Abstract
Objective:
Women who are currently using menopausal hormone therapy (MHT) have higher cerebrovascular reactivity when compared with postmenopausal women who are not taking MHT; however the effect of cessation of MHT on cerebrovascular reactivity is not known. Given that MHT can have structural and activational effects on vascular function, this study was performed to characterize cerebrovascular reactivity following cessation of MHT in women at low risk for cerebrovascular disease.
Methods:
Cerebrovascular reactivity measured in a subset of women from the Kronos Early Estrogen Prevention Study (KEEPS) three years after the cessation of the study drug [oral conjugated equine estrogen (oCEE); transdermal 17-β estradiol (tE2); or placebo (PLA)].
Results:
Age, body mass index, and blood pressure were comparable among groups. At rest, middle cerebral artery velocity (MCAv), cerebrovascular conductance index (CVCi), mean arterial pressure (MAP) and cerebral pulsatility index did not differ among groups. Slope-based summary measures of cerebrovascular reactivity did not differ significantly among groups. However, utilizing repeated measures modeling, there was a significant upward shift in MCAv responses (p=0.029) in the combined MHT group compared with PLA group.
Conclusion:
MHT has a marginal sustained effect on cerebrovascular reactivity when measured three years after cessation of hormone treatment.
Keywords: cerebral hemodynamics, menopause, estrogen, blood pressure, sex hormones
Introduction
The cerebrovasculature is highly sensitive to changes in partial pressure of carbon dioxide (CO2), vasodilating in response to hypercapnia, while vasoconstricting in response to hypocapnia. Cerebrovascular reactivity to hypercapnia is often used to estimate cerebral vascular responsiveness and has implications for cerebrovascular health.[1] Cross-sectional studies have shown that women experience an age-related decline in cerebrovascular reactivity after the 5th decade of life; however, this age-associated decline in cerebrovascular reactivity in women was attenuated in postmenopausal women using menopausal hormone therapy (MHT).[2] Previous studies that evaluated cerebral blood flow regulation in women in response to MHT used a short-term intervention or included a variety of MHT formulations in the same study. Long-term MHT use could affect the structure of blood vessels reflecting nuclear-directed changes in gene translation, gene transcription, and protein expression as well as activational effects on cerebrovascular endothelial or vascular smooth muscle cells through rapid membrane-associated receptor or enzyme systems.[3, 4] Therefore, these effects might be sustained to influence cerebral blood flow regulation following cessation of long-term MHT use. It is unclear whether MHT alters cerebral blood flow regulation beyond the years of hormonal treatment. Thus, the purpose of this study was to characterize cerebrovascular reactivity and cerebral pulsatility index (PI) three years after cessation of two different formulations of MHT in a well-characterized cohort of women who participated in the Kronos Early Estrogen Prevention Study (KEEPS).
Materials and Methods
Participants
In order to participate in KEEPS with randomization into treatment groups, women were required to be six months and three years past menopause (defined by serum measures of 17β estradiol and follicular stimulating hormone) and without prior cardiovascular events. Full inclusion criteria and clinical data for these participants are reported elsewhere. [5, 6] Postmenopausal women (n=74) who had participated in KEEPS at Mayo Clinic were invited to participate in the present follow-up study three years after their exit from the KEEPS. Inclusion criterial are included in the Supplement.
Sixty women qualified for and participated in the current study: 15 from the oral conjugated equine estrogen (oCEE) group, 22 from the transdermal 17-β estradiol (tE2) group, and 23 from the placebo (PLA) group. Data from two women in the tE2 group were excluded due to an unreliable Transcranial Doppler signal, resulting in a total of 58 women with complete data. All procedures were approved by the Institutional Review Board at the Mayo Clinic and were performed according to the Declaration of Helsinki. All participants gave written informed consent.
Screening
Blood samples were obtained after a 12-hour fast and analyzed for blood glucose, cholesterol, triglycerides, and high sensitivity C-reactive protein (hs-CRP) by the Clinical Laboratories at Mayo Clinic as previously described. [5] Prescription and over-the-counter medications were reviewed by a physician prior to the study day.
Experimental Protocol
Participants arrived at the Clinical Research Unit after a four-hour fast. They were asked to abstain from alcohol, caffeine, and chocolate for 24 hours prior to the study. After 10 minutes of rest, supine brachial cuff blood pressure was measured in triplicate (the means of these measures are reported in Table 1). Heart rate (HR) from a standard 3-lead ECG and oxygen saturation using pulse oximetry were monitored continuously throughout the study (Cardiocap/5, Datex-Ohmeda, Louisville, CO, USA). Beat-to-beat recording of mean arterial blood pressure (MAP) was monitored non-invasively using finger photoplethysmography (Nexfin, Edwards Lifesciences, Irvine, CA, USA). During stepped hypercapnia, breath-by-breath end-tidal CO2 (ETCO2) was measured using a nasal cannula.
Table 1.
Participant Characteristics
| PLA (n=23) |
tE2 (n=20) |
oCEE (n=15) |
P-value† | |
|---|---|---|---|---|
| Age, yr | 60 ± 3 | 60 ± 3 | 60 ± 3 | 0.930 |
| Height, cm | 165 ± 4 | 166 ± 4 | 161 ± 6 | 0.008 |
| Weight, kg | 77 ± 10 | 73 ± 15 | 72 ± 12 | 0.410 |
| BMI, kg/m2 | 28 ± 4 | 27 ± 5 | 28 ± 4 | 0.440 |
| Waist circumference, cm | 91 ± 10 | 87 ± 12 | 90 ± 11 | 0.373 |
| Systolic BP, mmHg | 125 ± 13 | 125 ± 11 | 124 ± 16 | 0.976 |
| Diastolic BP, mmHg | 77 ± 9 | 77 ± 7 | 77 ± 8 | 0.995 |
| Mean BP, mmHg | 93 ± 10 | 93 ± 8 | 93 ± 11 | 0.994 |
| Fasting glucose, mg/dL | 93 ± 9 | 95 ± 8 | 96 ± 6 | 0.685 |
| Total cholesterol, mg/dL | 210 ± 26 | 201 ± 30 | 208 ± 24 | 0.515 |
| HDL cholesterol, mg/dL | 64 ± 19 | 65 ± 13 | 67 ± 19 | 0.901 |
| LDL cholesterol, mg/dL | 126 ± 25 | 116 ± 31 | 117 ± 19 | 0.401 |
| Triglycerides, mg/dL | 98 ± 32 | 96 ± 39 | 119 ± 69 | 0.293 |
| hs-CRP, mg/dL | 0.16 ± 0.16 | 0.17 ± 0.15 | 0.31 ± 0.25 | 0.087‡ |
Mean ± SD. BMI = body mass index; BP = blood pressure; HDL = high density lipoprotein; LDL = low density lipoprotein; oCEE = oral conjugated equine estrogen; PLA = placebo; tE2 = transdermal 17-β estradiol.
P-value from one-way ANOVA. Participant characteristics were comparable among groups except for lower height in the oCEE group compared with PLA and tE2 groups (overall test P=0.008; oCEE vs. PLA, P=0.014; oCEE vs. tE2, P=0.003).
hsCRP values were log-transformed prior to statistical testing to satisfy normality assumptions.
Cerebral Blood Flow Velocity
To estimate middle cerebral artery (MCA) blood velocity and cerebral PI, the basal portion of the right MCA was insonated by placing the probe (2-MHz Doppler probe from Transcranial Doppler, Neurovision System, Multigon, Yonkers, NY, USA) over the temporal bone just above the zygomatic arch between the frontal process and the front of the ear. Once the optimal signal was determined, the probe was secured with a headband device to maintain proper position and angle throughout the protocol.
Hypercapnia Breathing Challenge
Cerebral blood flow velocity responses to hypercapnia were assessed using a steady state, open circuit technique.[7] Participants were positioned supine on a hospital bed with a mask covering the nose and mouth. The mask was attached to one-way Hans Rudolph valves to prevent rebreathing. After breathing room air for three minutes, stepwise CO2 elevations were applied by adding 2%, 4%, and 6% CO2, while the oxygen content was maintained at 21% and balanced by nitrogen.[1] The CO2 was elevated for three minutes at each level followed by a three-minute recovery period (breathing room air). Cerebrovascular reactivity was calculated from the slope of the relationship between MCA blood velocity (MCAv) and ETCO2. The coefficient of variation in calculated cerebrovascular reactivity between trials was 15±4%.[8] To minimize the effects of blood pressure on our MCAv measurements, we also calculated cerebrovascular conductance index (CVCi). To better understand the sympathoexciatory effects of hypercapnia, we also calculated MAP reactivity as the slope of the relationship between MAP and ETCO2. Because some quantitative information is lost when converting serial MCAv, CVCi, and MAP measurement into slopes, analysis of reactivity was considered using the raw measurements in repeated measures modeling.
Data Analysis and Statistics
Data was collected at 250Hz, stored on a laboratory computer and analyzed off-line with signal processing software (WinDaq, DATAQ Instruments, Akron, OH, USA). All variables of interest were continuously monitored throughout the hypercapnia trials. Beat-by-beat and breath-by breath measurements (MCAv, MAP, and ETCO2) were averaged over the final 60 seconds of room air breathing, at each level of hypercapnia, and during the last minute of recovery. CVCi was calculated as MCAv/MAP. Cerebral PI, a measure of the variability of blood velocity in a vessel within each cardiac cycle, was calculated as (systolic MCAv-diastolic MCAv) /mean MCAv for each stage of the breathing protocol. The linear slopes of the relationships between ETCO2 and MCAv, ETCO2 and CVCi, and ETCO2 and MAP were calculated as described below.
Data were analyzed with SAS statistical software, version 9.4 (SAS Institute, Cary, NC). Participant demographics and clinical characteristics were compared between prior treatments (PLA vs. oCEE vs. tE2) using one-way ANOVA. HsCRP values were skewed and log-transformed before analysis to satisfy normality assumptions. For our primary outcome variables, paired t-tests were used to compare response measurements at each level of hypercapnia with corresponding baseline measurement. Between-group differences in responses were also assessed separately by stage, with baseline differences analyzed via ANOVA and baseline-adjusted differences in response analyzed via ANCOVA.
For our primary analyses, repeated measurements for MCAv, CVCi, and MAP were reduced to single responses of cerebrovascular reactivity, each assuming a linear relationship between the raw measure and ETCO2 during hypercapnia and compared using one-way ANOVA tests. Additional statistical analysis is available in the Supplement.
Results
Clinical characteristics on the 58 participants are reported in Table 1 according to randomized treatment assignment from the previous KEEPS trial. At the time of the present study, the three groups were similar demographically, with comparable levels of cholesterol and blood pressure. Despite lower height in women previously randomized to oCEE, there were no significant differences in weight, BMI, or waist circumference among the groups.
At baseline (room air condition), MCAv, CVCi, MAP, and cerebral PI did not differ among the three groups (Supplemental Table 1), although the sensitivity analysis combining the hormone treatment groups showed a trend toward higher baseline MCAv, CVCi, and cerebral PI in the combined MHT group compared with PLA (Supplemental Table 2). Compared to baseline, MCAv, CVCi, and MAP increased in response to hypercapnia, with significant increases achieved during initial stages (2% or 4% hypercapnia) and continuing through the final stage (6% hypercapnia) for all groups. Cerebral PI decreased from baseline to 4% and 6% hypercapnia in the hormone groups and from baseline to 6% hypercapnia in the PLA group. With respect to the magnitude of change in these variables, there were no differences among the groups when analyzed separately by stage with adjustment for baseline values.
There were no differences in the single summary measures of MCAv or CVCi reactivity among the groups (Figure 1 & 2; Table 2), nor were there differences from the sensitivity analysis with the two MHT formulations combined (Table 2). Although the pattern of non-parallel lines suggests possible differences by treatment, there were no differences in MAP reactivity among groups (Figure 3; Table 2).
Figure 1.
The relationship between end-tidal CO2 (ETCO2) and middle cerebral artery velocity (MCAv) stratified by prior MHT assignment (Panel A) among 58 postmenopausal women (mean values and error bars showing 95% confidence intervals). There were no differences among the groups in MCAv reactivity (p=0.165; Panel B). The grey diamond indicates the mean and the grey bar indicates the median value on Figure 1B. oCEE = oral conjugated equine estrogen (green); PLA = placebo (blue); tE2 = transdermal 17-β estradiol (red).
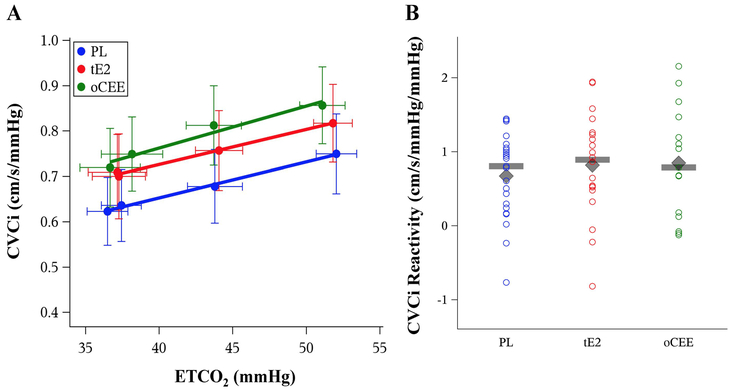
Figure 2.
The relationship between end-tidal CO2 (ETCO2) and cerebrovascular conductance index (CVCi) stratified by prior MHT assignment (Panel A) among 58 postmenopausal women (mean values and error bars showing 95% confidence intervals). There were no differences among the groups in CVCi reactivity (p=0.675; Panel B). The grey diamond indicates the mean and the grey bar indicates the median value on Figure 2B. oCEE = oral conjugated equine estrogen (green); PLA = placebo (blue); tE2 = transdermal 17-β estradiol (red).
Table 2.
Cerebrovascular Reactivity Measures According to Prior Treatment Group
| Summary Statistic Analysis | Repeated Measures Analysis |
|||||
|---|---|---|---|---|---|---|
| MHT | ||||||
| Variable | PLA | tE2 | oCEE | P-value† | P-value† (slope) |
P-value (shift) |
| MCAv Reactivity, cm/s/mmHg | ||||||
| Primary: PLA vs. tE2 vs. oCEE | 1.18 ± 0.71 | 1.44 ± 0.95 | 1.74 ± 0.98 | 0.165 | 0.094* | 0.064* |
| Secondary: PLA vs. MHT | 1.57 ± 0.96 | 0.107 | 0.070 | 0.029 | ||
| CVCi Reactivity, cm/s/mmHg/mmHg | ||||||
| Primary: PLA vs. tE2 vs. oCEE | 0.68 ± 0.57 | 0.82 ± 0.70 | 0.85 ± 0.75 | 0.675 | 0.853 | 0.192 |
| Secondary: PLA vs. MHT | 0.83 ± 0.71 | 0.378 | 0.574 | 0.075 | ||
| MAP Reactivity, mmHg/mmHg | ||||||
| Primary: PLA vs. tE2 vs. oCEE | 0.77 ± 0.83 | 0.77 ± 0.64 | 1.09 ± 0.70 | 0.342 | 0.051* | 0.742 |
| Secondary: PLA vs. MHT | 0.91 ± 0.67 | 0.493 | 0.063 | 0.946 | ||
Mean ± SD. MCAv=middle cerebral artery velocity; CVCi=cerebrovascular conductance index; MAP=mean arterial blood pressure; MHT= menopausal hormone therapy; oCEE = oral conjugated equine estrogen; PLA = placebo; tE2 = transdermal 17-β estradiol.
P-value from ANOVA of the differences in slope.
Individual contrast of oCEE vs. PLA was significant (P<0.05). Both the summary statistic (primary analysis) and the repeated measures (sensitivity analysis) methods were used to test for a difference in slopes (reactivity) across treatments. There were no significant differences in slope using the summary approach whereas the modeling approach, which capitalizes on the full information of repeated measurements, showed trends toward treatment-varying reactivity for MCAv and MAP. Furthermore, the repeated measure models provided tests for an overall shift effect from a treatment effect, demonstrating a borderline to significant overall effect in MCAv (overall 3-group comparison, P=0.064; 2-group comparison, P=0.029), and a trend toward an overall shift in CVCi in the PLA vs. MHT contrast (P=0.075).
Figure 3.
The relationship between end-tidal CO2 (ETCO2) and mean arterial pressure (MAP) stratified by prior MHT assignment (Panel A) among 58 postmenopausal women (mean values and error bars showing 95% confidence intervals). There was a modest, yet nonsignificant trend for a difference in MAP Reactivity between groups when analyzed with repeated measures modeling (p=0.051). The grey diamond indicates the mean and the grey bar indicates the median value on Figure 3B. oCEE = oral conjugated equine estrogen (green); PLA = placebo (blue); tE2 = transdermal 17-β estradiol (red).
In the repeated measures analysis, there was a trend for an interaction between treatment groups and MCAv both when analyzed with both distinct and pooled MHT formulations (Table 2). Additionally, there was marginal evidence of an overall shift in MCAv reactivity due to treatment group (P = 0.064), with overall higher MCAv levels in the combined MHT group (P = 0.029). A comparison of the CVCi response revealed no treatment interaction in agreement with the pattern of parallel lines in Figure 2A. However, there was a trend towards an overall higher CVCi in the combined MHT group (Table 2).
The repeated measures analysis also revealed a trend for a treatment interaction with MAP reactivity, indicating progressively larger MAP responses with increasing ETCO2 in the MHT group, particularly in oCEE, vs. the PLA group (Table 2). In addition, cerebral PI responses to hypercapnia, which declined quadratically with escalating CO2 level, showed a trend toward a treatment interaction suggesting progressively lower cerebral PI in the oCEE group (Supplemental Figure).
Discussion
Menopausal treatment with oCEE or tE2, when initiated early in menopause, appears to marginally impact cerebrovascular reactivity to hypercapnia years after cessation of treatment. For the summary indices of cerebrovascular reactivity, the effect of prior treatment group was not significant; however, in the repeated measures analysis, MCAv was overall statistically greater in the combined MHT group, and there were trends towards increased CVCi and overall MAP responses in the MHT groups. Similar trends were noted when comparisons were performed on all three groups, although no significant differences were detected. Taken together, these analyses suggest that MHT had marginal effects on cerebrovascular responses to hypercapnia when evaluated three years after treatment cessation. Importantly, this is the first study to evaluate the potential effect of MHT on cerebrovascular variables years after MHT use.
Following the publication of results from the Women’s Health Initiative in 2002, MHT was not recommended for the prevention of CV disease.[9] However, the major criticism of that study was that participants included women who were over a decade beyond the onset of menopause, many of whom had already developed atherosclerosis.[10, 11] Additionally, the generalization of results to all women regardless of age and MHT formulation was not appropriate. [12] Since then, additional analyses and studies suggest that MHT is not associated with an increase in absolute CV disease risk if used early after menopause, prior to the development of atherosclerosis, and at a low dose for a short duration.[11, 13] In addition, clinical trials on MHT, when initiated early in the menopausal transition, demonstrated an improvement in cardiovascular and neurological clinical outcomes in some women.[14, 15, 16]
The KEEPS was a randomized, placebo-controlled trial that was initiated to evaluate effects of MHT on progression of atherosclerosis when initiated within 3 years of menopause. The women of the present study participated in KEEPS at Mayo Clinic and a subset from this group were invited to participate in this follow-up study. We were specifically interested in whether or not previous use of MHT was associated with a significant effect on cerebral circulation in the women who previously participated in the KEEPS trial. Previous studies indicate that postmenopausal women have lower cerebrovascular reactivity than premenopausal women with similar biological age.[17] Furthermore, when postmenopausal women who were taking MHT were compared with those who had never taken MHT, those taking MHT had similar cerebrovascular reactivity to young premenopausal women.[2] Yet, a short-term intervention with two MHT formulations in postmenopausal women determined no difference in cerebrovascular reactivity.[18] To date, there is limited information regarding the long-term outcomes of MHT use, and that any potential MHT effects are activational, i.e. reversed in the absence of therapy. Thus, our study provides novel information regarding the effects of cessation of two different formulations of MHT and suggests that there is only a marginal sustained effect of the treatments after three years.
Estrogen causes dilation of blood vessels by enhancing the production of endothelial-derived nitric oxide (NO) and prostacyclin (PGI2) through an increase in the expression and activity of the enzymes endothelial NO synthase, cyclooxygenase, and PGI2 synthase [19, 20, 21, 22]. These estrogen-derived changes lead to a decrease in cerebral vascular tone in and an increase in cerebral blood flow.[20, 21, 22, 23] For example, estrogen administered in perimenopausal rats led to less vasoconstriction and increased dilation of the MCA; however, estrogen treatment in older reproductively senescent rats had the opposite effect.[20] Thus, estrogen administration in rats could have a negative consequences in the cerebrovasculature if administered late after menopause which is consistent with the “timing hypothesis”.[24] Importantly, MHT was initiated early in menopause (six months to three years) in KEEPS.
In addition to the vasodilatory response to hypercapnia, we also investigated cerebral PI. A higher cerebral PI reflects greater pulsatile flow in the cerebral circulation and may contribute to cerebral small vessel disease.[25] [26] Previous studies have reported that six months of MHT reduced cerebral PI with reversal of the effect within three months of cessation of MHT.[27] [28] Therefore, it seems that any beneficial decrease in cerebral PI that may occur during MHT treatment are not sustained long-term, and may even shift to higher PI. These results are consistent with the findings of the present study that showed no difference in baseline cerebral PI (room air condition) among groups in primary or secondary analysis. We did report an interesting trend for progressively lower cerebral PI in the oCEE group during hypercapnia, but these results should be interpreted with caution due to high heart rates during 6% hypercapnia.
Our data in the present study may have relevance for migraine. Migraine headaches are common in young women and the menopausal transition may increase migraine frequency.[29] Therefore, hormone therapy has been suggested for the management of migraine in postmenopausal women.[30] Yet, there are studies that report worsening migraine symptoms after MHT in postmenopausal women.[31] This controversy may be related to the study population (i.e. women with history of migraine vs. no history of migraine) or due to the acute and chronic effects of MHT on the cerebral circulation. Chronic effects of estrogen on the cerebral blood vessels may take several weeks [32] or several months.[33] In contrast, a single dose of intranasal estrogen may increase cerebral blood flow over several hours, suggesting that non-genomic mechanisms may be responsible for this transient change in cerebral hemodynamics.[34, 35]. Because we did not observe sustained differences in cerebral hemodynamics in the women treated with hormones, our study would suggest that effects of MHT may not sustain to alter migraine incidence or severity. However, additional studies are needed to determine the impact of MHT on cerebral hemodynamics in women with a history of migraine.
There are several methodological considerations with our experimental approach. First, transcranial Doppler was used to measure cerebrovascular reactivity, and therefore, blood velocity to was used to estimate blood flow of the MCA. Measuring MCAv using Doppler is an ideal approach to determine the beat-by-beat changes during hypercapnia. Changes in MCAv correspond to hypercapnia-related changes in cerebral blood flow to hypercapnia measured using other techniques [36, 37, 38]. Blood velocity is a reliable indicator of flow when diameter or cross-sectional area of the insonated vessel is constant. Accordingly, there are minimal changes in MCA cross-sectional area during hypercapnia when measured using MRI or angiography,[37, 39, 40, 41] although more recent studies have shown that vasodilation of the MCA may occur with large increases in ETCO2.[42] Furthermore, altered arterial CO2 (during hypercapnia) induces changes in the microvasculature downstream from the MCA allowing the use of MCA velocity as an indicator of cerebral blood flow.[37, 40] A previous study showed differences in the magnitude of change in MCA diameter between young and older adults, so it is important to note that the women in our study were of similar ages.[43]
In our analysis, we used the summary statistic approach tested on slope-based reactivity measures and repeated measures models fit for each outcome to further assess potential differences in cerebrovascular reactivity and examine the robustness of our results. Because these two analytic techniques result in slightly different conclusions for the combined MHT group, both are presented in detail (see Supplement). One explanation for the difference in statistical results is due to our small sample size, given that tests from repeated measures models are more sensitive than tests comparing univariate statistics for the hypercapnia responses. Reducing the four serial measurements to a single slope estimate per participant will inevitably result in loss of (potentially important) quantitative information. The repeated measures statistical analysis makes complete use of the available data and is considered more statistically efficient. An additional advantage of the repeated measures approach is the ability to test, not only whether reactivity differed among MHT groups, but also to test for an overall shift in the cerebrovascular measure (before and during hypercapnia) due to prior MHT use.
This study was a cross-sectional design as cerebrovascular measurements were not collected in participants either prior to or immediately after 48 months of MHT. While this information as part of a randomized control would be helpful to clarify the effect of current use of MHT, the information presented still allows us explore whether any effects of these lower dose MHT formulations persists up to three years beyond the treatment window.
Cerebral blood flow and its regulation is an important marker of future risk of stroke and lower cerebrovascular reactivity is associated with an increased risk of stroke and mortality, [44, 45] as well as cognitive decline.[46, 47, 48] Changes in cerebrovascular reactivity in women around menopause may contribute to the increased risk of stroke that is seen in postmenopausal women.[49] While MHT may increase cerebrovascular reactivity in postmenopausal women similar to that seen in premenopausal women,[2] the results of the present study show that in women who were part of the KEEPS, cerebrovascular reactivity to hypercapnia is only marginally sustained three years after discontinuation of two different formulations of MHT compared to placebo. Thus, the effect of previous use of MHT, when initiated recently after menopause, does not affect cerebrovascular variables years after cessation of treatment. Importantly, these findings may be especially relevant to cognition because many of the risk factors for cognitive decline (e.g. hypertension, diabetes, dyslipidemia, and physical inactivity) are augmented in the postmenopausal stage and are known to affect the microcirculation across several vascular beds. Thus, studies are thus needed to: 1) explore mechanisms that may lead to the age-dependent responses to MHT; and 2) incorporate new interventions that improve and maintain cerebral blood flow regulation in women after menopause.
Supplementary Material
Acknowledgements
The authors would like to thank the volunteers who participated in the study. In addition, the authors thank Lynne T. Shuster, Kim Jensen, Shelly K. Roberts, Sarah Wolhart, and Christopher P. Johnson for their technical assistance.
Sources of Funding
NIH grants AG044170, HL118154, and HL83497.
Footnotes
Disclosures
None.
References
- 1.Xie A, Skatrud JB, Morgan B, et al. Influence of cerebrovascular function on the hypercapnic ventilatory response in healthy humans. J Physiol. 2006. November 15;577(Pt 1):319–29. PubMed PMID: 16931556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kastrup A, Dichgans J, Niemeier M, et al. Changes of cerebrovascular CO2 reactivity during normal aging. Stroke. 1998. July;29(7):1311–4. PubMed PMID: 9660378. [DOI] [PubMed] [Google Scholar]
- 3.Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacol Rev. 2008. June;60(2):210–41. doi: 10.1124/pr.107.08002. PubMed PMID: 18579753; PubMed Central PMCID: PMCPMC2637768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol. 2011. August 16;7(12):715–26. doi: 10.1038/nrendo.2011.122. PubMed PMID: 21844907; PubMed Central PMCID: PMCPMC3474542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raz L, Jayachandran M, Tosakulwong N, et al. Thrombogenic microvesicles and white matter hyperintensities in postmenopausal women [Research Support, N.I.H., Extramural]. Neurology. 2013. March 5;80(10):911–8. doi: 10.1212/WNL.0b013e3182840c9f. PubMed PMID: 23408873; PubMed Central PMCID: PMC3653211. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller VM, Lahr BD, Bailey KR, et al. Longitudinal effects of menopausal hormone treatments on platelet characteristics and cell-derived microvesicles. Platelets. 2016. January;27(1):32–42. doi: 10.3109/09537104.2015.1023273. PubMed PMID: 25856160; PubMed Central PMCID: PMCPMC4732432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berkenbosch A, Bovill JG, Dahan A, et al. The ventilatory CO2 sensitivities from Read’s rebreathing method and the steady-state method are not equal in man. J Physiol. 1989. April;411:367–77. PubMed PMID: 2515274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnes JN, Schmidt JE, Nicholson WT, et al. Cyclooxygenase inhibition abolishes age-related differences in cerebral vasodilator responses to hypercapnia [Research Support, N.I.H., Extramural]. J Appl Physiol (1985). 2012. June;112(11):1884–90. doi: 10.1152/japplphysiol.01270.2011. PubMed PMID: 22442028; PubMed Central PMCID: PMC3379157. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002. July 17;288(3):321–33. PubMed PMID: 12117397. [DOI] [PubMed] [Google Scholar]
- 10.Naftolin F, Taylor HS, Karas R, et al. The Women’s Health Initiative could not have detected cardioprotective effects of starting hormone therapy during the menopausal transition. Fertil Steril. 2004. June;81(6):1498–501. doi: 10.1016/j.fertnstert.2004.02.095. PubMed PMID: 15193467. [DOI] [PubMed] [Google Scholar]
- 11.Clark JH. A critique of Women’s Health Initiative Studies (2002–2006). Nucl Recept Signal. 2006. October 30;4:e023. doi: 10.1621/nrs.04023. PubMed PMID: 17088939; PubMed Central PMCID: PMCPMC1630688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langer RD, Simon JA, Pines A, et al. Menopausal hormone therapy for primary prevention: why the USPSTF is wrong. Climacteric. 2017. October;20(5):402–413. doi: 10.1080/13697137.2017.1362156. PubMed PMID: 28805475. [DOI] [PubMed] [Google Scholar]
- 13.Raz L. Estrogen and cerebrovascular regulation in menopause. Mol Cell Endocrinol. 2014. May 25;389(1–2):22–30. doi: 10.1016/j.mce.2014.01.015. PubMed PMID: 24472522. [DOI] [PubMed] [Google Scholar]
- 14.Harman SM, Black DM, Naftolin F, et al. Arterial imaging outcomes and cardiovascular risk factors in recently menopausal women: a randomized trial. Ann Intern Med. 2014. August 19;161(4):249–60. doi: 10.7326/M14-0353. PubMed PMID: 25069991. [DOI] [PubMed] [Google Scholar]
- 15.Schierbeck LL, Rejnmark L, Tofteng CL, et al. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomised trial. BMJ. 2012. October 09;345:e6409. doi: 10.1136/bmj.e6409. PubMed PMID: 23048011. [DOI] [PubMed] [Google Scholar]
- 16.Scott E, Zhang QG, Wang R, et al. Estrogen neuroprotection and the critical period hypothesis. Front Neuroendocrinol. 2012. January;33(1):85–104. doi: 10.1016/j.yfrne.2011.10.001. PubMed PMID: 22079780; PubMed Central PMCID: PMCPMC3288697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matteis M, Troisi E, Monaldo BC, et al. Age and sex differences in cerebral hemodynamics: a transcranial Doppler study [Comparative Study]. Stroke; a journal of cerebral circulation. 1998. May;29(5):963–7. PubMed PMID: 9596243; eng. [DOI] [PubMed] [Google Scholar]
- 18.Bain CA, Walters MR, Lees KR, et al. The effect of HRT on cerebral haemodynamics and cerebral vasomotor reactivity in post-menopausal women. Hum Reprod. 2004. October;19(10):2411–4. doi: 10.1093/humrep/deh396. PubMed PMID: 15284214. [DOI] [PubMed] [Google Scholar]
- 19.Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol. 2004. February;286(2):R233–49. doi: 10.1152/ajpregu.00338.2003. PubMed PMID: 14707008. [DOI] [PubMed] [Google Scholar]
- 20.Deer RR, Stallone JN. Effects of estrogen on cerebrovascular function: age-dependent shifts from beneficial to detrimental in small cerebral arteries of the rat. Am J Physiol Heart Circ Physiol. 2016. May 15;310(10):H1285–94. doi: 10.1152/ajpheart.00645.2015. PubMed PMID: 26993224; PubMed Central PMCID: PMCPMC4895833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stirone C, Boroujerdi A, Duckles SP, et al. Estrogen receptor activation of phosphoinositide-3 kinase, akt, and nitric oxide signaling in cerebral blood vessels: rapid and long-term effects. Mol Pharmacol. 2005. January;67(1):105–13. doi: 10.1124/mol.104.004465. PubMed PMID: 15496504. [DOI] [PubMed] [Google Scholar]
- 22.Geary GG, Krause DN, Duckles SP. Estrogen reduces mouse cerebral artery tone through endothelial NOS- and cyclooxygenase-dependent mechanisms. Am J Physiol Heart Circ Physiol. 2000. August;279(2):H511–9. PubMed PMID: 10924048. [DOI] [PubMed] [Google Scholar]
- 23.Deer RR, Stallone JN. Effects of age and sex on cerebrovascular function in the rat middle cerebral artery. Biol Sex Differ. 2014;5:12. doi: 10.1186/s13293-014-0012-8. PubMed PMID: 25780555; PubMed Central PMCID: PMCPMC4360140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, menopause, estrogen, and cognitive aging: the timing hypothesis. Neurodegener Dis. 2010;7(1–3):163–6. doi: 10.1159/000289229. PubMed PMID: 20197698; PubMed Central PMCID: PMCPMC2859235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zarrinkoob L, Ambarki K, Wahlin A, et al. Aging alters the dampening of pulsatile blood flow in cerebral arteries. J Cereb Blood Flow Metab. 2016. September;36(9):1519–27. doi: 10.1177/0271678X16629486. PubMed PMID: 26823470; PubMed Central PMCID: PMCPMC5012521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell GF, van Buchem MA, Sigurdsson S, et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility--Reykjavik study [Research Support, N.I.H., Extramural Research Support, N.I.H., Intramural Research Support, Non-U.S. Gov’t]. Brain. 2011. November;134(Pt 11):3398–407. doi: 10.1093/brain/awr253. PubMed PMID: 22075523; PubMed Central PMCID: PMC3212721. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sendag F, Terek MC, Karadadas N, et al. Effects of oral and transdermal hormone replacement therapy on internal carotid artery pulsatility indices in postmenopausal women. A prospective, randomized, comparative study. J Reprod Med. 2001. November;46(11):962–8. PubMed PMID: 11762152. [PubMed] [Google Scholar]
- 28.Penotti M, Nencioni T, Gabrielli L, et al. Blood flow variations in internal carotid and middle cerebral arteries induced by postmenopausal hormone replacement therapy. Am J Obstet Gynecol. 1993. November;169(5):1226–32. PubMed PMID: 8238189. [DOI] [PubMed] [Google Scholar]
- 29.Wang SJ, Fuh JL, Lu SR, et al. Migraine prevalence during menopausal transition. Headache. 2003. May;43(5):470–8. PubMed PMID: 12752752. [DOI] [PubMed] [Google Scholar]
- 30.Shuster LT, Faubion SS, Sood R, et al. Hormonal manipulation strategies in the management of menstrual migraine and other hormonally related headaches. Curr Neurol Neurosci Rep. 2011. April;11(2):131–8. doi: 10.1007/s11910-010-0174-7. PubMed PMID: 21207200. [DOI] [PubMed] [Google Scholar]
- 31.Aegidius KL, Zwart JA, Hagen K, et al. Hormone replacement therapy and headache prevalence in postmenopausal women. The Head-HUNT study. Eur J Neurol. 2007. January;14(1):73–8. doi: 10.1111/j.1468-1331.2006.01557.x. PubMed PMID: 17222117. [DOI] [PubMed] [Google Scholar]
- 32.Gangar KF, Vyas S, Whitehead M, et al. Pulsatility index in internal carotid artery in relation to transdermal oestradiol and time since menopause. Lancet. 1991. October 5;338(8771):839–42. PubMed PMID: 1681213. [DOI] [PubMed] [Google Scholar]
- 33.Cacciatore B, Paakkari I, Toivonen J, et al. Randomized comparison of oral and transdermal hormone replacement on carotid and uterine artery resistance to blood flow. Obstet Gynecol. 1998. October;92(4 Pt 1):563–8. PubMed PMID: 9764629. [DOI] [PubMed] [Google Scholar]
- 34.Acar M, Cevrioglu AS, Haktanir A, et al. Effect of Aerodiol administration on cerebral blood flow volume in postmenopausal women. Maturitas. 2005. October 16;52(2):127–33. doi: 10.1016/j.maturitas.2005.01.006. PubMed PMID: 16186075. [DOI] [PubMed] [Google Scholar]
- 35.Ciccone MM, Scicchitano P, Gesualdo M, et al. Systemic vascular hemodynamic changes due to 17-beta-estradiol intranasal administration. J Cardiovasc Pharmacol Ther. 2013. July;18(4):354–8. doi: 10.1177/1074248413484385. PubMed PMID: 23624711. [DOI] [PubMed] [Google Scholar]
- 36.Bishop CC, Powell S, Rutt D, et al. Transcranial Doppler measurement of middle cerebral artery blood flow velocity: a validation study. Stroke; a journal of cerebral circulation. 1986. Sep-Oct;17(5):913–5. PubMed PMID: 3764963; eng. [DOI] [PubMed] [Google Scholar]
- 37.Poulin MJ, Robbins PA. Indexes of flow and cross-sectional area of the middle cerebral artery using doppler ultrasound during hypoxia and hypercapnia in humans. Stroke. 1996. December;27(12):2244–50. PubMed PMID: 8969788. [DOI] [PubMed] [Google Scholar]
- 38.Serrador JM, Picot PA, Rutt BK, et al. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke. 2000. July;31(7):1672–8. PubMed PMID: 10884472. [DOI] [PubMed] [Google Scholar]
- 39.Giller CA, Bowman G, Dyer H, et al. Cerebral arterial diameters during changes in blood pressure and carbon dioxide during craniotomy. Neurosurgery. 1993. May;32(5):737–41; discussion 741–2. PubMed PMID: 8492848; eng. [PubMed] [Google Scholar]
- 40.Bradac GB, Simon RS, Heidsieck CH. Angiographically verified transient alteration of the intracranial arteries and veins in dependence of different CO2 tensions. Neuroradiology. 1976;10(5):257–62. PubMed PMID: 945488; eng. [DOI] [PubMed] [Google Scholar]
- 41.Huber P, Handa J. Effect of contrast material, hypercapnia, hyperventilation, hypertonic glucose and papaverine on the diameter of the cerebral arteries. Angiographic determination in man. Invest Radiol. 1967. Jan-Feb;2(1):17–32. PubMed PMID: 6031628; eng. [DOI] [PubMed] [Google Scholar]
- 42.Coverdale NS, Gati JS, Opalevych O, et al. Cerebral blood flow velocity underestimates cerebral blood flow during modest hypercapnia and hypocapnia. J Appl Physiol (1985). 2014. November 15;117(10):1090–6. doi: 10.1152/japplphysiol.00285.2014. PubMed PMID: 25012027. [DOI] [PubMed] [Google Scholar]
- 43.Coverdale NS, Badrov MB, Shoemaker JK. Impact of age on cerebrovascular dilation versus reactivity to hypercapnia. J Cereb Blood Flow Metab. 2017. January;37(1):344–355. doi: 10.1177/0271678X15626156. PubMed PMID: 26759432; PubMed Central PMCID: PMCPMC5363751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silvestrini M, Pasqualetti P, Baruffaldi R, et al. Cerebrovascular reactivity and cognitive decline in patients with Alzheimer disease [Research Support, Non-U.S. Gov’t]. Stroke; a journal of cerebral circulation. 2006. April;37(4):1010–5. doi: 10.1161/01.STR.0000206439.62025.97. PubMed PMID: 16497984; eng. [DOI] [PubMed] [Google Scholar]
- 45.Portegies ML, de Bruijn RF, Hofman A, et al. Cerebral vasomotor reactivity and risk of mortality: the Rotterdam Study. Stroke. 2014. January;45(1):42–7. doi: 10.1161/STROKEAHA.113.002348. PubMed PMID: 24203842. [DOI] [PubMed] [Google Scholar]
- 46.Bangen KJ, Nation DA, Clark LR, et al. Interactive effects of vascular risk burden and advanced age on cerebral blood flow. Front Aging Neurosci. 2014;6:159. doi: 10.3389/fnagi.2014.00159. PubMed PMID: 25071567; PubMed Central PMCID: PMCPMC4083452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Catchlove SJ, Parrish TB, Chen Y, et al. Regional Cerebrovascular Reactivity and Cognitive Performance in Healthy Aging. J Exp Neurosci. 2018;12:1179069518785151. doi: 10.1177/1179069518785151. PubMed PMID: 30013388; PubMed Central PMCID: PMCPMC6043917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peng SL, Chen X, Li Y, et al. Age-related changes in cerebrovascular reactivity and their relationship to cognition: A four-year longitudinal study. Neuroimage. 2018. July 1;174:257–262. doi: 10.1016/j.neuroimage.2018.03.033. PubMed PMID: 29567504; PubMed Central PMCID: PMCPMC5949266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bushnell C, McCullough LD, Awad IA, et al. Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014. May;45(5):1545–88. doi: 10.1161/01.str.0000442009.06663.48. PubMed PMID: 24503673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.