Abstract
Background
Several studies reported that curcumin supplementation could improve non-alcoholic fatty liver disease (NAFLD). The aim of this study was to evaluate the efficacy of curcumin/turmeric supplementation on liver enzymes in patients with NAFLD.
Methods
PubMed, Scopus, Web of Science and Google Scholar were systematically searched until December 2017. We included randomized controlled trials (RCTs) which examined effect of curcumin/turmeric supplementation on NAFLD in adult participants. Main outcome was alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Potential risks of bias (ROB) were assessed by using Cochrane ROB tool.
Results
All included studies showed low ROB in most of item of Cochrane ROB tool. Meta-analysis of 4 randomized controlled trials including 228 subjects showed a trend toward significant reduction of ALT blood concentrations in subgroup with ≥1000 mg/day curcumin supplementation (–11.36 IU/L, 95% CI: –22.75 to 0.02; I2:51%). Meta-analysis showed a significant reduction of AST in studies with 8-weeks administration (–9.22 IU/L, 95% CI: –12.77 to –5.67; I2: 49%).
Conclusion
This review suggests that curcumin/turmeric might have a favorable effect on NAFLD in higher dosage. Further high-quality studies with large-scale and higher dosage are warranted.
Keywords: Curcumin, Non-alcoholic fatty liver, Alanine transaminase, Aspartate aminotransferases, Meta-analysis
1. Introduction
Nonalcoholic Fatty Liver Disease (NAFLD) is known to be the most prevalent hepatic disorder that characterized by excessive hepatic fat accumulation, in absence of remarkable alcohol consumption.1 It affected people around the world in range of 25–30% in developed and 6–35% in developing countries.2 Although many aspects of NAFLD pathogenesis are not yet fully understood, metabolic disturbances such as excessive fat accumulation and insulin resistance play an important roles in the pathogenesis of NAFLD.3 In modern medicine, adherence to life style and dietary modification is a first strategy for NAFLD management or/and prevention of disease progression to cirrhosis and hepatocellular carcinoma.4, 5 However, many patients fail to comply the lifestyle modification.5 Owing to the growing prevalence of NAFLD and paucity of beneficial remedy, a surge of interest to detect novel effective therapy for alleviating or preventing progression of this disease with minimal side-effect is required.6
In the last decades growing evidences showed that investigators are interesting to find effective natural alternatives therapy in treatment of numerous diseases.7 Although vary medical plants were used as traditional and self-care, there is lacked of sufficient information in efficacy and their possible side-effect on diseases and this issue made it one of the important problems faced by doctors.8
Turmeric (Curcuma longa) is a perennial herb belonging to ginger family (Zingiberaceae).9 The main biological activity of turmeric is related to curcumin which has commonly used as curry powder in Asian cuisine.10, 11 Curcumin has a polyphenol structure and has been traditionally used as a household treatment for various diseases.12 Several studies suggested that curcumin has antimicrobial,13 anti-inflammatory,14 antioxidant,15, 16 immunomodulatory,17 renoprotective,18 anti-cancer,19 hepatoprotective,20 phypoglycaemic21 properties which are acts through signaling pathways and regulating gene expression.22
Although a large body of evidence in in vitro and animal studies have supported hepatoprotective activity of curcumin,23, 24 results from single human study have remained inconclusive. Therefore, present review was aimed to provide summary and conclusive result for effect of curcumin/turmeric on NAFLD in compare with placebo in adult participants.
2. Methods
This study was registered on the PROSPERO (registration number: CRD42017077949) and the guideline of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement was followed in writing this report.25
2.1. Databases and search strategy
The online databases PubMed (MEDLINE), the Web of Science, Cochrane Library, and Google Scholar were systematically searched until October 2017. The search term was used in combination with the wild-card ‘*’ and Medical Subject Heading (MeSH) terms: (Curcumin OR Curcuminoid OR Curcuma OR Turmeric) AND (non-alcoholic fatty liver disease OR NAFLD OR nonalcoholic steatohepatitis OR NASH OR fatty liver OR fatty liver disease). References of selected studies and review articles were also checked to detect eligible trials that might have been missed.
2.2. Study selection
Two reviewers (A.H. and M.P.) screened all included study by title/abstract and selected potentially relevant studies for further assessment. Any discrepancy in study selection was resolved by third reviewer (F.J.). Studies were included if they were randomized control trials (RCTs) and assessed the effect of curcumin/turmeric on ALT or AST levels in compare with placebo in adult participants. Studies were excluded if curcumin or turmeric was administrated combined with other substances or lack of information about ALT or AST levels at baseline and/or endpoint.
2.3. Data extraction and risk of bias assessment
Eligible studies were reviewed by two researchers (A.H. and M.P.) independently and following data were abstracted: first author's last name, years of publication, study location, sample size, mean age of subjects, condition of participants, duration of study, dosage of intervention and main outcomes. Any disagreement was reached consensus through discussion with third investigator (F.J.). Furthermore, unclear information in included studies was unraveled by email to corresponding authors.
Risk of bias (ROB) in included citations were assessed using Cochrane Risk of Bias Tool.26 The items include adequacy of random sequence generation, allocation concealment, blinding as well as detection of incomplete outcome data, selective outcome reporting, and other potential sources of bias. Judgment of each item was “Low”, “High” or “Unclear” ROB.
2.4. Statistical analysis
Meta-analysis was performed by using the Cochrane Program Review Manager Version 5.1 (The Cochrane Collaboration, 2011, The Nordic Cochrane Centre, Copenhagen). To calculate effect size, ALT and AST concentrations were collated in UL/L. Heterogeneity was explored quantitatively by using Cochran's Q and I2 statistics. In this regard, I2 ≥ 50% (or P < 0.05) and ≥75% indicated substantial and considerable heterogeneity, respectively.27, 28 When high heterogeneity exists, a subgroup analysis was applied to find out potential sources of heterogeneity. Potential publication bias was not applied due to low number of included trials. P-values < 0.05 were considered statistically significant.
3. Result
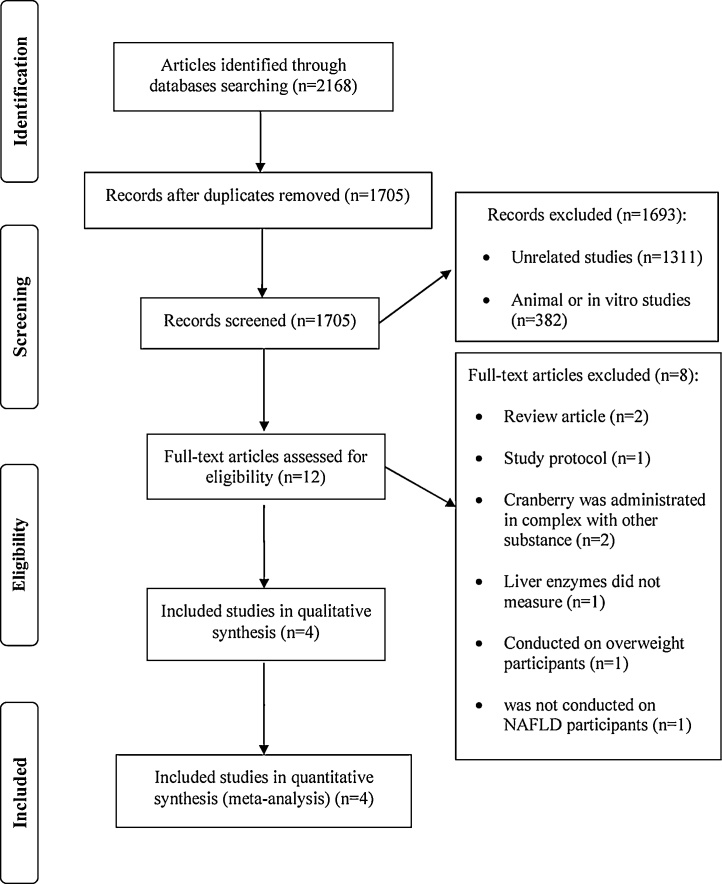
Primary search provided 2168 articles. After excluding duplicates, 1705 studies were screened by title/abstracts and 12 articles selected for further assessment. Of those, eight articles were excluded due to review articles (n = 2), curcumin administered in combination with other substance (n = 2), liver enzymes did not measure (n = 1), conducted on overweight participants (n = 1), study protocol (n = 1) and was not conducted on NAFLD (n = 1). Finally, four RCTs were included in analysis (Fig. 1).
Fig. 1.
Flow chart of the process of the study selection.
3.1. Characteristics of included studies
Characteristics of included studies are outlined in Table 1. Four RCTs29, 30, 31, 32 comprising 228 participants with 50 mean age were included. All trials had parallel design and were conducted in Iran.29, 30, 31, 32 Treatment duration ranged between 8 and 12 weeks. Dosage was varied and ranged from 80 to 3000 mg/day. Three studies supplemented with curcumin29, 31, 32 whilst one remains study30 administrated turmeric. Ultrasonography was used for diagnosis of NAFLD in all studies.
Table 1.
The Characteristics of Included Paralleled Randomized Controlled Trials
| First author (year) Ref location |
Mean age Diagnosis method for NAFLD |
Dose and type of intervention (number of participants) | Comparison group (number of participants) | Trials duration (weeks) | Main outcome | Results |
|---|---|---|---|---|---|---|
| Panahi )2016) Iran |
Intervention: 45.0 Control: 47.2 Ultrasonography |
1000 mg/day Curcumin (n = 44) |
NR Placebo (n = 43) |
8 | 1) ALT 2) AST |
1) P < 0.001 2) P < 0.001 |
| Rahmani (2016) Iran |
Intervention: 46.4 Control : 49.0 |
500 mg/day Curcumin (n = 37) |
500 mg/day Placebo (n = 40) |
8 | 1) ALT 2) AST |
1) P = 0.001 2) P = 0.002 |
| Navekar (2017) Iran |
Intervention: 42.1 Control : 40.4 |
3000 mg/day Turmeric (n = 21) |
3000 mg/day Placebo (n = 21) |
12 | 1) ALT 2) AST |
1) NS 2) NS |
| Moradi-Kelardeh (2017) Iran |
Intervention: 66.7 Control: 64.4 |
80 mg/day Curcumin (n = 11) | 80 mg/day Placebo (n = 11) |
12 | 1) ALT 2) AST |
1) NS 2) NS |
ALT: alanine aminotransferase; AST: aspartate aminotransferase; NR: not reported in details; NS: not significant.
3.2. Quantitative data synthesis
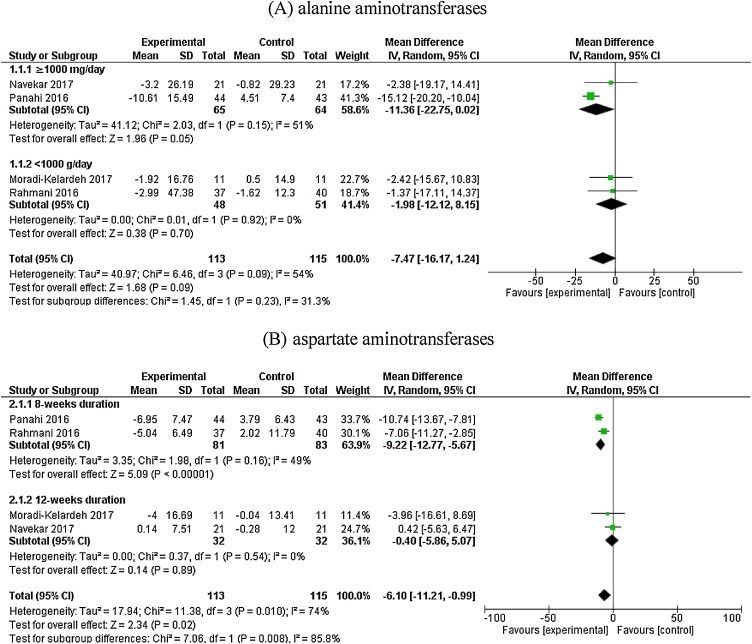
Meta-analysis of 4 RCTs did not indicate a significant effect of curcumin supplementation on reducing ALT blood levels (–7.47 IU/L, 95% CI: –16.17 to 1.24). However, a significant between study heterogeneity was observed (I2: 54%). Subgroup analysis showed a trend to significant reduction of ALT in subset in which dose of curcumin administration was ≥1000 mg/day (–11.36 IU/L, 95% CI: –22.75 to 0.02; I2 = 51). Whilst, the effect of curcumin on ALT was not significant in subgroup with <1000 mg/day curcumin supplementation (–1.98 IU/L, 95% CI: –12.12 to 8.15; I2 = 0). (Fig. 2 (A)). Overall result of meta-analysis showed a significant lowering effect of curcumin on AST blood concentration with significant between study heterogeneity (–6.10 IU/L, 95% CI: –11.21 to –0.99; I2 = 74). When studies were categorized based on duration of intervention, a significant reduction was observed in subgroup with 8-weeks study duration (–9.22 IU/L, 95% CI: –12.77 to –5.67; I2: 49%). No similar favorable effect was observed in other subgroup (–0.40 IU/L, 95% CI: –5.86 to 5.07; I2: 0%) (Fig. 2 (B)). Due to high within-subgroup heterogeneity in subset from other subgroup analysis, we confined to present only aforementioned stratified analysis.
Fig. 2.
Effect of Curcumin/Turmeric supplementation on alanine aminotransferases and aspartate aminotransferases (IU/L) blood concentrations.
No serious adverse effect related to curcumin administration was reported from included studies.
3.3. Risk of bias and adverse events
Summary of risk of bias assessment on each included study are presented in Table 2. Although random generation methodology was well-addressed in all studies, only 2 trials provided sufficient information about allocation concealment. Two studies reported adequate data for method which used for blinding design. All studies were showed low risk of bias according to incomplete outcome data and selective outcome reporting.
Table 2.
The Summary of Review Authors’ Judgments About Each Risk of Bias Item for Included Studies
| Study | Random sequence generation | Allocation concealment | Blinding | Incomplete outcome data | Selective reporting | Other bias |
|---|---|---|---|---|---|---|
| Panahi et al. 2016 | L | L | H | L | L | U |
| Rahmani et al. 2016 | L | L | L | L | L | U |
| Navekar et al. 2017 | L | H | L | L | L | U |
| Moradi-Kelardeh et al. 2017 | L | U | H | L | L | U |
H: high risk of bias; L: low risk of bias; U: unclear or unrevealed risk of bias. Criteria defined for risk of bias assessment are according to the Cochrane guidelines.
4. Discussion
The overall results of our meta-analysis suggested a possible efficacy of curcumin supplementation on management of elevated liver enzymes. Subgroup analysis showed a trend to significant reduction of ALT in sub-set with ≥1000 mg/day dosage of curcumin administration. It is possible that the favorable effect of curcumin may be dose-dependent and when administrated in high dose, it can affect as a NAFLD treatment. Also, subgroup analysis showed that curcumin can reduce AST in studies with 8-weeks intervention. Whilst, no significant effect was demonstrated in 12-weeks sub-set. This result might be due to optimal effect of curcumin in specific duration of supplementation. However, this finding erupted from low number of trials and should be interpreted with caution.
Present meta-analysis as a primary finding indicated curcumin might have potential favorable effect on elevated liver enzymes in higher dosage (≥1000 mg/day) and especial duration. It could be promising for improving of liver functions in patients who suffer from NAFLD. Although we analyzed only effect of curcumin on liver enzyme, there was few evidences that shows curcumin may have a potential favorable effect on body mass index (BMI)31, 32 and insulin30 among patients with NAFLD. Because of small number of available studies, we could not able to further subgroup analysis and define effect of other variable.
There are several plausible mechanisms that suggest favorable effect of curcumin on liver function. Curcumin might ameliorate hepatic steatosis and block fatty liver disease progression through inhibiting fatty acids synthesis and biosynthesis of unsaturated fatty acids such as stearic acid, oleic acid and linoleic acid.33 It can improve mitochondrial activity, facilitate β-oxidation and decrease lipogenesis.34 In addition, reports have indicated that oxidative stress and immune system disorder plays important roles in contribute to liver dysfunction such as NAFLD.35 In this case, curcumin can improve oxidative stress and prevent NAFLD by decrease production of reactive oxygen species, the hepatic protein expression of oxidative stress, pro-inflammatory cytokines, and chemokines such as interferon (IFN) γ, interleukin-1β and IFNγ-inducible protein 10.36, 37 Furthermore, it can inhibit liver damage in steatohepatitis via reducing the cytosolic and nuclear translocation of high mobility group box 1 (HMGB1) and nuclear factor kappa B (NF-κB) as well as inducing of peroxisome proliferator activated receptor-gamma (PPAR-γ).37, 38 Also, antioxidant property of curcumin is associated with inducing several anti-oxidant enzymes such as glutathione transferase, heme-oxygenase-1 and catalase.39, 40
Based on Food and Drug Administration (FDA) report, curcumin supplement has been characterized as a generally safe alternative medicine and no serious adverse event was documented by curcumin supplementation. However, there are some possible curcumin interaction evidences with antiplatelet drugs,41 camptothecin, mechlorethamine, doxorubicin, cyclophosphamide,42 celiprolol, midazolam43 and tacrolimus33 that should be considered when it was consumed.
To the best of authors’ knowledge, this is the first systematic review and meta-analysis of RTCs investigating the effect of curcumin on NAFLD treatment. However, some limitation should be considered when interpreting this meta-analysis. There were few eligible trials and the sample size was relatively small. Furthermore, the results of most studies were not adjusted for confounding factors. There are several evidences regarding the association between NAFLD and diet and lifestyle.44, 45, 46 Unfortunately, these variables did not assess in most included studies. Therefore, findings may be confounded by these parameters.
In conclusion, this review suggests that curcumin/turmeric might have a favorable effect on NAFLD in higher dosage. Further long-term studies with higher curcumin dosage supplementation are needed to confirm these results. Curcumin is a natural safe therapeutic with low cost/effective ratio that can provide an adjuvant treatment along with decrease in severity of adverse events caused by using common pharmacological therapy. This finding provided better insight into curcumin supplementation in NAFLD treatment and helped developing comprehensive information for future RCT with high quality design.
Author contributions
F.M.-G., M.P. and A.H. carried out the concept, design and drafting of this study. M.P., A.H. and F.J. searched databases, screened articles and extracted data. M.P. performed the acquisition, analysis, and interpretation of data. F.M.-G. critically revised the manuscript. All authors approved the final version of the manuscript. F.M.-G. and F.J. are the guarantors of this study.
Conflict of interest
There are no conflicts of interest to declare.
Contributor Information
Fariborz Mansour-Ghanaei, Email: ghanaie@yahoo.com.
Farahnaz Joukar, Email: farajov@gmail.com.
References
- 1.Williamson RM, Price JF, Glancy S, Perry E, Nee LD, Hayes PC. Prevalence of and risk factors for hepatic steatosis and nonalcoholic Fatty liver disease in people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes care. 2011;34:1139–1144. doi: 10.2337/dc10-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwenger KJ, Allard JP. Clinical approaches to non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20:1712. doi: 10.3748/wjg.v20.i7.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43(S1) doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 4.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 5.Hekmatdoost A, Shamsipour A, Meibodi M, Gheibizadeh N, Eslamparast T, Poustchi H. Adherence to the dietary approaches to stop hypertension (DASH) and risk of nonalcoholic fatty liver disease. Int J Food Sci Nutr. 2016;67:1024–1029. doi: 10.1080/09637486.2016.1210101. [DOI] [PubMed] [Google Scholar]
- 6.Zhang C, Yuan W, Fang J, Wang W, He P, Lei J. Efficacy of resveratrol supplementation against non-alcoholic fatty liver disease: a meta-analysis of placebo-controlled clinical trials. PLOS ONE. 2016;11:e0161792. doi: 10.1371/journal.pone.0161792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parikh NH, Parikh PK, Kothari C. Indigenous plant medicines for health care: treatment of Diabetes mellitus and hyperlipidemia. Chin J Nat Med. 2014;12:335–344. doi: 10.1016/S1875-5364(14)60041-8. [DOI] [PubMed] [Google Scholar]
- 8.Bahmani M, Mirhoseini M, Shirzad H, Sedighi M, Shahinfard N, Rafieian-Kopaei M. A review on promising natural agents effective on hyperlipidemia. Evid Based Complement Alternat Med. 2015;20:228–238. doi: 10.1177/2156587214568457. [DOI] [PubMed] [Google Scholar]
- 9.Priyadarsini KI. The chemistry of curcumin: from extraction to therapeutic agent. Molecules (Basel, Switzerland) 2014;19:20091–20112. doi: 10.3390/molecules191220091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luer SC, Goette J, Troller R, Aebi C. Synthetic versus natural curcumin: bioequivalence in an in vitro oral mucositis model. BMC Complement Altern Med. 2014;14:53. doi: 10.1186/1472-6882-14-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noorafshan A, Ashkani-Esfahani S. A review of therapeutic effects of curcumin. Curr Pharm Des. 2013;19:2032–2046. [PubMed] [Google Scholar]
- 12.Chattopadhyay I, Biswas K, Bandyopadhyay U, Banerjee RK. Turmeric and curcumin: Biological actions and medicinal applications. Curr Sci. 2004;87:44–53. [Google Scholar]
- 13.Zhu L, Ding X, Zhang D, Yuan C, Wang J, Ndegwa E. Curcumin inhibits bovine herpesvirus type 1 entry into MDBK cells. Acta Virol. 2015;59:221–227. doi: 10.4149/av_2015_03_221. [DOI] [PubMed] [Google Scholar]
- 14.Zhang N, Li H, Jia J, He M. Anti-inflammatory effect of curcumin on mast cell-mediated allergic responses in ovalbumin-induced allergic rhinitis mouse. Cell Immunol. 2015;298:88–95. doi: 10.1016/j.cellimm.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Meng B, Li J, Cao H. Antioxidant and antiinflammatory activities of curcumin on diabetes mellitus and its complications. Curr Pharm Des. 2013;19:2101–2113. [PubMed] [Google Scholar]
- 16.Kavakli HS, Koca C, Alici O. Antioxidant effects of curcumin in spinal cord injury in rats. Ulus Travma Acil Cerrahi Derg. 2011;17:14–18. [PubMed] [Google Scholar]
- 17.Jantan I, Bukhari SNA, Lajis NH, Abas F, Wai LK, Jasamai M. Effects of diarylpentanoid analogues of curcumin on chemiluminescence and chemotactic activities of phagocytes. J Pharm Pharmacol. 2012;64:404–412. doi: 10.1111/j.2042-7158.2011.01423.x. [DOI] [PubMed] [Google Scholar]
- 18.Trujillo J, Chirino YI, Molina-Jijón E, Andérica-Romero AC, Tapia E, Pedraza-Chaverrí J. Renoprotective effect of the antioxidant curcumin: Recent findings. Redox Biol. 2013;1:448–456. doi: 10.1016/j.redox.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Unlu A, Nayir E, Kalenderoglu MD, Kirca O, Ozdogan M. Curcumin (Turmeric) and cancer. J BUON. 2016;21:1050. [PubMed] [Google Scholar]
- 20.Kiso Y, Suzuki Y, Watanabe N, Oshima Y, Hikino H. Antihepatotoxic principles of Curcuma longa rhizomes. Planta Med. 1983;49:185–187. doi: 10.1055/s-2007-969845. [DOI] [PubMed] [Google Scholar]
- 21.Fujiwara H, Hosokawa M, Zhou X, Fujimoto S, Fukuda K, Toyoda K. Curcumin inhibits glucose production in isolated mice hepatocytes. Diabetes Res Clin Pract. 2008;80:185–191. doi: 10.1016/j.diabres.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Zhou H, Beevers CS, Huang S. Targets of curcumin. Curr Drug Targets. 2011;12:332–347. doi: 10.2174/138945011794815356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vera-Ramirez L, Perez-Lopez P, Varela-Lopez A, Ramirez-Tortosa M, Battino M, Quiles JL. Curcumin and liver disease. BioFactors (Oxford, England). 2013;39:88–100. doi: 10.1002/biof.1057. [DOI] [PubMed] [Google Scholar]
- 24.Darvesh AS, Aggarwal BB, Bishayee A. Curcumin and liver cancer: a review. Curr Pharm Biotechnol. 2012;13:218–228. doi: 10.2174/138920112798868791. [DOI] [PubMed] [Google Scholar]
- 25.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JP, Green S. John Wiley & Sons; 2011. Cochrane handbook for systematic reviews of interventions. [Google Scholar]
- 27.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1. 0 [updated March 2011]. The Cochrane Collaboration 2011. Available from: www.cochrane-handbook.orb. Cited April 11, 2012.
- 28.Dersimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 29.KB Moradi, Azarbayjani M, Peeri M, Mat Inhomaee H. 2016. Effect of curcum in supplementation and resistance TRA in ING in patients with nonalcoholic fatty liver disease. [Google Scholar]
- 30.Navekar R, Rafraf M, Ghaffari A, Asghari-Jafarabadi M, Khoshbaten M. Turmeric supplementation improves serum glucose indices and leptin levels in patients with nonalcoholic fatty liver diseases. J Am Coll Nutr. 2017;36:261–267. doi: 10.1080/07315724.2016.1267597. [DOI] [PubMed] [Google Scholar]
- 31.Panahi Y, Kianpour P, Mohtashami R, Jafari R, Simental-Mendía LE, Sahebkar A. Efficacy and safety of phytosomal curcumin in non-alcoholic fatty liver disease: a randomized controlled trial. Drug Res. 2017;67:244–251. doi: 10.1055/s-0043-100019. [DOI] [PubMed] [Google Scholar]
- 32.Rahmani S, Asgary S, Askari G, Keshvari M, Hatamipour M, Feizi A. Treatment of non-alcoholic fatty liver disease with curcumin: a randomized placebo-controlled trial. Phytother Res. 2016;30:1540–1548. doi: 10.1002/ptr.5659. [DOI] [PubMed] [Google Scholar]
- 33.Egashira K, Sasaki H, Higuchi S, Ieiri I. Food-drug interaction of tacrolimus with pomelo, ginger, and turmeric juice in rats. Drug Metab Pharmacokinet. 2012;27:242–247. doi: 10.2133/dmpk.dmpk-11-rg-105. [DOI] [PubMed] [Google Scholar]
- 34.Ferramosca A, Di Giacomo M, Zara V. Antioxidant dietary approach in treatment of fatty liver: new insights and updates. World J Gastroenterol. 2017;23:4146–4157. doi: 10.3748/wjg.v23.i23.4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inzaugarat ME, De Matteo E, Baz P, Lucero D, García CC, Gonzalez Ballerga E. New evidence for the therapeutic potential of curcumin to treat nonalcoholic fatty liver disease in humans. PLoS ONE. 2017;12:e0172900. doi: 10.1371/journal.pone.0172900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salomone F, Godos J, Zelber-Sagi S. Natural antioxidants for non-alcoholic fatty liver disease: molecular targets and clinical perspectives. Liver Int. 2016;36:5–20. doi: 10.1111/liv.12975. [DOI] [PubMed] [Google Scholar]
- 37.Afrin R, Arumugam S, Rahman A, Wahed MI, Karuppagounder V, Harima M. Curcumin ameliorates liver damage and progression of NASH in NASH-HCC mouse model possibly by modulating HMGB1-NF-kappaB translocation. Int Immunopharmacol. 2017;44:174–182. doi: 10.1016/j.intimp.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 38.Lin J, Tang Y, Kang Q, Feng Y, Chen A. Curcumin inhibits gene expression of receptor for advanced glycation end-products (RAGE) in hepatic stellate cells in vitro by elevating PPARgamma activity and attenuating oxidative stress. Br J Pharmacol. 2012;166:2212–2227. doi: 10.1111/j.1476-5381.2012.01910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iqbal M, Sharma SD, Okazaki Y, Fujisawa M, Okada S. Dietary supplementation of curcumin enhances antioxidant and phase II metabolizing enzymes in ddY male mice: possible role in protection against chemical carcinogenesis and toxicity. Pharmacol Toxicol. 2003;92:33–38. doi: 10.1034/j.1600-0773.2003.920106.x. [DOI] [PubMed] [Google Scholar]
- 40.Motterlini R, Foresti R, Bassi R, Green CJ. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic Biol Med. 2000;28:1303–1312. doi: 10.1016/s0891-5849(00)00294-x. [DOI] [PubMed] [Google Scholar]
- 41.Jantan I, Raweh SM, Sirat HM, Jamil S, Mohd Yasin YH, Jalil J. Inhibitory effect of compounds from Zingiberaceae species on human platelet aggregation. Phytomedicine. 2008;15:306–309. doi: 10.1016/j.phymed.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 42.Somasundaram S, Edmund NA, Moore DT, Small GW, Shi YY, Orlowski RZ. Dietary curcumin inhibits chemotherapy-induced apoptosis in models of human breast cancer. Cancer Res. 2002;62:3868–3875. [PubMed] [Google Scholar]
- 43.Zhang W, Tan TM, Lim LY. Impact of curcumin-induced changes in P-glycoprotein and CYP3A expression on the pharmacokinetics of peroral celiprolol and midazolam in rats. Drug Metab Dispos. 2007;35:110–115. doi: 10.1124/dmd.106.011072. [DOI] [PubMed] [Google Scholar]
- 44.Asrih M, Jornayvaz FR. Diets and nonalcoholic fatty liver disease: the good and the bad. Clin Nutr (Edinburgh, Scotland) 2014;33:186–190. doi: 10.1016/j.clnu.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Nseir W, Hellou E, Assy N. Role of diet and lifestyle changes in nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:9338–9344. doi: 10.3748/wjg.v20.i28.9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sofi F, Casini A. Mediterranean diet and non-alcoholic fatty liver disease: new therapeutic option around the corner? World J Gastroenterol. 2014;20:7339–7346. doi: 10.3748/wjg.v20.i23.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]