Abstract
Cellular immunity in insects is accompanied by change in hemocyte shape. This study hypothesizes that cytoskeletal rearrangement is accompanied by transmembrane water transport to change cell volume, thus changing cell shape. A water-transporting pore (=aquaporin:AQP) has been identified in the beet armyworm, Spodoptera exigua. Its expression was detected in all developmental stages and tissues, although its transcription levels were different between biotic and abiotic conditions. Heterologous expression of Se-AQP in Sf9 cells showed that Se-AQP was localized on cell membrane. RNA interference (RNAi) using double-stranded RNA effectively suppressed its transcript levels. Under different ionic concentrations, hemocytes of RNAi-treated larvae did not change cell volume presumably due to malfunction in water transportation. Se-AQP might participate in glycerol transport because up-regulation of hemolymph glycerol titer after rapid cold-hardening was prevented by RNAi treatment against Se-AQP expression. The inhibitory effect of RNAi treatment on change of cell shape significantly impaired cellular immune responses such as phagocytosis and nodule formation upon bacterial challenge. RNAi treatment also significantly interfered with immature development of S. exigua. These results indicate that Se-AQP plays a crucial role in cell shape change that is required for cellular immunity and other physiological processes.
Introduction
Aquaporins (AQPs) belong to major intrinsic proteins that form a diverse family consisting of more than 1,700 integral membrane proteins. They are responsible for transporting water and other neutral molecules through lipid bilayer membrane in almost all living organisms1. AQPs from both vertebrates and invertebrates have a similar structural organization with six transmembrane domains linked by five intra-helical loops. They are present as tetramer in the biological membrane. Each monomer of AQP contains two conserved Asn-Pro-Ala (NPA) signature motifs and an aromatic/arginine (Ar/R) constriction region for their selective permeability2–4.
There are 13 AQP genes denoted as AQP0-AQP12 in mammals. They are classified into two subfamilies5,6. The first subfamily is known as aquaporin that only permits water to pass through. The second subfamily is known as aquaglyceroporin that permits water and some other nonpolar small solutes such as urea and glycerol to pass through7. However, few AQPs have been discovered and characterized in insects. Phylogenetic analyses on insect AQPs have revealed the presence of six major subfamilies, including water/urea-transporting Pyrocoelia rufa integral proteins (PRIP), water-specific Drosophila intrinsic protein (DRIP), water and glycerol transporting aquaglyceroporin (Glp), glycerol-permeating entomoglyceroporin (Eglp), water-impermeable but cation-permeable Big Brain proteins (BIB), and unorthodox aquaporin (AQP12L)8.
Various roles of AQPs in mammalian systems have been reported. Besides their water transportation activity, they also play crucial roles in cell migration, cell proliferation, and adipocyte metabolism9. It has been reported that AQP1-deleted mice show growth retardation and reduced vascularity of implanted tumors10. Expression of AQP4 in brain astrocytes can induce their migration toward a chemotactic stimulus and increase glial scarring11,12. However, expression of AQP3 in skin and cornea can improve wound healing13,14 and colonic epithelial cell regeneration15. AQP7 and AQP9 have key metabolic regulatory functions in diabetes and obesity16.
In insects, AQPs play important roles in freeze tolerance, desiccation resistance, and heat tolerance17–20. An AQP identified from mosquito Aedes aegypti plays a crucial role in cell shape change by bidirectional water transport21. Change in cell shape is also required for cellular immune responses in insect immunity22. Especially, hemocytes exhibit spreading behavior to perform phagocytosis by increasing cell surface, in which cytoskeleton should be rearranged by F-actin growth and bundling23. Hemocyte-spreading behavior may also need cell volume change by bidirectional water transportation. Inhibition of water transport in hemocytes by treating ion channel inhibitor can impair hemocyte-spreading behavior24. The release of prophenoloxidase (PPO) for catalyzing melanization25 is also required for cell shape change to perform insect immunity. PPO is synthesized from a specific hemocyte, oenocytoid, and released into plasma by cell lysis in beet armyworm, Spodoptera exigua26. To trigger cell lysis, water should be transported into hemocytes presumably through AQP via an ion gradient established by sodium-potassium-chloride cotransporter activity27. Thus, the present study hypothesize that AQP activity is required for cell volume change of hemocytes to perform cellular immune responses.
In this study, an AQP gene (Se-AQP) was identified from S. exigua. Its expression patterns in different developmental stages and tissues including hemocytes were analyzed. After confirming its localization on cell membrane, physiological functions of Se-AQP associated with cellular immune responses were then assessed through RNA interference (RNAi).
Results
Molecular characterization and cellular location of Se-AQP
Se-AQP was predicted from a TSA transcriptome (GenBank accession number: GAOQ01010693.1) by using S. litura aquaporin sequence (GenBank accession number: KC999953.1) as a query. Its ORF consists of 843 bp encoding 280 amino acids. Se-AQP domain analysis showed two tandem structural repeats, each consisting of three transmembrane helices (TM1-3 and TM4-6). It also had a short α-helix in loops B and E, each containing an NPA motif predicted to line one side of the pore (Fig. 1A) known as the “aquaporin fold”28. Residues responsible for the Ar/R constriction region (Phe-92, His-216, Ser-226, and Arg-231) were found in Se-AQP. They were predicted to have function of establishing water selectivity (Fig. 1B). For efficient water selectivity, Ar/R constriction region was present at close proximity to NPA domains. These conserved NPAs formed a canonical structure in the center of the pore29, allowing water molecule for passing through the midpoint of the channel30. Se-AQP appeared on the biological membrane as tetramer with each containing two NPA domains (Fig. S1A). Se-AQP shared 42.2% amino acid sequence similarities with Homo sapiens AQP (PDB accession number: 4CSK). Protein-protein interaction maps of Se-AQP with other proteins in Drosophila melanogaster were prepared due to no information on the interaction map of S. exigua. In this bioinformatics analysis, Se-AQP was predicted to interact with glycerol kinase (Fig. S1B). Phylogenetic analysis showed six clusters including PRIP, DRIP, Eglp, BIB, Glp, and AQP12L. Se-AQP was clustered with DRIP (Fig. 1C).
Figure 1.
Molecular characterization of S. exigua aquaporin (Se-AQP). (A) Transmembrane domain analysis of Se-AQP. Domains of Se-AQP were predicted using TMHMM69,70. NPA domains are shown in red color while residues related to the Ar/R constriction region are shown in yellow color. (B) Organization of Ar/R constriction region. The structure depicted was from the extracellular side of the membrane. Classical NPA motifs are shown in red sphere. Ar/R selectivity residues regions (Phe-92, His-216, Ser-226, and Arg-231) are shown in blue balls and sticks. (C) Phylogenetic analysis of S. exigua aquaporin (Se-AQP, GenBank accession number: MH333284) with known insect AQPs. The analysis was performed using MEGA6. Bootstrapping values were obtained with 1,000 repetitions to support branching and clustering. Amino acid sequences of selected AQP genes were retrieved from GenBank. Accession numbers were added after species name.
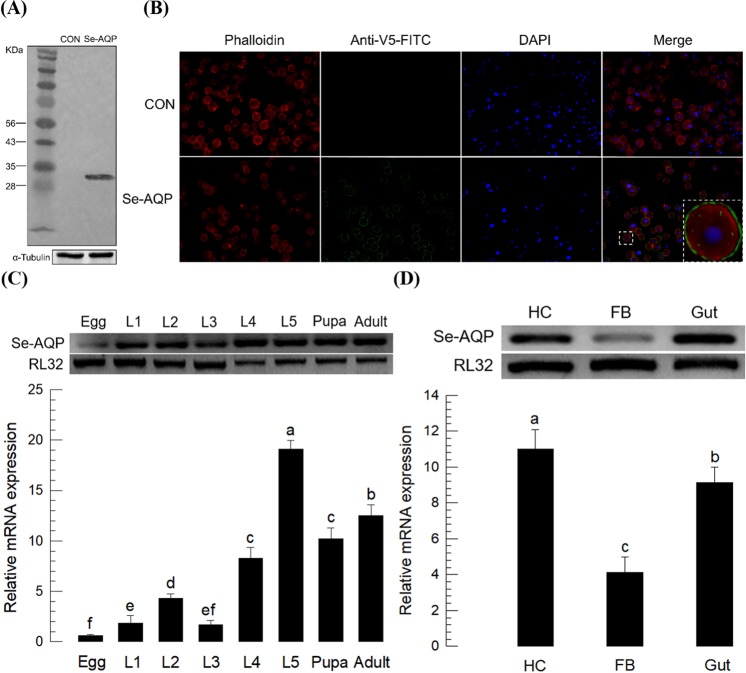
We transfected Sf9 cells with a eukaryotic expression vector containing Se-AQP to determine protein localization in expressed cells. Recombinant Se-AQP was heterologously expressed in Sf9 cells. The recombinant protein was confirmed by Western blot with expected size (~32 kDa) (Fig. 2A). Immunofluorescence assay showed that the recombinant Se-AQP protein was localized on the cell membrane of Sf9 cells (see a dotted big rectangle in Fig. 2B).
Figure 2.
Expression profile of Se-AQP. (A) Western blot analysis. Se-AQP was transfected into Sf9 cells. Protein size of recombinant Se-AQP was ~32 kDa. It was captured by V5 antibody. (B) Immunofluorescence assay for the detection of transient expression of Se-AQP in Sf9 cells. F-actin was specifically detected with Alexa Fluor 555 phalloidin while nucleus was stained with DAPI. To check transient expression, anti-V5-FITC antibody was used. (C) Expression patterns of Se-AQP in different developmental stages, including egg, first to fifth instar larvae (‘L1–L5’), pupa, and adult. (D) Expression patterns in indicated tissues of L5 larvae, including hemocyte (‘HC’), fat body (‘FB’), and gut (‘Gut’). A ribosomal gene RL32 was used as reference gene. Each treatment was replicated three times with independent tissue preparations. Different letters indicate significant differences among means at Type I error = 0.05 (LSD test).
Se-AQP expression and its down-regulation by RNAi
Expression of Se-AQP was analyzed under selected physiological conditions of S. exigua. Se-AQP was expressed in all developmental stages ranging from egg to adult, showing high expression levels during L5 larval and adult stages (Fig. 2C). Selected larval tissues were isolated and assessed for Se-AQP expression levels by RT-qPCR (Fig. 2D). Se-AQP exhibited the highest expression levels in hemocytes.
Se-AQP expression level was increased according to larval development, suggesting that its expression levels might be correlated with body size. To test this hypothesis, different body sized individuals in L4, L5, or pupal stage were assessed for expression levels of Se-AQP. Correlations between body weight and Se-AQP expression level were highly significant in L4 larvae (r = 0.88; P < 0.05), L5 larvae (r = 0.90; P < 0.05), and pupae (r = 0.70; P < 0.05) (Fig. S1A).
Body water content should be regulated according to physical climate conditions in insects. This raised a hypothesis that environmental factors such as temperature and humidity might influence the expression of Se-AQP. Results showed that expression levels of Se-AQP in larvae or pupae exposed to different temperatures for 6 h significantly fluctuated. They were different among developmental stages (F = 8.49; df = 2, 42; P < 0.001) (Fig. S1B). Expression levels of Se-AQP were significantly increased with increasing ambient temperature (F = 37.97; df = 1, 28; P < 0.001 for L4; F = 8.02; df = 1, 28; P < 0.01 for L5; F = 29.40; df = 1, 28; P < 0.001 for pupa). Humidity also influenced expression levels of Se-AQP in S. exigua. Their expression levels varied among developmental stages (F = 26.06; df = 2, 42; P < 0.001) (Fig. S2C). In both larval and pupal stages, Se-AQP expression levels were decreased with increasing relative humidity (F = 29.76; df = 1, 28; P < 0.001 for L4; F = 18.77; df = 1, 28; P < 0.01 for L5; F = 31.03; df = 1, 28; P < 0.001 for pupa).
RNAi was performed by injecting dsRNA specific to Se-AQP (Fig. S3). Test developmental stage was L5 larvae which took 5 days before pupation. The last 2 days of L5 larvae are considered a wandering phase for pupation while the first 3 days are considered its growing phase31. When 1 μg of dsRNA was injected to each of one day old L5 larvae, Se-AQP transcript levels were undetectable at 24 h PI in all three test tissues (Fig. S3A). Decreased levels of Se-AQP expression maintained at least for 3 days. To quantify RNAi efficacy, qPCR analyses were performed (Fig. S3B). dsRNA treatments resulted in significant reductions in Se-AQP expression in these tissues at 24 and 48 h PI. At 24 h PI, Se-AQP expression levels in hemocyte, fat body, and gut were decreased ~65.5%, ~84.1%, and ~68.8%, respectively. At 48 h PI, Se-AQP expression levels in hemocyte, fat body, and gut were decreased ~2.1, ~4.4, and ~14.4 fold, respectively, compared to controls. For subsequent functional analyses, dsRNA-treated larvae at 24 h PI were used.
Roles of Se-AQP in transporting water and glycerol
To determine the role of Se-AQP in water transport, two groups of hemocytes were used to compare their cell shapes under different osmotic environments (Fig. 3). One group of hemocytes was prepared from larvae treated with dsAQP at 24 PI as shown above. The other group was control hemocytes prepared from larvae treated with control dsRNA. In controls, hemocytes showed spreading, shrunk, and lysed behaviors under isotonic, hypertonic, and hypotonic environments, respectively (Fig. 3A). Under isotonic environment, most hemocytes were well spread. Their cell-spreading behavior was determined by extension of F-actin out of original cell boundary. Under hypertonic environment, hemocytes were distorted and shrunk probably due to loss of water from cellular content. Under hypotonic treatment, most hemocytes were lysed probably due to water intake. However, hemocytes collected from RNAi-treated larvae significantly (P < 0.05) prevented these cell shape changes (Fig. 3B). Hemocytes under dsAQP treatment were ~10.6 fold less spread under isotonic condition, ~5.5 fold less shrunk under hypertonic condition, and ~8.6 fold less lysed under hypotonic condition compared to control hemocytes.
Figure 3.
Role of Se-AQP in water transport. (A) Function of Se-AQP in regulating hemocyte shape. Hemocytes treated with gene specific dsRNA (‘dsAQP’) were exposed to isotonic, hypertonic, or hypotonic solution for 10 min. Hemocytes were observed under a fluorescence microscope at 400× magnification. Spread, shrunk, and lysed cells were indicated with white arrows. Hemocytic F-actin filaments were specifically recognized by FITC-tagged phalloidin (green). (B) Quantitative representation of spread, shrunk, and lysed hemocytes after exposure to isotonic, hypertonic, and hypotonic solutions, respectively. A GFP gene was used as a control dsRNA (‘dsCON’). Each treatment was independently replicated three times. Asterisk mark (*) on bars indicates significant differences among means at Type I error = 0.05 (LSD test).
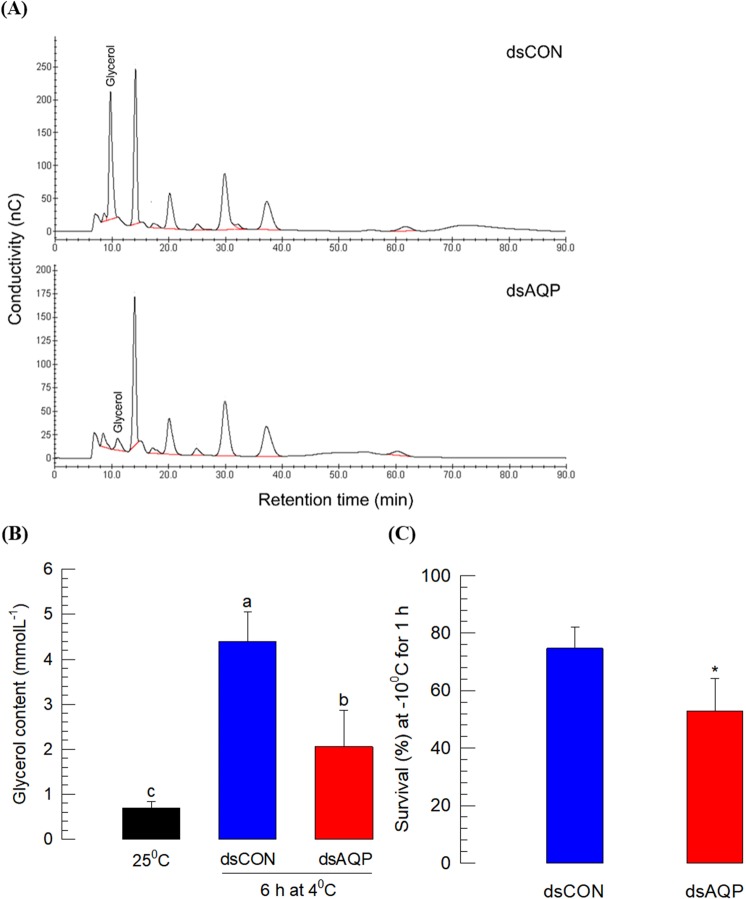
S. exigua is known to be freeze-susceptible. It needs rapid cold hardening (RCH) capacity for overwintering to supercool body water using cryoprotectant like glycerol32. Bioinformatics analysis indicated that Se-AQP was associated with glycerol kinase (Fig. S1B), suggesting that it might be involved in transport of glycerol during RCH. To test this hypothesis, RNAi was performed to knockdown expression of Se-AQP followed by RCH treatment (4 °C for 6 h) (Fig. 4). HPLC analysis showed that RCH treatment resulted in accumulation of glycerol in the hemolymph of control larvae. dsAQP treatment significantly decreased glycerol peak in the chromatogram (Fig. 4A). Results showed significant reduction (~2.6 fold) of glycerol content in the hemolymph of dsAQP-treated larvae after exposure to RCH treatment (Fig. 4B). This led us to determine whether Se-AQP might have a physiological function in cold tolerance. After RCH treatment, survivorship of RNAi-treated larvae was significantly reduced by ~1.5 folds compared to that of control larvae (Fig. 4C).
Figure 4.
Role of Se-AQP in glycerol transport. (A) Chromatograms showing reduced glycerol titer in hemolymph of RNAi treated fifth instar larvae in response to exposure to 4 °C for 6 h. (B) Changes in glycerol content in fifth instar larval hemolymph in response to exposure to 4 °C for 6 h. The eluent was 400 mM NaOH at a flow rate of 0.4 mL/min. (C) Suppression of cold tolerance after RNAi treatment of Se-AQP. Each treatment was independently replicated three times. Each replicate used 10 larvae. Different letters and asterisk mark (*) indicate significant differences among means at Type I error = 0.05 (LSD test).
Immune mediation by Se-AQP through cell shape change
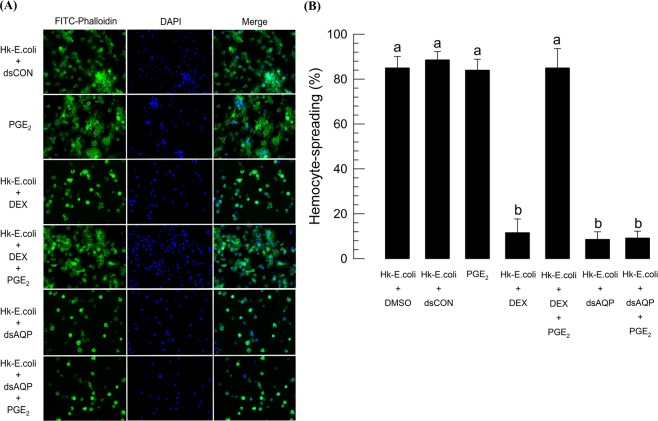
Hemocytes were spread along with F-actin growth upon immune challenge using heat-killed E. coli (Fig. 5A). PGE2 injection was also effective in inducing the hemocyte-spreading behavior as much as bacterial challenge (Fig. 5B). However, treatment with dexamethasone (DEX, a PLA2 inhibitor) significantly (P < 0.05) inhibited the hemocyte-spreading behavior upon immune challenge. Addition of arachidonic acid (AA, a catalytic product of PLA2) significantly (P < 0.05) rescued the inhibitory activity of DEX against hemocyte-spreading behavior. Hemocytes collected from dsAQP-treated larvae also significantly (P < 0.05) lost their spreading behavior upon immune challenge and did not even respond to the addition of PGE2.
Figure 5.
Role of Se-AQP in changing cell shape of hemocytes. For bacterial challenge, heat-killed (HK) E. coli (~3.2 × 104 cells/larva) in 1 µL were injected into larvae at 24 h after dsRNA treatment. (A) Effect of dsAQP on F-actin growth in response to bacterial challenge. At 2 h PI, hemocytes were observed under a fluorescence microscope at 400× magnification. Hemocytic F-actin filaments were specifically recognized by FITC-tagged phalloidin (green) while the nucleus was stained with DAPI (blue). (B) Quantitative representation of hemocyte spreading assay. Each treatment was independently replicated three times. Different letters indicate significant differences among means at Type I error = 0.05 (LSD test).
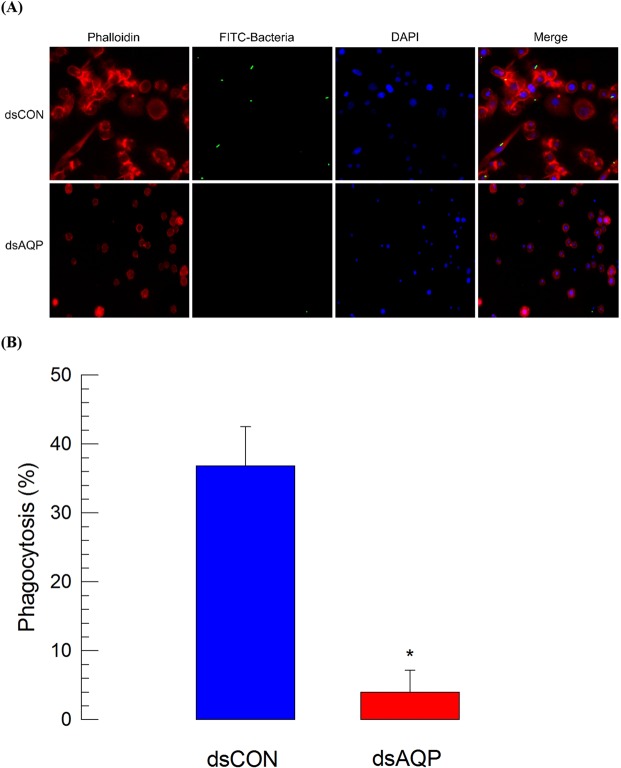
Cell shape change induced by Se-AQP activity might be required for cellular immunity of hemocytes in S. exigua. To test this hypothesis, hemocyte phagocytosis exhibiting cytoplasmic extension to form phagosome was assessed after injecting FITC-labeled E. coli to L5 larvae (Fig. 6). As expected, control hemocytes were well spread (Fig. 6A). Some (>35%) of hemocytes had FITC-labeled E. coli in their cytoplasm. However, phagocytosis was significantly (P < 0.05) lost in hemocytes collected from larvae treated with dsAQP, in which less than 4% hemocytes could perform cellular immunity (Fig. 6B).
Figure 6.
Influence of RNAi treatment of Se-AQP on hemocyte phagocytosis. (A) Effect of dsAQP on FITC-labeled E. coli. One microliter of heat-killed (HK) E. coli (~3.5 × 104 cells/larva) were injected into larvae at 24 h after dsRNA treatment. A GFP gene was used as a control dsRNA (‘dsCON’). (B) Quantitative representation of phagocytosis between dsCON and dsAQP treated hemocytes. Each treatment was independently replicated three times. Asterisk mark (*) on bars indicates significant differences among means at Type I error = 0.05 (LSD test).
In response to a large number of bacteria, hemocytes induced around 62 nodules per larva in S. exigua (Fig. 7A). DEX treatment along with bacterial infection significantly (P < 0.05) inhibited nodule formation. Addition of PGE2 significantly (P < 0.05) rescued such immunosuppression. Decrease of Se-AQP expression by RNAi significantly (P < 0.05) impaired nodule formation in response to bacterial challenge. Addition of PGE2 failed to rescue such immunosuppression.
Figure 7.
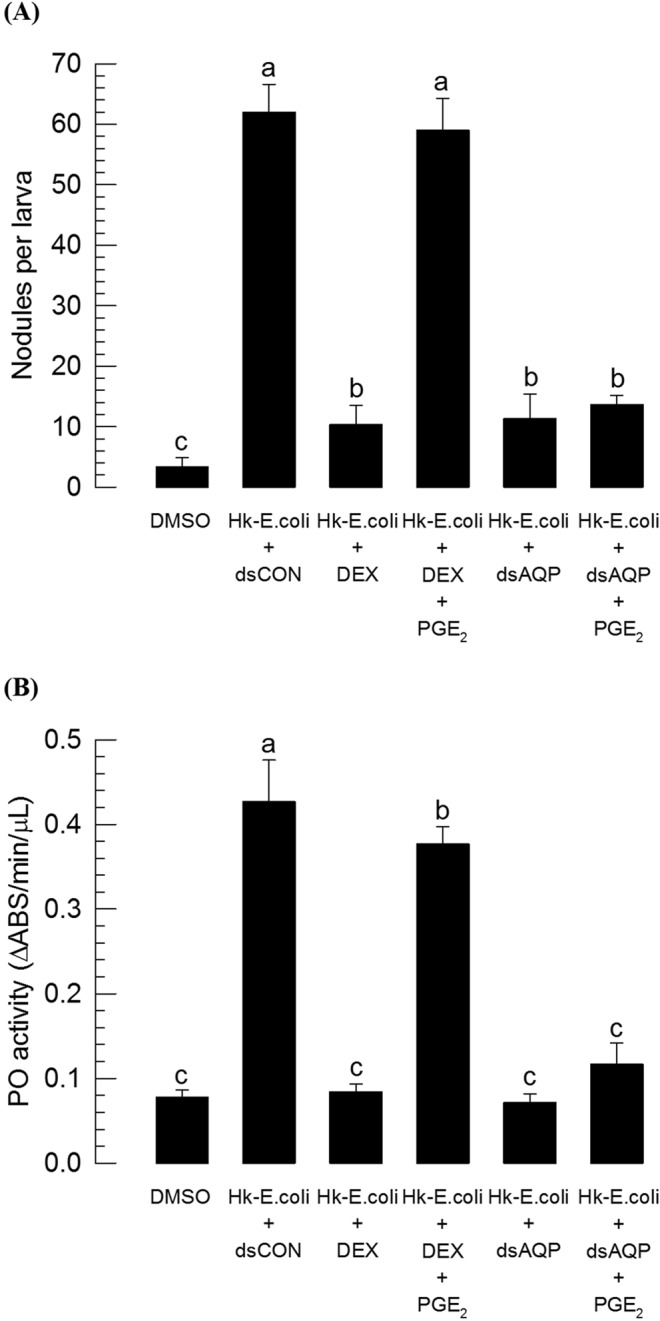
Influence of RNAi treatment of Se-AQP on nodulation and phenoloxidase (PO) activity. (A) Inhibitory effect of dsAQP on hemocyte nodule formation in response to bacterial challenge. One microliter of heat-killed (HK) E. coli (~4.2 × 104 cells/larva) were injected into larvae at 24 h after dsRNA treatment. At 8 h PI, numbers of nodules were assessed. (B) Inhibitory effect of dsAQP on PO activity in response to bacterial challenge. One microliter of heat-killed (HK) E. coli (~4.2 × 104 cells/larva) was injected into larvae at 24 h after dsRNA treatment. At 8 h PI of bacteria, PO activity was measured. A GFP gene was used as a control dsRNA (‘dsCON’). Each treatment was independently replicated three times. Each replicate used 10 larvae. Different letters indicate significant differences among means at Type I error = 0.05 (LSD test).
PO activity is required for nodule formation by catalyzing melanization to produce black nodules. It can be induced by the release of its precursor from oenocytoids through cell lysis after water influx26,27. Oenocytoid cell lysis resulted in PO activation via PG signaling as seen in PGE2 treatment (Fig. 7B). However, Se-AQP expression decreased by RNAi treatment prevented hemocytes from responding to PGE2 and inducing PO activation even under immune challenge.
Adverse effect of Se-AQP RNAi on immature development
Transport of water or small molecules through Se-AQP might be necessary for development of S. exigua. To test this hypothesis, larval and pupal developments of S. exigua were monitored to compare any difference between RNAi-treated and control individuals (Fig. 8). For larval instars L4 and L5 and pupae, developmental rates were significantly (P < 0.05) retarded after RNAi treatment to decrease Se-AQP expression. RNAi-treated individuals exhibited 1.11∼1.40 folds slower developmental rates compared to control (Fig. 8A). RNAi treatment also significantly reduced body size, resulting in only half size of larvae or 86% body weight of pupae compared to control (Fig. 8B). Adverse effects of RNAi treatment resulted in significant (P < 0.05) mortalities at larval (Fig. 8C) and pupal (Fig. 8D) stages. In addition, RNAi treatment resulted in malformed pupae. They could not emerge to adults (Fig. 8E).
Figure 8.
Influence of RNAi treatment of Se-AQP on larval and pupal developmental processes. One µg of dsCON or dsAQP was injected into larvae (within an hour after emerging into L4 and L5) or pupae (<4 h old) using a microsyringe. (A) Effect of dsAQP on developmental period of larvae and pupa. (B) Effect of dsAQP in decreasing body weights of larvae and pupa. (C) Pupation percentage in larvae after treatment with dsRNA. (D) Percentage of successful adult emergence in larvae and pupa after RNAi. (E) Detrimental effect of dsAQP on pupa. A GFP gene was used as a control dsRNA (‘dsCON’). Each treatment was independently replicated three times. Each replicate used 10 larvae. Asterisk mark (*) on bars indicates significant differences among means at Type I error = 0.05 (LSD test).
Discussion
An aquaporin gene, Se-AQP, of S. exigua was identified in this study. Its molecular structure has characters of other insect-specific AQPs. All developmental stages of S. exigua expressed Se-AQP. However, its expression varied with environmental conditions. Especially, its physiological function of transporting water and other small solutes allowed cell shape change of hemocytes to perform cellular immune responses. In addition, its expression was required for development of immature stages. This conclusion is supported by the following observations.
First, Se-AQP was homologous to insect DRIP type AQPs. Insect AQPs are composed of four distinct groups33. Group 1 AQPs include DRIP and PRIP subgroups, both of which are widespread in insects34,35. They have been characterized primarily in liquid-feeding insects such as green leafhopper36, buffalo fly37, yellow fever mosquito38, pea aphid39, whitefly40, and pest bugs41. In each of these species, DRIP is distributed mostly in hindgut (HG) and Malpighian tubule (MT). Mosquitoes also express PRIP in digestive and excretory tissues42,43. Both types of AQPs are water-specific44. They are presumed to perform osmoregulation of sap-feeding insects by extruding excess amounts of water or avoid salt overload through dietary intake45. Groups 2 and 4 AQPs are known as BIB and superaquaporins, respectively6,46. Group 3 AQPs are heterogeneous. Some of them have been characterized as Glp or Eglp that can transport glycerol in addition to water8,47–49. Transportation of water molecule through AQP is accomplished by projecting opposing NPA motifs containing two inverted helices on loop B and loop E50. Some insect AQPs can achieve glycerol transportability when they lose the first NPA classical motif 51. Transmembrane domain and structural analysis showed that Se-AQP had conserved characteristics of water-transporting activity. In vertebrate AQPs, Ar/R constriction region is composed of four conserved residues: Phe-58, His-182, Cys-191, and Arg-19752. In Se-AQP, this motif is conserved except that Cys-191 is replaced by Ser-226. Similar phenomenon has been observed for an AQP of Chilo suppressalis where Cys-191 is replaced by Ser-20353. Comparative analysis between vertebrate and insect AQPs has indicated that vertebrate Cys-191 is substituted by either Ser or Ala in insect DRIPs53,54. These bioinformatics analyses suggest that Se-AQP is insect-specific. It may perform water transportation.
Second, Se-AQP was expressed in all developmental stages from egg to adult. In larval stage, Se-AQP was expressed in all tested tissues including hemocytes. In other insects, different AQPs exhibit variations in their expression levels depending on development stages. For example, in Culex pipiens, mRNA levels of DRIP, Eglp, and AQP12L are significantly higher in larvae compared to those in pupae and adults whereas BIP mRNA levels are significantly lower in larvae compared to those in pupae and adults55. In contrast, in another mosquito species (Anopheles gambiae), mRNA levels of both PRIP and Eglp1 are similar between larvae and pupae51. Relatively low expression of AQP in fat body might be due to low AQP frequency in this tissue compared to its abundance in other tissues as seen for known AQPs in other insects. DRIPs are known to be present in fat body at low abundance17,33. In contrast, gut exhibited high expression of Se-AQP. This may be explained by crucial roles of gut in water homeostasis and osmoregulation carried out by two its components: HG and MT56. Likewise, the high expression level of Se-AQP in hemocytes suggests a high transporting activity of water and other small molecules through Se-AQP of hemocytes.
Variation in Se-AQP expression levels was observed in S. exigua having different body weights with the same developmental and environmental factors (ambient temperature and humidity). High correlation between body weight and expression levels of Se-AQP suggests that AQP is associated with metabolic activity. In Bombyx mori, DRIP and PRIP AQPs are expressed during egg development57. Although no specific locality is observed for PRIP AQPs, DRIP AQPs are localized in peripheral yolk granules in diapause-destined eggs during transition from vitellogenesis to choriogenesis while they are evenly distributed among medulla yolk granules in nondiapause-destined eggs. Furthermore, DRIP AQPs in diapause-destined eggs are inert, supporting the association of AQPs with metabolism. Increase of Se-AQP expression with ambient temperature may be explained by the increase in metabolic rate. The plasticity of expression level of Se-AQP may help S. exigua adapt to extreme conditions of relative humidity. In A. gambiae, dry conditions with relative humidity less than 20% can lead to a significant reduction in AQP gene expression to prevent water loss from the body58.
Third, RNAi treatment against Se-AQP impaired maintenance of hemocyte cell shape under different osmotic pressure and prevented up-regulation of hemolymph glycerol titer under RCH. The failure of hemocyte shape change under different osmotic pressure suggests inefficient water flux of hemocyte membrane due to knocking down of Se-AQP expression. This suggests that Se-AQP plays a role in water transportation of S. exigua. RCH did not up-regulate hemolymph glycerol titer in RNAi-treated larvae against Se-AQP, suggesting that Se-AQP might also transport glycerol. This is supported by the prediction of protein interaction between Se-AQP and glycerol kinase. S. exigua is classified as freeze-susceptible insect that possesses supercooling capacity59. Supercooling capacity can be achieved by accumulating high amounts of polyols or other forms of cryoprotectants60. In S. exigua, glycerol is accumulated during RCH, in which glycerol kinase catalyzes conversion of dihydroxyacetone-3-phosphate into glycerol32. Thus, under RCH, glycerol is produced by glycerol kinase near to Se-AQP, through which the newly synthesized glycerol may be secreted and accumulated in the hemolymph. However, RNAi treatment against Se-AQP expression prevented the glycerol movement into hemolymph. Thus, the glycerol titer in hemolymph was not up-regulated by RNAi treatment. This suggests that Se-AQP may play a crucial role in redistribution of water content in insects during RCH to tolerate freezing temperatures. In freeze-tolerant insect Eurosta solidaginis (goldenrod gall fly), AQPs appear to coordinate redistribution of water and glycerol transportation because mercuric chloride (a specific inhibitor against AQP activity) can prevent the freeze tolerance of this fly61.
Fourth, AQP modulated cell shape change of hemocytes by mediating their spreading behavior because hemocytes after AQP RNAi treatment failed to exhibit spreading behavior. Hemocyte-spreading behavior that extends cytoplasm in specific directions is required for cellular immune responses such as phagocytosis and nodule formation. It is triggered by several immune mediators for actin rearrangement62,63. It has been suggested that transmembrane water fluxes through AQPs play pivotal roles in cell shape change via local dilution by water along with actin polymerization64. In neural astrocytes, AQP4 activity is required for cytoplasmic extension along with F-actin growth65. Thus, the water transporting activity of Se-AQP is likely to be associated with hemocyte-spreading behavior. The role of Se-AQP in cell shape change of hemocytes was further supported by immunosuppression induced by RNAi treatment, resulting in significant impairments in phagocytosis and nodulation upon bacterial challenge or PGE2 treatment. Furthermore, PO activity was significantly reduced by RNAi treatment against Se-AQP. This is because its inactive precursor (PPO) is produced from oenocytoids and released to plasma via cell lysis to be activated by proteases26,66. A specific receptor for PGE2 can mediate cell lysis by activating sodium-potassium-chloride cotransporter to facilitate water influx27. These observations support the physiological role of Se-AQP in cellular immune responses by changing cell shape through transmembrane water fluxes.
Finally, RNAi treatment against Se-AQP expression resulted in developmental retardation and alteration of S. exigua immatures. Early intervention by RNAi treatment was much detrimental to later development to adults as seen in L4 larval treatment, resulting in the least percentage rate of adult emergence. This suggests that RNAi treatment can increase the severity, resulting in detrimental effect. Similar detriment effects of RNAi against AQP genes have been reported in Tribolium castaneum that expresses nine AQPs67. Knockdown of TcEglp3, TcEglp4, or TcDRIP killed ~20% to ~60% of larvae before pupation67. Because most AQPs of T. castaneum are expressed in HG and MT, the lethal effect of RNAi treatment can be explained by malfunctioning of excretory system67. This suggests that the high mortality observed in S. exigua treated by RNAi against Se-AQP expression might be also due to malfunctioning of excretory system in HG and MT where Se-AQP is likely to be highly expressed. This supports the prediction that AQPs might act as a potential molecular target for insect pest management39,44,68.
In summary, this study reports the first AQP of S. exigua with its gene structure and physiological functions. Its transporting function of water and small molecule is crucial for cell shape change which is required for cellular immune responses of hemocytes and development. The fact that hemocyte cell shape change induced by PGE2 treatment is prevented by RNAi of Se-AQP expression suggests that PG signaling is functionally associated with Se-AQP activity and cytoskeletal rearrangement. This opens a new area of PG signal transduction pathway that needs to be explored in a subsequent study.
Methods
Insect rearing and bacterial culture
Rearing of S. exigua followed published method31. Under rearing conditions, larval stage lasted about 13 days from first instar (L1) to fifth instar (L5) before pupation. Escherichia coli Top10 (Invitrogen, Carlsbad, CA, USA) was cultured in Luria-Bertani (LB) medium (BD, Franklin Lakes, NJ, USA) overnight at 37 °C with shaking at 180 rpm. For immune challenge, bacteria were heat-killed at 95 °C for 10 min. The number of bacterial cells was then counted using a hemocytometer under a phase contrast microscope (BX41, Olympus, Tokyo, Japan).
Chemicals
Prostaglandin E2 (PGE2: (5Z,11α,13E,15 S)-11,15-dihydroxy-9-oxoprosta-5,13-dienoic acid), dexamethasone (DEX: (11ß,16α)-9-fluoro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3), and L-dihydroxyphenylalanine (DOPA) were purchased from Sigma-Aldrich Korea (Seoul, Korea) and dissolved in dimethyl sulfoxide (DMSO). Fluorescein isothiocyanate (FITC)-tagged phalloidin (Alexa Fluor 488 phalloidin) and 4′,6-diamidino-2-phenylindole (DAPI) were purchased from Thermo Fisher Scientific Korea (Seoul, Korea). Anticoagulant buffer (ACB) was prepared in 186 mM NaCl, 17 mM Na2EDTA, and 41 mM citric acid. Its pH was then adjusted to 4.5 with HCl.
Bioinformatics and sequence analysis
Se-AQP sequence was analyzed using Lasergene EditSeq program (Ver. 7.1, DNASTAR, Madison, WI, USA) to predict open reading frame (ORF) and amino acid sequence. Its ORF sequence was deposited at GenBank with accession number of MH333284. Phylogenetic analysis was performed using MEGA6. Transmembrane domains of Se-AQP were predicted using TMHMM69,70. UCSF Chimera (https://www.cgl.ucsf.edu/chimera/) was used for protein motif analysis. Protein-protein interaction map was generated using STRING 10.0a (http://version10a.string-db.org).
Heterologous expression of Se-AQP in Sf9 cells
Using an eukaryotic expression vector pIB/V5-His (Invitrogen), a recombinant pIB/V5-His-Se-AQP was prepared and transiently expressed in Sf9 cell line by cationic lipid-mediated transfection using X-treme GENE 9 DNA transfection reagent (Roche, Mannheim, Germany). Transfection procedure and extraction of cellular proteins from Sf9 cells were performed according to the method described by Kumar and Kim23 and quantified using Bradford method71.
Western blotting
Extracted proteins (~100 µg/sample) were separated on 10% SDS-PAGE and subjected to western blotting according to the method described by Kumar and Kim23.
RNA extraction and RT-PCR
Total RNAs were extracted from different developmental stages of S. exigua using approximately 500 eggs, 30 individuals for L1 or L2, 5 individuals for L3 or L4, and one individual for L5 as an experimental unit (EU). To extract total RNAs from different tissues of L5 larvae, 3-day old L5 (L5D3) larvae were dissected in PBS. By cutting a proleg, hemolymph was collected while the remaining body was used to isolate fat body and gut. The collected hemolymph in ACB was centrifuged at 800 × g for 5 min. The resulting hemocyte pellet was used to extract total RNA with Trizol reagent (Invitrogen) according to the manufacturer’s instruction. After DNase treatment, RT-PCR was performed following the method described by Kumar and Kim23 with gene-specific primers (Table S1). Quantitative PCR (qPCR) was performed according to the general guideline suggested by Bustin et al.72. Ribosomal protein RL32 gene was used as a stably-expressed reference gene for qPCR with gene-specific primers (Table S1)73. Each treatment was replicated three times using independent RNA collections. Quantitative analysis of gene expression was done using the comparative CT (2−ΔΔCT) method74.
Humidity and temperature stress treatment
Three developmental stages (L4, L5, and pupa) after environmental stress treatment were analyzed and individual was assigned to each EU. For temperature stress assessment, each test individual was confined in a glass tube (25 × 50 mm) and exposed to different temperatures (10°, 16°, 20°, 25°, and 37 °C) for 6 h under 60 ± 10% RH. For humidity treatment, each test individual was kept in a small vented insect rearing box (73 × 73 × 73 mm) with different RH (10, 25, 60, 75, and 90%) in desiccators (ThermoFisher Scientific Korea) placed at 25 °C for 24 h. Different RH levels were prepared following Rockland method75. Each treatment was replicated three times.
Glycerol quantification in hemolymph and rapid cold hardening (RCH) bioassay
Hemolymph (~150 µL) from L5 larvae was collected into 1.7 mL tube containing 350 µL of ACB. Subsequent HPLC analysis followed the method described by Park and Kim32. For RCH bioassay, L5D3 larvae were randomly selected from the rearing stock. Test individuals were divided into two RNAi treatment groups. One group was exposed to 4 °C for 6 h prior to cold treatment (−10 °C for 1 h) while the other group was directly exposed to cold treatment without prior exposure to cool temperature. Subsequent bioassay followed the method described by Park and Kim32. Each treatment was replicated three times. Each replication used 10 individuals.
RNA interference (RNAi) of Se-AQP expression
Template DNA was amplified with gene-specific primers (Table S1) containing T7 promoter sequence at the 5′ end. Double-stranded RNA (dsRNA) encoding Se-AQP (‘dsAQP’) or control dsRNA (‘dsCON’) was then prepared following method described by Vatanparast et al.76. After mixing with a transfection reagent Metafectene PRO (Biontex, Plannegg, Germany) in 1:1 (v/v) ratio, the mixture was then incubated at 25 °C for 30 min to form liposomes to increase RNAi efficiency. To prepare dsCON, 500 bp fragment of green fluorescent protein (GFP) gene was synthesized. In every experiment, 1 µg of dsAQP was injected into larva or pupa using a microsyringe (Hamilton, Reno, NV, USA) equipped with a 26-gauge needle. RNAi efficiency was determined by RT-qPCR against Se-AQP expression at 24 and 48 h post-injection (PI). Each treatment was replicated three times using independent RNA preparations.
Immunofluorescence assay (IFA) for hemocyte-spreading behavior
IFA followed the method described by Kumar and Kim23. At 24 h PI of dsCON or dsAQP, L5 larvae was immune-challenged with 1 µL of heat-killed E. coli (~3.2 × 104 cells/larva). One microliter of DEX (1 µg/µL) or PGE2 (1 µg/µL) was injected separately along with heat-killed bacteria. In all cases, at 2 h PI of bacteria, hemocyte-spreading behavior was checked under a fluorescence microscope at 400× magnification. Hemocyte-spreading was determined by the extension of F-actin out of the original cell boundary. Hemocyte-spreading behavior was quantified by randomly assessing 100 cells. Each treatment was replicated three times.
Osmotic shock
At 24 h PI of dsCON or dsAQP, hemolymph was collected and fixed onto glass coverslip. After washing three times with PBS, cells were incubated with 10 µL of each of three different solutions including isotonic (TC100), hypertonic (10% glucose in TC100), and hypotonic (10 times diluted TC100 with deionized water) solutions for 10 min. After washing three times with PBS, cells were permeabilized with 0.2% Triton X-100 in PBS for 2 min at RT and subjected to IFA as described above. Hemocyte-spreading behavior was quantified as described above. Shrunk cells and hemocyte lysis were quantified separately by randomly checking 100 cells. Each treatment was replicated three times with independently prepared biological samples.
Phagocytosis
FITC-labeled E. coli were prepared with a general antibody-labeling method using ammonium chloride77. After confirmation of tagging under microscope, bacteria (1 µL) (~3.5 × 104 cells/larva) were injected to each L5D3 larva at 24 h PI of dsCON or dsAQP. After 10 min, hemolymph from each EU (5 larvae) was collected. Hemocytes were then collected in ACB as mentioned earlier and centrifuged at 180 × g for 2 min at 4 °C. These hemocytes were then washed three times in ice-cold PBS with 0.02% EDTA to stop phagocytosis and remove extracellular bacteria. The final cell pellet was resuspended in TC100 medium. IFA was then performed as described mentioned earlier except that F-actin of hemocytes was stained with 5% of Alexa Fluor 555 phalloidin (Invitrogen). The proportion of phagocytic cells was determined under a fluorescence microscope at 400× magnification.
Nodulation assay
Nodule counts followed the method described by Vatanparast et al.76. At 24 h PI of dsCON or dsAQP, L5D3 larvae were immune-challenged with 1 µL of heat-killed E. coli (~4.2 × 104 cells/larva). One microliter of DEX (1 µg/µL) or PGE2 (1 µg/µL) was injected separately along with bacteria. For all cases, at 8 h PI of bacteria, nodule formation was assessed. Each treatment used 10 larvae. Each treatment was independently replicated three times.
Phenoloxidase (PO) enzyme assay
Plasma PO activity was determined using DOPA as substrate. Activity was measured following the method described by Shrestha and Kim26. At 24 h PI of dsCON or dsAQP, each L5D3 larva was immune-challenged with 1 µL of heat-killed E. coli (~4.2 × 104 cells/larva). One microliter of DEX (1 µg/µL) or PGE2 (1 µg/µL) was injected separately along with heat-killed bacteria. For all cases, at 8 h PI of bacteria, PO activity was assessed. Each treatment consisted of three biologically independent replicates. Each replicate used 10 larvae.
Developmental assay
Developmental period was defined as elapsed time in days from injection (one day old L4 larvae) to pupation. One µg of dsCON or dsAQP was injected into larvae (within an hour after emerging into L4 and L5) or pupae (<4 h old) using a microsyringe. Each treatment was replicated three times. Each replicate used ten insects.
Supplementary information
Acknowledgements
This work was supported by a grant (No. 2017R1A2133009815) of the National Research Foundation (NRF) funded by the Ministry of Science, ICT and Future Planning, Republic of Korea.
Author Contributions
S.A. and Y.K. conceived, designed, and performed experiments, analyzed data, prepared reagents/materials/analysis tools, and wrote the paper.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-41541-2.
References
- 1.Abascal F, Irisarri I, Zardoya R. Diversity and evolution of membrane intrinsic proteins. Biochim. Biophys. Acta. 2014;1840:1468–1481. doi: 10.1016/j.bbagen.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Agre P, Saboori AM, Asimos A, Smith BL. Purification and partial characterization of the Mr 30,000 integral membrane protein associated with the erythrocyte Rh(D) antigen. J. Biol. Chem. 1987;262:17497–17503. [PubMed] [Google Scholar]
- 3.van Hoek AN, et al. Functional unit of 30 kDa for proximal tubule water channels as revealed by radiation inactivation. J. Biol. Chem. 1991;266:16633–16635. [PubMed] [Google Scholar]
- 4.Gonen T, Walz T. The structure of aquaporins. Q. Rev. Biophys. 2006;39:361–396. doi: 10.1017/S0033583506004458. [DOI] [PubMed] [Google Scholar]
- 5.Yasui M. Molecular mechanisms and drug development in aquaporin water channel diseases: structure and function of aquaporins. J. Pharm. Sci. 2004;96:260–263. doi: 10.1254/jphs.FMJ04004X4. [DOI] [PubMed] [Google Scholar]
- 6.Ishibashi K. Aquaporin subfamily with unusual NPA boxes. Biochem. Biophy. Acta. 2006;1758:989–993. doi: 10.1016/j.bbamem.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 7.Jensen MO, Tajkhorshid E, Schulten K. The mechanism of glycerol conduction in aquaglyceroporins. Structure. 2001;9:1083–1093. doi: 10.1016/S0969-2126(01)00668-2. [DOI] [PubMed] [Google Scholar]
- 8.Finn RN, Chauvigné F, Stavang JA, Belles X, Cerdà J. Insect glycerol transporters evolved by functional co-option and gene replacement. Nat. Commun. 2015;6:7814. doi: 10.1038/ncomms8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verkman AS. Aquaporins at a glance. J. Cell Sci. 2011;124:2107–2112. doi: 10.1242/jcs.079467. [DOI] [PubMed] [Google Scholar]
- 10.Saadoun S, Papadopoulos MC, Hara-Chikuma M, Verkman AS. Impairment of angiogenesis and cell migration by targeted of aquaporin-1 gene disruption. Nature. 2005;434:786–792. doi: 10.1038/nature03460. [DOI] [PubMed] [Google Scholar]
- 11.Saadoun S, et al. Involvement of aquaporin-4 in astroglial cell migration and glial scar formation. J. Cell Sci. 2005;118:5691–5698. doi: 10.1242/jcs.02680. [DOI] [PubMed] [Google Scholar]
- 12.Auguste KI, et al. Greatly impaired migration of implanted aquaporin-4-deficient astroglial cells in mouse brain toward a site of injury. FASEB J. 2007;21:108–116. doi: 10.1096/fj.06-6848com. [DOI] [PubMed] [Google Scholar]
- 13.Levin MH, Verkman AS. Aquaporin-3-dependent cell migration and proliferation during corneal re-epithelialization. Invest. Ophthalmol. Vis. Sci. 2006;47:4365–4372. doi: 10.1167/iovs.06-0335. [DOI] [PubMed] [Google Scholar]
- 14.Hara-Chikuma M, Verkman AS. Aquaporin-3 facilitates epidermal cell migration and proliferation during wound healing. J. Mol. Med. 2008;86:221–231. doi: 10.1007/s00109-007-0272-4. [DOI] [PubMed] [Google Scholar]
- 15.Thiagarajah JR, Zhao D, Verkman AS. Impaired enterocyte proliferation in aquaporin-3 deficiency in mouse models of colitis. Gut. 2007;56:1529–1535. doi: 10.1136/gut.2006.104620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maeda N, Hibuse T, Funahashi T. Role of aquaporin-7 and aquaporin-9 in glycerol metabolism; involvement in obesity. Handb. Exp. Pharmacol. 2009;190:233–249. doi: 10.1007/978-3-540-79885-9_12. [DOI] [PubMed] [Google Scholar]
- 17.Philip BN, Kiss AJ, Lee RE. The protective role of aquaporins in the freeze-tolerant insect Eurosta solidaginis: functional characterization and tissue abundance of EsAQP1. J. Exp. Biol. 2011;214:848–857. doi: 10.1242/jeb.051276. [DOI] [PubMed] [Google Scholar]
- 18.Benoit JB, et al. Aquaporins are critical for provision of water during lactation and intrauterine progeny hydration to maintain tsetse fly reproductive success. PLoS Negl. Trop. Dis. 2014;8:e2517. doi: 10.1371/journal.pntd.0002517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benoit JB, et al. Emerging roles of aquaporins in relation to the physiology of blood-feeding arthropods. J. Comp. Physiol. B. 2014;184:811–825. doi: 10.1007/s00360-014-0836-x. [DOI] [PubMed] [Google Scholar]
- 20.Drake LL, Rodriguez SD, Hansen IA. Functional characterization of aquaporins and aquaglyceroporins of the yellow fever mosquito, Aedes aegypti. Sci. Rep. 2015;5:7795. doi: 10.1038/srep07795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sreedharan S, Sankaranarayanan K. Water channel activity of putative aquaporin-6 present in Aedes aegypti. Arch. Insect Biochem. Physiol. 2019;100:e21519. doi: 10.1002/arch.21519. [DOI] [PubMed] [Google Scholar]
- 22.Lavine MD, Strand MR. Insect hemocytes and their role in immunity. Insect Biochem. Mol. Biol. 2002;32:1295–1309. doi: 10.1016/S0965-1748(02)00092-9. [DOI] [PubMed] [Google Scholar]
- 23.Kumar S, Kim Y. Glyceraldehyde-3-phosphate dehydrogenase is a mediator of hemocyte-spreading behavior and molecular target of immunosuppressive factor CrV1. Dev. Comp. Immunol. 2016;54:97–108. doi: 10.1016/j.dci.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Hepat R, Kim Y. JH modulates a cellular immunity of Tribolium castaneum in a Met-independent manner. J. Insect Physiol. 2014;63:40–47. doi: 10.1016/j.jinsphys.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Cerenius L, Söderhäll K. The prophenoloxidase-activating system in invertebrates. Immunol. Rev. 2004;198:116–126. doi: 10.1111/j.0105-2896.2004.00116.x. [DOI] [PubMed] [Google Scholar]
- 26.Shrestha S, Kim Y. Eicosanoids mediate prophenoloxidase release from oenocytoids in the beet armyworm Spodoptera exigua. Insect Biochem. Mol. Biol. 2008;38:99–112. doi: 10.1016/j.ibmb.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 27.Shrestha S, Park J, Ahn S, Kim Y. PGE2 mediates oenocytoid cell lysis via a sodium-potassium-chloride cotransporter. Arch. Insect Biochem. Physiol. 2015;89:218–229. doi: 10.1002/arch.21238. [DOI] [PubMed] [Google Scholar]
- 28.Murata K, et al. Structural determinants of water permeation through aquaporin-1. Nature. 2000;407:599–605. doi: 10.1038/35036519. [DOI] [PubMed] [Google Scholar]
- 29.Fu D, et al. Structure of a glycerol-conducting channel and the basis for its selectivity. Science. 2000;290:481–486. doi: 10.1126/science.290.5491.481. [DOI] [PubMed] [Google Scholar]
- 30.Ho JD, et al. Crystal structure of human aquaporin 4 at 1.8 Å and its mechanism of conductance. Proc. Natl. Acad. Sci. USA. 2009;106:7437–7442. doi: 10.1073/pnas.0902725106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goh HG, Lee SG, Lee BP, Choi KM, Kim JH. Simple mass-rearing of beet armyworm, Spodoptera exigua (Hübner) (Lepidoptera: noctuidae), on an artificial diet. Korean J. Appl. Entomol. 1990;29:180–183. [Google Scholar]
- 32.Park Y, Kim Y. RNA interference of glycerol biosynthesis suppresses rapid cold hardening of the beet armyworm, Spodoptera exigua. J. Exp. Biol. 2013;216:4196–4203. doi: 10.1242/jeb.092031. [DOI] [PubMed] [Google Scholar]
- 33.Kambara K, Takematsu Y, Azuma M, Kobayashi J. cDNA cloning of aquaporin gene expressed in the digestive tract of the Formosan subterranean termite, Coptotermes formosanus Shiraki (Isoptera: Rhinotermitidae) Appl. Entomol. Zool. 2009;44:315–321. doi: 10.1303/aez.2009.315. [DOI] [Google Scholar]
- 34.Azuma M, Nagae T, Maruyama M, Kataoka N, Miyake S. Two water-specific aquaporins at the apical and basal plasma membranes of insect epithelia: molecular basis for water recycling through the cryptonephric rectal complex of lepidopteran larvae. J. Insect Physiol. 2012;58:523–533. doi: 10.1016/j.jinsphys.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Kambara K, Nagae T, Ohmura W, Azuma M. Aquaporin water channel in the salivary glands of the Formosan subterranean termite Coptotermes formosanus is predominant in workers and absent in soldiers. Physiol. Entomol. 2014;39:94–102. doi: 10.1111/phen.12052. [DOI] [Google Scholar]
- 36.Le Cahérec F, et al. Aquaporin-related proteins in the filter chamber of homopteran insects. Cell Tissue Res. 1997;1:143–151. doi: 10.1007/s004410050916. [DOI] [PubMed] [Google Scholar]
- 37.Elvin CM, et al. Molecular cloning and expression in Escherichia coli of an aquaporin-like gene from adult buffalo fly (Haematobia irritans exigua) Insect Mol. Biol. 1999;8:369–380. doi: 10.1046/j.1365-2583.1999.83122.x. [DOI] [PubMed] [Google Scholar]
- 38.Drake LL, et al. The Aquaporin gene family of the yellow fever mosquito, Aedes aegypti. PLoS One. 2010;5:e15578. doi: 10.1371/journal.pone.0015578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shakesby AJ, et al. A water-specific aquaporin involved in aphid osmoregulation. Insect Biochem. Mol. Biol. 2009;39:1–10. doi: 10.1016/j.ibmb.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 40.Mathew LG, Campbell EM, Yool AJ, Fabrick JA. Identification and characterization of functional aquaporin water channel protein from alimentary tract of whitefly, Bemisia tabaci. Insect Biochem. Mol. Biol. 2011;41:178–190. doi: 10.1016/j.ibmb.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 41.Fabrick JA, Pei J, Hull JJ, Yool AJ. Molecular and functional characterization of multiple aquaporin water channel proteins from the western tarnished plant bug, Lygus hesperus. Insect Biochem. Mol. Biol. 2014;45:125–140. doi: 10.1016/j.ibmb.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Marusalin J, Matier BJ, Rheault MR, Donini A. Aquaporin homologs and water transport in the anal papillae of the larval mosquito, Aedes aegypti. J. Comp. Physiol. B. 2012;182:1047–1056. doi: 10.1007/s00360-012-0679-2. [DOI] [PubMed] [Google Scholar]
- 43.Tsujimoto H, Liu K, Linser PJ, Agre P, Rasgon JL. Organ-specific splice variants of aquaporin water channel AgAQP1 in the malaria vector Anopheles gambiae. PLoS One. 2013;8:e75888. doi: 10.1371/journal.pone.0075888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ibanez F, Hancock J, Tamborindeguy C. Identification and expression analysis of aquaporins in the potato psyllid, Bactericera cockerelli. PLoS One. 2014;9:e111745. doi: 10.1371/journal.pone.0111745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jing X, et al. Evolutionary conservation of candidate osmoregulation genes in plant phloem sap-feeding insects. Insect Mol. Biol. 2016;25:251–258. doi: 10.1111/imb.12215. [DOI] [PubMed] [Google Scholar]
- 46.Ishibashi K, Tanaka Y, Morishita Y. The role of mammalian superaquaporins inside the cell. Biochim. Biophys. Acta. 2014;1840:1507–1512. doi: 10.1016/j.bbagen.2013.10.039. [DOI] [PubMed] [Google Scholar]
- 47.Kataoka N, Miyake S, Azuma M. Aquaporin and aquaglyceroporin in silkworms, differently expressed in the hindgut and midgut of Bombyx mori. Insect Mol. Biol. 2009;18:303–314. doi: 10.1111/j.1365-2583.2009.00871.x. [DOI] [PubMed] [Google Scholar]
- 48.Kataoka N, Miyake S, Azuma M. Molecular characterization of aquaporin and aquaglyceroporin in the alimentary canal of Grapholita molesta (the oriental fruit moth)-comparison with Bombyx mori aquaporins. J. Insect Biotechnol. Sericol. 2009;78:81–90. [Google Scholar]
- 49.Wallace IS, et al. Acyrthosiphon pisum AQP2: a multifunctional insect aquaglyceroporin. Biochim. Biophys. Acta. 2012;1818:627–635. doi: 10.1016/j.bbamem.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 50.Wree D, Wu B, Zeuthen T, Beitz E. Requirement for asparagine in the aquaporin NPA sequence signature motifs for cation exclusion. FEBS J. 2011;278:740–748. doi: 10.1111/j.1742-4658.2010.07993.x. [DOI] [PubMed] [Google Scholar]
- 51.Liu K, Tsujimoto H, Huang Y, Rasgon JL, Agre P. Aquaglyceroporin function in the malaria mosquito Anopheles gambiae. Biol. Cell. 2016;108:294–305. doi: 10.1111/boc.201600030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sui H, Han BG, Lee JK, Walian P, Jap BK. Structural basis of water-specific transport through the AQP1 water channel. Nature. 2001;414:872–878. doi: 10.1038/414872a. [DOI] [PubMed] [Google Scholar]
- 53.Lu MX, et al. Identification and functional analysis of the first aquaporin from striped stem borer, Chilo suppressalis. Front. Physiol. 2018;9:57. doi: 10.3389/fphys.2018.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herraiz A, Chauvigné F, Cerdà J, Bellés X, Piulachs MD. Identification and functional characterization of an ovarian aquaporin from the cockroach Blattella germanica L. (Dictyoptera, Blattellidae) J. Exp. Biol. 2011;214:3630–3638. doi: 10.1242/jeb.057406. [DOI] [PubMed] [Google Scholar]
- 55.Yang L, Piermarini PM. Molecular expression of aquaporin mRNAs in the northern house mosquito, Culex pipiens. J. Insect Physiol. 2017;96:35–44. doi: 10.1016/j.jinsphys.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 56.Chawn, S. L. & Nicolson, S. W. Insect Physiological Ecology. (Oxford University Press, New York, 2004).
- 57.Maruyama M, Kambara K, Naka H, Azuma M. Insect water-specific aquaporins in developing ovarian follicles of the silk moth Bombyx mori: role in hydration during egg maturation. Biol. Bull. 2015;229:58–69. doi: 10.1086/BBLv229n1p58. [DOI] [PubMed] [Google Scholar]
- 58.Liu K, Tsujimoto H, Cha SJ, Agre P, Rasgon JL. Aquaporin water channel AgAQP1 in the malaria vector mosquito Anopheles gambiae during blood feeding and humidity adaptation. Proc. Natl. Acad. Sci. USA. 2011;108:6062–6066. doi: 10.1073/pnas.1102629108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim Y, Kim N. Cold hardiness in Spodoptera exigua (Lepidoptera: Noctuidae) Environ. Entomol. 1997;26:1117–1123. doi: 10.1093/ee/26.5.1117. [DOI] [Google Scholar]
- 60.Storey KB, Storey JM. Insect cold hardiness: metabolic, gene, and protein adaptation. Can. J. Zool. 2012;90:456–475. doi: 10.1139/z2012-011. [DOI] [Google Scholar]
- 61.Philip BN, Yi SX, Elnitsky MA, Lee RE. Aquaporins play a role in desiccation and freeze tolerance in larvae of the goldenrod gall fly, Eurosta solidaginis. J. Exp. Biol. 2008;211:1114–1119. doi: 10.1242/jeb.016758. [DOI] [PubMed] [Google Scholar]
- 62.Shim J, et al. Rab35 mediates transport of Cdc42 and Rac1 to the plasma membrane during phagocytosis. Mol. Cell Biol. 2010;30:1421–1433. doi: 10.1128/MCB.01463-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Srikanth K, Park J, Stanley DW, Kim Y. Plasmatocyte-spreading peptide influences hemocyte behavior via eicosanoids. Arch. Insect Biochem. Physiol. 2011;78:145–160. doi: 10.1002/arch.20450. [DOI] [PubMed] [Google Scholar]
- 64.Loitto VM, Karlsson T, Magnusson KE. Water flux in cell motility: expanding the mechanisms of membrane protrusion. Cell Motil. Cytoskeleton. 2009;66:237–247. doi: 10.1002/cm.20357. [DOI] [PubMed] [Google Scholar]
- 65.Nicchia GP, et al. Actin cytoskeleton remodeling governs aquaporin-4 localization in astrocytes. Glia. 2008;56:1755–1766. doi: 10.1002/glia.20724. [DOI] [PubMed] [Google Scholar]
- 66.Jiang R, et al. Three pairs of protease-serpin complexes cooperatively regulate the insect innate immune responses. J. Biol. Chem. 2009;284:35652–35658. doi: 10.1074/jbc.M109.071001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yao XX, Meng QW, Li GQ. RNA interference-mediated functional characterization of aquaporin genes in Tribolium castaneum. Insect Mol. Biol. 2018;27:234–246. doi: 10.1111/imb.12367. [DOI] [PubMed] [Google Scholar]
- 68.Tzin V, et al. RNA interference against gut osmoregulatory genes in phloem-feeding insects. J. Insect Physiol. 2015;79:105–112. doi: 10.1016/j.jinsphys.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 69.Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 2001;305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 70.Sonnhammer EL, von Heijne G, Krogh A. A hidden Markov model for predicting transmembrane helices in protein sequences. Proc. Int. Conf. Intell. Syst. Mol. Biol. 1998;6:175–182. [PubMed] [Google Scholar]
- 71.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye finding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 72.Bustin SA, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 73.Park Y, Kumar S, Kanumuri R, Stanley D, Kim Y. A novel calcium-independent cellular PLA2 acts in insect immunity and larval growth. Insect Biochem. Mol. Biol. 2015;66:13–23. doi: 10.1016/j.ibmb.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 74.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 75.Rockland LB. Saturated salt solutions for static control of relative humidity between 5° and 40°C. Anal. Chem. 1960;32:1375–1376. doi: 10.1021/ac60166a055. [DOI] [Google Scholar]
- 76.Vatanparast M, Ahmed S, Herrero S, Kim Y. A non-venomous sPLA2 of a lepidopteran insect: Its physiological functions in development and immunity. Dev. Comp. Immunol. 2018;89:83–92. doi: 10.1016/j.dci.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 77.Harlow, E. & Lane, D. Labeling Antibodies with Fluorochromes. In: Using Antibodies. [85–87] (Cold Spring harbor Laboratory Press, New York, 1998).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.