Abstract
Background:
Secondary metabolites from the group of isoprenoid compounds are widely distributed in mangrove plants. Polyisoprenoids (dolichol and polyprenol) are known to have benefits as anticancer agents. The present study was conducted to determine the cytotoxic potential of polyisoprenoids in leaves from seventeen selected mangrove species against colon cancer (WiDr) cells.
Methods:
Cytotoxic activity was evaluated by MTT assay in vitro using WiDr human colon cancer cells and 3T3 fibroblasts from Swiss albino mouse embryo tissue as controls. Mechanisms of action were approached by assessing apoptosis and the cell cycle using flow cytometry and fluorescence microscopy with annexin V-FITC, as well as expression of Bcl-2 and cyclin D1 by immunocytochemistry.
Results:
Polyisoprenoids from N. fruticans leaves demonstrated the highest anticancer activity, with an IC50 of 180.2 µg/mL, as compared to 397.7 µg/mL against 3T3 normal cells. Significant decrease in the expression of Bcl-2 and cyclin D1 was also noted, facilitating apoptosis and arrest of the cell cycle in the G0-G1 phase in WiDr cells. The present study showed for the first time that polyisoprenoids from N. fruticans exhibit concrete anticancer activity in vitro, decreasing cell proliferation and inducing apoptosis in colon cancer cells.
Conclusions:
Polyisoprenoids isolated from N. fruticans leaves may have promise as a source of anticancer agents.
Keywords: Antiproliferative, apoptosis, dolichol, nypa fruticans, Mangrove
Introduction
Cancer is one of the major health threats in the world (Miller et al., 2016). In the future, the number of people with cancer will continue to increase, largely due to lifestyle, nutrition and environmental conditions in developed countries (Soerjomataram et al., 2012). The current incidence of colon cancer may be due to a shift towards a high fat and low fibre diet. High fat consumption causes the absorbance of carcinogenic compounds in the body and slows transport time to the intestines (Siegel et al., 2017). Advances in cancer biology have resulted in the development of new treatment strategies, including new anticancer drugs that may act in one or more of the established pathways. Compounds that reactivate cell death mechanisms or decrease the proliferative ability of cancer cells show potential anticancer activity. However, most of the anticancer drugs currently used, such as doxorubicin, have undesirable side effects, such as cardiotoxicity and tumour drug resistance (Carvalho et al., 2009).
Several studies have focused on the potential of natural ingredients as chemopreventive agents, which are companions to chemotherapy. One of the plants that can be used for the treatment of cancer is the mangroven (Wu et al., 2008). Mangrove plants were sources of traditional medicine, as they are a source of bioactive compounds such as tannins, saponins, terpenoids, alkaloids, and steroids (Prabhu and Devaraj, 2016). Mangroves were also well known as a producer of secondary metabolite compounds, mainly from isoprenoid compounds (Basyuni et al., 2007). Polyisoprenoids are long-chain secondary metabolite compounds (> C50) found in almost all living cells. Long-chain polyisoprenoids occur in various plant tissues (Swiezewska and Danikiewicz, 2005). There are two types of polyisoprenoids:the first is polyprenol, and the second is dolichol. Polyisoprenoids are not toxic, and polyisoprenoids are known to show some pharmacological activity, as anticancer (Kuznecovs et al., 2007), antidyslipidaemic (Singh et al., 2007), anti-influenza, and antiviral agents (Safatov et al., 2005).
The distribution and diversity of polyisoprenoid compounds in the Okinawan and North Sumatran mangroves have been previously reported using two-dimensional thin layer chromatography. The distribution of polyprenols and dolichols were classified into three types: Type I displayed a predominance of dolichols over polyprenols, Type II had both polyprenol and dolichols, and Type III displayed a predominance of polyprenols over dolichols (Basyuni et al., 2016; 2017). The anticancer activity of polyisoprenoids from the leaves of seventeen mangrove and coastal species thriving in North Sumatra, Indonesia, based on the distribution and diversity of polyisoprenoids compounds as previously described (Basyuni et al., 2017, 2018), was screened by the MTT assay against WiDr cells.
The present study aimed to analyse the potential anticancer activity of polyisoprenoids from mangrove leaves against colon cancer. The mechanism of cytotoxicity was investigated through apoptosis, the cell cycle, and the induction of Bcl-2 and cyclin D1. This study allowed us to develop a new potential anticancer drug.
Matherials and Methods
Chemicals and reagents
Roswell Park Memorial Institute Medium (RPMI 1640), Dulbecco’s Modified Eagle’s Medium (DMEM), penicillin 1% and streptomycin 1% (v/v) were purchased from Gibco (Carlsbad, CA, USA). Dimethylsulfoxide (DMSO), MTT powder, foetal bovine serum (FBS), and sodium dodecyl sulfate were purchased from Sigma–Aldrich (St. Louis, MO,USA). Propidium iodide, and annexinV-fluorescein isothiocyanate (FITC) were obtained from BioLegend (San Diego, CA, USA). Antibodies against Bcl-2 and cyclin D1 were obtained from Santa Cruz Biotechnology (Dallas, TX, USA). Other chemicals were of analytical grade from commercial suppliers.
Plant materials
The leaves of the following seventeen mangrove species were collected from mangrove forest Lubuk Kertang, Langkat, North Sumatra province, Indonesia in February 2017: Acrostichum aureum, Avicennia lanata, Avicennia marina, Avicennia officinalis, Barringtonia asiatica, Bruguiera gymnorrhiza, Bruguiera gymnorrhiza yellow leaves, Calophyllum inophyllum, Ceriops tagal, Hibiscus tiliaceus, Nypa fruticans, Pandanus odoratissimus, Pongamia pinnata, Rhizophora mucronata, Ricinus communis, and Stachytarpheta jamaicensis.
Isolation of polyisoprenoids
Polyisoprenoids were isolated from mangrove leaves (abbreviated as PML hereafter) according to the method described previously (Sagami et al., 1992; Kurisaki et al., 1997; Basyuni et al., 2016). The mangrove leaves were dried at 60–75ºC for 1-2 days and then ground into powder. The powdered mangrove leaves were immersed in a mixture of chloroform and methanol (2:1, v/v) for 48 h. The lipid extract from the leaves was saponified at 65ºC for 24 h in 86% ethanol containing 2 M KOH. The nonsaponifiable lipids were extracted with hexane, and the organic solvent was evaporated and then redissolved in hexane.
Cell lines and culture conditions
Two cell lines (WiDr cells, human colon cancer cells isolated from the colon of a 78-year-old woman, and 3T3 cells, normal cells isolated from fibroblast Swiss albino mouse embryo tissue were obtained from the collection of the Parasitology Laboratory Faculty of Medicine, University of Gadjah Mada (UGM) (Yogyakarta, Indonesia). The WiDr cell line was cultured in RPMI 1640 medium, and the 3T3 cell line was cultured in DMEM medium. Both lines were supplemented with 10% (v/v) foetal bovine serum (FBS), 1% penicillin and streptomycin, 0.5% Fungizone, and were maintained in humidity 37°C incubator with 5% CO2.
Cytotoxic activity test with MTT assay
To evaluate the effects of the PML on cell viability and proliferation, WiDr cells were seeded onto 96-well microplates at a density of 5 × 103 cells/well and incubated for 24 h at 37°C with 5% CO2. Then, 100 μL of PML was added in a range of concentrations: 15.625, 31.25, 62.525, 125, 250, and 500 µg/mL. This step was repeated three times, and the samples were incubated again for 24 h. After incubation, 100 μL of MTT was added to each sample, and the samples were incubated for 4 h at 37°C with 5% CO2. Live cells react with MTT and turn purple. Reaction with MTT was halted by100 mL sodium dodecyl sulfate before overnight incubation at room temperature. The absorption was read at a wavelength of 595 nm on an ELISA plate reader (Bencmark Bio Rad). The most active extract was selected and tested with 3T3 cells (normal cells) at the same concentration. The results were presented as percent viability. Also, selective index (SI) value was calculated as the ratio of IC50 of cancer cells to the IC50 of normal cells.
Flowcytometry analysis of apoptosis and the cell cycle
Based on the IC50, polyisoprenoids from N. fruticans (abbreviated as PNF hereafter) was found to be the most potent towards colon cancer cell line (WiDr). Further experiment was then conducted by using PNF only. Tests for apoptosis and the cell cycle were performed using flow cytometry. WiDr cells were seeded onto a 6-well plate at a density of 1 ×106 cells/well and were incubated for 24 h at 37°C with 5% CO2. Then, the cells were treated with PNF at 1 IC50, 1/2 IC50, 1/5 IC50, 1/10 IC50 concentrations (180, 90, 36, 18 µg/mL). The negative control group received no treatment. Then, the cells were re-incubated for 24 h. After the incubation, the medium was removed from each well, and the cells were transferred to conical tubes and washed with PBS, which was then discarded. Trypsin (250 μL) was added to each well before incubation for 3 min at 37°C. Culture medium (1 mL) was added to each well, and then the contents were transferred back into conical tubes. The tubes were centrifuged for 5 min at 6000 rpm, and then the supernatant was discarded. PBS (1 mL) was added, and then the medium was transferred into a conical tube and centrifuged again at 2,000 rpm for 3 min, after which the supernatant was again discarded. Annexin V-FITC (5 μg/mL) and propidium iodide (5 μg/mL) were added to test for apoptosis, while propidium iodide alone was added to test for the cell cycle. Then, the samples were analysed with a flow cytometer by using FACSVerse (BD Biosciences).
Observed expression Bcl-2 and cyclin D1 protein with immunocytochemistry
The WiDr cells were seeded in a 24-well microplate at a density 5 x 104 cells/well and incubated for 24 h at 37°C with 5% CO2. The wells were treated with PNF at 1 IC50, 1/2 IC50, 1/5 IC50, 1/10 IC50 concentrations (180, 90, 36, 18 µg/mL), the negative control received no treatment and incubated at 37°C with 5% CO2 for 24 h. After, the medium was discarded, and the wells containing the cells were washed twice with PBS. The cover slip onto which the cells were loaded was lifted and placed in a 6 cm dish, and into the dish was dropped hydrogen peroxidase, then incubated at room temperature for 15 min. The cells were washed twice with PBS and was added monoclonal antibody of Bcl-2 and cyclin D1 into the cells and incubated for 1 h. The cells were washed twice with PBS and added with secondary antibody, incubated for 10 min, and washed twice with PBS. Added 3,3’-diaminobenzidine, as chromogen, to the cells, and incubated for 5 min. Then, the cells were washed with distilled water and added with hematoxylin solution, and incubated for 3 min. Immunocytochemical loading using Bcl-2- and cyclin D1-specific antibodies was observed using an inverted light microscope (Olympus, Tokyo, Japan), and documented. The data were expressed in terms of the percentage of cells expressing protein in 10 fields of view from each treatment group. Expression of Bcl-2 and cyclin D1 seen as brown in the cell nucleus and cytoplasm. Whereas cells with no protein expression appeared purple.
Statistical analysis
Data were expressed as the mean ± SD from at least three independent experiments. The IC50 concentration was calculated from the linear regression equations of dose response curve for each experiment. All statistical analyses were performed using the SPSS for Windows Version 23.
Results
Effect of polyisoprenoids from mangrove leaves on cell viability and proliferation in WiDr cell lines by MTT
The IC50 values are summarized in Table 1. The highest cytotoxic activity observed was in the N. fruticans extract, which had an IC50 value of 180.186 µg/mL, which in this research was used rounding concentration 180 ug/mL. To select the extracts and cell lines for use in the following experiments, two aspects were considered: 1) extracts should inhibit cell proliferation without significant direct cytotoxic effects, and 2) the IC50 value of the extract should be lower than 200 µg/mL. Having met these two criteria, the extract of N. fruticans was selected.
Table 1.
IC50 Values of Polyisoprenoids from Seventeen Mangrove Species
| No | Name of species | IC50 (µg/mL) |
|---|---|---|
| 1 | Acacia auriculiformis | 1,425.46 |
| 2 | Acrostichum aureum | 314.623 |
| 3 | Avicennia lanata | 305.928 |
| 4 | Avicennia marina | 209.693 |
| 5 | Avicennia officinalis | 1,444.45 |
| 6 | Barringtonia asiatica | 1,831.74 |
| 7 | Bruguiera gymnorrhiza | 350.395 |
| 8 | Bruguieragymnorrhiza yellow leaf | 1,853.57 |
| 9 | Calophyllum inophyllum | 275.829 |
| 10 | Ceriops tagal | 276.055 |
| 11 | Hibiscus tiliaceus | 409.821 |
| 12 | Nypa fruticans | 180.186 |
| 13 | Pandanus odoratissimus | 513.598 |
| 14 | Pongamia pinnata | 386.77 |
| 15 | Rhizophora mucronata | 278.335 |
| 16 | Ricinus communis | 1,890.00 |
| 17 | Stachytarpheta jamaicensis | 285.492 |
In relation to cell proliferation, WiDr cells treated with only medium (negative control) grew significantly throughout the incubation. The results showed that the addition of PNF decreased cell proliferation in a concentration-dependent manner in WiDr cells. Morphologically, living cells were spheroidal and pellucid in the middle, while dead cells had an irregular shape and were devoid of a nucleus. The results are shown in Figure 1. Control cells appear morphologically normal. The treatment of WiDr cells with PNF at various concentrations affected the morphology of the WiDr cells compared to the negative control.
Figure 1.
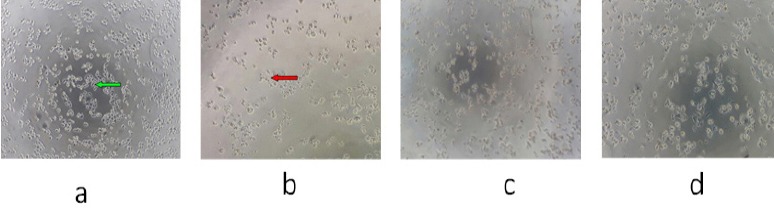
WiDr Colon-Cancer Cells were Treated with PNF. Description: a, control, WiDr cells without treatment; b, WiDr cells treated with PNF at a concentration of 500 µg/mL; c, WiDr cells treated with PNF at a concentrationof 250 µg/mL; d, WiDr cells treated with PNF at a concentration of 125 µg/mL. The living cells ares hown with a green arrow, and the dead cells areshown with a red arrow. 100× magnification.
The most active extract (PNF) was retested with 3T3 cells as a normal cell model for a comparison to the WiDr as a colon cancer cell model. Figure 2 shows that PNF inhibits proliferation of 3T3 cells but not as much as in WiDr cells. On the other hand, 3T3 cells line showing IC50 value of 397, 692 µg/mL and the selective index value of PNF to 3T3 cells line was 2,2.
Figure 2.
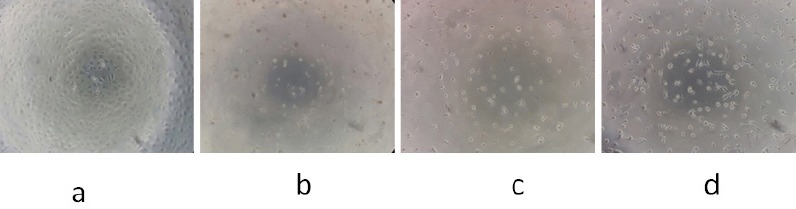
3T3 Cells (Normal Cells) were Treated with PNF. Description: a, control, 3T3 cells without treatment; b, 3T3 cellstreated with PNF at a concentrationof 500 µg/mL; c, 3T3 cellstreated with PNF at a concentration of 250 µg/mL; d, 3T3 cellstreated with PNF at a concentration of 125 µg/mL. 100× magnification.
Effects of polyisoprenoids from mangrove leaves on the cell cycle and apoptosis
The percentage of cycle arrest is shown in Table 2. The results suggest that polyisoprenoids from PNF induced cell cycle arrest of WiDr cell in the G0-G1 phase at concentrations of 18, 36, 90, and 180 µg/mL. When compared to the cell control, the cellular accumulation of WiDr with PNF in the G0-G1 phase was higer, so it can be said that the mechanism of inhibition of PNF on the cell cycle in WiDr cells is in the G0-G1 phase.
Table 2.
Distribution of WiDr Cell Cycle after Treatment with PNF
| Treatment | Concentration μg/mL | % Cell phase | ||
|---|---|---|---|---|
| G0-G1 | S | G2-M | ||
| Cell control | 0 | 46.18 ± 0.35 | 27.84 ± 0.27 | 26.96 ± 0.26 |
| Polyisoprenoids from N. fruticans | 18 (1/10 IC50) | 61.91 ± 0.18 | 21.66 ± 0.33 | 16.34 ± 0.29 |
| 36 (1/5 IC50) | 62.87 ± 0.23 | 18.57 ± 0.22 | 17.42 ± 0.24 | |
| 90 (1/2 IC50) | 69.82 ± 0.16 | 15.57 ± 0,31 | 14.31 ± 0,12 | |
| 180 (1 IC50) | 70.36 ± 0.12 | 14.64 ± 0.28 | 14.61 ± 0.14 | |
Data were expressed as mean ± SD (n=3)
After incubation for 24 h, apoptosis was tested for using flow cytometry. Data obtained from flow cytometry results in the percentage of live, apoptotic, or necrotic WiDr cells in the five groups (Figure 3). The mean percentages of apoptotic WiDr cells in the five groups are shown in Table 3.
Figure 3.
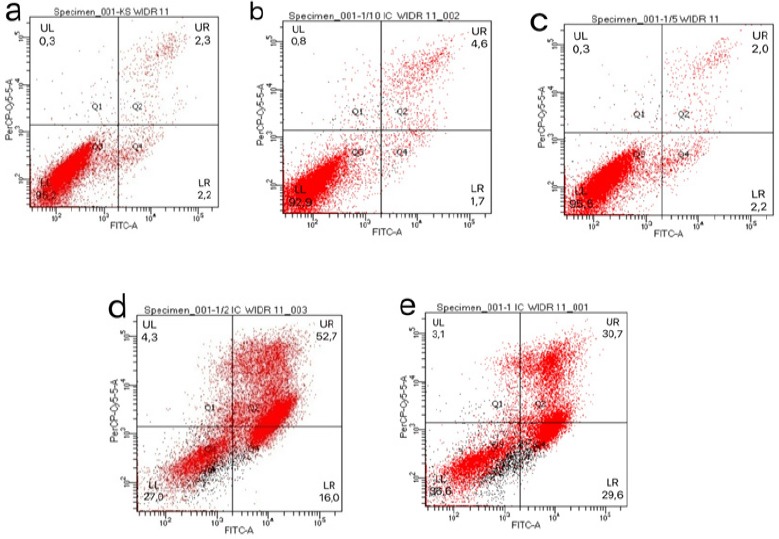
The Induction of Apoptosis in WiDr Cells by PNF. WiDr cells were treated with various concentrations of PNF for 24 h. The cells in the negative control group were untreated. The percentage of apoptotic cells was determined by annexin V/PI staining and flow cytometry. The cells generating an annexin V+/PI- (LR) signal were in the early apoptotic stage, while the cells generating an annexin V+/PI+(UR) signal were dead cells. Typical distribution of cell populations treated with 0/18/36/90/180 μg/mL PNF are shown in a-e, respectively.
Table 3.
The Mean Percentage of WiDr Cells that Develop Apoptosis after Incubation for 24 h with Various Concentrations of PNF
| Treatment | Concen-tration µg/mL | % of living cell (LL) | % of early apoptotic stage (LR) | % of late apoptotic stage and early necrosis (UR) | % of late necrosis (UL) | % of totally apoptotic cells (LR+UR) |
|---|---|---|---|---|---|---|
| Cell control | 0 | 95.20 ± 0.17 | 2.20 ± 0.49 | 2.30 ± 0.27 | 0.30 ± 0.12 | 4.5 |
| Polyiso-prenoids from N. fruticans | 18 (1/10 IC50) | 92.90 ± 0.13 | 1.70 ± 0.29 | 4.60 ± 0.32 | 0.80 ± 0.46 | 6.3 |
| 36 (1/5 IC50) | 95.50 ± 0.16 | 2.20 ± 0.31 | 2.00 ± 0.29 | 0.30 ± 0.29 | 4.2 | |
| 90 (1/2 IC50) | 27.00 ± 0.37 | 16.00 ± 0.43 | 52.70 ± 0.47 | 4.30 ± 0.23 | 68.7 | |
| 180 (1 IC50) | 36.60 ± 0.27 | 29.60 ± 0.24 | 30.70 ± 0.29 | 3.10 ± 0.32 | 60.3 |
Data were expressed as mean ± SD (n=3)
Observation of Bcl-2 and cyclin D1 protein expression
Bcl-2 and cyclin D1 expression in WiDr cells was observed using immunocytochemical methods with specific antibody binding principles. Qualitative observations were made by comparing the colour of treated WiDr cells with the WiDr cell control. The results showed that PNF decreased the expression of Bcl-2 and cyclin D1 expressionin WiDr cells (Figure 4 b, d) compared with control WiDr cells without PNF treatment (Figure 4 a, c). Treated cells and doxorubicin were stained as purple colour, appearing asignificantly different to control cells were stained as brown or dark colour. Percentage of Bcl-2 and cyclin D1 expression shown in Table 4.
Figure 4.
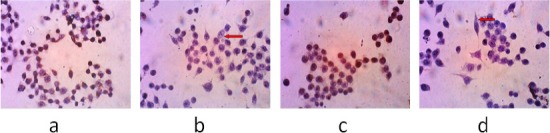
The Observation of Bcl-2 and Cyclin D1 Protein Expression in WiDr cells after Administration of PNF. The observations were made using an inverted microscope 400× magnification. (a), (c), The negative control, the group untreated with PNF; (b), cells incubated with PNF at a concentration of 90 µg/mL (1/2IC50) with antibodies against Bcl-2; (d), cells incubated with polyisoprenoids from N. fruticans at a concentration of 90 µg/mL (1/2 IC50) with antibodies against cyclin D1. The red arrow shows the negative result of Bcl-2 and cyclin D1 expression.
Discussion
Mangrove plants have long been used in traditional medicine to cure gastroenteritis and to treat cancer (Bandaranayake, 1998). The potential for these plants to be sources of anticancer agents prompted us to investigate whether polyisoprenoids from mangrove leaves would inhibit colon cancer using observations of apoptosis, the cell cycle, and specific protein expression. Our results clearly showed for the first time that polyisoprenoids from mangrove leaves suppressed the expression of specific proteins responsible for the apoptotic process and the cell cycle, i.e., Bcl-2 and cyclin D1.
The cytotoxic test was performed using the MTT method. The MTT method was chosen because it is accurate and it is possible to describe the relationship between the number of active cells and the absorbance values obtained (Behera et al., 2003). The smaller the value of IC50, the higher the potential of the extract to inhibit cell proliferation. The MTT assay measures the activity of mitochondrial dehydrogenase in converting MTT into formazan. The concentration of formazan, which has a blue colour, can be determined using a visible spectrophotometer. The concentration of formazan has a positive correlation with the number of living cells because the reduction event occurs only when mitochondrial reductase is produced by active mitochondria, indicating that the cell is alive (Van Meerloo et al., 2011).
The IC50 calculation was used to determine the IC50 of polyisoprenoids from the leaves of seventeen mangrove species. Extracts from some species showed selective cytotoxic effects, whereas some extracts showed non-selective cytotoxicity against the WiDr cell line or were not active. Negative IC50 values can be interpreted as direct and short-term cytotoxic effects of the extracts that may, in turn, indicate necrotic cell death. Positive values (between 0 and 100%) can be interpreted as inhibition of cell proliferation. This variant of the MTT assay allows a rapid and simple discrimination between inhibition of cell proliferation and cell death (Kiesslich et al., 2013).
Polyisoprenoids from mangrove leaves with the highest cytotoxic effect came from N. fruticans with an IC50 value of 180.186 μg/mL. The N. fruticans leaves have been reported to contain 100% dolichols (C75-C90) with no polyprenols detected (Basyuni et al., 2017). Mevalonic acid (a precursor in the biosynthesis of isoprenoid compounds, including cholesterol, dolichol, and ubiquinone) allows proper functioning and cellular repair of many proteins such as Ras and Rho. Treatment with dolichols significantly disrupted the function of the families of Ras and Rho GTPases, with mechanisms of inhibition acting on certain cyclin-dependent kinase (CDK) activity and the activation of CDK inhibitors (Jakobisiak and Golab, 2003). Previous studies by other researchers have showed that N. fruticans extract has selective cytotoxic and effects on HepG2 cells (Samarakoon et al., 2016) with known phytochemicals such as tannins, phenolics, and terpenoids (Prasad et al., 2013).
The cellular morphological change could be caused by apoptosis (Lee et al., 2014). The mechanism of apoptosis is usually used to describe the anticancer mechanism of various drug compounds. The WiDr cell growth was successfully inhibited by PNF. This could be caused by apoptotic mechanisms leading to cell morphological changes. Cellular morphological change associated with apoptosis involves the following steps: cell density decrease, chromatin condensation and fragmentation, and nucleus fragmentation (Rich et al., 2000). PNF may protect normal cells from becoming cancerous by binding to mitochondrial proteins of cancer cells, inducing apoptosis without lysing neighbouring cells (Selvendiran et al., 2003).
The accumulation of cells at different phases in the cell cycle is one of the main targets of anticancer agents. Cells in different phases in a normal cell cycle have differences in the number of sets of chromosomes, e.g., the number of sets of chromosomes in the G1 phase is 2n. In the S phase, the sets of chromosomes vary between 2n and 4n because replication occurs, whereas in the G2 and M phases, replication has already occurred, resulting in a 4n chromosome set (Golias et al., 2004). The regulation of the cell cycle causing a barrier in G0-G1 phase by PNF on WiDr cells occurs through decreasing expression of cyclin D1 (based on the immunocytochemical test), which acts to start the cell cycle. The occurrence of cell division depends on the complex bond between CDK and cyclin. The G0-G1 phase will be induced by CDK-4 and CDK-6 that form the complex with cyclin D and cyclin E. Therefore, when CDK is not functioning, cyclin does not form a complex with CDK and, as a result, the cell cycle stops (Owa et al., 2001). The cessation of the cell cycle in G0-G1 phase provides an opportunity for the cell to repair damaged DNA and, if that repair is unsuccessful, to continue to apoptosis. Checkpoint control is essential for maintaining genomic stability. An error atthe checkpoint will allow the cell cycle to continue, despite DNA damage, incomplete DNA replication, or a separate chromosome that will result in genetic damage. This is critical for the onset of cancer. Therefore, the cell cycle regulation process can play a role in cancer prevention (Ruddon, 2007).
WiDr cells were treated with PNF, which may stimulate apoptosis due to triterpenoids or steroids that may induce apoptosis (Petroneli et al., 2009). The apoptotic mechanism was also generated by PNF suppressing Bcl-2 expression, inducing apoptosis pathways (Pandanilam, 2003). Therefore, to better understand the expression of genes that are affected by PNF, immunocytochemical tests were performed using antibodies against proteins that play a role in the cell cycle and in apoptosis, i.e., Bcl-2 and cyclin D1. The decrease in cyclin D1 expression resulted in decreased formation of the cyclin D1-CDK complex, so the cell cycle stopped at the G0-G1 phase, inhibiting proliferation of WiDr cells. In line with results in the previous study (Das et al., 2016), we found that the extract from N. fruticans leaves may inhibit cell proliferation or induce apoptosis through reduced cyclin D1 and cyclin D1-CDK complex.
Apoptosis is a mechanism for controlling cell proliferation as part of the normal development process and will result in cell death if there is irreversible DNA damage (Xue et al., 2014). The capacity of the PNF extract to induce apoptosis could be due to the presence of secondary metabolites naturally produced by the mangrove leaves. Cell viability analysis and MTT tests showed that PNF might induce apoptosis in WiDr cells by an intrinsic pathway related to cytochrome c migration to the cytosol and the dissociation of the mitochondrial membrane (Gogvadze et al., 2006). Apoptosis has two main pathways, extrinsic and intrinsic. The intrinsic pathway is regulated by a group of proteins that belong to the B-cell lymphoma 2 protein (Bcl-2) family. There are two main groups of the Bcl-2 protein. The pro-apoptosis protein group includes proteins such as Bax, Bak, Bad, Bcl-Xs, Bid, Bik, Bim, and Hrk. The second group is a group of anti-apoptosis proteins, such as Bcl-2, Bcl-XL, Bcl-W, Bfl-1, and Mcl-1. The sensitivity of cells to apoptotic stimuli depends on the balance between pro-apoptosis and anti-apoptosis proteins (Wong, 2011). In cancer cells, there occurs an imbalance between pro-apoptosis (Bax) and anti-apoptosis (Bcl-2) proteins, where Bcl-2 levels are higher, inhibiting apoptosis. In this study, the treatment with PNF of colon cancer cells (WiDr) suppressed the expression of the Bcl-2 protein and therefore its ability to induce apoptosis. We tracked the apoptotic pathway in this study by observing the expression of Bcl-2 and cyclin D1 proteins, which was found to be decreased due to PNF treatment, showing a bluish cytoplasmic colour. Under normal conditions, pro-apoptosis and anti-apoptosis family members of Bcl-2 move equally. Decreased expression of Bcl-2 will increase pro-apoptosis protein (Bax, Bak, Bok) expression, which plays a role in opening the Pt-pore, so cytochrome C can exit the mitochondria. Released cytochrome C binds to the Apaf-1 protein and then the active Apaf-1 activates the caspase cascade, inducing cell death (apoptosis) (Petronelli et al., 2009).
This further confirms PNF as a chemopreventive agent that increases the cytotoxic activity on colon cancer cells by inhibiting the cell cycle and stimulating apoptosis by decreasing Bcl-2 and cyclin D1 protein expression. This result presents the opportunity to develop polyisoprenoids isolated from N. fruticans leaves in combination with standard chemotherapy for cancer therapy, especially for colon cancer. The combination of chemotherapy agents aims to improve the effectiveness of therapy and reduce the side effects of treatment. Appropriate combinational design is required to obtain a greater therapeutic benefit.
In summary, this study screened polyisoprenoids isolated from seventeen mangrove leaves for cytotoxic activity in colon cancer cells (WiDr). Polyisoprenoids from N. fruticans had the highest activity, with an IC50 value of 180.186 µg/mL. Our study showed for the first time that polyisoprenoids from N. fruticans have in vitro anticancer activity, decreasing cell proliferation and inducing cell death in colon cancer cells. Polyisoprenoids from N. fruticans were able to induce apoptosis and to suppress protein expression of Bcl-2 and cyclin D1 in the WiDr cell line.
Since Polyisoprenoids from N. fruticans exerted very potent cytotoxicity effect with lowest IC50 value, thus polyisoprenoids from N. fruticans could be used as a potential source of chemotherapeutic agents, but further work should be needed to confirm the cytotoxicity effect not only Bcl-2 and Cyclin D1 expression that was analysed in this study, but also in others protein expression, and to give a strong support to the apoptosis result, the inherent molecular targets and related signal transduction pathways need to be investigated in the future.
Acknowledgements
The authors are grateful to the Ministry of Research, Technology and Higher Education of the Republic of Indonesia through Excellent Research for Higher Education (PUPT) 2017 (No.003/SP2H/LT/DRPM/IV/2017).
References
- 1.Ashour OM, Elberry AA, Alahdal AM, et al. Protective effect of bilberry (Vaccinium myrtillus) against doxorubicin-induced oxidative cardiotoxicity in rats. Med Sci Monit. 2011;17:110–15. doi: 10.12659/MSM.881711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bandaranayake WM. Traditional and medicinal uses of mangroves. Mangroves Salt Marshes. 1998;2:133–48. [Google Scholar]
- 3.Basyuni M, Oku H, Baba S, Takara K, Iwasaki H. Isoprenoids of Okinawan mangroves as lipid input into estuarine ecosystem. J Oceanography. 2007;63:601–8. [Google Scholar]
- 4.Basyuni M, Sagami H, Baba S, Iwasakai H, Oku H. Diversity of Polyisoprenoids in ten Okinawan mangroves. Dendrobiology. 2016;75:167–75. [Google Scholar]
- 5.Basyuni M, Sagami H, Baba S, Oku H. Distribution and occurrence of new polyprenyl acetone and other polyisoprenoids in Indonesian mangroves. Dendrobiology. 2017;78:18–31. [Google Scholar]
- 6.Basyuni M, Wati R, Sagami H, et al. Diversity and abundance of polyisoprenoid composition in coastal plant species from North Sumatra, Indonesia. Biodiversitas. 2018;19:1–11. [Google Scholar]
- 7.Behera BC, Adawadkar B, Makhija U. Inhibitory activity of xanthine oxidase and superoxide-scavenging activity in some taxa of the lichen family Graphidaceae. Phytomedicine. 2003;10:536–43. doi: 10.1078/094471103322331511. [DOI] [PubMed] [Google Scholar]
- 8.Carvalho C, Santos RX, Cardoso S, et al. Doxorubicin:the good, the bad and the ugly effect. Curr Med Chem. 2009;16:3267–85. doi: 10.2174/092986709788803312. [DOI] [PubMed] [Google Scholar]
- 9.Das SK, Samantaray D, Patra JK, et al. Antidiabetic potential of mangrove plants:a review. Front Life Sci. 2016;9:75–88. [Google Scholar]
- 10.Golias CH, Charalabopoulos A, Charalabopoulos K. Cell proliferation and cell cycle control:a mini review. Int J Clin Pract. 2004;58:1134–41. doi: 10.1111/j.1742-1241.2004.00284.x. [DOI] [PubMed] [Google Scholar]
- 11.Gogvadze V, Orrenius S, Zhivotovsky B. Multiple pathways of cytochrome C release from mitochondria in apoptosis. Biochim Biophys Acta. 2006;1757:639–47. doi: 10.1016/j.bbabio.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Jakobisiak M, Golab J. Potential antitumor effects of statins. Int J Oncol. 2003;23:1055–69. [PubMed] [Google Scholar]
- 13.Kiesslich T, Gollmer A, Maisch T, et al. A comprehensive tutorial on in vitro characterization of new photosensitizers for photodynamic antitumor therapy and photodynamic inactivation of microorganisms. Biomed Res Int. 2013;2013:1–17. doi: 10.1155/2013/840417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurisaki A, Sagami H, Ogura K. Distribution of polyprenols and dolichols in soybean plant. Phytochemistry. 1997;44:45–50. [Google Scholar]
- 15.Kuznecovs S, Jegina K, Kuznecovs I. Inhibition of P-glycoprotein by polyprenol in human breast cancer cells. Breast J. 2007;16:515–21. [Google Scholar]
- 16.Lee YH, Cheng FY, Chiu HW, et al. Cytotoxicity, oxidative stress, apoptosis and the autophagic effects of silver nanoparticles in mouse embryonic fibroblasts. Biomaterials. 2014;35:4706–15. doi: 10.1016/j.biomaterials.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 17.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics. CA Cancer J Clin. 2016;66:271–89. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 18.Owa T, Yoshino H, Yoshimatsu K, et al. Cell cycle regulation in the G1 phase:a promising target for the development of new chemotherapeutic anticancer agents. Curr Med Chem. 2001;8:1487–503. doi: 10.2174/0929867013371996. [DOI] [PubMed] [Google Scholar]
- 19.Padanilam BJ. Cell death induced by acute renal injury:aperspective on the contributions of apoptosis dan necrosis. Am J Physiol Renal Physiol. 2003;284:608–27. doi: 10.1152/ajprenal.00284.2002. [DOI] [PubMed] [Google Scholar]
- 20.Prabhu V, Devaraj SN. Natural products from mangrove-potent inhibitors of lung cancer. Malaya J Biosci. 2016;3:23–30. [Google Scholar]
- 21.Petronelli A, Pannitteri G, Testa U. Triterpenoids as new promising anticancer drugs. Anticancer Drugs. 2009;20:880–92. doi: 10.1097/CAD.0b013e328330fd90. [DOI] [PubMed] [Google Scholar]
- 22.Prasad N, Yang B, Kong KW, et al. Phytochemicals and antioxidant capacity from Nypa fruticans Wurmb. fruit. Evid Based Complementary Altern Med. 2013;2013:1–9. doi: 10.1155/2013/154606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rich T, Allen RL, Wyllie AH. Defying death after DNA damage. Nature. 2000;407:777–83. doi: 10.1038/35037717. [DOI] [PubMed] [Google Scholar]
- 24.Ruddon RW. Cancer Biology. Fourth Edition. New York: Oxford University Press; 2007. pp. 151–6. [Google Scholar]
- 25.Safatov AS, Boldyrev AN, Bulychev LE, et al. Aprototype prophylactic anti-influenza preparation in aerosol form on the basis of Abies sibirica polyprenols. J Aerosol Med. 2005;18:55–62. doi: 10.1089/jam.2005.18.55. [DOI] [PubMed] [Google Scholar]
- 26.Sagami H, Kurisaki A, Ogura K, et al. Separation of dolichol from dehydrodolichol by a simple two-plate thin layer chromatography. J Lipid Res. 1992;33:1857–62. [PubMed] [Google Scholar]
- 27.Samarakoon SR, Shanmuganathan C, Ediriweera MK, et al. Screening of fifteen mangrove plants found in Sri Lanka for in vitro cytotoxic properties on breast (MCF-7) and hepatocellular carcinoma (HepG2) cells. Eur J Med Plants. 2016;14:1–11. [Google Scholar]
- 28.Selvendiran K, Sigh JPV, Krishnan D, et al. Cytoprotective effect of piperine against benzo[a]pyrene induced lung cancer with reference to lipid peroxidation and antioxidant system in Swiss albino mice. Fitoterapia. 2003;74:109–15. doi: 10.1016/s0367-326x(02)00304-0. [DOI] [PubMed] [Google Scholar]
- 29.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177–93. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 30.Singh G, Gupta P, Rawat P, et al. Anti dyslipidemic activity of polyprenol from Coccinia grandis in high-fat diet fed hamster model. Phytomedicine. 2007;14:792–8. doi: 10.1016/j.phymed.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Soerjomataram I, Tieulent JL, Parkin DM, et al. Global burden of cancer in 2008:a systematic analysis of disability-adjusted life-years in 12 world regions. Lancet. 2012;380:1840–50. doi: 10.1016/S0140-6736(12)60919-2. [DOI] [PubMed] [Google Scholar]
- 32.Swiezewska E, Danikiewicz W. Polyisoprenoids:Structure, biosynthesis, and func-tion. Prog Lipid Res. 2005;44:235–58. doi: 10.1016/j.plipres.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 33.Van Meerloo J, Kaspers GJ, Cloos J. Cell sensitivity assays:the MTT assay. Methods Mol Biol. 2011;731:237–45. doi: 10.1007/978-1-61779-080-5_20. [DOI] [PubMed] [Google Scholar]
- 34.Wong RSY. Apoptosis in cancer:from pathogenesis to treatment. J Exp Clin Cancer Res. 2011;30:87. doi: 10.1186/1756-9966-30-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu J, Xiao Q, Xu J, et al. Natural products from true mangrove flora:source, chemistry and bioactivities. Nat Prod Rep. 2008;25:955–81. doi: 10.1039/b807365a. [DOI] [PubMed] [Google Scholar]
- 36.Xue X, Yu JL, Sun DQ, et al. Curcumin induces apoptosis in SGC-7901 gastric adenocarcinoma cells via regulation of mitochondrial signaling pathways. Asian Pac J Cancer Prev. 2014;15:3987–92. doi: 10.7314/apjcp.2014.15.9.3987. [DOI] [PubMed] [Google Scholar]


