Abstract
Background:
Treating B–non-Hodgkin lymphoma (NHL) in lower-income countries is challenging because of imprecise diagnosis, the increased risk of fatal toxicity associated with advanced disease at presentation, and limited supportive care.
Procedure:
Central American patients with newly diagnosed stage I or II B-NHL received a modified BFM regimen including a prephase (prednisone, cyclophosphamide) followed by A/B/A courses (A: cytarabine, dexamethasone, etoposide, ifosfamide, methotrexate, and intrathecal therapy; B: cyclophosphamide, dexamethasone, doxorubicin, methotrexate, and intrathecal therapy). Those with stage III or IV NHL received additional courses (B/A/B), intensified for stage IV disease by additional vincristine and methotrexate doses. Patients in poor condition received a second prephase treatment before their chemotherapy courses.
Results:
Between March 2004 and June 2016, of 405 patients with B-NHL, 386 (109 females) were eligible for treatment. Immunohistochemistry was performed in 177 cases (47.4%) and characterized the disease as mature B-cell lymphoma. Stage distribution was as follows: I/II, 31 (8.1%); III, 252 (65.3%); IV, 93 (24.1%); 10 (2.6%) not available. The 3-year overall survival was 70% for the whole group (86% for stages I/II, 75% for stage III, 58% for stage IV). Events included death during induction (34 patients, 8.8%), relapse/progression (46, 11.9%), death in remission (9, 2.3%), second malignancy (1, 0.26%), and death of unknown cause (1, 0.26%). Twenty-three (6%) patients abandoned or refused therapy.
Conclusions:
Approximately 70% of children with B-NHL from Central America experienced long-term, disease-free survival with a modified BFM schedule. Toxic death and relapse/resistant disease were the main reasons for treatment failure.
Keywords: non-Hodgkin lymphoma, chemotherapy, global oncology, Burkitt lymphoma, treatment
1. INTRODUCTION
The prognosis of childhood mature B-cell malignant neoplasms has improved dramatically in high-income countries,1 and more than 90% of patients are now expected to survive. Intensive chemotherapy including rituximab, detailed supportive care, and well-designed clinical trials account for this success.1 However, the prognosis of this disease in settings with limited resources continues to be poorer, with disease-free survival rates being in the range of 60% to 70%.2–5 Although intense chemotherapy is critical for high survival rates, such treatment may not be feasible in limited-resource settings because of limitations in supportive care, poor patient performance status, and advanced disease at presentation, all of which are associated with high toxicity and mortality rates, primarily due to tumor lysis syndrome and bacterial or fungal infections during the early treatment period.6, 7
The clinical and biologic features and outcomes of mature B-cell neoplasms are not uniform worldwide.8–10 For example, the survival rates for endemic Burkitt lymphoma are substantially lower than those for sporadic Burkitt lymphoma.11, 12 The reasons for these differences are unknown, but they are probably related to access to optimal care rather than to the disease biology. No studies have yet addressed these issues.13 Most of the drugs used to treat non-Hodgkin lymphoma (NHL) are available in low- to middle-income countries (LMICs). However, because of deficient supportive care in these countries, it is a common practice to reduce the dose intensity of protocols used by cooperative groups in high-income countries.2–4, 14, 15 Even with such dose reductions, there are still many treatment-related deaths in LMICs.3, 7, 14 There is only sparse information available on the outcomes of patients with Burkitt lymphoma in countries16 such as those in Central America that have invested resources to improve supportive care and collaborate in establishing uniform treatment and supportive care guidelines. Previous experience with unmodified treatment regimens in our region showed unacceptable toxicity.17 For the past 20 years, pediatric oncologists from Guatemala, El Salvador, Honduras, Nicaragua, Costa Rica, Panama, the Dominican Republic, and Haiti (as members of AHOPCA, the Asociación de Hemato-Oncología Pediátrica de Centro América) have been working collaboratively to standardize treatment regimens across multiple institutions to improve therapy for children with cancer.18, 19 Here we report the results of a retrospective review of multi-site experience with a common treatment strategy adapted from the Berlin-Frankfurt-Münster (BFM) protocol for children with a clinico-pathologic diagnosis of B-NHL, as used by institutions in six Central American countries.
2. METHODS
2.1. Patients
This study included all patients aged up to 18 years with newly diagnosed, previously untreated B-NHL treated between March 2004 and June 2016. Patients with a history of previous therapy or malignant neoplasms, HIV infection, or congenital immunodeficiencies were not included in the analysis. Data were obtained from six institutions (in Tegucigalpa and San Pedro de Sula in Honduras, Guatemala City in Guatemala, San Salvador in El Salvador, Managua in Nicaragua, and Santo Domingo in the Dominican Republic). In all cases, the extent of disease was evaluated by computed tomography of clinically affected sites, bone marrow aspiration, and lumbar puncture before treatment. Serum lactate dehydrogenase levels were not mandatory for patient stratification and were not consistently obtained. The St. Jude–Murphy system was used for staging.20 The histopathologic diagnosis of B-NHL was performed by the local pathologist, using hematoxylin-eosin–stained slides. When available, immunohistochemical analysis was performed. All cases were classified histopathologically by the local pathologist but this classification was not used for calculating survival results. In some cases, the diagnosis was established by flow cytometric analysis of cells from the bone marrow or from peritoneal or pleural effusions. When the local pathologist was uncertain of the diagnosis, tumor samples were submitted to St. Jude Children’s Research Hospital for pathology consultation. A specifically designed case report form was created at the Pediatric Oncology Networked Database (www.POND4KIDS.org), and all data were uploaded to this database prospectively by a data manager at each center upon diagnosis and analyzed yearly by the first author for consistency. A final analysis was performed for this report in March 2018.
2.2. Treatment
The treatment used was based on the Berlin-Frankfurt-Münster (BFM) 90 strategy,21 adapted to local supportive care resources (Table 1). The treatment plan according to the disease stage is shown in Table 2. The patient stratification and the order of treatment courses are shown in Supplemental Figure S1, and the composition of the central nervous system (CNS) chemotherapy regimen is shown in Supplemental Table S1.
TABLE 1.
Summary of modifications in this guideline as compared to the original NHL BFM 90 protocol
| Treatment component | NHL BFM 90 | This study |
|---|---|---|
| Methotrexate dose in blocks AA and BB | 5 g/m2 in a 24-hour infusion | 1–3 g/m2 in a 3-hour infusion |
| Leucovorin rescue | 30 mg/m2 (hour 42 after the start of methotrexate) 15 mg/m2 (hours 48 and 54 after the start of methotrexate) Additional doses as per plasma methotrexate levels |
15 mg/m2/dose starting at 24 hours after the start of methotrexate) No plasma methotrexate levels |
| Dose of ifosfamide in blocks A and AA | 800 mg/m2/day on days 1 to 5 | 400 mg/m2/day on days 1 to 5 |
| Proposed interval between chemotherapy blocks | 14 days | 21 days |
| Treatment of stage IV (CNS positive) | Intraventricular chemotherapy | Intrathecal chemotherapy |
| Treatment of patients with incomplete response after induction | Blocks CC, AA, and BB Second-look surgery if persistent mass |
No block CC was given. Methotrexate dose was increased to 3 g/m2 in further blocks AA and BB No second-look surgery if persistent mass |
| Patient stratification | Stage, primary site location, and LDH levels | Stage |
| Second prephase in critical cases | No | Used for suspected or documented infections, unresolved renal and electrolyte disorders |
| Autologous stem cell rescue | For patients with persistent disease activity after CC block | Not available |
TABLE 2.
Therapy courses
| Drug | Dose | Day | ||
|---|---|---|---|---|
| Prephase | Prednisone | 30 mg/m2 | 1 to 5 | Orally in 3 divided doses |
| Cyclophosphamide | 200 mg/m2 | 1 to 5 | 1-hour IV infusion | |
| Intrathecal | Methotrexate | Age-adjusted | 1 or 1 and 4¥ | |
| Hydrocortisone | ||||
| Cytarabine | ||||
| Course A/AA | Dexamethasone | 10 mg/m2 | 1 to 5 | Orally in 3 divided doses |
| Ifosfamide/MESNA | 400 mg/m2 | 1 to 5 | 1-hour IV infusion | |
| Methotrexate | 1–3 g/m2 | 1 | 10% infused as a push and 90% in 3 hours | |
| Leucovorin | 15 mg/m2 (*) | 2 | First dose starting at 24 hours after the start of the infusion of methotrexate and continued every 6 hours for 12 doses. |
|
| Cytarabine | 150 mg/m2 | 4 and 5 | 30-minute IV infusion every 12 hours (4 doses). | |
| Etoposide | 100 mg/m2 | 4 and 5 | 1-hour IV infusion daily (2 doses) | |
| Vincristine(*) | 1.5 mg/m2 | 1 | IV push | |
| Intrathecal | Methotrexate | Age-adjusted | 1 or 4 doses weekly ¥ | |
| Hydrocortisone | ||||
| Cytarabine | ||||
|
Course B/BB Intrathecal |
Dexamethasone Cyclophosphamide Methotrexate Leucovorin Doxorubicin Vincristine (**) Methotrexate Hydrocortisone Cytarabine |
10 mg/m2 200 mg/m2 1–3 g/m2 15 mg/m2 (*) 25 mg/m2 1.5 mg/m2 Age-adjusted |
1 to 5 1 to 5 1 2 4 and 5 1 1 |
Divided into 3 doses, given orally 1-hour IV infusion 10% infused as a push and 90% in 3 hours First dose starting at 24 hours after the start of the infusion of methotrexate and continued every 6 hours for 12 doses. 1-hour IV infusion IV push |
Block AA only
Block BB only
Patients with central nervous system involvement
Imaging studies and bone marrow aspirates and/or lumbar punctures (when performed initially) were repeated after two cycles to assess the response to therapy. Patients with stage III disease with incomplete response after the first two cycles of chemotherapy (the A and B courses) received four more courses consisting of AA, BB, AA, and BB. No second-look surgery was scheduled.
All patients received uniform supportive care, including hyperhydration and allopurinol; however, rasburicase was not regularly available. Patients with suspected or documented infections, extensive abdominal surgery, or unresolved renal insufficiency or electrolyte disturbances at the time of completion of the prephase received a second prephase treatment before their chemotherapy courses.
Chemotherapy courses were scheduled every 3 weeks if hematopoietic reconstitution (ANC > 1000/mm3 and platelet count > 100,000/mm3) was attained. Methotrexate plasma level measurements were not available. Patients with initial CNS involvement received an intensified schedule of intrathecal therapy consisting of two applications during the prephase followed by four weekly applications during the first chemotherapy block. There was no change in CNS-directed therapy in the remaining courses.
Weekly online meetings were held via the St. Jude Cure4Kids website (www.Cure4kids.org) to discuss the management of newly diagnosed cases and complications arising during treatment.
This study was performed under the tenets of the Declaration of Helsinki, and written informed consent for general treatment was obtained from the parents or guardians of the patients. The study was approved by the institutional review board at the coordinating center, the Hospital Escuela-Universitario in Tegucigalpa, Honduras
2.3. Statistical analysis
Event-free survival (EFS) was calculated from the time of diagnosis until death from any cause, relapse, abandonment, or second malignancy and was censored at the date of last follow-up for patients with no adverse event. The EFS was calculated using the Kaplan-Meier method, and the difference between groups was estimated by the log-rank test. Early deaths were defined as those occurring during the prephase; deaths in induction as those occurring after the beginning of the first induction course but before remission was evaluated; and deaths in complete remission as those attributed to causes other than the primary tumor while the patient’s disease was in complete remission. Abandonment was defined as absence from scheduled visits during therapy for at least 4 weeks. Follow-up was updated as of September 1, 2017.
3. RESULTS
A clinico-pathologic diagnosis of B–non-Hodgkin lymphoma was made in 405 patients from six centers in five countries during the study period, and 386 patients (277 males and 109 females) were eligible for inclusion. Reasons for exclusion included previous treatment (11 patients), HIV infection (five patients), and a change in the diagnosis after review (three patients). Of the evaluable patients, 95 were from Honduras (46 from Tegucigalpa and 49 from San Pedro Sula), 90 from El Salvador, 76 from Guatemala, 70 from Nicaragua, and 55 from the Dominican Republic.
The diagnosis of B-NHL was established clinically and from tissue biopsy in 373 cases, of which 177 (47.4%) were further characterized as mature B-cell lymphoma by immunohistochemical analysis (Supplemental Table S2). In 13 cases, the diagnosis was established by cytomorphologic examination of the bone marrow or body fluids and was confirmed by flow cytometry. Thirty-two cases were submitted for pathology review at St. Jude Children’s Research Hospital. Of these, three were not confirmed as B-NHL and were excluded from further analysis. The pathologic diagnosis was consistent with Burkitt lymphoma in 319 cases (82.6%) and with diffuse B large-cell lymphoma in 24 (6.2%). Thirty cases (7.7%) were reported to be consistent with B-cell lymphoma by the local pathologist.
Thirty-one patients had stage I or II disease, 252 had stage III disease, and 93 had stage IV B-ALL (53 with bone marrow involvement, 23 with CNS involvement, and 17 with combined bone marrow and CNS involvement). Ten patients arrived at the hospital in very poor clinical condition and died before staging could be completed; they were included in the general EFS analysis but were excluded from the analysis by stage. The primary tumor sites included the abdomen in 263 patients (68.1%), the peripheral lymph nodes in 77 patients (19.9%), and extranodal sites in 26 patients (6.7%). The extranodal sites included the mandible in 14 patients (3.6%). Eleven patients (2.9%) were considered to have primary bone marrow involvement (B-ALL), and seven had mediastinal primary tumors (1.8%). In two cases (0.5%), the primary site could not be determined because the patients died before imaging studies could be obtained. The lactate dehydrogenase level obtained at diagnosis immediately before starting therapy was available in 263 cases, having a median value of 685 UI/L (range, 111 UI/L to 11,425 UI/L) (Table 3).
TABLE 3.
Selected clinical features of patients
| Features | 386 patients |
| Median age (range) | 6.9 years (2 to 17 years) |
| Sex Female Male |
109 (28.2%) 277 (71.8%) |
| Stage I/II III IV Bone marrow Central nervous system Combined Unknown |
31 (8%) 252 (65.3%) 93 (24.1%) 53 23 17 10 (2.6%) |
| Primary site Abdomen Peripheral lymph nodes Mandible Other extranodal Bone marrow (B-ALL) Mediastinum Unknown |
263 (68.1%) 77 (19.9%) 14 (3.6%) 12 (3.1%) 11 (2.9%) 7 (1.8%) 2 (0.5%) |
| Diagnostic method Biopsy Biopsy plus immunohistochemistry Cytology plus flow cytometry |
196 (50.8%) 177 (45.85%) 13 (3.36%) |
| Histology Burkitt Lymphoma Large cell lymphoma Non-specified Not applicable |
319 (82.6%) 24 (6.2%) 30 (7.7%) 13 (3.5%) |
| LDH (IU/L) <500 ≥500 Not available |
97 (25.1%) 166 (43%) 123 (31.9%) |
Eighty-nine patients (23.1%) received a second prephase treatment before the first chemotherapy block. In this group, the interval between the initial prephase treatment and the first block (n = 82) was 19 days (range, 5–73 days), which was comparable to that for patients receiving a single prephase treatment (20 days; range, 5–39 days). The median time between chemotherapy blocks was 25 days (range, 14–98 days). Twenty-two patients with stage III disease received intensified therapy because of an incomplete response after induction.
With a median follow-up of 49 months (range, 2–150 months), the 3-year probability of EFS was 70% (95% CI, 65%–74%) for the whole group (Figure 1A), 83% (95% CI, 65%–91%) for patients with stage I or II disease, 75% (95% CI, 69%–81%) for patients with stage III disease, and 55% (95% CI, 44%–64%) for patients with stage IV B-ALL (Figure 1B). For patients with stage III disease, the EFS of patients experiencing complete remission (n = 208; 84% EFS [95% CI, 79%–88%]) was significantly higher (P = 0.0001) than that of patients with a residual mass who underwent treatment intensification (n = 89; 50% EFS [95% CI, 32%–68%]) (Figure 1C). The pEFS at 3 years of patients who had immunohistochemistry confirmation of mature B-cell lymphoma was 0.77 (95% CI, 0.7–0.82) versus 0.69 (95% CI, 0.62–0.75) for those in whom no immunohistochemistry confirmation was performed (P = 0.09).
FIGURE 1.
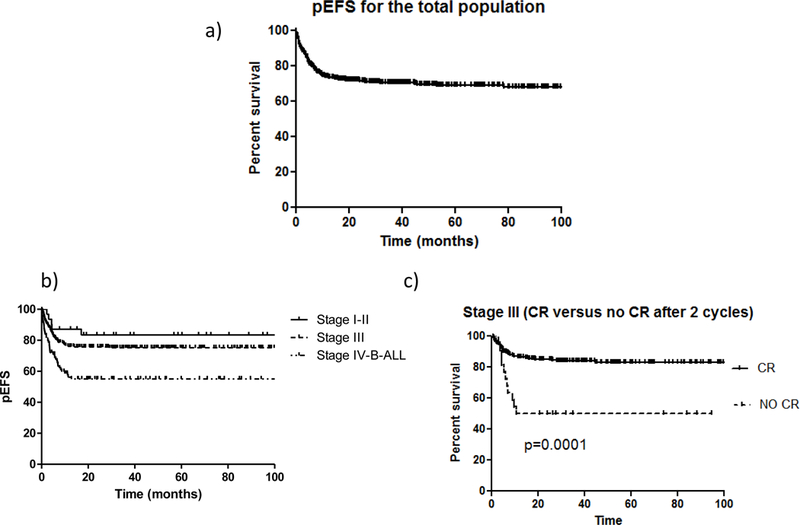
(A) Probability of 3-year event-free survival (EFS) for the 386 patients with NHL. (B) Probability of 3-year EFS according to disease stage. (C) Probability of 3-year EFS for 208 patients with stage III disease based on the evidence of residual disease after induction.
Adverse events included early deaths in 13 patients (3.3%): 11 of metabolic complications and two of infectious complications. Twenty-one patients (5.4%) died during induction from infectious complications (n = 18), metabolic complications (n = 2), or surgical complications (n = 1). Twenty-two patients (5.7%) experienced relapse (at the primary site in 13 patients, in the bone marrow in one patient, in the combined CNS and bone marrow in one patient, in the CNS alone in five patients, and in the testicles in two patients). The median time from diagnosis to relapse was 7.4 months (range, 2–29 months). Only one of the patients who experienced relapse remains alive, having been in second complete remission for 155 months. Twenty-four patients (6.2%) never experienced complete remission, and they died of progressive disease. Of these 24 patients, 13 received a second prephase treatment and one received a third prephase treatment. In one case, complications from abdominal surgery resulted in long delays in chemotherapy, and in another case, hepatic toxicity precluded chemotherapy administration. Nine patients (2.3%) died while in complete remission. Of these, eight died of septicemia during neutropenia and one of cardiogenic shock of unknown etiology. One child presented a disseminated lymphoblastic lymphoma 44 months after the diagnosis of the primary Burkitt lymphoma, and this was interpreted as a secondary lymphoma. One patient died at home of an unknown cause. Twenty-three patients abandoned or refused therapy (6%) during treatment and were considered to have died. The types of event according to disease stage are shown in Table 4.
TABLE 4.
Description of adverse events according to stage
|
Total N=114 (%) |
Stage II N=5 (%) |
Stage III N=61 (%) |
Stage IV/B- ALL N=41 (%) |
Unknown N=7 (%) |
|
|---|---|---|---|---|---|
| Early death | 13 (11) | 0 | 4 (7) | 5 (12) | 4 (57) |
| Death in Induction | 21 (18) | 2 (40) | 11 (18) | 7 (17) | 1 (14) |
| Relapse | 22 (19) | 1 (20) | 9 (15) | 12 (29) | 0 |
| Progressive disease | 24 (21) | 0 | 10 (16) | 14 (34) | 0 |
| Death in complete remission | 9 (8) | 1 (20) | 8 (13) | 0 | 0 |
| Abandonment or treatment refusal | 23 (20) | 1 (20) | 18 (30) | 2 (5) | 2 (29) |
| Other | 2 (2) | 0 | Second malignancy N=1 (2) |
Death of unknown cause N=1 (2) | 0 |
4. DISCUSSION
This collaborative study among institutions in several low-income countries has demonstrated that an effective reduced-intensity regimen for treating mature B-cell lymphoma is feasible. More than 70% of the children included in the study experienced EFS, which, given that only one case having an event could be salvaged, is equivalent to overall survival. Our findings support the concept that reduced-intensity chemotherapy regimens for mature B-cell lymphomas can be successfully implemented in low-resource settings2, 3 and are associated with disease-free survival in two-thirds of patients. Even in settings where minimal supportive care is available, such as Equatorial Africa, low-intensity therapy is still feasible and effective for up to 40% of cases,6 although the difficulty of long-term follow-up remains unresolved.
Our overall results are substantially inferior to those achieved in high-income countries, where the use of more intensive chemotherapy approaches in conjunction with improved supportive care has been associated with survival rates of 95% in children with mature B-cell malignant neoplasms.1 Further improving survival in the remaining 30% of children with mature B-cell lymphoma in our region will be challenging. In these settings, patients commonly present with advanced disease, and supportive care is suboptimal and variable among participating institutions. Even within the same institution, competing needs during a health crisis can affect the ability to deliver routine care. Thus, simply increasing the treatment intensity for all patients might result in unacceptable toxic mortality rates at those institutions that have inadequate infrastructure or are vulnerable to intermittent competing health-associated demands. This is consistent with reports showing that in low-income settings, increasing the intensity of chemotherapy regimens was associated with high toxic mortality rates that counterbalanced the improved antitumor effects.12, 22 Similarly, reports from referral institutions in upper middle-income countries such as Brazil, Argentina, and Venezuela, showed that the rates of toxic death there were still higher than those associated with the protocols from large cooperative groups in high-income countries, despite the patients being treated in institutions with better infrastructure than is found in lower-income settings.2, 3, 23 In this regard, the remission-induction mortality rates varied from 4.3% to 12.2% among the centers participating in our study.
The impact of adapting a uniform collaborative treatment regimen for participating institutions in countries or continents with substantial inequalities has yet to be addressed.4, 24, 25 As a first step, we suggest evaluating the conditions at each center and then tailoring the regimen intensity accordingly. Moreover, monitoring the results of the ongoing treatment for all patients enables treatment adaptations in real time. For example, a feasibility study conducted in Honduras revealed that an unmodified LMB89-based regimen was associated with unacceptable toxicity with a 45% toxic death rate in a small cohort.17 Based on this experience, treatment guidelines were adapted to the local resources2, 23 and evaluated every week through online case discussions that included the primary physicians and one or two NHL experts via the St. Jude Cure4Kids website. This enabled the identification of diverse medical and sociocultural circumstances and prompt intervention to address them.26
Despite these efforts, the 8.7% death rate in remission in our study is much higher than the rate of 1.4% reported by the BFM 95 protocol investigators27 and the rates in upper middle-income countries, which range from 1.8% to 4%.2, 3, 23 Because 90% of the patients included in our study cohort had stage III or IV B-ALL, compared to 66% of the patients treated on the BFM 95 protocol,27 our patients were at a higher risk of early complications or relapse. In fact, in our series, 10 patients died before any therapy was initiated because of their poor clinical condition at presentation. A further 5.4% died during induction chemotherapy. Because of the high tumor burden, tumor lysis, organ dysfunction, and infection complications in these patients, many of them could not begin the first chemotherapy block soon after the first prephase treatment. The median time from initiation of the first prephase treatment to the first block was 20 days, and there were no significant differences between those receiving a single prephase cycle and those receiving two cycles. In the original BFM protocol, the first block of intensive chemotherapy is scheduled immediately after the completion of the prephase. However, in this series, it was usually delayed because of concerns about infections and hematopoietic toxicity in a setting with high death rates during induction. Hence, the low early dose intensity may have allowed the expansion of resistant clones, which would partly explain why relatively more patients did not attain complete remission and experienced progression while undergoing chemotherapy. In our study, 6.2% of the patients had resistant or progressive disease, compared to 2.2% of those treated on the original BFM 95 protocol.27
Granulocyte colony-stimulating factor (G-CSF) was not used systematically in our cohort. Although a meta-analysis showed that its use in patients with chemotherapy-induced neutropenia had no effect on overall mortality, these data were mostly obtained from patient series in developed countries, where the toxic mortality rates are already low,28 and a different impact might be seen in settings like ours, with higher mortality rates during neutropenia. Administering G-CSF might also reduce the interval between cycles, thereby allowing higher-intensity early doses, and its use is under consideration by our group. Other strategies to reduce the intensity of therapy may have influenced the outcomes in our patients. The dose of methotrexate in courses AA and BB was reduced from 5 g/m2 via 24-hour infusion, as in the original BFM protocol, to 1–3 g/m2 via 3-hour infusion. We have not used the original “CC” BFM block, containing high-dose cytarabine, steroids, and etoposide, because of its higher hematopoietic and oro-intestinal toxicity. The BFM 95 protocol23 showed that shortening the duration of methotrexate infusion resulted in less toxicity, albeit accompanied by less activity in patients with advanced disease, than was seen with the 24-hour infusion.4, 27, 29 A reasonable option for enhancing the anti-lymphoma effect would be to add rituximab to the induction regimen.30 This approach could avoid the need to increase the dose intensity of drugs with multi-organ toxicity. A prospective study in Russian children showed that using rituximab in early therapy of mature B-cell neoplasms resulted in a 5% induction death rate and an overall survival of 82%.31 However, the relatively high cost of rituximab may restrict its use in limited-resource settings.
Abandonment of therapy has been a major determinant of outcome in many countries with limited resources, but it was relatively uncommon in our study patients, occurring in only 6% of cases. Central American countries have made efforts to understand the causes of abandonment and to intervene to prevent it.32 Because the treatment is relatively short and mature B-cell lymphoma has a high probability of cure, proving support to enable parents to adhere to the treatment schedule is effective in reducing abandonment.
There were limitations in the pathologic diagnosis of our cases. In a relatively high proportion of them, the diagnosis was based on hematoxylin-eosin staining, and other types of small blue cell tumors might have been included. However, among 30 of 194 cases in which the local pathologist was uncertain of the diagnosis and no immunohistochemical analysis was available, 27 cases were confirmed to be mature B-cell lymphoma. Moreover, the relapse pattern in our patients is comparable to that typically reported for mature B-cell lymphomas.33 However, in one case interpreted provisionally as a secondary lymphoma, a late relapse could not be ruled out molecularly. Finally, patients with limited-stage disease had very good outcomes, as reported with most series.2, 14, 15, 23
5. CONCLUSION
Seventy percent of children with B-NHL from poor areas of Central American countries experienced long-term, disease-free survival when treated with a modified BFM-based protocol for mature B-cell lymphoma. Modifications of the chemotherapy dose, the use of surrogate markers for risk assignment, and avoidance of regimens with higher-dose cytarabine and etoposide were implemented to complement the available supportive care capabilities of each collaborating institution. Actions to improve toxic death rates, especially during the induction period, and improved anti-lymphoma treatments based on better immunohistologic or molecular characterization would have the greatest impact in terms of improving results in our setting.
Supplementary Material
Patient stratification and treatment courses according to disease stage.
ACKNOWLEDGEMENTS
Raul C. Ribeiro and Monika L. Metzger are partially funded by NCI grant CA21765, the American Lebanese and Syrian Associated Charities (ALSAC), and the St. Jude Global Pediatric Medicine program. We thank Keith A. Laycock for expert review of the manuscript.
Abbreviation key:
- AHOPCA
Association of Pediatric Hematology-Oncology of Central America
- BFM
Berlin-Frankfurt-Münster
- CNS
Central nervous system
- EFS
Event-free survival
- G-CSF
Granulocyte colony-stimulating factor
- NHL
Non-Hodgkin lymphoma
- OS
Overall survival
References
- 1.Minard-Colin V, Brugieres L, Reiter A, et al. Non-Hodgkin Lymphoma in Children and Adolescents: Progress Through Effective Collaboration, Current Knowledge, and Challenges Ahead. J Clin Oncol. 2015;33: 2963–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chantada GL, Felice MS, Zubizarreta PA, Diaz L, Gallo G, Sackmann-Muriel F. Results of a BFM-based protocol for the treatment of childhood B-non-Hodgkin’s lymphoma and B-acute lymphoblastic leukemia in Argentina. Med Pediatr Oncol. 1997;28: 333–341. [DOI] [PubMed] [Google Scholar]
- 3.Acquatella G, Insausti CL, Garcia R, et al. Outcome of children with B cell lymphoma in Venezuela with the LMB-89 protocol. Pediatr Blood Cancer. 2004;43: 580–586. [DOI] [PubMed] [Google Scholar]
- 4.Harif M, Barsaoui S, Benchekroun S, et al. Treatment of B-cell lymphoma with LMB modified protocols in Africa--report of the French-African Pediatric Oncology Group (GFAOP). Pediatr Blood Cancer. 2008;50: 1138–1142. [DOI] [PubMed] [Google Scholar]
- 5.Gross TG, Biondi A. Paediatric non-Hodgkin lymphoma in low and middle income countries. Br J Haematol. 2016;173: 651–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hesseling P, Israels T, Harif M, Chantada G, Molyneux E, Pediatric Oncology in Developing C. Practical recommendations for the management of children with endemic Burkitt lymphoma (BL) in a resource limited setting. Pediatr Blood Cancer. 2013;60: 357–362. [DOI] [PubMed] [Google Scholar]
- 7.Cervio C, Barsotti D, Ibanez J, Paganini H, Sara Felice M, Chantada GL. Early mortality in children with advanced mature B-cell malignancies in a middle-income country. J Pediatr Hematol Oncol. 2012;34: e266–270. [DOI] [PubMed] [Google Scholar]
- 8.Ferreira JM, Klumb CE, de Souza Reis R, et al. Lymphoma subtype incidence rates in children and adolescents: first report from Brazil. Cancer Epidemiol. 2012;36: e221–226. [DOI] [PubMed] [Google Scholar]
- 9.Stefan DC, Lutchman R. Burkitt lymphoma: epidemiological features and survival in a South African centre. Infect Agent Cancer. 2014;9: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molyneux EM, Rochford R, Griffin B, et al. Burkitt’s lymphoma. Lancet. 2012;379: 1234–1244. [DOI] [PubMed] [Google Scholar]
- 11.Hesseling PB, Molyneux E, Tchintseme F, et al. Treating Burkitt’s lymphoma in Malawi, Cameroon, and Ghana. Lancet Oncol. 2008;9: 512–513. [DOI] [PubMed] [Google Scholar]
- 12.Buckle G, Maranda L, Skiles J, et al. Factors influencing survival among Kenyan children diagnosed with endemic Burkitt lymphoma between 2003 and 2011: A historical cohort study. Int J Cancer. 2016;139: 1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hesseling PB, Njume E, Kouya F, et al. The Cameroon 2008 Burkitt lymphoma protocol: improved event-free survival with treatment adapted to disease stage and the response to induction therapy. Pediatr Hematol Oncol. 2012;29: 119–129. [DOI] [PubMed] [Google Scholar]
- 14.Celkan TT, Baris S, Ozdemir N, et al. Treatment of pediatric Burkitt lymphoma in Turkey. J Pediatr Hematol Oncol. 2010;32: e279–284. [DOI] [PubMed] [Google Scholar]
- 15.Sun XF, Zhen ZJ, Lui DG, et al. Improved treatment outcome in Chinese children and adolescents with Burkitt’s lymphoma and large cell lymphoma by using the modified B-non-Hodgkin’s lymphoma-Berlin-Frankfurt-Munster-90 protocol. Eur J Haematol. 2006;77: 365–371. [DOI] [PubMed] [Google Scholar]
- 16.Prakash G, Sharma A, Raina V, Kumar L, Sharma MC, Mohanti BK. B cell non-Hodgkin’s lymphoma: experience from a tertiary care cancer center. Ann Hematol. 2012;91: 1603–1611. [DOI] [PubMed] [Google Scholar]
- 17.Howard SC, Ortiz R, Baez LF, et al. Protocol-based treatment for children with cancer in low income countries in Latin America: a report on the recent meetings of the Monza International School of Pediatric Hematology/Oncology (MISPHO)--part II. Pediatr Blood Cancer. 2007;48: 486–490. [DOI] [PubMed] [Google Scholar]
- 18.Castellanos EM, Barrantes JC, Baez LF, et al. A chemotherapy only therapeutic approach to pediatric Hodgkin lymphoma: AHOPCA LH 1999. Pediatr Blood Cancer. 2014;61: 997–1002. [DOI] [PubMed] [Google Scholar]
- 19.Ceppi F, Ortiz R, Antillon F, et al. Anaplastic Large Cell Lymphoma in Central America: A Report From the Central American Association of Pediatric Hematology Oncology (AHOPCA). Pediatr Blood Cancer. 2016;63: 78–82. [DOI] [PubMed] [Google Scholar]
- 20.Murphy SB, Fairclough DL, Hutchison RE, Berard CW. Non-Hodgkin’s lymphomas of childhood: an analysis of the histology, staging, and response to treatment of 338 cases at a single institution. J Clin Oncol. 1989;7: 186–193. [DOI] [PubMed] [Google Scholar]
- 21.Reiter A, Schrappe M, Tiemann M, et al. Improved treatment results in childhood B-cell neoplasms with tailored intensification of therapy: A report of the Berlin-Frankfurt-Munster Group Trial NHL-BFM 90. Blood. 1999;94: 3294–3306. [PubMed] [Google Scholar]
- 22.Magrath IT. Treatment of Burkitt lymphoma in children and adults: Lessons from Africa. Curr Hematol Malig Rep. 2006;1: 230–240. [DOI] [PubMed] [Google Scholar]
- 23.Klumb CE, Schramm MT, De Resende LM, et al. Treatment of children with B-cell non-Hodgkin’s lymphoma in developing countries: the experience of a single center in Brazil. J Pediatr Hematol Oncol. 2004;26: 462–468. [DOI] [PubMed] [Google Scholar]
- 24.Moleti ML, Al-Hadad SA, Al-Jadiry MF, et al. Treatment of children with B-cell non-Hodgkin lymphoma in a low-income country. Pediatr Blood Cancer. 2011;56: 560–567. [DOI] [PubMed] [Google Scholar]
- 25.Ahmad N, Zaidi A, Badar F, Maaz AU, Akram MS. Clinical characteristics and outcome analysis of pediatric B-cell non-Hodgkin’s lymphoma. Experience with FAB-LMB 96 and UKCCSG B-cell NHL guidelines in a developing country. Asia Pac J Clin Oncol. 2010;6: 49–56. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez-Galindo C, Friedrich P, Alcasabas P, et al. Toward the Cure of All Children With Cancer Through Collaborative Efforts: Pediatric Oncology As a Global Challenge. J Clin Oncol. 2015;33: 3065–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woessmann W, Seidemann K, Mann G, et al. The impact of the methotrexate administration schedule and dose in the treatment of children and adolescents with B-cell neoplasms: a report of the BFM Group Study NHL-BFM95. Blood. 2005;105: 948–958. [DOI] [PubMed] [Google Scholar]
- 28.Mhaskar R, Clark OA, Lyman G, Engel Ayer Botrel T, Morganti Paladini L, Djulbegovic B. Colony-stimulating factors for chemotherapy-induced febrile neutropenia. Cochrane Database Syst Rev. 2014: CD003039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kutluk T, Varan A, Akyuz C, Buyukpamukcu M. Clinical characteristics and treatment results of LMB/LMT regimen in children with non-Hodgkin’s lymphoma. Cancer Invest. 2002;20: 626–633. [DOI] [PubMed] [Google Scholar]
- 30.Meinhardt A, Burkhardt B, Zimmermann M, et al. Phase II window study on rituximab in newly diagnosed pediatric mature B-cell non-Hodgkin’s lymphoma and Burkitt leukemia. J Clin Oncol. 2010;28: 3115–3121. [DOI] [PubMed] [Google Scholar]
- 31.Samochatova EV, Maschan AA, Shelikhova LN, et al. Therapy of advanced-stage mature B-cell lymphoma and leukemia in children and adolescents with rituximab and reduced intensity induction chemotherapy (B-NHL 2004M protocol): the results of a multicenter study. J Pediatr Hematol Oncol. 2014;36: 395–401. [DOI] [PubMed] [Google Scholar]
- 32.Bonilla M, Rossell N, Salaverria C, et al. Prevalence and predictors of abandonment of therapy among children with cancer in El Salvador. Int J Cancer. 2009;125: 2144–2146. [DOI] [PubMed] [Google Scholar]
- 33.Jourdain A, Auperin A, Minard-Colin V, et al. Outcome of and prognostic factors for relapse in children and adolescents with mature B-cell lymphoma and leukemia treated in three consecutive prospective “Lymphomes Malins B” protocols. A Societe Francaise des Cancers de l’Enfant study. Haematologica. 2015;100: 810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patient stratification and treatment courses according to disease stage.


