Abstract
In this issue of Blood, Dunne et al report that platelets from type O subjects bound poorly to von Willebrand factor (VWF) of mixed ABOs under arterial shear stress, as compared with those from non-O subjects.1 The binding difference between O and non-O platelets was defined by several key biophysical measurements that govern the platelet reactivity toward VWF under physical forces. Platelets contain 2 receptors that bind VWF: the glycoprotein Ib (GPIb)-IX-V complex and the integrin αIIbβ3, but this ABO effect is likely on the GPIb-VWF interaction, which is regulated by fluid shear stress and exhibits “catch bond” characteristics. This study is important in several key aspects.
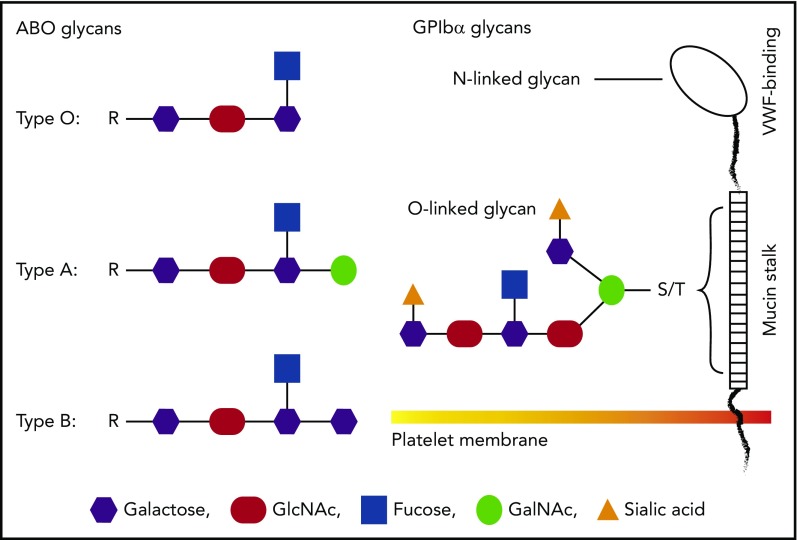
A schematic comparison between ABO and GPIb glycans. Glycans on GPIbα are most likely to be heavily modified by terminal sialic acids, whereas platelet ABO is less likely to be sialylated. There are 2 possible locations of ABO epitopes on platelets: the mucin-rich region of GPIbα and membrane glycolipids. Neither is in the VWF-binding region, suggesting that ABO indirectly regulates GPIb-VWF interaction. The 2 potential locations of ABO on platelets could also lead to different mechanisms of regulating GPIb-VWF interaction in flowing blood.
First, this is the first study to demonstrate that the platelet ABO regulates how GPIb interacts with VWF under hydrodynamic conditions that mimic blood flow. Platelets are known to express ABO,2,3 but its physiological relevance has been almost exclusively considered for platelet transfusion and related issues. The location of ABO epitopes on platelets is not known. ABO can be attached to the backbone of GPIbα (the VWF-binding subunit of the GPIb-IX-V complex), likely in the membrane-proximal mucin-rich region where O-linked glycans cluster (see figure). However, most, if not all, glycans on GPIb carry terminal sialic acids.4 In contrast, ABO is less likely to carry the monosaccharide because glycophorins on erythrocytes carry most sialic acid–containing glycans but only residual ABO,5 raising the questions as to whether (1) ABO epitopes on platelets are identical to those on red blood cells and (2) platelet ABO is modified by sialic acids. The answer to the first question is likely “no” because it has recently been shown that platelets from A2 subjects lack or express only very low levels of the A antigen.6 The answer to the second question requires additional experiments, but the abundant presence of sialic acids on GPIb glycans suggests that ABO may influence GPIb-VWF interaction through terminal sialic acids. This notion is supported by the report that ABO modulates sialic acid recognition on erythrocytes.7 The alternative to being GPIb anchored, ABO could also be attached to glycolipids on platelet membrane and thus regulate GPIb-VWF interaction by orienting the GPIb-IX-V complex differently.
Second, platelet-VWF interaction is the first step that tethers platelets to the subendothelial matrix exposed at the site of vessel injury to initiate hemostasis. The absence or weakening of this interaction results in a bleeding diathesis as seen in patients with genetic deficiencies of the GPIb-IX-V complex (Bernard-Soulier syndrome) or VWF (von Willebrand disease).8 The results from this study are consistent with an early observation that type O individuals have a longer bleeding time than non–type O individuals.9 In this regard, this study provides new information that is valuable for evaluating the bleeding risk of individuals who have low levels of plasma VWF antigen (eg, 30% to 50%).
Third, the findings from this study identify a new role of ABO in the development of thrombotic and thromboembolic diseases. The observation that low-ligand-receptor binding kinetics appears to be most significant between type O platelets and type O VWF suggests that ABO regulates both the receptor and the ligand. Consistent with that notion, VWF is also heavily glycosylated with carbohydrates accounting for >20% of its molecular mass and carries ABO antigens.10 It has been well established that type O individuals have the lowest levels of plasma VWF because of accelerated VWF clearance. This low VWF antigen is widely cited as the reason that type O subjects have a low risk for coronary heart disease and stroke. However, the findings from this study suggest that type O subjects could also be protected because their platelets are less active in binding VWF.
In summary, the study by Dunne et al demonstrates that platelets from type O subjects form a weaker interaction with VWF. The findings extend the relevance of platelet ABO beyond transfusion medicine, but require further validation. The findings also raise several key questions: (1) Do type O VWF multimers also have a weaker reactivity toward platelets, (2) Do type O platelets have a shorter life-span in circulation, and (3) Should the ABO-regulated platelet reactivity be considered when transfusing patients to arrest bleeding (eg, trauma resuscitation)? Answering these questions requires extensive follow-up studies.
Footnotes
Conflict-of-interest disclosure: The author declares no competing financial interests.
REFERENCES
- 1.Dunne E, Qi QM, Shaqfeh ES, et al. . Blood group alters platelet binding kinetics to von Willebrand factor and consequently platelet function. Blood. 2019;133(12):1371-1377. [DOI] [PubMed] [Google Scholar]
- 2.Santoso S, Kiefel V, Mueller-Eckhardt C. Blood group A and B determinants are expressed on platelet glycoproteins IIa, IIIa, and Ib. Thromb Haemost. 1991;65(2):196-201. [PubMed] [Google Scholar]
- 3.Hou M, Stockelberg D, Rydberg L, Kutti J, Wadenvik H. Blood group A antigen expression in platelets is prominently associated with glycoprotein Ib and IIb. Evidence for an A1/A2 difference. Transfus Med. 1996;6(1):51-59. [DOI] [PubMed] [Google Scholar]
- 4.Jansen AJ, Josefsson EC, Rumjantseva V, et al. . Desialylation accelerates platelet clearance after refrigeration and initiates GPIbα metalloproteinase-mediated cleavage in mice. Blood. 2012;119(5):1263-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Podbielska M, Fredriksson SA, Nilsson B, Lisowska E, Krotkiewski H. ABH blood group antigens in O-glycans of human glycophorin A. Arch Biochem Biophys. 2004;429(2):145-153. [DOI] [PubMed] [Google Scholar]
- 6.Cooling LL, Kelly K, Barton J, Hwang D, Koerner TA, Olson JD. Determinants of ABH expression on human blood platelets. Blood. 2005;105(8):3356-3364. [DOI] [PubMed] [Google Scholar]
- 7.Cohen M, Hurtado-Ziola N, Varki A. ABO blood group glycans modulate sialic acid recognition on erythrocytes. Blood. 2009;114(17):3668-3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.López JA. The platelet glycoprotein Ib-IX complex. Blood Coagul Fibrinolysis. 1994;5(1):97-119. [PubMed] [Google Scholar]
- 9.Moeller A, Weippert-Kretschmer M, Prinz H, Kretschmer V. Influence of ABO blood groups on primary hemostasis. Transfusion. 2001;41(1):56-60. [DOI] [PubMed] [Google Scholar]
- 10.Sodetz JM, Paulson JC, McKee PA. Carbohydrate composition and identification of blood group A, B, and H oligosaccharide structures on human Factor VIII/von Willebrand factor. J Biol Chem. 1979;254(21):10754-10760. [PubMed] [Google Scholar]