Abstract
Optimal ex vivo expansion protocols for adoptive cell therapy (ACT) must yield T cells able to effectively home to tumors and survive the inhospitable conditions of the tumor microenvironment (TME), while simultaneously exerting persistent anti-tumor effector functions. Our previous work has shown that ex vivo activation in the presence of IL-12 can induce optimal expansion of murine CD8+ T cells, thus resulting in significant tumor regression after ACT mostly via sustained secretion of IFN-γ. In this report, we further elucidate the mechanism of this potency, showing that IL-12 additionally counteracts the negative regulatory effects of autocrine IFN-γ. IL-12 not only downregulates PD-1 expression by T cells, thus minimizing the effects of IFN-γ-induced PD-L1 upregulation by tumor stromal cells, but also inhibits IFNγR2 expression, thereby protecting T cells from IFN-γ-induced cell death. Thus, the enhanced anti-tumor activity of CD8+ T cells expanded ex vivo in the presence of IL-12 is due not only to the ability of IL-12-stimulated cells to secrete sustained levels of IFN-γ, but also to the additional capacity of IL-12 to counter the negative regulatory effects of autocrine IFN-γ.
Electronic supplementary material
The online version of this article (10.1007/s00262-018-2280-3) contains supplementary material, which is available to authorized users.
Keywords: Melanoma/skin cancers, Tumor promotion and progression, Models of host–tumor interactions, Tumor microenvironment, Adoptive T cells transfer, PD-1
Introduction
A number of studies suggest that optimally manipulating T cells ex vivo prior to transfer is crucial for maximizing the clinical efficacy of ACT for cancer [1]. This can be achieved by providing the appropriate signals during T cell expansion. TCR engagement and co-stimulation are sufficient to induce robust expansion of T cells, though optimal effector capabilities are only achieved in the presence of inflammatory cytokine signals such as IL-12 and Type I IFN [2–4]. IL-12 stimulation during T cell activation induces the differentiation of effector and memory cells via direct modulation of genes regulating cell cycle, DNA synthesis and repair, protein translation, and metabolism [5–8]. Accordingly, we previously reported that ex vivo activation of tumor-reactive CD8+ T cells in the presence of antigen and IL-12 resulted in enhanced anti-tumor activity after adoptive transfer, which also correlated with superior tumor control and prolonged survival [9–11].
In this setting, the enhanced anti-tumor activity of IL-12-preconditioned CD8+ T cells was associated with sustained levels of intratumoral IFN-γ. However, because IFN-γ regulates CD8+ T cell homeostasis by both contracting activated T cell numbers and by promoting T cell exhaustion via PD-L1 induction on tumor stromal cells [12, 13], we further investigated how these conflicting activities of IL-12-induced IFN-γ expression coordinately regulate anti-tumor responses. While downregulation of PD-1 expression on tumor-infiltrating, adoptively transferred CD8+ T cells expanded ex vivo in the presence of IL-12 was reported previously by Gerner et al. [14], it was identified as a mechanism to circumvent T cell exhaustion mediated specifically by sustained exposure to an exogenous antigen, ovalbumin (OVA). Here we used an endogenous, ubiquitously expressed tumor antigen (Pmel/gp100) to show that PD-1 modulation on CD8+ T cells by IL-12 is not dependent on repeated exposure to antigen, suggesting instead that other inflammatory factors within a TME might play a role.
Our study also describes a second, more direct, mechanism of resistance to IFN-γ mediated by IL-12 that involves protection from apoptosis via the downregulation of the beta chain of the IFN-γ receptor (IFNγR2) on adoptively transferred CD8+ T cells. Like PD-1 inhibition, this response also occurs within the TME and results in enhanced T cell survival and improved anti-tumor activity.
Collectively, these findings suggest that the enhanced anti-tumor activity of CD8+ T cells expanded ex vivo in the presence of IL-12 not only involves the upregulation of effector functions such as IFN-γ secretion, but also the induction of mechanisms that protect them from the autocrine negative regulation induced by IFN-γ. These results support the rational use of IL-12 during the expansion of T cells for ACT, due to both its direct anti-tumor effect and indirect, T-cell protective activities, and provides novel insights into the regulatory roles of IFN-γ during T cell-mediated anti-tumor immune responses.
Materials and methods
Mice
IFNγR1 knockout and Pmel-1 transgenic animals were crossed and bred to produce homozygous IFNγR−/− Pmel animals.
Antibodies and flow cytometry
Human antibodies used were anti-CD8 (clone RPA-T8), anti-IFN-γ (clone B27) from BD Bioscience, and anti-CD279 (PD-1) (clone MIH4) from Thermo Fisher Scientific (Waltham, MA). Mouse antibodies were anti-CD8 (clone 53-6.7) and annexin V from BD Biosciences (San Jose, CA) and anti-CD90.1 (Thy1.1) (clone HIS51), anti-IFN- γ (clone XMG1.2) and anti-CD279 (PD-1) (clone J43) from Thermo Fisher Scientific. Titrated concentrations were used and stained cell samples were examined on a BD Biosciences LSRFortessa flow cytometer using FACSDiva v8.0.1. All analyses were performed using FlowJo 10.4 software (FLOWJO, LLC, Ashland, OR).
Melanoma culture and tumor growth
B16-F10 melanoma cells were cultured in RPMI 1640 containing 10% FBS, 0.1% penicillin/ streptomycin, 0.2% l-glutamine, 0.05% 2-mercaptoethanol, 0.01% sodium pyruvate, 0.1% HEPES, and 0.1% nonessential amino acids. Melanoma tumors were established by subcutaneous (s.c.) injection of 2.0 × 105 B16-F10 cells in the right flank. Tumors were measured using calipers on alternate days. Mice with tumors larger than 2000 mm3 were euthanized. Melanoma lung lesions were established after intravenous (i.v.) injection of 5 × 104 B16-F10 cells. Mice were killed 25 days after tumor cell injection, and lungs were harvested.
Ex vivo CD8+T cell activation and adoptive cell transfer
Cell suspensions from the spleens of Pmel-1 mice were adjusted to 1 × 106 cells/ml in complete RPMI and activated with 1 µg/ml of cognate peptide (KVPRNQDWL) (American Peptide; Sunnyvale, CA). Where indicated, the suspensions were subjected to a 3-day activation with 10 ng/ml IL-12 at the time of priming (source CHO cells, PeproTech, Rocky Hill, NJ). 1 day prior to ACT, wild-type C57BL6 mice bearing 7-day-old B16-F10 tumors were conditioned by a single intraperitoneal (i.p.) injection of 4 mg cyclophosphamide (Baxter Healthcare Corporation; Deerfield, IL). 24 h later, adoptive cell transfer (ACT) was performed by intravenous (i.v.) injection via tail vein of 5 × 106 Pmel cells that had been primed under various conditions. To determine the persistence of circulating donor Pmel-specific T cells, blood samples were stained with mAb against Thy1.1 and CD8, which distinguished the transferred Pmel T cells from the endogenous CD8+Thy1.1− cells upon flow cytometry analysis.
Culture of human tumor-infiltrating T cells (TILs)
Fresh primary melanoma tumors were digested using the Miltenyi Tumor Dissociation Kit, (human) following the manufacturer’s instructions (Miltenyi Biotec, Bergisch Gladbach, Germany). Tumor cell suspensions were adjusted to 1 × 106 cells/ml in complete RPMI containing 10% pretested FBS (Thermo Fisher Scientific), 0.1% gentamicin, 0.2% l-glutamine, 0.01% sodium pyruvate, and 0.1% nonessential amino acids (Life Technologies Thermo Fisher Scientific). The cells were activated at 37 °C with Dynabeads™ Human T-Activator CD3/CD28 (25 µl/ml) (Thermo Fisher Scientific) with or without IL-12 (10 ng/ml) (source CHO cells). Cells were harvested after 72 h and expression of PD-1 or IFN-γ by CD8+ T cells was detected by flow cytometry using FMO (Fluorescence Minus One) controls. The laboratory performing this assay operates under exploratory research principles. We report on the T cell assay conducted in MIATA compliance [15], and the MIATA checklist is included in the “Supplementary” files (http://miataproject.org/).
Cell viability and apoptosis assays
Cells were assayed using LIVE/DEAD® Viability/Cytotoxicity Assay Kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. Briefly, for the fluorescence microplate protocol, 100 µl of cultured cells were placed in a 96-well flat bottom plate and 100 µl of a working solution of 2 µM EthD-1 and 1 µM calcein AM was added to the cells. After 15-min incubation, the pictures were taken on an EVOS FL fluorescence microscope (Life Technologies Thermo Fisher Scientific). For flow cytometry, 1 × 106 cells in 1 ml were stained with 0.5 µl of 50 µM calcein AM and 0.5 µl of 2 µM EthD-1. The cells were acquired on an LRSFortessa (BD Bioscience) and analyzed using FlowJo software (FLOWJO, LLC). Apoptosis was assayed by annexin V staining followed by flow cytometry analysis.
Gene expression analysis
PmelAg+IL-12- and PmelAg-infiltrated tumors were harvested 3 and 9 days after adoptive transfer. Total RNA was isolated using TRI Reagent (Molecular Research Center, Inc., Cincinnati, OH) and cDNA was synthesized from 1 µg of total RNA using M-MLV Reverse Transcriptase (Promega; Madison, WI) per manufacturer’s instructions. Gene expression was analyzed utilizing SYBR Green quantitative PCR technology (qPCR) and measured on a StepOne Plus Real-time PCR System (Life Technologies Thermo Fisher Scientific) in a final reaction volume of 20 µl according to the manufacturer’s instructions. The relative quantification of the target transcripts was normalized to endogenous actin expression and relative changes in gene expression between samples were analyzed using the 2− ddCt method.
Statistical analyses
p values were calculated using Student’s t test and a significant difference among experimental groups was defined as a p value of < 0.05. Cumulative survival was calculated using a Kaplan–Meier curve using Prism (GraphPad, La Jolla, CA).
Results
Induction of tumor regression by PmelAg+IL-12 cells associates with sustained levels of IFN-γ
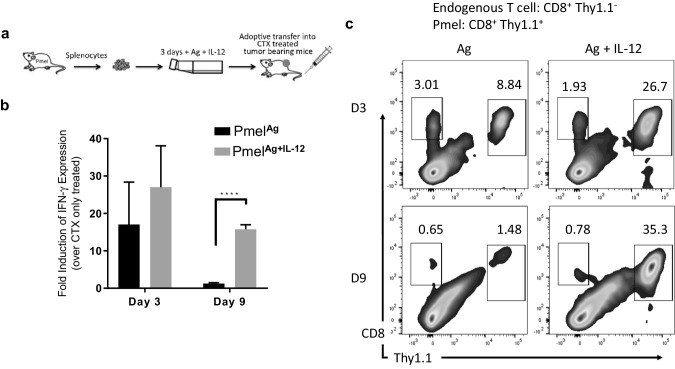
We previously reported that ex vivo expansion of tumor-reactive CD8+ T cells in the presence of IL-12 results in increased in vivo anti-tumor activity following adoptive transfer [9–11]. In our system, CD8+ T cells from Pmel mice—which are transgenic for a TCR that recognizes an epitope within the Pmel/gp100 protein expressed by normal melanocytes and melanoma cells [16]—were activated ex vivo in the presence of cognate peptide with or without IL-12. Expanded Pmel cells were adoptively transferred into transiently lymphopenic mice bearing B16 melanoma tumors (Fig. 1a). To further characterize the impact of these ex vivo expanded T cells on the TME, B16 melanoma tumors were allowed to grow to approximately 7 mm in diameter before treatment took place. Tumors from mice treated with cyclophosphamide (CTX) only, PmelAg, or PmelAg+IL-12 cells were collected at days 3 and 9 after adoptive transfer and analyzed for expression of IFN-γ by qPCR and for frequency of tumor-infiltrating Pmel cells by flow cytometry, based on their expression of Thy1.1. Figure 1b shows that there is a higher level of intratumoral IFN-γ at day 3 post-transfer in all mice that received adoptive T cells, irrespective of whether IL-12 was added to the expansion protocol. However, at day 9 post-transfer, a drastic reduction of intratumoral IFN-γ was observed in mice treated with the PmelAg cells. This decrease in IFN-γ levels correlated with a greater reduction in the frequency of tumor-infiltrating PmelAg cells than intratumoral PmelAg+IL-12 cells at 9 days post-transfer (Fig. 1c). These results suggest the possibility that IL-12 enhances the survival of antigen-activated T cells, thus allowing the sustained release of IFN-γ required for effective tumor regression.
Fig. 1.
Anti-tumor activity of PmelAg+IL-12 cells associates with sustained levels of IFN-γ. a Adoptive cell therapy approach involving the infusion of Pmel cells activated in the presence of antigen with or without IL-12 into a lymphopenic host. This approach mediates the regression of pre-established melanoma lesions which results in improved survival. b Tumors from mice treated with PmelAg or PmelAg+IL-12 were harvested 3 and 9 days after adoptive transfer. Expression of IFN-γ in RNA isolated from tumors was measured using SYBR green quantitative real-time PCR. The relative quantification of the target transcripts normalized to the endogenous control (actin) was determined by the comparative Ct method. Data are presented as average fold change ± SD (n = 3) relative to tumors from control mice that did not receive adoptive transfer. Two-tailed Student’s t test was used, *p < 0.05, **p < 0.005, and ***p < 0.0001. c Tumors were digested to single cell suspensions and analyzed for frequency of infiltrating Pmel cells (Thy1.1+) by flow cytometry. Data shown are representative of at least three independent experiments
IL-12-mediated modulation of PD-1 expression can occur in self-reactive CD8+ T cells
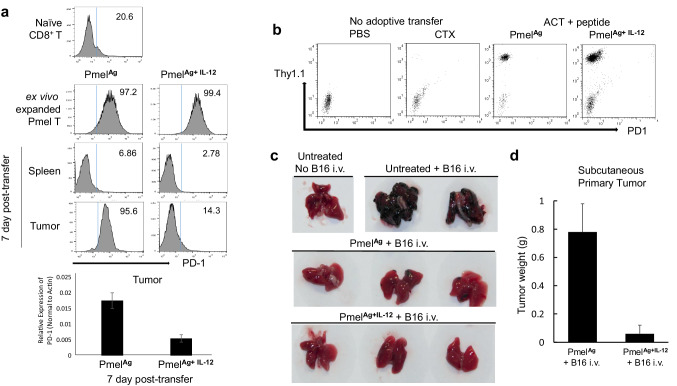
One mechanism by which IFN-γ negatively regulates T cell function is reported to be via the induction of PD-L1 on tumor stromal cells [13, 17]. Thus, the inhibition of PD-1 expression on CD8+ T cells could be a means by which IL-12 maintains T cell activity. Indeed, this notion is supported by earlier observations of lower PD-1 levels on intratumoral CD8+ T cells that were activated in the presence of IL-12 [14], even though the lower PD-1 levels were originally ascribed to the ability of IL-12 to protect T cells from exhaustion following repeated exposure to a transfected, exogenous tumor antigen, ovalbumin. Since physiologically relevant tumor antigens are mostly homologous to endogenous antigens—we sought to determine if IL-12 would also preserve the activity of T cells directed against chronically and ubiquitously expressed endogenous tumor antigen (gp100, also known as Pmel). Consistent with previous reports, naïve Pmel CD8+ T cells did not initially express PD-1, though the receptor was upregulated within 72 h of in vitro stimulation with cognate peptide, even when the stimulation took place in the presence of IL-12 (Fig. 2a, top two rows). Seven days following transfer, however, Pmel cells in the spleens of recipient mice had decreased PD-1 expression levels, regardless of whether the T cells were stimulated in the presence or absence of IL-12 (Fig. 2a, third row). Interestingly, there were strikingly different outcomes for T cells within the tumor microenvironment. Here, Pmel cells that had been expanded ex vivo in the presence of antigen alone showed complete PD-1 upregulation, while those expanded in the presence of both antigen and IL-12 (PmelAg+IL-12) expressed lower amounts of PD-1 (Fig. 2a, bottom row).
Fig. 2.
IL-12 downregulates PD-1 expression on adoptively transferred TILs independently of antigen. a Expression of PD-1 among Pmel cells activated in the presence of antigen plus or minus IL-12 prior to and 7 days after adoptive transfer in cell suspensions from spleen and tumor was determined by flow cytometry. Decreased levels of PD-1 expression among tumor-infiltrating PmelAg+IL-12 was confirmed by quantitative real-time PCR. b Mice bearing B16 melanoma tumors treated with PmelAg, PmelAg+IL-12, or CTX or PBS alone were injected i.p with 50 µg of cognate peptide every other day for 10 days. At day 11, blood was collected and expression of PD-1 was assessed on circulating Pmel (Thy1.1+) cells. c 10 days after transfer, mice bearing B16 melanoma tumors untreated or treated with PmelAg or PmelAg+IL-12 cells were injected i.v. with 5 × 104 B16 melanoma cells. 25 days after transfer mice were killed and lungs excised and examined for the presence of B16 nodules. d At the same time, primary tumors were excised and weighed. Tumor size is presented as the average weight ± SD (n = 3). Data shown are representative of at least three independent experiments
IL-12-mediated modulation of PD-1 expression can occur independently of repeated antigen exposure
Previous work using the OT1 model attributed the minimal expression of PD-1 by circulating transferred CD8+ T cells to their lack of exposure to ubiquitous antigen; the authors contended that in this model peripheral blood CD8+ T-cells are spared from exhaustion because the OVA antigen is highly restricted to the tumor. Arguing against this explanation, however, is the fact that we find PD-1 is also minimally expressed on circulating Pmel cells, in spite of their continuous exposure to antigens ubiquitously expressed by melanocytes in the periphery. To further explore the role of systemic antigen exposure on the expression of PD-1 by T cells, murine recipients of antigen-stimulated Pmel cells were injected i.p. with an additional 50 µg of cognate peptide every other day for 10 days, prior to the circulating Pmel cells being assessed for PD-1 levels 24 h after the last treatment. Figure 2b shows that the levels of PD-1 on circulating Pmel expanded either in the presence or absence of IL-12 remained low despite repeated systemic exposure to antigen. The lack of PD-1 upregulation after repeated antigen stimulation demonstrates that chronic antigen exposure is not the sole factor governing enhanced PD-1 expression by tumor-reactive activated T cells.
Differential expression of PD-1 by circulating or tumor-infiltrating Pmel cells determines their anti-tumor activity
We next determined whether differential PD-1 expression by adoptively transferred intratumoral or circulating Pmel cells is associated with the ability of those lymphocytes to control the spread of tumor cells. Mice already bearing B16 melanoma tumors were adoptively transferred with: saline, PmelAg, or PmelAg+IL-12, and 10 days later the mice were intravenously injected with an additional 5 × 104 B16 melanoma cells. Lungs were harvested 15 days after injection and analyzed for tumor lesions. Figure 2c shows that mice receiving ACT were able to inhibit the development of lung lesions regardless of whether they received PmelAg or PmelAg+IL-12. In other words, the effective peripheral control of B16 spread by circulating PmelAg was comparable to that mediated by circulating PmelAg+IL-12. In contrast, within the tumor microenvironment, only the intratumoral PmelAg+IL-12 cells were able to control progression of primary tumors, and tumors borne by mice injected with PmelAg alone continued growing robustly (Fig. 2d). These results are consistent with the differential expression of PD-1 on Pmel cells in the periphery versus the tumor microenvironment in the absence of IL-12.
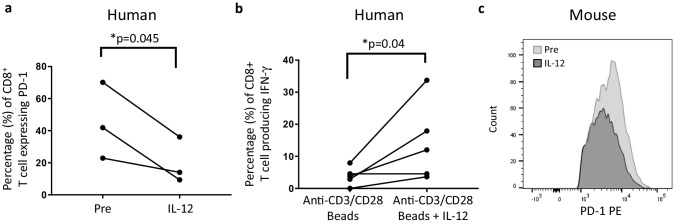
IL-12 downregulates PD-1 expression on CD8+ T cells infiltrating human melanoma tumors resulting in enhanced secretion of IFN-γ
Next, we determined whether IL-12 could directly modulate the expression of PD-1 on human tumor-infiltrating lymphocytes (TILs). To this end, tumor cell suspensions from melanoma patients were treated in vitro with 10 ng/ml of human IL-12 for 72 h, and PD-1 expression by CD8+ T cells was assessed before and after IL-12 treatment. Figure 3a shows that exposure of TILs to IL-12 resulted in the downregulation of PD-1 expression. We then asked whether IL-12 would enhance IFN-γ secretion if provided during anti-CD3/CD28 activation of T cells. Figure 3b shows a significant increase in the percentage of IFN-γ-producing CD8+ T cells when activated with both anti-CD3/CD28-coated beads and IL-12 compared to with the beads alone. Downregulation of PD-1 expression after culture with IL-12 was also observed, though to a lesser extent, in T cells infiltrating a murine B16 tumor (Fig. 3c). These results suggest that IL-12 directly modulates the expression of PD-1 on human TILs, and that such decreases in PD-1 expression could contribute to the restoration of IFN-γ secretion by TILs in response to reduced signaling by PD-1.
Fig. 3.
IL-12 can directly downregulate the expression of PD-1 on human TILs. a Tumor cell suspensions from human melanoma tumors were incubated with 10 ng/ml human IL-12 for 72 h, and the expression of PD-1 among CD8+ T cells was determined by flow cytometry before and after culture. (n = 3). b The same tumor cell suspensions were cultured in the presence of anti-CD3/CD28 beads with or without IL-12, and production of IFN-γ among CD8+ T cells was determined by flow cytometry (n = 5). One-tailed paired Student’s t test was used, *p < 0.05, **p < 0.005, and ***p < 0.0001. c Cell suspensions from a B16 melanoma tumor were incubated with 10 ng/ml murine IL-12 for 72 h, and the expression of PD-1 among CD8+ T cells was determined by flow cytometry
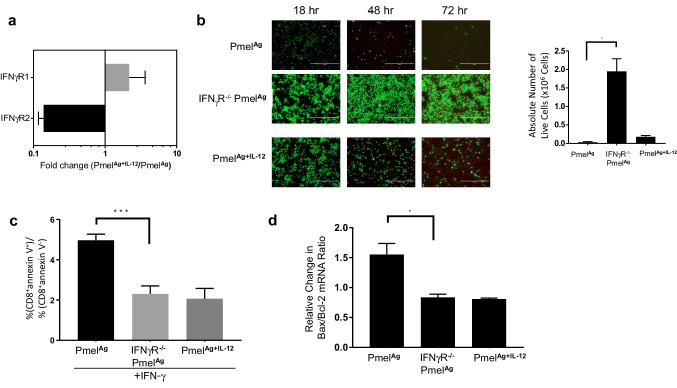
Ex vivo activation of CD8+ T cells in the presence of IL-12 stimulates the downregulation of IFNγR2 expression
Next, we explored whether the enhanced persistence of intratumoral PmelAg+IL-12 cells was based on their reduced sensitivity to the cytotoxic effects of IFN-γ. Because the apoptotic signal can be greatly influenced by an overabundance of IFNγR2 [18], we first determined whether IL-12 modulated the expression of that receptor component on activated Pmel cells. To this end, tumor-infiltrating PmelAg and PmelAg+IL-12 cells were sorted from B16 melanoma tumor cell suspensions based on their expression of Thy1.1. Figure 4a demonstrates that as compared to PmelAg, PmelAg+IL-12 cells express slightly more of the IFNγR1 chain but significantly lower levels of IFNγR2, both of which are required to form the functional, heterodimeric IFN-γ receptor. To assess the functional consequences of IL-12-mediated decreased sensitivity to IFN-γ, we generated a homozygous IFNγR1−/− strain on the background of Pmel-1 transgenic strain (IFNγR−/− Pmel). Although in this transgenic mouse, the receptor component targeted is IFNγR1 rather than IFNγR2, the lack of IFNγR1 completely abrogates response to IFN-γ signaling [19]. Therefore, this system allowed us to test whether the lack of IFN-γ responsiveness could affect the survival of activated CD8+ T cells exposed to IFN-γ. To this end, equal numbers of ex vivo expanded wild-type PmelAg, PmelAg+IL-12, and IFNγR−/− PmelAg cells were cultured in fresh media. Cell viability was determined at 18, 48, and 72 h after reculture using a LIVE/DEAD® Assay Kit. As shown in Fig. 4b, there was a significantly greater population of viable IFNγR−/− PmelAg cells as compared to wild-type PmelAg cells following reculture. This increase in viability was likely due to a decrease in apoptosis, as evidenced by lower annexin V positivity among IFNγR−/− PmelAg when compared to its wild-type counterparts after 24 h of reculture (Fig. 4c), and by a decrease in Bax/Bcl-2 ratio which is indicative of reduced apoptosis [20] (Fig. 4d). These results strongly suggest that a lack of IFN-γ sensitivity protects activated CD8+ T cells from IFN-γ-induced apoptosis.
Fig. 4.
Lack of IFN-γ sensitivity protects activated CD8 + T cells from IFN-γ-induced apoptosis. a Adoptively transferred Thy1.1+ Pmel cells were sorted from cell suspensions prepared from similar size tumors (n = 3) 5 days after treatment with PmelAg or PmelAg+IL-12 cells, and analyzed for IFNγR1 and IFNγR2 expression by qPCR. Data are expressed as fold expression of PmelAg+IL-12 over PmelAg ±SD (p = 0.07). 1 × 106 ex vivo expanded wild-type PmelAg or PmelAg+IL-12, and IFNγR−/− PmelAg cell were recultured in fresh media. b Cell viability was determined after 24, 48, and 72 h of culture using the LIVE/DEAD® assay. c Apoptosis was measured by annexin V stain 24 h after culture. Data are presented as the average ratio of CD8+ annexin V+ over CD8+ annexin V− cells ± SD (n = 3). d RNA isolated from cells after 24 h of culture was analyzed by qPCR for expression of Bcl-2 and Bax. These values were used to calculate Bax/Bcl-2 ratio which directly associates with apoptosis [20]. Data are presented as the average change in Bax/Bcl-2 ratio relative to their respective baseline measurement ± SD (n = 3). Two-tailed Student’s t test was used, *p < 0.05, **p < 0.005, and ***p < 0.0001. Data shown are representative of at least three independent experiments
IFN-γ unresponsiveness enhances the in vivo survival and anti-tumor activity of adoptively transferred Pmel cells
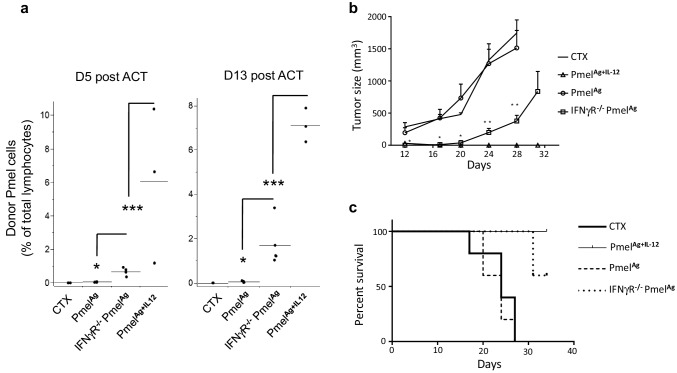
We next tested the impact of diminished IFN-γ sensitivity on the function of adoptively transferred PmelAg or PmelAg+IL-12 in vivo. To determine if decreased sensitivity to IFN-γ signaling would enhance in vivo T cell survival, we transferred IFNγR−/− PmelAg, wild-type PmelAg, and PmelAg+IL-12 to animals bearing 7-day post-injection melanoma tumors (palpable lesions). We first determined the frequency of Pmel cells in the blood, since we and others have previously reported that circulating levels of adoptively transferred T cells is an accurate determinant of their persistence in the periphery as well as within the tumor [9, 21]. At day 5 post-ACT, IFNγR−/− PmelAg accounted for over 0.5% of total circulating lymphocytes, a slight but significant increase in frequency as compared to their wild-type PmelAg counterparts. This difference in persistence was even more marked at day 13 after transfer, at which time IFNγR−/− PmelAg cells remained at a frequency 1–3.4% of total lymphocytes (Fig. 5a). We then determined whether this enhanced T cell persistence due to IFN-γ insensitivity also translated into superior tumor control. Figure 5b shows the significant delay in tumor progression that occurred in mice treated with IFNγR−/− PmelAg cells as compared to animals treated with wild-type PmelAg or CTX alone. As expected, this tumor delay resulted in a survival advantage for the mice (Fig. 5c). These results suggest that lack of IFN-γ responsiveness in T cells enhances both their survival and anti-tumor activity upon adoptive transfer.
Fig. 5.
Adoptively transferred ex vivo expanded Pmel cells unable to respond to IFN-γ stimuli have longer in vivo survival and superior anti-tumor activity. a Circulating levels of adoptively transferred PmelAg or IFNγR−/− PmelAg ex vivo expanded in the presence of antigen plus or minus IL-12 at days 5 and 13 after infusion into wild-type mice bearing B16 melanoma tumors. Data are presented as the average percent of CD8+/Thy1.1+ cells of total lymphocytes ± SD. (n = 4–5). b Tumor progression and c overall survival of mice treated with CTX alone or with either PmelAg, PmelAg+12 or IFNγR−/− PmelAg cells. Two-tailed Student’s t test was used, *p < 0.05, **p < 0.005, and ***p < 0.0001. Cumulative survival was calculated using a Kaplan–Meier curve using GraphPad Prism (n = 5). Data shown are representative of at least three independent experiments
Discussion
The clinical success of ACT is contingent on a synergy between the anti-tumor activity of the T cells and the durability of their function. Here, we show that the superior anti-tumor activity of CD8+ T cells activated ex vivo in the presence of IL-12 associates with both enhanced effector function via sustained secretion of IFN-γ and extended in vivo survival. Because of the known regulatory functions of IFN-γ, we wanted to explore whether resistance to the negative regulation of IFN-γ played a role in the ability of Pmel cells activated ex vivo in the presence of IL-12 to maintain sustained secretion of IFN-γ.
The ability of IFN-γ to induce PD-L1 expression within the TME is one potential mechanism by which IFN-γ might be negatively regulating T cell responses. CD8+ T cells activated ex vivo in the presence of IL-12, however, downregulate PD-1 expression upon entering the TME, conferring protection against PD-L1/PD-1-mediated suppression.
Elevated PD-1 expression by TILs has been attributed to their chronic exposure to high cognate antigen loads within the tumor bed, based on experiments using ovalbumin-transfected tumors as the experimental tumor antigen [14]. Under these manipulated conditions, tumor antigen expression is strictly limited to the tumor, a situation distinct from the more physiological scenario in which tumor-reactive T cells are often exposed to peripheral antigens homologous to the endogenous tumor proteins. Using the Pmel model in which the identical antigen is expressed by the peripheral melanocytes as well as by tumor melanoma cells, we demonstrated that circulating T cells exposed to ubiquitous, peripheral antigens, do not upregulate their PD-1 expression. This result suggests that tumor antigen load alone may not be the main driver of PD-1 upregulation on tumor-reactive T cells. Indeed, other factors independent of chronic antigen exposure have been implicated in the induction of T cell exhaustion, such as certain cytokines and suppressive immune cell types. Molecules associated with exhaustion include immunosuppressive cytokines such as IL-10 and transforming growth factor-β, as well as proinflammatory cytokines such as type I interferons and IL-6. Suppressive cell subtypes such as Tregs, myeloid-derived suppressor cells (MDSCs), suppressive NK, and CD8+ T cells also contribute to the inhibition of T cell responses [22, 23]. The disproportionate accumulation of all these exhaustion-inducing factors within tumors, as compared to the periphery, may explain the differential expression of PD-1 that distinguishes circulating- from tumor-infiltrating tumor-reactive T cells.
Our findings also suggest that the inhibitory effect that IL-12 has on PD-1 expression can occur as a direct effect on TILs, which also results in the restoration of IFN-γ secretion. A similar effect by IL-12 has been recently described in exhausted HBV-specific CD8+ T cells. In this study, in vitro exposure to IL-12 resulted in the downregulation of PD-1 expression and restoration of IFN-γ secretion [24].
IFN-γ can also contribute to the modulation of activated CD8+ T cell activity by directly inducing T cell death, i.e., by effectively promoting activation-induced cell death (AICD) [25, 26]. Conversely, IFN-γ is also known to promote cell proliferation. The net response to IFN-γ, whether it induces proliferation or apoptosis, depends on the relative expression of the β chain of the IFNγR (also known as IFNγR2); when it is upregulated, T cell apoptosis occurs [27]. Our data suggest that the protective effect of IL-12 on tumor-infiltrating activated T cells from IFN-γ-induced apoptosis could be in part mediated by downregulating the expression of IFNγR2. Experiments using IFNγR−/− Pmel mice confirmed the contribution of reduced IFN-γ sensitivity to enhancing T cell viability, as it prolonged survival and improved anti-tumor activity upon adoptive transfer. Receptor modification to induce selective resistance to IFN-γ stimuli has also been described during Th1/Th2 generation. It has been reported that the differential ability of IFN-γ to inhibit the proliferation of Th2 cells, but not that of Th1 cells, is due to the disproportional upregulation of IFNγR2 on Th2 cells.
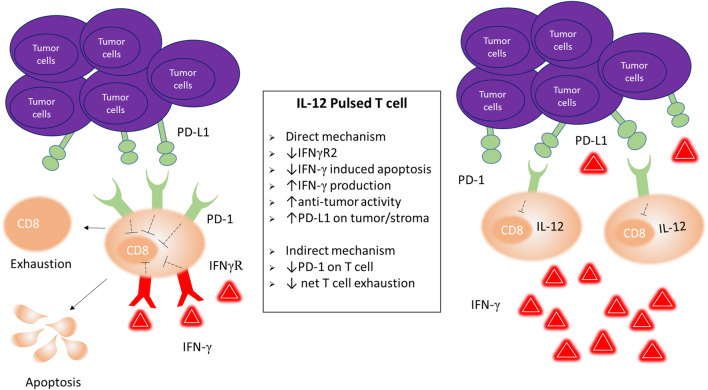
In summary, our data suggest that the sustained secretion of IFN-γ induced by ex vivo conditioning with IL-12 is crucial for optimal tumor control and depends, at least in part, on the ability of activated CD8+ T cells to counteract the deleterious effects of IFN-γ (Fig. 6).These results have important implications for cancer immunotherapy because they suggest that strategies aimed at curbing the negative regulatory effects of IFN-γ could prolong the persistence of activated TILs, and thus enhance their anti-tumor activity. Furthermore, given the mounting evidence on the pivotal role of IFN-γ in the clinical response to checkpoint blockade, a better understanding of the regulatory effects of IFN-γ would aid in elucidating the reasons behind the lack of response to checkpoint blockade by a significant fraction of patients treated.
Fig. 6.
IL-12-induced mechanisms to counter negative feedback by IFN-γ Exposure to IL-12 during ex vivo expansion protects tumor-infiltrating transferred CD8+ T cells from negative feedback by IFN-γ, directly by downregulating IFNγR2 which results in reduced apoptosis, and indirectly by downregulating PD-1 hence circumventing the impact of IFN-γ-induced PD-L1 upregulation on tumor stromal cells
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- EthD-1
Ethidium homodimer-1
- qPCR
Quantitative polymerase chain reaction
Author contributions
LL performed most of the experiments, analyzed the data, and drafted the manuscript. PR and PGP Jr. assisted and/or performed some of the experiments, generated figures, and revised the manuscript. CT, TH, AM, and JF contributed to the design and interpretation of the experiments, and revised the manuscript. JK, BG, and ME oversaw the consent and collection of human samples, contributed to the design and analysis of the experiments, and revised the manuscript. CMD-M coordinated the design and execution of the experiments, data analysis, and manuscript drafting and revision.
Funding
This work was supported by National Cancer Institute Grants K01CA134927, R21CA188767 and by a Velosano Pilot Research Award.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All animals were housed under specific pathogen-free conditions in accordance with Institutional and Federal guidelines at the Cleveland Lerner Research Institution and the experiments were approved by the local Institutional Animal Care and Use Committee (IACUC). Human samples were collected under IRB-approved tissue collection protocol number 3164.
Informed consent
Informed consent was obtained prior to sample collection.
Animal source
C57BL/6 (Thy1.1−), Pmel-1 transgenic (Thy1.1+Vβ13+), IFNγR1 knockout mice were purchased from Jackson Laboratory (Bar Harbor, ME).
Cell line authentication
B16-F10 cells, derived from a gp100+ spontaneous murine melanoma cell line, were obtained from American Type Culture Collection (ATCC) (Manassas, VA). Cell culture was performed under standardized protocols to ensure that phenotypically similar cells are implanted during each experiment. In our laboratory, cell lines are routinely tested for pathogens, and expanded to produce stock aliquots frozen at the same passage.
References
- 1.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8(4):299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK, Mescher MF. Inflammatory cytokines provide a third signal for activation of naive CD4 + and CD8+ T cells. J Immunol. 1999;162(6):3256–3262. [PubMed] [Google Scholar]
- 3.Valenzuela J, Schmidt C, Mescher M. The roles of IL-12 in providing a third signal for clonal expansion of naive CD8 T cells. J Immunol. 2002;169(12):6842–6849. doi: 10.4049/jimmunol.169.12.6842. [DOI] [PubMed] [Google Scholar]
- 4.Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, Popescu F, Xiao Z. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 5.Chowdhury FZ, Ramos HJ, Davis LS, Forman J, Farrar JD. IL-12 selectively programs effector pathways that are stably expressed in human CD8+ effector memory T cells in vivo. Blood. 2011;118(14):3890–3900. doi: 10.1182/blood-2011-05-357111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henry CJ, Ornelles DA, Mitchell LM, Brzoza-Lewis KL, Hiltbold EM. IL-12 produced by dendritic cells augments CD8+ T cell activation through the production of the chemokines CCL1 and CCL17. J Immunol. 2008;181(12):8576–8584. doi: 10.4049/jimmunol.181.12.8576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pearce EL, Shen H. Generation of CD8 T cell memory is regulated by IL-12. J Immunol. 2007;179(4):2074–2081. doi: 10.4049/jimmunol.179.4.2074. [DOI] [PubMed] [Google Scholar]
- 8.Starbeck-Miller GR, Xue HH, Harty JT. IL-12 and type I interferon prolong the division of activated CD8 T cells by maintaining high-affinity IL-2 signaling in vivo. J Exp Med. 2014;211(1):105–120. doi: 10.1084/jem.20130901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diaz-Montero CM, Naga O, Zidan AA, Salem ML, Pallin M, Parmigiani A, Walker G, Wieder E, Komanduri K, Cole DJ, Montero AJ, Lichtenheld MG. Synergy of brief activation of CD8 T-cells in the presence of IL-12 and adoptive transfer into lymphopenic hosts promotes tumor clearance and anti-tumor memory. Am J Cancer Res. 2011;1(7):882–896. [PMC free article] [PubMed] [Google Scholar]
- 10.Diaz-Montero CM, Zidan AA, Pallin MF, Anagnostopoulos V, Salem ML, Wieder E, Komanduri K, Montero AJ, Lichtenheld MG. Understanding the biology of ex vivo-expanded CD8 T cells for adoptive cell therapy: role of CD62L. Immunol Res. 2013 doi: 10.1007/s12026-013-8456-1. [DOI] [PubMed] [Google Scholar]
- 11.Diaz-Montero CM, El Naggar S, Al Khami A, El Naggar R, Montero AJ, Cole DJ, Salem ML. Priming of naive CD8+ T cells in the presence of IL-12 selectively enhances the survival of CD8(+)CD62L (hi) cells and results in superior anti-tumor activity in a tolerogenic murine model. Cancer Immunol Immunother. 2008;57(4):563–572. doi: 10.1007/s00262-007-0394-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tewari K, Nakayama Y, Suresh M. Role of direct effects of IFN-gamma on T cells in the regulation of CD8 T cell homeostasis. J Immunol. 2007;179(4):2115–2125. doi: 10.4049/jimmunol.179.4.2115. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Diaz A, Shin DS, Moreno BH, Saco J, Escuin-Ordinas H, Rodriguez GA, Zaretsky JM, Sun L, Hugo W, Wang X, Parisi G, Saus CP, Torrejon DY, Graeber TG, Comin-Anduix B, Hu-Lieskovan S, Damoiseaux R, Lo RS, Ribas A. Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell Rep. 2017;19(6):1189–1201. doi: 10.1016/j.celrep.2017.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerner MY, Heltemes-Harris LM, Fife BT, Mescher MF. Cutting edge: IL-12 and type I IFN differentially program CD8 T cells for programmed death 1 re-expression levels and tumor control. J Immunol. 2013;191(3):1011–1015. doi: 10.4049/jimmunol.1300652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Britten CM, Janetzki S, Butterfield LH, Ferrari G, Gouttefangeas C, Huber C, Kalos M, Levitsky HI, Maecker HT, Melief CJ, O’Donnell-Tormey J, Odunsi K, Old LJ, Ottenhoff TH, Ottensmeier C, Pawelec G, Roederer M, Roep BO, Romero P, van der Burg SH, Walter S, Hoos A, Davis MM. T cell assays and MIATA: the essential minimum for maximum impact. Immunity. 2012;37(1):1–2. doi: 10.1016/j.immuni.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Overwijk WW, Tsung A, Irvine KR, Parkhurst MR, Goletz TJ, Tsung K, Carroll MW, Liu C, Moss B, Rosenberg SA, Restifo NP. gp100/pmel 17 is a murine tumor rejection antigen: induction of “self’’-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J Exp Med. 1998;188(2):277–286. doi: 10.1084/jem.188.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abiko K, Matsumura N, Hamanishi J, Horikawa N, Murakami R, Yamaguchi K, Yoshioka Y, Baba T, Konishi I, Mandai M. IFN-gamma from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br J Cancer. 2015;112(9):1501–1509. doi: 10.1038/bjc.2015.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernabei P, Coccia EM, Rigamonti L, Bosticardo M, Forni G, Pestka S, Krause CD, Battistini A, Novelli F. Interferon-gamma receptor 2 expression as the deciding factor in human T, B, and myeloid cell proliferation or death. J Leukoc Biol. 2001;70(6):950–960. [PubMed] [Google Scholar]
- 19.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel RM, Aguet M. Immune response in mice that lack the interferon-gamma receptor. Sci (N Y) 1993;259(5102):1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 20.Kim JW, Tsukishiro T, Johnson JT, Whiteside TL. Expression of pro- and antiapoptotic proteins in circulating CD8+ T cells of patients with squamous cell carcinoma of the head and neck. Clin Cancer Res. 2004;10(15):5101–5110. doi: 10.1158/1078-0432.CCR-04-0309. [DOI] [PubMed] [Google Scholar]
- 21.Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3(9):666–675. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang Y, Li Y, Zhu B. T-cell exhaustion in the tumor microenvironment. Cell Death Dis. 2015;6:e1792. doi: 10.1038/cddis.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15(8):486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schurich A, Pallett LJ, Lubowiecki M, Singh HD, Gill US, Kennedy PT, Nastouli E, Tanwar S, Rosenberg W, Maini MK. The third signal cytokine IL-12 rescues the anti-viral function of exhausted HBV-specific CD8 T cells. PLoS Pathog. 2013;9(3):e1003208. doi: 10.1371/journal.ppat.1003208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Refaeli Y, Van Parijs L, Alexander SI, Abbas AK. Interferon gamma is required for activation-induced death of T lymphocytes. J Exp Med. 2002;196(7):999–1005. doi: 10.1084/jem.20020666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lohman BL, Welsh RM. Apoptotic regulation of T cells and absence of immune deficiency in virus-infected gamma interferon receptor knockout mice. J Virol. 1998;72(10):7815–7821. doi: 10.1128/jvi.72.10.7815-7821.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Novelli F, Bernabei P, Ozmen L, Rigamonti L, Allione A, Pestka S, Garotta G, Forni G. Switching on of the proliferation or apoptosis of activated human T lymphocytes by IFN-gamma is correlated with the differential expression of the alpha- and beta-chains of its receptor. J Immunol. 1996;157(5):1935–1943. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.