Highlights
-
•
Ai Chi can be used to treat arm volume for breast cancer related lymphoedema.
-
•
For the circumstances seen here, the main effect of Ai Chi is in the short term.
-
•
After 1 h, the effect of Ai Chi was similar to conventional aquatic therapy.
-
•
Participants were satisfied with both, Ai Chi and conventional aquatic therapy.
Keywords: Ai Chi, aquatic exercise, Lymphoedema, Volumetry, Bio-impedance, Physical therapy
Abstract
Objective
To identify whether slow aquatic exercise in the form of modified Ai Chi is more effective than conventional (faster pace) aquatic therapy at reducing arm volume in women with or at risk of breast cancer related lymphoedema.
Methods
Randomized, cross-over controlled trial with concealed allocation and blinded assessment. Eighteen women with a history of breast cancer related lymphoedema were recruited. Participants received two intervention sessions (randomized order) with one week apart. Interventions were a 50 min conventional aquatic intervention or a 50 min modified Ai Chi. Arm volume was measured as the difference between affected and unaffected arm; bio-impedance was measured as an index of extracellular fluid; satisfaction was measured via a 12 question form. Outcomes were measured before, immediately after and one hour after intervention.
Results
Comparison between interventions showed larger decreased arm volume of 140 mL (95%CI 17–263) immediately after intervention in favor of the Ai Chi intervention, however it was not sustained at 1 h follow-up. A post hoc analysis showed 72% of participants had a decrease in arm volume immediately after Ai Chi compared to 28% immediately after conventional aquatic therapy; with a number needed to treat of 3 (95%CI 1.4–6.6). There were no differences between interventions for bio-impedance. Satisfaction was good for both interventions.
Conclusion
Slow pace aquatic exercise is more effective than conventional aquatic exercise immediately after intervention for arm volume. Also, undesirable increase in arm volume seems to subside after 1 h, which can be beneficial if therapy does not address arm volume.
Trial registration: ACTRN12614000557639 (https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12614000557639)
Introduction
Lymphoedema is a condition of abnormal accumulation of lymph in the arm, shoulder, breast or thoracic area that causes discomfort, pain, impaired function, and emotional distress in breast cancer survivors and is best managed at the earliest opportunity.1, 2 Lymphoedema is one of the possible complications following treatment for breast cancer affecting approximately 21% of survivors.3 The risk of developing lymphoedema varies considerably, with the extent of intervention to the axilla by surgery and or radiotherapy, being a major determinant. Data shows that 0–3% of patients undergoing sentinel node biopsy (SNB) to up to 70% after modified radical mastectomy with regional lymph node radiation develop lymphoedema.3 In this cohort of patients, it is common and indeed expected that there will be fluctuations in the arm volume. The fluctuation is often associated with exercise, use or not of compression therapy, diligence in undertaking self-management, heat, general variations in hydration and diet.1, 4, 5
The Best Practice for the Management of Lymphoedema International Consensus Document lists exercise/movement as number one management strategy to enhance lymphatic and venous flow.6 In 2010 the American College of Sports Medicine developed exercise guidelines for cancer survivors, suggesting that exercise training is safe during and after cancer treatments and results in improvements in physical functioning, quality of life and cancer related fatigue in several cancer groups including breast cancer.7
Complex decongestive physical therapy is the mainstay of treatment for lymphoedema; this is a multimodal treatment protocol with exercise as one component.8 Aquatic therapy is frequently used as exercise treatment for edema management as it uses the principles of hydrostatic forces during immersion along with exercises in thermo-neutral water to stimulate the circulatory system.9 The hydrostatic pressure will create an upward squeezing action thereby encouraging the central flow of fluid.10
There are different approaches to aquatic therapy, from community aqua-aerobic classes to hospital programs. One modality less commonly known is Ai Chi, a slow pace water based activity first described by Sova and Konno.11 Ai Chi is an aquatic exercise sequence with the purpose of relaxation, balance and pain management.11, 12 Tidhar et al., 13 have described another slow aquatic therapy approach of teaching women with breast cancer related lymphoedema (BCRL) a routine they term aqua lymphatic therapy (ALT) where patients do self massage in a hydrotherapy pool and this was shown to reduce affected arm oedema over the 14 month trial period.13 ALT was also described by Letellier et al.,14 in 2014 and was shown in their study to be beneficial in the reports of pain intensity. No differences in oedema volume were shown but no worsening either, showing that this program of aquatic therapy was safe in the BCRL population.14
There is some evidence for conventional hydrotherapy regimes however for the slowly performed activity of Ai Chi there has been no clinical trials published in the “at risk” or BCRL population. As Ai Chi uses diaphragmatic breathing, which has been shown to be effective in increasing lymphatic drainage through the thoracic duct4 and combines it with slow movements in the water, it is possible that this hydrotherapy modality would benefit patients with lymphoedema. Furthermore, different protocols (conventional or slow aquatic therapy) increase the options of therapy for therapists and clients to choose from. Clinicians specialized in treating women with lymphoedema after breast cancer treatment have anecdotally reported that arm volume increases when water exercise intervention is of a faster pace, and the same event is not reported after slower pace similar interventions. Therefore the anecdotal evidence seen in the clinical setting was the basis for the design of the current study. Thus the current study aimed to investigate whether slow aquatic exercise in the form of Ai Chi modified to include extra diaphragmatic breathing and lymph node massage is more effective than conventional (faster pace) aquatic therapy at reducing arm volume in women with breast cancer related lymphoedema. The intention of the study was to look at the short term effects of the intervention.
Methods
Design
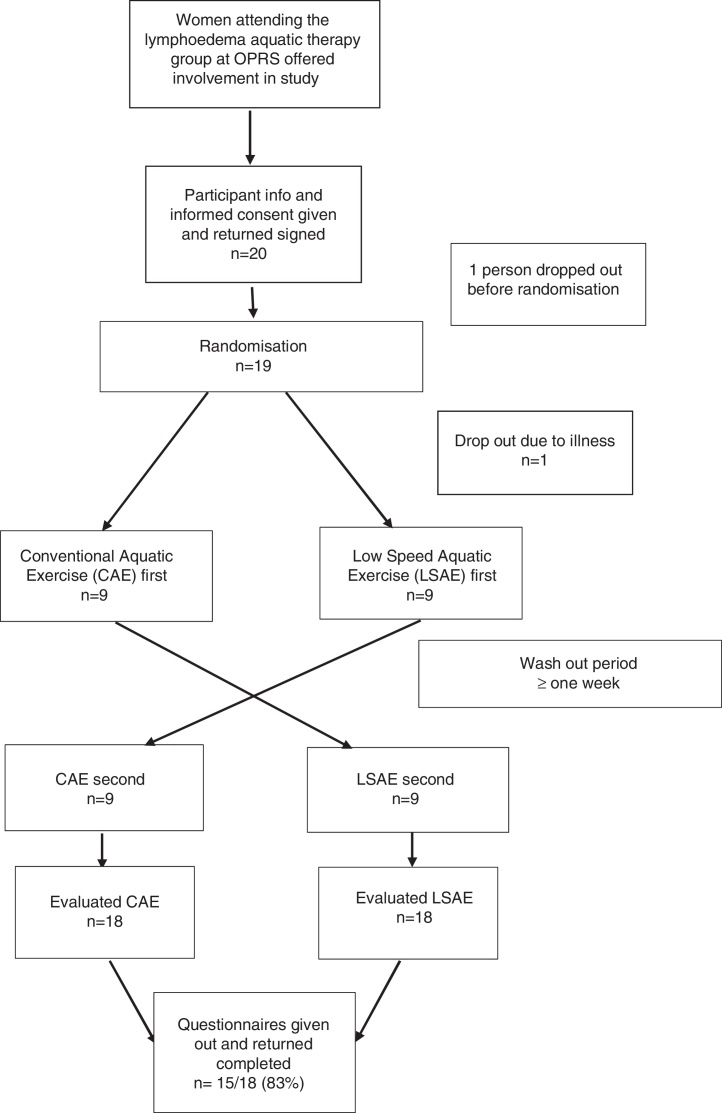
This was a cross-over randomized controlled trial. Participants received two aquatic therapy interventions on two different days, a low speed aquatic exercise (LSAE) session and a conventional aquatic exercise (CAE) session. Both interventions were group based (10–16 participants), led by a physical therapist and an allied health assistant. Sessions were at least one week apart and that was considered the wash-out period (Fig. 1). One week was chosen because it follows the treatment routine from Bendigo Health and because the effects of a single aquatic exercise session are expected to subside within this time due to the fluctuant characteristics of the lymphoedema addressed in this study. The order in which sessions were delivered was randomized by computer operated by an administration officer not involved in the study, in a concealed fashion.
Figure 1.
Flow diagram for randomization: cross-over controlled trial.
Participants
We recruited 18 women (age range 52–81 years) who met the inclusion criteria of having a history of breast cancer with unilateral axillary lymph node excision and who had been attending the aquatic exercise group at Bendigo Health Outpatient Rehabilitation Service (OPRS); participants with either SNB or axillary clearance (AC) or both were accepted. Participants with a metastatic disease and unable to attend both intervention sessions were excluded from the study. Participants had a history of breast cancer related lymphoedema after surgery (between 1 and 35 years from surgery) and used hydrotherapy as part of their lymphoedema self-management regime. They had no involvement in any other treatment other than their usual self-management at the time of the study.
All participants agreed to participate in the study after reading the information and signing consent documentation. Participant's lymphoedema was classified according to the International Society of Lymphology (ISL). Lymphoedema staging where stage 0 is subclinical; stage 1 is considered a transient and early stage; stage 2 is when pitting is manifest and the edema rarely reduces; and late stage 2 where pitting and tissue fibrosis is evident.15 Assessments regarding stage of lymphoedema prior to the start of the study were performed by experienced lymphoedema practitioners (2 physical therapists and 2 occupational therapists) with between 2 and 25 years of experience treating and assessing patients with lymphoedema. All 4 practitioners had advanced lymphoedema management training accredited through the Australasian Lymphology Association. All participants with SNB (22.2%) had stage 1 lymphoedema, with one having fluctuant arm lymphoedema stage 1 (6%). From the participants with axillary clearance, two (11%) had fluctuating stage 1 lymphoedema, 6 (33%) stage 1 and 6 (33%) stage 2 lymphoedema. All participants with stage 2 lymphoedema still had some degree of pitting indicating fluctuant mobile lymphoedema (Table 1).
Table 1.
Participants characteristics.
| Age (yr), mean (range) | 67.3 (range 52–81yrs) |
|---|---|
| Surgical proceduresa | |
| Wide local excision (n) | 14 |
| Total mastectomy (n) | 4 |
| Sentinel node biopsy (SNB) (n) | 3 |
| Axillary clearance (AC) (n) | 13 |
| Both SNB and AC (n) | 2 |
| SL stage of lymphoedema | |
| Transient (stage 1) UL lymphoedema | 6 |
| Early (stage 2) UL lymphoedema | 5 |
| Late (stage 2) UL lymphoedema | 3 |
| Breast and transient UL lymphoedema | 4 |
| Regular usage of compression garment | 9 |
| BMI | |
| <25 | 6 |
| >25 and <40 | 7 |
| ≥40 | 5 |
Some participants presented with a combination of surgical procedures. UL, upper limb; BMI, body mass index; ISL, International Society of Lymphology.
All participants had been attending this group as part of their post breast cancer self-management which was offered as a means of encouraging them to maintain a regular exercise habit. Participant's characteristics are presented in Table 1. The study was approved by the Human Research Ethics Committee from Bendigo Health (LNR/14/BHCG/6), Bendigo, Australia. The trial was also prospectively registered in the Australian New Zealand Clinical Trials Registry under the number ACTRN12614000557639
Procedures
The study was conducted at OPRS between July 2014 and February 2016. On each of the two intervention days data collection took place prior to intervention (Pre-I), immediately after (Post-I) and one hour after intervention (1h-I). Outcomes used for analyses were arm volumetry (measured via water displacement, Fig. 2) and body composition (bio-impedance). We also collected data on satisfaction (via a feedback form) after completion of the trial. Participants were given instructions regarding attending each of the interventions well hydrated and with an empty bladder, to avoid caffeine and alcohol on the measurement days, and to continue to use their compression garment between interventions if that was their normal practice. If it was normal practice for them to wear compression, the garment was removed for the first measurement each day and was not reapplied until after the third measurement. All assessors involved in the measurements were blinded to which intervention the participant had received that day. The therapists were blinded as to who was being assessed on any particular day.
Figure 2.

Volumetry tank with position taken by participants for measuring water displacement.
Interventions
Interventions took place in the same hydrotherapy pool which is maintained with water temperature of 34 degree Celsius and ambient air temperature of 28–30 degree Celsius. The pool measures 8 × 15 m and ranges from 1 to 1.5 m in depth with access via steps or a hoist if required. Interventions were conducted predominately in the deeper section of the pool so participants could be immersed neck deep with slightly flexed knees for LSAE. During the CAE the mid pool was also used for walking and other activities.
The intervention was conducted on a weekly basis with only one participant from the group under treatment being measured in each week. On the day of measurement/intervention the participant arrived 20 min prior to the intervention to allow time for Pre-I. The CAE was a 50 min conventional intervention including lymph node massage, diaphragmatic breathing, warm ups and stretches, a small component of aerobic activities and then a cool down period. This format was familiar to most participants as many had been attending for a year or more.13, 16
The LSAE was 50 min of Ai Chi. The Ai chi was modified from the routine used and well documented by Sova and Konno,12 by adding extra diaphragmatic breathing and lymph node massage at intervals during the intervention. Ai Chi is performed in neck deep water in time with the breath. It is a slow, relaxing and gentle exercise regime in contrast to the slightly faster conventional program.11, 12 Therefore the interventions were designed to have similar levels of lymph clearance and diaphragmatic breathing, however substantial difference in pattern and speed in which movements were performed, along with the extended time of immersion for the LSAE in comparison to the CAE.
Outcome measures
Arm volumetry. Both upper limbs were measured. Participants had a red line drawn in wax pencil 15 cm down from the acromion. In standing, participants immersed one of the arms (randomly decided) in a transparent volumeter (dimensions 19 × 21 × 76 cm) that had water to the level of a spout (Fig. 2). The displaced water was collected in a separate container and weighed. The difference in volume between affected and unaffected sides was noted. Water displacement is considered a reliable measure, with an intraclass correlation coefficient of 0.99.17, 18, 19
Bio-impedance. We used the ImpediMed L-Dex U400 machine and the protocol used was the one suggested in the instruction manual. Three dual tab electrodes were used for each measurement.20, 21 The positioning of the participants was standardized with the participants lying in supine with jewellery removed, palms down and limbs slightly abducted to ensure the skin between the limbs and trunk was not touching. The electrode sites were cleaned with alcohol wipes and then the electrode placed via the standard protocol.22 Bioimpedance is measured as an index of extracellular fluid of which lymph is a primary component and the normal range is between −10 and 10.22 The U400 unit measures bio-impedance values in each arm from the wrist to the shoulder and is represented on the unit as an “L-dex”score.
Satisfaction. After completion of the data collection, participants received a feedback form to return in a reply paid envelope or immediately into the collection box at reception. The feedback form comprised of 12 questions in 5 categories on a single page, with eleven questions rated on a 5 point Likert scale varying from strongly disagree to strongly agree. Categories addressed enjoyment, time commitment, reasons for attending, intervention preference, and the final question was an open ended question on suggestions for changes. Data on satisfaction is presented in a descriptive fashion.
Statistical analysis
All the analyses followed the intention-to-treat principle. We calculated that a sample size of 18 participants were necessary for this study, assuming a minimum difference of 10% in arm lymphoedema volume between interventions, with a SD of 10%, power of 0.8 and alpha of 0.5. For that, arm lymphoedema volume was calculated as the difference in volume between the arm with lymphedema and the contralateral arm, in milliliters (mL). Data from Letellier et al.14 was used as reference. Mann–Whitney U test was used to investigate the difference between LSAE and CAE as data did not present normal distribution; a difference was considered present when p < 0.05. We also calculated the mean difference (and 95%CI) for the comparison between LSAE and CAE in change of arm volume and bio-impedance. Statistical analyses were processed using the software SPSS version 21. We also ran a post hoc analysis to calculate the number needed to treat (NNT) based on volume change of the affected limb only. Data was dichotomized based on whether the participants affected arm improved (decreased arm volume) or did not improve (no change or increased arm volume).
Results
Baseline characteristics for participants are presented in Table 1. Participants had a mean age of 67.3 years; body mass index (BMI) varied from normal to extremely obese. Presentation of lymphoedema varied in stages (1–2; Table 1) and surgical procedure (wide local excision, mastectomy, sentinel node biopsy and axillary clearance; Table 1). Twenty participants agreed to be involved in the study however one participant dropped out of the study due to illness soon after randomization and one participant was unable to partake due to personal reasons and therefore was not randomized.
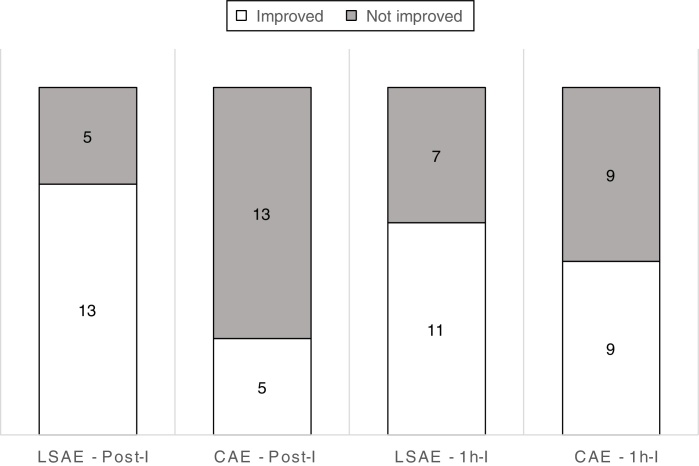
Volume of lymphoedema decreased for LSAE in comparison to CAE by an average of 140 mL (95%CI 17 mL to 263 mL) immediately after intervention, however the difference was not sustained at 1 h after the intervention (Table 2). For bioimpedance, the results showed no difference between LSAE and CAE either for the measurements Post-I and 1h-I (Table 2). Unfortunately, data from three participants, for all three assessments were not included due to equipment failure. For the dichotomous analysis (post hoc), 72% of participants after the LSAE intervention decreased arm volume compared to 28% after the CAE intervention at Post-I; with a NNT of 3 (95%CI 1.4–6.6). For the analysis at 1h-I, 61% of participants after the LSAE intervention decreased arm volume compared to 50% after the CAE intervention; with a NNT of 9 (95%CI −21.2 to 43.4; Fig. 3).
Table 2.
Primary outcomes – arm volume (mL) and bio-impedance. Comparison between LSAE and CAE. Arm volume was calculated as the difference between the affected and unaffected side. Bioimpedance was measured as an index of extracellular fluid.
| LSAE median/mean (SD) | CAE median/mean (SD) | Mean difference in change between interventions (95%CI) | Mann–Whitney U p |
||
|---|---|---|---|---|---|
| Arm volume | Pre-intervention | 161/292 (458) | 88.5/243 (445) | – | – |
| Immediately post-intervention | 62/198 (478) | 95/289 (469) | 140 (17 to 263)* | 0.037 | |
| One hour after intervention | 109.5/250 (447) | 57/298 (494) | 97 (−26 to 221) | 0.17 | |
| Bio-impedance | Pre-intervention | 2.5/12.7 (26.0) | 3.3/13.9 (27.7) | – | – |
| Immediately post-intervention | 4.4/13.5 (27.8) | 3.5/14.3 (26.1) | 0.3 (−3.3 to 2.6) | 0.78 | |
| One hour after intervention | 2.6/13.3 (26.8) | 3.8/13.4 (26.9) | 0.59 (−2.4 to 3.6) | 0.49 |
LSAE, low speed aquatic exercise; CAE, conventional aquatic exercise.
means significant difference between interventions.
Figure 3.
Number of participants that improved or not, immediately (Post-I) and 1 hour (1h-I) after low speed aquatic exercise (LSAE) and conventional aquatic exercise (CAE). Data was calculated only for the affected arm.
Return rate for the survey was 83%, with 15/18 returned. One respondent was strongly dissatisfied with the time commitment and her involvement in the LSAE part of the study. The remaining 93% of respondents agreed or strongly agreed that the time commitment and involvement in the project was acceptable. Most respondents (73%) agreed or strongly agreed they would prefer to attend a full class of Ai Chi or a class with Ai Chi as the cool down; 66% agreed or strongly agreed that they enjoyed the CAE intervention with standard cool down. Some comments regarding the Ai Chi were related to the feeling of getting cold when only exercising at slower speeds and a few participants mentioned that they preferred more active classes.
Discussion
The current trial was set out to determine the effect of a slower pace aquatic intervention compared to the conventional, faster pace aquatic intervention, as usually adopted in rehabilitation settings. The results of the current study show that slow pace aquatic therapy, as presented in this study (Ai Chi combined with additional diaphragmatic breathing and lymph node massage) is a viable and more effective form of therapy to reduce arm volume in women with BCRL when compared to conventional aquatic therapy, immediately after therapy.
One possible explanation for the better results for the LSAE intervention is combination of diaphragmatic breathing and hydrostatic pressure. There is evidence suggesting that diaphragmatic breathing enhances central flow of venous and lymphatic fluids,23, 24 thereby reducing the peripheral volume.25, 26, 27 Also, during the LSAE participants are immersed neck deep during the entire session, with arms fully submerged. In contrast, during the CAE participants are generally chest deep, with occasions when participants have their arms completely out of water. As a consequence the LSAE utilizes the positive benefits of hydrostatic pressure for longer periods, which is known to reduce peripheral volume.4, 26, 27 Therefore, although diaphragmatic breathing was present in both interventions, the combination of diaphragmatic breathing and hydrostatic pressure was likely a stronger feature in the LSAE.
Another possibility is that the speed of exercise in itself may account for the differences seen. The LSAE intervention included a rhythmic, slow and gentle intervention in time with the breath, while for the CAE participants were exercising at intermittent rates involving, walking, punching, cycling and jumping actions, all of which are faster and likely to increase blood flow. Although these water activities can be beneficial for other aspects of health, they could potentially be ineffective or increase arm volume.
Unfortunately, the results showed that the positive effect seen immediately after the LSAE intervention was not carried over after 1 h, which can be seen as a negative outcome. However after looking at the post hoc analysis (Fig. 3), it seems that what could have happened is that during CAE, patients increase their arm volume, however after 1 h that increase tends to subside to a condition prior to the intervention. If the water exercises are being conducted with the aim to improve other health aspects (for example cardiorespiratory fitness and general muscle strength), our findings could be an indication that undesirable increases in arm volume are unlikely to still be present 1 h after the end of the treatment. This could be used as an incentive for these population to engage in water exercise with the aim to improve their general health. Further investigation should be conducted to confirm this possibility.
Interestingly the results from the bioimpedance analyses did not show the same benefits seen in volumetry. One possible explanation for this result is that sample size calculation was based on volumetry data, not on bioimpedance data. It is possible that our study was underpowered to find changes in bioimpedance, especially after the current results showed very small changes in bioimpedance. Future studies with larger samples could increase statistical power and give a clearer understanding of data from bioimpedance and the effects of the interventions over time.
Some study limitations need to be acknowledged. One possible limitation is the pragmatic approach to inclusion criteria. Our inclusion criteria were based on patients who are seen in the Bendigo Health Outpatient Rehabilitation Service (OPRS) as part of the routine service provision. This had the aim to increase external validity of the study, and although we may have achieved that, the large variation in participants characteristics could have decreased the level of control over the results, impacting interpretation of findings. Some of the characteristics that largely varied were time since surgery and regular use of compression garments. Regarding the use of compression garments, the pragmatic approach in this study could have also influenced results. The only instructions between Post-I and 1h-I was to have a non-caffeinated drink and to avoid sleeve use (if this was their usual habit). These could be considered as minimum control during the intervention and the hour awaiting for the 1h-I measurement. It is possible that the intervention would be more or less effective depending on how regularly the patient wore compression garment. Another limitation was that the equipment used for bioimpedance assessment was only capable of measuring extracellular fluid of the arm. Perhaps the use of an equipment with the capability of measuring extracellular fluid of other parts of the body would have given us a better understanding of how fluids shifted after the intervention. Also, the small sample size was another limitation. Increasing participant numbers to add to our data would certainly be beneficial for future studies, as would undertaking repeat measures over a longer period. However our intention was to look at immediate effects in this study. Another important point noted was that the level of lymphoedema prior to the interventions was generally smaller than expected (Table 2) and that could have an influence in the final results of the study. However, we were still able to identify a difference in volume between the groups, showing the positive effects of LSAE.
In this cohort of participants, there was a high proportion of participants with BMI greater than 25, including several with BMI above 40. Thermoregulation in these participants is likely to be compromised due to body heat retention and increased plasma sodium levels, which are related to lymphoedema volume.28 Had this study been undertaken on patients who had only recently commenced their management and possibly had more mobile lymphoedema, we may have seen more notable changes in the bioimpedance. Finally, future studies could be designed to investigate whether breathing alone or submersion alone would have an effect on the oedema. Also, larger studies focusing on comparing LSAE to land-based interventions such as yoga and tai chi (integration of breathing with slow body movements) could further enhance the understanding of the effects of low speed water exercise.
Conclusion
LSAE is a viable therapeutic option for women with breast cancer related lymphoedema as it has a positive immediate effect in reducing arm volume when compared to conventional aquatic therapy. However any difference in arm volume due to aquatic therapy seems to subside after 1 h, which can be beneficial when water exercise does not aim to address arm volume.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors would like to acknowledge Dr Sally Ruston and Margaret Burke for their participation in implementation and data collection for the study; Carol Williams and Louise Coppock for administration support.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
This paper is part of a Special Issue on Women's Health Physical Therapy.
References
- 1.Yüksel A., Gürbüz O., Velioğlu Y., Kumtepe G., Şenol S. Management of lymphoedema. VASA Z Gefasskrankheiten. 2016;45(4):283–291. doi: 10.1024/0301-1526/a000539. [DOI] [PubMed] [Google Scholar]
- 2.Kwan M.L., Cohn J.C., Armer J.M., Stewart B.R., Cormier J.N. Exercise in patients with lymphedema: a systematic review of the contemporary literature. J Cancer Surviv Res Pract. 2011;5(4):320–336. doi: 10.1007/s11764-011-0203-9. [DOI] [PubMed] [Google Scholar]
- 3.DiSipio T., Rye S., Newman B., Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14(6):500–515. doi: 10.1016/S1470-2045(13)70076-7. [DOI] [PubMed] [Google Scholar]
- 4.Moseley A., Piller N. Exercise for limb lymphoedema: evidence that it is beneficial. J Lymphoedema. 2008;3(1):51–56. [Google Scholar]
- 5.Kayıran O., De La Cruz C., Tane K., Soran A. Lymphedema: from diagnosis to treatment. Turk J Surg. 2017;33(2):51. doi: 10.5152/turkjsurg.2017.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.International Consensus; 2006. Lymphoedema Framework. Best Practice for the Management of Lymphoedema. http://www.lympho.org/portfolio/lymphoedema-frameworks-the-way-forward/. Accessed 03.03.17. [Google Scholar]
- 7.Schmitz K.H., Courneya K.S., Matthews C. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 8.Fu M.R., Ridner S.H., Armer J. Post-breast cancer lymphedema: Part 2. J ET Nurs. 2009;109(8):34–41. doi: 10.1097/01.NAJ.0000358492.91678.78. [DOI] [PubMed] [Google Scholar]
- 9.Tidhar D., Katz-Leurer M. Aqua lymphatic therapy in women who suffer from breast cancer treatment-related lymphedema: a randomized controlled study. Support Care Cancer. 2009;18(3):383–392. doi: 10.1007/s00520-009-0669-4. [DOI] [PubMed] [Google Scholar]
- 10.Wilcock I.M., Cronin J.B., Hing W.A. Physiological response to water immersion: a method for sport recovery? Sports Med Auckl NZ. 2006;36(9):747–765. doi: 10.2165/00007256-200636090-00003. [DOI] [PubMed] [Google Scholar]
- 11.Sova R., Konno J. DSL Ltd; Port Washington: 1999. Ai Chi Balance Harmony and Healing. [Google Scholar]
- 12.Skinner E.H., Dinh T., Hewitt M., Piper R., Thwaites C. An Ai Chi-based aquatic group improves balance and reduces falls in community-dwelling adults: a pilot observational cohort study. Physiother Theory Pract. 2016;32(8):581–590. doi: 10.1080/09593985.2016.1227411. [DOI] [PubMed] [Google Scholar]
- 13.Tidhar D., Shimony A., Drouin J. Aqua lymphatic therapy for postsurgical breast cancer lymphedema. Rehabil Oncol. 2004;22(3):6–14. [Google Scholar]
- 14.Letellier M.-E., Towers A., Shimony A., Tidhar D. Breast cancer-related lymphedema: a randomized controlled pilot and feasibility study. Am J Phys Med Rehabil Assoc Acad Physiatr. 2014;93(9):751–759. doi: 10.1097/PHM.0000000000000089. quiz 760–61. [DOI] [PubMed] [Google Scholar]
- 15.International Society of Lymphology The diagnosis and treatment of peripheral lymphedema. 2009 Concensus Document of the International Society of Lymphology. Lymphology. 2009;42(2):51–60. [PubMed] [Google Scholar]
- 16.Becker B.E. Aquatic therapy: scientific foundations and clinical rehabilitation applications. PM R. 2009;1(9):859–872. doi: 10.1016/j.pmrj.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 17.Megens A.M., Harris S.R., Kim-Sing C., McKenzie D.C. Measurement of upper extremity volume in women after axillary dissection for breast cancer. Arch Phys Med Rehabil. 2001;82(12):1639–1644. doi: 10.1053/apmr.2001.26822. [DOI] [PubMed] [Google Scholar]
- 18.Sander A.P., Hajer N.M., Hemenway K., Miller A.C. Upper-extremity volume measurements in women with lymphedema: a comparison of measurements obtained via water displacement with geometrically determined volume. Phys Ther. 2002;82(12):1201–1212. [PubMed] [Google Scholar]
- 19.Deltombe T., Jamart J., Recloux S. Reliability and limits of agreement of circumferential, water displacement, and optoelectronic volumetry in the measurement of upper limb lymphedema. Lymphology. 2007;40(1):26–34. [PubMed] [Google Scholar]
- 20.Chen Y.-W., Tsai H.-J., Hung H.-C., Tsauo J.-Y. Reliability study of measurements for lymphedema in breast cancer patients. Am J Phys Med Rehabil. 2008;87(1):33–38. doi: 10.1097/PHM.0b013e31815b6199. [DOI] [PubMed] [Google Scholar]
- 21.Cornish B.H., Chapman M., Hirst C. Early diagnosis of lymphedema using multiple frequency bioimpedance. Lymphology. 2001;34(1):2–11. [PubMed] [Google Scholar]
- 22.Cornish B. Bioimpedance analysis: scientific background. Lymphat Res Biol. 2006;4(1):47–50. doi: 10.1089/lrb.2006.4.47. [DOI] [PubMed] [Google Scholar]
- 23.Sumner D.S. Hemodynamics and pathophysiology of venous disease. Vasc Surg. 1989;2:1483–1504. [Google Scholar]
- 24.Shields J.W. Central lymph propulsion. Lymphology. 1980;13(1):9–17. [PubMed] [Google Scholar]
- 25.Moseley A.L., Piller N.B., Carati C.J. The effect of gentle arm exercise and deep breathing on secondary arm lymphedema. Lymphology. 2005;38(3):136–145. [PubMed] [Google Scholar]
- 26.McKenzie D.C., Kalda A.L. Effect of upper extremity exercise on secondary lymphedema in breast cancer patients: a pilot study. J Clin Oncol Off J Am Soc Clin Oncol. 2003;21(3):463–466. doi: 10.1200/JCO.2003.04.069. [DOI] [PubMed] [Google Scholar]
- 27.Box R., Marnes T., Robertson V. 5th Australasian Lymphology Association Conference Proceedings. 2004. Aquatic physiotherapy and breast cancer related lymphoedema; pp. 47–49. [Google Scholar]
- 28.Eijsvogels T.M.H., Veltmeijer M.T.W., Schreuder T.H.A., Poelkens F., Thijssen D.H.J., Hopman M.T.E. The impact of obesity on physiological responses during prolonged exercise. Int J Obes. 2011;35(11):1404–1412. doi: 10.1038/ijo.2010.277. [DOI] [PubMed] [Google Scholar]