zubıllaga M, Weill R, Postaire E, Goldman C, Caro R, Boccio J. Effect of Probiotics and Functional Foods and Their use in Different Diseases. Nutr Res. 2001;21:569–79. doi: 10.1016/S0271-5317(01)00281-0. [DOI] [Google Scholar]
- 36.Vinderola G, Perdigon G, Duarte J, Thangavel D, Farnworth E, Matar C. Effects of kefir fractions on innate immunity. Immunobiology. 2006;211:149–56. doi: 10.1016/j.imbio.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Brigidi P, Swennen E, Vitali B, Rossi M, Matteuzzi D. PCR detection of Bifidobacterium strains and Streptococcus thermophilus in feces of human subjects after oral bacteriotherapy and yogurt consumption. Int J Food Microbiol. 2003;81:203–9. doi: 10.1016/S0168-1605(02)00245-3. [DOI] [PubMed] [Google Scholar]
- 38.Manichanh C, Rigottier-Gois L, Bonnaud E, et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut. 2006;55:205–11. doi: 10.1136/gut.2005.073817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gophna U, Sommerfeld K, Gophna S, Doolittle WF, Veldhuyzen van Zanten SJ. Differences between tissue-associated intestinal microfloras of patients with Crohn’s disease and ulcerative colitis. J Clin Microbiol. 2006;44:4136–41. doi: 10.1128/JCM.01004-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andoh A, Imaeda H, Aomatsu T, et al. Comparison of the fecal microbiota profiles between ulcerative colitis and Crohn’s disease using terminal restriction fragment length polymorphism analysis. J Gastroenterol. 2011;46:479–86. doi: 10.1007/s00535-010-0368-4. [DOI] [PubMed] [Google Scholar]
- 41.Anonymous. Tarım ve Köy İşleri Bakanlığı Derg. 2001. Muhafaza Süresince Kefirin Kimyasal, Mikrobiyolojik ve Duyusal Niteliklerindeki Değişmeler; p. 137. [Google Scholar]
- 42.Medici M, Vinderola CG, Weill R, Perdigón G. Effect of fermented milk containing probiotic bacteria in the prevention of an enteroinvasive Escherichia coli infection in mice. J Dairy Res. 2005;72:243–9. doi: 10.1017/S0022029905000750. [DOI] [PubMed] [Google Scholar]
- 43.Wszolek M, Tamime AY, Muir DD, Barclay MNI. Properties of Kefir made in Scotland and Poland using Bovine, Caprine and Ovine Milk with Different Starter Cultures. Food Sci Tech. 2001;34:251–61. doi: 10.1006/fstl.2001.0773. [DOI] [Google Scholar]
- 44.Nagendra P, Shah Ã. Functional cultures and health benefits. Int Dairy J. 2007;17:1262–77. doi: 10.1016/j.idairyj.2007.01.014. [DOI] [Google Scholar]
- 45.Rahimi R, Nikfar S, Rahimi F, et al. Meta-analysis on the efficacy of probiotics for maintenance of remission and prevention of clinical and endoscopic relapse in Crohn’s disease. Dig Dis Sci. 2013;53:2524–31. doi: 10.1007/s10620-007-0171-0. [DOI] [PubMed] [Google Scholar]
- 46.Gupta P. Is Lactobacillus GG helpful in children with Crohn’s disease? Results of a preliminary, open-label study. J Pediatr Gastroenterol Nutr. 2000;31:453–7. doi: 10.1097/00005176-200010000-00024. [DOI] [PubMed] [Google Scholar]
- 47.Bousvaros A. A randomized, double-blind trial of Lactobacillus GG versus placebo in addition to Standard maintenance therapy for children with Crohn’s disease. Inflammatory Bowel Dis. 2005;11:833–9. doi: 10.1097/01.MIB.0000175905.00212.2c. [DOI] [PubMed] [Google Scholar]
- 48.Steed H, Mafarlane GT, Blackett KL, Bahrami B, Reynolds N, Walsh SV. Clinical trial: the microbiological and immunological effects of synbiotic consumption, a randomized double blind placebo controlled study inactive Crohn’s disease. Aliment Pharmacol Ther. 2010;32:872–83. doi: 10.1111/j.1365-2036.2010.04417.x. [DOI] [PubMed] [Google Scholar]
- 49.Sood A, Midha V, Makharia GK. The probiotic preparation, VSL#3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clin Gastroenterol Hepatol. 2009;7:1202–9. doi: 10.1016/j.cgh.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 50.Purchiaroni F, Tortora A, Gabrielli M, et al. The role of intestinal microbiota and the immune system. Eur Rev Med Pharmacol Sci. 2013;17:323–33. [PubMed] [Google Scholar]
- 51.Zheng Y, Lu Y, Wang J, Yang L, Pan C, Huang Y. Probiotic properties of Lactobacillus strains isolated from Tibetan kefir grains. PLoS One. 2013;8:e69868. doi: 10.1371/journal.pone.0069868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen J, Zuo ZX, Mao AP. Effect of probiotics on inducing remission and maintaining therapy in ulcerative colitis, Crohn’s disease, and pouchitis: meta-analysis of randomized controlled trials. Inflamm Bowel Dis. 2014;20:21–35. doi: 10.1097/01.MIB.0000437495.30052.be. [DOI] [PubMed] [Google Scholar]
- 53.Vahabzadeh S, Özpınar H. Investigation of some biochemical properties, antimicrobial activity and antibiotic resistances of kefir supernatants and Lactococcus lactis ssp. lactis strains isolated from raw cow milk and cheese samples. Kafkas Univ Vet Fak Derg. 2018;24:443–50. [Google Scholar]
- 54.Dogan M, Ozpinar H. Investigation of probiotic features of bacteria isolated from some food products. Kafkas Univ Vet Fak Derg. 2017;23:555–62. [Google Scholar]


