Abstract
BACKGROUND:
Blacks have a high prevalence of hypertension and uncontrolled blood pressure (BP), each of which may be partially explained by untreated sleep apnea. We investigated the association of sleep apnea with uncontrolled BP and resistant hypertension in blacks.
METHODS:
Between 2012 and 2016, Jackson Heart Sleep Study participants (N=913) underwent an in-home Type 3 sleep apnea study, clinic BP measurements, and anthropometry. Moderate or severe obstructive sleep apnea (OSA) was defined as a respiratory event index ≥15, and nocturnal hypoxemia was quantified as percent sleep time with <90% oxyhemoglobin saturation. Prevalent hypertension was defined as either a systolic BP ≥130 mm Hg or diastolic BP >80mm Hg, use of antihypertensive medication, or self-report of a diagnosis of hypertension. Controlled BP was defined as systolic BP <130 mm Hg and diastolic BP <80 mm Hg; uncontrolled BP as systolic BP ≥130 mm Hg or diastolic BP ≥80 mm Hg with use of 1 to 2 classes of antihypertensive medication; and resistant BP as systolic BP ≥130 mm Hg or diastolic BP ≥80 mm Hg with the use of ≥3 classes of antihypertensive medication (including a diuretic) or use of ≥4 classes of antihypertensive medication regardless of BP level. Multinomial logistic regression models were fit to determine the association between OSA severity and uncontrolled BP or resistant hypertension (versus controlled BP) after multivariable adjustment.
RESULTS:
The analytic sample with hypertension (N=664) had a mean age of 64.0 (SD,10.6) years, and were predominately female (69.1%), obese (58.6%), and college educated (51.3%). Among the sample, 25.7% had OSA, which was untreated in 94% of participants. Overall, 48% of participants had uncontrolled hypertension and 14% had resistant hypertension. After adjustment for confounders, participants with moderate or severe OSA had a 2.0 times higher odds of resistant hypertension (95% confidence interval [CI], 1.14–3.67). Each standard deviation higher than <90% oxyhemoglobin saturation was associated with an adjusted odds ratio for resistant hypertension of 1.25 (95% CI 1.01–1.55). OSA and <90% oxyhemoglobin saturation were not associated with uncontrolled BP.
CONCLUSION:
Untreated moderate or severe OSA is associated with increased odds of resistant hypertension. These results suggest that untreated OSA may contribute to inadequate BP control in blacks.
Keywords: blood pressure, hypertension, sleep apnea
Blacks have the highest prevalence of hypertension of any race/ethnic group in the United States and have a 90% higher odds of uncontrolled blood pressure (BP) compared with non-Hispanic whites.1,2 The reasons for these disparities are not well understood. Identifying modifiable risk factors for uncontrolled BP in blacks will facilitate the design of interventions aimed at improving BP control. Untreated sleep disorders and insufficient sleep disproportionately affect minority populations and may contribute to the disparity in BP control2–4; however, there have been few studies on the association between sleep disorders and BP control in blacks.
The prevalence of sleep disorders, including sleep apnea, are high and a growing public health challenge.5 Adverse sleep patterns (eg, short and long sleep duration and poor sleep quality) and sleep disorders are associated with a host of cardiovascular health outcomes including heart disease, high blood pressure, obesity, diabetes mellitus, stroke, and all-cause mortality.6–8 Blacks have a higher risk for not only these health conditions but also sleep disorders and poor sleep patterns.3,4 In the Multi-Ethnic Study of Atherosclerosis (MESA), 84% of black participants who had sleep-disordered breathing, as measured by polysomnography, did not report a physician diagnosis of sleep-disordered breathing, suggesting a high prevalence of untreated obstructive sleep apnea (OSA) in this population.3 Data have shown that severe OSA contributes to uncontrolled BP in non-Hispanic whites.9 To our knowledge, only 1 study has evaluated the association between OSA and uncontrolled BP among a population of blacks; this study of 1035 blacks with metabolic syndrome found that uncontrolled BP independently increased the odds of OSA 2-fold.10 However, the previous study assessed OSA risk based on questionnaire assessment as opposed to objective overnight assessments. Although previous studies support an association between OSA and hypertension among non-Hispanic whites,11–15 similar evidence is needed for blacks using objective measurements of OSA. Because blacks are most at risk for severe OSA and uncontrolled BP, studies exploring the impact of OSA on BP in this population are warranted.
Using data from the Jackson Heart Sleep Study (JHSS), we investigated objectively measured sleep apnea (based on the respiratory event index) and a key physiological stress of OSA, nocturnal hypoxemia, with uncontrolled BP and resistant hypertension among blacks with hypertension. We hypothesized that OSA and its related hypoxemia burden would associate with more severe forms of hypertension.
METHODS
Data used in this analysis were produced and used in accordance with the policies of the Jackson Heart Study under contracts from the National, Heart, Lung, and Blood Institute and are not the domain of the authors but that of the Jackson Heart Study. These data are available to other researchers for purposes of reproducing the results or replicating the procedures by submitting a manuscript proposal16 to the Jackson Heart Study at jhspub@umc.edu. Data updates for the Jackson Heart Study are also deposited regularly in the National Institutes of Health data repositories, dbGaP17 and BioLincc.18
The Jackson Heart Study (JHS) is a longitudinal study of 5306 black adults, aged 21 to 95 years, enrolled from 3 counties in Jackson, Mississippi (Hinds, Madison, and Rankin) between 2000 and 2004. JHS was designed to study the cause of cardiovascular disease among blacks, as detailed previously.19 Three core examinations have been conducted to date. The current analyses use data from the JHSS, an ancillary study conducted between December 2012 and May 2016 after the third JHS examination. Institutional Review Board approval was obtained from the University of Mississippi Medical Center and Partners Research Committee, and written informed consent was obtained from all participants.
In total, 913 participants were recruited to participate in JHSS. The details of JHSS were previously published.20 In brief, participants who completed the third JHS examination (N=3609) or other ancillary studies were eligible for participation. Potential participants were contacted by phone or mail (N=3015) with an invitation to participate. Participants with documented use of continuous positive airway pressure (CPAP; N=70) and those who were first-degree relatives of a consenting participant (N=10) were not eligible for the study. Participants attended a clinic visit and underwent in-home sleep apnea testing, 1-week wrist actigraphy, fasting venipuncture, anthropometry, blood pressure and other vascular studies, and completed interviewer administered sleep and health questionnaires.
For the current analysis, we restricted the study sample to participants with hypertension (high BP, use of antihypertensive medication, or self-reported diagnosis; N=773). We then further restricted the analysis to exclude participants without valid in-home sleep apnea test (≥3 hours of data from the oximeter, nasal pressure cannula and one or more respiratory band; N=51) or those with missing data on hypertension, measured BP, or number of antihypertensive medications and diuretic use (N=58). The final analytic sample consisted of 664 participants.
Sleep Measures
Sleep apnea was assessed with a validated Type 3 home sleep apnea device (Embletta-Gold device; Embla, Broomfield, CO)21,22, recording nasal pressure (measuring airflow), thoracic and abdominal inductance plethysmography, finger pulse oximetry, body position, and electrocardiography. Sleep studies were scored according to published guidelines.23 Sleep onset was identified based on reduction of movement artifact, heart rate, and assumption of rhythmic breathing, and sleep offset was identified by the appearance of sustained movement activity or increased heart rate.24 Obstructive apneas were identified when the amplitude (peak to trough) of the nasal pressure signal was flat or nearly flat for >10 seconds and accompanied by respiratory effort on the abdominal or thoracic inductance plethysmography bands.23 Central apneas were identified if no displacement was noted on both thoracic and the abdominal inductance channels in addition to a flat nasal pressure signal. Hypopneas were identified if a ≥30% reduction of amplitude was visualized in the nasal pressure signal or, if unclear, in the respiratory inductance bands, for ≥10 seconds. Events were further classified based on the degree of associated desaturation (≥3% and ≥4%). All scoring was conducted by registered polysomnologists at the Harvard Sleep Reading Center; intraand interscorer reliability, for respiratory events evaluated on an ongoing basis, exceeds 0.92. The Respiratory Event Index (REI) was derived as the sum of all apneas plus hypopneas associated with 3% (REI3%) or 4% (REI4%) oxygen desaturation divided by the estimated sleep time.24 Sleep apnea was characterized by the standard REI categories: <5 (unaffected), ≥5 to <15 (mild), ≥15 to <30 (moderate), and ≥30 (severe). For the current study, we further categorized moderate or severe OSA based on the REI ≥15 using the definition of hypopneas that required ≥ 4% associated desaturation (REI4%), and in secondary analysis used a hypopnea definition defined by ≥3% desaturation (REI3%). Nocturnal hypoxemia was quantified as % sleep time with <90% oxyhemoglobin saturation (%Sat<90%). Secondary analyses explored the oxygen desaturation index (ODI), based on all desaturations of ≥4%, and the obstructive apnea index (OAI), all obstructive apneas per hour of sleep.
Participants also underwent 7-day actigraphy using a GT3X+ Activity Monitor on the nondominant wrist for 7 consecutive days along with completing a sleep diary.25 Actigraphic data during 60-s epochs were scored as sleep or wake by ActiLife version 6.13 analysis software (ActiGraph Corp., Pensacola, FL) using a validated algorithm (Cole-Kripke).26
Although CPAP use identified during screening was an exclusion criterion, 8 participants reported CPAP use on the sleep questionnaire and are included in this analysis.
Blood Pressure
During a visit to the JHSS research clinic, seated BP measurements were obtained using an Omron HEM907XL BP monitor after 5 minutes of rest. Three BP readings were taken 1 minute apart, and the last 2 measurements were averaged. Use of antihypertensive medication was determined by self-report, and classes of antihypertensive medication were ascertained by a medication bottle review. High BP was defined as systolic BP ≥ 130 mm Hg or diastolic BP ≥ 80 mm Hg.27 Controlled hypertension was defined as systolic BP <130 mm Hg and diastolic BP <80 mm Hg. Uncontrolled BP was defined as having high BP with use of 1 or 2 classes of antihypertensive medications. Resistant hypertension was defined as having high BP while on ≥3 classes of antihypertensive medications with 1 class being a diuretic or the use of >4 classes of antihypertensive medications regardless of BP control. Uncontrolled BP and resistant hypertension were analyzed as mutually exclusive outcome categories.
Covariates
Participants’ age, sex, education, and smoking status were ascertained by self-report. Education was categorized as less than high school, high school or general equivalency diploma (GED), some college, or college degree (bachelor or higher). Body mass index was calculated in kg/m2 using measurements of weight and height, and obesity was defined as a body mass index ≥ 30 kg/m2. Diabetes mellitus was defined as a fasting glucose ≥126 mg/dL, use of antidiabetes medication, or self-reported diabetes mellitus diagnosis.28 Depressive symptoms were assessed with the Center for Epidemiological Studies of Depression (CES-D) scale and modeled as a continuous variable.29 Perceived stress was measured according to Cohen’s 10-item Perceived Stress Scale, which measured the degree to which someone appraised his or her life as unpredictable, uncontrollable, and overloading in the last month and modeled as a continuous variable.30 Sleep duration and efficiency were obtained from 7-day wrist actigraphy. We computed the average values for total sleep time and sleep efficiency (ratio of total sleep time to total time in bed; continuously and dichotomized at 85%) from actigraphy data.
Statistical Analysis
Frequency and summary statistics of demographics, health characteristics, and sleep symptoms were calculated within each sleep apnea severity category (none or mild versus moderate or severe). We conducted χ2 and Kruskal–Wallis tests to evaluate the statistical significance of the differences in the distribution of categorical and continuous variables across hypertension groups, respectively.
Multinomial logistic regression models with a generalized logit link function were used to estimate odds ratios of having uncontrolled or resistant hypertension, separately, versus controlled hypertension associated with sleep apnea measures, and 95% confidence intervals (CI). We expressed percent sleep time in hypoxemia according to standard deviation units (eg, odds ratios represent one standard deviation higher percent sleep time in hypoxemia). Separate models evaluated uncontrolled or resistant hypertension each as outcomes, with moderate or severe OSA (REI ≥ 15), OSA severity categories, and percent sleep time in hypoxemia as predictors. We used a sequential modeling approach with Model 1 unadjusted, Model 2 adjusted for age, sex, obesity defined as a body mass index ≥ 30 kg/m2, education, smoking (current versus ever or never), and diabetes mellitus, and Model 3 adjusted for the variables in Model 2 and depressive symptoms, perceived stress, sleep duration, and sleep efficiency. In the models with categories of OSA severity, we tested for the linear component of trend by analyzing REI as an ordinal variable. Also, we modeled REI, ODI, and OAI as continuous variables with hypertension control groups. We conducted a secondary analysis that evaluated the association of sleep parameters with uncontrolled and resistant hypertension as a single outcome variable. We performed all analyses with SAS version 9.4 (SAS Institute Inc, Cary, NC).
RESULTS
Study characteristics by sleep apnea severity are shown in Table 1. Of the sample with hypertension, 25.7% had moderate or severe OSA (REI4%), and of those 6% had a physician diagnosis of OSA (data not shown). Of this sample, 48.2% of participants had uncontrolled hypertension and 14.5% had resistant hypertension. Compared with participants with no or mild OSA, those with moderate or severe OSA were more likely to be male (39.8% versus 27.8%), obese (71.0% versus 54.4%), to be taking a higher number of antihypertensive medication classes (1.9 [1.3] versus 1.7 [1.2]), and had a higher percent time with oxygen saturation <90% (3.4 [6.0] versus 0.2 [0.9]). Although our study sample consisted of hypertensive participants, we provided some baseline characteristics comparing normotensive, controlled hypertension, and resistant hypertension groups in Table I in the online-only Data Supplement. Of note, the nonhypertensive group had a lower prevalence of OSA than the hypertensive group.
Table 1.
Participant Characteristics, by Sleep Apnea Severity Category (n=664)
| Characteristics | Total (n=664) | REI4% <15 (n=493) | REI4% 215 (n=171) | P Value |
|---|---|---|---|---|
| Age (y), mean±SD | 64.9±10.6 | 63.9±10.6 | 64.4±10.6 | 0.83 |
| Male sex, n (%) | 205 (30.9) | 137 (27.8) | 68 (39.8) | <0.01 |
| Education, n (%) | 0.28 | |||
| < High school | 64 (9.8) | 51 (10.5) | 13 (7.8) | |
| High school or graduate equivalency diploma | 99 (15.2) | 76 (15.7) | 23 (13.8) | |
| Some college/training | 154 (23.7) | 106 (21.9) | 48 (28.7) | |
| College degree | 334 (51.3) | 251 (51.9) | 83 (49.7) | |
| Body mass index, kg/m2 | ||||
| Mean±SD | 32.3±6.9 | 31.4±6.4 | 35.0±7.5 | <0.01 |
| ≥30 | 387 (58.6) | 267 (54.4) | 120 (71.0) | <0.01 |
| Current/past smoker, n (%) | 213 (34.2) | 160 (34.7) | 53 (32.7) | 0.65 |
| Alcohol use, yes vs no, n (%) | 224 (34.3) | 165 (33.9) | 59 (35.5) | 0.70 |
| Depression symptoms, mean±SD | 8.6±7.8 | 8.7±8.0 | 8.3±7.5 | 0.77 |
| Perceived stress, mean±SD | 11.2±7.0 | 11.3 + 7.2 | 11.0±6.5 | 0.73 |
| Class of antihypertensive medications, n (%) | ||||
| α-Blockers | 19 (2.9) | 14 (2.9) | 5 (3.0) | 0.94 |
| β-Blockers | 129 (19.7) | 93 (19.1) | 36 (21.4) | 0.51 |
| Calcium-channel blockers | 247 (37.6) | 180 (36.9) | 67 (39.6) | 0.52 |
| Diuretics | 333 (50.1) | 243 (49.3) | 90 (52.6) | 0.45 |
| Angiotensin-converting enzyme inhibitors/ angiotensin II receptor blockers /renin inhibitors | 336 (51.1) | 242 (49.6) | 94 (55.6) | 0.17 |
| Peripheral vasodilators | 10 (1.5) | 6(1.2) | 4 (2.4) | 0.29 |
| Number of antihypertensive medications, mean±SD | 1.8±1.2 | 1.7±1.2 | 1.9±1.3 | 0.04 |
| Diabetes mellitus, n (%) | 187 (28.2) | 132 (26.8) | 55 (32.2) | 0.18 |
| Sleep duration <7 h, n (%) | 376 (59.4) | 277 (58.7) | 99 (61.5) | 0.53 |
| Sleep efficiency ≥85%, n (%) | 447 (70.6) | 339 (71.8) | 108 (67.1) | 0.25 |
| Percent time in hypoxemia, median ± interquartile range | 0.4±2.1 | 0.2±0.9 | 3.4±6.0 | <0.01 |
| Hypertension category, n (%) | 0.03 | |||
| Controlled | 248 (37.3) | 191 (38.7) | 57 (33.3) | |
| Uncontrolled | 320 (48.2) | 241 (48.9) | 79 (46.2) | |
| Resistant | 96 (14.5) | 61 (12.4) | 35 (20.5) | |
REI indicates respiratory event index.
Unadjusted odds of uncontrolled and resistant hypertension associated with moderate or severe OSA and %Sat<90% are shown in Table 2. Moderate or severe OSA (REI4% ≥15) was associated with a 1.92-times higher odds of resistant hypertension (odds ratio [OR], 1.92; 95% CI, 1.15–3.20). A standard deviation increase in %Sat<90% was associated with a higher odds of resistant hypertension (OR, 1.30; 95% CI, 1.05–1.61).
Table 2.
Unadjusted Odds Ratios (95% Confidence Intervals) of Uncontrolled or Resistant Hypertension Versus Controlled Hypertension
| Variable | Uncontrolled (n=320) | Resistant (n=96) | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | |
| REI4%* ≥15 vs REI4% <15 | 1.10 (0.74–1.62) | 0.64 | 1.92 (1.15–3.20) | 0.01 |
| Sleep apnea (4%) | ||||
| Mild sleep apnea vs none (4%) | 0.84 (0.58–1.24) | 0.39 | 1.65 (0.93–2.93) | 0.09 |
| Moderate sleep apnea vs none (4%) | 0.94 (0.57–1.53) | 0.79 | 1.83 (0.90–3.70) | 0.09 |
| Severe sleep apnea vs none (4%) | 1.12 (0.58–2.17) | 0.74 | 3.50 (1.54–7.91) | 0.003 |
| P trend | 0.99 | 0.003 | ||
| Percent time in hypoxemia | 1.03 (0.82–1.28) | 0.82 | 1.30 (1.05–1.61) | 0.01 |
| Age (per 10 y) | 0.81 (0.69–0.95) | 0.01 | 1.21 (0.96–1.52) | 0.11 |
| Male (yes vs no) | 1.34 (0.93–1.93) | 0.12 | 1.35 (0.81–2.25) | 0.25 |
| Education | 1.08 (0.92–1.28) | 0.35 | 0.76 (0.61–0.95) | 0.02 |
| Obese (yes vs no) | 1.01 (0.72–1.42) | 0.93 | 1.10 (0.68–1.78) | 0.70 |
| Diabetes (yes vs no) | 0.53 (0.36–0.77) | 0.001 | 1.14 (0.70–1.86) | 0.61 |
| Current/past smoker (yes vs no) | 1.14 (0.79–1.63) | 0.49 | 0.81 (0.48–1.37) | 0.44 |
| Depressive symptoms | 1.00 (0.98–1.02) | 0.98 | 1.04 (1.01–1.07) | 0.007 |
| Perceived stress | 1.01 (0.99–1.04) | 0.31 | 1.04 (1.00–1.07) | 0.04 |
| Sleep duration, min | 1.00 (1.00–1.00) | 0.22 | 1.00 (1.00–1.00) | 0.74 |
| Sleep efficiency, % | 1.00 (0.96–1.03) | 0.92 | 0.99 (0.94–1.04) | 0.78 |
4% oxygen desaturation. Percent time in hypoxemia was standardized to a mean of 0 and standard deviation of 1. The referent group is controlled hypertension (n=248).
In fully adjusted models, moderate or severe OSA (REI4% ≥15) and %Sat<90% were not associated with uncontrolled hypertension (Table 3). However, moderate or severe OSA (REI4%≥ 15) and %Sat<90% were associated with resistant hypertension (OR, 2.04; 95% CI, 1.14–3.67; OR, 1.25; 95% CI, 1.01–1.55), respectively, after adjustment for covariates. Also, OSA based on REI3% was not associated with uncontrolled or resistant hypertension (Table II in the online-only Data Supplement).
Table 3.
Odds Ratios (95% Confidence Intervals) of Uncontrolled or Resistant Hypertension Versus Controlled Hypertension Associated With Moderate or Severe Sleep Apnea and Percent Time in Hypoxemia
| Uncontrolled (n=320) | Resistant (n=96) | |||
|---|---|---|---|---|
| Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | |
| REI4%* ≥15 vs. REI4% <15 | ||||
| Model 1 | 1.10 (0.74–1.62) | 0.64 | 1.92 (1.15–3.20) | 0.01 |
| Model 2 | 1.09 (0.71–1.66) | 0.70 | 1.85 (1.06–3.21) | 0.03 |
| Model 3 | 1.09 (0.70–1.69) | 0.71 | 2.04 (1.14–3.67) | 0.02 |
| Percent time in hypoxemia | ||||
| Model 1 | 1.03 (0.82–1.28) | 0.82 | 1.30 (1.05–1.61) | 0.01 |
| Model 2 | 1.02 (0.82–1.27) | 0.87 | 1.27 (1.03–1.58) | 0.03 |
| Model 3 | 1.01 (0.81–1.27) | 0.92 | 1.25 (1.01–1.55) | 0.04 |
Model 1: Unadjusted. Model 2: Adjusted for age, sex, obese, education, smoking, and diabetes mellitus. Model 3: Adjusted for age, sex, obese, education, smoking, diabetes, depressive symptoms, perceived stress, sleep duration, and sleep efficiency.
4% oxygen desaturation. Percent time in hypoxemia was standardized to a mean of 0 and standard deviation of 1. The referent group is controlled hypertension (n=248).
Sleep Apnea Severity
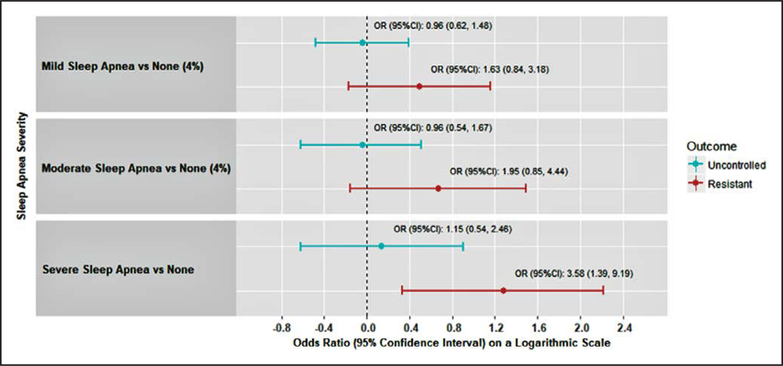
Severe (REI4% ≥30) compared with no OSA (REI4% < 5) was associated with a 3.50-times higher odds of resistant hypertension (OR, 3.50; 95% CI, 1.54–7.91). After adjustment for covariates, severe versus no OSA was associated with a higher odds of resistant hypertension (OR, 3.58; 95% CI, 1.39–9.19; Figure). A statistically significant trend with a graded increase in severity of OSA was associated with a higher prevalence of resistant hypertension (Ptrend=0.008). No associations were present between sleep apnea severity and uncontrolled hypertension.
Figure. Adjusted odds ratios (95% confidence intervals) for uncontrolled and resistant hypertension separately, associated with sleep apnea severity categories.
REI4%<5 (none), REI4%≥5 to <15 (mild), REI4%≥15 to <30 (moderate), and REI4%≥30 (severe). CI indicates confidence interval; and OR, odds ratio.
Secondary Analyses
Secondary analyses showed a similar association for the REI4% and for the oxygen desaturation index (ODI4%) when each are modeled as continuous variables (Table III in the online-only Data Supplement) with resistant hypertension (OR, 1.03; 95% CI, 1.01–1.05 and OR, 1.03; 95% CI, 1.01–1.05; REI4% and ODI4%, respectively). Similar associations also were found for the OAI. The OAI was associated with resistant (OR, 1.03; 95% CI, 1.00–1.06) but not uncontrolled (OR, 1.01; 95% CI, 0.98–1.03) hypertension (Table III in the online-only Data Supplement).
OSA severity and %Sat<90% were not associated with the composite outcome of uncontrolled and resistant hypertension (Table IV in the online-only Data Supplement).
DISCUSSION
In the current study of blacks with hypertension, moderate or severe OSA (REI4%>15) was very common, with a prevalence of 26%, and was largely undiagnosed (6% of participants with OSA reported a previous diagnosis). Resistant hypertension was present in 15% of the sample. Moderate or severe OSA and overnight hypoxemia were associated with resistant hypertension after adjustment for potential confounders. Along with experimental data showing that OSA-related physiological stressors may contribute to resistant hypertension, these data suggest that untreated OSA may contribute to the high burden of resistant hypertension in blacks. Moreover, future studies should test whether diagnosis and treatment of OSA may be interventions for improving BP control and decreasing the burden of resistant hypertension among blacks.
It is estimated that 9% of U.S. adults have resistant hypertension,31 with estimates among blacks in the U.S. of 19%,31 which is similar to the prevalence reported in the current study. Epidemiological studies have demonstrated that resistant hypertension is associated with increased risk for coronary heart disease and all-cause mortality32; therefore, there is a clear need to identify modifiable determinants of resistant hypertension. Previous studies have reported an association between OSA and resistant hypertension.11–15 Several studies have shown that the prevalence of OSA is high among individuals with resistant hypertension.14,33 A case– control study of 63 patients found that OSA (apnea-hypopnea index≥10) was independently associated with resistant hypertension.12 An observational study of 125 participants also reported that OSA (apnea-hypopnea index>15) was associated with resistant hypertension.15 The current results are in accordance with prior studies showing that OSA is associated with resistant hypertension, particularly among blacks, a population most-atrisk but underrepresented in studies.
There are several pathways by which OSA may contribute to BP control. It is hypothesized that OSA increases BP through several mechanisms, including endothelial dysfunction, the autonomic nervous system, and neurohumoral mechanisms.34 Also, untreated sleep apnea may affect energy, cognition, and mood, which may influence adherence to BP medications.35 Participants with resistant hypertension had both higher levels of REI and higher percent time spent in hypoxemia compared with participants with uncontrolled hypertension. Moreover, the association between OSA and resistant hypertension was present when hypopneas were defined using the 4% but not the 3% minimal desaturation criteria for event detection, suggesting that event-related hypoxemia contributes to impaired BP control. The latter is also supported by secondary analyses that showed similar associations between resistant hypertension and the ODI, a measurement of episodic desaturations commonly used as an index for intermittent hypoxemia. Although the mechanisms by which OSA contributes to the development of resistant hypertension have not been fully elucidated, it has been hypothesized that intermittent hypoxemia during sleep leads to overactivation of the sympathetic nervous system36 with concomitant acute increases in BP and subsequent vascular damage, leading to chronic elevations in BP.37–39 These chronic elevations in BP may be mediated by central or peripheral resetting of chemoreceptors and baroreceptors that control BP.40 Additionally, intermittent hypoxemia also activates the endothelin system which leads to vasoconstriction41,42 and arterial stiffness.43 Over time, the vasculature may become less compliant and resistant to alteration by antihypertensive therapy. Aldosterone excess, which is an independent cause of resistant hypertension, has also been shown to contribute to the severity of OSA.11,44 Furthermore, blacks are more likely to be salt-sensitive and respond less well to β-blockers and ACE inhibitors than other racial groups.45,46 Whether differences in BP medication responses relate to OSA is poorly understood.
OSA was not associated with uncontrolled hypertension (high BP while taking < 3 classes of antihypertensive medications) nor with uncontrolled BP and resistant hypertension combined. OSA may be most strongly associated with the most severe forms of hypertension. In the current study, the association was mainly pronounced when comparing participants with severe OSA with those with no or minimal OSA (ie, 3.5-times higher odds). Similarly, Walia et al9 reported an ≈4-times higher odds of resistant hypertension with severe OSA in a group of patients with cardiovascular disease risk factors. The current study extends these findings to a large community-based cohort of blacks.
Results from cross-sectional studies have shown that indices of sleep-disordered breathing, such as reduced slow wave sleep and higher arousal index, are associated with high BP,47,48 postulated to be attributable to alterations in autonomic nervous system activation during sleep. Decreased slow wave sleep and increased arousals are characteristic features of more severe sleep apnea. Because of lack of EEG data, we cannot evaluate the contributions of changes in sleep architecture compared with hypoxemia to resistant hypertension. However, the similarity of the association of resistant hypertension with the ODI4%, as with the REI4%, suggests that simple measurements from oximetry may be useful for identifying hypertension-related risk in this population.
We also explored the OAI as an alternative exposure variable and showed that the association with BP was in a similar pattern as REI. Although obstructive apneas are scored regardless of associated desaturation, these events tend to be associated with desaturation. However, they also reflect events with large intrathoracic swings, which can contribute to adverse cardiovascular responses.
Results from observational studies as well as randomized trials have shown that treatment of OSA may reduce BP.20,21 Findings from the HeartBEAT study demonstrated that CPAP, the most common sleep apnea treatment, led to small but significant improvements in 24-hour and nocturnal blood pressure in patients with sleep apnea and cardiovascular disease.49 Additionally, among patients with OSA and resistant hypertension enrolled in a randomized, controlled trial, CPAP treatment compared with control resulted in a decrease in 24-hour mean and diastolic BP and improved nocturnal dipping BP.50 Meta-analyses of randomized, controlled trials of CPAP treatment in resistant hypertension estimate that systolic and diastolic BP improve by −6.74 mm Hg and −5.94 mm Hg.51 It is plausible that treating OSA could improve resistant hypertension among blacks who are most-at-risk for severe OSA and uncontrolled BP.
The strengths of this study include the use of a large community-based sample of blacks, BP measurement following a standardized protocol, and use of home sleep testing to objectively assess OSA. However, these results should be interpreted in the context of potential limitations. Although this study enrolled a large community-based sample of blacks, the sample is not representative of blacks in the United States. More specifically, participants were recruited from 3 counties in the Jackson, MS metropolitan area and, as reported before, enrolled individuals have a higher education level compared with the U.S. population and larger proportions of females. The JHHS cohort was comparable with the baseline JHS cohort, except that the proportion of JHSS participants with hypertension and a college degree were higher in comparison with the full cohort. BP was measured at a single visit, whereas it is recommended to obtain measurements at a minimum of 2 separate visits.52 In particular, ambulatory BP monitoring should be performed to confirm the diagnosis of resistant hypertension and to better understand the relationship of OSA with diurnal BP patterns. Participants self-reported medication use, and we are unable to address adherence with medications. It is plausible that individuals with OSA were less adherent to medications, which may have been a factor that contributed to BP control. Given that ambulatory BP monitoring was not conducted, we cannot rule out white coat hypertension. Although we cannot assess associations with secondary hypertension, our population was generally healthy and the low frequency of these conditions (eg, primary aldosteronism, renal artery stenosis) in the general population makes it unlikely that these conditions explain the association between resistant hypertension and OSA. This analysis was based on cross-sectional data. However, despite these limitations, the study provides important insights on the association between sleep apnea measures and BP control.
Significance
Based on our findings of an association between OSA and resistant hypertension, future studies should explore whether patients whose BP is not controlled on ≥3 medications or who require the use of ≥4 medications regardless of BP control would benefit from an overnight sleep study or oximetry evaluation, particularly if they exhibit risk factors associated with OSA. Additionally, these findings suggest that untreated OSA may contribute to uncontrolled BP in blacks and, thus, treatment (eg, CPAP) may improve BP control among blacks. These findings are particularly important given that most adults with OSA are undiagnosed and untreated.5
Conclusion
Moderate or severe OSA and hypoxemia was associated with a >2-times higher odds of having resistant hypertension. In particular, severe sleep apnea determined by an elevated REI4% (an index that included hypopneas with ≥4% desaturations) but not REI3% (hypopneas with ≥3% desaturations) was associated with resistant hypertension, suggesting that indices derived from events associated with more severe desaturation may be helpful for identifying adverse BP-related outcomes. These findings are consistent with other studies suggesting that overnight hypoxemia may mediate some of the cardiovascular and metabolic consequences of sleep apnea.53 Furthermore, 94% of participants with OSA were not diagnosed and therefore not treated for sleep disorders. These results suggest that untreated OSA may contribute to hard-to-control BP in blacks. Assessment and treatment of OSA should be considered in this population and may assist in narrowing cardiovascular health disparities.
Supplementary Material
Clinical Perspective.
What Is New?
The current study shows that obstructive sleep apnea (OSA) defined by an elevated respiratory event index or overnight hypoxemia is associated with resistant hypertension among blacks.
Moderate or severe OSA was associated with resistant hypertension but not uncontrolled blood pressure (high blood pressure while taking <3 classes of antihypertensive medication), suggesting that OSA is associated with more severe forms of hypertension.
What Are the Clinical Implications?
The current study suggests that untreated OSA may contribute to hard-to-control blood pressure in blacks.
The results of the current study support hypertension guidelines which encourage OSA screening in patients with hypertension, particularly among those who require the use of ≥4 medications to control their blood pressure.
Acknowledgments
The authors thank the staff and participants of the Jackson Heart Study.
Sources of Funding
Research reported in this article was supported by the National Heart, Lung, and Blood Institute grants R01HL110068, 3R01HL110068-03S2, T32HL007901-18, and K01HL138211. Dr Thomas was supported in part by American Heart Association grant 15SFRN2390002. Dr Abdalla was supported in part by National Heart, Lung, and Blood Institute grant KL2TR001874. Dr Wilson was supported by National Institute of General Medical Sciences grant U54GM115428. Dr Muntner was supported in part by National Heart, Lung, and Blood Institute grant R01HL117323. Dr Redline was supported in part by 5R35HL135818. The Jackson Heart Study is supported and conducted in collaboration with Jackson State University (HHSN268201800013I), Tougaloo College (HHSN268201800014I), the Mississippi State Department of Health (HHSN268201800015I/HHSN26800001) and the University of Mississippi Medical Center (HHSN268201800010I, HHSN268201800011I and HHSN268201800012I) contracts from the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute for Minority Health and Health Disparities (NIMHD). The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Footnotes
Disclosures
Dr Muntner receives funding from Amgen Incorporated. The remaining authors report no conflicts.
Contributor Information
Dayna A. Johnson, Division of Sleep and Circadian Disorders, Brigham and Women’s Hospital, Boston, MA; Division of Sleep Medicine, Harvard Medical School, Boston, MA; Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, GA.
S. Justin Thomas, Departments of Psychiatry, University of Alabama at Birmingham. Department of Medicine, Columbia University, New York, NY.
Marwah Abdalla, Department of Medicine, Beth Israel Deaconess Medical Center, Boston; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Harvard University, Boston, MA; University of Alabama at Birmingham. Department of Medicine, Columbia University, New York, NY.
Na Guo, Division of Sleep and Circadian Disorders, Brigham and Women’s Hospital, Boston, MA.
Yuichiro Yano, Department of Community and Family Medicine, Duke University, Durham, NC.
Michael Rueschman, Division of Sleep and Circadian Disorders, Brigham and Women’s Hospital, Boston, MA.
Rikki M. Tanner, Departments of Epidemiology, University of Alabama at Birmingham. Department of Medicine, Columbia University, New York, NY.
Murray A. Mittleman, Department of Medicine, Beth Israel Deaconess Medical Center, Boston, MA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Harvard University, Boston, MA.
David A. Calhoun, Departments of Medicine, University of Alabama at Birmingham. Department of Medicine, Columbia University, New York, NY.
James G. Wilson, Department of Physiology and Biophysics, University of Mississippi Medical Center, Jackson.
Paul Muntner, University of Alabama at Birmingham. Department of Medicine, Columbia University, New York, NY; Departments of Epidemiology, University of Alabama at Birmingham. Department of Medicine, Columbia University, New York, NY; Departments of, Medicine, University of Alabama at Birmingham. Department of Medicine, Columbia University, New York, NY.
Susan Redline, Division of Sleep and Circadian Disorders, Brigham and Women’s Hospital, Boston, MA; Division of Sleep Medicine, Harvard Medical School, Boston, MA; Department of Medicine, Beth Israel Deaconess Medical Center, Boston, MA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Harvard University, Boston, MA.
REFERENCES
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152 [DOI] [PubMed] [Google Scholar]
- 2.Redmond N, Baer HJ, Hicks LS. Health behaviors and racial disparity in blood pressure control in the national health and nutrition examination survey. Hypertension. 2011;57:383–389. doi: 10.1161/HYPERTENSIONAHA.110.161950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen X, Wang R, Zee P, Lutsey PL, Javaheri S, Alcántara C, Jackson CL, Williams MA, Redline S. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep. 2015;38:877–888. doi: 10.5665/sleep.4732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Redline S, Tishler PV, Hans MG, Tosteson TD, Strohl KP, Spry K. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med 1997;155:186–192. doi: 10.1164/ajrccm.155.1.9001310 [DOI] [PubMed] [Google Scholar]
- 5.Institute of Medicine Committee on Sleep Medicine and Research. The National Academies Collection: Reports funded by National Institutes of Health; Colten HR, Altevogt BM, editors. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Washington (DC): National Academies Press (US) National Academy of Sciences; 2006. [PubMed] [Google Scholar]
- 6.Knutson KL, Van Cauter E, Rathouz PJ, Yan LL, Hulley SB, Liu K, Lauderdale DS. Association between sleep and blood pressure in midlife: the CARDIA sleep study. Arch Intern Med. 2009;169:1055–1061. doi: 10.1001/archinternmed.2009.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Cauter E, Holmback U, Knutson K, Leproult R, Miller A, Nedeltcheva A, Pannain S, Penev P, Tasali E, Spiegel K. Impact of sleep and sleep loss on neuroendocrine and metabolic function. Horm Res. 2007;67 Suppl 1:2–9. doi: 10.1159/000097543 [DOI] [PubMed] [Google Scholar]
- 8.Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J 2011;32:1484–1492. doi: 10.1093/eurheartj/ehr007 [DOI] [PubMed] [Google Scholar]
- 9.Walia HK, Li H, Rueschman M, Bhatt DL, Patel SR, Quan SF, Gottlieb DJ, Punjabi NM, Redline S, Mehra R. Association of severe obstructive sleep apnea and elevated blood pressure despite antihypertensive medication use. J Clin Sleep Med 2014;10:835–843. doi: 10.5664/jcsm.3946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seixas A, Ravenell J, Williams NJ, Williams SK, Zizi F, Ogedegbe G, Jean-Louis G. Uncontrolled blood pressure and risk of sleep apnea among blacks: findings from the Metabolic Syndrome Outcome (MetSO) study. J Hum Hypertens 2016;30:149–152. doi: 10.1038/jhh.2015.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calhoun DA, Nishizaka MK, Zaman MA, Harding SM. Aldosterone excretion among subjects with resistant hypertension and symptoms of sleep apnea. Chest. 2004;125:112–117. doi: 10.1378/chest.125.1.112 [DOI] [PubMed] [Google Scholar]
- 12.Gonçalves SC, Martinez D, Gus M, de Abreu-Silva EO, Bertoluci C, Dutra I, Branchi T, Moreira LB, Fuchs SC, de Oliveira AC, Fuchs FD. Obstructive sleep apnea and resistant hypertension: a case-control study. Chest 2007;132:1858–1862. doi: 10.1378/chest.07-1170 [DOI] [PubMed] [Google Scholar]
- 13.Khan A, Patel NK, O’Hearn DJ, Khan S. Resistant hypertension and obstructive sleep apnea. Int J Hypertens. 2013;2013:193010. doi: 10.1155/2013/193010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Logan AG, Perlikowski SM, Mente A, Tisler A, Tkacova R, Niroumand M, Leung RS, Bradley TD. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens. 2001;19:2271–2277. [DOI] [PubMed] [Google Scholar]
- 15.Pedrosa RP, Drager LF, Gonzaga CC, Sousa MG, de Paula LK, Amaro AC, Amodeo C, Bortolotto LA, Krieger EM, Bradley TD, Lorenzi-Filho G. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension 2011;58:811–817. doi: 10.1161/HYPERTENSIONAHA.111.179788 [DOI] [PubMed] [Google Scholar]
- 16.Jackson Heart Study. Research - Publications. Jackson Heart Study Manuscript Proposal. https://www.jacksonheartstudy.org/Research/Publications#submitmanuscript. 2018. Accessed November 8, 2018.
- 17.National Institutes of Health. The database of Genotypes and Pheno-types (dbGaP). https://www.ncbi.nlm.nih.gov/gap/. 2018. Accessed November 8, 2018.
- 18.National Institutes of Health. National Heart, Lung, and Blood Institute. Biologic Specimen and Data Repository Information Coordinating Center, BioLincc. https://biolincc.nhlbi.nih.gov/home/. 2018. Accessed November 8, 2018.
- 19.Fuqua SR, Wyatt SB, Andrew ME, Sarpong DF, Henderson FR, Cunningham MF, Taylor HA Jr. Recruiting African-American research participation in the Jackson Heart Study: methods, response rates, and sample description. Ethn Dis 2005;15(4 Suppl 6):S6–18. [PubMed] [Google Scholar]
- 20.Johnson DA, Guo N, Rueschman M, Wang R, Wilson G and Redline S. Prevalence and correlates of obstructive sleep apnea among African-Americans: the Jackson Heart Sleep Study. Sleep 2018; 41: doi: 10.1093/sleep/zsy15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oldenburg O, Lamp B, Horstkotte D. Cardiorespiratory screening for sleep-disordered breathing. Eur Respir J 2006;28:1065–1067. doi: 10.1183/09031936.00084406 [DOI] [PubMed] [Google Scholar]
- 22.Dingli K, Coleman EL, Vennelle M, Finch SP, Wraith PK, Mackay TW, Douglas NJ. Evaluation of a portable device for diagnosing the sleep apnoea/hypopnoea syndrome. Eur Respir J. 2003;21:253–259. [DOI] [PubMed] [Google Scholar]
- 23.Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, Marcus CL, Mehra R, Parthasarathy S, Quan SF, Redline S, Strohl KP, Davidson Ward SL, Tangredi MM; American Academy of Sleep Medicine. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2012;8:597–619. doi: 10.5664/jcsm.2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao YY, Weng J, Mobley DR, Wang R, Kwon Y, Zee PC, Lutsey PL, Redline S. Effect of Manual Editing of Total Recording Time: Implications for Home Sleep Apnea Testing. J Clin Sleep Med 2017;13:121–126. doi: 10.5664/jcsm.6404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgenthaler T, Alessi C, Friedman L, Owens J, Kapur V, Boehlecke B, Brown T, Chesson A Jr, Coleman J, Lee-Chiong T, Pancer J, Swick TJ; Standards of Practice Committee; American Academy of Sleep Medicine. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep. 2007;30:519–529. doi: 10.1093/sleep/30.4.519 [DOI] [PubMed] [Google Scholar]
- 26.Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep 1992;15:461–469. doi: 10.1093/sleep/15.5.461 [DOI] [PubMed] [Google Scholar]
- 27.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127–e248. doi: 10.1016/j.jacc.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 28.American Diabetes Association. Screening for type 2 diabetes. Diabetes Care. 2004;27:s11–s14. [DOI] [PubMed] [Google Scholar]
- 29.Zimmerman M, Posternak MA, Chelminski I. Using a self-report depression scale to identify remission in depressed outpatients. Am J Psychiatry 2004;161:1911–1913. doi: 10.1176/ajp.161.10.1911 [DOI] [PubMed] [Google Scholar]
- 30.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. doi: 10.2307/2136404 [DOI] [PubMed] [Google Scholar]
- 31.Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension 2011;57:1076–1080. doi: 10.1161/HYPERTENSIONAHA.111.170308 [DOI] [PubMed] [Google Scholar]
- 32.Irvin MR, Booth JN III, Shimbo D, Lackland DT, Oparil S, Howard G, Safford MM, Muntner P, Calhoun DA. Apparent treatment-resistant hypertension and risk for stroke, coronary heart disease, and all-cause mortality. J Am Soc Hypertens 2014;8:405–413. doi: 10.1016/j.jash.2014.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pratt-Ubunama MN, Nishizaka MK, Boedefeld RL, Cofield SS, Harding SM, Calhoun DA. Plasma aldosterone is related to severity of obstructive sleep apnea in subjects with resistant hypertension. Chest. 2007;131:453–459. doi: 10.1378/chest.06-1442 [DOI] [PubMed] [Google Scholar]
- 34.Dopp JM, Reichmuth KJ, Morgan BJ. Obstructive sleep apnea and hypertension: mechanisms, evaluation, and management. Curr Hypertens Rep 2007;9:529–534. [DOI] [PubMed] [Google Scholar]
- 35.Mensah GA. Eliminating disparities in cardiovascular health: six strategic imperatives and a framework for action. Circulation. 2005;111:1332–1336. doi: 10.1161/01.CIR.0000158134.24860.91 [DOI] [PubMed] [Google Scholar]
- 36.Marcus JA, Pothineni A, Marcus CZ, Bisognano JD. The role of obesity and obstructive sleep apnea in the pathogenesis and treatment of resistant hypertension. Curr Hypertens Rep. 2014;16:411. doi: 10.1007/s11906-013-0411-y [DOI] [PubMed] [Google Scholar]
- 37.Parati G, Lombardi C, Hedner J, Bonsignore MR, Grote L, Tkacova R, Lévy P, Riha R, Bassetti C, Narkiewicz K, Mancia G, McNicholas WT; EU COST Action B26 members. Recommendations for the management of patients with obstructive sleep apnoea and hypertension. Eur Respir J. 2013;41:523–538. doi: 10.1183/09031936.00226711 [DOI] [PubMed] [Google Scholar]
- 38.Punjabi NM, Newman AB, Young TB, Resnick HE, Sanders MH. Sleep-disordered breathing and cardiovascular disease: an outcome-based definition of hypopneas. Am J Respir Crit Care Med. 2008;177:1150–1155. doi: 10.1164/rccm.200712-1884OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D’Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829 [DOI] [PubMed] [Google Scholar]
- 40.Fletcher EC. The relationship between systemic hypertension and obstructive sleep apnea: facts and theory. Am J Med. 1995;98:118–128. doi: 10.1016/S0002-9343(99)80395-7 [DOI] [PubMed] [Google Scholar]
- 41.Seo B, Oemar BS, Siebenmann R, von Segesser L, Lüscher TF. Both ETA and ETB receptors mediate contraction to endothelin-1 in human blood vessels. Circulation. 1994;89:1203–1208. [DOI] [PubMed] [Google Scholar]
- 42.Yanagisawa M, Kurihara H, Kimura S, Goto K, Masaki T. A novel peptide vasoconstrictor, endothelin, is produced by vascular endothelium and modulates smooth muscle Ca2+ channels. J Hypertens Suppl. 1988;6:S188–S191. [DOI] [PubMed] [Google Scholar]
- 43.Dhaun N, Goddard J, Webb DJ. The endothelin system and its antagonism in chronic kidney disease. J Am Soc Nephrol. 2006;17:943–955. doi: 10.1681/ASN.2005121256 [DOI] [PubMed] [Google Scholar]
- 44.Dudenbostel T, Calhoun DA. Resistant hypertension, obstructive sleep apnoea and aldosterone. J Hum Hypertens 2012;26:281–287. doi: 10.1038/jhh.2011.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elijovich F, Weinberger MH, Anderson CA, Appel LJ, Bursztyn M, Cook NR, Dart RA, Newton-Cheh CH, Sacks FM, Laffer CL; American Heart Association Professional and Public Education Committee of the Council on Hypertension; Council on Functional Genomics and Translational Biology; and Stroke Council. Salt sensitivity of blood pressure: a Scientific Statement from the American Heart Association. Hypertension. 2016;68:e7–e46. doi: 10.1161/HYP.0000000000000047 [DOI] [PubMed] [Google Scholar]
- 46.Wright JT Jr, Dunn JK, Cutler JA, Davis BR, Cushman WC, Ford CE, Haywood LJ, Leenen FH, Margolis KL, Papademetriou V, Probstfield JL, Whelton PK, Habib GB; ALLHAT Collaborative Research Group. Outcomes in hypertensive black and nonblack patients treated with chlorthalidone, amlodipine, and lisinopril. JAMA. 2005;293:1595–1608. doi: 10.1001/jama.293.13.1595 [DOI] [PubMed] [Google Scholar]
- 47.Javaheri S, Redline S. Sleep, slow-wave sleep, and blood pressure. Curr Hypertens Rep. 2012;14:442–448. doi: 10.1007/s11906-012-0289-0 [DOI] [PubMed] [Google Scholar]
- 48.Sulit L, Storfer-Isser A, Kirchner HL, Redline S. Differences in polysomnography predictors for hypertension and impaired glucose tolerance. Sleep. 2006;29:777–783. doi: 10.1093/sleep/29.6.777 [DOI] [PubMed] [Google Scholar]
- 49.Gottlieb DJ, Punjabi NM, Mehra R, Patel SR, Quan SF, Babineau DC, Tracy RP, Rueschman M, Blumenthal RS, Lewis EF, Bhatt DL, Redline S. CPAP versus oxygen in obstructive sleep apnea. N Engl J Med. 2014;370:2276–2285. doi: 10.1056/NEJMoa1306766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martínez-García MA, Capote F, Campos-Rodríguez F, Lloberes P, Díaz de Atauri MJ, Somoza M, Masa JF, González M, Sacristán L, Barbé F, Durán-Cantolla J, Aizpuru F, Mañas E, Barreiro B, Mosteiro M, Cebrián JJ, de la Peña M, García-Río F, Maimó A, Zapater J, Hernández C, Grau SanMarti N, Montserrat JM; Spanish Sleep Network. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA. 2013;310:2407–2415. doi: 10.1001/jama.2013.281250 [DOI] [PubMed] [Google Scholar]
- 51.Iftikhar IH, Valentine CW, Bittencourt LR, Cohen DL, Fedson AC, Gíslason T, Penzel T, Phillips CL, Yu-sheng L, Pack AI, Magalang UJ. Effects of continuous positive airway pressure on blood pressure in patients with resistant hypertension and obstructive sleep apnea: a meta-analysis. J Hypertens. 2014;32:2341–50; discussion 2350. doi: 10.1097/HJH.0000000000000372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2 [DOI] [PubMed] [Google Scholar]
- 53.Dewan NA, Nieto FJ, Somers VK. Intermittent hypoxemia and OSA: implications for comorbidities. Chest 2015;147:266–274. doi: 10.1378/chest.14-0500 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.