Abstract
Total hip arthroplasty (THA) alleviates hip pain and improves joint function. Current implant design permits long-term survivorship of THAs, but certain metal-on-metal (MoM) articulations can portend catastrophic failure due to adverse local tissue reactions (ALTR). Here, we identified biological and molecular differences between periacetabular synovial tissues of patients with MoM THA failure undergoing revision THA compared to patients undergoing primary THA for routine osteoarthritis (OA) Analysis of tissue biopsies by RNA-sequencing (RNA-seq) revealed that MoM patient samples exhibit significantly increased expression of immune response genes but decreased expression of genes related to extracellular matrix (ECM) remodeling. Thus, interplay between local tissue inflammation and ECM degradation may account for the pathology and compromised clinical outcomes in select patients with MoM implants. We conclude that adverse responses of host tissues to implant materials result in transcriptomic modifications in patients with MoM implants that permit consideration of strategies that could mitigate ECM damage.
Keywords: Total hip arthroplasty, metallosis, adverse local tissue reaction, metal-on-metal, cell biology, molecular genetics
INTRODUCTION
Total hip arthroplasty (THA) is widely considered one of the most effective surgical procedures in modern medicine, transforming the management of patients with end-stage degenerative hip disease [1, 2]. The annual number of primary THAs is expected to increase 174% by 2030, and the number of revision THAs is projected to double by 2026 [3]. Currently, fewer than 1% of implanted THA constructs have metal-on-metal (MoM) articulations [4, 5]. However, prior to important discoveries documenting increased rates of revision due to adverse local tissue reaction (ALTRs) [6–9], MoM implants were common, and currently constitute roughly 35% of all in situ THAs in the United States [10, 11]. Historically, MoM wear rates were thought to be 60 times lower than wear rates for other prostheses and were once believed to have greater implant survivorship [12, 13]. However, national joint registry data have demonstrated that 20% of MoM THAs need to be revised at 10–13 years, while only 4% of metal-on-polyethylene (MoP) implants require revision at similar follow-up [4, 5]. This represents a substantial cohort as approximately 1 million MoM prostheses have been implanted, mostly in the last 15 years [10]. Reasons for THA failure of MoM implants include chronic pain [14], metallosis [6], pseudotumor formation [7], aseptic loosening [15], and systemic metal toxicities [16, 17]. Analysis of the biological and molecular consequences of MoM wear debris on capsular tissues permits assessment of adverse effects on the joint tissues of patients.
Patients with MoM constructs carry a greater risk of THA failure relative to patients with other designs, reinforcing the importance of in vivo implant monitoring. Previous studies have demonstrated that cobalt-chromium (CoCr) implant wear debris can induce systemic toxicity leading to catastrophic implant failure [18]. In the absence of patient-reported symptoms, clinical assessments rely on elevated serum Co or Cr levels, or radiographic evidence of implant failure. These tests provide limited guidance to the potential onset of local adverse events while monitoring MoM THAs [19]. While the blood concentrations of metals are used as a proxy for implant wear and predictors of systemic toxicity, they do not offer consistent prognostic value for the management of these patients [8, 10, 20, 21]. Furthermore, a clear correlation between systemic metal ion levels and orthopedic implant wear has not been established [22], in part because patients exhibit a broad range of tolerances to metal exposure (both locally and systemically) [23, 24]. Accurate identification of patients at higher risk for the development of complications prior to the onset of symptoms would significantly improve clinical care for MoM THA patients.
High-throughput next generation sequencing of mRNAs (RNA-seq) can enhance the clinical characterization of ALTR in patients with failed MoM THAs. For example, RNA-seq provides the basis for the identification of differentially expressed genes in cells and tissues. Specifically, we have applied RNA-seq to establish molecular differences in pericyte-like mesenchymal stromal cells, which could have a role in arthrofibrosis, as well as tissues of patients with total knee arthroplasty instability [25, 26]. Hence, RNA-seq comparison of periacetabular synovial tissues that surround metal-based prosthetics can be compared to that of patients with native, otherwise normal tissue. The long-term presence of metal debris generated by physiologic implant corrosion (i.e., particulate metal and cations derived from metal oxidation) may influence the surrounding joint tissue’s reactivity to osteolysis, as well as its ability to regulate peri-capsular tissue repair and remodeling. In particular, MoM implants are known to generate wear particles and/or the metallic debris that accumulates in surrounding tissues and visceral organs [16, 17]. In order to maintain metal homeostasis in the joint space and avoid hypersensitivity reactions and cytotoxicity [21], cytoprotective proteins such as metallothioneins (MTs) are crucial for the maintenance of cellular functions after extracellular exposure to metal ions. Previously published studies have shown systemic upregulation of MTs in vitro and in vivo using animal models [27–29]; however, none have directly assessed in vivo processes in patients with MoM THAs using CoCr articulations.
The goals of our study were to investigate the differential gene expression patterns of periacetabular synovial tissue in patients with failed CoCr MoM THAs compared to implant-naïve control patients (with primary THAs) by utilizing RNA-sequencing. We characterized expression patterns of immune cell markers and metal homeostasis proteins in the local tissue of patients with MoM THAs that may enhance detection strategies to clinical screening and patient management methods for implant failure.
MATERIALS & METHODS
Patient Enrollment & Tissue Handling
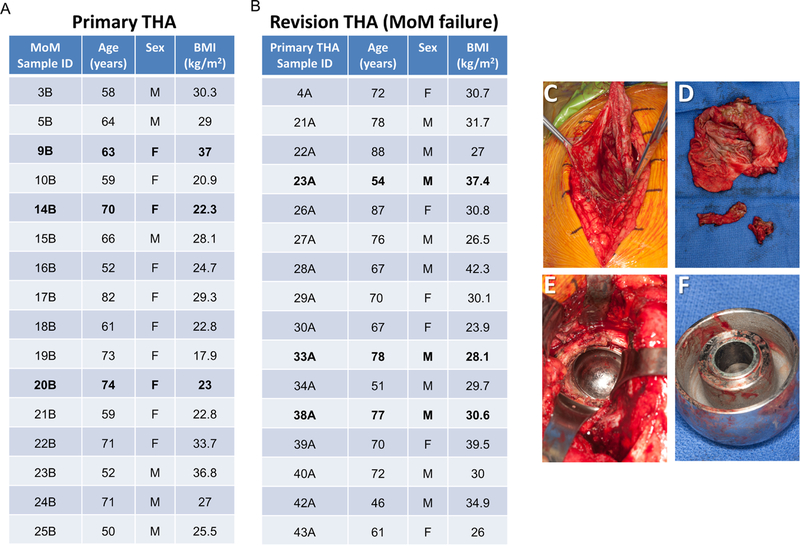
In accordance with our approved IRB protocol (09–000115, Mayo Clinic, Rochester, MN) all patients were identified, verbally informed, and signature-consented prior to enrolling in the study. When possible, patient clinical data were screened and matched based on age (±7.5 years), sex, and body mass index (BMI) (±7.5 kg/m2). We collected a total of 32 patient samples; 16 primary THAs (Figure 1A) and 16 revision THA for MoM failure (Figure 1B). At the time of revision surgery, periacetabular synovial tissue (either revision MoM THA or control) was carefully dissected by the operative surgeon using a scalpel. After rinsing with sterile PBS, tissues were placed in RNase/DNase-free polycon containers, immediately flash-frozen in liquid nitrogen, and stored at −80°C until used for RNA extraction. To avoid unnecessary risks to patients, capsular tissues were only collected for research when sufficient quantities of adjacent tissues were removed during the normal course of surgery and tissue removal was determined to not cause any adverse harm to the patient (Figure 1C-F).
Figure 1.
Overview of the patient cohort and tissue biopsies used for RNA-seq (samples analyzed by RNA-seq are presented in bold font) (A-B). Surgical photograph of periacetabular tissue sampling in diseased patient (C) and surgical photograph of removed tissue sent for tissue processing and RNA-seq analysis (D). Intraoperative photograph of metal corrosion from metal-on-metal cobalt-chromium implant from acetabular component (E) and from explanted femoral head component (F) at the time of revision THA.
mRNA Isolation
Frozen biopsy samples were pulverized into powder using a cold mortar and pestle. Crushed samples were then placed into Qiazol reagent and homogenized using the TissueLyser LT (Qiagen, Hilden, Germany). Genomic RNA extractions were performed using the miRNeasy mini kit (Qiagen, Venlo, Netherlands) according to the manufacturer’s protocol, and eluted in 50µL total volume. A NanoDropper (Thermo Fisher Scientific, Waltham, MA) was then used to measure concentrations and purity ratios. Additionally, the Mayo Clinic Gene Expression Core (Advanced Genomics Technology Center, Mayo Clinic, Rochester, MN) provided RNA integrity (RIN) scores for prioritization of samples best suited to RNA-seq data acquisition.
Next Generation mRNA-sequencing (RNA-seq)
RNA sequencing and subsequent bioinformatic analysis were performed in collaboration with the Mayo Clinic RNA sequencing and bioinformatics cores as previously described [25, 30]. In brief, library preparation was performed using the TruSeq RNA library preparation kit (Illumina, San Diego, CA). Polyadenylated mRNAs were selected using oligo dT magnetic beads. TruSeq Kits were used for indexing to permit multiplex sample loading on the flow cells. Paired-end sequencing reads were generated on the Illumina HiSeq 2000 sequencer. Quality control for concentration and library size distribution was performed using an Agilent Bioanalyzer DNA 1000 chip and Qubit fluorometry (Invitrogen, Carlsbad, CA). Sequence alignment of reads and determination of normalized gene counts were performed using the MAP-RSeq (v.1.2.1) workflow, utilizing TopHat 2.0.6[30] and HTSeq [31]. Normalized read counts were expressed as reads per kilobasepair per million mapped reads (RPKM). Count data for these samples has been made available under GEO Accession Number GSE119331.
Computational Analysis and Statistics
Normalized read counts in the form of RPKM were used for all subsequent bioinformatics analyses. Genes with RPKM values < 0.3 in both samples were excluded from the dataset. Principal component analysis was conducted using the ClustVis online tool (cited below). Hierarchical clustering was performed using Morpheus matrix visualization and analysis software (Broad Institute) after a Log2 adjustment was made for each gene row. Discovery of differentially expressed genes (MoM vs. controls) was conducted using Microsoft Excel 2010. A differential expression plot was generated using GraphPad Prism version 7.03 for Windows. Protein-protein interaction networks were generated using STRING Database version 10.5[32, 33].
RESULTS
We collected 16 samples of MoM THAs with CoCr articulations being revised for ALTR (Figure 1B). During each procedure, we collected periacetabular synovial tissue at the time of surgery for laboratory analysis (Figure 1C-F). During the same timeframe of this study, we also collected periacetabular synovial tissue from patients undergoing primary THA for end-stage degenerative osteoarthritis (Figure 1A). It is important to note that control patients had never received a CoCr hip implant, and for the intent of this study were therefore considered CoCr implant-naïve patients.
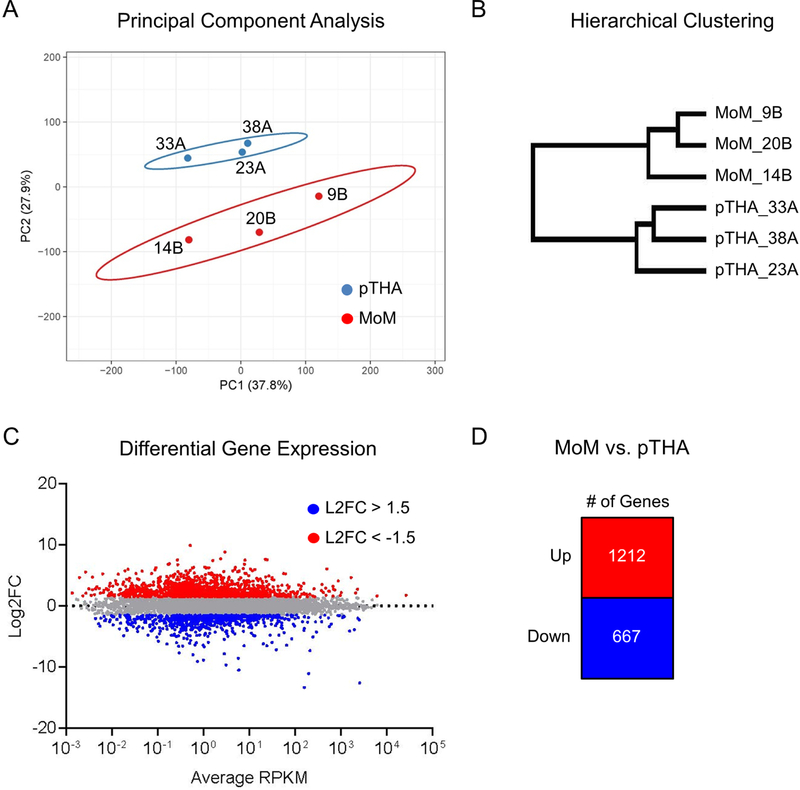
We isolated mRNA from all periacetabular synovial tissues that were collected and chose 3 primary THAs and 3 revision THAs specifically revised for MoM THAs to be analyzed by RNA-Seq (Figure 1A-B, bold). We first assessed whether or not the two groups (primary THA or MoM) displayed distinct gene expression profiles. Unsupervised principal component analysis was performed using all genes with expression values RPKM value > 0.3 (Figure 2A). We note distinct clustering of the MoM and primary THA samples. Additionally, unsupervised hierarchical clustering was performed using the same gene sets (Figure 2B), which displayed distinct branching between the two groups. To better understand the gene signatures driving this differential clustering, we performed a comparative analysis of MoM vs primary THAs to generate an MA plot of average RPKM vs. log2FC (Figure 2C). In doing so, we generated lists of genes that were upregulated (n=1212) or downregulated (n=667) greater than 1.5 fold in MoM samples compared to primary THA samples (Figure 2D, Supplemental Table 1). Together, these data demonstrate that the MoM patient samples possess a unique gene expression profile distinct from the primary THA controls.
Figure 2.
Synovial tissue derived from metallosis patients has a distinct gene expression profile. Unsupervised principal component analysis (A) and hierarchical clustering (B) was performed using expression profiles of genes expressed > 0.3 RPKM (n= 15,158) across all six specimens. Comparative analysis of expression data in metallosis patients (n=3) versus normal control (n=3) yields sets of upregulated (red) and downregulated (blue) genes (C) as summarized in (D).
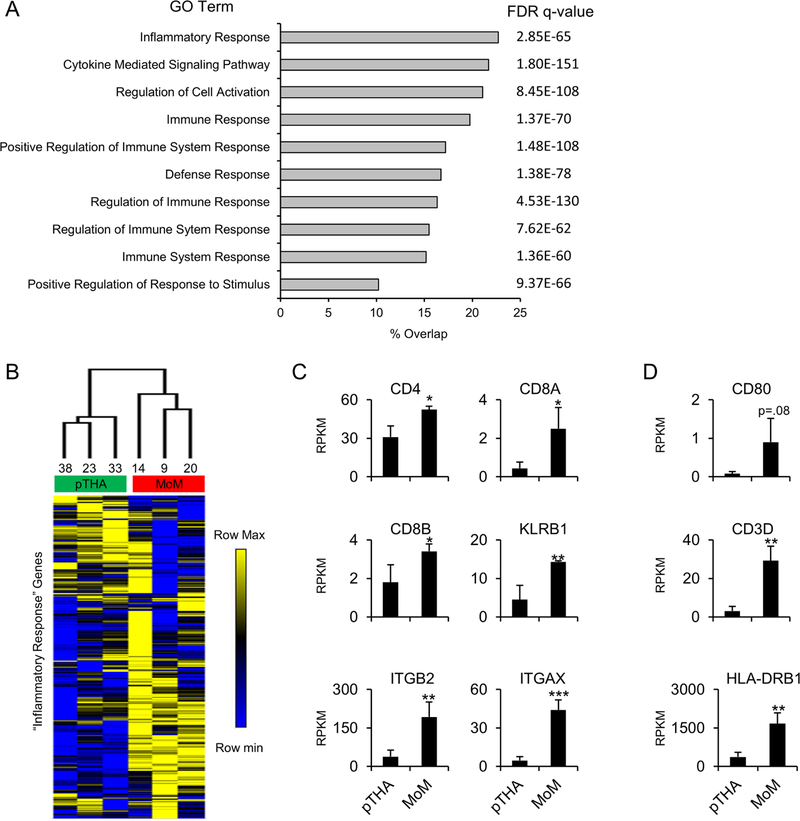
To better understand the biological significance of genes upregulated in MoM samples (Figure 2D), we utilized the gene set enrichment analysis (GSEA) molecular signatures database (MSigDB) to compute the overlap of our gene list (n=1212) with all gene ontology (GO) gene sets. Results of this analysis demonstrated significant enrichment for GO terms associated with immune response such as “Inflammatory Response” and “Cytokine Mediated Signaling Pathway” (Figure 3A). The “Inflammatory Response” gene list had the greatest overlap with our gene set (23%). Therefore, we filtered our gene expression matrix for these genes (Supplemental Table 2) and performed hierarchical clustering (Figure 3B). We note distinct clustering of the primary THA and MoM samples when assed over the “Inflammatory Response” genes suggesting a unique immune reaction signature in the MoM patients. General immune response genes (CD4, CD8A, CD8B, KLRB1, ITGB2, and ITGAX) all demonstrate significant upregulation in the MoM patients (Figure 3C). Additionally, we note an increase in M1 macrophage markers (CD80, CD3D, and HLA-DRB1) in the MoM samples (Figure 3D). Together, these findings indicate that the upregulated gene set that distinguishes the MoM samples from the primary THA samples is largely comprised of immune response genes.
Figure 3.
Inflammatory and immune response genes are upregulated in tissue derived from metallosis patients. Gene set enrichment analysis was performed on the 1,212 upregulated genes identified in Fig. 1D. Overlap of this gene set with curated gene sets was calculated and the 10 most enriched gene lists are displayed with false discover rate (FDR) q-value shown (A). Hierarchical clustering was performed using the RPKM values for the genes associated with the “Inflammatory Response” gene set (n=454 genes) (B). Average RPKM value (n=3) for control (pTHA) and metallosis (MoM) tissues for general inflammatory response genes (C) and specific M1 macrophage markers (D). Statistical significance of MoM compared to pTHA is indicated when appropriate (*: p<0.05, **: p<0.01, ***: p<0.001, n.s.: not significant).
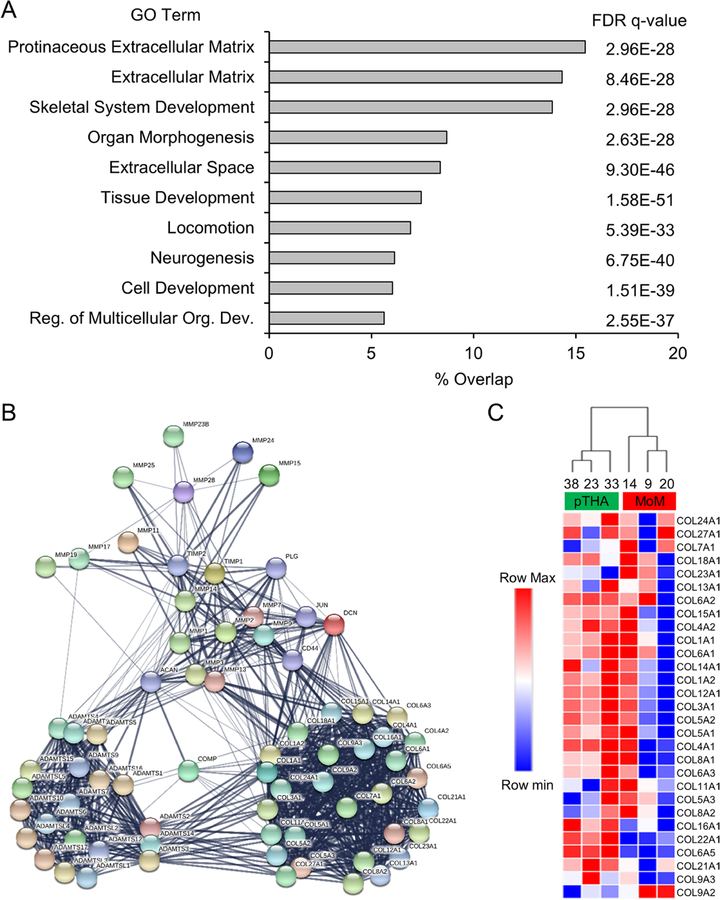
To evaluate the set of downregulated genes identified in the MoM samples (Figure 2D), we again utilized the GSEA MSigDB to compute the overlap of the downregulated gene list (n=667) with all GO gene sets. Interestingly, gene lists with the most overlap included GO terms associated with ECM and tissue development (Figure 4A). As ECM appeared in several of the enriched gene lists, we selected the gene ID’s within the “Extracellular Matrix” gene list (overlap=14%) for further analysis. Genes encoding structural ECM proteins and ECM remodeling enzymes (i.e. Collagens and Matrix Metalloproteinases) were selected from this list and used for STRING protein-protein interaction analysis (Figure 4B). We note a distinct node of collagens within the interaction matrix. Using these collagen genes, we filtered our gene expression matrix and performed hierarchical clustering (Figure 4C). A significant reduction of these collagen genes in the MoM samples suggest decreased ECM deposition and compromised local tissue integrity in the periarticular tissue.
Figure 4 A-C.
Metallosis patients demonstrate downregulation of extracellular matrix proteins and maintenance enzymes. Gene set enrichment analysis was performed on the 667 downregulated genes identified in Fig. 1D. Overlap of this gene set with curated gene sets was calculated and the 10 most enriched gene lists are displayed with false discover rate (FDR) q-value shown (A). STRING protein-protein interaction analysis was conducted with a subset of genes (n=69) associated with the “Extracellular Matrix” gene list (B). All collagen genes within this gene list were extracted, and hierarchical clustering was performed using the RPKM values for these genes after a log2 adjustment (C).
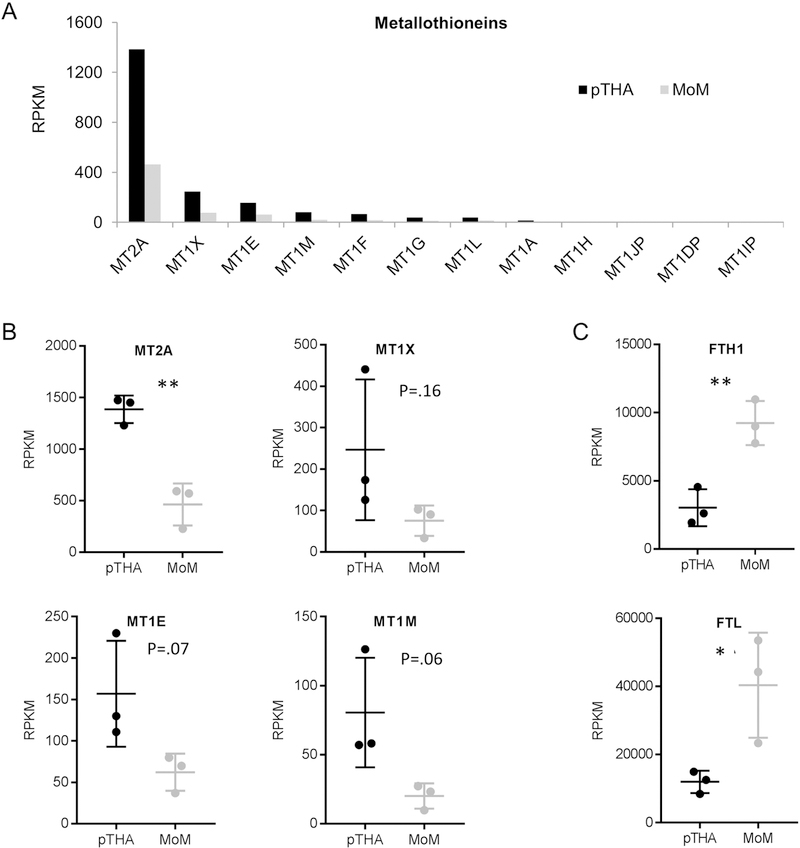
Heavy metal exposure may induce expression of metal ion detoxifying enzymes, including a family of distinct MT proteins. Therefore, we examined the expression levels of these genes in our patient samples that received CoCr implants (Figure 5A-B). Interestingly, we observe decreased expression of the MTs in the MoM patients. We also assessed expression levels of the ferritin protein subunits (FTH1 and FTL) as independent indicators of cobalt toxicity and find a significant increase in both FTH1 and FTL expression in the MoM patients (Figure 5C).
Figure 5 A-C.
mRNA expression levels of the metallothionein (MT) proteins determined by RNA-Seq (A). Box and whisker plot of the mRNA expression levels for the four MTs with the highest RPKM value for each sample (n=3 in each group) (B). Box and whisker plot of the mRNA expression levels for ferritin heavy chain 1 (FTH1) and ferritin light chain (FTL) for each sample (n=3 in each group) (C). Statistical significance of MoM compared to pTHA is indicated when appropriate (*: p<0.05, **: p<0.01, ***: p<0.001)
The results of our analyses indicate that the synovial samples collected from MoM patients demonstrate a unique gene expression profile. Specifically, these tissues have an increase in immune response genes and a decrease in ECM structural and maintenance genes. Using RNA sequencing, we identified augmented expression of several immune and inflammatory signaling markers, metallothioneins, collagens and extracellular matrix remodeling enzymes, and heavy metal stress markers responsible for metal homeostasis in our analyzed MoM tissue samples.
DISCUSSION
This study indicates that the underlying pathology of failed MoM THAs in patients with CoCr articulation incites an inflammatory response. This response activates a positive feedback like-mechanism provoking the destruction of surrounding connective tissue which, in turn, invokes further inflammation and tissue degradation. If left untreated, patients with CoCr implants will likely experience the clinical symptoms characteristic of ALTR and require revision THA.
All metals implanted in the body undergo corrosion and liberate ions which activate natural protective cellular mechanisms[21]. Metals commonly found in implants can induce a Type IV, or ‘delayed type’ hypersensitivity reaction in 10–15% of the population[34]. The majority of immune mediators in this cell-mediated process are macrophages or T-cells primed by circulating antigen presenting cells; only 5% are antigen-specific T-cells[35]. T-cell, myelocyte, leukocyte, and lymphocyte suppression can also lead to serum Co and Cr levels five-fold higher than the upper limits of normal [36–39]. Systemic metal toxicity, defined differently for each type of metal, leads to a host of issues including ALTRs, commonly referred to as hypersensitivity reactions. As our results demonstrate, the significant upregulation of gene families involved in inflammatory responses, cytokine mediated signaling pathways, and immune system responses suggests there is a substantial immunological process initiated by the failure to adequately remove metal ions from the periarticular extracellular environment of the hip joint. Hence, our results provide an important advance in our knowledge, by identifying which genes are specifically involved in the clinical pathology of MoM THA.
It has been proposed that particulate debris released from CoCr implants activates macrophages, causing the release of IL-1 and other immune mediators promoting the phagocytosis of the metal debris [40]. Over time, the physiologic corrosion of metal produces metal ion debris and its subsequent uptake by macrophages can result in grey/black discoloration of the surrounding tissue, which is referred to as ‘metallosis’ (Figure 1E-F) [41, 42]. The aforementioned complications arise from binding of metal debris to albumin and other proteins, decreases in myeloid and lymphoid cell lines, and aberrant immune system activation[43]. Complications associated with metal toxicity affect almost every organ system including the nervous system[44, 45], musculoskeletal system, ocular, auditory[46], renal, hepatic, and many others[47, 48]. Not only does metallic debris affect organisms on a systemic level, but cytotoxicity, carcinogenesis and mutagenesis, are also well documented effects of metal exposure on the cellular level [49, 50].
Previous publications have demonstrated that metal implants lead to debris accumulation and increased expression of metallothioneins, particularly MT1 and MT2A in surrounding local tissue, peripheral blood, and visceral organs, regardless of wear pattern[21, 27, 29]. Remarkably, our RNA-seq data show that the metallothioneins were significantly downregulated in the MoM patients, suggesting that decreased gene expression of key MTs may preclude an effective endogenous response to mitigate the presence of non-physiological levels of metal ions. The role of MTs, specifically the MT1 family, in protecting the body against free metal ions and damage from reactive oxygen species, has been previously described [28]. In the absence of MTs, cells exposed to metal debris respond similarly to those exposed to low-dose radiation treatment [51]. This may be the etiology in the MoM THA failures described here, as downregulated gene expressions of MTs may compromise cytoprotective mechanisms and contribute to the pathological onset of metallosis. In support of this hypothesis, MT1/2-null mice have been shown to be more susceptible to CdCl2 bone injury [52].
Studies have shown that MoM implants generate 1012–1014 alloy particles per year, far more than MoP THA implants [29, 53]. Additional literature has demonstrated the metal debris generated by MoM constructs falls within the nanometer size range, much smaller than MoP and other THA systems [35]. This smaller size decreases the potential for osteolysis and aseptic loosening, but increases the spread of metal particles via the lymphatic circulation, where metals consumed by macrophages tend to accrue in the reticulo-endothelial system (liver, spleen, bone marrow) and lymph nodes [16, 17, 35, 54]. It is for this reason that MoM THA patients often present with pseudotumor, reactive skin changes and pain rather than aseptic loosening of the joint [41, 42].
A substantial number of collagen genes and MMPs were also demonstrated to be significantly lower in the MoM patients. These proteins are critical for ECM structural integrity and remodeling as well as wound healing and the stimulation of osteoblast growth and proliferation. Impaired function of local reduction of these proteins may impair regeneration of the injured joint tissue [55, 56]. Further, a compromised ECM may reduce the capacity for the local synovial tissue to cope with metal ion toxicity created by the MoM implant. Together, this contributes to an unstable soft-tissue environment prone to aseptic loosening and painful degradation of the joint tissue.
Current guidelines for management recommend monitoring asymptomatic MoM THA recipients with sequential assessments of serum metal ion levels [42, 57]. For patients who develop symptoms, advanced imaging studies are often indicated to evaluate the state of soft-tissues and assess pseudotumor formation [42, 57]. The results from our study provide a framework for the categorization of MoM THA patients into subgroups that reflects their overall risk of developing a symptomatic THA failure. Our transcriptomic data highlight key differences between patients with failed CoCr MoM implants and “CoCr naïve” patients, it may be possible to provide patients with an opportunity to monitor their THA using a personalized approach and precision medicine management. For example, determining to what extent elevated immune response or heavy metal stress gene expression is correlated to a tissue metallosis or pseudotumor formation severity, synovial fluid markers, or inflammatory markers such as CRP and ESR values would be useful. Further studies that categorize MoM failure mechanisms (radiographic evidence of aseptic loosening, or MRI detection of pseudotumor fluid collection) and assess their relative heavy metal response levels, may provide additional information about this relationship. Our approach using periacetabular tissue biopsy depicts the local tissue response and could aid in the detection of ALTRs before blood metal levels are detected as abnormal or toxic.
While metallothioneins were initially thought to play a predominant role in the physiologic reaction to metal debris, our present investigation reveals a critical role of the immunologic response to metal debris and its subsequent initiation of extracellular matrix deposition and maintenance. Interestingly, as indicated by our present findings, the genes encoding the subunits of the ferritin protein complex may be better indicators of CoCr toxicity in affected MoM patients. Future studies on the epigenetic regulation of bone or capsular tissue response to metal exposure may provide information on how metal ion or nanoparticles interfere with DNA-methylation or affect histone-modification and contribute to the failure process in vitro and in vivo studies. Understanding the role of the host immune system and the individual genetic variations therein will help predict which patients are at an increased risk of developing adverse reactions to MoM THAs.
Research assessing the long-term risks of metal wear debris from implants in joint replacement has translational application in other medical disciplines [51]. Devices made of metals, plastics, and other composite materials have long been used in many medical procedures, including orthopedic surgery, cardiology, and dentistry. The long-term monitoring of the biocompatibility of these devices is often difficult, requiring a longitudinal study of the complications and failure patterns that is rarely known. Development of diagnostic tools to monitor these implants prior to the overt detection of symptoms is relevant to MoM THA where the risk of complication is well described [6–8]. This information may be helpful in identifying patients at risk for ALTR prior to the onset of clinical symptoms, as many metal-on-polyethylene arthroplasty constructs in use today contain CoCr femoral heads, which have been demonstrated to release metal ions into the joint space, causing periprosthetic hypersensitivity reactions and metal toxicity [58–60]. As precision medicine trends toward customized modular approaches, the classification of MoM THA patients into molecular sub-classes that indicate relative risk of THA complication would revolutionize our ability to treat these patients while ensuring appropriate use of clinical resources (e.g., relevant tests) that directly align with the needs of a patient.
Supplementary Material
Highlights.
Tissues exposed to CoCr debris exhibit a distinct gene expression profile
CoCr exposure increased expression of genes involved in inflammatory response
Gene expression of ECM proteins is reduced in the presence of CoCr debris
CoCr presence results in elevated expression of genes encoding Ferratin subunits
Gene expression analysis may provide a diagnostic tool to monitor metallic implants
ACKNOWLEDGMENTS
We thank members of the Abdel and van Wijnen laboratories, and in particular Anthony Viste, Nic Reina, Carter Jones, Jie (‘Jay’) Yao and Scott Riester for stimulating discussions and/or assistance with reagents and procedures. Research reported in this publication was supported by the National Institutes of Arthritis and Musculoskeletal and Skin Disease of the National Institute of Health under Award Numbers R01 AR072597 for MPA, R01 AR049069 for AJVW, and F32 AR068154 for EAL. The Mayo Clinic Center for Regenerative Medicine also provided a Post-doctoral Career Development Award to Roman Thaler. We also appreciate the generous philanthropic support of Anna-Maria and Stephen Kellen Foundation and William H. and Karen J. Eby, and the charitable foundations in their names. Matthew P. Abdel, M.D. is a paid consultant for Stryker. Daniel J. Berry, M.D. receives royalties from DePuy, and David G. Lewallen, M.D. receives royalties from Zimmer-Biomet.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No other authors have conflicts of interest to disclosure.
REFERENCES
- [1].Learmonth ID, Young C, Rorabeck C, The operation of the century: total hip replacement, Lancet (London, England), 370 (2007) 1508–1519. [DOI] [PubMed] [Google Scholar]
- [2].Pivec R, Johnson AJ, Mears SC, Mont MA, Hip arthroplasty, The Lancet, 380 (2012) 1768–1777. [DOI] [PubMed] [Google Scholar]
- [3].Kurtz S, Ong K, Lau E, Mowat F, Halpern M, Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030, The Journal of bone and joint surgery American volume, 89 (2007) 780–785. [DOI] [PubMed] [Google Scholar]
- [4].Peters CL, Erickson JA, Gililland JM, Clinical and radiographic results of 184 consecutive revision total knee arthroplasties placed with modular cementless stems, J Arthroplasty, 24 (2009) 48–53. [DOI] [PubMed] [Google Scholar]
- [5].Eriksen J, Christensen J, Solgaard S, Schroder H, The cementless AGC 2000 knee prosthesis: 20-year results in a consecutive series, Acta Orthop Belg, 75 (2009) 225–233. [PubMed] [Google Scholar]
- [6].Gruber FW, Bock A, Trattnig S, Lintner F, Ritschl P, Cystic lesion of the groin due to metallosis: a rare long-term complication of metal-on-metal total hip arthroplasty, J Arthroplasty, 22 (2007) 923–927. [DOI] [PubMed] [Google Scholar]
- [7].Clayton RA, Beggs I, Salter DM, Grant MH, Patton JT, Porter DE, Inflammatory pseudotumor associated with femoral nerve palsy following metal-on-metal resurfacing of the hip. A case report, The Journal of bone and joint surgery American volume, 90 (2008) 1988–1993. [DOI] [PubMed] [Google Scholar]
- [8].Pandit H, Glyn-Jones S, McLardy-Smith P, Gundle R, Whitwell D, Gibbons CL, Ostlere S, Athanasou N, Gill HS, Murray DW, Pseudotumours associated with metal-on-metal hip resurfacings, The Journal of bone and joint surgery British volume, 90 (2008) 847–851. [DOI] [PubMed] [Google Scholar]
- [9].Curfman GD, Redberg RF, Medical Devices — Balancing Regulation and Innovation, New England Journal of Medicine, 365 (2011) 975–977. [DOI] [PubMed] [Google Scholar]
- [10].Sampson B, Hart A, Clinical usefulness of blood metal measurements to assess the failure of metal-on-metal hip implants, Annals of clinical biochemistry, 49 (2012) 118–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bozic KJ, Kurtz S, Lau E, Ong K, Chiu V, Vail TP, Rubash HE, Berry DJ, The epidemiology of bearing surface usage in total hip arthroplasty in the United States, The Journal of bone and joint surgery American volume, 91 (2009) 1614–1620. [DOI] [PubMed] [Google Scholar]
- [12].Schmalzried TP, Peters PC, Maurer BT, Bragdon CR, Harris WH, Long-duration metal-on-metal total hip arthroplasties with low wear of the articulating surfaces, J Arthroplasty, 11 (1996) 322–331. [DOI] [PubMed] [Google Scholar]
- [13].Cuckler JM, The rationale for metal-on-metal total hip arthroplasty, Clinical orthopaedics and related research, 441 (2005) 132–136. [DOI] [PubMed] [Google Scholar]
- [14].Iqbal HJ, Al-Azzani WAK, Jackson-Taylor E, Clatworthy E, John A, Outcome of revision arthroplasty for failed metal-on-metal total hip replacements; is there a relation with metal ions?, Hip international : the journal of clinical and experimental research on hip pathology and therapy, 27 (2017) 235–240. [DOI] [PubMed] [Google Scholar]
- [15].Lohmann CH, Singh G, Goldau G, Müller T, Feuerstein B, Rütschi M, Meyer H, Aseptic Loosening of Metal-on-Metal (MOM) Total Hip Arthroplasties (THA) with Large-Diameter Heads, in: Knahr K (Ed.) Total Hip Arthroplasty: Tribological Considerations and Clinical Consequences, Springer Berlin Heidelberg, Berlin, Heidelberg, 2013, pp. 141–151. [Google Scholar]
- [16].Urban RM, Jacobs JJ, Tomlinson MJ, Gavrilovic J, Black J, Peoc’h M, Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacement, The Journal of bone and joint surgery American volume, 82 (2000) 457–476. [DOI] [PubMed] [Google Scholar]
- [17].Case CP, Langkamer VG, James C, Palmer MR, Kemp AJ, Heap PF, Solomon L, Widespread dissemination of metal debris from implants, The Journal of bone and joint surgery British volume, 76 (1994) 701–712. [PubMed] [Google Scholar]
- [18].Cheung AC, Banerjee S, Cherian JJ, Wong F, Butany J, Gilbert C, Overgaard C, Syed K, Zywiel MG, Jacobs JJ, Mont MA, Systemic cobalt toxicity from total hip arthroplasties: review of a rare condition Part 1 - history, mechanism, measurements, and pathophysiology, The bone & joint journal, 98-b (2016) 6–13. [DOI] [PubMed] [Google Scholar]
- [19].Drummond J, Tran P, Fary C, Metal-on-Metal Hip Arthroplasty: A Review of Adverse Reactions and Patient Management, Journal of functional biomaterials, 6 (2015) 486–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Langton DJ, Jameson SS, Joyce TJ, Hallab NJ, Natu S, Nargol AV, Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: A consequence of excess wear, The Journal of bone and joint surgery British volume, 92 (2010) 38–46. [DOI] [PubMed] [Google Scholar]
- [21].Hallab N, Merritt K, Jacobs JJ, Metal sensitivity in patients with orthopaedic implants, The Journal of bone and joint surgery American volume, 83-a (2001) 428–436. [DOI] [PubMed] [Google Scholar]
- [22].Yao JJ, Lewallen EA, Trousdale WH, Xu W, Thaler R, Salib CG, Reina N, Abdel MP, Lewallen DG, van Wijnen AJ, Local Cellular Responses to Titanium Dioxide from Orthopedic Implants, BioResearch open access, 6 (2017) 94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jantzen C, Jorgensen HL, Duus BR, Sporring SL, Lauritzen JB, Chromium and cobalt ion concentrations in blood and serum following various types of metal-on-metal hip arthroplasties: a literature overview, Acta orthopaedica, 84 (2013) 229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].MacDonald SJ, Can a safe level for metal ions in patients with metal-on-metal total hip arthroplasties be determined?, J Arthroplasty, 19 (2004) 71–77. [DOI] [PubMed] [Google Scholar]
- [25].Dudakovic A, Camilleri E, Riester SM, Lewallen EA, Kvasha S, Chen X, Radel DJ, Anderson JM, Nair AA, Evans JM, Krych AJ, Smith J, Deyle DR, Stein JL, Stein GS, Im HJ, Cool SM, Westendorf JJ, Kakar S, Dietz AB, van Wijnen AJ, High-resolution molecular validation of self-renewal and spontaneous differentiation in clinical-grade adipose-tissue derived human mesenchymal stem cells, Journal of cellular biochemistry, 115 (2014) 1816–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lewallen EA, Salib CG, Trousdale WH, Berry CE, Hanssen GM, Robin JX, Tibbo ME, Viste A, Reina N, Morrey ME, Sanchez-Sotelo J, Hanssen AD, Berry DJ, van Wijnen AJ, Abdel MP, Molecular pathology of total knee arthroplasty instability defined by RNA-seq, Genomics, (2017). [DOI] [PMC free article] [PubMed]
- [27].Jakobsen SS, Danscher G, Stoltenberg M, Larsen A, Bruun JM, Mygind T, Kemp K, Soballe K, Cobalt-chromium-molybdenum alloy causes metal accumulation and metallothionein up-regulation in rat liver and kidney, Basic & clinical pharmacology & toxicology, 101 (2007) 441–446. [DOI] [PubMed] [Google Scholar]
- [28].Fujie T, Murakami M, Yoshida E, Yasuike S, Kimura T, Fujiwara Y, Yamamoto C, Kaji T, Transcriptional Induction of Metallothionein by Tris(pentafluorophenyl)stibane in Cultured Bovine Aortic Endothelial Cells, International journal of molecular sciences, 17 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gajski G, Jelcic Z, Orescanin V, Geric M, Kollar R, Garaj-Vrhovac V, Physico-chemical characterization and the in vitro genotoxicity of medical implants metal alloy (TiAlV and CoCrMo) and polyethylene particles in human lymphocytes, Biochimica et biophysica acta, 1840 (2014) 565–576. [DOI] [PubMed] [Google Scholar]
- [30].Kalari KR, Nair AA, Bhavsar JD, O’Brien DR, Davila JI, Bockol MA, Nie J, Tang X, Baheti S, Doughty JB, Middha S, Sicotte H, Thompson AE, Asmann YW, Kocher JP, MAP-RSeq: Mayo Analysis Pipeline for RNA sequencing, BMC bioinformatics, 15 (2014) 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Metsalu T, Vilo J, ClustVis: a web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap, Nucleic acids research, 43 (2015) W566–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, Kuhn M, Bork P, Jensen LJ, von Mering C, STRING v10: protein-protein interaction networks, integrated over the tree of life, Nucleic acids research, 43 (2015) D447–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P, Jensen LJ, von Mering C, The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible, Nucleic acids research, 45 (2017) D362–d368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Thyssen JP, Johansen JD, Menne T, Nielsen NH, Linneberg A, Nickel allergy in Danish women before and after nickel regulation, The New England journal of medicine, 360 (2009) 2259–2260. [DOI] [PubMed] [Google Scholar]
- [35].Brown C, Fisher J, Ingham E, Biological effects of clinically relevant wear particles from metal-on-metal hip prostheses, Proceedings of the Institution of Mechanical Engineers. Part H, Journal of engineering in medicine, 220 (2006) 355–369. [DOI] [PubMed] [Google Scholar]
- [36].Granchi D, Savarino L, Ciapetti G, Cenni E, Rotini R, Mieti M, Baldini N, Giunti A, Immunological changes in patients with primary osteoarthritis of the hip after total joint replacement, The Journal of bone and joint surgery British volume, 85 (2003) 758–764. [PubMed] [Google Scholar]
- [37].Yang J, Black J, Competitive binding of chromium, cobalt and nickel to serum proteins, Biomaterials, 15 (1994) 262–268. [DOI] [PubMed] [Google Scholar]
- [38].Merritt K, Rodrigo JJ, Immune response to synthetic materials. Sensitization of patients receiving orthopaedic implants, Clinical orthopaedics and related research, (1996) 71–79. [PubMed]
- [39].Dahlstrand H, Stark A, Wick MC, Anissian L, Hailer NP, Weiss RJ, Comparison of metal ion concentrations and implant survival after total hip arthroplasty with metal-on-metal versus metal-on-polyethylene articulations, Acta orthopaedica, (2017) 1–6. [DOI] [PMC free article] [PubMed]
- [40].al Saffar N, Revell PA, Interleukin-1 production by activated macrophages surrounding loosened orthopaedic implants: a potential role in osteolysis, British journal of rheumatology, 33 (1994) 309–316. [DOI] [PubMed] [Google Scholar]
- [41].Fehring KA, Fehring TK, Modes of failure in metal-on-metal total hip arthroplasty, The Orthopedic clinics of North America, 46 (2015) 185–192. [DOI] [PubMed] [Google Scholar]
- [42].Chalmers BP, Perry KI, Taunton MJ, Mabry TM, Abdel MP, Diagnosis of adverse local tissue reactions following metal-on-metal hip arthroplasty, Current reviews in musculoskeletal medicine, 9 (2016) 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Du Z, Wang S, Yue B, Wang Y, Wang Y, Effects of wear particles of polyether-ether-ketone and cobalt-chromium-molybdenum on CD4- and CD8-T-cell responses, Oncotarget, 9 (2018) 11197– 11208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Green B, Griffiths E, Almond S, Neuropsychiatric symptoms following metal-on-metal implant failure with cobalt and chromium toxicity, BMC Psychiatry, 17 (2017) 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Mao X, Wong AA, Crawford RW, Cobalt toxicity--an emerging clinical problem in patients with metal-on-metal hip prostheses?, The Medical journal of Australia, 194 (2011) 649–651. [DOI] [PubMed] [Google Scholar]
- [46].Catalani S, Rizzetti MC, Padovani A, Apostoli P, Neurotoxicity of cobalt, Human & experimental toxicology, 31 (2012) 421–437. [DOI] [PubMed] [Google Scholar]
- [47].Germain MA, Hatton A, Williams S, Matthews JB, Stone MH, Fisher J, Ingham E, Comparison of the cytotoxicity of clinically relevant cobalt-chromium and alumina ceramic wear particles in vitro, Biomaterials, 24 (2003) 469–479. [DOI] [PubMed] [Google Scholar]
- [48].Tower SS, Arthroprosthetic cobaltism: neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty: a case report, The Journal of bone and joint surgery American volume, 92 (2010) 2847–2851. [DOI] [PubMed] [Google Scholar]
- [49].Leonard A, Lauwerys R, Mutagenicity, carcinogenicity and teratogenicity of cobalt metal and cobalt compounds, Mutation research, 239 (1990) 17–27. [DOI] [PubMed] [Google Scholar]
- [50].Leonard A, Lauwerys RR, Carcinogenicity and mutagenicity of chromium, Mutation research, 76 (1980) 227–239. [DOI] [PubMed] [Google Scholar]
- [51].Coen N, Kadhim MA, Wright EG, Case CP, Mothersill CE, Particulate debris from a titanium metal prosthesis induces genomic instability in primary human fibroblast cells, Br J Cancer, 88 (0000) 548–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Habeebu SS, Liu J, Liu Y, Klaassen CD, Metallothionein-null mice are more susceptible than wild-type mice to chronic CdCl(2)-induced bone injury, Toxicological sciences : an official journal of the Society of Toxicology, 56 (2000) 211–219. [DOI] [PubMed] [Google Scholar]
- [53].Doorn PF, Campbell PA, Worrall J, Benya PD, McKellop HA, Amstutz HC, Metal wear particle characterization from metal on metal total hip replacements: transmission electron microscopy study of periprosthetic tissues and isolated particles, Journal of biomedical materials research, 42 (1998) 103–111. [DOI] [PubMed] [Google Scholar]
- [54].Willert HG, Semlitsch M, Tissue reactions to plastic and metallic wear products of joint endoprostheses, Clinical orthopaedics and related research, (1996) 4–14. [PubMed]
- [55].Cassuto J, Folestad A, Gothlin J, Malchau H, Karrholm J, The key role of proinflammatory cytokines, matrix proteins, RANKL/OPG and Wnt/beta-catenin in bone healing of hip arthroplasty patients, Bone, 107 (2018) 66–77. [DOI] [PubMed] [Google Scholar]
- [56].Takagi M, Konttinen YT, Kemppinen P, Sorsa T, Tschesche H, Blaser J, Suda A, Santavirta S, Tissue inhibitor of metalloproteinase 1, collagenolytic and gelatinolytic activity in loose hip endoprostheses, The Journal of rheumatology, 22 (1995) 2285–2290. [PubMed] [Google Scholar]
- [57].Berber R, Skinner JA, Hart AJ, Management of metal-on-metal hip implant patients: Who, when and how to revise?, World Journal of Orthopedics, 7 (2016) 272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Eltit F, Assiri A, Garbuz D, Duncan C, Masri B, Greidanus N, Bell R, Sharma M, Cox M, Wang R, Adverse reactions to metal on polyethylene implants: Highly destructive lesions related to elevated concentration of cobalt and chromium in synovial fluid, Journal of biomedical materials research. Part A, 105 (2017) 1876–1886. [DOI] [PubMed] [Google Scholar]
- [59].Oliveira CA, Candelária IS, Oliveira PB, Figueiredo A, Caseiro-Alves F, Metallosis: A diagnosis not only in patients with metal-on-metal prostheses, European Journal of Radiology Open, 2 (2015) 3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Esposito CI, Wright TM, Goodman SB, Berry DJ, Clinical B, Bioengineering TBW Study Groups from Carl, What is the trouble with trunnions?, Clinical orthopaedics and related research, 472 (2014) 3652–3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.