Abstract
HIV pre-exposure prophylaxis (PrEP) strategies have the potential to prevent millions of incident HIV infections each year. However, the efficacy of PrEP strategies has been plagued by issues of non-adherence, likely because of the difficulty in motivating otherwise healthy people to adhere to treatment regimens that require significant behavioral changes and daily discipline. An alternative approach to PrEP is to focus on strategies that fit in to normal, and even desirable, sexual behaviors, such as the use of cleansing enemas by men who have sex with men (MSM) prior to receptive anal intercourse (RAI). Here, we describe preclinical efforts toward optimizing a tenofovir (TFV)-based enema formulation for rectal PrEP. Using a murine model, we compared the plasma and tissue pharmacokinetics of TFV and various TFV prodrugs, including tenofovir disoproxil fumarate (TDF), tenofovir alafenamide (TAF), and hexadecyloxypropyl tenofovir (CMX157), after dosing as enema formulations with varying osmolality and ion content. We observed that the enema vehicle composition played a more important role than the TFV prodrug properties in achieving rapid and therapeutically relevant tenofovir diphosphate (TFV-DP) concentrations in mouse colorectal tissue. Our results support the next steps, which are further preclinical (non-human primate) and clinical development of a hypo-osmolar TFV enema product for rectal PrEP.
Keywords: hypotonic, sodium bicarbonate, rectal douche, Fleet, rectal gel
1. Introduction
In 2016, UNAIDS reported that the number of new HIV infections among adults has remained static at an estimated 1.9 million per year since 2010 [1]. The CDC similarly reported in 2017 that the HIV incidence rate in men who have sex with men (MSM) in the US remained steady between 2008–2014, accounting for 70% of all new HIV infections in the US [2, 3]. The risk of colorectal HIV transmission after unprotected receptive anal intercourse (URAI) is 10–20 fold higher than vaginal transmission [4], and US MSM reported not using condoms in their last sexual encounter 38–65% of the time [5–7]. Thus, strategies for preventing HIV infection associated with URAI are strongly needed.
The effectiveness of HIV pre-exposure prophylaxis (PrEP) approaches has varied widely, largely due to lack of adherence to the treatment regimen [8]. Particularly for a generally healthy target population, a treatment strategy must not only be effective when used, but easy to remember, convenient, and attractive to use. A recent literature review of rectal douching in association with receptive anal intercourse (RAI) found that up to 88% of men who practiced RAI ever douched for cleansing purposes, of which 43–64% reported recent douching [9]. Thus, a rectal douche/enema containing a microbicidal agent may be a behaviorally congruent approach for HIV PrEP in MSM [10, 11].
We previously found in mice that the osmolality and ion composition of the enema vehicle itself has a profound impact on epithelial distribution and drug delivery [12]. Namely, mildly hypotonic, sodium ion (Na+) containing fluids caused the colorectal epithelium to absorb water, drawing mucoinert nanoparticles and even water soluble, small molecule drugs like tenofovir (TFV) evenly over the epithelial surface [12]. Further, similar to what was described for Na+ containing fluids in the intact human colon, active pumping of Na+ from the lumen results in water absorption even for iso-osmolar fluids, such as normal saline (NS) [12, 13]. We hypothesized that water absorption from the colorectum would also facilitate rapid uptake and phosphorylation of TFV, and that because TFV has a relatively long intracellular half-life [14], a hypotonic TFV enema would be a means for achieving local protection from colorectal HIV transmission in the timeframe relevant for URAI after enema use. Further, we aimed to explore whether modulation of the ion content and osmolality of the enema vehicle, as well as the use of several TFV prodrugs (tenofovir disoproxil fumarate (TDF), tenofovir alafenamide (TAF), and hexadecyloxypropyl tenofovir (CMX157)), could further improve upon colorectal TFV delivery. Here, we describe optimization of procedures for screening various enema formulations in mice with the aim of selecting formulations of interest for further clinical testing.
2. Materials and methods
2.1. Materials
TFV (FT104801501) and TDF (FT280311499) were both sourced from Carbosynth (Compton, UK). CMX157 (lot 021; potassium salt) was generously provided by Chimerix (Chimerix, Durham, NC). TAF (GS-7340; lot C11/019W) was provided by Gilead Sciences (Gilead, Foster City, CA). Normal saline was obtained from Quality Biological, Inc and UltraPure distilled water was obtained from Invitrogen. Sodium bicarbonate, sodium carbonate and sodium chloride powders were obtained from Sigma-Aldrich.
2.2. Animals
Male CF-1 mice (age 5–6 weeks) were purchased from Harlan (Indianapolis, IN). All protocols involving animals were approved by the Johns Hopkins Medical Institutions Animal Care and Use Committee.
2.3. Optimization of administration and processing
Final dosing and processing conditions were as follows. Male 5–6 week old CF-1 mice were housed in cages with wire mesh bottoms to prevent coprophagia and food was withheld for 16–24 h to reduce the number of pellets present in the colon. Mice were anesthetized with isoflurane and given a 200 μL normal saline (Quality Biological) pre-cleansing enema administered with a plastic flexible feeding tube (Instech Laboratories; 22 G X 25 mm), which was optimized to be sufficient volume to clear the distal colon of pellets prior to dosing. Mice were allowed to regain consciousness for 10 min ambulatory time. Mice were then anesthetized to administer 50 μL of drug solution using a 100 μL WireTrol (Drummond) inserted at a depth of 2 cm into the mouse colon. At designated times, blood was collected into BD Microtainer tubes with K2EDTA, and plasma was obtained by centrifugation (1300 rcf for 10 min). The distal 4 cm of colon was excised, bisected open to remove pellets as necessary, and cut in half longitudinally with a scalpel. The tissue was stored in cryogenic tubes and flash frozen in liquid nitrogen. All samples were stored at −80˚C after being flash frozen in liquid nitrogen.
2.4. Enema formulations
TFV powder was added to normal saline (NS) or a 1:1 mixture of NS and UltraPure distilled water (½ NS) with sufficient 5 M NaOH to fully dissolve and bring to near-neutral pH. Alternately, TFV powder was added to 1 mg/ml solution of NaHCO3 and NaCl powder was added at a concentration of 0.9% to make a saline-based enema. Prodrug enemas were formulated as molar equivalents to TFV to facilitate direct comparisons of the resulting TFV-DP concentrations in tissue achieved. TAF and CMX157 potassium salt were directly dissolved in NS or ½ NS at a molar equivalent to TFV (3.28 and 3.72 mg/mL, respectively). The acidic TDF molar equivalent (3.89 mg/mL) formulation was made by adding TDF directly to NS. The near-neutral formulation was made by adding TDF powder to a solution of 2.5 mg/ml of NaHCO3, followed by NaCl powder added at a concentration of 0.9%. Osmolality was measured with a Vapro vapor pressure osmometer (model 5600). pH was measured with a Mettler Toledo EL20 pH meter and Microelectrodes, Inc micro-combination pH needle electrode (MI-411-P). The properties for each enema are detailed in Table 1.
Table 1.
Enema formulation properties. Abbreviations: tenofovir (TFV), tenofovir disoproxil fumarate (TDF), tenofovir alafenamide (TAF), hexadecylpropyl tenofovir (CMX157), sodium hydroxide (NaOH), sodium bicarbonate (NaHCO3), normal saline (NS), normal saline diluted 1:1 with water (½ NS).
| Drug | Conc. (mg/mL) | Base | Vehicle | Osmolality (mOsm/kg) | pH |
|---|---|---|---|---|---|
| TFV | 1.76 | NaOH | ½ NS | 163 | 7.4 |
| TFV | 1.76 | NaOH | NS | 288 ± 1.0 | 7.4 |
| TFV | 1.76 | NaHCO3 | NS | 287–289# | 6.6–7*# |
| TDF | 3.89 | NaHCO3 | ½ NS | 194 ± 0.3 | 7* |
| TDF | 3.89 | NaHCO3 | NS | 322–328# | 7.1–7.6# |
| TAF | 3.28 | none | ½ NS | 143 ± 0.9 | 7* |
| TAF | 3.28 | none | NS | 292–300# | 6.8–6.9# |
| CMX | 3.72 | none | ½ NS | 127 ± 0.6 | 7.2 |
| CMX | 3.72 | none | NS | 277–294# | 7.3–7.7# |
indicates measurement with pH paper.
indicates range of average measured values for different batches of the same formulation used in in animal experiments on different days or doses administered at different times on the same day. Osmolality data presented as averages ± standard deviation where possible (otherwise a single measurement was made).
2.5. Sample Analysis
Systemic and compartmentalized TFV and TFV-DP drug concentrations were determined by previously described liquid chromatographic-tandem mass spectrometric (LC-MS/MS) assays [15, 16]. The lower limits of quantification (LLOQ) of TFV in plasma and homogenized rectal tissue lysates are 0.31 ng/ml and 0.05 ng/sample, respectively. The LLOQ for the TFV-DP assay is 50 fmol/sample. Results for TFV and TFV-DP in tissue specimens were normalized to tissue weights. Results were reported in ng/mg or fmol/mg. All assays were validated in accordance with the FDA’s Guidance for Industry: Bioanalytical Method Validation recommendations [17].
2.6. Pharmacokinetic studies
All enema solutions were made fresh prior to dosing and used within 1 h. Groups of 5 mice were randomized to each treatment group at each time point. Mice were held under anesthesia for 15 min and either sacrificed or returned to their cages for a total of 4, 8 or 24 h exposure time. Food was re-supplied to the 24 h group after a total of 24 h starvation time. Areas under the curve from t = 0 to the last measurable concentration (AUClast) were calculated using noncompartmental analysis in Phoenix 64 WinNonlin software.
2.7. Statistical analysis
One-way analysis of variance (ANOVA) with post-hoc Tukey test was used for comparing three or more groups and Student’s t-test (two-tailed distribution, unpaired) was used for comparing two groups in Graphpad Prism 7. Pairwise comparisons of calculated AUClast values was done using Bailer’s method [18].
3. Results
3.1. Optimization of administration and processing
We first had to optimize the procedure for enema administration to compare enema formulations without variation in the volume of enema successfully administered and the retention time before enema fluid expulsion. The healthy mouse colon generally contains a series of hard pellets, and they defecate roughly every 5–10 mins. Thus, we explored the effect of pre-cleansing enemas to clear a consistent segment of the descending colon prior to dosing of the microbicide enema formulations and the duration of anesthesia to facilitate retention of the administered fluid on TFV levels in the plasma (Figure S1A) and colon tissue (Figure S1B). We further assessed the timing of tissue collection to ensure that TFV diphosphate (TFV-DP) was accumulating in the colon tissue and that the accumulation was rapid enough after dosing to potentially provide protection (Figure S1C/D). Of note, early measurements of the mesenteric lymph node also indicated that enema administration led to TFV delivery to the draining lymph (Figure S1E), though this was not pursued further. We further explored the use of tissue washing steps to remove luminal drug (Figure S2A), though this introduced more variability in the measurements of TFV levels (Figure S2B). Sectioning the colon tissue to a mass more similar to a human tissue biopsy further increased extraction and detection of TFV-DP (Figure S2B).
3.2. TFV dose ranging and comparison to clinical data
Topical administration of TFV has typically been achieved by gels containing 1% (10 mg/mL) TFV. However, enema volumes administered are much higher than rectal gel volumes. For example, a Fleet® enema delivers 118 mL, whereas clinical studies have been performed where 4 mL of TFV gel was dosed to the colorectum [19, 20]. Thus, we aimed to identify relevant concentration ranges for the TFV containing enemas. As shown in Figure 1, we dosed enemas with TFV concentrations ranging from 10 mg/mL to 0.085 mg/mL (Table S1) and measured TFV and TFV-DP concentrations in colon tissue 15 min after dosing. We compared these data to the reported median values for human colorectal biopsies obtained 30 min after rectal dosing of the original vaginal gel formulation of TFV (TFV VF) [21], shown as red dotted lines in Figure 1. Similar median parent TFV levels were achieved in mice with as low as 0.17 mg/mL enema concentration (9.3 ng/mg vs 5.81 ng/mg) (Figure 1A), whereas similar median TFV-DP levels were achieved with a 1.8 mg/mL TFV enema (312 fmol/mg vs. 176 fmol/mg) (Figure 1B). Importantly, we also confirmed that dosing the TFV VF gel to mice resulted in similar median TFV-DP colorectal tissue concentrations to what was observed clinically (173 fmol/mg vs. 176 fmol/mg) (Figure 1B). In coordination with parallel planning for clinical studies, an enema concentration of 1.76 mg/mL was chosen to move forward for comparisons of various enema compositions and TFV prodrugs.
Figure 1.
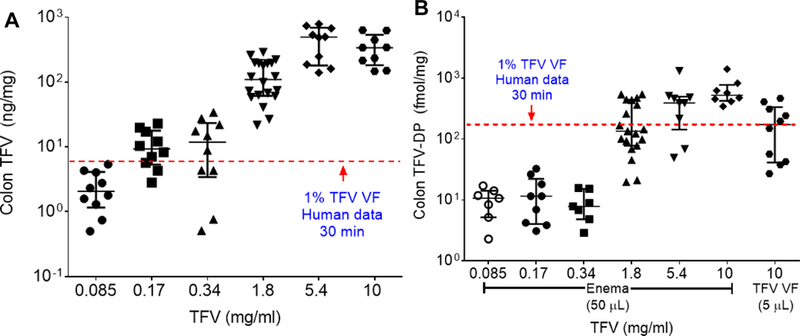
Colon tissue (A) TFV levels and (B) colon tissue TFV-DP levels measured 15 min after enema dosing with a range of TFV enema concentrations (0.085–10 mg/ml). The red dotted lines in both figures represent the median levels measured in a human study 30 min after a single dose of 10 mg/ml (1%) TFV gel (vaginal formulation, VF) [22]. Part (B) also includes TFV-DP levels measured in mouse colon tissue 15 min after administration of the TFV VF gel.
3.3. TFV prodrug enema preparation and dosing
We next sought to formulate TFV prodrug enemas containing the same molar equivalent of TFV parent drug and with a near neutral pH. TAF and CMX157 could be directly dissolved into NS without pH adjustment. Similar to TFV, dissolving TDF acidifies the solution, requiring the use of base to neutralize the pH. However, we found that pH adjustment of TDF solution with NaOH resulted in significant degradation (Figure S3). In contrast, bases such as Na2CO3 and NaHCO3 resulted in a more stable TDF solution for up to 5 h, with NaHCO3 being the most mild of the three bases (Figure S3). We then compared enema administration of TDF solution without pH adjustment (pH 3.4) and with the pH adjustment with NaHCO3 (pH 7), and found that there was no difference in plasma concentrations (Figure S4A) but a significant increase in median TFV-DP concentrations in colon tissue 15 min after dosing with the pH 7 formulation (144 fmol/mg vs 944 fmol/mg) (Figure S4B). Thus, we moved forward with the TDF/NaHCO3 formulation.
To mirror the concurrently developing clinical studies (to be detailed in a future publication), we next tested the various TFV prodrugs at molar equivalent concentrations in NS and NS diluted 1:1 with water (½ NS) as the vehicles. As shown in Figure 2A, there was no significant difference in plasma TFV concentrations 15 min after dosing, with the exception of much lower concentrations with the CMX157 formulations. In colon tissue, there was a small but significant decrease in parent TFV concentrations when dosing TFV in the ½ NS vehicle, and significantly lower TFV concentrations associated with the CMX157 enema formulations (Figure 2B). For colon TFV-DP concentrations, the only significant difference at 15 min for any drug when comparing the ½ NS and NS vehicle was a slight reduction in TFV-DP when dosing TDF in the ½ NS vehicle (Figure 2C). However, there was a trend toward increased median TFV-DP levels for TDF (994 fmol/mg) and TAF (363 fmol/mg) compared to TFV (312 fmol/mg) (Figure 2C). In an effort to understand whether the increased tissue TFV-DP concentrations associated with the TDF enema was due to the physicochemical properties of the prodrug or the addition of NaHCO3 for pH adjustment, we compared the colon TFV-DP concentrations in colorectal tissue after administration of TFV pH-adjusted with NaHCO3. As shown in Figure S5, we saw an increase in median TFV-DP tissue concentrations 15 min after administering a TFV/NaHCO3 enema (584 fmol/mg) compared to TFV/NaOH (312 fmol/mg). Thus, we moved forward to compare TFV/NaOH, TFV/NaHCO3, TDF/NaHCO3, TAF and CMX157 in the NS vehicle for pharmacokinetics.
Figure 2.
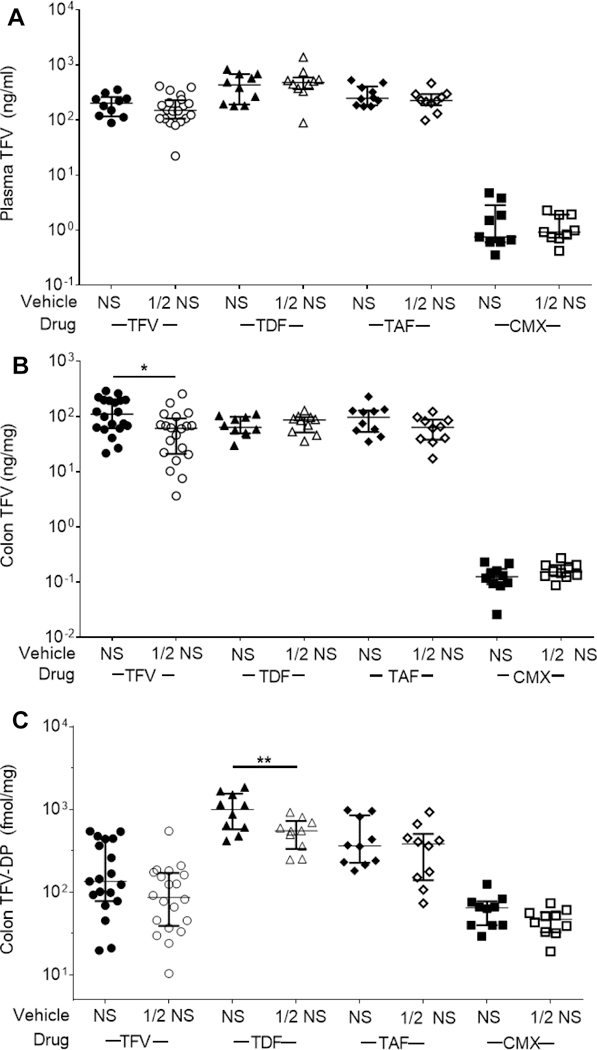
(A) Plasma TFV, (B) colon TFV, and (C) colon TFV-DP concentrations 15 min after enema dosing. Normal saline (NS) and NS diluted 1:1 with water (1/2 NS) were compared as vehicles. TFV prodrugs were formulated at molar equivalents: TFV (1.76 mg/ml), TDF (3.89 mg/ml), TAF (3.28 mg/ml), CMX157 (3.72 mg/ml). * p < 0.05, ** p < 0.01, *** p < 0.001
3.4. Pharmacokinetics
We next dosed individual groups of mice with the various NS enema formulations for blood and tissue collection at 15 min, 4 h, 8 h, and 24 h after administration. As shown in Figure 3A, peak median plasma TFV concentrations were measured at 15 min after dosing. The TDF/NaHCO3 formulation provided a significantly increased plasma TFV AUC (1.2 × 103 ng*h/ml) compared to all other enema formulations, while the plasma TFV AUC for the CMX enema (17 ng*h/ml) was significantly lower than all other formulations (Figure 3B, Table 2). Peak median TFV colon concentrations also occurred at 15 min after dosing for all formulations except CMX157 (Figure 3C). Similarly, the only statistically different calculated colon TFV AUC was for CMX, which was lower than for all other formulations (Figure 3D, Table 3). In contrast, there was more variation in median colon TFV-DP concentrations and calculated AUCs for the various enema formulations. As shown in Figure 3E, three formulations (TFV/NaOH, TFV/NaHCO3, TDF/NaHCO3) were associated with an increase in median colon TFV-DP from 15 min to 4 h, whereas two formulations (TAF, CMX) were associated with peak median TFV-DP concentrations at 15 min. The TFV/NaHCO3 enema formulation resulted in a significantly increased AUC (1.7 × 105 fmol*h/mg) compared to all other formulations, followed by the TDF/NaHCO3 formulation (4.5 × 104 fmol*h/mg) (Figure 3F, Table 4).
Figure 3.
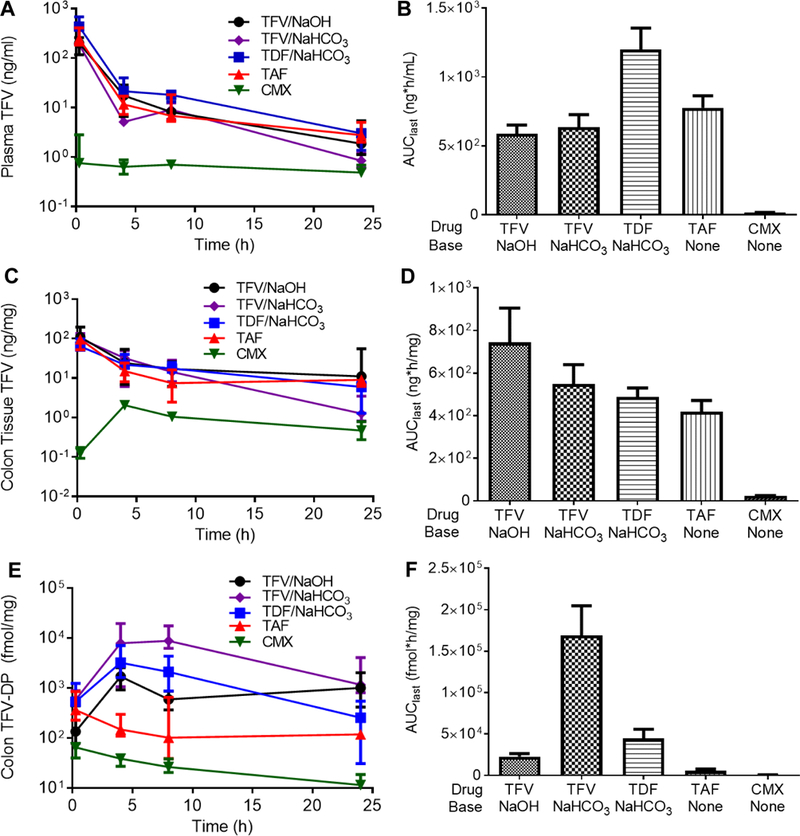
Pharmacokinetics for a single enema dose of TFV pH adjusted with NaOH (TFV/NaOH), TFV pH adjusted with NaHCO3 (TFV/NaHCO3), TDF pH adjusted with NaHCO3 (TDF/NaHCO3), TAF, or CMX157. (A) Time course of plasma TFV concentrations after enema dosing with corresponding (B) AUClast calculations. (C) Time course of colon TFV concentrations after enema dosing with corresponding (D) AUClast calculations. (E) Time course of colon TFV-DP concentrations after enema dosing with corresponding (F) AUClast calculations. Comparisons of AUC values is shown in Tables 2–4
Table 2.
Fold changes in plasma TFV AUClast when comparing various enema formulations. Values in the table represent the fold increase when comparing row > column. N/A represents redundant comparisons that are omitted for clarity.
| TAF | TFV/NaHCO3 | TFV/NaOH | CMX | |
|---|---|---|---|---|
| TDF/NaHCO3 | 1.5* | 1.9* | 2.0* | 69* |
| TAF | N/A | 1.2 | 1.3 | 45* |
| TFV/NaHCO3 | N/A | N/A | 1.1 | 37* |
| TFV/NaOH | N/A | N/A | N/A | 34* |
p < 0.05
Table 3.
Fold changes in colon TFV AUClast when comparing various enema formulations. Values in the table represent the fold increase when comparing row > column. N/A represents redundant comparisons that are omitted for clarity.
| TFV/NaHCO3 | TDF/NaHCO3 | TAF | CMX | |
|---|---|---|---|---|
| TFV/NaOH | 1.4 | 1.5 | 1.8 | 32* |
| TFV/NaHCO3 | N/A | 1.1 | 1.3 | 24* |
| TDF/NaHCO3 | N/A | N/A | 1.2 | 21* |
| TAF | N/A | N/A | N/A | 18* |
p < 0.05
Table 4.
Fold changes in colon TFV-DP AUClast when comparing various enema formulations. Values in the table represent the fold increase when comparing row > column. N/A represents redundant comparisons that are omitted for clarity.
| TDF/NaHCO3 | TFV/NaOH | TAF | CMX | |
|---|---|---|---|---|
| TFV/NaHCO3 | 3.8* | 7.5* | 30* | 256* |
| TDF/NaHCO3 | N/A | 2.0 | 7.9* | 68* |
| TFV/NaOH | N/A | N/A | 4.0* | 34* |
| TAF | N/A | N/A | N/A | 8.5* |
p < 0.05
3.5. Interspecies comparison
In parallel work in rhesus macaques, we recently reported that a hypo-osmolar 5.28 mg/ml TFV enema formulation provided increased TFV-DP levels in colon biopsies and CD4+ cells from colon tissue compared to an iso-osmolar TFV enema [22]. The increased TFV-DP levels achieved with the hypo-osmolar TFV formulation also correlated with increased protection against ex vivo SIV/SHIV infection [22]. Although we did not observe a significant benefit here for the ½ NS vehicle compared to the NS vehicle at 15 min after administration in mice, there are several differences to consider. When making interspecies comparisons, the median TFV-DP levels achieved with the ½ NS TFV enema formulation were more disparate between species at the 1.76 mg/ml concentration (Figure S6A) than at the higher (5.28 mg/ml in macaques, 5.4 mg/ml in mice) TFV concentration (Figure S6B). Figure 1B indicates that TFV-DP concentrations in mouse colon tissue did not scale directly with TFV enema concentration, so it is possible that the benefit for using the ½ NS vehicle would have been seen at a higher TFV enema concentration, as in the rhesus macaque studies. Studies conducted in mice over a full range of enema osmolality (Table S1) suggested that the ½ NS vehicle provided increased colon TFV delivery at an enema concentration of 10 mg/ml TFV (Figure S7), though TFV-DP measurements were not obtained. Regardless, we did observe in mice that the ion composition of the enema vehicle has a dramatic effect on the resulting colon TFV-DP concentrations, and that the prodrugs did not provide any clear benefit as an enema formulation.
4. Discussion
Despite the clear protective potential of PrEP strategies, adherence to the PrEP regimen has often been highlighted as the crux for achieving high levels of efficacy [8]. Given the common practice of rectal douching with an enema prior to RAI, an enema formulation presents a unique opportunity requiring little to no behavioral change compared to oral and other topical approaches [9, 10]. Further, as we have learned from contraceptives, providing a variety of options is important for meeting the needs of a larger portion of the target population [23]. Thus, microbicide enemas have potential as an alternative to the highly effective daily oral PrEP regimens that may be more attractive to those who participate in RAI. However, similar to other PrEP strategies, condom use must be recommended to provide protection against other sexually transmitted infections.
Many TFV prodrugs have been developed to improve delivery to target cells and tissues after oral administration. TDF was first developed to increase bioavailability of TFV [24]. In vitro, TDF was associated with >100-fold increased antiretroviral activity and >1,000-fold increased intracellular TFV-DP concentrations compared to TFV [25]. TAF was shown to have increased stability in the blood and enhanced intracellular delivery compared to TDF [26, 27], and the in vitro anti-HIV activity of TAF was shown to be >1,000-fold greater than TFV [27]. HIV-infected patients on oral TAF (25 mg) had 7-fold increased TFV-DP concentrations in peripheral blood mononuclear cells (PBMCs) compared to patients on oral TDF (300 mg) [28]. CMX157 is a lipid conjugate designed to remain intact in the blood and exploit natural lipid uptake pathways to achieve high intracellular TFV-DP. In vitro, CMX157 was >300-fold more active than TFV against multiple viruses in several different cell systems [29]. Due to these various demonstrations of increased potency of TDF, TAF, and CMX157 compared to TFV, we explored whether the prodrugs would provide pharmacokinetic benefits upon enema administration. Surprisingly, there was little variation in the colorectal TFV levels for TFV enemas compared to TDF and TAF enemas, and only the TDF formulation provided a significant increase in plasma TFV AUC compared to the TFV formulations. Further, there was no apparent benefit in using either TDF or TAF in achieving increased colorectal TFV-DP concentrations. Plasma and colon TFV concentrations and colon TFV-DP concentrations were the lowest overall with the CMX157 enema, suggesting that there may be impaired conversion of CMX157 to TFV in mice. Ultimately, we found that the highest overall TFV-DP exposure in the colon was achieved with a TFV enema formulation containing both Na+ and bicarbonate ions, suggesting that ion transport and the associated water absorption was a primary driving factor for drug absorption in the colon. Indeed, the colon absorbs large amounts of water daily to dry the feces, which is facilitated by active ion transport [30]. It has been demonstrated in both humans and mice that water absorption occurs in the colon even from iso-osmolar solutions when Na+ is present [12, 13]. However, because the measurements of parent TFV in unwashed, homogenized colon tissue do not distinguish between drug that is intracellular versus in the extracellular space, we cannot directly quantify if there is a difference in the intracellular TFV:TFV-DP ratios for different enema formulations. Thus, we cannot rule out whether increased TFV-DP concentrations is a result of another factor, such as enzyme kinetics. Regardless, for these water soluble TFV drugs, increased cell permeability in vitro and improved biodistribution upon oral administration did not lead to improved delivery to the colon via enema.
The fact that TFV alone is sufficient in an enema product is advantageous for further development, as TFV is off-patent and simpler to synthesize than the prodrugs. Further, a peri-coital enema formulation will likely reduce the amount of drug that is used compared to daily oral PrEP strategies. Less frequent drug exposure will limit the potential for toxicity, and less systemic drug absorption will further reduce the risk of systemic side effects. Our parallel work in macaques also found no evidence of acute mucosal toxicity with the enema product [22], and future work will include evaluating the colorectal mucosa after multiple doses. However, one drawback of intermittent drug exposure has the potential to generate drug resistance in people with undiagnosed active HIV infections [31]. For this reason, oral PrEP is only prescribed after confirming HIV negative status, and confirmatory testing continues while using PrEP [31]. An enema product is likely to be implemented using a similar strategy.
A key next step in our enema development program will be confirming improved protection against mucosal infection in vivo. Although we demonstrate that TFV-DP concentrations in colon tissue achieved by enema in mice are in the target range for what has been associated with protection in humans with oral PrEP, it is still unclear where viral infection is initially established following rectal transmission. In the case of oral drug dosing, drug partitions into the blood stream and then to the mucosa, whereas it is opposite in the case of enema dosing. Thus, the ratio of drug concentrations in the plasma compared to the mucosal compartments is much higher with oral dosing, which increases the risk of systemic side effects, but may also increase the amount of drug that reaches other key sites for preventing infection. It is possible that the draining lymph is an important site for drug delivery, which appears to be accessible by enema (Figure S1E), though delivery to the draining lymph was not carefully studied here. Thus, it will be important to determine the correlation between colon tissue TFV-DP concentration and protection from infection, and whether there is a similar correlation for oral and enema dosing. Further, as with any peri-coital prevention strategy, determining the duration of protection and whether it aligns with typical timeframes for use will be important. Incorporating a sustained-release element, such as a nanoparticle, into the enema formulation could extend the duration of time in which drug concentrations remain within the therapeutic window. Importantly, our preclinical results described here and recently in rhesus macaques [22] strongly support further development of enema-based microbicide products for rectal PrEP.
5. Conclusion
We demonstrated that tenofovir (TFV) enema formulations are a promising option for rectal pre-exposure prophylaxis (PrEP). By modulating the enema vehicle ion composition to achieve rapid water absorption by the colorectal epithelia, therapeutically relevant concentrations of tenofovir diphosphate (TFV-DP) in colon tissues were obtained. Further, despite the documented improvements in oral pharmacokinetics provided by TFV prodrugs, we did not observe any discernible comparative advantage over TFV for enema administration. Rather, including both sodium (Na+) and bicarbonate (HCO3-) ions in the enema vehicle led to the highest concentrations of TFV-DP in mouse colon tissue. Interspecies comparisons with rhesus macaques further support the rationale for clinical development of TFV-based microbicide enemas for rectal PrEP.
Supplementary Material
Acknowledgments
We acknowledge the rest of the DREAM team for their support and collaboration. We thank the JHMI animal husbandry and veterinary staff. We thank Michelle Rudek for advising on pharmacokinetic calculations and statistical tests. This work was funded by NIH/NIAID grants U19AI113127 and R01DK107806.
References
- [1].Prevention Gap Report, Joint United Nations Programme on HIV/AIDS (UNAIDS), 2016.
- [2].HIV Among Gay and Bisexual Men, Centers for Disease Control and Prevention (CDC), 2017.
- [3].CDC Fact Sheet: HIV among Gay and Bisexual Men, Centers for Disease Control and Prevention, 2017.
- [4].Baggaley RF, White RG, Boily MC, HIV transmission risk through anal intercourse: systematic review, meta-analysis and implications for HIV prevention, Int J Epidemiol, 39 (2010) 1048–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Anderson PL, Glidden DV, Liu A, Buchbinder S, Lama JR, Guanira JV, McMahan V, Bushman LR, Casapia M, Montoya-Herrera O, Veloso VG, Mayer KH, Chariyalertsak S, Schechter M, Bekker LG, Kallas EG, Grant RM, iPrEx Study T, Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men, Sci Transl Med, 4 (2012) 151ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Calabrese SK, Rosenberger JG, Schick VR, Novak DS, Reece M, An event-level comparison of risk-related sexual practices between black and other-race men who have sex with men: condoms, semen, lubricant, and rectal douching, AIDS Patient Care STDS, 27 (2013) 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rosenberger JG, Reece M, Schick V, Herbenick D, Novak DS, Van Der Pol B, Fortenberry JD, Sexual behaviors and situational characteristics of most recent male-partnered sexual event among gay and bisexually identified men in the United States, J Sex Med, 8 (2011) 3040–3050. [DOI] [PubMed] [Google Scholar]
- [8].Hendrix CW, Exploring concentration response in HIV pre-exposure prophylaxis to optimize clinical care and trial design, Cell, 155 (2013) 515–518. [DOI] [PubMed] [Google Scholar]
- [9].Carballo-Dieguez A, Lentz C, Giguere R, Fuchs EJ, Hendrix CW, Rectal Douching Associated with Receptive Anal Intercourse: A Literature Review, AIDS Behav, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Carballo-Dieguez A, Bauermeister J, Ventuneac A, Dolezal C, Mayer K, Why Rectal Douches May Be Acceptable Rectal-Microbicide Delivery Vehicles for Men Who Have Sex With Men, Sex Transm Dis, 37 (2010) 228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Leyva FJ, Bakshi RP, Fuchs EJ, Li LY, Caffo BS, Goldsmith AJ, Ventuneac A, Carballo-Dieguez A, Du Y, Leal JP, Lee LA, Torbenson MS, Hendrix CW, Isoosmolar Enemas Demonstrate Preferential Gastrointestinal Distribution, Safety, and Acceptability Compared with Hyperosmolar and Hypoosmolar Enemas as a Potential Delivery Vehicle for Rectal Microbicides, Aids Res Hum Retrov, 29 (2013) 1487–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Maisel K, Ensign L, Reddy M, Cone R, Hanes J, Effect of surface chemistry on nanoparticle interaction with gastrointestinal mucus and distribution in the gastrointestinal tract following oral and rectal administration in the mouse, J Control Release, 197 (2015) 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Billich CO, Levitan R, Effects of sodium concentration and osmolality on water and electrolyte absorption form the intact human colon, J Clin Invest, 48 (1969) 1336–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Louissaint NA, Cao YJ, Skipper PL, Liberman RG, Tannenbaum SR, Nimmagadda S, Anderson JR, Everts S, Bakshi R, Fuchs EJ, Hendrix CW, Single dose pharmacokinetics of oral tenofovir in plasma, peripheral blood mononuclear cells, colonic tissue, and vaginal tissue, AIDS Res Hum Retroviruses, 29 (2013) 1443–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hendrix CW, Andrade A, Bumpus NN, Kashuba AD, Marzinke MA, Moore A, Anderson PL, Bushman LR, Fuchs EJ, Wiggins I, Radebaugh C, Prince HA, Bakshi RP, Wang R, Richardson P, Shieh E, McKinstry L, Li X, Donnell D, Elharrar V, Mayer KH, Patterson KB, Dose Frequency Ranging Pharmacokinetic Study of Tenofovir-Emtricitabine After Directly Observed Dosing in Healthy Volunteers to Establish Adherence Benchmarks (HPTN 066), Aids Res Hum Retrov, 32 (2016) 32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hendrix CW, Chen BA, Guddera V, Hoesley C, Justman J, Nakabiito C, Salata R, Soto-Torres L, Patterson K, Minnis AM, Gandham S, Gomez K, Richardson BA, Bumpus NN, MTN-001: Randomized Pharmacokinetic Cross-Over Study Comparing Tenofovir Vaginal Gel and Oral Tablets in Vaginal Tissue and Other Compartments, Plos One, 8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Guidance for Industry: Bioanalytical Method Validation, in: U.S.D.o.H.a.H. Services (Ed.), 2001. [Google Scholar]
- [18].Bailer AJ, Testing for the equality of area under the curves when using destructive measurement techniques, J Pharmacokinet Biopharm, 16 (1988) 303–309. [DOI] [PubMed] [Google Scholar]
- [19].Anton PA, Cranston RD, Kashuba A, Hendrix CW, Bumpus NN, Richardson-Harman N, Elliott J, Janocko L, Khanukhova E, Dennis R, Cumberland WG, Ju C, Carballo-Dieguez A, Mauck C, McGowan I, RMP-02/MTN-006: A phase 1 rectal safety, acceptability, pharmacokinetic, and pharmacodynamic study of tenofovir 1% gel compared with oral tenofovir disoproxil fumarate, AIDS Res Hum Retroviruses, 28 (2012) 1412–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].McGowan I, Cranston RD, Duffill K, Siegel A, Engstrom JC, Nikiforov A, Jacobson C, Rehman KK, Elliott J, Khanukhova E, Abebe K, Mauck C, Spiegel HM, Dezzutti CS, Rohan LC, Marzinke MA, Hiruy H, Hendrix CW, Richardson-Harman N, Anton PA, A Phase 1 Randomized, Open Label, Rectal Safety, Acceptability, Pharmacokinetic, and Pharmacodynamic Study of Three Formulations of Tenofovir 1% Gel (the CHARM-01 Study), PLoS One, 10 (2015) e0125363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yang KH, Hendrix C, Bumpus N, Elliott J, Tanner K, Mauck C, Cranston R, McGowan I, Richardson-Harman N, Anton PA, Kashuba AD, A multi-compartment single and multiple dose pharmacokinetic comparison of rectally applied tenofovir 1% gel and oral tenofovir disoproxil fumarate, PLoS One, 9 (2014) e106196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Xiao P, Gumber S, Marzinke MA, Date AA, Hoang T, Hanes J, Ensign LM, Wang L, Rohan L, Fuchs EJ, Hendrix C, Villinger F, Hypo-osmolar Formulation of Tenofovir (TFV) Enema Promotes Uptake and Metabolism of TFV in Tissues, Leading to Prevention of SHIV/SIV Infection, Antimicrob Agents Chemother, 62 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Rodriguez J, Abutouk M, Roque K, Sridhar A, Personalized contraceptive counseling: helping women make the right choice, Open Access J Contracept, 7 (2016) 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Barditch-Crovo P, Deeks SG, Collier A, Safrin S, Coakley DF, Miller M, Kearney BP, Coleman RL, Lamy PD, Kahn JO, McGowan I, Lietman PS, Phase i/ii trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults, Antimicrob Agents Chemother, 45 (2001) 2733–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Robbins BL, Srinivas RV, Kim C, Bischofberger N, Fridland A, Anti-human immunodeficiency virus activity and cellular metabolism of a potential prodrug of the acyclic nucleoside phosphonate 9-R-(2-phosphonomethoxypropyl)adenine (PMPA), Bis(isopropyloxymethylcarbonyl)PMPA, Antimicrob Agents Chemother, 42 (1998) 612–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Eisenberg EJ, He GX, Lee WA, Metabolism of GS-7340, a novel phenyl monophosphoramidate intracellular prodrug of PMPA, in blood, Nucleosides Nucleotides Nucleic Acids, 20 (2001) 1091–1098. [DOI] [PubMed] [Google Scholar]
- [27].Lee WA, He GX, Eisenberg E, Cihlar T, Swaminathan S, Mulato A, Cundy KC, Selective intracellular activation of a novel prodrug of the human immunodeficiency virus reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue, Antimicrob Agents Chemother, 49 (2005) 1898–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ruane PJ, DeJesus E, Berger D, Markowitz M, Bredeek UF, Callebaut C, Zhong L, Ramanathan S, Rhee MS, Fordyce MW, Yale K, Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of tenofovir alafenamide as 10-day monotherapy in HIV-1-positive adults, J Acquir Immune Defic Syndr, 63 (2013) 449–455. [DOI] [PubMed] [Google Scholar]
- [29].Lanier ER, Ptak RG, Lampert BM, Keilholz L, Hartman T, Buckheit RW Jr., Mankowski MK, Osterling MC, Almond MR, Painter GR, Development of hexadecyloxypropyl tenofovir (CMX157) for treatment of infection caused by wild-type and nucleoside/nucleotide-resistant HIV, Antimicrob Agents Chemother, 54 (2010) 2901–2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sandle GI, Salt and water absorption in the human colon: a modern appraisal, Gut, 43 (1998) 294–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Pre-exposure prophylaxis for the prevention of HIV in the United States - 2014: A clinical practice guideline, in: C.f.D.C.a. Prevention (Ed.), 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


