Abstract
Episodic memory deficits are consistently documented as a core aspect of cognitive dysfunction in schizophrenia patients, present from the onset of the illness and strongly associated with functional disability. Over the past decade research using approaches from experimental cognitive neuroscience revealed disproportionate episodic memory impairments in schizophrenia (Sz) under high cognitive demand relational encoding conditions and relatively unimpaired performance under item-specific encoding conditions. These specific deficits in component processes of episodic memory reflect impaired activation and connectivity within specific elements of frontal-medial temporal lobe circuits, with a central role for the dorsolateral prefrontal cortex (DLPFC), relatively intact function of ventrolateral prefrontal cortex and variable results in the hippocampus. We propose that memory deficits can be understood within the broader context of cognitive deficits in Sz, where impaired DLPFC related cognitive control has a broad impact across multiple cognitive domains. The therapeutic implications of these findings are discussed.
Keywords: episodic memory, cognitive control, schizophrenia, frontal-medial temporal lobe networks, DLPFC
Cognitive impairments have long been accepted as core features of schizophrenia (Sz) that contribute significantly to disability and are generally treatment refractory 1. Indeed, Keefe 2 recently recommended including cognitive impairment in the formal diagnostic criteria for this illness and highlighted the need for research on developing treatments to improve cognitive abilities in Sz. Numerous neuropsychological studies demonstrate a broad range of measurable cognitive deficits in Sz including impairments in attention, working memory (WM), episodic memory, processing speed and executive functions 3. Among these traditional cognitive domains, episodic memory has frequently been highlighted as showing the largest effect sizes among cognitive deficits in Sz 4, regardless of factors such as antipsychotic medication treatment 5, duration of illness 6, stage of illness 7 or level of psychotic symptoms 8, and of being particularly relevant for the functional deficits that characterize the illness 9.
In recent years, episodic memory has been extensively studied in Sz using fine grained cognitive neuroscience methods to identify the specific components of memory systems that are impaired in the illness. These studies have led the field to two important conclusions, both of which have implications for our understanding of the illness and for the development of novel therapeutics. Firstly, these studies, reviewed in detail below, suggest that specific aspects of episodic memory are either spared or impaired, in contrast to the more global learning and memory impairment seen in amnestic disorders 10. Secondly, deficits in episodic memory may be best understood in the context of a broader pattern of deficits in higher cognitive functions in Sz that are often accompanied by dorsolateral prefrontal (DLPFC) dysfunction, and most likely to be observed when organizational and cognitive control demands are high.
Cognitive models of episodic memory deficits in Schizophrenia
Experimental cognitive studies of episodic memory in schizophrenia have generally focused on the function of recall and recognition mechanisms in the brain. In healthy subjects the use of recall paradigms has revealed many discrete component memory processes including the serial position effect (e.g. primacy and recency) 11, 12, the impact of levels of processing (deep and shallow encoding) 13 and the effects of self-initiated organizational encoding strategies 14, 15. Schizophrenia patients have been shown to exhibit comparable serial position effects as healthy controls (HC), suggesting intact shallow encoding, and are able to benefit from instructions to use semantic strategies to support encoding 16–19. However, they fail to self-initiate these semantic encoding strategies, suggesting impaired deep encoding mechanisms 20. These results, in aggregate, highlight the view that there are specific strategic memory deficits that limit encoding into episodic memory under high levels of processing demand in the illness, overlaid on a set of basically intact more automatic learning and memory mechanisms.
To obtain a deeper understanding of encoding and retrieval mechanisms, cognitive neuroscience researchers employed a variety of recognition paradigms, where individuals learn material during a study phase and are then probed as to whether it is “old” or “new” during a test phase. One common approach has been to examine recognition performance using incidental encoding paradigms in which individuals alternate between two types of encoding: item-specific (e.g. words denoting living things) or relational (e.g. beer and milk from beverage category). Several analytic methods can then be used to pull apart two forms of retrieval: familiarity (e.g., novelty detection) or recollection (e.g., associative recognition) 21. Performance on old/new recognition tests has been modelled using either signal-detection theory or threshold model approaches. Signal-detection assumes that recognition judgements fall on a continuously distributed memory strength variable (e.g. old items with high familiarity fall on the high end and new items with low familiarity fall on the low end) 22, 23. The threshold model assumes discrete mental states where only items with sufficient episodic details to describe the state of memory (over threshold) can be recalled 24, 25. A third “dual-process” modelling approach obtains confidence ratings that are examined using a receiver operator characteristics (ROC) analysis to generate quantitative and orthogonal recollection and familiarity parameter estimates 26, 27. Recollection involves a sense of “remembering” and is accompanied by retrieval of qualitative aspects of the encoding event, such as context-item associations or source details. Conversely, familiarity involves a sense of “knowing” based on a global (or gist-like) sense of memory strength or novelty, and is not accompanied by retrieval of qualitative details of the encoding context.
In a series of studies using a dual-process modelling approach in Sz, Ragland and colleagues demonstrated that familiarity is unimpaired when item encoding is utilized, familiarity is severely impaired when a relational encoding strategy is required, and recollection is severely impaired regardless of encoding processes used (Figure 1) 28–30. In relation to these findings, a meta-analysis which reviewed nineteen studies of recollection and familiarity in Sz using a variety of quantitative methods including remember/know/new, process dissociation, and ROC modeling found that effect sizes of impairments in Sz are medium to large on recollection and small to medium on familiarity estimates 31. Thus it appears that Sz patients can successfully utilize encoding strategies that focus on item features to support a sense of familiarity, however, relational encoding strategies do not promote successful familiarity processes and recollection shows the most pervasive impairment regardless of what encoding strategy is applied.
Figure 1:
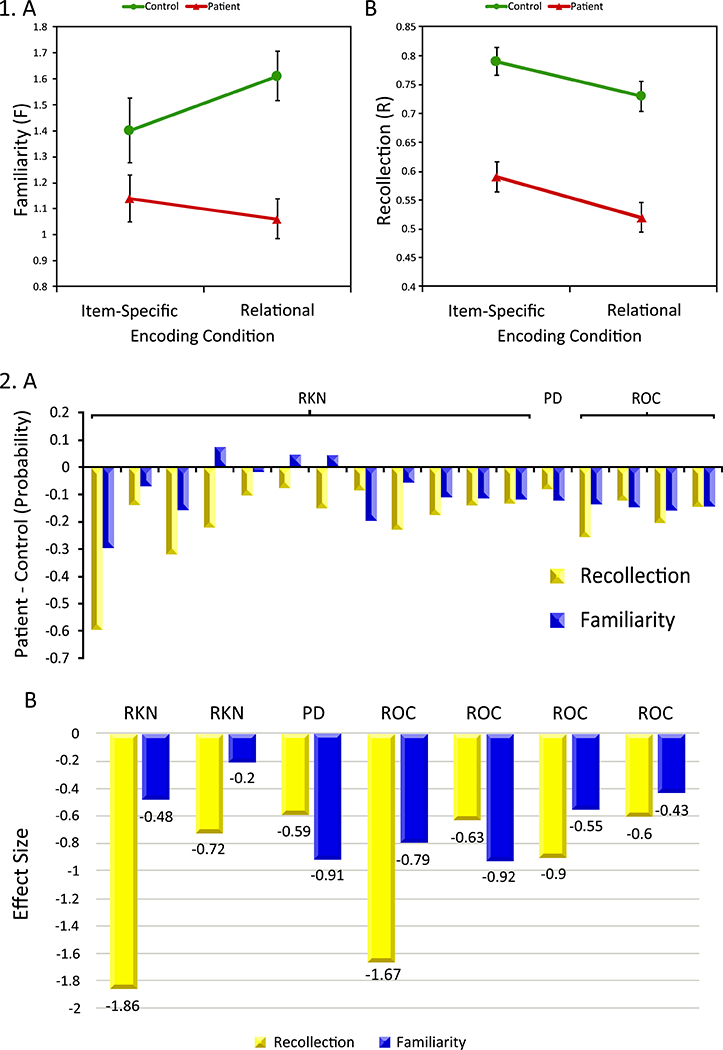
Panel 1, Mean (±SEM) familiarity and recollection in healthy controls (blue circles) and Sz patients (red triangles). (A) Familiarity reveals a group by encoding interaction, with more severe patient deficits following relational versus item-specific encoding, (B) Recollection reveals a main effect of group across both encoding conditions, with lower patient versus control performance. See Ragland, Ranganath 28 for additional results. Panel 2, Meta-analysis of recollection and familiarity deficits across 19 previous studies utilizing remember/know/new (RKN), receiver-operator characteristic (ROC), or process dissociation (PD) methods. (A) Recollection and familiarity estimates were recalculated with three probabilities models from three types of studies respectively, showing more impaired recollection in Sz patients than controls across studies. (B) Effect sizes of Sz on recollection were slightly larger than on familiarity. See 31 for study references.
Neural circuitry dysfunction underlying episodic memory deficits in Schizophrenia
While early neuropychological studies of episodic memory focused on the critical role of the medial temporal lobe (MTL), and specifically the hippocampus, in episodic memory 32, 33, with the advent of whole brain functional imaging in the 1990’s it became clear that encoding and retrieving information in episodic memory depends on dynamic interactions between mutliple prefrontal and medial temporal lobe systems 34, 35. It is not suprising that functional imaging studies in schizophrenia pointed to alterations in the function of these networks during memory performance. The overall pattern of results in these studies suggest that, as in behavioral studies of component memory mechanisms, there is a specificity of these effects to distinct elements of neural circuitry that has implications for our understanding of the illness and future therapeutics.
The majority of previous functional imaging studies of episodic memory encoding processes in Sz reported reduced DLPFC activation during deep encoding e.g. 36, Meta-analysis: 37, while one study by Bonner-Jackson 18 found increased DLPFC and inferior frontal gyrus activation during deep encoding in Sz patients compared to HC that was interpreted as a compensative hyperactivation. Guimond and colleagues 19 further illustrated that hypoactivation of the left DLPFC plays a critical role in the impairment of self-initiating semantic encoding strategies in Sz. Interestingly, these studies also showed unimpaired activation in the ventrolateral prefrontal cortex (VLPFC) when patients were provided item-specific encoding strategies 37, 38. With regard to the MTL component of episodic memory circuitry, to date, fMRI studies in Sz have yielded heterogeneous results with hyper- or hypoactivation of MTL structures during novel stimuli encoding, such as hypoactivation in the right posterior hippcampus and hyperactivation in the right anterior hippocampus 39, hyperactivation in the left inferior temporal gyrus, right MTL and bilateral parahippocampus 40, and hypoactivation in the bilateral parahippocampal and hippocampal-parietal network 41. Such contradictory results may reflect whether a given study controls for encoding success 42, on the types of materials being encoded 18 and whether deep or shallow levels of processing are examined. When Ragland and colleagues 38 utilized a deep versus shallow encoding paradigm to control for strategic memory deficits, they found unimpaired VLPFC activation during shallow encoding, and reduced DLPFC and increased hippocampal and thalamic activation during deep-encoding. In summary, these combined results point to consistently reduced DLPFC activation during deep encoding, and when no encoding strategies are provided, relatively intact VLPFC activation during shallow or item-specific encoding, and mixed findings of both under- and over-activation or no group differences in the MTL and hippocampus.
To understand neural circuits underlying episodic memory recognition processes, Ragland et al. 43 demonstrated DLPFC hypoactivation in Sz patients compared to controls during old/new recognition of items following relational vs item-specific encoding, whereas no group differences were found in VLPFC activation regardless of encoding condition. However, Lepage et al. 44 revealed hypoactivation in left DLPFC, right VLPFC, and two medial frontal cortices in Sz patients compared to controls during recognition following relational versus item encoding. Across both studies, reduced DLPFC activity was associated with recognition deficits in Sz, whereas VLPFC results were more variable – possibly due to whether or not encoding strategies were provided 45 or had to be self-initiated 44. However, at a meta-analytic level 37, there is an absence of VLPFC group differences, while the DLPFC appears consistently impaired. Functional connectivity evidence of reduced hippocampal to DLPFC connectivity and enhanced hippocampal to VLPFC connectivity 46 suggests that the VLPFC may be engaged in patients to support item encoding and familiarity-based retrieval in an effort to compensate for more intractable DLPFC and hippocampal dependent relational encoding and recollective memory deficits.
Given the strong association between the hippocampus and episodic memory in classical neuropsychology it is not surprising that this has been a primary focus in fMRI studies of episodic memory impairment in Sz See review: 47. However, accumulating inconsistent evidence of hippocampal hyperactivation, hypoactivation and no differences in Sz patients compared to controls have complicated our understanding of the role of the hippocampus in these deficits. An early PET study demonstrated that Sz patients exhibited increased regionalcerebral blood flow (rCBF) in the hippocampus during recall after perceptual encoding and decreased hippocampal rCBF during recall after semantic encoding compared to controls 48, suggesting a dysfunction of cortico-hippocampal network integration in Sz 49. However, two subsequent meta-analyses have revealed that Sz patients exhibit abnormal brain activation in the prefrontal cortex during encoding but not in the medial temporal lobe during either encoding or retrieval 37, 50. More recent studies have revisited the role of hippocampal dysfunction in Sz by utilizing paradigms that require recollection and associative processing or by examining differences along the longitudinal axis of this structure. Regarding this longitudinal axis, the anterior hippocampus has fairly consistently shown increased resting state activity in Sz (e.g. cerebral blood volume (CBV) and cerebral metabolic rate of oxygen (CMRO2)) See book chapter: 51, See review: 52 in SZ. For example, Schobel et al. 53 reported increased CBV in anterior subfield of hippocampus (CA1) and the orbitofrontal cortex in Sz patients without a history of antipsychotic medication exposure. The same group extended this result in the early prodromal stages of the illness with a follow-up study, in which individuals at ultra-high risk (UHR) of psychosis exhibited increased CBV in the left anterior hippocampus (CA1) and spreads to anterior subiculum after psychosis onset 54. Thus, CA1 hypermetabolism has been considered as a regional vulnerability characterizing psychotic symptoms in Sz See review: 55. Furthermore, a substantial body of work in basic and behavioral neuroscience suggests that anterior and posterior divisions subserve different functions in episodic memory (figure 2)56, See review: 57, e.g. 58, 59. Specifically, posterior hippocampal subregions are more likely to be involved in visual spatial memory encoding 60, whereas anterior hippocampal subregions are more involved in other complex behaviors (e.g., anxiety related behaviors 61and stress 62). When Ragland and colleagues 63 recently examined this using an eye-tracking fMRI paradigm that manipulated either item information or spatial information in previously studied scenes, they found that the task-specific impairment (worse relational versus item memory) was explained by a reduction in posterior hippocampal activation for relational versus item changes. However, the anterior hippocampus, unexpectedly, showed hyperactivation in Sz patients for the item change condition. If the above findings of differential impacts of Sz on anterior versus posterior hippocampal function are confirmed in future studies, it suggests that looking separately at the head and tail of the hippocampus may help to reconcile some of the current variability in the literature.
Figure 2:
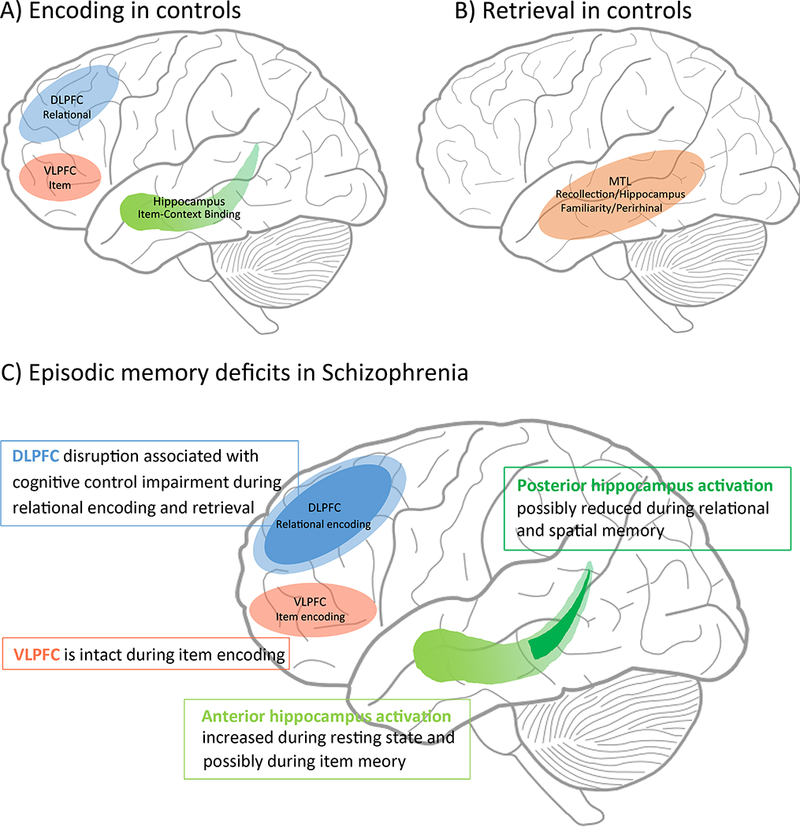
In controls, DLPFC provides control of relational encoding and VLPFC controls item-specific semantic processing, and hippocampus binds associations between item and context information during encoding (panel A). At retrieval, recollection engages the hippocampus and familiarity engages mainly perirhinal cortex, with little hippocampal engagement (See 30, 33, 118 for theoretical models) (panel B). Panel C illustrates disrupted DLPFC (blue) activation during encoding and retrieval in Sz, while VLPFC (orange) remains intact during item encoding, suggesting an important role for cognitive control deficits underlying episodic memory impairment in Sz. On the other hand, relational and spatial memory dysfunction in Sz is associated with reduced posterior hippocampal activation in Sz, whereas increased anterior hippocampal activation has been associated with item memory.
Cognitive impairments in schizophrenia as a context for understanding deficits in episodic memory
As noted above, episodic memory is just one of a range of cognitive domains that are impaired in Sz e.g. 64, 65. For example, Keefe et al. 66 demonstrated that Sz patients performed at 2.5 standard deviation below controls in 7 domains: speed of processing, attention/vigilance, working memory, verbal learning and memory, visual learning and memory, reasoning/problem solving and social cognition, indicating a profound cognitive impairment in schizophrenia. Across the cognitive domains that have been interrogated from an experimental cognitive neuroscience perspective, attention and working memory were also shown to be robustly impaired in the illness. In the following section we will briefly review findings in these two higher cognitive domains and then introduce the concept of cognitive control, which may provide an integrative framework for understanding impaired congition in Sz and a link between the specific impairments in episodic memory that are seen in the illness and the broader range of congitive deficits that are also observed.
Attention
In examining component processes of attention, selective attention can be examined by Stroop Color and Word task 67, wherein participants are required to name the ink color of color words (e.g., the word “green” written in red ink). Altered Stroop task performance in Sz, reflected by increased errors or reaction times has been reported by many investigators 68–70. Subsequent neuroimaging studies suggested that these deficits are associated with reduced activity in frontal-parietal attentional control systems and anterior cingulate related conflict monitoring 71. Sustained attention/vigilance can be examined by various types of continuous performance tests (CPT) 72, See review: 73. In general, patients have shown no performance difference on vigilance versions of the CPT compared to controls e.g. 74, 75, 76. When task difficulty is increased through the use of degraded stimuli 77, patients are reliably impaired. However, whether this reflects a pure vigilance deficit or the contribution of increased perceptual discrimination on decision making is unclear. In neuroimaging studies, Sz patients have exhibited reduced activation in ACC and inferior frontal gyrus, increased activation in inferior parietal lobule and mixed results in subcortical regions during various types of CPT tasks e.g. 78, 79–83. These contrasting results may reflect interaction of sustained attention and other cognitive demands. Indeed, Roth and colleagues 84 revealed that both X-CPT and identical pairs (IP)-CPT reliably detected more impaired sustained attention in Sz smokers compared to nonsmokers when IP-CPT exhibited larger effect size to detect group differences than X-CPT, suggesting IP-CPT taxes working memory.
Working memory
Working memory impairments in Sz are characterized by reduced accuracy and increased response time, which have been documented in various stages of Sz, such as UHR See meta-analysis: 85, early onset of schizophrenia patients 86, first-episode schizophrenia patients See meta-analysis: 87 and especially in chronic Sz 88. The majority of neuroimaging findings suggest that regions comprising the dorsal frontal-parietal network are affected in Sz, and that this neuropathology may underlie WM impairments in Sz 89. It has also been proposed that the inverted U–shaped relation of DLPFC activation to WM loads is shifted to the left side in Sz patients compared to controls e.g. 89, 90, 91, 92. Similar abnormal brain activation is also revealed in the dorsal parietal cortex in Sz suggesting a frontal-parietal dysfunction underlying high cognitive demands during WM 93.
Cognitive control
Cognitive control is defined as the ability to actively maintain contextual information (including information related to stimuli, task rules and goals) in order to guide task-relevant responding 94, 95. Cognitive control is domain general, supporting a wide variety of cognitive domains, including attention, working memory and episodic memory e.g. 96, 97–99. Cognitive control is supported by a distinct neural network in which the DLPFC serves as a central hub See meta-analysis: 100, 101. Theoretical models of cognitive control have dissociated proactive (or endogeneously guided) and reactive (in response to conflict, errors and other indicators of negative utility) elements subserved by both distinct and overlapping neural systems 102, 103. In schizophrenia research, there is considerable evidence that DLPFC hypoactivation is related to impaired proactive control in first episode Sz 104, 105, medication naive Sz 106 and chronic Sz 71. Moreover, Sz patients show a significant reduction of DLPFC-related functional connectivity (e.g. fronto-parietal functional connectivity) under conditions requiring high cognitive control 107, See review: 108.
Cognitive control and episodic memory deficits in Schizophrenia
Drawing on the cognitive model of episodic memory outlined at the beginning of this review, the pattern of impaired and preserved component processes of episodic memory seen in Sz suggests that memory impairments may be most severe when DLPFC mediated cognitive control demands are greatest. Thus, a general cognitive control deficit model provides a parsimonious link between the prominent deficits in episodic memory and the broader range of higher cognitive deficits including impaired attention and working memory. As reviewed above, the design of relational versus item memory task was intended to distinguish high and low cognitive control conditions by introducing paired versus item specific cues, while the majority of studies revealed a strong link of deficits in the DLPFC with the more cognitive control demanding associated relational encoding condition 28, 37, 109. This view is also supported by the results of the meta-analysis by Ragland et al. 37, which showed the most prominent prefrontal deficits in Sz during both encoding and retrieval of episodic memory tasks. Specifically, persistent DLPFC deficits are not secondary to providing semantic encoding strategies, suggesting a general impairment of cognitive control. The hypothesis that there is impaired cognitive control of episodic memory in Sz has also been directly tested using a directed forgetting paradigm (DF), where HC are required to successfully encode and retrieval target stimuli into long-term memory while intentionally preventing non-target stimuli from being encoded. During a subsequent recognition phase, participants are required to respond to a probe of to-be-remembered (TBR) or to-be-forgotten (TBF) stimuli See review: 110. Behavioral data index the engagement of cognitive control, as reflected by a longer reaction time to TBF than TBR stimuli and greater recognition of TBR than TBF stimuli (DF effect) e.g. 111. fMRI neuroimaging studies consistently demonstrate that intentional forgetting engages DLPFC and parietal regions, suggesting that inhibition of encoding a recent stimuli is effortful 112, 113. Moreover, brain lesion studies also support a causal relationship of frontal lobe lesions with reduced DF effects and abnormally high recognition of TBF stimuli 114. In Sz, behavioral studies reported reduced DF effect in patients compared to controls 115, 116. During fMRI, Ragland and colleagues 117 found that increased DLPFC activation was associated with increased cognitive control during intentional forgetting trails in controls, but not in Sz patients. Sz patients show prominent deficits in some aspects of episodic memory (e.g. relational encoding, intentional forgetting) while other aspects appear to be spared, which is a pattern of selective deficits that is seen across a range of cognitive domains including attention, working memory and cognitive control. Common elements across the neural systems that support these “domain specific” deficits include the DLPFC, which must integrate with parietal, cingulate and medial temporal networks under demands for high levels of contextual processing and cognitive control. As a central, and domain general, hub in these domain specific networks the DLPFC can be understood as a critical target for efforts to restore cognitive functioning, including episodic memory, in Sz.
Conclusion
In the current review, we have argued that memory deficits in Sz are specific to encoding and retrieval conditions with high cognitive demands and which depend on cognitive control and relational memory functions of the DLPFC as it participates in frontal-MTL network function. Such deficits provide a link for understanding the relationship between episodic memory deficits and other cognitive deficits, such as attention and WM in the illness. Therapeutically, these conclusions support the development of treatments that target the function of the DLPFC, pharmacologically or through neurostimulation. Such an approach should have generalizable benefits for memory as well as other kinds of cognitive deficits in Sz.
Acknowledgements
We sincerely thank our colleague Vanessa Zarubin for her diligent proofreading.
Footnotes
Conflict of interest statement
We warrant that the article is the authors’ original work. All authors have seen and approved the manuscript being submitted. The article has not received prior publication and is not under consideration for publication elsewhere.
Reference
- 1.Bora E, Yucel M, Pantelis C. Cognitive impairment in schizophrenia and affective psychoses: implications for DSM-V criteria and beyond. Schizophrenia bulletin 2010; 36(1): 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keefe RS. Should cognitive impairment be included in the diagnostic criteria for schizophrenia? World Psychiatry 2008; 7(1): 22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kahn RS, Keefe RS. Schizophrenia is a cognitive illness: time for a change in focus. JAMA psychiatry 2013; 70(10): 1107–1112. [DOI] [PubMed] [Google Scholar]
- 4.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology 1998; 12(3): 426–445. [DOI] [PubMed] [Google Scholar]
- 5.Goldberg TE, Weinberger DR. Effects of neuroleptic medications on the cognition of patients with schizophrenia: a review of recent studies. The Journal of clinical psychiatry 1996; 57 Suppl 9: 62–65. [PubMed] [Google Scholar]
- 6.Rushe TM, Woodruff PW, Murray RM, Morris RG. Episodic memory and learning in patients with chronic schizophrenia. Schizophr Res 1999; 35(1): 85–96. [DOI] [PubMed] [Google Scholar]
- 7.Hoff AL, Riordan H, O’Donnell DW, Morris L, DeLisi LE. Neuropsychological functioning of first-episode schizophreniform patients. The American journal of psychiatry 1992; 149(7): 898–903. [DOI] [PubMed] [Google Scholar]
- 8.Aleman A, Hijman R, de Haan EH, Kahn RS. Memory impairment in schizophrenia: a meta-analysis. The American journal of psychiatry 1999; 156(9): 1358–1366. [DOI] [PubMed] [Google Scholar]
- 9.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? The American journal of psychiatry 1996; 153(3): 321–330. [DOI] [PubMed] [Google Scholar]
- 10.Ragland JD, Cools R, Frank M, Pizzagalli DA, Preston A, Ranganath C et al. CNTRICS final task selection: long-term memory. Schizophrenia bulletin 2009; 35(1): 197–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murdock BB Jr. The serial position effect of free recall. Journal of Experimental Psychology 1962; 64(5): 482–488. [Google Scholar]
- 12.Deese J, Kaufman RA. Serial effects in recall of unorganized and sequentially organized verbal material. J Exp Psychol 1957; 54(3): 180–187. [DOI] [PubMed] [Google Scholar]
- 13.Craik FIM, Lockhart RS. Levels of processing: A framework for memory research. Journal of Verbal Learning and Verbal Behavior 1972; 11(6): 671–684. [Google Scholar]
- 14.Bousfield WA. The Occurrence of Clustering in the Recall of Randomly Arranged Associates. The Journal of General Psychology 1953; 49(2): 229–240. [Google Scholar]
- 15.Tulving E Subjective organization in free recall of “unrelated” words. Psychological Review 1962; 69(4): 344–354. [DOI] [PubMed] [Google Scholar]
- 16.Ragland JD, Moelter ST, McGrath C, Hill SK, Gur RE, Bilker WB et al. Levels-of-processing effect on word recognition in schizophrenia. Biological psychiatry 2003; 54(11): 1154–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koh SD, Peterson RA. Encoding orientation and the remembering of schizophrenic young adults. Journal of abnormal psychology 1978; 87(3): 303–313. [PubMed] [Google Scholar]
- 18.Bonner-Jackson A, Haut K, Csernansky JG, Barch DM. The influence of encoding strategy on episodic memory and cortical activity in schizophrenia. Biological psychiatry 2005; 58(1): 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guimond S, Hawco C, Lepage M. Prefrontal activity and impaired memory encoding strategies in schizophrenia. Journal of psychiatric research 2017; 91: 64–73. [DOI] [PubMed] [Google Scholar]
- 20.Brebion G, Amador X, Smith MJ, Gorman JM. Mechanisms underlying memory impairment in schizophrenia. Psychological medicine 1997; 27(2): 383–393. [DOI] [PubMed] [Google Scholar]
- 21.Murray LJ, Ranganath C. The dorsolateral prefrontal cortex contributes to successful relational memory encoding. The Journal of neuroscience: the official journal of the Society for Neuroscience 2007; 27(20): 5515–5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lockhart RS, Murdock BB. Memory and the theory of signal detection. Psychological Bulletin 1970; 74(2): 100–109. [Google Scholar]
- 23.Banks WP. Signal detection theory and human memory. Psychological Bulletin 1970; 74(2): 81–99. [Google Scholar]
- 24.Memory Kintsch W. and decision aspects of recognition learning. Psychological Review 1967; 74(6): 496–504. [DOI] [PubMed] [Google Scholar]
- 25.Macmillan N, Douglas Creelman C. Response bias: Characteristics of detection theory, threshold theory, and “nonparametric” indexes, vol. 1071990, 401–413pp. [Google Scholar]
- 26.Yonelinas AP. Receiver-operating characteristics in recognition memory: evidence for a dual-process model. Journal of experimental psychology Learning, memory, and cognition 1994; 20(6): 1341–1354. [DOI] [PubMed] [Google Scholar]
- 27.Yonelinas AP, Dobbins I, Szymanski MD, Dhaliwal HS, King L. Signal-detection, threshold, and dual-process models of recognition memory: ROCs and conscious recollection. Consciousness and cognition 1996; 5(4): 418–441. [DOI] [PubMed] [Google Scholar]
- 28.Ragland JD, Ranganath C, Barch DM, Gold JM, Haley B, MacDonald AW 3rd, et al. Relational and Item-Specific Encoding (RISE): task development and psychometric characteristics. Schizophrenia bulletin 2012; 38(1): 114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Owoso A, Carter CS, Gold JM, MacDonald AW 3rd, Ragland JD, Silverstein SM et al. Cognition in schizophrenia and schizo-affective disorder: impairments that are more similar than different. Psychological medicine 2013; 43(12): 2535–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ragland JD, Blumenfeld RS, Ramsay IS, Yonelinas A, Yoon J, Solomon M et al. Neural correlates of relational and item-specific encoding during working and long-term memory in schizophrenia. NeuroImage 2012; 59(2): 1719–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Libby LA, Yonelinas AP, Ranganath C, Ragland JD. Recollection and familiarity in schizophrenia: a quantitative review. Biological psychiatry 2013; 73(10): 944–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn Sci 2007; 11(9): 379–386. [DOI] [PubMed] [Google Scholar]
- 33.Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annual review of neuroscience 2007; 30: 123–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiss AP, Heckers S. Neuroimaging of declarative memory in schizophrenia. Scandinavian journal of psychology 2001; 42(3): 239–250. [DOI] [PubMed] [Google Scholar]
- 35.Preston AR, Eichenbaum H. Interplay of hippocampus and prefrontal cortex in memory. Current biology: CB 2013; 23(17): R764–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barch DM, Csernansky JG, Conturo T, Snyder AZ. Working and long-term memory deficits in schizophrenia: is there a common prefrontal mechanism? Journal of abnormal psychology 2002; 111(3): 478–494. [DOI] [PubMed] [Google Scholar]
- 37.Ragland JD, Laird AR, Ranganath C, Blumenfeld RS, Gonzales SM, Glahn DC. Prefrontal activation deficits during episodic memory in schizophrenia. The American journal of psychiatry 2009; 166(8): 863–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ragland JD, Gur RC, Valdez JN, Loughead J, Elliott M, Kohler C et al. Levels-of-processing effect on frontotemporal function in schizophrenia during word encoding and recognition. The American journal of psychiatry 2005; 162(10): 1840–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jessen F, Scheef L, Germeshausen L, Tawo Y, Kockler M, Kuhn KU et al. Reduced hippocampal activation during encoding and recognition of words in schizophrenia patients. The American journal of psychiatry 2003; 160(7): 1305–1312. [DOI] [PubMed] [Google Scholar]
- 40.Ragland JD, Gur RC, Valdez J, Turetsky BI, Elliott M, Kohler C et al. Event-related fMRI of frontotemporal activity during word encoding and recognition in schizophrenia. The American journal of psychiatry 2004; 161(6): 1004–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rasetti R, Mattay VS, White MG, Sambataro F, Podell JE, Zoltick B et al. Altered hippocampal-parahippocampal function during stimulus encoding: a potential indicator of genetic liability for schizophrenia. JAMA psychiatry 2014; 71(3): 236–247. [DOI] [PubMed] [Google Scholar]
- 42.Pirnia T, Woods RP, Hamilton LS, Lyden H, Joshi SH, Asarnow RF et al. Hippocampal dysfunction during declarative memory encoding in schizophrenia and effects of genetic liability. Schizophr Res 2015; 161(2–3): 357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ragland JD, Ranganath C, Harms MP, Barch DM, Gold JM, Layher E et al. Functional and Neuroanatomic Specificity of Episodic Memory Dysfunction in Schizophrenia: A Functional Magnetic Resonance Imaging Study of the Relational and Item-Specific Encoding Task. JAMA psychiatry 2015; 72(9): 909–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lepage M, Montoya A, Pelletier M, Achim AM, Menear M, Lal S. Associative memory encoding and recognition in schizophrenia: an event-related fMRI study. Biological psychiatry 2006; 60(11): 1215–1223. [DOI] [PubMed] [Google Scholar]
- 45.Ragland JD, Ranganath C, Phillips J, Boudewyn MA, Kring AM, Lesh TA et al. Cognitive Control of Episodic Memory in Schizophrenia: Differential Role of Dorsolateral and Ventrolateral Prefrontal Cortex. Frontiers in human neuroscience 2015; 9: 604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolf RC, Vasic N, Hose A, Spitzer M, Walter H. Changes over time in frontotemporal activation during a working memory task in patients with schizophrenia. Schizophr Res 2007; 91(1–3): 141–150. [DOI] [PubMed] [Google Scholar]
- 47.Heckers S Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus 2001; 11(5): 520–528. [DOI] [PubMed] [Google Scholar]
- 48.Heckers S, Rauch SL, Goff D, Savage CR, Schacter DL, Fischman AJ et al. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nature neuroscience 1998; 1(4): 318–323. [DOI] [PubMed] [Google Scholar]
- 49.Fletcher P The missing link: a failure of fronto-hippocampal integration in schizophrenia. Nature neuroscience 1998; 1(4): 266–267. [DOI] [PubMed] [Google Scholar]
- 50.Achim AM, Lepage M. Episodic memory-related activation in schizophrenia: meta-analysis. The British journal of psychiatry: the journal of mental science 2005; 187: 500–509. [DOI] [PubMed] [Google Scholar]
- 51.Bauman MD, Ragland JD, Schumann CM Feeling and Remembering: Effects of Psychosis on the Structure and Function of the Amygdala and Hippocampus In: Tamminga EII Carol A., Reininghaus Ulrich, Jim van Os (ed). Psychosis: Transdiagnostic Conceptualizations and Implications for TreatmentIn press. [Google Scholar]
- 52.Heckers S, Konradi C. GABAergic mechanisms of hippocampal hyperactivity in schizophrenia. Schizophr Res 2015; 167(1–3): 4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schobel SA, Lewandowski NM, Corcoran CM, Moore H, Brown T, Malaspina D et al. Differential Targeting of the CA1 Subfield of the Hippocampal Formation by Schizophrenia and Related Psychotic Disorders. Archives of general psychiatry 2009; 66(9): 938–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schobel SA, Chaudhury NH, Khan UA, Paniagua B, Styner MA, Asllani I et al. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a pathogenic driver. Neuron 2013; 78(1): 81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nature reviews Neuroscience 2011; 12(10): 585–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramos JM. Long-term spatial memory in rats with hippocampal lesions. The European journal of neuroscience 2000; 12(9): 3375–3384. [DOI] [PubMed] [Google Scholar]
- 57.Ramos JM. Hippocampal damage impairs long-term spatial memory in rats: comparison between electrolytic and neurotoxic lesions. Physiology & behavior 2008; 93(4–5): 1078–1085. [DOI] [PubMed] [Google Scholar]
- 58.Broadbent NJ, Gaskin S, Squire LR, Clark RE. Object recognition memory and the rodent hippocampus. Learning & memory 2010; 17(1): 5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cohen SJ, Munchow AH, Rios LM, Zhang G, Asgeirsdottir HN, Stackman RW Jr., The rodent hippocampus is essential for nonspatial object memory. Current biology: CB 2013; 23(17): 1685–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Small SA, Nava AS, Perera GM, DeLaPaz R, Mayeux R, Stern Y. Circuit mechanisms underlying memory encoding and retrieval in the long axis of the hippocampal formation. Nature neuroscience 2001; 4(4): 442–449. [DOI] [PubMed] [Google Scholar]
- 61.Bannerman DM, Rawlins JNP, McHugh SB, Deacon RMJ, Yee BK, Bast T et al. Regional dissociations within the hippocampus—memory and anxiety. Neuroscience & Biobehavioral Reviews 2004; 28(3): 273–283. [DOI] [PubMed] [Google Scholar]
- 62.Fanselow MS, Dong H-W. Are The Dorsal and Ventral Hippocampus functionally distinct structures? Neuron 2010; 65(1): 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Evan Ragland JL;Hannula Deborah; Niendam Tara; Lesh Tyler; Solomon Marjorie; Carter Cameron; Ranganath Charan. A Gradient of Hippocampal Function and Dysfunction During Relational Memory in People With Schizophrenia Schizophrenia bulletin 2016. [Google Scholar]
- 64.Keefe RS, Harvey PD. Cognitive impairment in schizophrenia. Handb Exp Pharmacol 2012; (213): 11–37. [DOI] [PubMed] [Google Scholar]
- 65.Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. The Journal of clinical psychiatry 2006; 67(10): e12. [PubMed] [Google Scholar]
- 66.Keefe RSE, Fox KH, Harvey PD, Cucchiaro J, Siu C, Loebel A. Characteristics of the MATRICS Consensus Cognitive Battery in a 29-site antipsychotic schizophrenia clinical trial. Schizophrenia Research 2011; 125(2): 161–168. [DOI] [PubMed] [Google Scholar]
- 67.Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology 1935; 18(6): 643–662. [Google Scholar]
- 68.Carter CS, Robertson LC, Nordahl TE. Abnormal processing of irrelevant information in chronic schizophrenia: Selective enhancement of Stroop facilitation. Psychiatry research 1992; 41(2): 137–146. [DOI] [PubMed] [Google Scholar]
- 69.Carter CS, Mintun M, Cohen JD. Interference and facilitation effects during selective attention: an H215O PET study of Stroop task performance. NeuroImage 1995; 2(4): 264–272. [DOI] [PubMed] [Google Scholar]
- 70.Carter CS, Mintun M, Nichols T, Cohen JD. Anterior cingulate gyrus dysfunction and selective attention deficits in schizophrenia: [O-15]H2O PET study during single-trial Stroop task performance. American Journal of Psychiatry 1997; 154(12): 1670–1675. [DOI] [PubMed] [Google Scholar]
- 71.Kerns JG, Cohen JD, MacDonald AW, Johnson MK, Stenger VA, Aizenstein H et al. Decreased conflict- and error-related activity in the anterior cingulate cortex in subjects with schizophrenia. American Journal of Psychiatry 2005; 162(10): 1833–1839. [DOI] [PubMed] [Google Scholar]
- 72.Riccio CA, Reynolds CR, Lowe P, Moore JJ. The continuous performance test: a window on the neural substrates for attention? Archives of Clinical Neuropsychology 2002; 17(3): 235–272. [PubMed] [Google Scholar]
- 73.Borgaro S, Pogge DL, DeLuca VA, Bilginer L, Stokes J, Harvey PD. Convergence of different versions of the continuous performance test: clinical and scientific implications. Journal of clinical and experimental neuropsychology 2003; 25(2): 283–292. [DOI] [PubMed] [Google Scholar]
- 74.Addington J, Addington D. Attentional vulnerability indicators in schizophrenia and bipolar disorder. Schizophr Res 1997; 23(3): 197–204. [DOI] [PubMed] [Google Scholar]
- 75.Buchanan RW, Strauss ME, Breier A, Kirkpatrick B, Carpenter WT, Jr. Attentional impairments in deficit and nondeficit forms of schizophrenia. The American journal of psychiatry 1997; 154(3): 363–370. [DOI] [PubMed] [Google Scholar]
- 76.Sponheim SR, McGuire KA, Stanwyck JJ. Neural anomalies during sustained attention in first-degree biological relatives of schizophrenia patients. Biological psychiatry 2006; 60(3): 242–252. [DOI] [PubMed] [Google Scholar]
- 77.Nuechterlein K, Parasuraman R, Jiang Q. Visual sustained attention: image degradation produces rapid sensitivity decrement over time. Science 1983; 220(4594): 327–329. [DOI] [PubMed] [Google Scholar]
- 78.Volz HP, Gaser C, Häger F, Rzanny R, Pönisch J, Mentzel HJ et al. Decreased frontal activation in schizophrenics during stimulation with the Continuous Performance Test - a functional magnetic resonance imaging study. European Psychiatry 1999; 14(1): 17–24. [DOI] [PubMed] [Google Scholar]
- 79.Honey GD, Pomarol-Clotet E, Corlett PR, Honey RA, McKenna PJ, Bullmore ET et al. Functional dysconnectivity in schizophrenia associated with attentional modulation of motor function. Brain: a journal of neurology 2005; 128(Pt 11): 2597–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liddle PF, Laurens KR, Kiehl KA, Ngan ET. Abnormal function of the brain system supporting motivated attention in medicated patients with schizophrenia: an fMRI study. Psychological medicine 2006; 36(8): 1097–1108. [DOI] [PubMed] [Google Scholar]
- 81.Gur RE, Turetsky BI, Loughead J, Snyder W, Kohler C, Elliott M et al. Visual attention circuitry in schizophrenia investigated with oddball event-related functional magnetic resonance imaging. The American journal of psychiatry 2007; 164(3): 442–449. [DOI] [PubMed] [Google Scholar]
- 82.Eyler LT, Olsen RK, Jeste DV, Brown GG. Abnormal brain response of chronic schizophrenia patients despite normal performance during a visual vigilance task. Psychiatry Research: Neuroimaging 2004; 130(3): 245–257. [DOI] [PubMed] [Google Scholar]
- 83.Morey RA, Inan S, Mitchell TV, Perkins DO, Lieberman JA, Belger A. Imaging frontostriatal function in ultra-high-risk, early, and chronic schizophrenia during executive processing. Archives of general psychiatry 2005; 62(3): 254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roth M, Hong LE, McMahon RP, Fuller RL. Comparison of the Effectiveness of Conners’ CPT and the CPT-Identical Pairs at Distinguishing Between Smokers and Nonsmokers with Schizophrenia. Schizophrenia research 2013; 148(0): 29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fusar-Poli P, Deste G, Smieskova R, Barlati S, Yung AR, Howes O et al. Cognitive functioning in prodromal psychosis: a meta-analysis. Archives of general psychiatry 2012; 69(6): 562–571. [DOI] [PubMed] [Google Scholar]
- 86.White T, Schmidt M, Karatekin C. Verbal and visuospatial working memory development and deficits in children and adolescents with schizophrenia. Early Interv Psychiatry 2010; 4(4): 305–313. [DOI] [PubMed] [Google Scholar]
- 87.Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology 2009; 23(3): 315–336. [DOI] [PubMed] [Google Scholar]
- 88.Ho BC, Wassink TH, O’Leary DS, Sheffield VC, Andreasen NC. Catechol-O-methyl transferase Val158Met gene polymorphism in schizophrenia: working memory, frontal lobe MRI morphology and frontal cerebral blood flow. Molecular psychiatry 2005; 10(3): 229, 287–298. [DOI] [PubMed] [Google Scholar]
- 89.Deserno L, Sterzer P, Wustenberg T, Heinz A, Schlagenhauf F. Reduced prefrontal-parietal effective connectivity and working memory deficits in schizophrenia. The Journal of neuroscience: the official journal of the Society for Neuroscience 2012; 32(1): 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Perlstein WM, Carter CS, Noll DC, Cohen JD. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. The American journal of psychiatry 2001; 158(7): 1105–1113. [DOI] [PubMed] [Google Scholar]
- 91.Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R et al. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cerebral cortex (New York, NY: 1991) 2000; 10(11): 1078–1092. [DOI] [PubMed] [Google Scholar]
- 92.Cannon TD, Glahn DC, Kim J, Van Erp TG, Karlsgodt K, Cohen MS et al. Dorsolateral prefrontal cortex activity during maintenance and manipulation of information in working memory in patients with schizophrenia. Archives of general psychiatry 2005; 62(10): 1071–1080. [DOI] [PubMed] [Google Scholar]
- 93.Barch DM, Csernansky JG. Abnormal parietal cortex activation during working memory in schizophrenia: verbal phonological coding disturbances versus domain-general executive dysfunction. The American journal of psychiatry 2007; 164(7): 1090–1098. [DOI] [PubMed] [Google Scholar]
- 94.Cohen JD, Servan-Schreiber D. Context, cortex, and dopamine: A connectionist approach to behavior and biology in schizophrenia. Psychological Review 1992; 99(1): 45–77. [DOI] [PubMed] [Google Scholar]
- 95.Posner MI, & Snyder CRR Attention and cognitive control In: Solso RL (ed). Information Processing and Cognition: Loyola Symposium. Erlbaum Associates.: New Jersey, 1975. [Google Scholar]
- 96.Koechlin E, Summerfield C. An information theoretical approach to prefrontal executive function. Trends in Cognitive Sciences 2007; 11(6): 229–235. [DOI] [PubMed] [Google Scholar]
- 97.Goulas A, Uylings HBM, Stiers P. Mapping the Hierarchical Layout of the Structural Network of the Macaque Prefrontal Cortex. Cerebral Cortex 2014; 24(5): 1178–1194. [DOI] [PubMed] [Google Scholar]
- 98.Badre D, D’Esposito M. Functional Magnetic Resonance Imaging Evidence for a Hierarchical Organization of the Prefrontal Cortex. Journal of Cognitive Neuroscience 2007; 19(12): 2082–2099. [DOI] [PubMed] [Google Scholar]
- 99.Ridderinkhof KR, van den Wildenberg WP, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain and cognition 2004; 56(2): 129–140. [DOI] [PubMed] [Google Scholar]
- 100.Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 Functional Neuroimaging Studies of Executive Function in Schizophrenia. Archives of general psychiatry 2009; 66(8): 811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ray KL, Lesh TA, Howell AM, Salo TP, Ragland JD, MacDonald AW et al. Functional network changes and cognitive control in schizophrenia. NeuroImage Clinical 2017; 15: 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kerns JG, Cohen JD, MacDonald AW 3rd Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science 2004; 303(5660): 1023–1026. [DOI] [PubMed] [Google Scholar]
- 103.Braver TS, Cohen JD, & Barch DM The Role of Prefrontal Cortex in Normal and Disordered Cognitive Control: A Cognitive Neuroscience Perspective In: IDTSRT Knight (ed). Principles of frontal lobe function. Oxford University Press: Oxford, England, 2002, pp 428–448. [Google Scholar]
- 104.Lesh TA, Westphal AJ, Niendam TA, Yoon JH, Minzenberg MJ, Ragland JD et al. Proactive and reactive cognitive control and dorsolateral prefrontal cortex dysfunction in first episode schizophrenia. Neuroimage-Clin 2013; 2: 590–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yoon JH, Minzenberg MJ, Ursu S, Ryan Walter BS, Wendelken C, Ragland JD et al. Association of dorsolateral prefrontal cortex dysfunction with disrupted coordinated brain activity in schizophrenia: relationship with impaired cognition, behavioral disorganization, and global function. The American journal of psychiatry 2008; 165(8): 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.MacDonald AW 3rd, Carter CS, Kerns JG, Ursu S, Barch DM, Holmes AJ et al. Specificity of prefrontal dysfunction and context processing deficits to schizophrenia in never-medicated patients with first-episode psychosis. The American journal of psychiatry 2005; 162(3): 475–484. [DOI] [PubMed] [Google Scholar]
- 107.Fornito A, Yoon J, Zalesky A, Bullmore ET, Carter CS. General and specific functional connectivity disturbances in first-episode schizophrenia during cognitive control performance. Biological psychiatry 2011; 70(1): 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lesh TA, Niendam TA, Minzenberg MJ, Carter CS. Cognitive control deficits in schizophrenia: mechanisms and meaning. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 2011; 36(1): 316–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Blumenfeld RS, Parks CM, Yonelinas AP, Ranganath C. Putting the pieces together: the role of dorsolateral prefrontal cortex in relational memory encoding. J Cogn Neurosci 2011; 23(1): 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Anderson MC, Hanslmayr S. Neural mechanisms of motivated forgetting. Trends Cogn Sci 2014; 18(6): 279–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fawcett JM, Taylor TL. Forgetting is effortful: Evidence from reaction time probes in an item-method directed forgetting task. Memory & Cognition 2008; 36(6): 1168–1181. [DOI] [PubMed] [Google Scholar]
- 112.Wylie GR, Foxe JJ, Taylor TL. Forgetting as an Active Process: An fMRI Investigation of Item-Method–Directed Forgetting. Cerebral Cortex 2008; 18(3): 670–682. [DOI] [PubMed] [Google Scholar]
- 113.Nowicka A, Marchewka A, Jednoróg K, Tacikowski P, Brechmann A. Forgetting of Emotional Information Is Hard: An fMRI Study of Directed Forgetting. Cerebral Cortex 2011; 21(3): 539–549. [DOI] [PubMed] [Google Scholar]
- 114.Conway MA, Fthenaki A. Disruption of Inhibitory Control of Memory Following Lesions to the Frontal and Temporal Lobes. Cortex 2003; 39(4): 667–686. [DOI] [PubMed] [Google Scholar]
- 115.Racsmány M, Conway MA, Garab EA, Cimmer C, Janka Z, Kurimay T et al. Disrupted memory inhibition in schizophrenia. Schizophrenia Research 2008; 101(1): 218–224. [DOI] [PubMed] [Google Scholar]
- 116.Müller U, Ullsperger M, Hammerstein E, Sachweh S, Becker T. Directed forgetting in schizophrenia. European archives of psychiatry and clinical neuroscience 2005; 255(4): 251–257. [DOI] [PubMed] [Google Scholar]
- 117.Ragland JD, Ranganath C, Phillips J, Boudewyn MA, Kring AM, Lesh TA et al. Cognitive Control of Episodic Memory in Schizophrenia: Differential Role of Dorsolateral and Ventrolateral Prefrontal Cortex. Frontiers in human neuroscience 2015; 9(604). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends in Cognitive Sciences 2007; 11(9): 379–386. [DOI] [PubMed] [Google Scholar]


