Abstract
Copper-mediated radiofluorination provides a quick and versatile approach for 18F-labeling of arenes and heteroarenes. However, this method is known to be base-sensitive which has been a barrier for preparative scale radiosynthesis. In this report, we provide an approach for copper-mediated radiofluorination without azeotropic drying or adding a base. [18F]Fluoride trapped on a PS-HCO3 Sep-Pak was quantitatively eluted with a solution of 4-dimethylaminopyridinium trifluoromethanesulfonate (DMAP·OTf) in anhydrous N,N-dimethylformamide (DMF). The eluted solution was directly used for copper-mediated radiofluorination. Twelve boronic ester substrates were tested, yielding fluorinated products in 27 – 83% radiochemical yield based on HPLC analysis. This approach was successfully applied to the radiosynthesis of [18F]flumazenil, a well-known PET tracer for imaging central benzodiazepine receptors, with a radiochemical yield of 47%. This highly efficient protocol significantly augments the powerful copper-mediated radiofluorination approach.
1. Introduction
Positron emission tomography (PET) is one of the leading imaging techniques in both clinical and research settings.1-4 It provides valuable functional information about a living subject, which helps the physicians and researchers for disease diagnosis, therapy monitoring, and drug evaluation. Among the PET radionuclides, fluorine-18 is undoubtedly the most popular isotope.4, 5 It has a convenient half-life of 110 min, which allows multi-step syntheses and regional dose transportation. In addition, fluorine-18 has a clean decay profile (97% positron emission) and low β+ decay energy (0.633 MeV) which is optimal for high resolution PET imaging.6
The traditional radiofluorination strategy involves electrophilic or nucleophilic substitution, with the latter being commonly used for decades.4, 7-9 However, radiofluorination of electron rich arenes or heteroarenes remains challenging.10, 11 In the past decade, numerous approaches have been developed to prepare fluorine-18 labeled arenes or heteroarenes, including iodonium salt, 12-14 ylide,15, 16 and transition metal mediated approaches.17-24 Among these, the copper-mediated radiofluorination approach developed by Gouverneur group has gained a great deal of attention due to the tolerance of a wide range of functional groups on both electron-rich or electron-deficient substrates.22 By treating the pinacolyl arylboronate (arylBPin) precursor with Cu(OTf)2py4 and [18F]KF/K222, a number of 18F-labeled arenes and heteroarenes were readily prepared.22 Further optimization of this efficient method has been performed by several research groups. For example, a “low-base” protocol was developed to efficiently produce PET tracers on a preparative scale.25 Other substrates such as boronic acids and arylstannanes have also been successfully evaluated.23, 26 However, the excess base and phase transfer agent (PTA) can significantly diminish the conversion, since the Cu catalyst possess low stability under basic conditions.23, 25 It is still challenging to apply this method on scale-up applications and routine productions of PET tracers. Recently, Mossine et al reported the use of an organic base, 4-(dimethylamino)pyridine (DMAP), and a customized elution technique for Cu-mediated radiofluorination with up to 58% radiochemical yield (RCY) based on radio-TLC.27 However, the isolated yield is relatively low, possibly due to activity loss during elution and azeotropic drying. In another recent publication a Et4N·OTf elution approach was developed for successful 18F-fluorodestannylation through the copper-mediated process.28 Overall, Cu-mediated radiofluorination is a significant improvement to the library of radiofluorination methods, and its full potential is being discovered to benefit radiochemistry communities.
Our group recently developed a “Radiofluorination on the Sep-Pak” method to quickly and efficiently prepare 6-[18F]fluoronicotinic acid-2,3,5,6-tetrafluorophenyl ester, a prosthetic group for peptide/protein labeling, without the addition of base or azeotropic drying.29, 30 Considering the base-sensitivity of the Cu-mediated radiofluorination process, a similar 18F-elution process could significantly aid this transformation. In this work, [18F]fluoride trapped on the Sep-Pak was eluted with an anhydrous organic solution of pyridinium salt such as pyridinium trifluoromethanesulfonate (Py·OTf). This approach does not require azeotropic drying, as no water was used for the elution of fluoride. Moreover, the absence of base increases the stability of both precursor and the copper reagent leading to better radiochemical yield. In this paper, we report our findings for an improved Cu-mediated radiofluorination through a no-base-added, azeotropic drying- free 18F-elution process.
2. Results and Discussion
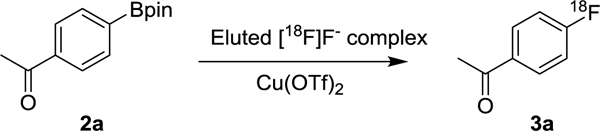
To begin our study, the elution of [18F]fluoride from PS-HCO3 Sep-Pak (Figure 1) and subsequent Cu-mediated fluorination was first tested with Py-OTf. Quantitative elution of [18F]fluoride was obtained (Table 1, entry 1) by slowly passing a DMF solution of Py·OTf through the Sep-Pak containing [18F]fluoride at 0.5 mL/min speed. The eluted [18F]pyridinium fluoride solution was subjected to the model reaction of 4-[18F]fluoroacetophenone synthesis via Cu-mediated radiofluorination. However, no product was obtained. Addition of pyridine as a co-eluent showed no effect on [18F]fluoride elution or radiochemical conversion (entry 2). To further optimize the protocol, 4-(dimethylamino)pyridinium trifluoromethanesulfonate (DMAP·OTf) was tested, as DMAP has been proven more efficient (10-fold higher efficiency) than pyridine in Cu-mediated radiofluorination.27 DMAP was readily converted to the trifluoromethanesulfonic acid salt, DMAP·OTf, by mixing DMAP with trifluoromethanesulfonic acid, and used without purification. The fluoride elution efficiency (95%) was comparable with Py·OTf. More importantly, the desired 4-[18F]fluoroacetophonone (entry 3) was prepared with a radiochemical yield of 41%.
Figure 1.
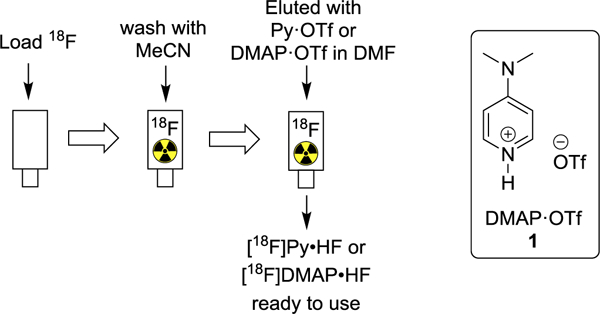
[18F]fluoride elution approach from PS-HCO3 cartridge.
Table 1.
Model reaction of Cu-mediated fluorination.a
 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Eluting agent | Amount (mg) |
Co-eluent | Solvent | Elution efficiency (%) |
T (°C) | RCY b (%) |
| 1 | Py·OTf | 10 | n/a | DMF | >95 | 120 | 0 |
| 2 | Py·OTf | 10 | Py c | DMF | >95 | 120 | 0 |
| 3 | DMAP·OTf | 10 | n/a | DMF | >95 | 110 | 41 |
| 4 | DMAP·OTf | 10 | DMAP d | DMF | >95 | 110 | 9 |
| 5 | DMAP·OTf | 10 | n/a | DMF | >95 | 120 | 51 ± 2 (n = 3) |
| 6 | DMAP·OTf | 5 | n/a | DMF | 83 | 120 | 49 |
| 7 | DMAP·OTf | 15 | n/a | DMF | >95 | 120 | 43 |
| 8 | DMAP·OTf | 10 | n/a | DMF | >95 | 105 | 40 |
| 9 | DMAP·OTf | 10 | n/a | DMF | >95 | 130 | 51 |
| 10 | DMAP·OTf | 10 | n/a | DMA | >95 | 120 | 53 |
Note: 10 mg ArBpin precursor (40 μmol), 0.37 - 0.76 GBq of [18F]fluoride were used for each reaction.
RCY were determined by analytical HPLC (method A) and radio-TLC.
10 μL of pyridine.
5 mg of DMAP.
This result prompted us to further optimize the radiosynthesis. Additional DMAP in the eluting solution was not productive, as it reduced the yield to 9% (entry 4). Increasing the reaction temperature to 120 °C improved the yield (entry 5). The effect of various amounts of DMAP·OTf was also tested for [18F]fluoride elution (entry 5-7). When 5 mg DMAP·OTf was used, the elution efficiency was slightly decreased to 83% (entry 6). However, larger amounts of DMAP·OTf (15 mg) reduced the radiochemical yield (entry 7). Therefore, we decided to perform the elution of [18F]fluoride with 10 mg of DMAP·OTf as this provided optimal balance between elution efficiency and yield. Various reaction temperatures were also evaluated, and slightly increased yield was observed at higher temperatures (entry 5 and entry 8). Further increasing the temperature to 130 °C had no effect on radiochemical yield (entry 9). Changing the solvent to N,N-dimethylacetamide (DMA) similarly showed no effect on radiochemical yield (entry 10). Based on the model reaction, we settled on a reaction temperature of 120 °C in DMF or DMA as the optimal conditions for the following studies. Variation of reaction time was not investigated in this optimization. All reactions were heated for 20 min, since it was the standardized time for most related works.22, 25, 27, 31
The optimized protocol was tested on a substrate scope study of various boronic esters (Figure 2). The selected substrates contain electron deficient (3a-b) and electron rich arenes (3d, 3g, 3j-l), as well as heteroarenes such as quinoline, indole and pyridine moieties (3c, 3e-f, 3h-i). Using the optimized conditions, medium to high RCY was obtained for most substrates (up to 83%). The RCYs for few compounds such as 3c and 3i are significantly higher than previously reported, possibly due to the improved stability of the precursor under the no-base-added conditions.27, 31 The side-by-side comparison of their synthesis under literature “high-base” condition vs. this protocol was performed.25, 31 For 3c, the literature method gave 31% RCY, whereas the RCY for the elution protocol was 83 ± 2%. Similar result was obtained for 3i, which gave <2% RCY for the literature method vs. 27% for the elution protocol. The solvent effect was studied for compound 3h, since the reaction in DMF resulted in a low RCY (19%). Switching the solvent to DMA improved the yield to 40%. Similar yield difference in DMF vs DMA was reported in the literature,11, 31 possibly due to the undesired reaction of precursor with dimethylamine (resulting from thermal decomposition of DMF). RCY was determined by analytical HPLC of the crude product and the identity of product was confirmed by co-injection with the standard compounds.
Figure 2.
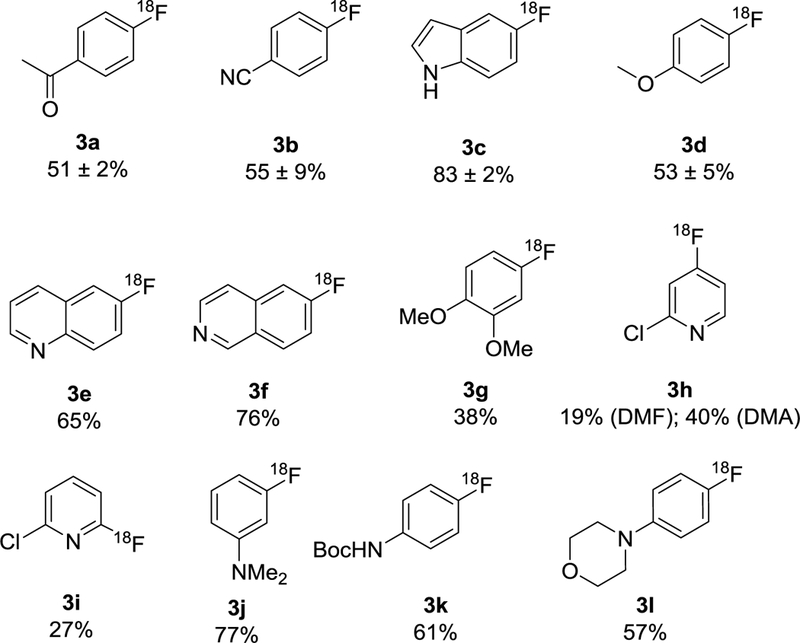
Substrate scope study. Reaction condition: precursor (10 mg), DMAP·OTf (10 mg), Cu(OTf)2 (3 mg), DMF or DMA (1.3 mL), 120 °C, 20 min.
Fluorine-18 labeled flumazenil is a well-known tracer for quantitative evaluation of central benzodiazepine receptors (Scheme 1).32, 33 Recently, Preshlock et al. reported the radiosynthesis of this tracer via copper-mediated radiofluorination with a RCY of 19%.11 In this study, we tested the labeling efficiency with the DMAP·OTf elution method. The reaction was optimized with low amounts of [18F]fluoride (0.37 - 0.76 GBq, 10-20 mCi) at varying temperatures, a 51% RCY was obtained at 110 °C with DMAP·OTf in DMA as eluent. When the radiosynthesis was performed on a production scale (6.7 - 7.4 GBq) with HPLC purification, [18F]flumazenil was successfully obtained with 26 - 47% RCY (isolated, n =3) in 55-60 min. The molar activity is 100 - 126 GBq/μmol at the end of synthesis. The identity of [18F]flumazenil was confirmed by co-injection with an authentic nonradioactive standard on the analytical HPLC (Figure 3).
Scheme 1.
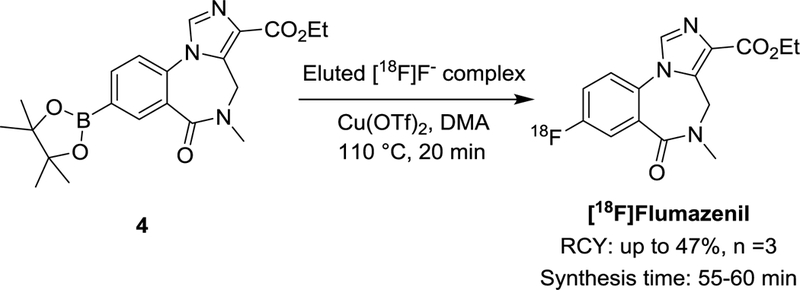
Radiosynthesis of [18F]flumazenil with DMAP·OTf elution method.
Figure 3.

HPLC analysis of a) [18F]flumazenil; b) [18F]flumazenil, co-injected with the nonradioactive standard using Method B. black line, in-line radiodetector; gray line, UV detector at 254 nm.
We believe our protocol increased the stability of both the precursor and the copper reagent. As a result, less mazenil pinacol boronate (4) and Cu(OTf)2 were needed in the radiosynthesis, which will significantly reduce the cost of the overall procedure. In our improved [18F]flumazenil synthesis, 2 mg of mazenil pinacol boronate precursor and 3 mg of Cu(OTf)2 were sufficient to give the purified product in 47% RCY. Low reagent loading and fewer side reactions should generally simplify the purification process, especially for reactions with base-sensitive precursors.
3. Conclusions
Copper-mediated radiofluorination is an efficient method of incorporating fluorine-18 on both electron-rich and electron-deficient substrates. However, this method require base to elute fluorine-18 from Sep-Pak and azeotropic drying. The current method requires neither the base to elute fluorine-18 from Sep-Pak nor azeotropic drying. Fluorine-18 eluted from the Sep-Pak with DMAP·OTF can be used directly for radiolabeling. This method will be beneficial for base sensitive precursors and labeled tracers. Moreover, stability of the copper reagent will be higher under this reaction conditions. The current method was successfully validated on 12 aryl- and heteroaryl-boronic ester substrates with a range of electronic properties. Using this approach, the radiosynthesis of [18F]flumazenil was achieved with 47% isolated RCY in 55-60 min synthesis time. The total synthesis time is shorter than the literature reported method (75-80 min) due to no need of azeotropic drying of fluorine-18.33 This highly efficient method will significantly boost the application scope of the powerful copper-mediated radiofluorination. Further evaluation of this method on other PET tracers is in progress.
4. Methods
4.1. General
Unless otherwise noted, all chemicals and solvents were purchased from Sigma-Aldrich (Milwaukee, WI, USA) or Combi-blocks (San Diego, CA, USA) and used without further purification. Flumazenil and its boronate precursor were purchased from ABX GmbH (Radeberg, Germany). Non-carrier added [18F]fluoride was obtained from the National Institutes of Health cyclotron facility (Bethesda, MD, USA). Chromafix PS-HCO3 anion-exchange Sep-Pak cartridges were purchased from Synthra (Hamburg, Germany) and the packing material was reduced to half (~20 mg) for better elution efficiency. Ultra-pure water was produced with the Milli-Q® Integral water purification system (Billerica, MA, USA). The Fusion 100 syringe pump was purchased from Chemyx (Stafford, TX, USA).
NMR data were recorded on a Bruker 400 MHz spectrometer (Billerica, MA, USA). High resolution mass spectrometry (HRMS) was carried out on an Agilent TOF mass spectrometer (Santa Clara, CA, USA). Radio-TLC was performed on an Eckert-Ziegler AR-2000 radio-TLC Imaging Scanner (Hopkinton, MA, USA). High performance liquid chromatography (HPLC) purification and analytical HPLC were conducted on the Agilent 1260 HPLC system equipped with multi-wavelength UV detector along with a flow count radiodetector (Eckert & Ziegler, B-FC-3500 diode).
HPLC conditions:
Method A: Phenomenex Luna C18 (2) column, 100×4.6 mm, 5 μm. Mobile phase: A: water (0.1% NH4OH); B: acetonitrile (0.1% NH4OH). Gradient: 20 – 80% B in 10 min; 1 mL/min.
Method B: Phenomenex Luna C18 (2) column, 100×4.6 mm, 5 μm. Mobile phase: 25% acetonitrile in water (0.1% formic acid); 1.0 mL/min.
Method C: Phenomenex Luna C18 (2) column, 250×10 mm, 5 μm. Mobile phase: 22% acetonitrile in water (25 mM NH4OAc); 4 mL/min.
4.2. Chemical synthesis
4-Dimethylaminopyridinium trifluoromethanesulfonate (DMAP·OTf)
A solution of trifluoromethanesulfonic acid (735 mg, 4.9 mmol) in dichloromethane (10 mL) was cooled to 0 °C. A solution of 4-dimethylaminopyridine (598 mg, 0.49 mmol) in dichloromethane (5 mL) was added dropwise and the mixture was stirred for 15 min. The precipitated product was collected by vacuum filtration and washed with cold dichloromethane (10 mL). The product was further dried under high vacuum overnight (1.3 g, quantitative yield). No further purification was necessary. 1H NMR (400 MHz, DMSO) δ 13.15 (s, 1H), 8.21 (d, J = 7.6 Hz, 2H), 6.98 (d, J = 7.7 Hz, 2H), 3.19 (s, 6H). 13C NMR (101 MHz, DMSO) δ 157.43, 139.56, 121.15 (d, J = 322.4 Hz), 107.41, 40.09. HRMS (ESI): Calcd for C7H10N2 (M+H)+ 123.0922, found 123.0919.
4.3. Radiochemistry
General procedure for the Cu-mediated radiofluorination via DMAP·OTf elution
[18F]fluoride (0.37 – 0.76 GBq, 10 – 20 mCi) in target water was diluted with 2 mL water and passed through a short PS-HCO3 cartridge (see Section 4.1). The cartridge was washed with anhydrous acetonitrile (6 mL) and dried for 2 min. The [18F]fluoride on the cartridge was eluted (0.5 mL/min, manually or via syringe pump) with a DMAP·OTf (10 mg) solution in DMF or DMA (1 mL) to a reaction vial containing the boronate precursor (10 mg) and Cu(OTf)2 (3 mg). The cartridge was further eluted with DMF or DMA (0.3 mL) to the same vial. The reaction mixture was heated at 120 °C for 20 min, an aliquot (~50 μL) was diluted with water (50 μL) and analyzed by analytical HPLC (method A) and radio-TLC for RCY determination. The identity of the product was confirmed by co-injection with unlabeled standard compound.
General procedure for the Cu-mediated radiofluorination via traditional azeotropic drying process for 3c and 3i
[18F]fluoride (0.37 - 0.76 GBq, 10 - 20 mCi) in target water was diluted with 2 mL water and passed through a short PS-HCO3 cartridge (see Section 4.1). [18F]fluoride was eluted from the cartridge with the eluent (2.8 mg K2CO3, 12 mg K222 in 1 mL acetonitrile and 280 μL water). The solution was azeotropically dried under vacuum and nitrogen flow at 110 ˚C. The azeotropic drying process was repeated by adding acetonitrile (1 mL × 3). To the dried activity was added the boronic ester precursor (60 μmol), tetrakis(pyridine)copper(II) triflate (Cu(OTf)2(py)4, 15 mg, 22 μmol) in DMF or DMA (0.3 mL). The resulting mixture was heated under 110 °C for 20 min. An aliquot (~50 μL) was diluted with water (50 μL) and analyzed by analytical HPLC (method A). The identity of the product was confirmed by co-injection with unlabeled standard compound.
Radiosynthesis of [18F]flumazenil
[18F]fluoride (6.7 GBq, 188 mCi) in target water was diluted with 2 mL water and passed through a short PS-HCO3 cartridge. The cartridge was washed with anhydrous acetonitrile (6 mL) and dried for 2 min. The [18F]fluoride on the cartridge was eluted (0.5 mL/min) with a DMAP·OTf (10 mg) solution in DMA (1 mL) to a reaction vial containing mazenil pinacol boronate (2 mg) and Cu (OTf)2 (3 mg). The cartridge was further eluted with DMA (0.3 mL) to the same vial. The reaction mixture was heated at 110 °C for 20 min, then diluted with aqueous NH4OAc solution (10 mM, 2 mL), and purified by HPLC using semi-preparative column (method C). The product peak was collected between 26 - 28 min. The radiosynthesis including [18F]fluoride catch on the Sep-Pak, radiofluorination, and purification is completed in 55-60 min.
Acknowledgements
This work was supported by the Intramural Research Program of the NIH. Intramural research funds for the Imaging Probe Development Center were administered by the National Heart, Lung, and Blood Institute. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. We thank Dr. Carolyn Woodroofe for her assistance in proofreading.
References
- [1].Ametamey SM, Honer M, and Schubiger PA. Molecular imaging with PET. Chem Rev 2008;108:1501–16. [DOI] [PubMed] [Google Scholar]
- [2].Phelps ME. Positron emission tomography provides molecular imaging of biological processes. Proc Natl Acad Sci 2000;97:9226–9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Matthews PM, Rabiner EA, Passchier J, and Gunn RN. Positron emission tomography molecular imaging for drug development. Br J Clin Pharmacol 2012;73:175–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Miller PW, Long NJ, Vilar R, and Gee AD. Synthesis of 11C, 18F, 15O, and 13N radiolabels for positron emission tomography. Angew Chem Int Ed Engl 2008;47:8998–9033. [DOI] [PubMed] [Google Scholar]
- [5].van der Born D, Pees A, Poot AJ, Orru RVA, Windhorst AD, and Vugts DJ. Fluorine-18 labelled building blocks for PET tracer synthesis. Chem Soc Rev 2017;46:4709–4773. [DOI] [PubMed] [Google Scholar]
- [6].Levin CS and Hoffman EJ. Calculation of positron range and its effect on the fundamental limit of positron emission tomography system spatial resolution. Phys Med Biol 1999;44:781–99. [DOI] [PubMed] [Google Scholar]
- [7].Alauddin MM. Positron emission tomography (PET) imaging with 18F-based radiotracers. Am J Nucl Med Mol Imaging 2012;2:55–76. [PMC free article] [PubMed] [Google Scholar]
- [8].Jacobson O, Kiesewetter DO, and Chen X. Fluorine-18 radiochemistry, labeling strategies and synthetic routes. Bioconjug Chem 2015;26:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cai L, Lu S, and Pike VW. Chemistry with [18F]fluoride ion. Eur J Org Chem 2008;2008:2853–2873. [Google Scholar]
- [10].Tredwell M and Gouverneur V. 18F-labeling of arenes. Angew Chem Int Ed Engl 2012;51:11426–37. [DOI] [PubMed] [Google Scholar]
- [11].Preshlock S, Tredwell M, and Gouverneur V. 18F-Labeling of arenes and heteroarenes for applications in positron emission tomography. Chem Rev 2016;116:719–66. [DOI] [PubMed] [Google Scholar]
- [12].Pike VW and Aigbirhio FI. Reactions of cyclotron-produced [18F]fluoride with diaryliodonium salts-a novel single-step route to no-carrier-added [18]fluoroarenes. J Chem Soc Chem Commun 1995:2215–2216. [Google Scholar]
- [13].Shah A W Pike V, and A. Widdowson D. The synthesis of [18F]fluoroarenes from the reaction of cyclotron-produced [18F]fluoride ion with diaryliodonium salts. J Chem Soc Perkin Trans 1 1998:2043–2046. [Google Scholar]
- [14].Ross TL, Ermert J, Hocke C, and Coenen HH. Nucleophilic 18F-fluorination of heteroaromatic iodonium salts with no-carrier-added [18F]fluoride. J Am Chem Soc 2007;129:8018–8025. [DOI] [PubMed] [Google Scholar]
- [15].Cardinale J, Ermert J, Humpert S, and Coenen HH. Iodonium ylides for one-step, no-carrier-added radiofluorination of electron rich arenes, exemplified with 4-(([18F]fluorophenoxy)-phenylmethyl)piperidine NET and SERT ligands. RSC Advances 2014;4:17293–17299. [Google Scholar]
- [16].Rotstein BH, Stephenson NA, Vasdev N, and Liang SH. Spirocyclic hypervalent iodine(III)-mediated radiofluorination of non-activated and hindered aromatics. Nat Commun 2014;5:4365. [DOI] [PubMed] [Google Scholar]
- [17].Lee E, Kamlet AS, Powers DC, Neumann CN, Boursalian GB, Furuya T, et al. A fluoride-derived electrophilic late-stage fluorination reagent for PET imaging. Science 2011;334:639–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kamlet AS, Neumann CN, Lee E, Carlin SM, Moseley CK, Stephenson N, et al. Application of palladium-mediated 18F-fluorination to PET radiotracer development: overcoming hurdles to translation. PLoS One 2013;8:e59187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lee E, Hooker JM, and Ritter T. Nickel-mediated oxidative fluorination for PET with aqueous [18F]fluoride. J Am Chem Soc 2012;134:17456–17458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ren H, Wey HY, Strebl M, Neelamegam R, Ritter T, and Hooker JM. Synthesis and imaging validation of [18F]MDL100907 enabled by Ni-mediated fluorination. ACS Chem Neurosci 2014;5:611–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ye Y, Schimler SD, Hanley PS, and Sanford MS. Cu(OTf)2-mediated fluorination of aryltrifluoroborates with potassium fluoride. J Am Chem Soc 2013;135:16292–5. [DOI] [PubMed] [Google Scholar]
- [22].Tredwell M, Preshlock SM, Taylor NJ, Gruber S, Huiban M, Passchier J, et al. A general copper-mediated nucleophilic 18F-fluorination of arenes. Angew Chem Int Ed Engl 2014;53:7751–5. [DOI] [PubMed] [Google Scholar]
- [23].Mossine AV, Brooks AF, Makaravage KJ, Miller JM, Ichiishi N, Sanford MS, et al. Synthesis of [18F]arenes via the copper-mediated [18F]fluorination of boronic acids. Org Lett 2015;17:5780–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sergeev ME, Morgia F, Lazari M, Wang C, Jr., and van Dam RM. Titania-catalyzed radiofluorination of tosylated precursors in highly aqueous medium. J Am Chem Soc 2015;137:5686–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zlatopolskiy BD, Zischler J, Krapf P, Zarrad F, Urusova EA, Kordys E, et al. Copper-mediated aromatic radiofluorination revisited: efficient production of PET tracers on a preparative scale. Chemistry 2015;21:5972–9. [DOI] [PubMed] [Google Scholar]
- [26].Makaravage KJ, Brooks AF, Mossine AV, Sanford MS, and Scott PJ. Copper-mediated radiofluorination of arylstannanes with [18F]KF. Org Lett 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mossine AV, Brooks AF, Ichiishi N, Makaravage KJ, Sanford MS, and Scott PJH. Development of customized [18F]fluoride elution techniques for the enhancement of copper-mediated late-stage radiofluorination. Sci Rep 2017;7:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zarrad F, Zlatopolskiy BD, Krapf P, Zischler J, and Neumaier B. A practical method for the preparation of 18F-labeled aromatic amino acids from nucleophilic [18F]fluoride and stannyl precursors for electrophilic radiohalogenation. Molecules 2017;22: 2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Basuli F, Zhang X, Jagoda EM, Choyke PL, and Swenson RE. Facile room temperature synthesis of fluorine-18 labeled fluoronicotinic acid-2,3,5,6-tetrafluorophenyl ester without azeotropic drying of fluorine-18. Nucl Med Biol 2016;43:770–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Basuli F, Zhang X, Woodroofe CC, Jagoda EM, Choyke PL, and Swenson RE. Fast indirect fluorine-18 labeling of protein/peptide using the useful 6-fluoronicotinic acid-2,3,5,6-tetrafluorophenyl prosthetic group: A method comparable to direct fluorination. J Label Compd Radiopharm 2017;60:168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Taylor NJ, Emer E, Preshlock S, Schedler M, Tredwell M, Verhoog S, et al. Derisking the Cu-mediated 18F-fluorination of heterocyclic positron emission tomography radioligands. J Am Chem Soc 2017;139:8267–8276. [DOI] [PubMed] [Google Scholar]
- [32].Ryzhikov NN, Gomzina NA, Fedorova OS, Vasil’ev DA, Kostikov AP, and Krasikova RN. Preparation of [18F]flumazenil, a potential radioligand for PET imaging of central benzodiazepine receptors, by isotope exchange. Radiochemistry 2004;46:290–294. [Google Scholar]
- [33].Ryzhikov NN, Seneca N, Krasikova RN, Gomzina NA, Shchukin E, Fedorova OS, et al. Preparation of highly specific radioactivity [18F]flumazenil and its evaluation in cynomolgus monkey by positron emission tomography. Nucl Med Biol 2005;32:109–116. [DOI] [PubMed] [Google Scholar]


