Abstract
Abstract:
Gastric cancer is one of the most common upper gastrointestinal malignancies. Some Iranian provinces, such as in the northern and northwestern areas, are at a high risk, whereas the central and western provinces are at a medium and the southern regions at low risk. This study was carried out to estimate the impact of the expression patterns of ASIC1 and IL-6 genes and the IL-6rs-174 and ASIC1rs 75624685 polymorphisms in the pathogenesis of gastric cancer.
Materials and methods:
Tetra-ARMS PCR was employed to analyze the polymorphism status of the ASIC1 and IL-6 genes with 85 paraffin-embedded tissue blocks from cases and 117 normal blood samples as controls. We also investigated mRNA expression levels of these genes in 12 cases and controls using real-time PCR.
Results:
Our results showed a significant association between expression of ASIC1 and elevated risk of gastric cancer (p<0.001).
Keywords: Gastric cancer, polymorphism, expression, IL-6, ASIC1
Introduction
Gastric cancer is the fourth most ordinary cancer worldwide and approximately two-thirds of all cases occur in developing countries. It is the fourth most common cancer in men, and the fifth most common in women. Despite a steady decline of its incidence in developed countries, in less developed countries such as those of the Middle and Eastern Asia, South America and Eastern Europe gastric cancer is an important burden on public health (Lee et al., 2013). Most of the northern and northwestern regions of Iran such as Ardabil, Semnan, Golestan, East Azerbaijan, and Tehran are at a high risk of gastric cancer, but the southern provinces show a lower level of incidence (Malekzadeh et al., 2009; Mansour-Ghanaei et al., 2012). It is a multi-factorial disease and develops because of a continuous cell damage caused by life-long exposure to different carcinogens. Environmental and behavioral factors including smoking, salt and salted food, diet, obesity, alcohol, radiation exposure, low socioeconomic status, and Helicobacter pylori infection are involved in the pathogenesis of gastric cancer. However, some people are at a high risk of gastric cancer (Kolahdoozan et al., 2010; Norouzinia et al., 2012). Adenocarcinomas, 90% of gastric tumors, subdivided into 2 histologic types: (i) well-differentiated and (ii) undifferentiated (Lin et al., 2007). In terms of genetics, a variety of genes such as MCC, APC, and p53 tumor suppressor genes increases the risk of gastric cancer, while a calcium-dependent adhesion molecule, E-cadherin, has been identified in a large percentage of gastric cancers (Zali et al., 2011; Kordi-Tamandani et al., 2014).
IL-6 is a pleiotropic cytokine that has significant roles in both acute and chronic inflammation and several effects on many immune and non-immune cell types. Macrophages and T-cells secrete this cytokine to stimulate an immune response. Its gene is located on the short arm of chromosome7 (Van der Poll et al., 1997). In various diseases such as inflammatory, autoimmune and malignant diseases, IL-6 has pathological roles (Kishimoto, 2010; Leech et al., 2013). Immunohistochemistry and in-situ hybridization have shown that IL-6 is highly expressed and is secreted from both gastricepithelial cells and inflammatory cells infiltrating the gastric mucosa in H. pylori infection (Furukawa et al., 1998; Kordi-Tamandani et al., 2014). There have been several reports examining IL-6 induction by H. pylori infection from nonepithelial cells (Gobert et al., 2004). This cytokine is involved in multiple signaling cascades such as NF-κB, STAT3, and HIF-1α. IL-6 is essentially required for the differentiation of Th17 cells (Wemmie et al., 2003). Acid-Sensing Ion Channels (ASICs) belong to the amiloride-sensitive cation channels that are expressed in the central and peripheral nervous systems. In human, the ASIC family comprises ASIC1, ASIC2, ASIC3, and ASIC4 subunit channels. ASICs consist of 500 to 560 amino acids. ASICs are homo or heterotrimers of homologous subunits (Leonard et al., 2003; Lingueglia et al., 2013). These channels have two transmembrane chains, an extracellular domain with several conserved cysteines, and intracellular N and C termini. ASICs are permeable to Na+ and Ca2+ ions and an external pH variation activate these channels. A variety of tissues expresses ASICs. The coding gene for ASIC1 is located on the long arm of chromosom12 (Gründer and Chen, 2010). IL-6 has a-174 G/C polymorphism in its promoter region and its gene is located on chromosome No. 7 (7p 15.3).
Materials and Methods
Samples
This study was performed with 85 samples (20 females and 65 males) of paraffin-embedded tissues of gastric adenocarcinoma, procured from the pathology service of Alzahra Hospital in Isfahan, Iran, and 117 normal blood samples as healthy controls. The samples belonged to patients who underwent curative gastrectomy between 2009 and 2014. The control subjects comprised the margins of tumor site that were unaffected and pathologist confirmed them to be healthy. The demographic, pathological and clinical characterizations of participates were cited by Kordi et al., (2012).
Genomic DNA extraction
Genomic DNA was extracted from the paraffin-embedded tissues using the phenol-chloroform DNA extraction method. Blood samples were collected in EDTA-coated tubes for DNA extraction and genotypic analysis, as previously described. For the DNA extraction, a Cinnapure DNA purification kit was used. Polymorphisms were identified by PCR using the Tetra Amplification Refractory Mutation System (T-ARMS), which is a simple and rapid detection method for different types of mutations. Amplification of IL-6 and ASIC1 genes was set according to the following PCR conditions: 10 µl premix master mix (including Taq, dNTP, Buffer, and Mg2+) 1 µl of each outer primer and 1.5 µl of each inner primer (10 mmol/l), and 3.5 µl of RNase-free double distilled water. The PCR was heated at 95°C for 10 min., followed by 40 cycles at 95°C for 30s, annealing at 54°C (ASIC1 rs75624685), and 57°C (IL-6174) for 30 s, extension at 72°C for the 30 s, and the final extension by incubation at 72°C for 10 min.
Tetra-primer amplification refractory mutation system PCR (T-ARMS-PCR)
The polymorphisms of the ASIC1 (rs75624685) and IL-6 174 genes were genotyped employing the T-ARMS-PCR technique. The sequences of inner and outer primers are listed in Table 1 in addition to PCR product sizes and annealing temperatures. The PCR products were evaluated by electrophoresis on a 2% agarose gel and visualized by ethidium bromide staining.
Table 1.
Primers Sequences and Annealing Temperatures
| Genes | Seqences (5’-3’) | Product size (bp) | Annealing temp (°C) |
|---|---|---|---|
| ASIC1 | F: ATGGAAAGTGCTACACGTTCAA | 192 | 60 |
| R: GTTCATCCTGACTATGGATCTGC | |||
| IL-6 | F: ACTCACCTCTTCAGAACGAATTG | 149 | 60 |
| R: CCATCTTTGGAAGGTTCAGGTTG | |||
| RNA 18S | F: GTAACCCGTTGAACCCCATT | 112 | 60 |
| R: CCATCCAATCGGTAGTAGCG | |||
| ASIC1 rs75624685 | Fo: CTACAGTTGGTCGCTCTTTCTCAAGTGAA | 332 | 54 |
| Ro: GAACCATTTAGGAGGAATGGTGATGATG | |||
| Fi: TGTGCTAAGATAAAAACTAAAACATGTCTG | 204 (G allele) | ||
| Ri: GCTGGCAGGTGCTGTGGAGTTGACAG | 176 (C allele) | ||
| IL-6-174 G/C rs1800795 | Fo: GACTTC AGCTTT ACTCTTTGTCAAGACA | 326 | 57 |
| Ro: GAATGAGCCTCAGACATCTCCAGTCCTA | |||
| Fi: GCACTT TTCCCC CTAGTTGTGTCTTCCG | 205 (G allele) | ||
| Ri: ATTGTGCAATGTGACGTCCTTTAGCTTG | 184 (C allele) |
RNA extraction and RT-PCR
The isolation of total RNA from paraffin-embedded tissues was performed using Cinna pure RNA, cat.No.PR891620 (sinaclon.com) following the manufacturer’s protocol. Subsequently, the first-strand cDNA was synthesized from 1-10μg of total RNA using a 2-step RT-PCR kit (vivantis, www.vivantechnologies.com) according to the supplier’s protocol. Real-time PCR was performed with Applied Biosystem Real-Time PCR and in 16μl-reaction volume with Power SYBR Green PCR Master Mix (Applied Biosystem) under the following conditions: 1 cycle of 95°C for 10 min; 40 cycles of 95°C for 15 s, 60°C for 30 s, and 72°C for 40 s; finally, 1 cycle of 72°C 10 min. for extension, and 1 cycle of 60-95°C for the melting curve. The PCR products were tested gel in electrophoresis with 2% agarose and visualized with ethidium bromide staining. The primer sequences used for the expression study are listed in Table 2. The expression of ASIC1 and IL-6 were normalized by rRNA18s and was given by2-∆∆CT.
Table 2.
Comparison of Relative Gene Expression for ASIC1and IL-6 Genes between Cases and Controls
| Genes | No. | Mean±SD | P-value | |
|---|---|---|---|---|
| ASIC1 | Cases | 12 | 34.98±1.81 | 0.001 |
| Controls | 12 | 31.23±2.62 | ||
| IL-6 | Cases | 12 | 32.84±5.33 | 0.6 |
| Controls | 12 | 32.47±2.62 |
Statistical analysis
SPSS version 20.0 (SPSS, Chicago, IL) and Epical Info were used for all statistical analyses. The significant P value was set at less than 0.05. Categorical data were analyzed by Pearson’s x2.
Results
No statistically significant differences were observed in the two genotypes IL-6rs1800795G/C promoter and ASIC1rs75624685 between cases and controls (Figures 1, 2). The predominant genotype in both IL-6rs1800795 and ASIC1 r s75624685 polymorphism was GG in the cases and the controls; also, the GC genotype was absent in both polymorphisms. The evaluated mRNA expression levels of ASIC1 was remarkably different in patients and healthy controls (p<0.001). We did not find any association between the expressions of IL-6 and gastric cancer (p=0.6) (Table 2).
Figure 1.
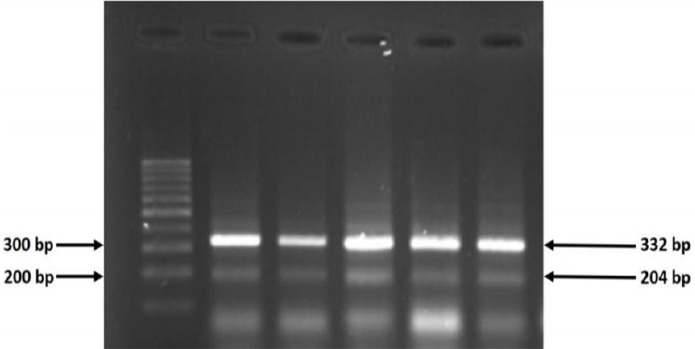
The Products of Tetra-ARMS PCR for ASIC1 (rs75624685). Control band 332bp and GG band 204bp.
Figure 2.
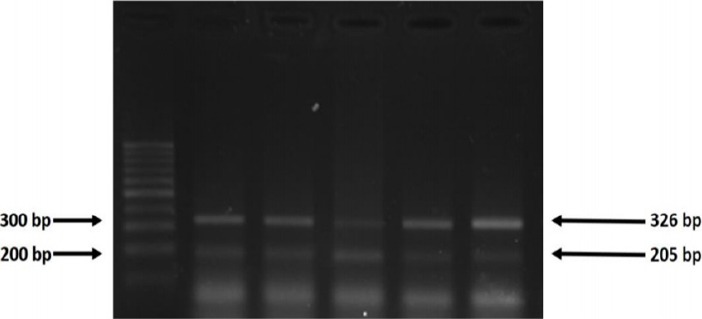
The Products of Tetra-ARMS PCR for IL-6 174. Control band 326 bp and G band 204bp.
Discussion
Interleukin (IL) genes are the most studied genes for gastric cancer (Gianfagna et al., 2008). All cancers are associated with inflammation for example, in gastric inflammation; epithelium starts cellular transformation and, by inducing immune responses and utilizing signaling cascades, contributes to invasion. Hallmarks of cancer such as DNA mutation, proliferation, angiogenesis, apoptosis, and invasion are related to inflammation. Through two intrinsic and extrinsic pathways, cancer and inflammation are connected, in extrinsic pathway, secreting chemokines, cytokines increase the risk of cancer, while genetic alterations such as activation of proto-oncogenes, inactivation of tumor suppressor genes, and instability of chromosomes are involved in the intrinsic pathway (Amjadi et al., 2014). Several studies reported that gastric carcinoma cells secrete many cytokines that play key roles in both cellular and immune responses (Ohta et al., 2003; Kai et al., 2005). Also, these authors showed that gastric cancer tissues contain high levels of the proinflammatory cytokines IL-1‚ and IL-6, and that the levels of these cytokines are associated with the tumor growth pattern (Takeda et al., 1991; Akira et al., 1993; Ueda et al., 1994). IL-6 activates the target genes involved in differentiation, survival, apoptosis, and proliferation. It also manipulates the process of tumorigenesis and the progression of the tumor (Weidle et al., 2010; Li et al., 2016). As mentioned above, the IL-6/IL-6R signal pathway promotes tumor growth, angiogenesis, invasion and migration in various cancers, such as epithelial ovarian cancer (Johnston et al., 2015). Studies by Lin et al., (2007) showed that the expression of IL-6 was related to the promotion of human gastric cancer invasion and migration of AGS cells. IL-6 signaling was exerted by the interaction of IL-6 with the gp130/IL6R complex in addition to by interaction of soluble IL6R/IL6 complex with gp130. The result of these interactions is JAK/STAT3 activation and MEK/ERK signaling, respectively (Weidle et al., 2010). In the tumor microenvironment, the production of IL-6 via HNSCC cells and stromal cells can activate STAT3 and contributes to tumor progression and drug resistance. Investigations by Chen et al., (2015) showed the effect of the vasoactive intestinal peptide on gastric cancer via tumor-associated macrophages by suppressing the expression levels of TNFα, IL-6, IL-12, and iNOS. VIP treatment significantly depressed TAM activation. In addition, TNFα, IL-6, IL-12 and iNOS expression in TAM were depressed with VIP treatment, and the VIP treated TAM depressed the gastric cancer cells. The presence of the C allele is associated with increased cancer risk and aggressive tumor behavior. In line with our results, a meta-analysis by Yin et al., (2012) showed that IL-6 gene-174C/G and-572C/G polymorphisms were not associated with the risk of gastric cancer. Our results also are consistent with the Gatti et al., (2007) findings; they did find any association between the frequencies of -174 SNP with the histological type of gastric adenocarcinoma, but the G allele at -174 was significantly higher in gastricadenocarcinoma than in patients with chronic gastritis. Shu et al., (2015) showed that compared to open gastrectomy (OG), laparoscopic-assisted gastrectomy (LAG) was associated with significantly lower serum levels of IL-6. Hence, LAG, among Asian populations, carries a markedly lower risk of adverse inflammatory reactions in GC patients. Investigation by Wang et al., (2012) showed that polymorphisms of IL-6-174 G/C, -572 G/C and -597 G/A were not associated with the risk of gastric cancer. In the present study, we focused on ASIC1, the most abundant ASIC subunit in the mammalian central nervous system (CNS). ASICs contribute to gastric acid hypersensitivity and pain under conditions of gastritis and peptic ulceration but also cause colonic hypersensitivity to mechanical stimuli (distension) under conditions of irritation that are not necessarily associated with overt inflammation. Investigation by Wong et al., (2008) on the Huntington disease showed that low-level of ASIC was impressiveness to the chance of a cure. Studies by Yifeng et al., (2011) show that a low-level expression of ASIC is related to the remedy of ischemic-brain attack. According to a report by Wu et al., (2014) high level of expression of ASIC is related to the progression of cancer. Previous studies had observed significant differences in the frequencies of IL-6 polymorphism in the−174 G/C loci in breast cancer, uterine leiomyosarcoma, and multiple myeloma. The result of Litovkin et al., (2007) in the distribution of this polymorphism in Ukrainian patients with breast cancers or uterine leiomyosarcoma compared with those in Macedonia, Italy, and Finland showed significant differences in the−174 G/C allele. Analysis of the G allele frequency by Duch et al., (2007) in patients with multiple myeloma revealed that its frequency in the Brazilian population was higher than in the European population. In addition, another study showed a lower frequency of the G allele in Taiwanese patients with CRC than Caucasian populations (Yeh et al., 2010). The genetic characteristic of this polymorphism may be different in diverse disease. Our findings indicate for the first time an association of the ASIC1 gene expression with the risk of gastric cancer. Ultimately, we are suggesting a verification of the present data, conducting extensive studies with large sample sizes in various genetic populations.
Acknowledgments
The present authors are especially grateful to all the patients and controls who took part in this study. Financial support was provided by the University of Sistan and Baluchestan, under of DMKT-94.
References
- 1.Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol. 1993;54:1–78. doi: 10.1016/s0065-2776(08)60532-5. [DOI] [PubMed] [Google Scholar]
- 2.Amjadi O, Rafiei A, Ajami A, Hosseini V, Asgarian-Omran H. Inflammation, a key factor in cancer ambush. Res Mol Med. 2014a;2:1–15. [Google Scholar]
- 3.Chen L, Yuan W, Chen Z, et al. Vasoactive intestinal peptide represses activation of tumor-associated macrophages in gastric cancer via regulation of TNFα, IL-6, IL-12 and iNOS. Int J Oncol. 2015;47:1361–70. doi: 10.3892/ijo.2015.3126. [DOI] [PubMed] [Google Scholar]
- 4.Duch CR, Figueiredo MS, Ribas C, et al. Analysis of polymorphism at site -174 G/C of interleukin-6 promoter region in multiple myeloma. Braz J Med Biol Res. 2007;40:265–7. doi: 10.1590/s0100-879x2007000200014. [DOI] [PubMed] [Google Scholar]
- 5.Furukawa K, Takahashi T, Arai F, Matsushima K, Asakura H. Enhanced mucosal expression of interleukin-6 mRNA but not of interleukin-8 mRNA at the margin of gastric ulcer in Helicobacter pylori-positive gastritis. J Gastroenterol. 1998;33:625–33. doi: 10.1007/s005350050148. [DOI] [PubMed] [Google Scholar]
- 6.Gatti LL, Burbano RR, Zambaldi-Tunes M. Interleukin-6 polymorphisms, Helicobacter pylori infection in adult Brazilian patients with chronic gastritis and gastric adenocarcinoma. Arch Med Res. 2007;38:551–6. doi: 10.1016/j.arcmed.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Gianfagna F, De Feo E, van Duijn CM, Ricciardi G, Boccia S. A systematic review of meta-analyses on gene polymorphisms and gastric cancer risk. Curr Genomics. 2008;9:361–74. doi: 10.2174/138920208785699544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gobert AP, Bambou JC, Werts C, et al. Helicobacter pylori heat shock protein 60 mediates interleukin-6 production by macrophages via a toll-like receptor (TLR)-2-, TLR-4-, and myeloid differentiation factor 88-independent mechanism. J Biol Chem. 2004;279:245–50. doi: 10.1074/jbc.M307858200. [DOI] [PubMed] [Google Scholar]
- 9.Gründer S, Chen X. Structure, function, and pharmacology of acid-sensing ion channels (ASICs):focus on ASIC1a. Int J Physiol Pathophysiol Pharmacol. 2010;2:73–94. [PMC free article] [PubMed] [Google Scholar]
- 10.Holzer P. Acid-sensing ion channels in gastrointestinal function. Neuropharmacology. 2015;94:72–9. doi: 10.1016/j.neuropharm.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnston PA, Sen M, Hua Y, et al. HCS campaign to identify selective inhibitors of IL-6-induced STAT3 pathway activation in head and neck cancer cell lines. Assay Drug Dev Technol. 2015;13:356–76. doi: 10.1089/adt.2015.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kai H, Kitadai Y, Kodama M, et al. Involvement of proinflammatory cytokines IL-1beta and IL-6 in progression of human gastric carcinoma. Anticancer Res. 2005;25:709–13. [PubMed] [Google Scholar]
- 13.Kolahdoozan S, Sadjadi A, Radmard AR, Khademi H. Five common cancers in Iran. Arch Iran Med, 13, Kishimoto T. (2010). IL-6:from its discovery to clinical applications. Int Immunol. 2010;22:347–52. doi: 10.1093/intimm/dxq030. [DOI] [PubMed] [Google Scholar]
- 14.Kordi-Tamandani DM, Davani SK, Baranzehi T, Hemati S. Analysis of promoter methylation, polymorphism and expression profile of cytotoxic T-lymphocyte-associated antigen-4 in patients with gastric cancer. J Gastrointestin Liver Dis. 2014;23:249–53. doi: 10.15403/jgld.2014.1121.233.dmkt. [DOI] [PubMed] [Google Scholar]
- 15.Kordi-Tamandani DM, Hashemi M, Sharifi N, Kaykhaei MA, Torkamanzehi A. Association between paraoxonase-1 gene polymorphisms and risk of metabolic syndrome. Mol Biol Rep. 2012;39:937–43. doi: 10.1007/s11033-011-0819-x. [DOI] [PubMed] [Google Scholar]
- 16.Lee YY, Derakhshan MH. Environmental and lifestyle risk factors of gastric cancer. Arch Iran Med. 2013;16:358–65. [PubMed] [Google Scholar]
- 17.Leech MD, Barr TA, Turner DG, et al. Cutting edge:IL-6-dependent autoimmune disease:dendritic cells as a sufficient, but transient, source. J Immunol. 2013;190:881–5. doi: 10.4049/jimmunol.1202925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leonard AS, Yermolaieva O, Hruska-Hageman A, et al. cAMP-dependent protein kinase phosphorylation of the acid-sensing ion channel-1 regulates its binding to the protein interacting with C-kinase-1. Proc Natl Acad Sci U S A. 2003;100:2029–34. doi: 10.1073/pnas.252782799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li F, Zhang Z, Cheng T, et al. SPECT imaging of interleukin-6 receptor in ovarian tumor xenografts with a novel radiotracer of 99mTc-HYNIC-Aca-LSLITRL. Amino Acids. 2016;48:91–101. doi: 10.1007/s00726-015-2060-8. [DOI] [PubMed] [Google Scholar]
- 20.Lim CS, Zheng S, Kim YS, et al. The --174 G to C polymorphism of interleukin-6 gene is very rare in koreans. Cytokine. 2002;19:52–4. doi: 10.1006/cyto.2002.1951. [DOI] [PubMed] [Google Scholar]
- 21.Lin MT, Lin BR, Chang CC, et al. IL-6 induces AGS gastric cancer cell invasion via activation of the c-Src/RhoA/ROCK signaling pathway. Int J Cancer. 2007;120:2600–8. doi: 10.1002/ijc.22599. [DOI] [PubMed] [Google Scholar]
- 22.Lingueglia E, Lazdunski M. Pharmacology of ASIC channels. WIREs Membr Transp Signal. 2013;2:155–71. [Google Scholar]
- 23.Litovkin KV, Domenyuk VP, Bubnov VV, Zaporozhan VN. Interleukin-6 -174G/C polymorphism in breast cancer and uterine leiomyoma patients:a population-based case control study. Exp Oncol. 2007;29:295–8. [PubMed] [Google Scholar]
- 24.Malekzadeh R, Derakhshan MH, Malekzadeh Z. Gastric cancer in Iran:epidemiology and risk factors. Arch Iran Med. 2009;12:576–83. [PubMed] [Google Scholar]
- 25.Mansour-Ghanaei F, Joukar F, Soati F, Mansour-Ghanaei A, Naserani SB. Knowledge about gastric carcinoma in North of Iran, a high prevalent region for gastric carcinoma:a population-based telephone survey. Asian Pac J Cancer Prev. 2012;13:3361–6. doi: 10.7314/apjcp.2012.13.7.3361. [DOI] [PubMed] [Google Scholar]
- 26.Norouzinia M, Asadzadeh H, Shalmani HM, Al Dulaimi D, Zali MR. Clinical and histological indicators of proximal and distal gastric cancer in eight provinces of Iran. Asian Pac J Cancer Prev. 2012;13:5677–9. doi: 10.7314/apjcp.2012.13.11.5677. [DOI] [PubMed] [Google Scholar]
- 27.Ohta M, Kitadai Y, Tanaka S, et al. Monocyte chemoattractant protein-1 expression correlates with macrophage infiltration and tumor vascularity in human gastric carcinomas. Int J Oncol. 2003;22:773–8. [PubMed] [Google Scholar]
- 28.Shu ZB, Cao HP, Li YC, Sun LB. Influences of laparoscopic-assisted gastrectomy and open gastrectomy on serum interleukin-6 levels in patients with gastric cancer among Asian populations:a systematic review. BMC Gastroenterol. 2015;15:52. doi: 10.1186/s12876-015-0276-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeda K, Fujii N, Nitta Y, et al. Murine tumor cells metastasizing selectively in the liver:ability to produce hepatocyte-activating cytokines interleukin-1 and/or -6. Jpn J Cancer Res. 1991;82:1299–308. doi: 10.1111/j.1349-7006.1991.tb01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ueda T, Shimada E, Urakawa T, et al. Serum levels of cytokines in patients with colorectal cancer:possible involvement of interleukin-6 and interleukin-8 in hematogenous metastasis. J Gastroenterol. 1994;29:423–9. doi: 10.1007/BF02361238. [DOI] [PubMed] [Google Scholar]
- 31.Van der Poll T, Keogh CV, Guirao X, et al. Interleukin-6 gene-deficient mice show impaired defense against pneumococcal pneumonia. J Infect Dis. 1997;176:439–44. doi: 10.1086/514062. [DOI] [PubMed] [Google Scholar]
- 32.Wang J, He W, Liu J, et al. Association of IL-6 polymorphisms with gastric cancer risk:evidences from a meta-analysis. Cytokine. 2012;59:176–83. doi: 10.1016/j.cyto.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 33.Weidle UH, Klostermann S, Eggle D, Krüger A. Interleukin 6/interleukin 6 receptor interaction and its role as a therapeutic target for treatment of cachexia and cancer. Cancer Genomics Proteomics. 2010;7:287–302. [PubMed] [Google Scholar]
- 34.Wemmie JA, Askwith CC, Lamani E, et al. Acid-sensing ion channel 1 is localized in brain regions with high synaptic density and contributes to fear conditioning. J Neurosci. 2003;23:5496–502. doi: 10.1523/JNEUROSCI.23-13-05496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong HK, Bauer PO, Kurosawa M, et al. Blocking acid-sensing ion channel 1 alleviates Huntington's disease pathology via an ubiquitin-proteasome system-dependent mechanism. Hum Mol Genet. 2008;17:3223–35. doi: 10.1093/hmg/ddn218. [DOI] [PubMed] [Google Scholar]
- 36.Wu FR, Pan CX, Rong C, et al. Inhibition of acid-sensing ion channel 1a in hepatic stellate cells attenuates PDGF-induced activation of HSCs through MAPK pathway. Mol Cell Biochem. 2014;395:199–209. doi: 10.1007/s11010-014-2125-0. [DOI] [PubMed] [Google Scholar]
- 37.Yifeng M, Bin W, Weiqiao Z, et al. Neuroprotective effect of sophocarpine against transient focal cerebral ischemia via down-regulation of the acid-sensing ion channel 1 in rats. Brain Res. 2011;1382:245–51. doi: 10.1016/j.brainres.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 38.Yin YW, Sun QQ, Hu AM, et al. Associations between interleukin-6 gene -174–C/G and -572–C/G polymorphisms and the risk of gastric cancer:a meta-analysis. J Surg Oncol. 2012;106:987–93. doi: 10.1002/jso.23199. [DOI] [PubMed] [Google Scholar]
- 39.Zali H, Rezaei-Tavirani M, Azodi M, et al. Gastric cancer:prevention, risk factors and treatment. Gastroenterol Hepatol Bed Bench. 2011;4:175–85. [PMC free article] [PubMed] [Google Scholar]


