Abstract
Background:
Castleman disease (CD) is an uncommon lymphoproliferative disorder that is rare in pediatric populations; literature describing this population is sparse. We sought to describe pediatric CD, including unicentric CD (UCD) and human herpes virus-8 (HHV8)-negative multicentric CD (MCD) in a multi-institutional cohort.
Methods:
We retrospectively reviewed 24 patients, aged 0–26 years at diagnosis, who were diagnosed with CD between January 1, 2005 and May 16, 2017 at two tertiary children’s hospitals. Demographic and clinical data were collected.
Results:
Most patients (75%, 18/24) presented with UCD. All patients with MCD were HHV8-negative. The most common histopathologic variant was hyaline vascular (75%, 18/24). Plasma cell variant occurred in 33% (2/6 [95% CI 4–78%]) of patients with HHV8-negative MCD and 17% (3/18 [95% CI 4–41%]) of patients with UCD. Systemic symptoms were present in 4/6 of patients with HHV8-negative MCD and 8/18 of patients with UCD. Anemia and laboratory inflammation occurred in both UCD and MCD patients, with non-significantly higher rates of anemia and elevated C-reactive protein in MCD patients. All but two UCD patients underwent gross total resection as definitive therapy. Among HHV8-negative MCD patients, a combination of resection, chemotherapy, and immunotherapy was used. No UCD patients and 3/6 HHV8-negative MCD patients experienced disease progression/relapse prior to lasting remission. There were no deaths.
Conclusion:
Pediatric patients with CD most commonly have unicentric, hyaline vascular variant disease. Pediatric patients with both UCD and MCD commonly have systemic inflammation and, despite risk of progression/relapse in MCD patients, ultimately have excellent survival.
Keywords: Castleman disease, lymphoproliferative disorder, pediatrics
Introduction
Castleman disease (CD) is a rare polyclonal lymphoproliferative disorder characterized by enlarged hyperplastic lymph node(s) with several different characteristic histopathologic variants and clinical presentations.1 Historically, CD has been described by disease centricity, including unicentric CD (UCD), or multicentric CD (MCD) and by histopathologic variant.1,2 Variants include 1) Hyaline vascular (HV) variant, with hyalinized regressed germinal centers, widened mantle zones and small lymphocytes; 2) Plasma cell variant (PCV), featuring hyperplastic germinal centers and an abundance of interfollicular plasma cells; 3) Mixed variant, with features of HV and PCV; and 4) Plasmablastic variant, with PCV features in addition to plasmablasts harboring human herpes virus-8 (HHV8).1 More recently, CD has been divided into three distinct entities by disease centricity and pathophysiology; specifically, MCD can be further subdivided by whether it is associated with HHV8 infection (HHV8-positive MCD) or not (HHV8-negative MCD, or idiopathic MCD), while UCD remains a single entity representing roughly three quarters of all CD.2–4 UCD and HHV8-negative MCD can demonstrate HV, PCV, or mixed histopathological variants while HHV8-positive MCD typically only demonstrates plasmablastic histopathology.5 In addition to describing etiology, these subtypes confer information about prognosis in adults: patients with HHV8-positive MCD have worse survival than patients with HHV8-negative MCD, and patients with UCD nearly all survive.6
While complete surgical resection is typically curative for patients with UCD,6–8 patients with MCD or unresectable UCD disease have significantly worse prognosis due to lack of consistently effective therapies, with no consensus regarding first line treatment until recently.7–11 New consensus guidelines recommend use of anti-interleukin-6 (IL-6) monoclonal antibody (siltuximab or tocilizumab) with or without corticosteroids as first-line therapy for idiopathic MCD.11 Use of rituximab in place of anti-IL-6 therapy, or the addition of conventional cytotoxic chemotherapy, should also be considered.9,10
Much of the CD literature is focused on an adult population, with pediatric CD described primarily in small case series12–15, and one review focusing on MCD in HHV8-endemic regions.16 Even among the adult literature, there is a focus on HHV8-positive disease, which is likely less common in the western pediatric population.17 Additionally, because of the rarity of pediatric CD of all types, and the wide variation in treatment, continued descriptions of treatment and outcomes in this population are needed.
In this multi-institutional review, we describe the presentation, treatment and outcomes of a pediatric and adolescent/young adult (AYA) cohort with Castleman disease.
Methods
A retrospective cohort study was performed at two large tertiary academic children’s hospitals, Children’s Hospital Colorado (CHCO) and Cincinnati Children’s Hospital Medical Center (CCHMC). All pediatric and AYA patients who were diagnosed with CD between January 1, 2005 and May 16, 2017 were identified using an existing pathology database (CHCO) or billing/coding records (CCHMC). Diagnosis of CD was confirmed by manual chart review, and demographic and clinical data were collected similarly. Inclusion criteria were 1) Age 0–26 at time of diagnosis, and 2) Pathologic diagnosis of CD based on interpretation at one of the two institutions. Patients were excluded if there was ambiguity in the pathologic diagnosis. The cohort consisted of 24 patients, with 13 from CHCO and 11 from CCHMC. This study was approved by the University of Colorado and CCHMC Institutional Review Boards with a waiver of informed consent.
Clinical data was extracted from the medical record including demographics, disease characteristics (unicentric [defined by presence of enlarged lymph nodes ≥1 cm in one lymph node station] versus multicentric [presence of enlarged lymph nodes (≥1 cm in short-axis diameter) in ≥2 lymph node stations]), primary disease site, histopathological variant [plasma cell, hyaline vascular, mixed, plasmablastic]), clinical symptoms, and treatments. One patient was initially diagnosed with UCD and for months later found to have MCD; this patient was analyzed in the MCD category and the date of diagnosis, as well as laboratory and radiology results are summarized from date of MCD diagnosis. In order to evaluate for idiopathic MCD (iMCD) by consensus criteria,2 histopathology of MCD patients was reviewed by a single board certified pediatric pathologist at each site. Imaging studies were reviewed by a single board certified pediatric radiologist at each site. Reviewing radiologists reported three-dimensional linear measurements of the largest three sites of disease; sonographic features (echogenicity, presence of flow on Doppler); enhancement characteristics on computed tomography (CT) and magnetic resonance imaging (MRI) (presence of enhancement following administration of intravenous contrast material, intensity of enhancement relative to skeletal muscle); maximum standardized uptake value (SUVmax) on 18F fluorodeoxyglucose positron emission tomography (FDG-PET). Imaging studies were included if available for review, obtained within two months before and one week after diagnosis, and obtained prior to resection.
Laboratory data at presentation were also collected, including complete blood counts (CBC), erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), IL-6 level, HHV8 quantification by polymerase chain reaction (PCR), and human immunodeficiency virus (HIV) status. The time interval for each of these values was within six weeks prior to two weeks after diagnosis except for HIV status, which was accepted if measured within two months of diagnosis. Hemoglobin and mean cellular volume were categorized as normal if they were within two standard deviations of the age and sex adjusted norm (extrapolated from the definition of anemia and encompassing 95% of observed population variation).18Treatment data, including surgeries, chemotherapy, immunotherapy, and radiation, was collected. A surgery was defined as a biopsy if the procedure was removal of a portion of the mass exclusively for diagnostic purposes, whereas resection was defined as an attempt of tumor removal. Resection was divided into gross total resection (GTR) if there was complete removal of all sites of disease or partial resection if disease at any site remained (i.e. portion of resected mass remains, or other sites of disease were not resected).
The primary outcome for this study was response to upfront systemic therapy, if given, or relapse/progression after primary surgical resection. Response to therapy was defined as radiologically stable, improved or remitted disease and resolution of any systemic inflammation. Relapse was defined as a recurrence of lymphadenopathy or systemic inflammation after initial remission or stable disease, and progression was defined as lymph node enlargement or increasing systemic inflammation while receiving therapy. Additionally, treatment-associated toxicity and mortality were collected for descriptive purposes.
Statistical analysis was performed using R version 3.5.1 (https://www.r-project.org/). Summary statistics were reported as medians, interquartile ranges (IQR), and ranges for continuous variables and counts and proportions for categorical variables. Exact 95% binomial confidence intervals (CI) were reported for all proportions. Variables of interest were summarized for patients with UCD vs. HHV8-negative MCD.
Results
Unicentric Castleman disease
18/24 patients in this cohort were diagnosed with UCD. The median age of UCD patients was 13.2 years (range 4.9–18.4), 56% were male and the majority were white (Table 1). The median length of follow-up for these patients was 0.6 years (IQR 0.05–3.3), with many patients seen only by pediatric surgery.
TABLE 1.
Patient and Disease Characteristics
| Characteristic | UCD (n=18) |
HHV8 negative MCD (n=6) |
|---|---|---|
| N (%) or Median (IQR) | ||
| Sex | ||
| Female | 8 (44%) | 1 (17%) |
| Male | 10 (56%) | 5 (83%) |
| Age (years) | 13.2 (9.4, 15.7) (range: 4.9–18.4) |
10.8 (4.8, 14.3) (range: 1.8–14.7) |
| Ethnicity | ||
| Hispanic | 2 (11%) | 1 (17%) |
| Non-Hispanic | 14 (78%) | 5 (83%) |
| Unknown | 2 (11%) | 0 (0%) |
| Race | ||
| Black or African American | 1 (6%) | 0 (0%) |
| White | 14 (82%) | 5 (83%) |
| Hispanic | 1 (6%) | 0 (0%) |
| Other | 1 (6%) | 1 (17%) |
| Pathologic diagnosis | ||
| Hyaline vascular | 14 (78%) | 4 (67%) |
| Plasma Cell Variant | 3 (17%) | 2 (33%) |
| Mixed | 1 (6%) | 0 (0%) |
| Primary disease site | ||
| Head/Neck | 8 (44%) | 1 (17%) |
| Chest | 2 (11%) | 1 (17%) |
| Abdomen | 5 (28%) | 0 (0%) |
| Pelvis | 0 (0%) | 2 (33%) |
| Extremity | 1 (6%) | 1 (17%) |
| Diffuse lymphadenopathy | 0 (0%) | 1 (17%) |
| Other | 2 (11%) | 0 (0%) |
| Systemic symptoms | 8 (44%) | 4 (67%) |
No patients in this cohort had HHV8-positive MCD
The most common disease site was head/neck (8/18, 44%), followed by abdomen (5/18, 28%) (Table 1). 8/18 (44%) UCD patients presented with systemic symptoms, with fatigue (7/18) and weight loss (6/18) being most common. In contrast, 8/18 presented with painless palpable masses, one patient presented with a painful palpable mass, and one mass was found incidentally. No patients had comorbidities associated with lymphadenopathy.
Most (14/18, 78%) UCD patients had HV variant histopathology (Table 1); representative histopathology is shown in Figure 1. A CBC was performed in 15/18 UCD patients (Table 3). Among these patients, five (33%) had anemia and four (27%) had red cell microcytosis (Table 3). CRP was measured in 10 UCD patients and while the median was normal (median 0.9 mg/dL [IQR 0.5–7.4; range <0.29–15.8]), five (50%) patients had an abnormal CRP. Similarly, ESR was measured in 11 UCD patients and five (45%) of these values were elevated (>20 mm/h) (median 10 mm/h [IQR 8.5–37; range 4- >145.0]). Ten patients had both CRP and ESR measurements, and 8/10 of these patients had values in agreement (both elevated or both normal). Serum IL-6 was elevated in 2/6 (33%) patients measured. Serum HHV8 and HIV were negative in the five UCD patients who were evaluated for each.
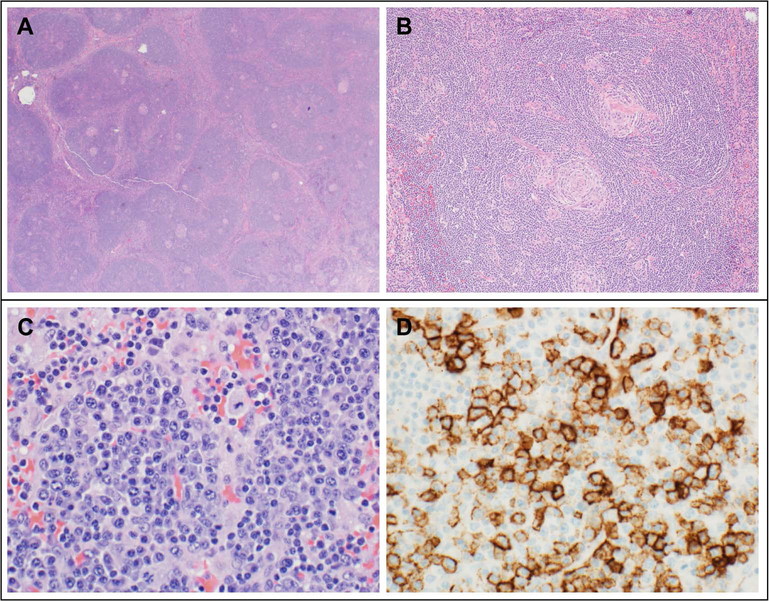
FIGURE 1. Representative histopathologic findings in Castleman disease.
(A) Hyaline vascular (HV) variant Castleman Disease (CD) in a 14 year old female with right neck mass: Low magnification reveals a broad mantle zone with several small and regressed germinal centers in lymphoid follicles. (B) HV disease (same patient): At a higher magnification, lymphoid follicles show a broad mantle zone with concentric rings (onion skin pattern) and small, regressed and hyalinized germinal centers radially penetrated by a blood vessel forming a lollipop pattern. (C) Plasma cell variant (PCV) CD in a 14 year old female with TAFRO (thrombocytopenia, anasarca, myelofibrosis, renal failure, and organomegaly) based on thrombocytopenia, anasarca, inflammation and lymphadenopathy: Lymph node biopsy reveals sheets of benign-appearing plasma cells. (D) PCV CD (same patient): Immunohistochemical stain CD138 highlights plasma cells.
TABLE 3.
Comparison of laboratory and radiology results at diagnosis, and outcome, by disease subtype
| Clinical Characteristic | UCD (n=18) |
HHV8-Negative MCD (n=6) |
|---|---|---|
| N (%) [95% CI] or Median (IQR) | ||
| Hemoglobin (g/dL)** | ||
| Normal | 8/15 (53%) [27–79%] |
2/5 (40%) [5–85%] |
| Low | 5/15 (33%) [12–62%] |
3/5 (60%) [15–95%] |
| High | 2/15 (13%) [2–40%] |
0/5 (0%) [0–52%] |
| Hemoglobin Z-score | −0.5 (−2.9, 0.17) | −2.2 (−5.6, −0.53) |
| MCV | ||
| Normal | 11/15 (73%) [45–92%] |
4/5 (80%) [28–99%] |
| Low | 4/15 (27%) [8–55%] |
1/5 (20%) [0.5–72%] |
| MCV Z-score | −1.04 (−2.35, −0.32) | −0.08 (−0.76, 0.08) |
| WBC Count (x103/μL) | 7.6 (5.8, 9.06) | 8.1 (5.0, 9.5) |
| Platelet count (x103/μL) | 330 (294, 401) | 216 (176, 274) |
| CRP Abnormal |
5/10 (50%) [19–81%] |
3/4 (75%) [19–99%] |
| ESR Abnormal |
5/11 (45%) [17–77%] |
1/4 (25%) [0.6–81%] |
| Blood HHV Positive |
0/5 (0%) [0–52%] |
0/5 (0%) [0–52%] |
| Serum IL-6 Abnormal |
2/6 (33%) [4–78%] |
2/4 (50%) [7–93%] |
| Size of largest site (cm3)a | 53.0 (26.6, 71.1) | 14.8 (11.8, 30.4) |
| Size of 2nd largest site (cm3) | NA | 8.7 (6.8, 9.7) |
| Size of 3rd largest site (cm3) | NA | 3.3 (3.2, 4.2) |
| PET SUVmaxb | 3.9 (3.0, 5.2) | 2.6 (2.4, 2.9) |
| Relapse/Progression | 0/10 [0–31%] |
3/6 (50%) [12–88%] |
Hemoglobin was categorized using two standard deviations above/below the age and sex adjusted norm
Volumetric measurement was available for 12 UCD and all MCD patients.
PET SUVmax was available for 7 UCD and 2 MCD patients.
UCD, unicentric Castleman disease; MCD, multicentric Castleman disase; HHV8 human herpes virus-8; CI, confidence interval; IQR, interquartile range; MCV, mean cellular volume; WBC, white blood cell; CRP, C-reactive protein; ESR, erythryocyte sedimentation rate;IL-6, interleukin-6; cm3, cubic centimeters; PET SUVmax, positron emission tomography maximum standardized uptake value
Imaging studies of any type were performed at the time of diagnosis in 14/18 UCD patients and representative imaging is show in Figure 2. Among patients with upfront imaging, patients with UCD had a median volume of the mass of 53.0 cubic centimeters (cm3) (IQR 26.6–71.1). All lesions imaged with CT and MRI showed enhancement, typically homogenous and greater than skeletal muscle following contrast material administration. FDG-PET was performed in eight patients and the median SUVmax of all lesions was 3.9 (IQR 3.0–5.2; range 2.8–8.2). Four UCD patients had ultrasound exams, all demonstrating hypoechoic masses with increased vascularity on Doppler interrogation.
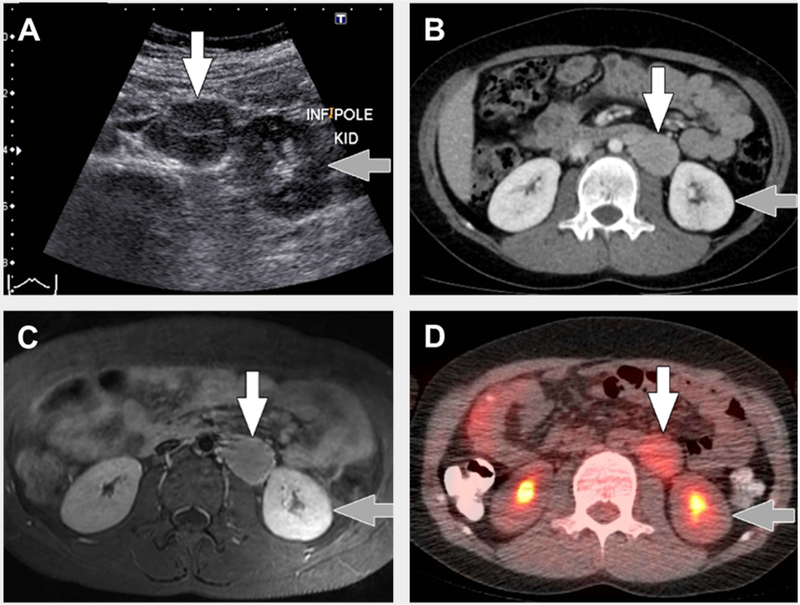
FIGURE 2. Typical imaging findings in unicentric Castleman disease.
8 year old with unicentric Castleman disease. (A) Transverse greyscale ultrasound image shows a hypoechoic mass (white arrow) in the left retroperitoneum near the lower pole of the left kidney (grey arrow). (B) Axial computed tomography (CT) with intravenous contrast only shows the mass (white arrow) adjacent to the lower pole of the left kidney (grey arrow) to be homogenously hyperenhancing relative to muscle. (C) Transverse T1-weighted magnetic resonance imaging with intravenous gadolinium-based contrast material shows the mass (white arrow) adjacent to the lower pole of the left kidney (grey arrow) to be homogenously hyperenhancing relative to muscle. (D) Axial fused 18F-fluorodeoxyglucose positron emission tomography (FDG)/CT image shows uniform FDG uptake, SUVmax=3, in the mass (white arrow) adjacent to the lower pole of the left kidney (grey arrow).
Patient treatment and outcome are summarized in Table 2. Following diagnosis, one UCD patient was lost to follow-up prior to any therapy. Two additional UCD patients did not receive any therapy (including resection); one had stable disease and one was subsequently lost to follow-up. GTR was the most common treatment and performed in 15/18 (83%) UCD patients. No UCD patients were treated with chemotherapy and there were no deaths.
TABLE 2.
Diagnoses, treatments and outcomes by patient
| Pati-ent | Subtype | Path. Variant | Upfront Therapy | Initial Remission/Stable Disease | Relapse/Progress-ion | Second Line Therapy | LTFU |
|---|---|---|---|---|---|---|---|
| 1 | UCD | HV | Resection | Y | N | N/A | N |
| 2 | UCD | Mixed | Resection | Y | N | N/A | N |
| 3 | UCD | HV | Resection | Y | N | N/A | Y |
| 4 | UCD | PCV | Resection | Y | N | N/A | N |
| 5 | UCD | HV | None | Y | N | N/A | N |
| 6 | UCD | PCV | Resection | Y | Unknown | N/A | Y |
| 7 | UCD | HV | Resection | Y | N | N/A | N |
| 8 | UCD | PCV | Resection | Y | N | N/A | Y |
| 9 | UCD | HV | Resection | Y | N | N/A | N |
| 10 | UCD | HV | Resection | Y | Unknown | Unknown | Y |
| 11 | UCD | HV | Unknown | Unknown | Unknown | Unknown | Y |
| 12 | UCD | HV | Resection | Y | Unknown | Unknown | Y |
| 13 | UCD | HV | Resection | Y | Unknown | Unknown | Y |
| 14 | UCD | HV | Resection | Y | Unknown | Unknown | Y |
| 15 | UCD | HV | Resection | Y | N | N/A | N |
| 16 | UCD | HV | Resection | Y | N | N/A | N |
| 17 | UCD | HV | Resection | Y | Unknown | Unknown | Y |
| 18 | UCD | HV | None | Unknown | Unknown | Unknown | Y |
| 1 | HHV8-neg MCD | PCV | R-CVP, tocilizumab | Y | N | N/A | N |
| 2 | HHV8-neg MCD | HV | Partial resection, R-CHOP, tocilizumab | Y | N | N/A | N |
| 3 | HHV-8 neg MCD | PCV | Partial resection, steroids | Y | N | N/A | N |
| 4 | HHV-8 neg MCD | HV | Partial resection, rituximab, steroids | N | Y | Tocilizumab (no improvement), followed by CHOP | N |
| 5 | HHV8-neg MCD | HV | Partial resection, MTX, thalidomide, steroids | N | Y | Resection | Y |
| 6 | HHV-8 neg MCD | HV | Partial resection | Y | Y | Partial Resection | N |
Path, pathologic; HV, hyaline vascular; PCV, plasma cell variant; LTFU, lost to follow-up; UCD, unicentric Castleman disease; MCD, multicentric Castleman disease; R-CVP, rituximab, cyclophosphamide, vincristine, predisone; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone.
HHV8-negative multicentric Castleman disease
Six patients had MCD and all had HHV8-negative disease: 4/6 had negative immunohistochemistry (IHC) staining for LANA1 on biopsy and the other two MCD patients were found to be negative for HHV8 by serum PCR. The median age of HHV8-negative MCD patients was 10.8 years (range 1.8–14.7), 83% were male, and the majority of patients were white (Table 1). The median length of follow-up for these patients was 3.8 years (IQR 3.5–4.1). HHV8-negative MCD patients were reviewed against the consensus criteria for iMCD2 and while all met major criteria, only 3/6 met minor and exclusion criteria. The fourth met minor criteria but did not have enough laboratory or clinical data to conclusively meet exclusion criteria for infection-related disorders and the fifth did not meet minor criteria or have enough data to meet exclusion criteria. The sixth patient did not meet exclusion criteria because of concern that his MCD was triggered by Epstein bar virus (EBV). While serologies were consistent with past EBV infection, recurrent low-level positive PCR in the setting of recurrent autoimmune neutropenia raise concern for chronic active EBV. Further, there was no staining for EBER on lymph node biopsy, making it impossible to rule out EBV-association. Other than this patient, there were no co-morbidities in this cohort.
Among HHV8-negative MCD patients, 4/6 (67%) presented with systemic symptoms. In addition, one patient initially presented in cardiac failure with restrictive cardiomyopathy due to mediastinal CD and subsequently required heart transplantation. One patient met criteria for TAFRO (thrombocytopenia, anasarca, myelofibrosis, renal failure, and organomegaly) syndrome by modified consensus criteria.19,20 No patients had polyneuropathy, which is described in adult populations.6,9,19,20 Four had HV disease and two had PCV disease (Table 1).
Five HHV8-negative MCD patients had a CBC at diagnosis: three had anemia and one had microcytosis (Table 3). One patient (who presented with TAFRO) had thrombocytopenia with platelets of 25. CRP was measured in four patients (median 11.3 mg/dL [IQR 4.2–20.0; range <0.29–28.5]) and elevated in 3/4 patients. ESR was elevated in 1/4 patients (overall median 12.0 mm/h [IQR 5.3–45.5; range 3–128]). IL-6 was elevated in 2/4 patients measured.
Among patients with HHV8-negative MCD, the median volume of the largest mass per patient was 14.8 cm3 (IQR 11.8–30.4) (Table 3). These patients had similar imaging features by CT and MRI to those with UCD. FDG-PET was available for two patients, with a median SUVmax of 2.6 (IQR 2.4–2.9), which was lower than in the UCD cohort.
Due to the lack of consensus in treatment of MCD at the institution level and nationally until recently,11 treatment was left to physician discretion, resulting in significant heterogeneity. Among patients with HHV8-negative MCD, three successfully achieved upfront lasting remission; these two received combined chemotherapy and immunotherapy (R-CVP [rituximab, cyclophosphamide, vincristine, prednisone] or R-CHOP) with tocilizumab, and one of these patients also underwent partial resection. The third patient to achieve upfront lasting remission was the EBV-associated MCD patient, who was treated with partial resection and steroids. One patient was initially diagnosed with UCD (based on ultrasound only) and treated with what was incorrectly believed to be GTR. This patient was subsequently diagnosed with MCD four months later and failed treatment with steroids and rituximab, then tocilizumab, before successful treatment with CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) chemotherapy. Interestingly, this patient developed anthracycline-related dilated cardiomyopathy requiring heart transplantation after a total anthracycline dose of 400 mg/m2. One patient, HHV8-negative MCD patient 5 (Table 2) was initially treated with methotrexate, thalidomide and steroids without achieving a stable disease status; as disease was limited to two nodal stations, this patient then underwent GTR to achieve clinical and radiologic remission. Finally, HHV8-negative MCD patient 6 was initially treated with partial resection (one of two involved nodal stations) with initial stable disease but subsequent progression and spread (four nodal stations); lasting stable disease was then achieved with partial resection (one nodal station resected). Of these two patients, one had inflammatory markers at time of diagnosis, which were negative. No patients died.
Presentation by disease centricity
Analysis was performed comparing presentation and outcomes of patients with UCD versus HHV8-negative MCD. While statistical tests were not conducted due to small sample size, there were some noticeable differences between these groups. UCD patients had larger disease sites (median 53.0 cm3 [IQR 26.6–71.1]) compared to MCD patients’ largest sites (14.8 cm3 [IQR 11.8–30.4]). Patients with HHV8-negative MCD had a slightly higher frequency of plasma cell variant pathology (33% [95% CI 4–78%]) than patients with UCD (17% [95% CI 4–41%]), more patients with anemia (60% [95% CI 15–95%] versus 33% [95% CI 12–62%]), a slightly lower median platelet count (216 × 103/μL [IQR 176–274] versus 330 × 103/μL [IQR 294–401]), a higher median CRP (11.3 mg/dL [IQR 4.2–20.0] versus 0.9 mg/dL [IQR 0.5–7.4]), and more patients with systemic symptoms (67% [95% CI 22–96%] versus (44% [95% CI 22–69%]). Median ESR (12.0 mm/h [IQR 6.3–45.5] versus 10.0 mm/h [IQR 8.5–37]) was not appreciably higher in the MCD group, however. No patients with UCD required systemic therapy, whereas 5/6 with MCD did. Lastly, while no patients with UCD experienced relapse/progression, three of six patients (50% [95% CI 12–88%]) with MCD experienced relapse.
Discussion
This retrospective study describes the largest published cohort of pediatric/AYA patients with Castleman disease. Among pediatric patients with both UCD and HHV8-negative MCD, there is a high proportion of patients with systemic inflammation at presentation and while there is a significant risk of relapse/progression among MCD patients, there is excellent overall survival.
As in adult populations, HV histopathology was most common in our cohort for both UCD and HHV8-negative MCD patients.10 In adults, histopathologic variant is associated with variation in presentation. Adults with the HV variant are likely to have UCD and often lack systemic or constitutional symptoms; when adults with HV variant present with MCD, it is often associated TAFRO and inflammatory milieu.6,7,10,20 In contrast, patients with the PCV, regardless of centricity, often have fevers, diaphoresis, arthralgia, neuropathies, and laboratory derangements such as elevated IL-6, hypoalbuminemia, microcytic anemia, elevated ESR, amyloidosis, and association with HHV8.1,13 Similarly, in our pediatric/AYA cohort, systemic symptoms were more common among MCD patients than UCD patients, although this difference in presentations by centricity is less striking than in adults. For example, 44% presented with systemic symptoms, which is above the upper range of the reported 10–40% rate of systemic symptoms reported in adult UCD reports.6,7,10 Among HHV8-negative MCD patients in this cohort, 4/6 (67%) had systemic symptoms, which is at the lower end of the 60–80% range reported in adult cohorts.6,7,9,21
In addition to symptoms of inflammation, our cohort demonstrated a high frequency of laboratory inflammation (CRP, ESR and IL-6) among patients with both UCD and HHV8-negative MCD. While laboratory inflammation is expected among MCD patients, our UCD population appeared to have increased inflammation compared to adult cohorts. For example, while in our cohort, 45% of UCD patients had an elevated ESR, in an adult UCD cohort this rate was 19%,7 and 50% had an elevated CRP.6
Even with this high rate of systemically symptomatic UCD in our population, roughly half of patients did present with an asymptomatic palpable mass, suggesting that UCD should remain on the differential diagnosis when a pediatric/AYA patient presents with a palpable mass without systemic symptoms. Given our findings that disease centricity cannot be predicted by clinical or laboratory evaluation at diagnosis, cross-sectional body imaging should be considered as part of the staging evaluation for any pediatric/AYA CD patient. The necessity of considering upfront cross-sectional body imaging in CD patients is highlighted by HHV8-negative MCD Patient 4 (Table 2), who initially was thought to have UCD but likely had a missed diagnosis MCD due to limited upfront imaging. Typical imaging features in our cohort include nodal masses that appear uniformly hypoechoic on ultrasound and homogeneously hyper-enhancing on CT and MRI. By FDG-PET, lesions showed variable, mild to moderate avidity, which has also been shown in adult studies.6,7 Of note, UCD patients tended to have larger lesions than MCD patients.
This pediatric cohort also differed from adult MCD literature in that that no patients had HHV8- or HIV-associated disease. In contrast, a systematic review of all literature describes an MCD population in which 42% of MCD patients had HHV8- and/or HIV-associated disease21 and Robinson et al. reported a United States population in which 17% of MCD patients had HHV8 and 14% had HIV (extent of overlap not reported).22 Our data is consistent, however, with Leroy et al.’s systematic review of pediatric MCD cases, in which the 22% of patients who were tested for HHV8 were all negative, even despite 50% of patients being from HHV8 endemic countries;16 in combination, these studies suggest that viral associations may be less important in development of pediatric MCD.
As in adults, management and prognosis of Castleman disease in this pediatric/AYA cohort was defined by disease centricity. Patients who cannot undergo GTR should receive some combination of surgery, corticosteroids, chemotherapies, radiation, or immunotherapies such as rituximab, anti-IL-6 therapy, and anti-viral therapies.8,10,12,23–25 Among our cohort, all patients with UCD achieved either initial remission with GTR or stable disease without treatment. Among patients in our cohort with HHV8-negative MCD, 5/6 required treatment with chemotherapy and/or immunotherapy to achieve lasting remission. One of these patients did only require steroid therapy, which may be due to his EBV-related disease; known disease was limited to two nodal stations, although this patient also had plasmacytosis in his bone marrow and tonsils. Because of the diverse immunosuppressive or chemotherapeutic regimens administered to our patients, conclusions about them is not possible. However, as evidenced by MCD patients 5 and 6, resection (GTR or partial resection) may play a role in patients with limited disease, lack of systemic inflammation, or where GTR is possible.
HHV8-negative MCD patients were much more likely to experience relapse/progression (50%) than UCD patients (none). Even so, no patients in our cohort died. In contrast, the adult 5-year overall survival (OS) rate for patients with UCD is 91–98%6,7,9 and the 5-year OS for patients with MCD has been reported to be as low as 55–77%.7,9,26–28 The discrepancy between adult and pediatric survival may be related to the aforementioned lower rate of underlying viral illness associated with CD in pediatrics, since HHV8 and HIV-associated CD have significantly worse two-year survival than patients with idiopathic MCD.6 In addition, our small sample size may contribute to our lack of deaths; to this end, one institution has since had a patient die of MCD.
The retrospective nature of this study is associated with several limitations. The small sample size in our study resulted in large variability in many of the outcomes and while the results were clinically interesting and relevant, statistical testing was not possible. Further investigation into some of the observed trends using a larger cohort will be of value. Furthermore, since a standard of care consensus for management of MCD is lacking until recently, there was significant variability in management, limiting conclusions. In addition, due to the retrospective nature of this study, we are limited by the available data; for example, one HHV8-negative MCD patient did not have any available laboratory results at diagnosis, and several others did not have inflammatory markers. There was a moderate amount of loss to follow-up, largely among UCD patients who undergo GTR; it is possible that some of these patients subsequently recurred and sought treatment at a different institution, although this is unlikely both because of the nature of UCD but also given that the participating centers are the dominant pediatric practices in their respective areas.
In summary, our cohort of pediatric/AYA patients with CD presented similarly to adults in terms of disease centricity and pathology but, unlike in adults, there are high rates of systemic inflammation even among UCD patients. Because patients with MCD typically require systemic therapy, a standardized approach to disease evaluation with CBC, inflammatory markers, and cross-sectional imaging is recommended. While treatment approaches in pediatric/AYA CD are similar to those in adults, overall survival is favorable.
Acknowledgements
This work was supported by the National Institutes of Health Paul Calabresi Clinical Oncology Scholar program (grant K12CA086913). Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Abbreviation Key
- AYA
Adolescent/young adult
- CBC
Complete blood count
- CD
Castleman disease
- CHCO
Children’s Hospital Colorado
- CI
Confidence intervals
- CRP
C-reactive protein
- CT
Computed tomography
- cm3
Cubic centimeters
- ESR
Erythrocyte sedimentation rate
- FDG-PET
18F fluorodeoxyglucose positron emission tomography
- GTR
Gross total resection
- HHV8
Human herpes virus 8
- HIV
Human immunodeficiency virus
- HV
Hyaline vascular
- IL-6
Interleukin-6
- iMCD
Idiopathic multicentric Castleman disease
- IQR
Interquartile range
- MCD
Multicentric Castleman disease
- MRI
Magnetic resonance imaging
- OS
Overall survival
- PCR
Polymerase chain reaction
- PCV
Plasma cell variant
- R-CHOP
Rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone
- R-CVP
Rituximab, cyclophosphamide, vincristine, prednisone
- SUVmax
Maximum standardized uptake value
- TAFRO
Thrombocytopenia, anasarca, myelofibrosis, renal failure, and organomegaly
- UCD
Unicentric Castleman disease
Footnotes
Conflict of Interest statement
All authors declare no competing financial interests.
References
- 1.El-Osta HE, Kurzrock R. Castleman’s disease: from basic mechanisms to molecular therapeutics. Oncologist. 2011;16(4):497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fajgenbaum DC, Uldrick TS, Bagg A, et al. International, evidence-based consensus diagnostic criteria for HHV-8-negative/idiopathic multicentric Castleman disease. Blood. 2017;129(12):1646–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munshi N, Mehra M, van de Velde H, Desai A, Potluri R, Vermeulen J. Use of a claims database to characterize and estimate the incidence rate for Castleman disease. Leuk Lymphoma. 2015;56(5):1252–1260. [DOI] [PubMed] [Google Scholar]
- 4.Fajgenbaum DC, van Rhee F, Nabel CS. HHV-8-negative, idiopathic multicentric Castleman disease: novel insights into biology, pathogenesis, and therapy. Blood. 2014;123(19):2924–2933. [DOI] [PubMed] [Google Scholar]
- 5.Dupin N, Diss TL, Kellam P, et al. HHV-8 is associated with a plasmablastic variant of Castleman disease that is linked to HHV-8-positive plasmablastic lymphoma. Blood. 2000;95(4):1406–1412. [PubMed] [Google Scholar]
- 6.Oksenhendler E, Boutboul D, Fajgenbaum D, et al. The full spectrum of Castleman disease: 273 patients studied over 20 years. Br J Haematol. 2018;180(2):206–216. [DOI] [PubMed] [Google Scholar]
- 7.Yu L, Tu M, Cortes J, et al. Clinical and pathological characteristics of HIV- and HHV-8-negative Castleman disease. Blood. 2017;129(12):1658–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Talat N, Belgaumkar AP, Schulte KM. Surgery in Castleman’s disease: a systematic review of 404 published cases. Ann Surg. 2012;255(4):677–684. [DOI] [PubMed] [Google Scholar]
- 9.Dispenzieri A, Armitage JO, Loe MJ, et al. The clinical spectrum of Castleman’s disease. Am J Hematol. 2012;87(11):997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casper C The aetiology and management of Castleman disease at 50 years: translating pathophysiology to patient care. Br J Haematol. 2005;129(1):3–17. [DOI] [PubMed] [Google Scholar]
- 11.van Rhee F, Voorhees P, Dispenzieri A, et al. International, evidence-based consensus treatment guidelines for idiopathic multicentric Castleman disease. Blood. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karapinar TH, Tufekci O, Gozmen S, Yilmaz S, Irken G, Oren H. Multicentric plasma cell type of castleman disease in a child: difficulty in diagnosis and treatment. J Pediatr Hematol Oncol. 2013;35(7):e306–308. [DOI] [PubMed] [Google Scholar]
- 13.Baserga M, Rosin M, Schoen M, Young G. Multifocal Castleman disease in pediatrics: case report. J Pediatr Hematol Oncol. 2005;27(12):666–669. [DOI] [PubMed] [Google Scholar]
- 14.Farruggia P, Trizzino A, Scibetta N, et al. Castleman’s disease in childhood: report of three cases and review of the literature. Ital J Pediatr. 2011;37:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J, Li C, Lv L, et al. Clinical and experimental study of Castleman disease in children. Pediatr Blood Cancer. 2015;62(1):109–114. [DOI] [PubMed] [Google Scholar]
- 16.Leroy S, Moshous D, Cassar O, et al. Multicentric Castleman disease in an HHV8-infected child born to consanguineous parents with systematic review. Pediatrics. 2012;129(1):e199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Renwick N, Dukers NH, Weverling GJ, et al. Risk factors for human herpesvirus 8 infection in a cohort of drug users in the Netherlands, 1985–1996. J Infect Dis. 2002;185(12):1808–1812. [DOI] [PubMed] [Google Scholar]
- 18.Orkin SH. Nathan and Oski’s hematology and oncology of infancy and childhood (ed Eighth edition.). Philadelphia, PA: Elsevier/Saunders; 2015. [Google Scholar]
- 19.Kawabata H, Takai K, Kojima M, et al. Castleman-Kojima disease (TAFRO syndrome): a novel systemic inflammatory disease characterized by a constellation of symptoms, namely, thrombocytopenia, ascites (anasarca), microcytic anemia, myelofibrosis, renal dysfunction, and organomegaly: a status report and summary of Fukushima (6 June, 2012) and Nagoya meetings (22 September, 2012). J Clin Exp Hematop. 2013;53(1):57–61. [DOI] [PubMed] [Google Scholar]
- 20.Masaki Y, Kawabata H, Takai K, et al. Proposed diagnostic criteria, disease severity classification and treatment strategy for TAFRO syndrome, 2015 version. Int J Hematol. 2016;103(6):686–692. [DOI] [PubMed] [Google Scholar]
- 21.Liu AY, Nabel CS, Finkelman BS, et al. Idiopathic multicentric Castleman’s disease: a systematic literature review. Lancet Haematol. 2016;3(4):e163–175. [DOI] [PubMed] [Google Scholar]
- 22.Robinson D, Jr., Reynolds M, Casper C, et al. Clinical epidemiology and treatment patterns of patients with multicentric Castleman disease: results from two US treatment centres. Br J Haematol. 2014;165(1):39–48. [DOI] [PubMed] [Google Scholar]
- 23.Rokx C, Rijnders BJ, van Laar JA. Treatment of multicentric Castleman’s disease in HIV-1 infected and uninfected patients: a systematic review. Neth J Med. 2015;73(5):202–210. [PubMed] [Google Scholar]
- 24.Nishimoto N, Kanakura Y, Aozasa K, et al. Humanized anti-interleukin-6 receptor antibody treatment of multicentric Castleman disease. Blood. 2005;106(8):2627–2632. [DOI] [PubMed] [Google Scholar]
- 25.Bandera B, Ainsworth C, Shikle J, Rupard E, Roach M. Treatment of unicentric Castleman disease with neoadjuvant rituximab. Chest. 2010;138(5):1239–1241. [DOI] [PubMed] [Google Scholar]
- 26.Shin DY, Jeon YK, Hong YS, et al. Clinical dissection of multicentric Castleman disease. Leuk Lymphoma. 2011;52(8):1517–1522. [DOI] [PubMed] [Google Scholar]
- 27.Melikyan AL, Egorova EK, Kovrigina capital A CEMC, et al. [Clinical and morphological features of different types of Castleman’s disease]. Ter Arkh. 2015;87(7):64–71. [DOI] [PubMed] [Google Scholar]
- 28.Seo S, Yoo C, Yoon DH, et al. Clinical features and outcomes in patients with human immunodeficiency virus-negative, multicentric Castleman’s disease: a single medical center experience. Blood Res. 2014;49(4):253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]