Abstract
Introduction:
Biotransformation is important in the metabolism of endobiotics and xenobiotics. This process comprises the activity of phase I and phase II enzymes. Estrogen sulfotransferase (SULT1E1 or EST) is a phase II conjugating enzyme that belongs to the family of cytosolic sulfotransferases. The expression of SULT1E1 can be detected in many tissues, including the liver. SULT1E1 catalyzes the transfer of a sulfate group from 3’-phosphoadenosine-5’-phosphosulfate (PAPS) to any available hydroxyl group in estrogenic molecules. The substrates of SULT1E1 include the endogenous and synthetic estrogens. Upon SULT1E1-mediated sulfation, the hydrosolubility of estrogens increases, preventing the binding between the sulfated estrogens and the estrogen receptor (ER). This sulfated state of the estrogens is not irreversible, as the steroid sulfatase (STS) can convert sulfoconjugated estrogens to free estrogens. The expression of SULT1E1 is inducible by several diseases that involve tissue inflammation, such as type 2 diabetes, sepsis, and ischemia-reperfusion injury.
Areas covered:
This systematic literature review aims to summarize the role of SULT1E1 in the metabolism of estrogenic drugs and xenobiotics, and the role of SULT1E1 in the pathogenesis of several diseases, including cancer, metabolic disease, sepsis, liver injury, and cystic fibrosis. Meanwhile, ablation or pharmacological inhibition of SULT1E1 can affect the outcomes of the aforementioned diseases.
Expert opinion:
In addition to its role in metabolizing estrogenic drugs, SULT1E1 is unexpectedly being unveiled as a mediator for the disease effect on estrogen metabolism and homeostasis. Meanwhile, because the expression and activity of SULT1E1 can affect the outcome of diseases, the same sulfotransferase and the reversing enzymes STS can be potential therapeutic targets to prevent or manage diseases. Accumulating evidence suggest that the effects of SULT1E1 can be estrogen-independent and it is necessary to elucidate what other possible substrates may be recognized by the enzyme. Moreover, human studies are paramount to confirm the human relevance of the animal studies.
Keywords: Estrogen sulfotransferase, Estrogen, Estrogenic drugs, Diseases, Metabolism, Phase II enzymes
1. Introduction
1.1. Phase II enzymes
Metabolism is a crucial mechanism to inactivate and excrete both endobiotics and xenobiotics. This process occurs in the gut, liver, and kidneys, and is divided into hydrolytic and oxido-redox (Phase I) reactions, as well as conjugation reactions (Phase II). The phase I enzymes are responsible for N- and O-dealkylation, aliphatic and aromatic hydroxylation, N- and S-oxidation, and deamination of any lipophilic compounds. Ester hydrolases and the cytochrome P450 enzymes are involved primarily in the hydroxylation, the latter being the most important and extensively studied phase I enzymes [1].
The phase II enzymes also play a crucial role in drug metabolism. These enzymes are responsible for the conjugation of oxidized or hydrolyzed compounds, therefore making them more hydrosoluble and suitable for excretion. Phase II enzymes are mostly transferases that transfer small molecular weight, organic donor molecules such as 3-phosphoadenosine 5’-phosphosulfate ((PAP)-sulfate, also called PAPS), glutathione, UDP-glucuronic acid, or acetyl-coenzyme A [1, 2, 3, 4]. These conjugation reactions are catalyzed by PAPS-sulfotransferase (SULT), glutathione S-Transferase (GST), UDP-glucuronosyltransferase (UGT), and N-Acetyltransferase (NAT).
1.2. Sulfotransferases, their functions and tissue distributions
Sulfotransferases (SULT) comprise a gene family of enzymes responsible for catalyzing a reversible sulfation of low molecular weight compounds via the transfer of a negatively charged sulfonate group (SO3-) from the universal donor 3-phosphoadenosine 5’-phosphosulfate (PAPS) to a nucleophilic group of their substrates [5, 6, 7, 8, 9, 10, 11]. PAPS is produced by the reaction between inorganic sulfate, uptake from the extracellular medium to the cytosol [10], and two molecules of ATP, which can be mediated by both ATP sulfurylase and two forms of adenosine 5’-phosphosulfate kinase (APS kinases), PAPSS1 and PAPSS2 [5, 6, 7, 11]. Both PAPS and APS kinases are conserved among species and their absence culminates with lethality, because sulfotransferases are vital for homeostasis [11, 12]. SULT enzymes are divided into membrane-bound, Golgi-residing [9, 13], and soluble cytosolic enzymes [9]. The Golgi-located SULTs conjugate proteins, carbohydrates, and proteoglycans, whereas the cytosolic enzymes sulfate essentially small hydrophobic molecules, such as phenols, xenobiotics (including drugs, dietary chemicals, and environmental contaminants [11, 14]) and steroids [9, 11, 14]. Although some sulfated chemicals remain metabolically active, sulfation is majorly a vital step for detoxification and reduction of biological activity, as it increases hydrosolubility, enabling the molecule to be excreted from the body via urine and/or bile [14]. At least thirteen isoforms of human cytosolic SULTs have been identified [5, 8, 15], but out of those, SULT1 and SULT2 families are responsible for sulfonating the largest number of xeno- and endobiotics, making them the most important isoforms for drug metabolism [5, 8, 9, 11]. Their isoforms comprise phenol sulfotransferases (SULT1A1 and SULT1A2), catecholamine phenol sulfotransferase (SULT1A3/4), thyroid hormone sulfotransferase (SULT1B1), estrogen sulfotransferase (EST/SULT1E1), and hydroxysteroid sulfotransferase (SULT2A1) [14]. For the substrates, SULT 1A1 and 1A2 are responsible for the metabolism of phenolic compounds, whereas SULT1A3/4 is responsible for the conjugation of catecholamines. SULT1B1 conjugates the thyroid hormone substrates, tyrosine, and DOPA, whereas SULT2A1 and SULT1E1 have steroid substrates, with estrogens as the preferred substrates of SULT1E1 [16]. The expression of SULT1 and SULT2 isoforms vary among tissue types, and the expression is subject to the regulation by tissue development and hormonal influence [5, 8, 9].
Among SULTS, SULT1A1 is one of the most studied SULT isoforms. It has been suggested that polymorphisms in its gene may have accounted for variations in inter-individual susceptibility to cancers [5, 7, 8] due to the fact that SULT1A1 activates environmental mutagens and carcinogens found in well-done meat [5, 8, 15]. A study conducted by Riches et al. analyzed the expression of all major human SULTS within different organs. Their results demonstrated that out of all human hepatic SULT enzymes, 53% of them are SULT1A1, followed by SULT2A1 (27%), SULT1B1 (14%), and SULT1E1 (6%). In the gastrointestinal tract, SULT1B1 accounted for 36% of all SULTs, followed by SULT1A3 (31%), SULT1A1 (19%), SULT1E1 (8%), and SULT2A1 (6%). In the same report, the authors also observed that SULT1E1 was the main isoform expressed in the lung (40%), followed by SULT1A1 (20%), SULT1A3 (19%), SULT1B1 (12%), and SULT2A1 (9%). Meanwhile, SULT1A1, SULT1B1, and SULT1A3 were abundantly expressed in the kidney, constituting 40, 31, and 28% of all SULTs, respectively [14]. Although the renal expression of SULT1E1 was not detectable in the study, Miki et al. have previously shown that this enzyme is also expressed in the tubular cells of the human kidney, and in several other tissues, including trachea, lung, esophagus, spleen, pancreas, adrenal gland, thyroid, urinary bladder, kidney [17], placenta, testis and ovaries [18, 19]. On the other hand, the evaluation of SULT tissue distributions in mice showed some discrepancies compared to the human isoforms. As reported by Alnouti and colleagues, the Sult1e1 mRNA was only expressed in the gonadal organs [20]. In contrast to their findings, several studies have since been published showing that Sult1e1 is expressed in mouse extragonadal tissues, such as the liver and adipose tissue [21, 22, 23, 24].
1.3. SULT1E1
The circulating estrogens are predominantly synthesized in premenopausal women’s ovaries. Upon menopause, the ovary discontinues the estrogen production, but extragonadal tissues such as the breast [25], adipose tissue, and brain maintain the production of estrogens. In males as well as females, testosterone and androstenedione can serve as substrates of the brain and testis CYP19A1 enzyme (aromatase) to synthesize estrogens [26]. Most, if not all, cellular effects of estrogens are mediated by the nuclear receptors estrogen receptor alpha (ERα) and -beta (ERß) with a high ligand-receptor binding affinity (Kd ˜1nM) [27, 28, 29]. Although estrogens have been reported to be the substrates of multiple SULTs including SULT1A1 and SULT2A1 [30], SULT1E1 exhibits the highest affinity for these hormones, especially the 3-hydroxyl position of E2 [5, 29], to which it binds with a Michaelis-Menten constant (Km) of 0.27 nM and with a turnover number (kcat) of 10s−1×103/nM respectively [31, 32]. This sulfoconjugation of estrogens can be reversed by the deconjugation reaction catalyzed by the steroid sulfatase (STS) [33].
In COS-1 cells, SULT1E1 was able to sulfoconjugate dehydroepiandrosterone (DHEA), but with a low affinity of 850 nM [34]. Interestingly, at the concentration of 1.8 nM E1 is sulfonated by SULT1E1; however, at 40 nM this compound inhibits the SULT1E1 activity [32, 35]. The structure of SULT1E1 is majorly formed by an α/ß motif that comprises a ß sheet of five parallel ß-strands involved by two lateral α-helices and a preserved helix, which accommodate the PAPS binding site [36]. Petrotchenko and colleagues demonstrated that E2 firmly adheres to the Tyrosine (Tyr)-81/Phenilalanine (Phe)-142 residues of SULT1E1 via van der Waals interactions and is placed horizontally in the cylindrical hydrophobic binding pocket [31]. It has been reported that the amino acids Histinine (His)107 and Lysine (Lys)105 are crucial for the catalytic reaction between E2 and PAPS, which begins by His107-mediated deprotonation of the hydroxyl group of E2, enabling its nucleophilic oxygen to attack PAPS’ sulfur atom [31, 37]. Then, mediated by Lys105 and Lys47, the 5’-sulfonate group of PAPS is hydrolyzed and transferred to the 3’-phenolic hydroxyl group of E2, consequentially forming hydrogen bonds between His/Lys and the hydroxyl group [31, 37]. Mutations in these residues lead to reduced E2 sulfonation [31]. A ligand binding study performed by Zhang et al. showed that two different molecules of E2 may independently attach to the SULT1E1 binding pocket, either via an allosteric or a catalytic site, suggesting a random Bi Bi mechanism with two dead-end complexes [29]. The resulting sulfonated estrogens are more hydrosoluble, the 1-octanol/water partition coefficients (PC) of E2 and E2-sulfate are 490 and <0.01, respectively [8, 38]. Therefore, sulfonated estrogens are unable to bind the receptors located inside the hydrophobic nuclear envelope and thus lose their hormonal activity.
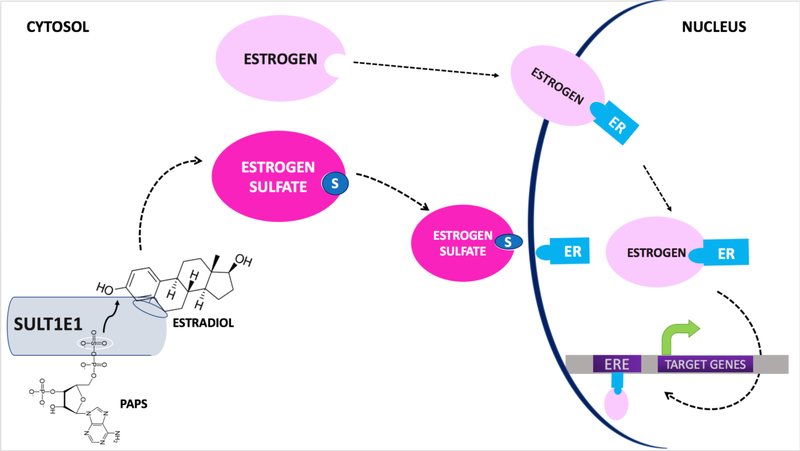
Figure 1 depicts the major findings regarding the structure and physiological role of SULT1E1.
Figure 1. Physiological role of EST in the sulfoconjugation of estrogens.
Endogenous or synthetic estrogens bind to either the catalytic or allosteric domains of the EST dimer. Two molecules may bind independently. EST catalyzes the transfer of a sulfate group from the universal donor 3’-phosphoadenosine-5’-phosphosulfate (PAPS) to the 3’-hydroxy group of E2. Upon sulfonation, estrogens become hydrosoluble and unable to bind to the estrogen receptor (ER) ⍺ nor β. Consequently, unlike the parent estrogens, the sulfonated estrogens cannot translocate to the nucleus and cause ER and estrogen response element (ERE) mediated regulation of target genes.
SULT1E1-mediated sulfoconjugation and deactivation of estrogens is a reversible reaction, because the hormonally inactive estrogen sulfates can be desulfonated and re-activated by steroid sulfatase (STS). STS is responsible for catalyzing the hydrolysis of steroid sulfates and generating hydroxysteroids. This enzyme is present in several tissues, especially in the liver, where the metabolism of circulating steroid hormones mainly happens [39]. In patients with chronic inflammatory liver diseases, inflammation-mediated activation of NF-kB in hepatocytes stimulates STS and consequently the levels of circulating estrogens rise, which mitigates this inflammatory response [40]. Moreover, STS has been implicated in a sex-linked role in energy homeostasis, because transgenic overexpression of human STS in adipose tissue or the liver of male and female mice resulted in different responses to high fat diet (HFD)-induced obesity and type 2 diabetes mellitus (T2DM). In female mice, the inflammatory profile and metabolic functions were improved due to increased estrogenic activity whereas in male mice the metabolic response was worsened [41, 42].
2. Transcriptional regulation of SULT1E1 by nuclear hormone receptors and its implications in drug-hormone interactions
The expression of SULT1E1 is subject to the transcriptional regulation by nuclear hormone receptors, a family of ligand-dependent transcriptional factors. The regulation of SULT1E1 by nuclear receptors provides a mechanism for the drug/hormone-hormone interactions that lead to compromised estrogen activities. Several classes of drugs may indirectly modulate SULT1E1 activity. For instance, glucocorticoids, such as dexamethasone (Dex), have been shown to reduce estrogenic activity in vivo and in vitro by increasing the expression of SULT1E1. The induction of SULT1E1 and the resultant inhibition of estrogen activity by Dex were consistent with previous reports that glucocorticoids can inhibit estrogen responses [43, 44, 45, 46, 47, 48]. Treatment with Dex attenuated the estrogen-induced uterine expression of insulin-like growth factor-I (IGF-I) [47]. DEX also blocked the stimulatory effect of estrogen on MCF-7 cell proliferation [46, 48]. Mechanistically, Dex interacts with the glucocorticoid receptor (GR), which acts as a transcriptional factor that promotes the upregulation of its transcriptional target, SULT1E1; consequently, SULT1E1 induction is responsible for dramatically reducing the levels of active estrogens [46]. Similarly, cholesterol-derived oxysterols, or synthetic agonists such as GW3965, bind to the isoforms α and ß of the liver X receptor (LXR); this ligand-receptor complex, in turn, stimulates the transcription of hepatic SULT1E1, resulting in an increased estrogen sulfonation and decreased estrogen activity [49].
The regulation of SULT1E1 by nuclear receptors can be both gender-specific and species-specific. As an example of gender specificity, in female mice, the constitutive androstane receptor (CAR) agonist, TCBOPOP, was shown to induce liver Sult1e1, whereas in male mice the induction was not observed [50]. As an example of species specificity, although the regulation of EST by LXR has been reported in mice, the same regulation is yet to be verified in humans. In addition to the positive regulation, some nuclear receptors are associated with a decreased activity of EST. Retinoid-related orphan receptor alpha (RORα) is a negative regulator of EST in hepatocytes, therefore, the effects of agonists, such as cholesterol- and lipid-sulfates, and antagonists of RORα can modulate the activity of this steroid enzyme [51]. Another nuclear receptor agonist that downregulates SULT1E1 is the antibiotic rifampicin, a known activator of the human pregnane X receptor (PXR). Rifampicin represses the transcription of SULT1E1 in hepatocytes via an interaction with hepatocyte nuclear factor 4α (HNF4α) [52]. Recently, Wang and colleagues also showed that, in HepG2 cells, the farnesoid X receptor (FXR) agonists, 3-(2,6-Dichlorophenyl)-4-(3’-carboxy-2-chlorostilben-4-yl) oxymethyl-5-isopropylisoxazole (GW4064) and chenodeoxycholic acid (CDCA), indirectly downregulated SULT1E1 via the prevention of peroxisome proliferator-activated receptor-γ coactivator 1α (PGC1α) binding to HNF4α [53]. The role of nuclear receptors in SULT1E1 regulation is summarized in Figure 2.
Figure 2. Regulation of EST by nuclear receptors.
Normally, EST has a low expression in some tissues, such as the liver. Upon the presence of a ligand (L), that can be either dexamethasone, GW3965, or TCPOPBOP, the nuclear receptors GR, LXR, or CAR, respectively, can bind to the promoter region of the EST gene and increase its expression in the liver. * indicates that the induction only occurs in livers of female mice. In contrast, retinoid X receptor alpha (RXRα) suppresses the expression of EST in the presence of its agonists Bexarotene (BEX). Similarly, upon the binding of its agonist rifampicin (RIF), pregnane X receptor (PXR) binds to the transcription factor hepatocyte nuclear factor 4α (HNF4α), which culminates with the downregulation of EST. Additionally, agonists for farnesoid X receptor (FXR), such as GW4064, prevent the binding of PGC1α to HNF4α, also leading to EST downregulation.
3. Metabolism of estrogenic drugs by SULT1E1
3.1. Estrogenic drugs that are SULT1E1 substrates
Synthetic oral contraceptives are widely used among fertile women. Norethindrone (NET) and ethynylestradiol (EE) are the major active compounds in this medication group [54]. Cytochrome P450 (CYP) enzymes are responsible for the phase I metabolism of endo- and xenobiotics. CYP3A4 and CYP2C9, followed by CYP2C8, CYP2C19, and CYP3A5, are the major enzymes responsible for oxidizing EE into 2-hydroxy-EE, which accounts for more than 90% of all metabolites [54]. Sulfation at the 3-O group of 2-hydroxy-EE accounts for up to 60% of EE’s first pass metabolism. The proportion of intestinal over hepatic SULTs effective in this metabolism is approximately 2:1 [55]. Inhibition analysis with the SULT1A1 inhibitor quercetin and the SULT1E1 inhibitor 2,6-dichloro p-nitrophenol (DCNP) showed that SULT1E1 is the SULT enzyme with the highest affinity for EE, with a Km value of 6.7 nM, being responsible for 75–80% of the sulfoconjugation [35]. Moreover, the metabolism of EE may be altered when co-administered with agents that reduce its plasma levels, such as rifampicin [56], or agents that increase EE levels, such as acetaminophen, fluconazole, and ascorbic acid[57, 58, 59]. On the other hand, the acetylenic group of EE may act as an inhibitor of several CYP enzymes [60, 61], such as CYP2B1, CYP2B6, and CYP3A4 [62, 63]. Additionally, in human intestinal mucosa, EE was shown to indirectly inhibit sulfation considerably, as in the case of the progestogen (progesterone) oral contraceptive desogestrel, whose sulfation was inhibited by up to 48% in the presence of EE [64].
Selective estrogen receptor modulators (SERMs) inhibit the effects of estrogens in breast tissue. Tamoxifen and Raloxifene (Evista®) are SERMs widely used to decrease the risk of developing hormone-receptor-positive breast cancer in susceptible postmenopausal women. Sulfation assays demonstrate that, at therapeutic levels, SULT1E1 has a high affinity for the tamoxifen metabolite, 4-hydroxytamoxifen (4-OHT), with a Km of 0.2 μM. Kinetic assays of raloxifene sulfation showed most SULT enzymes recognized this substrate; nonetheless, SULT1E1 was the only SULT able to generate raloxifene monosulfates and disulfates. An affinity docking algorithm further demonstrated that in both rings of the molecule, the nucleophilic hydroxyls are placed in a crucial region for catalysis, predicting many possible interactions at two different positions. The analysis suggests this enzyme has a flexible active site that adjusts to accommodate reactive groups [65]. Hormonal replacement therapy (HRT) is broadly used in postmenopausal women to reduce uncomfortable symptoms of menopause, including hot flashes, disturbed sleep, and vaginal dryness. Tibolone is a synthetic steroid used as a HRT agent to modulate bone loss, menopause symptoms, and libido, possibly due to a selective sulfatase inhibition. Once absorbed, Tibolone binds to ER, progesterone receptor (PG), and androgen receptor (AR), and is rapidly metabolized into 3α-OH and 3ß-OH-tibolone, which can be further metabolized into Δ4-tibolone. Tibolone metabolism occurs mainly by steroid SULTs. SULT1E1 sulfoconjugates the 3-OH position of both 3α-OH-tibolone and 3ß-OH-tibolone with a Km of 2.1 μM and 6.6 μM, respectively [65, 66].
Among estrogenic drugs, the clinical use of conjugated equine estrogens (CEEs) also involves SULT1E1 and the estrogen re-activating enzyme STS. As a natural formulation of an extraction from pregnant mares’ urine, CEEs represent one of the most prescribed estrogen production for postmenopausal HRT either alone or in combination with a progestin. CEEs is not a single estrogen but a complex containing 10 different estrogens in their sulfate esters, with estrone sulfate and equilin sulfate as the main constituents [67]. Since estrogens exert their biological effect only in their unconjugated forms, SULT1E1 and STS are reasonably believed to be involved in CEEs metabolic process. Similar to estradiol which is also widely used in HRT, CEEs have been proven to benefit postmenopausal women, such as improvement of osteoporosis, with no increased risk of cardiovascular disease and invasive breast cancer [68, 69]. However, several randomized controlled trials (RCTs) have revealed significant declines in cognitive function as well as a higher incidence of probable dementia in patients receiving CEEs alone or in combination with medroxyprogesterone acetate (MPA) compared with placebo [70, 71, 72], whereas transdermal E2, in comparison to placebo, was found to play no effect on cognition [73]. Additionally, in postmenopausal women with an increased risk of Alzheimer’s disease, continued or discontinued use of estradiol could improve attention/working memory/processing speed (P =0.04) and verbal memory (P = 0.01) domains compared with continued or discontinued CEE use for 2years [74]. On the other hand, as the enzyme activating sulfated estrogen, STS also highly expresses in the brain. Evidence has shown that a STS inhibitor is related to up-regulation of endogenous dehydroepiandrosterone sulfate (DHEAS) which acts as γ-aminobutyric acidA receptor antagonists, resulting in a memory-enhancing effect [75]. Although these results might provide a possible link between estrogen sulfate and SULT1E1/STS in mental disorders, there is no solid evidence to support this view. The potential association and underlying mechanism need to be further evaluated.
3.2. Chemicals that inhibit the SULT1E1 activity
Polychlorinated biphenyls (PCBs) are environmental pollutants with estrogenic or anti-estrogenic properties that have gained increasing attention due to their effects on animal reproduction and sexual development. The human exposure to PCBs has been associated with an increased incidence of testicular cancer and diminished semen quality and sperm counts [76]. It has been suggested that hydroxylated metabolites of PCBs (PCB-OHs) exert most of the hormonal properties of these compounds [77]. Kester et al investigated if PCB-OHs were inhibitors of E2 metabolism and discovered that low concentrations of PCB-OHs (0.1 nM) were sufficient to bind to and inhibit human SULT1E1 with an affinity higher than the endogenous estrogens. Their results suggest that PCB-OHs may increase local estrogenic activity in reproduction-related organs by suppressing SULT1E1-mediated estrogen sulfation and deactivation. Moreover, the authors observed that a hydroxyl group in the para position of PCB-OHs with two nearby chloride substituents was required for interacting with the SULT1E1 enzyme and that the inhibitory effect was increased per the number of halogen groups in the molecule. Phenolic OH groups in PCB-OHs were also shown to be non-competitive inhibitors of E2 sulfation since they do not bind to the active site, but the allosteric site of SULT1E1 [78].
Additionally, phytochemicals, such as isoflavonoids, flavonoids, stilbenes and lignans, are a class of SERMs that recently have also been implicated in the changes of estrogenic metabolism [79]. Isoflavones are enriched in soybeans and heavily consumed in Eastern diets. Genistein and daidzein, as well daidzein metabolite equol, are also widely studied isoflavones [79, 80]. An in vitro study using human liver cytosols showed that equol and genistein can inhibit the SULT1E1 activity at the active site with Ki values of 400 nM and 500 nM, respectively. Their inhibitions at the allosteric site have Ki of 2,000 nM and 5,000 nM, respectively. However,, isoflavones have not been shown to inhibit SULT1E1 at concentrations that would occur in vivo [80].
Similarly, using recombinant human SULT enzymes, Miksits and colleagues demonstrated that although SULT1A1 was the major enzyme, SULT1E1 had a minor role in the sulfoconjugation of 3,4’,5-trihydroxy-trans-stilbene (Resveratrol) [81, 82]. This is a polyphenol chemical present in the herb Polygonum cuspidatum, that among many functions also has estrogenic activity and competitively inhibits SULT1E1 with a Ki value of 360 nM [81, 83]. The substrate inhibition profiles of the resveratrol metabolites, trans-resveratrol-3-O-sulfate (M1) and trans-resveratrol-4’-O-sulfate (M2) on SULT1E1 had a Ki value of 337 nM and 1310 nM, respectively [81]. Quercetin is another phytochemical known to competitively inhibit the human SULT1E1 with a Ki value of approximately 580 nM [83].
Additionally, Triclosan is also established SULT1E1 inhibitor. Triclosan is a chlorinated phenolic compound that was used as an anti-microbial agent in hand soap and other personal care agents. Triclosan has been detected in human blood, urine, and breast milk [84, 85]. Stoker et al. evaluated the effects of Triclosan in female Wistar rats. They found that in pubertal mice this agent resulted in a premature vaginal opening, whereas in weaning mice Triclosan changed the degree of reproduction development and increased uterine response to EE [86]. In sheep, Triclosan was reported as a potent inhibitor of placental SULT1E1 by competing with E2 molecules for the enzyme’s substrate binding site with a competitive inhibitory constant (Kic) of 0.09nM [32]. Besides the competitive inhibition, Triclosan also displays an uncompetitive inhibition of the E2-SULT1E1 interaction, with an uncompetitive inhibitory constant (Ki) of approximately 5.2 nM. In the same study, another PCB, 4’OH-CB79, also demonstrated competitive E2- SULT1E1 inhibition with a Ki of 0.89 nM [32]. Table 1 summarizes all SULT1E1 substrates and inhibitors and their enzyme binding affinity.
Table 1:
Binding affinity of substrates and inhibitors of EST within different species.
| COMPOUNDS | APPROXIMATE AFFINITY | SPECIES | ROLES | REFERENCES |
|---|---|---|---|---|
| 17ß-Estradiol (E2) | 0.27 nM | Mouse | Substrate | [31] |
| Estrone (E1) | 1.8 nM | Sheep | Substrate | [32] |
| Estrone (E1) | 40 nM | Human | Inhibitor | [35] |
| Ethynylestradiol (EE) | 6.7 nM | Human | Substrate | [35] |
| 4-Hydroxytamoxifen | 200 nM | Human | Substrate | [65] |
| DHEA | 850 nM | Human | Substrate | [34] |
| 3α-OH-Tibolone | 2,100 nM | Human | Substrate | [66] |
| 3ß-OH-Tibolone | 6,600 nM | Human | Substrate | [66] |
| Tibolone | 19,500 nM | Human | Substrate | [66] |
| 4’OH-CB79 | 0.89 nM | Sheep | Competitive Inhibitor | [32] |
| Triclosan | 0.09 nM | Sheep | Competitive Inhibitor | [32] |
| Triclosan | 5.2 nM | Sheep | Uncompetitive Inhibitor | [32] |
| Resveratrol | 360 nM | Human | Competitive Inhibitor | [83] |
| Equol | 400 nM | Human | Competitive Inhibitor | [80] |
| Genistein | 500 nM | Human | Competitive Inhibitor | [80] |
| Quercetin | 580 nM | Human | Competitive Inhibitor | [83] |
| Equol | 2,000 nM | Human | Uncompetitive Inhibitor | [80] |
| Trans-resveratrol-3-O-sulfate (M1) | 3,370 nM | Human | Inhibitor | [81] |
| Genistein | 5,000 nM | Human | Uncompetitive Inhibitor | [80] |
| Trans-resveratrol-4’-O-sulfate (M2) | 13,100 nM | Human | Inhibitor | [81] |
4. The mutual effects between diseases and the expression and activity of SULT1E1
Variations in the activity of SULT1E1 are responsible for differences in inter-individual response to hormonal-related diseases. Expression of SULT1E1 in the human liver, although showing no gender differences, presents significant variations between alcohol consumers, as well as among different individuals, where it can vary up to 25-fold. The causes behind such variations are not fully elucidated but it is believed they could happen as a result of SULT1E1 polymorphisms and exogenous administration of estrogens [87]. Three nonsynonymous SULT1E1 coding single nucleotide polymorphisms (cSNPs) that modify the expression of the amino acids Asp22Tyr, Ala32Val, and Pro253His, have been characterized in COS-1 cells. Constructs containing the three cSNPs evidenced a decline in SULT1E1 activity for the allozymes Tyr22 and Val32, which suggest such polymorphisms may be partly responsible for the advancement of estrogenic diseases and metabolic alterations of estrogenic drugs [88]. Using genomic DNA extracted from buccal samples, Rebbeck and collaborators conducted a population based case-control study that evidenced an association between the chance of developing endometrial cancer and the G → A polymorphism at position −64 (−64G>A; rs3736599) of SULT1E1’s promoter region [89]. Although only a few studies have been conducted so far to understand the origin of such variations, lack of sex-specific expression changes suggest the role of SULT1E1 in homeostasis may go beyond a simple estrogen inactivation.
The genes encoding SULT1E1 are highly conserved in humans and mice, because the mouse Sult1e1 shares 77% homology in amino acids with the human enzyme [18]. As a result, various mouse models have been used to further understand the impact of diseases on this enzyme, and vice-versa. Noticeably, the use of Sult1e1 loss of function and gain of function models permitted the advancement of studies regarding the role of this enzyme in estrogen homeostasis and disease pathogenesis. In animals, experiments using female Sult1e1 null mice showed the importance of this enzyme as a regulator of estrogen levels, especially during pregnancy as the fetal loss was a common feature and the surviving offspring were smaller and had excessive levels of estrogens [90].
4.1. Sult1e1 in reproductive tissues and cancers
In humans, postmenopausal women receiving HRT have a higher risk of presenting serious side effects like pulmonary embolism, stroke, coronary heart disease, and cancer [91, 92, 93, 94]. Cancer-focused studies have shown that the activity of SULT1E1 has been correlated with a reduction in breast, endometrial, and ovarian cancer recurrence and improved survival [89, 95, 96, 97], whereas SULT1E1-negative breast tumors may be associated with a poor prognosis due to a rise in in situ estrogens [17, 98]. Moreover, treatment of a lung cancer xenograft with Dex has shown to reduce tumor volume due to Dex-mediated induction of SULT1E1 [99]. These phenomena are in accordance with the finding that an increased sulfation of E2 has been linked to decreased proliferation rates of hormone-sensitive malignant cells [100]. Endometriosis is manifested by abnormal growth of endometrial tissue ectopically of the uterus. Biopsy specimens of women with endometriosis presented a diminished expression of SULT1E1 and an augmented expression of STS in accordance with the dependence of endometriosis on female sex hormone [101].
4.2. Sult1e1 in adipocyte differentiation
The adipose tissue plays an important role in lipid storage, energy balance and insulin response; nonetheless, the mechanisms surrounding adipogenesis are not fully understood. We reported that Sult1e1 was highly expressed in 3T3-L1 pre-adipocytes and at the time of cellular differentiation to mature adipocytes this expression was decreased considerably. Furthermore, upon Sult1e1 overexpression in 3T3-L1 cells, adipocyte differentiation was diminished due to an ERK1/2 MAPK-dependent inhibition of insulin signaling, whereas Sult1e1 ablation in adipocytes conferred differentiation. The enzymatic activity of Sult1e1 was required for the inhibitory effect of Sult1e1 on adipogenesis, because an enzyme-dead Sult1e1 mutant failed to inhibit adipocyte differentiation. An in vivo investigation using transgenic female mice overexpressing Sult1e1 specifically in adipose tissue further confirmed that the adipocytes’ diameters were reduced. Interestingly, physiological concentrations of E2 had little effect on 3T3-L1 differentiation. Their results suggest that Sult1e1 is a negative regulator of adipogenesis in an estrogen-independent manner. The authors used transient transfection and luciferase reporter gene assay to examine other candidate substrates for Sult1e1, such as thyroid hormones, testosterone, glucocorticoids, and peroxisome proliferator-activated receptor gamma (PPARγ) ligands, but none of them were shown to be metabolized by this enzyme [102]. As such, the Sult1e1 substrate(s) responsible for the effect of Sult1e1 on mouse adipocyte differentiation remain to be defined.
Curiously, the effect of Sult1e1 in adipocyte differentiation is species specific. We conducted a study using pre-adipocytes isolated from obese and non-obese subjects, combined with Sult1e1 loss of function and gain of function manipulations. Our results showed that Sult1e1 positively regulates adipogenesis via loss of estrogenic activity, and that the enzyme expression is positively correlated with the body mass index [23]. Moreover, human adipogenesis was affected by estrogen treatment.
4.3. Sult1e1 in metabolic disease
Type 2 Diabetes Mellitus (T2DM) is a metabolic syndrome associated with insulin resistance. The diabetic mouse model (db/db) presents a liver induction of Sult1e1 [103]. We showed that the hepatic expression of Sult1e1 was also markedly induced in the ob/ob mice, another genetic model of obesity and type 2 diabetes. In determining the functional relevance of Sult1e1 and its regulation by metabolic disease, we showed that ablation of Sult1e1 in female ob/ob (termed obe) mice resulted in improved metabolic function due to a rise in hepatic estrogenic activity, as ovariectomy abolished the protection. Interestingly, the effect of Sult1e1 ablation on obesity and type 2 diabetes was sex-specific, because Sult1e1 ablation in male ob/ob mice worsened their phenotype, which was accounted for by the ß-cell loss due to the boosted macrophage infiltration and inflammation in the white adipose tissue (WAT) [104].
We initially thought the loss of expression and induction of hepatic Sult1e1 in the male obe mice was responsible for the worsened metabolic function. In a follow-up study, we were surprised to find that transgenic reconstitution of Sult1e1 in the adipose tissue, but not in the liver, attenuated diabetic phenotype in obe males. Mechanistically, adipose reconstitution of Sult1e1 in obe mice resulted in reduced local and systemic inflammation, improved insulin sensitivity, and increased energy expenditure. At the molecular level, adipose induction of lipocalin-2 (Lcn2) in male obe mice that bear the adipose reconstitution of Sult1e1 (oae mice) may have contributed to the inhibition of inflammation, because the level of Lcn2 was negatively associated with Tnfα expression, and treatment of differentiated adipocytes with Lcn2 antagonized Tnfα-responsive inhibition of insulin signaling. The metabolic benefit of adipose reconstitution of Sult1e1 was sex-specific, because adipose reconstitution of Sult1e1 in obe females had little effect. Interestingly, although reconstitution of Sult1e1 in obe males improved metabolic phenotype, these mice were not protected from ß cell mass loss. Their results suggest Sult1e1 is crucial for WAT homeostasis in an estrogen and ß cell-independent manner [105].
4.4. Sult1e1 in liver injury induced by sepsis and ischemia and reperfusion
Sepsis is a major cause of mortality in the intensive care unit (ICU). Although sepsis and its associated inflammation are known to decrease the expression and activity of many drug-metabolizing enzymes, we found that upon bacterial lipopolysaccharide (LPS) treatment or subjecting mice to the cecum ligation and puncture (CLP), the hepatic expression of Sult1e1 was highly upregulated via the activation of the NF-kB pathway. The mouse Sult1e1 gene was established as a NF-kB target gene. The sepsis-responsive induction of Sult1e1 was sufficient to compromise the estrogen activity. Interestingly, not only sepsis can induce Sult1e1- the expression and activity of Sult1e1 can impact the clinical outcome of sepsis. Specifically, we showed that Sult1e1 ablation or pharmacological inhibition of Sult1e1 by Triclosan sensitizes mice to sepsis-induced death in an estrogen dependent manner. Mechanistically, Sult1e1 ablation attenuates sepsis-induced inflammatory responses due to compromised estrogen deactivation, leading to increased sepsis lethality. The reciprocal regulation of inflammation and Sult1e1 may represent a yet to be explored mechanism of endocrine regulation of inflammation, which has an impact on the clinical outcome of sepsis [24].
Liver ischemia-reperfusion injury (LIRI) is another liver injury condition that can regulate Sult1e1. Sult1e1 has also been studied in inflammation-based conditions. LIRI is caused by hepatic blood flow blockage or reduction and is a common feature after organ transplantation, abdominal surgeries, massive trauma, hemorrhagic- and cardiogenic shock. LIRI is directly associated with oxidative stress and inflammation. We reported that LIRI induced Sult1e1 in the mouse liver, and that upon Sult1e1 ablation the female mice were protected from the injury in an estrogen dependent manner, whereas the male mice were further sensitized in an androgen-dependent manner. The LIRI responsive induction of Sult1e1 is dependent on the nuclear factor erythroid 2-related factor 2 (Nrf2), but independent of the hypoxia-inducible factor 1 (HIF-1). Sult1e1 was established as a Nrf2 target gene [21].
4.5. SULT1E1 in cystic fibrosis (CF)
Cystic fibrosis (CF) is an autosomal recessive condition characterized by mutations in both copies of the cystic fibrosis transmembrane receptor (CFTR) and manifested by pulmonary abnormalities. However, loss of CFTR is often linked to distant organ injury. The liver is one of the organs affected, which accounts for the high mortality observed in children with CF [106, 107]. Additionally, animal models of CF, characterized by both CFTR-deficient and CFTR KO mice, do not present pulmonary disease but manifest CF-associated gastrointestinal and reproduction comorbidities, such as severe growth retardation and mediocre weight gains [108]. Furthermore, CF patients present growth shortfalls that are associated with a decline in IGF-1 plasma levels [109, 110]. Li and colleagues have demonstrated that hepatic Sult1e1 is induced in CFTR KO mice [111] and that in Sult1e1-transfected HepG2 cells, Sult1e1 played a role in inhibiting both growth hormone-mediated signal transducers and activators transcription 5b (STAT5b) phosphorylation and insulin-like growth factor-1 (IGF-1) synthesis in an estrogen-dependent aspect [100]. As a result of estrogenic decline, the expression of the hepatic detoxifying enzymes, glutathione S-transferase P1 and carbonic anhydrase, are also downregulated in CFTR mice [111]. In addition, Falany and colleagues showed that co-transfection of HepG2 hepatocytes with human MMNK-1 cholangiocytes transfected with CFTR siRNA resulted in SULT1E1 induction in an LXR-dependent manner due to changes in cholesterol biosynthesis [112]. Therefore, SULT1E1 induction in CF patients may be responsible for growth retardation and indicate a disrupted paracrine regulatory mechanism that may help elucidate the reasons behind CF-dependent liver damage.
A general association between disease onset and SULT1E1 regulation is summarized in Table 2.
Table 2:
Disease onsets that were shown to regulate EST expression within different species.
| DISEASE STATE | EST REGULATION | SPECIES | REFERENCES |
|---|---|---|---|
| Endometrial Cancer | Downregulated | Human | [83] |
| Ovarian Cancer | Downregulated | Human | [85] |
| Breast Cancer | Downregulated | Human | [86] |
| Endometriosis | Downregulated | Human | [89] |
| Metabolic Disease | Upregulated | Mouse | [96] |
| Sepsis | Upregulated | Mouse | [98] |
| Ischemia-Reperfusion | Upregulated | Mouse | [99] |
| Cystic Fibrosis | Upregulated | Mouse | [105] |
5. Expert opinion
SULT1E1 has long been appreciated as a phase II metabolizing enzyme whose primary function is to sulfonate and deactivate estrogens, so SULT1E1 is implicated in the metabolism of estrogenic drugs, including drugs used in oral contraceptives, SERMs, and hormone replacement therapy (HRT). Interestingly, SULT1E1 has been shown to be inhibited by several polichlroynated biphenyls (PCBs), phytochemicals, especially isoflavones, as well as the chlorinated phenolic compound Triclosan. We believe more classes of compounds will be revealed as relevant substrates or inhibitors of SULT1E1.
In humans, SULT1E1, although showing no expression differences between genders, may present inter-individual variations as a result of the gene polymorphisms. The expression and activity of the human SULT1E1 can also be impacted by environmental factors, such as the alcoholism. Furthermore, SULT1E1 plays an important role as a mediator in a myriad of diseases.
In several estrogen-dependent carcinomas, cancer cells activate mechanisms to decrease the expression of the deactivating enzyme SULT1E1 and increase the expression of the re-activating enzyme, providing a mechanism for the initiation and progression of estrogen-dependent cancers. Furthermore, patients with polymorphisms that lead to enzyme inactivation have a correlation with a worse prognosis.
However, the physiological function of SULT1E1 remains to be fully elucidated yet, as several studies have suggested both estrogen-dependent and -independent roles of this enzyme in physiology and pathophysiology. Moreover, the enzyme is expressed in several non-gonadal organs, such as the liver, tubular renal cells, trachea, lung, esophagus, spleen, pancreas, adrenal gland, thyroid, urinary bladder, kidney, and placenta. These expression pattern hints that the function of SULT1E1 is beyond reproduction.
As the mouse shares a 77% amino acidic homology with the human enzyme, this rodent specie has been widely used as a model to study the effects of diseases on SULT1E1 and vice-versa. Early studies based on the use of Sult1e1 null mice suggested a role of Sult1e1 in reproduction. In the past 10 years, results from our laboratory and other laboratories have pointed to functions of this enzyme beyond reproduction. The expression of Sult1e1 can be regulated by nuclear receptors and diseases. Sult1e1 is implicated in adipogenesis and in several inflammation-mediated conditions such as sepsis, ischemia-induced injuries, diabetes mellitus and cystic fibrosis, at least in mice. In the disease effect on Sult1e1, there are a number of interesting observations: 1) Not only the expression of Sult1e1 can be regulated by diseases but genetic ablation or pharmacological inhibition of Sult1e1 can impact the outcome of the mouse models of these diseases; 2) Both the disease effect on Sult1e1 and the Sult1e1 effect on diseases can be tissue-specific and/or sex-specific; 3) Tissue-specific regulation of Sult1e1 can have a systemic effect through the circulating estrogens as well as other yet to be defined mediators for the organ crosstalk; 4) The Sult1e1 effect on disease is not always sex hormone dependent, suggesting Sult1e1 substrates besides estrogens.
To our knowledge, loss of Sult1e1 by genetic ablation or enzymatic inhibition prevents the rise of inflammation-based diseases in animal models, whereas enzyme absence may increase the risk of estrogen-dependent cancers. However, another outstanding challenge is the species specificity of the Sult1e1 functions. The transcriptional regulation of Sult1e1 can be species specific. It remains to be determined whether many of the disease effects on Sult1e1 can be recapitulated in humans, an area that warrants more studies. With the advance of studies focusing on SULT1E1, it is our hope to target this enzyme to prevent the occurrence of various human disease conditions.
Article highlights.
Estrogen sulfotransferase (SULT1E1, also previously known as EST) is a phase II cytosolic enzyme that sulfoconjugates and deactivates estrogens.
Both endogenous and synthetic estrogens, such as those used as hormone replacement therapies (HRTs) and oral contraceptives, can be metabolized by SULT1E1. Meanwhile, some selective estrogen receptor modulators (SERMs) metabolites and environmental pollutants like polychlorinated biphenyls, may act as inhibitors of the enzyme.
The transcription of SULT1E1 is subjected to regulation by nuclear receptors and disease states.
Both the disease effect on SULT1E1 and the SULT1E1 effect on diseases can be tissue-specific and/or sex-specific.
Although the enzyme was initially implicated in reproduction, several studies conducted in rodents indicate SULT1E1 has crucial roles in non-gonadal organs and possibly has substrates other than the estrogens.
Acknowledgements
The original research of ours described in this article was supported in part by National Institutes of Health (NIH) grant ES023438 and DK083952. W Xie was supported in part by the Joseph Koslow Endowed Chair Professorship from the University of Pittsburgh School of Pharmacy. M Huang was supported in part by the Chinese Ministry of Education 111 Project Grant No. B16047.
Funding
This paper was not funded.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- 1.Jancova P, Anzenbacher P, Anzenbacherova E. Phase II drug metabolizing enzymes. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2010;154(2):103–116. [DOI] [PubMed] [Google Scholar]
- 2.Hines RN, McCarver DG. The ontogeny of human drug-metabolizing enzymes: phase I oxidative enzymes. Journal of Pharmacology and Experimental Therapeutics. 2002;300(2):355–360. [DOI] [PubMed] [Google Scholar]
- 3.McCarver DG, Hines RN. The ontogeny of human drug-metabolizing enzymes: phase II conjugation enzymes and regulatory mechanisms. Journal of Pharmacology and Experimental Therapeutics. 2002;300(2):361–366. [DOI] [PubMed] [Google Scholar]
- 4.Pompeo F, Brooke E, Kawamura A, et al. The pharmacogenetics of NAT: structural aspects. Pharmacogenomics. 2002;3(1):19–30. [DOI] [PubMed] [Google Scholar]
- 5.Kauffman FC. Sulfonation in pharmacology and toxicology. Drug Metabolism Reviews. 2004;36(3–4):823–843. [DOI] [PubMed] [Google Scholar]
- 6.Prakash C, Vaz AD. Drug metabolism: significance and challenges. John Wiley & Sons: Hoboken, NJ, USA; 2009. [Google Scholar]
- 7.Coughtrie M Sulfation through the looking glass—recent advances in sulfotransferase research for the curious. The Pharmacogenomics Journal. 2002;2(5):297. [DOI] [PubMed] [Google Scholar]
- 8.Glatt H Sulfotransferases in the bioactivation of xenobiotics. Chemico-Biological Interactions. 2000;129(1–2):141–170. [DOI] [PubMed] [Google Scholar]
- 9.Mueller JW, Gilligan LC, Idkowiak J, et al. The regulation of steroid action by sulfation and desulfation. Endocrine Reviews. 2015;36(5):526–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hemmerich S, Verdugo D, Rath VL. Strategies for drug discovery by targeting sulfation pathways. Drug Discovery Today. 2004;9(22):967–975. [DOI] [PubMed] [Google Scholar]
- 11.Chapman E, Best MD, Hanson SR, et al. Sulfotransferases: structure, mechanism, biological activity, inhibition, and synthetic utility. Angewandte Chemie International Edition. 2004;43(27):3526–3548. [DOI] [PubMed] [Google Scholar]
- 12.Superti-Furga A A defect in the metabolic activation of sulfate in a patient with achondrogenesis type IB. American Journal of Human Genetics. 1994;55(6):1137. [PMC free article] [PubMed] [Google Scholar]
- 13.Goettsch S, Badea RA, Mueller JW, et al. Human TPST1 transmembrane domain triggers enzyme dimerisation and localisation to the Golgi compartment. Journal of Molecular Biology. 2006;361(3):436–449. [DOI] [PubMed] [Google Scholar]
- 14.Riches Z, Stanley EL, Bloomer JC, et al. Quantitative evaluation of the expression and activity of five major sulfotransferases (SULTs) in human tissues: the SULT “pie”. Drug Metabolism and Disposition. 2009;37(11):2255–2261.* This paper describes the tissue distribution of the major sulfotransferases in humans.
- 15.Miller JA. Sulfonation in chemical carcinogenesis—history and present status. Chemico-Biological Interactions. 1994;92(1–3):329–341. [DOI] [PubMed] [Google Scholar]
- 16.Gamage N, Barnett A, Hempel N, et al. Human sulfotransferases and their role in chemical metabolism. Toxicological Sciences. 2005;90(1):5–22. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki T, Nakata T, Miki Y, et al. Estrogen sulfotransferase and steroid sulfatase in human breast carcinoma. Cancer Research. 2003;63(11):2762–2770. [PubMed] [Google Scholar]
- 18.Song W, Moore R, McLachlan J, et al. Molecular characterization of a testis-specific estrogen sulfotransferase and aberrant liver expression in obese and diabetogenic C57BL/KsJ-db/db mice. Endocrinology. 1995;136(6):2477–2484. [DOI] [PubMed] [Google Scholar]
- 19.Hobkirk R, Cardy CA, Saidi F, et al. Development and characteristics of an oestrogen sulphotransferase in placenta and uterus of the pregnant mouse. Comparison between mouse and rat. Biochemical Journal. 1983;216(2):451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alnouti Y, Klaassen CD. Tissue distribution and ontogeny of sulfotransferase enzymes in mice. Toxicological Sciences. 2006;93(2):242–255.* This paper describes the tissue distribution of the major sulfotransferases in mice.
- 21.Guo Y, Hu B, Huang H, et al. Estrogen sulfotransferase is an oxidative stress-responsive gene that gender-specifically affects liver ischemia/reperfusion injury. Journal of Biological Chemistry. 2015;290(23):14754–14764.** First paper to mechanistically demonstrate the induction of Sult1e1 in mouse liver ischemia and reperfusion injury as a result of Nrf-2 activation.
- 22.Cole GB, Keum G, Liu J, et al. Specific estrogen sulfotransferase (SULT1E1) substrates and molecular imaging probe candidates. Proceedings of the National Academy of Sciences. 2010;107(14):6222–6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ihunnah CA, Wada T, Philips BJ, et al. Estrogen sulfotransferase/SULT1E1 promotes human adipogenesis. Molecular and Cellular Biology. 2014;34(9):1682–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chai X, Guo Y, Jiang M, et al. Oestrogen sulfotransferase ablation sensitizes mice to sepsis. Nature Communications. 2015;6.** First paper to demonstrate the essential role of Sult1e1 in sepsis response.
- 25.Macedo LF, Sabnis G, Brodie A. Aromatase inhibitors and breast cancer. Annals of the New York Academy of Sciences. 2009;1155(1):162–173. [DOI] [PubMed] [Google Scholar]
- 26.Simpson ER, Mahendroo MS, Means GD, et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocrine Reviews. 1994;15(3):342–355. [DOI] [PubMed] [Google Scholar]
- 27.Purohit A, Woo LL, Potter BV. Steroid sulfatase: a pivotal player in estrogen synthesis and metabolism. Molecular and Cellular Endocrinology. 2011;340(2):154–160. [DOI] [PubMed] [Google Scholar]
- 28.Mungenast F, Thalhammer T. Estrogen biosynthesis and action in ovarian cancer. Frontiers in Endocrinology. 2014;5:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang H, Varmalova O, Vargas FM, et al. Sulfuryl transfer: the catalytic mechanism of human estrogen sulfotransferase. Journal of Biological Chemistry. 1998;273(18):10888–10892.** This paper describes the estrogen substrate binding mechanism of SULT1E1.
- 30.Hernández JS, Watson R, Wood TC, et al. Sulfation of estrone and 17 beta-estradiol in human liver. Catalysis by thermostable phenol sulfotransferase and by dehydroepiandrosterone sulfotransferase. Drug Metabolism and Disposition. 1992;20(3):413–422. [PubMed] [Google Scholar]
- 31.Petrotchenko EV, Doerflein ME, Kakuta Y, et al. Substrate gating confers steroid specificity to estrogen sulfotransferase. Journal of Biological Chemistry. 1999;274(42):30019–30022.** First paper to describe the gate that confers estrogen specificity to SULT1E1.
- 32.James MO, Li W, Summerlot DP, et al. Triclosan is a potent inhibitor of estradiol and estrone sulfonation in sheep placenta. Environment International. 2010;36(8):942–949.** First paper to describe the role of Triclosan as a potent SULT1E1 inhibitor.
- 33.Dao TL, Hayes C, Libby PR. Steroid sulfatase activities in human breast tumors. Proceedings of the Society for Experimental Biology and Medicine. 1974;146(2):381–384. [DOI] [PubMed] [Google Scholar]
- 34.Aksoy IA, Wood TC, Weinshilboum R. Human liver estrogen sulfotransferase: identification by cDNA cloning and expression. Biochemical and Biophysical Research Communications. 1994;200(3):1621–1629.* First paper to describe the cloning of human liver SULT1E1.
- 35.Schrag ML, Cui D, Rushmore TH, et al. Sulfotransferase 1E1 is a low km isoform mediating the 3-O-sulfation of ethinyl estradiol. Drug Metabolism and Disposition. 2004;32(11):1299–1303. [DOI] [PubMed] [Google Scholar]
- 36.Pedersen LC, Petrotchenko E, Shevtsov S, et al. Crystal structure of the human estrogen sulfotransferase-paps complex evidence for catalytic role of Ser137 in the sulfuryl transfer reaction. Journal of Biological Chemistry. 2002;277(20):17928–17932. [DOI] [PubMed] [Google Scholar]
- 37.Rakers C, Schumacher F, Meinl W, et al. In silico prediction of human sulfotransferase 1E1 activity guided by pharmacophores from molecular dynamics simulations. Journal of Biological Chemistry. 2016;291(1):58–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Landsiedel R, Engst W, Seidel A, et al. Physico-chemical properties and mutagenicity of benzylic compounds. Experimental and Toxicologic Pathology. 1996;48:215–221. [Google Scholar]
- 39.Reed M, Purohit A, Woo LL, et al. Steroid sulfatase: molecular biology, regulation, and inhibition. Endocrine Reviews. 2005;26(2):171–202. [DOI] [PubMed] [Google Scholar]
- 40.Jiang M, Klein M, Zanger UM, et al. Inflammatory regulation of steroid sulfatase: A novel mechanism to control estrogen homeostasis and inflammation in chronic liver disease. Journal of Hepatology. 2016;64(1):44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bi Y, Jiang M, Guo W, et al. Sex-Dimorphic and Sex Hormone–Dependent Role of Steroid Sulfatase in Adipose Inflammation and Energy Homeostasis. Endocrinology. 2018;159(9):3365–3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang M, He J, Kucera H, et al. Hepatic over-expression of steroid sulfatase ameliorates mouse models of obesity and type 2 diabetes through sex-specific mechanisms. Journal of Biological Chemistry. 2014:jbc. M113. 535914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bever AT, Hisaw FL, Velardo JT. Inhibitory action of desoxycorticosterone acetate, cortisone acetate, and testosterone on uterine growth induced by estradiol-17beta. Endocrinology. 1956. August;59(2):165–9. [DOI] [PubMed] [Google Scholar]
- 44.Campbell PS. The mechanism of the inhibition of uterotrophic responses by acute dexamethasone pretreatment. Endocrinology. 1978. September;103(3):716–23. [DOI] [PubMed] [Google Scholar]
- 45.Rhen T, Grissom S, Afshari C, et al. Dexamethasone blocks the rapid biological effects of 17beta-estradiol in the rat uterus without antagonizing its global genomic actions. Faseb J. 2003. October;17(13):1849–70. [DOI] [PubMed] [Google Scholar]
- 46.Gong H, Jarzynka MJ, Cole TJ, et al. Glucocorticoids antagonize estrogens by glucocorticoid receptor–mediated activation of estrogen sulfotransferase. Cancer Research. 2008;68(18):7386–7393.** First paper to demonstrate that the mouse and human SULT1E1 are transcriptional targets of GR.
- 47.Sahlin L Dexamethasone attenuates the estradiol-induced increase of IGF-I mRNA in the rat uterus. J Steroid Biochem Mol Biol. 1995. October;55(1):9–15. [DOI] [PubMed] [Google Scholar]
- 48.Zhou F, Bouillard B, Pharaboz-Joly MO, et al. Non-classical antiestrogenic actions of dexamethasone in variant MCF-7 human breast cancer cells in culture. Mol Cell Endocrinol. 1989. October;66(2):189–97. [DOI] [PubMed] [Google Scholar]
- 49.Gong H, Guo P, Zhai Y, et al. Estrogen deprivation and inhibition of breast cancer growth in vivo through activation of the orphan nuclear receptor liver X receptor. Molecular Endocrinology. 2007;21(8):1781–1790.** First paper to demonstrate that SULT1E1 is a transcriptional target of LXR.
- 50.Alnouti Y, Klaassen CD. Regulation of sulfotransferase enzymes by prototypical microsomal enzyme inducers in mice. Journal of Pharmacology and Experimental Therapeutics. 2008;324(2):612–621.** First paper to demonstrate that Sult1e1 is a transcriptional target of CAR in female mice.
- 51.Kang HS, Angers M, Beak JY, et al. Gene expression profiling reveals a regulatory role for RORα and RORγ in phase I and phase II metabolism. Physiological Genomics. 2007;31(2):281–294. [DOI] [PubMed] [Google Scholar]
- 52.Kodama S, Hosseinpour F, Goldstein JA, et al. Liganded pregnane X receptor represses the human sulfotransferase SULT1E1 promoter through disrupting its chromatin structure. Nucleic Acids Research. 2011;39(19):8392–8403.** First paper to mechanistically demonstrate how PXR mediates SULT1E1 repression.
- 53.Wang S, Yuan X, Lu D, et al. farnesoid X receptor regulates Sult1e1 expression through inhibition of Pgc1α binding to Hnf4α. Biochemical Pharmacology. 2017;145:202–209.** First paper to mechanistically demonstrate how FXR mediates SULT1E1 repression.
- 54.Wang B, Sanchez RI, Franklin RB, et al. The involvement of CYP3A4 and CYP2C9 in the metabolism of 17α-ethinylestradiol. Drug Metabolism and Disposition. 2004. [DOI] [PubMed] [Google Scholar]
- 55.Back D, Breckenridge A, MacIver M, et al. The gut wall metabolism of ethinyloestradiol and its contribution to the pre‐systemic metabolism of ethinyloestradiol in humans. British Journal of Clinical Pharmacology. 1982;13(3):325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bolt H, Bolt M, Kappus H. Interaction of rifampicin treatment with pharmacokinetics and metabolism of ethinyloestradiol in man. Acta Endocrinologica. 1977;85(1):189–197. [DOI] [PubMed] [Google Scholar]
- 57.Back D, Breckenridge A, MacIver M, et al. Interaction of ethinyloestradiol with ascorbic acid in man. British Medical Journal (Clinical research ed). 1981;282(6275):1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rogers SM, Back D, Stevenson P, et al. Paracetamol interaction with oral contraceptive steroids: increased plasma concentrations of ethinyloestradiol. British Journal of Clinical Pharmacology. 1987;23(6):721–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sinofsky FE, Pasquale SA. The effect of fluconazole on circulating ethinyl estradiol levels in women taking oral contraceptives. American Journal of Obstetrics & Gynecology. 1998;178(2):300–304. [DOI] [PubMed] [Google Scholar]
- 60.De Montellano PO, Kunze KL. Self-catalyzed inactivation of hepatic cytochrome P-450 by ethynyl substrates. Journal of Biological Chemistry. 1980;255(12):5578–5585. [PubMed] [Google Scholar]
- 61.De Montellano PO, Kunze KL, Yost GS, et al. Self-catalyzed destruction of cytochrome P-450: covalent binding of ethynyl sterols to prosthetic heme. Proceedings of the National Academy of Sciences. 1979;76(2):746–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kent UM, Mills DE, Rajnarayanan RV, et al. Effect of 17-α-ethynylestradiol on activities of cytochrome P450 2B (P450 2B) enzymes: characterization of inactivation of P450s 2B1 and 2B6 and identification of metabolites. Journal of Pharmacology and Experimental Therapeutics. 2002;300(2):549–558. [DOI] [PubMed] [Google Scholar]
- 63.Lin H-l, Kent UM, Hollenberg PF. Mechanism-based inactivation of cytochrome P450 3A4 by 17α-ethynylestradiol: evidence for heme destruction and covalent binding to protein. Journal of Pharmacology and Experimental Therapeutics. 2002;301(1):160–167. [DOI] [PubMed] [Google Scholar]
- 64.Madden S, Back D, Martin C, et al. Metabolism of the contraceptive steroid desogestrel by the intestinal mucosa. British Journal of Clinical Pharmacology. 1989;27(3):295–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Falany JL, Pilloff DE, Leyh TS, et al. Sulfation of raloxifene and 4-hydroxytamoxifen by human cytosolic sulfotransferases. Drug Metabolism and Disposition. 2006;34(3):361–8. [DOI] [PubMed] [Google Scholar]
- 66.Falany JL, Macrina N, Falany CN. Sulfation of tibolone and tibolone metabolites by expressed human cytosolic sulfotransferases. The Journal of Steroid Biochemistry and Molecular Biology. 2004;88(4–5):383–391. [DOI] [PubMed] [Google Scholar]
- 67.Bhavnani BR, Stanczyk FZ. Pharmacology of conjugated equine estrogens: efficacy, safety and mechanism of action. The Journal of Steroid Biochemistry and Molecular Biology. 2014;142:16–29. [DOI] [PubMed] [Google Scholar]
- 68.Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. New England Journal of Medicine. 2003;349(6):523–534. [DOI] [PubMed] [Google Scholar]
- 69.Gambacciani M, Levancini M. Hormone replacement therapy and the prevention of postmenopausal osteoporosis. Przeglad menopauzalny= Menopause review. 2014;13(4):213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rapp SR, Espeland MA, Shumaker SA, et al. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289(20):2663–2672. [DOI] [PubMed] [Google Scholar]
- 71.Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291(24):2947–2958. [DOI] [PubMed] [Google Scholar]
- 72.Shumaker SA, Legault C, Rapp SR, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289(20):2651–2662. [DOI] [PubMed] [Google Scholar]
- 73.Yaffe K, Vittinghoff E, Ensrud KE, et al. Effects of Ultra–Low-Dose Transdermal Estradiol on Cognition and Health-Related Quality of Life. Archives of Neurology. 2006;63(7):945–950. [DOI] [PubMed] [Google Scholar]
- 74.Wroolie TE, Kenna HA, Williams KE, et al. Cognitive effects of hormone therapy continuation or discontinuation in a sample of women at risk for Alzheimer disease. The American Journal of Geriatric Psychiatry. 2015;23(11):1117–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Park-Chung M, Malayev A, Purdy RH, et al. Sulfated and unsulfated steroids modulate γ-aminobutyric acidA receptor function through distinct sites. Brain Research. 1999;830(1):72–87. [DOI] [PubMed] [Google Scholar]
- 76.Skakkebék NE, Meyts ERD, Jørgensen N, et al. Germ cell cancer and disorders of spermatogenesis: an environmental connection? APMIS. 1998;106(1‐6):3–12. [DOI] [PubMed] [Google Scholar]
- 77.Safe SH. Polychlorinated biphenyls (PCBs): environmental impact, biochemical and toxic responses, and implications for risk assessment. Critical Reviews in Toxicology. 1994;24(2):87–149. [DOI] [PubMed] [Google Scholar]
- 78.Kester MH, Bulduk S, Tibboel D, et al. Potent inhibition of estrogen sulfotransferase by hydroxylated PCB metabolites: a novel pathway explaining the estrogenic activity of PCBs. Endocrinology. 2000;141(5):1897–1900. [DOI] [PubMed] [Google Scholar]
- 79.Basly J-P, Lavier M-CC. Dietary phytoestrogens: potential selective estrogen enzyme modulators? Planta Medica. 2005;71(04):287–294. [DOI] [PubMed] [Google Scholar]
- 80.Harris R, Wood D, Bottomley L, et al. Phytoestrogens are potent inhibitors of estrogen sulfation: implications for breast cancer risk and treatment. The Journal of Clinical Endocrinology & Metabolism. 2004;89(4):1779–1787. [DOI] [PubMed] [Google Scholar]
- 81.Miksits M, Maier-Salamon A, Aust S, et al. Sulfation of resveratrol in human liver: evidence of a major role for the sulfotransferases SULT1A1 and SULT1E1. Xenobiotica. 2005;35(12):1101–1119. [DOI] [PubMed] [Google Scholar]
- 82.Walle T, Hsieh F, DeLegge MH, et al. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metabolism and Disposition. 2004. [DOI] [PubMed] [Google Scholar]
- 83.Otake Y, Nolan AL, Walle UK, et al. Quercetin and resveratrol potently reduce estrogen sulfotransferase activity in normal human mammary epithelial cells. The Journal of Steroid Biochemistry and Molecular Biology. 2000;73(5):265–270. [DOI] [PubMed] [Google Scholar]
- 84.Adolfsson-Erici M, Pettersson M, Parkkonen J, et al. Triclosan, a commonly used bactericide found in human milk and in the aquatic environment in Sweden. Chemosphere. 2002;46(9–10):1485–1489. [DOI] [PubMed] [Google Scholar]
- 85.Calafat AM, Ye X, Wong L-Y, et al. Urinary concentrations of triclosan in the US population: 2003–2004. Environmental Health Perspectives. 2007;116(3):303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stoker TE, Gibson EK, Zorrilla LM. Triclosan exposure modulates estrogen-dependent responses in the female wistar rat. Toxicological Sciences. 2010;117(1):45–53. [DOI] [PubMed] [Google Scholar]
- 87.Song W-C, Qian Y, Li AP. Estrogen sulfotransferase expression in the human liver: marked interindividual variation and lack of gender specificity. Journal of Pharmacology and Experimental Therapeutics. 1998;284(3):1197–1202. [PubMed] [Google Scholar]
- 88.Adjei AA, Thomae BA, Prondzinski JL, et al. Human estrogen sulfotransferase (SULT1E1) pharmacogenomics: gene resequencing and functional genomics. British Journal of Pharmacology. 2003;139(8):1373–1382.** First paper to identify SULT1E1 cSNPs in COS-1 cells that are associated with the enzyme’s loss of funtion.
- 89.Rebbeck TR, Troxel AB, Wang Y, et al. Estrogen sulfation genes, hormone replacement therapy, and endometrial cancer risk. Journal of the National Cancer Institute. 2006;98(18):1311–1320. [DOI] [PubMed] [Google Scholar]
- 90.Tong MH, Jiang H, Liu P, et al. Spontaneous fetal loss caused by placental thrombosis in estrogen sulfotransferase—deficient mice. Nature Medicine. 2005;11(2):153.** First paper to demonstrate the generation of Sult1e1 KO mouse and characterize the physiological effects of Sult1e1 ablation.
- 91.Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA. 1998;280(7):605–613. [DOI] [PubMed] [Google Scholar]
- 92.Cancer CGoHFiB. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52 705 women with breast cancer and 108 411 women without breast cancer. The Lancet. 1997;350(9084):1047–1059. [PubMed] [Google Scholar]
- 93.Nabulsi AA, Folsom AR, White A, et al. Association of hormone-replacement therapy with various cardiovascular risk factors in postmenopausal women. New England Journal of Medicine. 1993;328(15):1069–1075. [DOI] [PubMed] [Google Scholar]
- 94.Daly E, Vessey MP, Hawkins MM, et al. Risk of venous thromboembolism in users of hormone replacement therapy. The Lancet. 1996;348(9033):977–980. [DOI] [PubMed] [Google Scholar]
- 95.Šmuc T, Rižner TL. Aberrant pre-receptor regulation of estrogen and progesterone action in endometrial cancer. Molecular and Cellular Endocrinology. 2009;301(1–2):74–82. [DOI] [PubMed] [Google Scholar]
- 96.Ren X, Wu X, Hillier SG, et al. Local estrogen metabolism in epithelial ovarian cancer suggests novel targets for therapy. The Journal of Steroid Biochemistry and Molecular Biology. 2015;150:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pasqualini JR. Estrogen sulfotransferases in breast and endometrial cancers. Annals of the New York Academy of Sciences. 2009;1155(1):88–98. [DOI] [PubMed] [Google Scholar]
- 98.Sasano H, Miki Y, Nagasaki S, et al. In situ estrogen production and its regulation in human breast carcinoma: from endocrinology to intracrinology. Pathology International. 2009;59(11):777–789. [DOI] [PubMed] [Google Scholar]
- 99.Wang L-j, Li J, Hao F-r, et al. Dexamethasone suppresses the growth of human non-small cell lung cancer via inducing estrogen sulfotransferase and inactivating estrogen. Acta Pharmacologica Sinica. 2016;37(6):845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li L, He D, Wilborn TW, et al. Increased SULT1E1 activity in HepG2 hepatocytes decreases growth hormone stimulation of STAT5b phosphorylation. Steroids. 2009;74(1):20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Piccinato CA, Neme RM, Torres N, et al. Effects of steroid hormone on estrogen sulfotransferase and on steroid sulfatase expression in endometriosis tissue and stromal cells. The Journal of Steroid Biochemistry and Molecular Biology. 2016;158:117–126. [DOI] [PubMed] [Google Scholar]
- 102.Wada T, Ihunnah CA, Gao J, et al. Estrogen sulfotransferase inhibits adipocyte differentiation. Molecular Endocrinology. 2011;25(9):1612–1623.** First paper to demonstrate the role of Sult1e1 in adipocyte differentiation.
- 103.Leiter EH, Chapman HD. Obesity-induced diabetes (diabesity) in C57BL/KsJ mice produces aberrant trans-regulation of sex steroid sulfotransferase genes. The Journal of Clinical Investigation. 1994;93(5):2007–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gao J, He J, Shi X, et al. Sex-specific effect of estrogen sulfotransferase on mouse models of type 2 diabetes. Diabetes. 2012;61(6):1543–1551.** First paper to demonstrate that hepatic Sult1e1 is induced in type 2 Diabetes Mellitus and that Sult1e1 ablation ammeliorates the metabolic function in a sex-specific manner.
- 105.Garbacz WG, Jiang M, Xu M, et al. Sex-and tissue-specific role of estrogen sulfotransferase in energy homeostasis and insulin sensitivity. Endocrinology. 2017;158(11):4093–4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Durie PR, Kent G, Phillips MJ, et al. Characteristic multiorgan pathology of cystic fibrosis in a long-living cystic fibrosis transmembrane regulator knockout murine model. The American Journal of Pathology. 2004;164(4):1481–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kerem B-s, Rommens JM, Buchanan JA, et al. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989;245(4922):1073–1080. [DOI] [PubMed] [Google Scholar]
- 108.Rosenberg LA, Schluchter MD, Parlow AF, et al. Mouse as a model of growth retardation in cystic fibrosis. Pediatric Research. 2006;59(2):191. [DOI] [PubMed] [Google Scholar]
- 109.Ozen M, Cokugras H, Ozen N, et al. Relation between serum Insulin‐like growth factor‐I and insulin‐like growth factor‐binding protein‐3 levels, clinical status and growth parameters in prepubertal cystic fibrosis patients. Pediatrics International. 2004;46(4):429–435. [DOI] [PubMed] [Google Scholar]
- 110.Boguszewski MC, Kamoi TO, Radominski RB, et al. Insulin-like growth factor-1, leptin, body composition, and clinical status interactions in children with cystic fibrosis. Hormone Research in Paediatrics. 2007;67(5):250–256. [DOI] [PubMed] [Google Scholar]
- 111.Li L, Falany CN. Elevated hepatic SULT1E1 activity in mouse models of cystic fibrosis alters the regulation of estrogen responsive proteins. Journal of Cystic Fibrosis. 2007;6(1):23–30.** First paper to demonstrate that Sult1e1 is induced in a mouse models of cystic fibrosis.
- 112.Falany CN, He D, Li L, et al. Regulation of hepatic sulfotransferase (SULT) 1E1 expression and effects on estrogenic activity in cystic fibrosis (CF). The Journal of Steroid Biochemistry and Molecular Biology. 2009;114(1–2):113–119.** First paper to mechanistically demonstrate how CFTR ablation induces Sult1e1 in mouse models of cystic fibrosis.