Abstract
Background:
Recurrence after curative resection of hepatocellular carcinoma (HCC) is associated with early death and poor prognosis. Microvascular invasion (mVI) is strongly associated with disease recurrence. Although many studies have examined the relationship between various serum inflammatory indices and post-treatment prognosis, little is known about preoperative predictors of microvascular invasion in HCC.
Methods:
Patients who underwent curative hepatic resection for HCC at our institute from January 2006 to December 2016 were retrospectively reviewed. The associations between mVI and various potential risk factors, including tumor size, hepatitis B and C virus infection, Child–Pugh scores, platelet-to-lymphocyte ratio, and neutrophil-to-lymphocyte ratio, were analyzed. Optimal cut-off values were determined using receiver operating characteristic curves.
Results:
A total of 330 HCC patients were enrolled in this study, of whom 74 (22.4%) had tumors with mVI. After univariate analysis, two parameters were significantly associated with mVI after hepatic resection: platelet-to-lymphocyte ratio ≥102 (odds ratio [OR] 2.385, p = 0.001) and tumor size ≥5 cm (OR 4.29, p < 0.001). Both variables remained significant risk factors for mVI after multivariate analysis: platelet-to-lymphocyte ratio ≥102 (OR 1.831, p = 0.034) and tumor size ≥5 cm (OR 3.791, p < 0.001).
Conclusions:
Large tumor size (≥5 cm) and high platelet-to-lymphocyte ratio (≥102) are independent predictive factors for mVI in HCC.
Keywords: Hepatocellular carcinoma, risk factors, platelet-to-lymphocyte, prognosis, microvascular
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide (Torre et al., 2015). Many treatment options are available for HCC, depending on the stage of disease and the presence of liver cirrhosis (Bruix et al., 2016), but liver transplantation is considered the optimal approach because it treats both the tumor and background liver disease simultaneously. However, in Thailand, resection is the main treatment option because of the limited availability of donor organs. Although the overall morbidity and mortality associated with surgical treatment of HCC have declined in recent years, the rate of cancer recurrence remains high (Fan et al., 1999; Imamura et al., 2003; Regimbeau et al., 2004; Liu et al., 2015). Indeed, recurrent intrahepatic disease is the most common cause of death from HCC (Lim et al., 2012; Bruix et al., 2016).
Microvascular invasion (mVI), large tumor size, multifocality, high serum alpha-fetoprotein (AFP) concentration, and liver cirrhosis are among the known risk factors for poor prognosis in HCC (Poon et al., 2000b; Cha et al., 2003; Regimbeau et al., 2004; Kaibori et al., 2009; Kamiyama et al., 2012). Of these, mVI is the most commonly reported predictor of poor prognosis of HCC after curative resection (Poon et al., 2000a; Lim et al., 2011; Rodriguez-Peralvarez et al., 2013). The American Joint Committee on Cancer Staging manual, 7th edition (Compton Cc, 2006), classifies HCC with mVI as T2, regardless of the tumor size. A second form of vascular invasion is macrovascular (Sumie et al., 2014), which is defined as tumor growth into a major vessel that can be identified by macroscopic examination or preoperative radiological imaging. According to the Barcelona Clinic Liver Cancer Classification system, macrovascular invasion is a contraindication for liver transplantation due to very high recurrence rate (Bruix et al., 2016). In contrast, mVI, which is defined as the presence of tumor emboli in a portal pedicle vein, a large capsule vessel, or a vascular space lined by endothelial cells, does not preclude transplantation (Rodriguez-Peralvarez et al., 2013).
Various predictors of mVI in HCC have been reported, including tumor size, tumor capsule, tumor multifocality, and smooth tumor margin in computed tomography (Pawlik et al., 2005; Kim et al., 2008; Eguchi et al., 2010; Suh et al., 2012; Rodriguez-Peralvarez et al., 2013; Yamamura et al., 2014; Pote et al., 2015; Xia et al., 2015; Goh et al., 2016; Yang et al., 2017). In addition, several recent studies have identified several serum inflammatory factors associated with post-treatment prognosis of HCC, including platelet-to-lymphocyte ratio (PLR) (Tian et al., 2016; Huang et al., 2017; Yang et al., 2017), neutrophil-to-lymphocyte ratio (NLR) (Goh et al., 2016; Jin et al., 2017; Urabe et al., 2017), and prognostic nutritional index (PNI) (Chan et al., 2015; Wu et al., 2016). However, only a few studies have examined the relationship between inflammatory indices and mVI (Gomez et al., 2008; Chan et al., 2015; Zheng et al., 2017). The aim of this study was to identify preoperative predictors of mVI by evaluating the correlation between preoperative clinicopathological features, including serum inflammatory indices, and mVI.
Materials and Methods
Patients and methods
This was a retrospective study of 330 consecutive patients who underwent liver resection for pathologically proven HCC at the Department of Surgery at our hospital between January 2006 and December 2016. The Institutional Review Board of Ramathibodi Hospital approved the study.
Patient demographic and clinicopathological data collected included age, gender, indocyanine green retention value at 15 min (ICG-R15), AFP, alcohol consumption, and Child–Pugh score. All patients underwent preoperative cross-sectional dynamic imaging using either triple-phase computed tomography or magnetic resonance imaging. Liver biopsy was not routinely performed, except in cases with an inconclusive diagnosis after preoperative imaging. The tumor size was derived from preoperative imaging. Routine blood examinations included complete blood count, coagulogram, liver and kidney function tests, hepatitis B and C virus (HBV, HCV) infection, and serum AFP concentration. A preoperative ICG-R15 was also performed. NLR was calculated as neutrophil count divided by the lymphocyte count. PLR was calculated as the platelet count divided by the lymphocyte count. PNI was calculated as ([albumin {g/L} + 0.005] × [total lymphocyte count {/µL}]). HCC was diagnosed preoperatively based on characteristic findings on computed tomography or magnetic resonance imaging. In our center, patients are selected for curative resection based on the Makuuchi criteria (Miyagawa et al., 1995). The extent of liver resection was individualized according to the patient’s liver functional reserve, which was mainly assessed using the Makuuchi criteria, including preoperative ascites volume, Child–Pugh score, ICG-R15, and, occasionally, volumetric computed tomography analysis.
Surgery was performed laparoscopically or by open laparotomy. Prophylactic antibiotics were routinely injected up to 30 min before skin incision. The incision type depended on the surgeon’s preference. The Pringle maneuver was performed using intermittent clamping (clamp for 15 min, de-clamp for 5 min). Intraoperative ultrasound was routinely used to stage the disease and to guide parenchymal transection, which was performed using a Cavitron ultrasonic aspirator or the clamp crushing technique, depending on the surgeon’s preference. Blood loss was estimated by an anesthesiologist, who also assessed the need for blood transfusion. The operative time was defined as the period from the start of incision until closure of the abdominal wound.
Pathological specimens were reviewed by a pathologist to confirm the diagnosis of HCC. Patients with combined cholangiocarcinoma, other kinds of malignancies, or with incomplete data were excluded from the study. mVI was defined as the presence of tumor cells in the microvasculature.
Statistical analysis
Categorical and numerical variables were analyzed using Pearson’s χ2 test and the Mann–Whitney test, respectively. Univariate and multivariate analyses were conducted using the logistic regression model. Odds ratios (OR) and 95% confidence intervals (CI) were computed to assess the strength of the associations between the various factors and the outcome. A p value of <0.05 was considered statistically significant. Analyses were performed using STATA program version 14 (StataCorp, College Station, TX, USA). The cut-off value for high and low PLR, 102, was determined by receiver operating characteristic (ROC) curve analysis.
Results
Patient characteristics
A total of 330 patients underwent curative resection for HCC from January 2006 to December 2016, of whom 74 (22.4%) had mVI. For analyses, the patients were divided into mVI+ (n = 74) and mVI− (n = 256) groups. The clinicopathological characteristics of the two groups are shown in Table 1. The mVI+ group had significantly higher median platelet counts than the mVI− group (2.07 × 105/μL [range 0.94–8.5] vs 1.92 × 105/μL [0.14–6.90], p = 0.009) and median PLR (129.2 vs 94.1, p = 0.001). The median tumor size was also higher in the mVI+ group than the mVI− group (6.8 vs 4.0 cm, p < 0.001). In the mVI+ group (n = 74), there was a significant difference in the number of patients with tumor size <5 cm compared with ≥5 cm (19 vs 55, p < 0.001) and in the number of patients with PLR <102 compared with PLR ≥102 (47 vs 27, p = 0.001; Table 1). There were no significant differences between the two groups with respect to age, gender, HBV, HCV, ICG-R15, AFP, international normalized ratio (INR), NLR, PNI, alcohol consumption, Child–Pugh score, number of tumors, or multifocality.
Table 1.
Preoperative Characteristics of Patients Stratified by Microvascular Invasion
| Characteristics | Total (n=330) | mVI− (n=256) | mVI+ (n=74) | p value |
|---|---|---|---|---|
| Age (years), mean±sda | 58.12 (10.65) | 58.32 (10.31) | 57.44 (11.76) | 0.536 |
| Platelets (×105 /μL) | 1.95 (0.14–8.50) | 1.92 (0.14–6.90) | 2.07 (0.94–8.5) | 0.009 |
| INR (U/L) | 1.06 (0.06–1.70) | 1.06 (0.06–1.70) | 1.04 (0.84–1.28) | 0.238 |
| AFP (ng/mL) | 15.20 (0.89–82392) | 13.56 (0.89–60500) | 33.5 (0.9–82392) | 0.09 |
| ICG-R15 (%) | 14.70 (0.10–51.60) | 14.90 (0.50–51.60) | 13.95 (0.10–33.80) | 0.247 |
| Tumor size (cm) | 4.5 (0.1–26.5) | 4 (0.10–19) | 6.80 (1.50–26.50) | 0 |
| Lymphocytes (/μL) | 1,900.8 (480–21060) | 1,938.4 (480–21060) | 1,803 (630–4893) | 0.266 |
| PLR | 101.7 (6.89–536.83) | 94.1 (6.9–536.8) | 129.2 (38.6–370.5) | 0.001 |
| Neutrophils (/μL) | 3,451 (1064–11620) | 3,440 (1064–10600) | 3,583 (1575–11620) | 0.476 |
| PNI | 95.37 (24.4–1053.3) | 97.3 (24.4–1053.3) | 90.6 (31.8–245.0) | 0.272 |
| NLR | 1.81 (0.33–10.62) | 1.78 (0.50–10.62) | 2.03 (0.33–6.38) | 0.162 |
| PNI, n (%) (n = 312) | ||||
| <95 | 153 (49.04) | 114 (47.30) | 39 (54.93) | 0.259 |
| >95 | 159 (50.96) | 127 (52.70) | 32 (45.07) | |
| NLR, n (%) (n= 310) | ||||
| <1.8 | 153 (49.35) | 122 (50.62) | 31 (44.93) | 0.404 |
| >1.8 | 157 (50.65) | 119 (49.38) | 38 (55.07) | |
| Tumor size, n (%) | ||||
| <5 cm | 172 (52.12) | 153 (59.77) | 19 (25.68) | 0 |
| >5 cm | 158 (47.88) | 103 (40.23) | 55 (74.32) | |
| PLR, n (%) | ||||
| <102 | 175 (53.03) | 148 (57.81) | 27 (36.49) | 0.001 |
| >102 | 155 (46.97) | 108 (42.19) | 47 (63.51) | |
| Gender, n (%) | ||||
| Male | 127 (38.48) | 101 (39.45) | 26 (35.14) | 0.501 |
| Female | 203 (61.52) | 155 (60.55) | 48 (64.86) | |
| HBV, n (%) | ||||
| Negative | 147 (44.55) | 109 (42.58) | 38 (51.35) | 0.181 |
| Positive | 183 (55.45) | 147 (57.72) | 36 (48.65) | |
| HCV, n (%) | ||||
| Negative | 271 (82.12) | 212 (82.81) | 59 (79.73) | 0.542 |
| Positive | 59 (17.88) | 44 (17.19) | 15 (20.27) | |
| Alcohol consumption, n (%) (n=299) | ||||
| None | 167 (55.85) | 132 (51.56) | 35 (47.30) | 0.572 |
| Occasional | 70 (23.41) | 51 (19.92) | 19 (25.68) | |
| Alcoholism | 62 (20.74) | 47 (18.36) | 15 (20.27) | |
| Child–Pugh, n (%) (n = 309) | ||||
| A | 293 (94.82) | 229 (95.02) | 64 (94.12) | 0.767 |
| B | 16 (5.18) | 12 (4.98) | 4 (5.88) | |
| Multifocal tumor, n (%) | ||||
| Single | 255 (77.27) | 200 (78.13) | 55 (74.32) | 0.492 |
| Multiple | 75 (22.73) | 56 (21.88) | 19 (25.68) | |
, Age is presented as the mean ± standard deviation (sd). All other data are presented as the median (range) or number (%) of patients; AFP, alpha-fetoprotein; CI, confidence interval; HCV, hepatitis C virus; ICG, indocyanine green retention; INR, international normalized ratio; mVI−/+, microvascular invasion absent/present; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; PNI prognostic nutritional index, PTT, partial thromboplastin time; sd, standard deviation.
Univariate and multivariate analyses
The results of the univariate and multivariate analyses of potential predictors of mVI are shown in Table 2. Univariate analysis identified two variables significantly associated with mVI after hepatic resection: PLR ≥102 (OR 2.385, p = 0.001) and tumor size ≥5 cm (OR 4.29, p < 0.001). In multivariate analysis, these variables remained significant predictive indicators of mVI: PLR ≥102 (OR 1.831, p = 0.034) and tumor size ≥5 cm (OR 3.791, p < 0.001).
Table 2.
Univariate and Multivariate Analysis of Predictors of Microvascular Invasion
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age (years) | 0.992 (0.96–1.02) | 0.535 | ||
| INR | 0.418 (0.04–4.15) | 0.457 | ||
| AFP (<400 ng/mL) | ||||
| >400 ng/mL | 1.557 (0.91–2.65) | 0.102 | ||
| PLR (<102) | ||||
| >102 | 2.385 (1.39–4.07) | 0.001 | 1.831(1.05–3.21) | 0.034 |
| PNI (<95) | ||||
| >95 | 0.736 (0.43–1.25) | 0.259 | ||
| NLR (<1.8) | ||||
| >1.8 | 1.167 (0.96–1.42) | 0.121 | ||
| Tumor size (<5cm) | ||||
| >5 cm | 4.299 (2.41–7.66) | 0 | 3.791(2.1–6.84) | 0 |
| Gender (Male) | ||||
| Female | 1.202 (0.70–2.06) | 0.502 | ||
| HBV (Negative) | ||||
| Positive | 0.702 (0.42–1.18) | 0.182 | ||
| HCV (Negative) | ||||
| Positive | 1.224 (0.64–2.35) | 0.543 | ||
| Alcohol (None) | ||||
| Occasional | 1.405 (0.74–2.68) | 0.302 | ||
| Alcoholism | 1.203 (0.60–2.40) | 0.599 | ||
| Child–Pugh score (A) | ||||
| B | 1.193 (0.37–3.82) | 0.767 | ||
| Multifocal tumor (Single) | ||||
| zMultiple | 1.233 (0.68–2.25) | 0.492 | ||
AFP, alpha-fetoprotein; CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; INR, international normalized ratio; NLR, neutrophil-to-lymphocyte ratio; OR, odds ratio; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutritional index.
Discussion
The presence of mVI is strongly associated with HCC recurrence and early disease-related death (Poon et al., 2000a; Poon et al., 2007; Lim et al., 2011). Several studies have shown that mVI+ patients have poorer prognosis than mVI− patients (Pawlik et al., 2005; Yamamoto et al., 2015; Hirokawa et al., 2016; Shimoda et al., 2016). Although there is currently no consensus definition of mVI, Rodriguez-Peralvarez (2013) recently proposed the following criteria: (i) the presence of tumor cells forming plug or polyp and (ii) tumor thrombus partially or totally covered by endothelial cells in either the portal vein or hepatic vein branches. They also suggested that tumor cells or small clusters of tumor cells free-floating inside isolated vessels but not covered by endothelium should not be considered part of the criteria (Rodriguez-Peralvarez et al., 2013). In the present study, we defined mVI according to these criteria. The rate of mVI in this study was 22.4%, comparable to that seen in previous studies (Kim et al., 2008; Eguchi et al., 2010; Rodriguez-Peralvarez et al., 2013; Shimoda et al., 2016). Since mVI cannot be identified preoperatively, knowledge of significant risk factors could be of great help to the surgeon for preoperative preparation. From univariate and multivariate analyses, we identified tumor size ≥5 cm and PLR ≥102 as preoperative predictors of mVI.
Angiogenesis is the main mechanism of mVI in HCC. Rodriguez-Peralvarez (2013) reported that vascular invasion results when tumor cells develop a sufficiently evolved phenotype to invade blood vessels and potentially begin to metastasis. The production of proteases and reduced expression of E-cadherin result in loss of tissue integrity and further facilitate tumor invasion (Wu et al., 2011; Rodriguez-Peralvarez et al., 2013). Pawlik (2005) reported that the incidence of mVI positively correlated with increasing tumor size, and that mVI was twice as common in patients with tumors ≥5 cm vs <5 cm.
Although it is well-established that tumor size is associated with the outcome after resection in HCC (Shirabe et al., 2014; Hwang et al., 2015; Lei et al., 2016) and that large tumor size is associated with mVI, there is some controversy about the optimal tumor size for risk stratification (Pawlik et al., 2005; Shindoh et al., 2013). In the present study, we identified a cut-off value of 5 cm for predicting mVI. Chen et al. reported that patients with smaller HCC tumors (<5 cm) have better prognosis (Chen et al., 2011). In addition, several large population studies in HCC have identified a relationship between mVI and >5 cm tumors (Pawlik et al., 2005; Kaibori et al., 2009; Huang et al., 2015; Hwang et al., 2015). In the Barcelona Clinic Liver Cancer staging system, patients with tumor sizes <5 cm are classified as having early stage HCC (Bruix et al., 2016). A single tumor of size ≤5 cm is one of the Milan criteria for liver transplantation (Mazzaferro et al., 2008). In accordance with these findings, we suggest a cut-off value of 5 cm for the association between tumor size and mVI.
Systemic inflammatory responses play critical roles in the pathogenesis and progression of cancer (Grivennikov et al., 2010). Inflammation promotes tumor angiogenesis, invasion, and metastasis through regulation of a subset of regulatory T lymphocytes and chemokines (Mantovani et al., 2008; Fan et al., 2015). Recently, Zhou and Templeton (Templeton et al., 2014; Zhou et al., 2014) reported that PLR is a significant biomarker of poor prognosis for many cancers, including HCC. Moreover, in patients who underwent hepatic resection, transplantation, and transarterial chemoembolization, high PLR levels were associated with poor prognosis and disease recurrence (Suh et al., 2012; Xue et al., 2015; Goh et al., 2016; Song et al., 2016; Yang et al., 2017).
Few studies have investigated the relationship between PLR and mVI (Ma et al., 2016; Zheng et al., 2017). Ma (2016) performed a meta-analysis of the prognostic value of PLR in HCC and found no association with vascular invasion; however, this conclusion was based on only three eligible studies, and vascular invasion was defined as including both macrovascular invasion and mVI. The Memorial Sloan Kettering Center group (Zheng et al., 2017) investigated the utility of serum inflammatory markers as predictors of mVI. They found that the mVI+ group had higher mean PLR values than the mVI−, but univariate and multivariate analyses identified significant associations between mVI and AFP, albumin, and radiologic tumor size, but not PLR. In contrast, PLR was significantly higher in the mVI+ group than mVI− group in our study, and univariate and multivariate analysis demonstrated that PLR >102 was an independent prognostic factor for mVI. These apparently discrepant results could be due to the higher proportion of HBV-infected patients (55.5% vs 25%) and alcoholic patients (44.3% vs 11%) in our study vs the Memorial Sloan Kettering Center study (Zheng et al., 2017). Inflammation plays a role in the severity of chronic HBV infection through antigen-nonspecific inflammatory cell enhancement of cytotoxic T lymphocyte-mediated liver damage, and platelets facilitate intrahepatic accumulation of cytotoxic T lymphocytes (Iannacone et al., 2005; Seki and Schwabe, 2015). In two studies examining inflammatory indices in patients with chronic HBV infection, Zhao and Wang (Wang et al., 2015; Zhao et al., 2017) both reported an association between PLR and liver fibrosis and disease severity.
Although the relationship between PLR and mVI has previously been investigated, no consensus cut-off value was obtained (Yamamura et al., 2014; Spolverato et al., 2015; Goh et al., 2016; Ma et al., 2016; Yang et al., 2017). Our finding that PLR >102 is an independent risk factor for mVI is consistent with previous studies, most of which identified PLR cut-off values of >100 (Ma et al., 2016). Notably, we showed that the combination of tumor size ≥5 cm and PLR ≥102 had superior predictive sensitivity and specificity compared with either measure (Figure 1).
Figure 1.
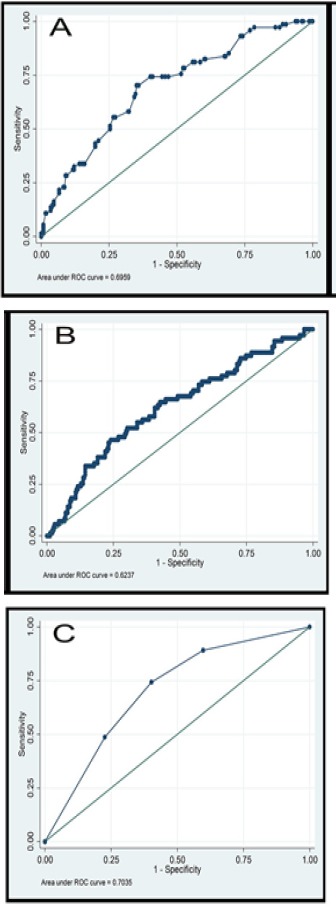
Receiver Operating Characteristic Curves for Predicting Microvascular Invasion. (A) Tumor size. (B) Platelet-to-lymphocyte ratio. (C) Tumor size ≥5 cm and platelet-to-lymphocyte ratio ≥102.
This study has some limitations. First, because of its retrospective nature, the study design could have some selection bias. Second, some preoperative inflammatory indices, such as the Glasgow Prognostic Score, could not be included because our center does not routinely measure C-reactive protein, which is a component of the Glasgow Prognostic Score.
In conclusions in this study, we demonstrated that large tumor size and high PLR are independent predictive risk factors for mVI. The suggested cut-off values are >5 cm for tumor size and 102 for PLR. When used in combination, these factors showed better discriminatory performance than either measure alone for predicting mVI. These findings may be helpful for surgeons in preoperative counselling for the patients undergoing HCC resection.
Author contributions
NR: study design, data collection and interpretation, writing and drafting of the manuscript; SM: data collection and analysis; PT: data collection and analysis; PM: data collection; WS: data collection; SA: data collection and analysis.
Fundings statement
The authors report no funding in this work.
Acknowledgments
We thank Napaphat Proprom for help with the statistical analysis and Anne M. O’Rourke, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
References
- 1.Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150:835–53. doi: 10.1053/j.gastro.2015.12.041. [DOI] [PubMed] [Google Scholar]
- 2.Cha C, Fong Y, Jarnagin WR, et al. Predictors and patterns of recurrence after resection of hepatocellular carcinoma. J Am Coll Surg. 2003;197:753–8. doi: 10.1016/j.jamcollsurg.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Chan AW, Chan SL, Wong GL, et al. Prognostic nutritional index (PNI) predicts tumor recurrence of very early/early stage hepatocellular carcinoma after surgical resection. Ann Surg Oncol. 2015;22:4138–48. doi: 10.1245/s10434-015-4516-1. [DOI] [PubMed] [Google Scholar]
- 4.Chen YL, Ko CJ, Chien SY, et al. Tumor size as a prognostic factor in resected small hepatocellular carcinoma:a controversy revisited. J Gastroenterol Hepatol. 2011;26:851–7. doi: 10.1111/j.1440-1746.2010.06595.x. [DOI] [PubMed] [Google Scholar]
- 5.Compton CC, Garcia-Aguilar J, Kurtzman SH, Olawaiye A, Washington MK. AJCC cancer staging atlas:A companion to the seventh editions of the AJCC cancer staging manual and handbook. New York: Springer; 2006. pp. 241–9. [Google Scholar]
- 6.Eguchi S, Takatsuki M, Hidaka M, et al. Predictor for histological microvascular invasion of hepatocellular carcinoma:A lesson from 229 consecutive cases of curative liver resection. World J Surg. 2010;34:1034–8. doi: 10.1007/s00268-010-0424-5. [DOI] [PubMed] [Google Scholar]
- 7.Fan ST, Lo CM, Liu CL, et al. Hepatectomy for hepatocellular carcinoma:toward zero hospital deaths. Ann Surg. 1999;229:322–30. doi: 10.1097/00000658-199903000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan W, Zhang Y, Wang Y, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as predictors of survival and metastasis for recurrent hepatocellular carcinoma after transarterial chemoembolization. PLoS One. 2015;10:e0119312. doi: 10.1371/journal.pone.0119312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goh BK, Kam JH, Lee SY, et al. Significance of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and prognostic nutrition index as preoperative predictors of early mortality after liver resection for huge (>/=10 cm) hepatocellular carcinoma. J Surg Oncol. 2016;113:621–7. doi: 10.1002/jso.24197. [DOI] [PubMed] [Google Scholar]
- 10.Gomez D, Farid S, Malik HZ, et al. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. World J Surg. 2008;32:1757–62. doi: 10.1007/s00268-008-9552-6. [DOI] [PubMed] [Google Scholar]
- 11.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirokawa F, Hayashi M, Asakuma M, et al. Risk factors and patterns of early recurrence after curative hepatectomy for hepatocellular carcinoma. Surg Oncol. 2016;25:24–9. doi: 10.1016/j.suronc.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Huang GQ, Zheng JN, Zou TT, et al. Stratified platelet-to-lymphocyte ratio:A novel target for prognostic prediction of hepatocellular carcinoma after curative liver resection. J Clin Transl Hepatol. 2017;5:35–42. doi: 10.14218/JCTH.2016.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang WJ, Jeng YM, Lai HS, et al. Tumor size is a major determinant of prognosis of resected stage I hepatocellular carcinoma. Langenbecks Arch Surg. 2015;400:725–34. doi: 10.1007/s00423-015-1329-4. [DOI] [PubMed] [Google Scholar]
- 15.Hwang S, Lee YJ, Kim KH, et al. The impact of tumor size on long-term survival outcomes after resection of solitary hepatocellular carcinoma:Single-institution experience with 2558 patients. J Gastrointest Surg. 2015;19:1281–90. doi: 10.1007/s11605-015-2849-5. [DOI] [PubMed] [Google Scholar]
- 16.Iannacone M, Sitia G, Isogawa M, et al. Platelets mediate cytotoxic T lymphocyte-induced liver damage. Nat Med. 2005;11:1167–9. doi: 10.1038/nm1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imamura H, Seyama Y, Kokudo N, et al. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138:1198–206. doi: 10.1001/archsurg.138.11.1198. discussion 206. [DOI] [PubMed] [Google Scholar]
- 18.Jin C, Li C, Peng W, et al. Changes of platelet times neutrophil to lymphocyte ratio predict BCLC stage A hepatocellular carcinoma survival. Medicine (Baltimore) 2017;96:e7821. doi: 10.1097/MD.0000000000007821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaibori M, Ishizaki M, Saito T, et al. Risk factors and outcome of early recurrence after resection of small hepatocellular carcinomas. Am J Surg. 2009;198:39–45. doi: 10.1016/j.amjsurg.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 20.Kamiyama T, Nakanishi K, Yokoo H, et al. Analysis of the risk factors for early death due to disease recurrence or progression within 1 year after hepatectomy in patients with hepatocellular carcinoma. World J Surg Oncol. 2012;10:107. doi: 10.1186/1477-7819-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim BK, Han KH, Park YN, et al. Prediction of microvascular invasion before curative resection of hepatocellular carcinoma. J Surg Oncol. 2008;97:246–52. doi: 10.1002/jso.20953. [DOI] [PubMed] [Google Scholar]
- 22.Lei Z, Li J, Wu D, et al. Nomogram for preoperative estimation of microvascular invasion risk in hepatitis B virus-related hepatocellular carcinoma within the Milan criteria. JAMA Surg. 2016;151:356–63. doi: 10.1001/jamasurg.2015.4257. [DOI] [PubMed] [Google Scholar]
- 23.Lim KC, Chow PK, Allen JC, et al. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg. 2011;254:108–13. doi: 10.1097/SLA.0b013e31821ad884. [DOI] [PubMed] [Google Scholar]
- 24.Lim KC, Chow PK, Allen JC, et al. Systematic review of outcomes of liver resection for early hepatocellular carcinoma within the Milan criteria. Br J Surg. 2012;99:1622–9. doi: 10.1002/bjs.8915. [DOI] [PubMed] [Google Scholar]
- 25.Liu W, Zhou JG, Sun Y, et al. Hepatic resection improved the long-term survival of patients with BCLC stage B hepatocellular carcinoma in Asia:a systematic review and meta-analysis. J Gastrointest Surg. 2015;19:1271–80. doi: 10.1007/s11605-015-2811-6. [DOI] [PubMed] [Google Scholar]
- 26.Ma W, Zhang P, Qi J, et al. Prognostic value of platelet to lymphocyte ratio in hepatocellular carcinoma:a meta-analysis. Sci Rep. 2016;6:35378. doi: 10.1038/srep35378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 28.Mazzaferro V, Chun YS, Poon RT, et al. Liver transplantation for hepatocellular carcinoma. Ann Surg Oncol. 2008;15:1001–7. doi: 10.1245/s10434-007-9559-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyagawa S, Makuuchi M, Kawasaki S, et al. Criteria for safe hepatic resection. Am J Surg. 1995;169:589–94. doi: 10.1016/s0002-9610(99)80227-x. [DOI] [PubMed] [Google Scholar]
- 30.Pawlik TM, Delman KA, Vauthey JN, et al. Tumor size predicts vascular invasion and histologic grade:Implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11:1086–92. doi: 10.1002/lt.20472. [DOI] [PubMed] [Google Scholar]
- 31.Poon RT, Fan ST, Lo CM, et al. Difference in tumor invasiveness in cirrhotic patients with hepatocellular carcinoma fulfilling the Milan criteria treated by resection and transplantation:impact on long-term survival. Ann Surg. 2007;245:51–8. doi: 10.1097/01.sla.0000225255.01668.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poon RT, Fan ST, Ng IO, et al. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2000a;89:500–7. [PubMed] [Google Scholar]
- 33.Poon RTP, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000b;232:10–24. doi: 10.1097/00000658-200007000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pote N, Cauchy F, Albuquerque M, et al. Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J Hepatol. 2015;62:848–54. doi: 10.1016/j.jhep.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Regimbeau JM, Abdalla EK, Vauthey JN, et al. Risk factors for early death due to recurrence after liver resection for hepatocellular carcinoma:Results of a multicenter study. J Surg Oncol. 2004;85:36–41. doi: 10.1002/jso.10284. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez-Peralvarez M, Luong TV, Andreana L, et al. A systematic review of microvascular invasion in hepatocellular carcinoma:diagnostic and prognostic variability. Ann Surg Oncol. 2013;20:325–39. doi: 10.1245/s10434-012-2513-1. [DOI] [PubMed] [Google Scholar]
- 37.Seki E, Schwabe RF. Hepatic inflammation and fibrosis:functional links and key pathways. Hepatology. 2015;61:1066–79. doi: 10.1002/hep.27332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimoda M, Tago K, Shiraki T, et al. Risk factors for early recurrence of single lesion hepatocellular carcinoma after curative resection. World J Surg. 2016;40:2466–71. doi: 10.1007/s00268-016-3529-7. [DOI] [PubMed] [Google Scholar]
- 39.Shindoh J, Andreou A, Aloia TA, et al. Microvascular invasion does not predict long-term survival in hepatocellular carcinoma up to 2 cm:reappraisal of the staging system for solitary tumors. Ann Surg Oncol. 2013;20:1223–9. doi: 10.1245/s10434-012-2739-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shirabe K, Toshima T, Kimura K, et al. New scoring system for prediction of microvascular invasion in patients with hepatocellular carcinoma. Liver Int. 2014;34:937–41. doi: 10.1111/liv.12459. [DOI] [PubMed] [Google Scholar]
- 41.Song W, Wang K, Zhong FP, et al. Clinicopathological and prognostic significance of platelet-to-lymphocyte ratio in patients with hepatocellular carcinoma. Oncotarget. 2016;7:81830–8. doi: 10.18632/oncotarget.13244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spolverato G, Maqsood H, Kim Y, et al. Neutrophil-lymphocyte and platelet-lymphocyte ratio in patients after resection for hepato-pancreatico-biliary malignancies. J Surg Oncol. 2015;111:868–74. doi: 10.1002/jso.23900. [DOI] [PubMed] [Google Scholar]
- 43.Suh YJ, Kim MJ, Choi JY, et al. Preoperative prediction of the microvascular invasion of hepatocellular carcinoma with diffusion-weighted imaging. Liver Transpl. 2012;18:1171–8. doi: 10.1002/lt.23502. [DOI] [PubMed] [Google Scholar]
- 44.Sumie S, Nakashima O, Okuda K, et al. The significance of classifying microvascular invasion in patients with hepatocellular carcinoma. Ann Surg Oncol. 2014;21:1002–9. doi: 10.1245/s10434-013-3376-9. [DOI] [PubMed] [Google Scholar]
- 45.Templeton AJ, Ace O, McNamara MG, et al. Prognostic role of platelet to lymphocyte ratio in solid tumors:a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2014;23:1204–12. doi: 10.1158/1055-9965.EPI-14-0146. [DOI] [PubMed] [Google Scholar]
- 46.Tian XC, Liu XL, Zeng FR, et al. Platelet-to-lymphocyte ratio acts as an independent risk factor for patients with hepatitis B virus-related hepatocellular carcinoma who received transarterial chemoembolization. Eur Rev Med Pharmacol Sci. 2016;20:2302–9. [PubMed] [Google Scholar]
- 47.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 48.Urabe M, Yamashita H, Watanabe T, et al. Comparison of prognostic abilities among preoperative laboratory data indices in patients with resectable gastric and esophagogastric junction adenocarcinoma. World J Surg. 2018;42:185–94. doi: 10.1007/s00268-017-4146-9. [DOI] [PubMed] [Google Scholar]
- 49.Wang Q, Blank S, Fiel MI, et al. The severity of liver fibrosis influences the prognostic value of inflammation-based scores in hepatitis B-associated hepatocellular carcinoma. Ann Surg Oncol. 2015;22:1125–32. doi: 10.1245/s10434-015-4598-9. [DOI] [PubMed] [Google Scholar]
- 50.Wu SJ, Lin YX, Ye H, et al. Lymphocyte to monocyte ratio and prognostic nutritional index predict survival outcomes of hepatitis B virus-associated hepatocellular carcinoma patients after curative hepatectomy. J Surg Oncol. 2016;114:202–10. doi: 10.1002/jso.24297. [DOI] [PubMed] [Google Scholar]
- 51.Wu SM, Huang YH, Yeh CT, et al. Cathepsin H regulated by the thyroid hormone receptors associate with tumor invasion in human hepatoma cells. Oncogene. 2011;30:2057–69. doi: 10.1038/onc.2010.585. [DOI] [PubMed] [Google Scholar]
- 52.Xia W, Ke Q, Wang Y, et al. Predictive value of pre-transplant platelet to lymphocyte ratio for hepatocellular carcinoma recurrence after liver transplantation. World J Surg Oncol. 2015;13:60. doi: 10.1186/s12957-015-0472-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xue TC, Jia QA, Ge NL, et al. The platelet-to-lymphocyte ratio predicts poor survival in patients with huge hepatocellular carcinoma that received transarterial chemoembolization. Tumour Biol. 2015;36:6045–51. doi: 10.1007/s13277-015-3281-x. [DOI] [PubMed] [Google Scholar]
- 54.Yamamoto Y, Ikoma H, Morimura R, et al. Optimal duration of the early and late recurrence of hepatocellular carcinoma after hepatectomy. World J Gastroenterol. 2015;21:1207–15. doi: 10.3748/wjg.v21.i4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamamura K, Sugimoto H, Kanda M, et al. Comparison of inflammation-based prognostic scores as predictors of tumor recurrence in patients with hepatocellular carcinoma after curative resection. J Hepatobiliary Pancreat Sci. 2014;21:682–8. doi: 10.1002/jhbp.114. [DOI] [PubMed] [Google Scholar]
- 56.Yang HJ, Jiang JH, Liu QA, et al. Preoperative platelet-to-lymphocyte ratio is a valuable prognostic biomarker in patients with hepatocellular carcinoma undergoing curative liver resection. Tumour Biol. 2017;39:1–8. doi: 10.1177/1010428317707375. [DOI] [PubMed] [Google Scholar]
- 57.Zhao Z, Liu J, Wang J, et al. Platelet-to-lymphocyte ratio (PLR) and neutrophil-to-lymphocyte ratio (NLR) are associated with chronic hepatitis B virus (HBV) infection. Int Immunopharmacol. 2017;51:1–8. doi: 10.1016/j.intimp.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 58.Zheng J, Seier K, Gonen M, et al. Utility of serum inflammatory markers for predicting microvascular invasion and survival for patients with hepatocellular carcinoma. Ann Surg Oncol. 2017;24:3706–37. doi: 10.1245/s10434-017-6060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou X, Du Y, Huang Z, et al. Prognostic value of PLR in various cancers:a meta-analysis. PLoS One. 2014;9:e101119. doi: 10.1371/journal.pone.0101119. [DOI] [PMC free article] [PubMed] [Google Scholar]


