Abstract
Vaccines consisting of synthetic peptides representing cytotoxic T-lymphocyte (CTL) epitopes have long been considered as a simple and cost-effective approach to treat cancer. However, the efficacy of these vaccines in the clinic in patients with measurable disease remains questionable. We believe that the poor performance of peptide vaccines is due to their inability to generate sufficiently large CTL responses that are required to have a positive impact against established tumors. Peptide vaccines to elicit CTLs in the clinic have routinely been administered in the same manner as vaccines designed to induce antibody responses: injected subcutaneously and in many instances using Freund’s adjuvant. We report here that peptide vaccines and poly-ICLC adjuvant administered via the unconventional intravenous route of immunization generate substantially higher CTL responses as compared to conventional subcutaneous injections, resulting in more successful antitumor effects in mice. Furthermore, amphiphilic antigen constructs such as palmitoylated peptides were shown to be better immunogens than long peptide constructs, which now are in vogue in the clinic. The present findings if translated into the clinical setting could help dissipate the wide-spread skepticism of whether peptide vaccines will ever work to treat cancer.
Electronic supplementary material
The online version of this article (10.1007/s00262-018-02294-5) contains supplementary material, which is available to authorized users.
Keywords: Peptide vaccines, Route of injection, CD8 T cells, Melanoma
Introduction
The field of tumor immunotherapy has dramatically changed the way cancer is treated today. In particular, antibodies that function as immune checkpoint inhibitors (ICIs) have achieved remarkable success in the clinic [1–4]. Unfortunately, this success is limited to a low proportion of patients and to specific cancer types. The effectiveness of ICIs depends on the existence of antigen-specific T cells at the tumor site, which in many instances are scarce or absent [5, 6]. In view of this, cytotoxic T-lymphocyte (CTL)-inducing cancer vaccines have the potential of broadening the applicability and increasing the effectiveness of ICIs.
Amongst various types of therapeutic cancer vaccines, those composed of synthetic peptides have gained popularity for their low cost and ease of manufacturing. The first examples of peptide vaccines contained CTL epitopes from self-tissue differentiation antigens (e.g., melanosomal gp100 and MART1/MelanA [7]), cancer/testis antigens (e.g., MAGE and NY-ESO-1 [8, 9]), or oncogenic viral proteins (e.g., HPV16-E7 [10]). Regrettably, these vaccines failed to induce CTL responses of sufficient magnitude and quality necessary to demonstrate a substantial therapeutic effect, generating skepticism that these vaccines would ever work [7]. The failure of these vaccines targeting conventional tumor antigens was attributed to immune tolerance to self-antigens [7] and to peptide size, where small minimal peptide epitopes that could be presented by non-professional antigen-presenting cells would also induce tolerance [11]. Recent advances in genomic sequencing have facilitated the discovery of mutational-derived CTL epitopes (neoantigens), which supposedly will not be subject to immune tolerance when injected as long-peptides (LPs [12–14]). Personalized LP vaccines utilizing patient specific neoantigens have recently shown some glimpse of success in the clinic in early stage melanoma patients [15]. Nevertheless, the magnitude of CTL responses generated by these vaccines, compared to how the immune system responds to viral infections is substantially lower, questioning whether these vaccines will be effective against more advanced cancer stages.
Peptide-based cancer vaccination approaches in the clinic utilize strategies originally developed for generating antibody responses such as subcutaneous (s.c.) injections and the use of depot-based adjuvants such as incomplete Freund’s adjuvant (IFA). Although this approach is successful in generating protective antibody titers, it is clearly ineffective and perhaps detrimental at generating substantial CTL responses [16]. In view of these problems, we have advocated that developing optimized peptide vaccination strategies that generate strong CTL responses such as those that occur during viral infections should be a research priority [17].
The immunogenicity of cancer vaccines depends on many factors including peptide composition, immune adjuvants, and the generation of memory T cells capable of responding to vaccine boosts that enable further T-cell expansion. Although research has been done to optimize the peptide composition and formulation [10, 17, 18] and selecting potent adjuvants [19–22], less attention has been paid to address the impact of the route of administration of peptide-based vaccines. Herein, we established that the route of vaccine administration has a significant impact on the potency and antitumor efficacy of peptide-based vaccines. Our results show that systemic intravenous (i.v.) vaccination is far superior to the conventional s.c. vaccination route at inducing large T-cell responses in mice. Peptide vaccines administered i.v. promote the systemic dissemination of antigen throughout the lymphoid system for delivery to large numbers of antigen-presenting cells (APCs) allowing efficient recruitment of the low number of antigen-specific CTL precursors into the immune response. These findings could have a significant impact in the success of therapeutic peptide vaccines that target both conventional tumor antigens or mutationally-derived neoantigens.
Materials and methods
Mice and cell lines
C57BL/6 (WT-B6) and B6-Ly5.1 (CD45.1) mice were used throughout this work. TnTR1 TCR-transnuclear mice (Trp1455–463 specific CTLs in a RAG-KO background) were described [23]. Transgenic mice that express the TCR specific for gp10025–33 (pmel-1 mice) [24], or Ova257–264 restricted TCR (OT-1 mice) were purchased from The Jackson Laboratory. Trp1-KO B6 mice were generated in our facility by breeding F1 mice of TRP-1 TCR Bw RAG mice (Jackson Laboratory Stock No. 8684) with WT-B6 mice, with each other and selecting for Trp1 deficient (brown coat), RAG1+, and Trp1-TCR− mice. B16F10 murine melanoma was obtained from the American-Type Culture Collection.
Peptides, antibodies, and reagents
Synthetic peptides used in this study were purchased from A&A Laboratories (San Diego, CA): minimal Trp1455–463/9M (TAPDNLGYM), long Trp1455–473/9M (LP-Trp1; TAPDNLGYMYEVQWPGQEF), palmitoylated Trp1455–463/9M (pam-Trp1; pam2KMFVTAPDNLGYM), long human gp10018–33 (LP-hgp100; LLAVGATKVPRNQDWL), minimal hgp10025–33 (KVPRNQDWL), and palmitoylated hgp10025–33 (pam-hgp100; pam2KMFVKVPRNQDWL). The identity and purity of peptides were determined by the vendor using mass spectrometry analysis and high-performance liquid chromatography. All peptides were solubilized at 20 mg/ml in DMSO-TFA (99.9%/0.1%) and stored at − 80 °C. Poly-ICLC is poly-IC stabilized with poly-l-lysine and carboxymethyl cellulose (Hiltonol, Oncovir, Inc.). Rat anti-mouse CD40 mAb (clone FGK4.5) was purchased from Bio X Cell (West Lebanon, NH). PE-conjugated H-2Db/Trp1455–463/9M and hgp10025–33 tetramers were provided by the NIH Tetramer Core Facility at Emory University (Atlanta, GA, USA). Fluorochrome-labeled antibodies were purchased from Thermo Fisher Scientific (San Diego, CA, USA).
Immunization protocol
Mice received adoptive transfers via the tail vein of various numbers (as indicated in the figure legends) TnTR1 or pmel-1 CTLs and 1 day later were immunized with one single injection of one of the following vaccines: TriVax (containing 120 µg pam peptide or 200 µg LP, 100 µg anti-CD40 mAb, and 50 µg poly-ICLC) or BiVax (peptide and 50 µg poly-ICLC). In some experiments, mice received a booster dose with the same vaccine formulation. Endogenous CTL responses were measured in mice not receiving CTL-adoptive transfers.
Evaluation of cellular immune responses
For measuring antigen-specific CTL responses, either peripheral blood samples (~ 3 to 5 drops) taken from the submandibular vein, or splenocytes were stained with FITC-anti-MHC class II mAb, PerCP Cy5.5-anti-CD8a mAb, PE-conjugated tetramers, APC-anti-CD45.2 mAb or Thy1.1 mAb were also included to differentiate endogenous CTL responses from the ACTs. Fluorescence was measured using an LSR II flowcytometer (BD Biosciences) and analyzed using FlowJo software (Ashland, OR, USA). Results are presented as % antigen-specific CTLs in blood or spleen or total CTL numbers per spleen.
Evaluation of in vivo antigen presentation after vaccination
OT-1 mice were vaccinated with pam-Trp1/TriVax through the intravenous, intramuscular, or subcutaneous route. 24 h later, lymph nodes (inguinal, mesenteric, axillary) and spleens were harvested, and cells from these organs were used as antigen-presenting cells in the EliSpot assay. TnTR1 cells, previously preactivated with CD3/CD28 beads (Thermo Fisher) and human IL-2 (50 IU/ml, PeproTech) for 1 week, were used as effector CTLs in the EliSpot assays. IFN-γ EliSpot assays were performed as described [22].
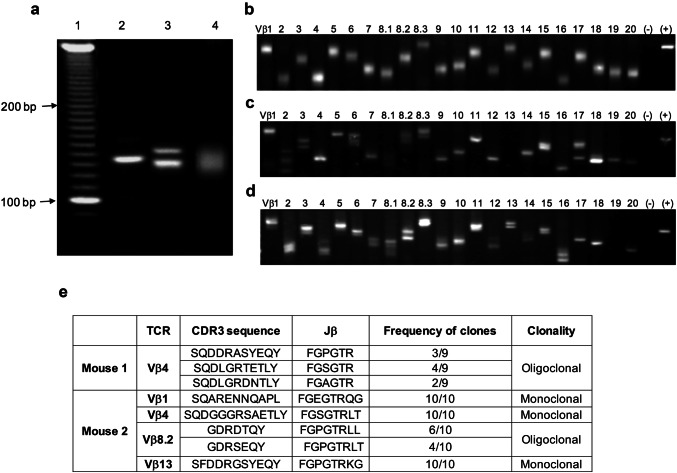
Clonality of antigen-specific CTLs
TCR-Vβ repertoire analysis was examined using a TCRExpress™ clonality detecting kit (BioMed ImmunoTech, Alachua, FL, USA). Briefly, cDNA was prepared from flowcytometer-sorted CD8 T cells from naïve WT-B6 mice or Trp1455 tetramer+ CTLs from mice that received pam-Trp1 TriVax (7 days before the analysis). The primary PCR amplification was performed using Vβ chain primer pre-coated plates, followed by nested PCR amplification using Vβ chain primer pre-coated plates. The PCR products were separated by electrophoresis on high-resolution 4% agarose gels and detected with ethidium bromide. In some instances, PCR products were purified from gel bands and sequenced by PCR.
Identification of the precursor frequency of antigen-specific CD8 T-cell numbers
The numbers of precursor antigen-specific CD8 T cells were enumerated by counting Trp1455–463 tetramer+ CD8 T cells in the spleens and lymph nodes (inguinal, mesenteric, and axillary) of WT-B6 and Trp1-KO mice. The accuracy of this method was confirmed by mixing pmel-1 CD8 T cells and TnTR1 CD8 T cells in different ratios (1:0.3–1:0, Supplemental Fig. 3). The percentage of Trp1455–463 tetramer+ CD8 T cells was reliable only when the percentage was > 0.05%.
Therapeutic tumor model
WT-B6 mice were inoculated s.c. with B16F10 (3 × 105/mouse) and 7 days later mice received 1 × 105 TnTR1 cells i.v. followed by pam-Trp1/TriVax or LP-Trp1/TriVax administered by various routes. A booster immunization was given 12 days later. The tumor growth was monitored every 2–3 days. Results are presented as the mean tumor size (area in mm2) ± SD.
Statistical analyses
All experiments were repeated at least twice to ensure reproducibility. Statistical significance to assess numbers of antigen-specific CD8 T cells and surface markers’ expression were performed using Student’s t tests or one-way ANOVA as appropriate. Results are presented as mean ± SD (*< 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, and ns = not significant). For the therapeutic tumor model, a two-way ANOVA was used to evaluate significance between treatment groups. Statistical analyses were performed using GraphPad Prism (v7).
Results
The impact of route of administration of the vaccine on T-cell responses
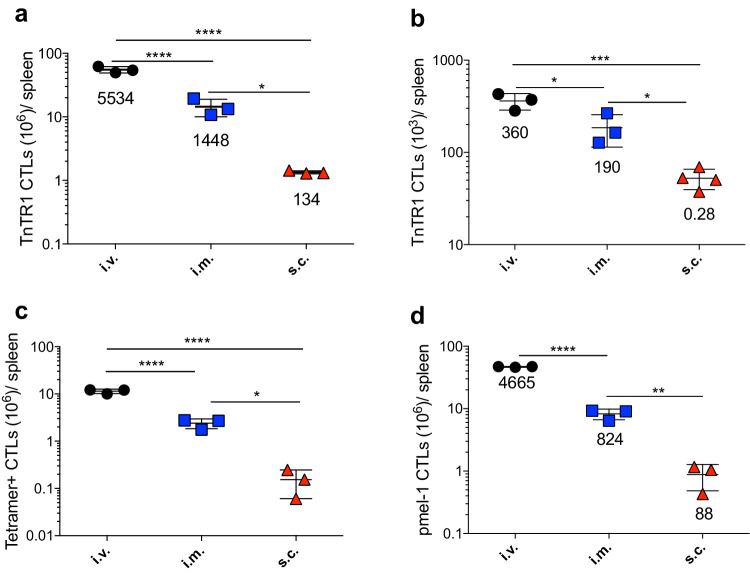
In previous studies, we reported that peptide vaccines administered with poly-ICLC adjuvant (TLR3 and MDA5 agonist) in a prime-boost protocol-induced vast CD8 T-cell responses that lead to significant antitumor effects in mice [17, 25–27]. Amphiphilic peptides such as those conjugated with palmitic acid chains (pam peptides), which self-assemble into nanoparticles, were more immunogenic than minimal peptide epitopes or long peptides (LPs) [17]. In these studies, there was an indication that the vaccines injected intravenously (i.v.) elicited stronger CTL responses as compared to the same vaccines administered via the subcutaneous (s.c.) or intramuscular (i.m.) routes. However, the mechanism by which the i.v. administered vaccines elicited enhanced antitumor effects and induced larger CTL responses remained unclear. We hypothesized that i.v. immunizations disseminate the peptide throughout the lymphoid organs allowing the recruitment of the majority of low numbers of antigen-reactive CTL precursors into the response and that expansion into large CTL numbers would be required for an effective antitumor effect. To ensure that the precursor frequency of antigen-specific cells was similar among different animals, we used adoptive transfers of low numbers (10,000 cells) of naive antigen-specific CTLs from TCR-transnuclear TnTR1 mice specific for the Trp1455–463 epitope [23] into WT-B6 mice followed by i.v., i.m., or s.c. immunizations with an amphiphilic (palmitoylated) Trp1 peptide (pam-Trp1) in combination with poly-ICLC and costimulatory anti-CD40 mAb (pam-Trp1/TriVax). Seven days after a single vaccination, the total numbers of TnTR1 CTLs were quantitated in spleens. Intravenous vaccinations were clearly more efficient in expanding antigen-specific CTLs as compared to s.c. vaccines (Fig. 1a). Although i.m. immunizations were less efficient (~ threefold) than i.v. injections, these were still tenfold better than s.c. vaccinations. The expansion of the adoptively transferred TnTR1 CTLs required peptide antigen, because poly-ICLC and αCD40 alone failed to expand the cells (Supplemental Fig. 1). Furthermore, using pam-Trp1/BiVax (consisting solely of pam-Trp1 and poly-ICLC), the i.v. and i.m. routes elicited a 360- and 190-fold CTL expansion, respectively, while the s.c. vaccines failed to expand the TnTR1 CTLs (Fig. 1b). To compare the impact of route of vaccine administration in expanding endogenous Trp1455–463-specific CTLs, WT-B6 mice were vaccinated with one single dose of pam-Trp1/TriVax administered either i.v., i.m., or s.c. Similarly, the i.v. and i.m. administered vaccines were more effective than the s.c. vaccine at inducing and expanding the CTL response (Fig. 1c). Similar findings were obtained when comparing the three modes of vaccine administration using CTLs from pmel-1 TCR transgenic mice specific for the melanoma gp10025–33 epitope using pam-gp100/TriVax immunizations (Fig. 1d). Notably, the expression of the exhaustion markers PD-1, KLRG1, and LAG3 in the pmel-1 CTLs from mice vaccinated s.c. was higher as compared to the CTLs from mice vaccinated i.v. or i.m. (Supplemental Fig. 2a).
Fig. 1.
Systemic vaccination is superior to subcutaneous immunization. a Congenic CD45.1 WT-B6 mice received 10,000 naïve TnTR1 CTLs followed by vaccination with pam-Trp1/TriVax administered i.v., i.m., or s.c. and numbers of TnTR1 cells (CD45.2+) in spleens were measured 7 days after the vaccination. b Similar experiment to (a) except mice was vaccinated with pam-Trp1/BiVax. c WT-B6 mice were vaccinated with pam-Trp1/TriVax administered i.v., i.m., or s.c. and numbers of endogenous Trp1 specific CTLs in spleens were measured 7 days after the vaccination. d WT-B6 mice (Thy1.2) received 10,000 naïve pmel-1 CTLs (Thy.1.1) followed by vaccination with pam-hgp100/TriVax administered i.v., i.m. or s.c. and numbers of pmel-1 cells (Thy1.1+) in spleens were measured 7 days after the vaccination. Results in (b) are presented as individual mice (each symbol) with the mean ± SD for each group. Numbers below each group represent the average CTL expansion (total numbers of CTL in spleen/number of CTLs adoptively transferred). These experiments were repeated 2–3 times with similar results
Not all long peptides are similarly immunogenic
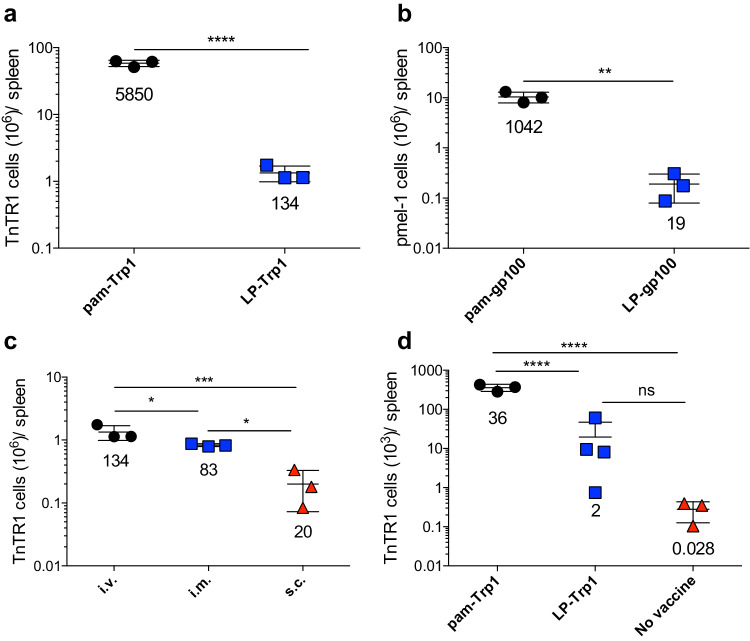
It has been reported that vaccines using LPs are more effective than vaccines using the minimal peptide epitopes, because they force antigen presentation to T cells by professional antigen-presenting cells such as dendritic cells, preventing the induction of T-cell tolerance or anergy [11]. In this respect, the pam peptides used in our studies behave like LPs, but their amphiphilic nature allows them to self-associate into nanoparticles, which could potentially affect their immunogenicity. Indeed, both pam-Trp1 and pam-gp100 peptides were considerably more efficient as compared to the corresponding LPs in promoting the expansion of TnTR1 and pmel-1 CTLs after i.v. TriVax immunization (Fig. 2a, b). On a cell per cell basis, no major phenotype and functional differences were observed between the CTLs derived from pam peptide or LP immunizations (Supplemental Fig. 2b, c). LP vaccines have been traditionally administered via the s.c. route [11, 13, 15]; thus, we compared the three modes of vaccine administration using LP-Trp1 for their ability to expand adoptively transferred TnTR1 CTLs. As with pam peptides, the i.v. and i.m. administrations of LP-Trp1/TriVax were significantly more effective as compared to the s.c. vaccines (Fig. 2c). Moreover, when comparing pam-Trp1 and LP-Trp1 i.v. vaccines using BiVax, pam-Trp1 was approximately 18-fold more effective than LP-Trp1 at inducing CTL expansion (Fig. 2d). So far, these results indicate that i.v. and i.m. vaccinations using amphiphilic pam peptides or LPs are more effective as compared to the traditionally used s.c. route of vaccine administration.
Fig. 2.
Amphiphilic peptides are more immunogenic than long peptides. a Congenic CD45.1 WT-B6 mice received 10,000 naïve TnTR1 CTLs followed by vaccination with pam-Trp1/TriVax or LP-Trp1/TriVax and numbers of TnTR1 cells (CD45.2+) in spleens were measured 7 days after the vaccination. b WT mice received 10,000 naïve pmel-1 cells followed by vaccination with pam-hgp100/TriVax or LP-hgp100 TriVax and numbers of pmel-1 cells (Thy1.1+) in spleens were measured 7 days after the vaccination. c CD45.1 WT-B6 mice received 10,000 naïve TnTR1 CTLs followed by vaccination with LP-Trp1/TriVax administered i.v., i.m., or s.c. and numbers of TnTR1 cells (CD45.2+) in spleens were measured 7 days after the vaccination. d CD45.1 WT-B6 mice received 10,000 naïve TnTR1 CTLs followed pam-Trp1/BiVax or LP-Trp1/BiVax and numbers of TnTR1 cells (CD45.2+) in spleens were measured 7 days after the vaccination. Results are presented as individual mice (each symbol) with the mean ± SD for each group. Numbers below each group represent the average CTL expansion. These experiments were repeated 2–3 times with similar results
Systemic vaccination disperses antigen throughout the immune system
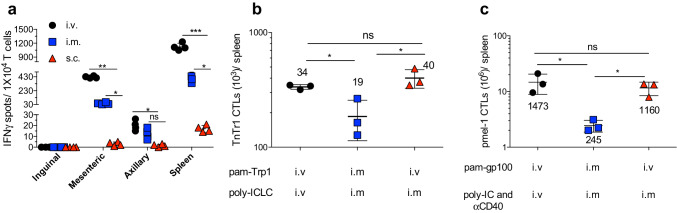
The ability of systemic immunization to expand CTL responses could be explained by its capacity to deliver the antigen to large numbers of antigen-presenting cells (APCs) allowing the recruitment of a maximum number of naïve T cells scattered throughout the lymphoid system. To test the ability of systemic immunization to disseminate the antigen throughout the body, APCs isolated from spleens and different lymph nodes from mice injected (i.v., i.m. or s.c.) with pam-Trp1/TriVax were used to stimulate TnTR1 cells in vitro using an EliSpot assay. Pam-Trp1/TriVax given i.v. or i.m. was more efficient than s.c. vaccination in delivering antigen to APCs in spleen and some of the lymph nodes tested, with the exception of inguinal lymph nodes (Fig. 3a). When the pam peptide and poly-ICLC were administered separately using pam-Trp1/BiVax either via i.v. or i.m. routes, it became apparent that while i.v. peptide administration was more efficient than i.m. injection for promoting CTL expansion, the adjuvant was equally effective when administered i.v. or i.m. (Fig. 3b). Similar findings were observed using pam-gp100/TriVax (Fig. 3c).
Fig. 3.
Systemic vaccine induces APC-T-cell interactions at various sites. a OT-I mice were vaccinated with pam-Trp1/TriVax administered i.v., i.m., or s.c. and 24 h later, the cells from vaccinated mice were collected (inguinal, mesenteric, and axillary lymph nodes, and spleen) and cocultured with TnTR1 CTLs. The reactivity to the Trp1 epitope was examined using an IFN-γ ELISPOT assay. b CD45.1 WT-B6 mice received 10,000 naïve TnTR1 CTLs followed by pam-Trp1 peptide and poly-ICLC administered i.v. or i.m. as shown and numbers of TnTR1 cells (CD45.2+) in spleens were measured 7 days after the vaccination. c WT-B6 mice received 10,000 naïve pmel-1 cells followed by pam-gp100 peptide, poly-ICLC/antiCD40 administered i.v. or i.m. as shown and numbers of pmel-1 CTLs (Thy1.1+) in spleens were measured 7 days after the vaccination. Results are presented as individual mice (each symbol) with the mean ± SD for each group. Numbers below or above each group represent the average CTL expansion. These experiments were repeated 2–3 times with similar results
Intravenous vaccination effectively recruits low number of antigen-specific CTLs
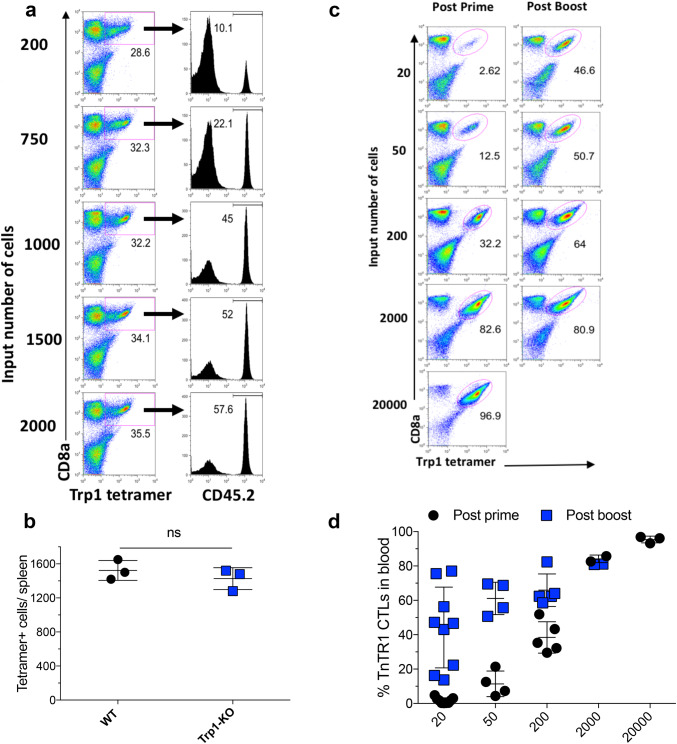
In most instances, the naïve precursor frequency of tumor antigen-specific CTLs is very low [28]. In view of this, an efficient vaccination strategy should stimulate and expand the majority of these cells. Thus, we assume that systemic vaccination should be capable of recruiting most antigen-specific naïve T cells into the immune response. To test this hypothesis, we initially estimated the precursor frequency of Trp1455–463 reactive CTLs in WT-B6 mice using two different methods. First, we evaluated the ability of different numbers of adoptively transferred TnTR1 cells to generate a response of a similar magnitude to the endogenous CTL response to pam-Trp1/TriVax in the same host. The assumption was that when ~ 50% of the CTLs expanded by TriVax were derived from the TnTR1 cells and ~ 50% from the endogenous repertoire, the number of naïve TnTr1 cells injected into the mice would be close to the number of naïve Trp1455–463-reactive CTLs in the host. Using this approach, we estimated that the precursor frequency of naïve Trp1455–463-reactive CTLs in WT-B6 mice is between 1000 and 1500 cells per mouse (Fig. 4a). It is possible that the endogenous and adoptively transferred anti-Trp1 CTLs proliferate at the same rate. In this case, the endogenous precursors would display a diverse TCR repertoire, with various affinities for the antigen. To validate these findings, we measured the number of Trp1455–463 tetramer-binding CD8 T cells in naïve WT-B6 mice and Trp1-knockout (Trp1-KO) mice. The sensitivity of the tetramer analysis was tested by mixing various numbers of TnTR1 CTLs with purified CD8 T cells from pmel-1 mice, where we estimated that it was possible to accurately detect a low frequency of Trp1455–463 tetramer positive cells between 0.1 and 0.03% of the total CD8 cells (Supplemental Fig. 3a, b). Using this method, we assessed that the spleens of naive WT-B6 and Trp1-KO mice contain approximately 1500 Trp1455–463-specific CD8 T cells (Fig. 4b). Overall, these results indicate that a single dose of pam-Trp1/TriVax administered i.v. is sufficiently effective to stimulate and expand ~ 1500 cells to ~ 10 × 106 cells (~ 6600-fold) in a 7-day period, assuming that all the naïve T-cell precursors were stimulated. This level of expansion is similar to the results obtained with adoptive transfers of TnTR1 CTLs (Figs. 1a, 2a). This result together with the finding that the precursor frequency between WT-B6 and Trp1-KO mice was not different indicates that there is no immune tolerance to this particular CD8 T-cell epitope derived from a melanosomal protein in B6 mice.
Fig. 4.
Intravenous vaccination is able to recruit low numbers of antigen-specific CTL precursors. a CD45.1 WT-B6 mice received 200–2000 naïve TnTR1 CTLs followed by vaccination with pam-Trp1/TriVax administered i.v and percentages of TnTR1 cells (CD45.2) and endogenous responses (CD45.1) were assessed in the Trp1 tetramer + populations. b Number of Trp1 tetramer+ CTL precursors in naïve WT-B6 and Trp1-KO splenocytes. c, d OT-I mice received 20–20,000 TnTR1 naïve CTLs followed by vaccination with 2 doses of pam-Trp1/TriVax (administered 21 days apart) injected i.v. and percentages of TnTR1 cells in blood were measured 7 days after each vaccination. Results are presented as individual mice (each symbol) with the mean ± SD for each group. Numbers below the tetramer + gates represent the percentage positive CD8 T cells. These experiments were repeated 2–3 times with similar results
To further evaluate the ability of the i.v. route of immunization using pam-Trp1/TriVax to recruit low numbers of CTLs, we performed adoptive transfers of various numbers of TnTR1 CTLs into TCR transgenic OT-I mice to avoid competing endogenous CTL responses to Trp1455–463 and the effects of homeostatic proliferation, which would occur if the experiment was performed in RAG deficient mice. As shown in Fig. 4c, d, the i.v. vaccine administered in a prime-boost protocol was able to generate substantial CTL responses in mice that received as few as 20 TnTR1 cells. Altogether, these findings demonstrate that peptide vaccines administered via the unconventional i.v. route are more effective in generating substantial CTL responses perhaps, because these are able to recruit larger numbers of precursor CTLs into the response as compared to the conventional s.c. route of administration. In addition, our results indicate that pam peptides are markedly more efficient in eliciting these responses as compared to the more commonly used LPs, even in circumstances when both are administered via the i.v. route.
The large endogenous CTL response obtained with pam-Trp1/TriVax could be explained by two different mechanisms: one potential scenario is that the vaccine efficiently stimulates and expands a small proportion of the pool of naïve CTL precursors (e.g., 5% of the 1500 cells), which would generate an oligoclonal response. The second scenario is that a large proportion of the CTL precursors are effectively recruited into a polyclonal response. To distinguish between these two possibilities, we performed TCR clonality assays on flow cytometry sorted tetramer positive CTLs from pam-Trp1/TriVax immunized mice. This high-resolution gel electrophoresis assay (Fig. 5a) based on PCR products identifies 22 different TCR-Vβ family members and can also differentiate lengths of the CDR3-Jβ region, making it possible to assess whether more than one clone exists for each TCR-Vβ type (Fig. 5a, b). A large diversity of TCR-Vβ chain usage was clearly evident within the Trp1 tetramer positive population, where most of the TCR-Vβ families were found to be represented in the pool of antigen-specific CTLs (Fig. 5c, d). Moreover, in the majority of the TCR-Vβ family members, more than one clonotype was present. In addition, when single bands were analyzed for CDR3 and Jβ sequences, further TCR diversity was observed (Fig. 5e). Thus, these results indicate that the i.v. vaccine generates a highly polyclonal CTL response resulting from the stimulation of a large number of the naïve CTL precursor repertoire.
Fig. 5.
Systemic immunization induces large diversity of polyclonal antigen-specific T cells. a Example showing the capacity of the high-resolution agarose gels to distinguish different sizes of oligonucleotides. b TCR-Vβ chain usage of CD8 spleen T cells from WT-B6 mice (note diffuse bands). c WT-B6 mice received pam-Trp1/TriVax and 7 days later Trp1 tetramer + spleen CTLs were sorted and TCR-Vβ chain usage was examined. d WT-B6 mice received pam-Trp1/TriVax and TCR-Vβ chain usage was examined 15 months later as in (c). e CDR3 and Jβ gene sequences from PCR products of gel bands excised from 2 mice immunized as described in (c). Results presented in (b–d) are from individual mice, representative of 4 mice in each treatment group
The route of vaccine administration determines the magnitude of antitumor effects
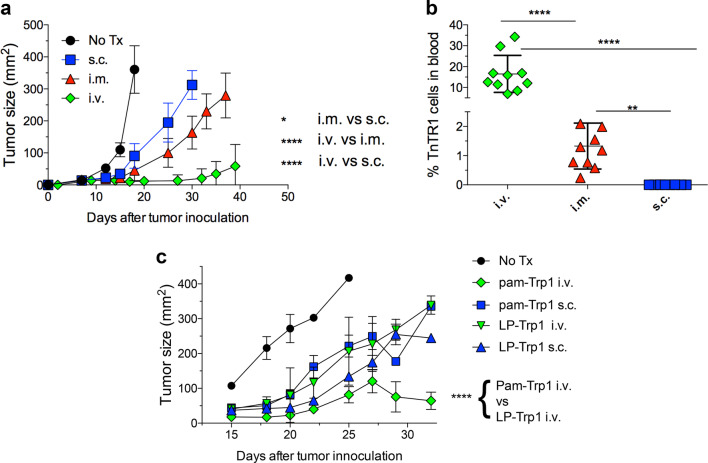
Next, we evaluated the impact of route of vaccine administration on the therapeutic antitumor effects of peptide-based vaccines. WT-B6 mice bearing 7-day established B16 melanomas were adoptively transferred with naive TnTR1 CTLs followed by 2 doses of Pam-Trp1/TriVax (14 days apart). Although s.c immunization was able to slightly slow the rate of tumor growth, the antitumor effect of i.v. vaccination was significantly more effective (Fig. 6a). The i.m. administered vaccine elicited an intermediate antitumor effect. The large difference in the antitumor effects between different routes of immunization correlated with the magnitude of the CTL responses generated in the tumor-bearing mice by the vaccines (Fig. 6b). Finally, we compared the antitumor efficacy of Pam-Trp1/TriVax with LP-Trp1/TriVax administered either i.v. or s.c. While both pam-Trp1 and LP-Trp1 had similar antitumor efficacies when administered s.c., the i.v. pam-Trp1 peptide exhibited a significantly higher antitumor effect as compared to the i.v. LP-Trp1 vaccine (Fig. 6c).
Fig. 6.
Route of vaccine administration dictates the antitumor effects of peptide vaccines. CD45.1 WT-B6 mice were inoculated s.c. with 3 × 105 B16F10 melanoma cells and 7 days later, when tumors were measurable the mice received 10,000 TnTR1 CTLs followed by 2 doses of pam-Trp1/TriVax administered i.v, i.m or s.c. a Mean tumor sizes in each group (n = 10 mice). b Percentages of TnTR1 CTLs in blood from (a) at day 25. c Similar experiment as in (a), but comparing the antitumor effects of pam-Trp1/TriVax and LP-Trp1/TriVax administered either i.v. or s.c. (n = 10 mice). Results in (b) are presented as individual mice (each symbol) with the mean ± SD for each group. These experiments were repeated 2–3 times with similar results
Collectively, the overall results show that the systemic administration of palmitoylated peptide-based vaccines is essential to recruit, activate, and expand low number of antigen-specific cells and exert maximal antitumor effects.
Discussion
The low effectiveness of therapeutic vaccines using conventional tumor-associated antigens (TAAs) in their ability to elicit substantial CTL responses and generate clinical responses has been attributed in great part to potential immune tolerance, since these antigens are also expressed by normal tissues (e.g., melanosomal antigens such as Trp1 and gp100). One strategy to overcome tolerance has been to utilize subdominant CTL epitopes by focusing on intermediate MHC-binding peptides, which contain a non-canonical anchor residue and modify the epitope sequence to generate a heteroclitic immunizing peptide. For example, the HLA-A2 CTL epitopes gp100209, ITDQVPFSV and gp100280, YLEPGPVYA were modified at the suboptimal MHC-binding anchor to make the heteroclitic variants gp100209/2M, IMDQVPFSV and gp100280/9V, YLEPGPVYV, which were used to immunize melanoma patients, where some levels of immune responses were detected [29]. In the present studies, we modified the sequences of two subdominant H-2Db CTL epitopes, Trp1455, TAPDNLGYA and gp10025, EGSRNDQWL to produce the heteroclitic epitopes Trp1455/9M, TAPDNLGYM and gp10025/1K/2V3P, KVPRNDQWL to produce the peptide vaccines. In spite of heteroclitic peptides being more immunogenic than the corresponding natural sequences, clinical antitumor responses in vaccine trials have been in general suboptimal, raising doubts that tolerance can be circumvented with the use of the modified peptide vaccines. However, the results presented here and previous work by our group demonstrate that peptide vaccination using heteroclitic melanoma epitopes is effective in treating mice with established tumors, but to obtain optimal therapeutic effects (i.e., rejections), vaccination must be followed with either PD-1 blockade or sustained IL-2 administration [17, 30]. Furthermore, no significant differences in the Trp1455 CTL precursor frequencies between WT-B6 and Trp1-KO mice were observed (Fig. 4b), indicating that the precursor frequency of these CTLs is not reduced in mice expressing the Trp1 antigen. In addition, the present results indicate that the therapeutic effect of the vaccine requires a vast CTL response, which was achieved with systemic immunizations using amphiphilic peptides.
Recently, the use of neoantigen cancer vaccines has been proposed as an alternative strategy to the use of TAAs to circumvent the potential immune tolerance and improve antitumor efficacy [13, 15, 31]. Although initial clinical studies with LP vaccines showed promising results preventing recurrences in melanoma patients after surgical resections, the magnitude of the immune responses was not substantial, requiring in vitro antigen stimulation of the blood lymphocytes to detect T-cell activity [15]. Thus, the potential clinical benefit of these vaccines in more advance disease stages would be questionable based on our results indicating that the antitumor effects correlate with the magnitude of the CTL response (Fig. 6). In these studies, LP mixtures containing predicted CD8 epitopes were administered with poly-ICLC via the s.c. route. Based on the results presented here in mice, we predict that systemic (i.v.) vaccination using pam-peptide constructs would generate far greater CTL responses that could be effective even in patients with evidence of disease. We are cognizant of potential safety concerns regarding the i.v. administration of antigenic peptides and poly-ICLC, which could deter the implementation of this vaccination strategy in the clinic. There are reports of LP vaccines inducing IgG-mediated anaphylactic reactions in mice when repetitively administered in solution, which was avoided by IFA emulsification [32]. However, by design, these LPs contained strong CD4 T-helper cell epitopes resulting in the induction of robust anti-peptide IgG antibody responses. Thus, one should ensure that any i.v. administered peptide in combination with strong adjuvants such as poly-ICLC should lack T-helper epitopes to avoid this complication. There are also concerns with respect to the i.v. injections of poly-ICLC due to reported toxic reactions especially when administered at high doses, which are diminished when the drug is injected i.m [33–36]. As we have shown here, CTL responses induced by pam peptide i.v. immunization were similar when poly-ICLC was administered i.v. or i.m., indicating that antigen and adjuvant can be administered separately and that one can decrease potential toxicity of i.v. poly-ICLC injections.
Collectively, the present results show that the route of vaccine administration and the amphiphilic nature of the synthetic peptide bear huge impact on the immunogenicity and the antitumor effects of peptide-based vaccines in mice. We hope that these findings will be considered by clinical researchers when designing vaccine trials in human cancer patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- ACT
Adoptive cell transfer
- APC
Antigen-presenting cell
- CTL
Cytotoxic T lymphocyte
- ICIs
Immune checkpoint inhibitors
- IFA
Incomplete Freund’s adjuvant
- KO
Knockout
- LP
Long peptide
- mAb
Monoclonal antibody
- MDA5
Melanoma differentiation-associated protein 5
- MHC-I
MHC class I
- MHC-II
MHC class II
- pam
Palmitoylated
- poly-IC
Polyinosinic–polycytidylic acid
- poly-ICLC
Poly-IC stabilized with poly-lysine and carboxymethyl cellulose
- TAA
Tumor-associated antigen
- TCR
T-cell receptor for antigen
- TLR3
Toll-like receptor 3
- Trp1
Tyrosinase-related protein 1
- WT
Wild type
Author contributions
HS, TK, TN, and JW performed research and analyzed the data. EC designed, supervised, and analyzed the experiments and wrote the manuscript. AMS provided reagents and discussed results.
Funding
This work was supported by National Cancer Institute Grant R01CA157303 and by start-up funds from Augusta University, Georgia Cancer Center and the Georgia Research Alliance (GRA).
Compliance with ethical standards
Conflict of interest
Andres M. Salazar is President and CEO of Oncovir, Inc. and is developing poly-ICLC (Hiltonol ™) for the clinic. Esteban Celis is a consultant for Oncovir, Inc. and has filed patent applications based on the use of synthetic peptides and poly-IC combinatorial vaccines. The rights of the patent applications have been transferred to the Moffitt Cancer Center (Tampa, FL). Other authors declare no conflict of interest.
Animal sources and statement on the welfare of animals
C57BL/6 (WT-B6) and B6-Ly5.1 (CD45.1) mice were purchased from the National Cancer Institute (Wilmington, MA). TnTR1 TCR-transnuclear, pmel-1 and Trp1-KO mice were bred at the Georgia Cancer Center animal facility. All procedures performed in the experiments involving animals were in accordance with the ethical standards of the Augusta University Institutional Animal Care and Use Committee, where all the studies were conducted (Protocol No. 2013-0598, approved on 11/21/2016).
Cell authentication
The B16F10 murine melanoma cell line was obtained from the American Type Culture Collection.
Footnotes
Hussein Sultan, Takumi Kumai, and Toshihiro Nagato contributed equally to the work.
References
- 1.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 2.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 3.Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro G, Jr, Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 4.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 5.Melero I, Rouzaut A, Motz GT, Coukos G. T-cell and NK-cell infiltration into solid tumors: a key limiting factor for efficacious cancer immunotherapy. Cancer Discov. 2014;4:522–526. doi: 10.1158/2159-8290.CD-13-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coulie PG, Karanikas V, Lurquin C, Colau D, Connerotte T, Hanagiri T, Van Pel A, Lucas S, Godelaine D, Lonchay C, Marchand M, Van Baren N, Boon T. Cytolytic T-cell responses of cancer patients vaccinated with a MAGE antigen. Immunol Rev. 2002;188:33–42. doi: 10.1034/j.1600-065X.2002.18804.x. [DOI] [PubMed] [Google Scholar]
- 9.Gnjatic S, Nishikawa H, Jungbluth AA, Gure AO, Ritter G, Jager E, Knuth A, Chen YT, Old LJ. NY-ESO-1: review of an immunogenic tumor antigen. Adv Cancer Res. 2006;95:1–30. doi: 10.1016/S0065-230X(06)95001-5. [DOI] [PubMed] [Google Scholar]
- 10.Melief CJ, van Hall T, Arens R, Ossendorp F, van der Burg SH. Therapeutic cancer vaccines. J Clin Invest. 2015;125:3401–3412. doi: 10.1172/JCI80009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melief CJ, van der Burg SH. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nat Rev Cancer. 2008;8:351–360. doi: 10.1038/nrc2373. [DOI] [PubMed] [Google Scholar]
- 12.Matsushita H, Vesely MD, Koboldt DC, Rickert CG, Uppaluri R, Magrini VJ, Arthur CD, White JM, Chen YS, Shea LK, Hundal J, Wendl MC, Demeter R, Wylie T, Allison JP, Smyth MJ, Old LJ, Mardis ER, Schreiber RD. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature. 2012;482:400–404. doi: 10.1038/nature10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, Ivanova Y, Hundal J, Arthur CD, Krebber WJ, Mulder GE, Toebes M, Vesely MD, Lam SS, Korman AJ, Allison JP, Freeman GJ, Sharpe AH, Pearce EL, Schumacher TN, Aebersold R, Rammensee HG, Melief CJ, Mardis ER, Gillanders WE, Artyomov MN, Schreiber RD. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tran E, Robbins PF, Rosenberg SA. ‘Final common pathway’ of human cancer immunotherapy: targeting random somatic mutations. Nat Immunol. 2017;18:255–262. doi: 10.1038/ni.3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, Zhang W, Luoma A, Giobbie-Hurder A, Peter L, Chen C, Olive O, Carter TA, Li S, Lieb DJ, Eisenhaure T, Gjini E, Stevens J, Lane WJ, Javeri I, Nellaiappan K, Salazar AM, Daley H, Seaman M, Buchbinder EI, Yoon CH, Harden M, Lennon N, Gabriel S, Rodig SJ, Barouch DH, Aster JC, Getz G, Wucherpfennig K, Neuberg D, Ritz J, Lander ES, Fritsch EF, Hacohen N, Wu CJ. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017 doi: 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hailemichael Y, Dai Z, Jaffarzad N, Ye Y, Medina MA, Huang XF, Dorta-Estremera SM, Greeley NR, Nitti G, Peng W, Liu C, Lou Y, Wang Z, Ma W, Rabinovich B, Sowell RT, Schluns KS, Davis RE, Hwu P, Overwijk WW. Persistent antigen at vaccination sites induces tumor-specific CD8(+) T cell sequestration, dysfunction and deletion. Nat Med. 2013;19:465–472. doi: 10.1038/nm.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho HI, Barrios K, Lee YR, Linowski AK, Celis E. BiVax: a peptide/poly-IC subunit vaccine that mimics an acute infection elicits vast and effective anti-tumor CD8 T-cell responses. Cancer Immunol Immunother. 2013;62:787–799. doi: 10.1007/s00262-012-1382-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slingluff CL., Jr The present and future of peptide vaccines for cancer: single or multiple, long or short, alone or in combination? Cancer J. 2011;17:343–350. doi: 10.1097/PPO.0b013e318233e5b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho HI, Celis E. Optimized peptide vaccines eliciting extensive CD8 T-cell responses with therapeutic antitumor effects. Cancer Res. 2009;69:9012–9019. doi: 10.1158/0008-5472.CAN-09-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Llopiz D, Dotor J, Zabaleta A, Lasarte JJ, Prieto J, Borras-Cuesta F, Sarobe P. Combined immunization with adjuvant molecules poly(I:C) and anti-CD40 plus a tumor antigen has potent prophylactic and therapeutic antitumor effects. Cancer Immunol Immunother. 2008;57:19–29. doi: 10.1007/s00262-007-0346-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park H, Adamson L, Ha T, Mullen K, Hagen SI, Nogueron A, Sylwester AW, Axthelm MK, Legasse A, Piatak M, Jr, Lifson JD, McElrath JM, Picker LJ, Seder RA. Polyinosinic-polycytidylic acid is the most effective TLR adjuvant for SIV Gag protein-induced T cell responses in nonhuman primates. J Immunol. 2013;190:4103–4115. doi: 10.4049/jimmunol.1202958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumai T, Lee S, Cho HI, Sultan H, Kobayashi H, Harabuchi Y, Celis E. Optimization of peptide vaccines to induce robust antitumor CD4 T-cell responses. Cancer Immunol Res. 2017;5:72–83. doi: 10.1158/2326-6066.CIR-16-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dougan SK, Dougan M, Kim J, Turner JA, Ogata S, Cho HI, Jaenisch R, Celis E, Ploegh HL. Transnuclear TRP1-specific CD8 T cells with high or low affinity TCRs show equivalent antitumor activity. Cancer Immunol Res. 2013;1:99–111. doi: 10.1158/2326-6066.CIR-13-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, Dellemijn TA, Antony PA, Spiess PJ, Palmer DC, Heimann DM, Klebanoff CA, Yu Z, Hwang LN, Feigenbaum L, Kruisbeek AM, Rosenberg SA, Restifo NP. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barrios K, Celis E. TriVax-HPV: an improved peptide-based therapeutic vaccination strategy against human papillomavirus-induced cancers. Cancer Immunol Immunother. 2012;61:1307–1317. doi: 10.1007/s00262-012-1259-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sultan H, Fesenkova VI, Addis D, Fan AE, Kumai T, Wu J, Salazar AM, Celis E. Designing therapeutic cancer vaccines by mimicking viral infections. Cancer Immunol Immunother. 2017;66:203–213. doi: 10.1007/s00262-016-1834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sultan H, Wu J, Kumai T, Salazar AM, Celis E. Role of MDA5 and interferon-I in dendritic cells for T cell expansion by anti-tumor peptide vaccines in mice. Cancer Immunol Immunother. 2018 doi: 10.1007/s00262-018-2164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rizzuto GA, Merghoub T, Hirschhorn-Cymerman D, Liu C, Lesokhin AM, Sahawneh D, Zhong H, Panageas KS, Perales MA, Altan-Bonnet G, Wolchok JD, Houghton AN. Self-antigen-specific CD8+ T cell precursor frequency determines the quality of the antitumor immune response. J Exp Med. 2009;206:849–866. doi: 10.1084/jem.20081382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pass HA, Schwarz SL, Wunderlich JR, Rosenberg SA. Immunization of patients with melanoma peptide vaccines: immunologic assessment using the ELISPOT assay. Cancer J Sci Am. 1998;4:316–323. [PubMed] [Google Scholar]
- 30.Sultan H, Kumai T, Fesenkova VI, Fan AE, Wu J, Cho HI, Kobayashi H, Harabuchi Y, Celis E. Sustained persistence of IL2 signaling enhances the antitumor effect of peptide vaccines through T-cell expansion and preventing PD-1 inhibition. Cancer Immunol Res. 2018;6:617–627. doi: 10.1158/2326-6066.CIR-17-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Lower M, Bukur V, Tadmor AD, Luxemburger U, Schrors B, Omokoko T, Vormehr M, Albrecht C, Paruzynski A, Kuhn AN, Buck J, Heesch S, Schreeb KH, Muller F, Ortseifer I, Vogler I, Godehardt E, Attig S, Rae R, Breitkreuz A, Tolliver C, Suchan M, Martic G, Hohberger A, Sorn P, Diekmann J, Ciesla J, Waksmann O, Bruck AK, Witt M, Zillgen M, Rothermel A, Kasemann B, Langer D, Bolte S, Diken M, Kreiter S, Nemecek R, Gebhardt C, Grabbe S, Holler C, Utikal J, Huber C, Loquai C, Tureci O. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547:222–226. doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- 32.Quakkelaar ED, Fransen MF, van Maren WW, Vaneman J, Loof NM, van Heiningen SH, Verbeek JS, Ossendorp F, Melief CJ. IgG-mediated anaphylaxis to a synthetic long peptide vaccine containing a B cell epitope can be avoided by slow-release formulation. J Immunol. 2014;192:5813–5820. doi: 10.4049/jimmunol.1302337. [DOI] [PubMed] [Google Scholar]
- 33.Levine AS, Sivulich M, Wiernik PH, Levy HB. Initial clinical trials in cancer patients of polyriboinosinic-polyribocytidylic acid stabilized with poly-l-lysine, in carboxymethylcellulose [poly(ICLC)], a highly effective interferon inducer. Cancer Res. 1979;39:1645–1650. [PubMed] [Google Scholar]
- 34.Bever CT, Jr, Salazar AM, Neely E, Ferraraccio BE, Rose JW, McFarland HF, Levy HB, McFarlin DE. Preliminary trial of poly ICLC in chronic progressive multiple sclerosis. Neurology. 1986;36:494–498. doi: 10.1212/WNL.36.4.494. [DOI] [PubMed] [Google Scholar]
- 35.Butowski N, Chang SM, Junck L, DeAngelis LM, Abrey L, Fink K, Cloughesy T, Lamborn KR, Salazar AM, Prados MD. A phase II clinical trial of poly-ICLC with radiation for adult patients with newly diagnosed supratentorial glioblastoma: a North American Brain Tumor Consortium (NABTC01-05) J Neurooncol. 2009;91:175–182. doi: 10.1007/s11060-008-9693-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenfeld MR, Chamberlain MC, Grossman SA, Peereboom DM, Lesser GJ, Batchelor TT, Desideri S, Salazar AM, Ye X. A multi-institution phase II study of poly-ICLC and radiotherapy with concurrent and adjuvant temozolomide in adults with newly diagnosed glioblastoma. Neuro Oncol. 2010;12:1071–1077. doi: 10.1093/neuonc/noq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.