Abstract
The ability to rapidly discriminate successive auditory stimuli within tens-of-milliseconds is crucial for speech and language development, particularly in the first year of life. This skill, called Rapid Auditory Processing (RAP), is altered in infants at familial risk for language and learning impairment (LLI) and is a robust predictor of later language outcomes. In the present study, we investigate the neural substrates of RAP, i.e., the underlying neural oscillatory patterns, in a group of Italian 6-month-old infants at risk for LLI (FH+, n = 24), compared to control infants with no known family history of LLI (FH−, n = 32). Brain responses to rapid changes in fundamental frequency and duration were recorded via high-density electroencephalogram during a non-speech double oddball paradigm. Sources of event-related potential generators were localized to right and left auditory regions in both FH+ and FH− groups. Time-frequency analyses showed variations in both theta (Ɵ) and gamma (ɣ) ranges across groups. Our results showed that overall RAP stimuli elicited a more left-lateralized pattern of oscillations in FH− infants, whereas FH+ infants demonstrated a more right-lateralized pattern, in both the theta and gamma frequency bands. Interestingly, FH+ infants showed reduced early left gamma power (starting at 50 ms after stimulus onset) during deviant discrimination. Perturbed oscillatory dynamics may well constitute a candidate neural mechanism to explain group differences in RAP. Additional group differences in source location suggest that anatomical variations may underlie differences in oscillatory activity. Regarding the predictive value of early oscillatory measures, we found that the amplitude of the source response and the magnitude of oscillatory power and phase synchrony were predictive of expressive vocabulary at 20 months of age. These results further our understanding of the interplay among neural mechanisms that support typical and atypical rapid auditory processing in infancy.
Keywords: Infants, Auditory processing, Language and learning impairment, EEG/ERP, Neural sources, Time-frequency analysis oscillations
Abbreviations: RAP, Rapid Auditory Processing; LLI, language learning impairment; FH+/−, family history positive/negative; ISI, interstimulus interval; TSE, temporal spectral evolution; ITPL, intertrial phase locking; LAC, left auditory cortex; RAC, right auditory cortex
Highlights
-
•
Neural sources of RAP in infancy were identified at right/left auditory regions.
-
•
FH− infants demonstrated a mature left-lateralized pattern of neural oscillations.
-
•
FH+ infants demonstrated a more right-lateralized pattern of neural oscillations.
-
•
FH+ infants showed reduced left gamma power during rapid auditory discrimination.
-
•
Source and oscillatory measures are both associated with later language skills.
1. Introduction
1.1. Electrophysiological correlates of rapid auditory processing in infancy
The ability to perform fine-grained acoustic analyses of the incoming auditory input is a critical aspect of language acquisition (e.g., Aslin, 1989; Benasich and Tallal, 2002). Within the speech stream, crucial information is coded by detecting brief, rapid and successive events that change over a time range as little as tens of milliseconds (Tallal, 2004). This ability to process and categorize fast acoustic changes over time is called Rapid Auditory Processing (RAP). RAP deficits are characteristic of individuals with developmental language and reading disorders (for a review, see Tallal and Gaab, 2006), affecting not only basic speech processing but also higher-level linguistic skills, such as language comprehension and later reading ability (e.g., Banai et al., 2005; Cantiani et al., 2010; Gaab et al., 2007; Tallal, 1980). Developmental disorders affecting language and reading skills are often comorbid and aggregate in families suggesting a genetic etiology (Plomin and Kovas, 2005). Up to 67% of children with language impairment in preschool-age continue on to develop reading problems by elementary school (Bishop and Snowling, 2004). In addition, bivariate genetic analyses, which are based on cross-trait correlations in twin pairs and assess the proportion of phenotypic covariance attributable to common genetic contributions, have shown that genetic effects on language strongly correlate with genetic effects on reading in twins from the general population (r = 0.67–1.0) (Hohnen and Stevenson, 1999) and in affected twins (r = 0.53–0.86) (Bishop, 2001), supporting the view that language and reading (dis)abilities share common genes and are not etiologically distinct. For these reasons, the term “language-learning impairment” (LLI) has become increasingly popular and been used to characterize children with disorders affecting either or both language and reading skills. Thus, we will use the term LLI in the present study to describe our familial risk sample.
Converging evidence based on behavioral and electrophysiological measures suggests that RAP proficiency is impacted in young infants with an elevated risk of LLI (Benasich et al., 2002; Cantiani et al., 2016; Choudhury et al., 2007; Choudhury and Benasich, 2011) by virtue of a family history for the disorder (Hayiou-Thomas, 2008; Tomblin and Buckwalter, 1998). Further, studies suggest that RAP impairment in infancy is a robust predictor of language and reading abilities at later ages (Benasich et al., 2002; Cantiani et al., 2016; Choudhury et al., 2007; Choudhury and Benasich, 2011; Guttorm et al., 2005; Hämäläinen et al., 2013; Leppänen et al., 2010; Lohvansuu et al., 2018; Molfese et al., 2001; van Zuijen et al., 2012). Within this framework, several studies have investigated the electrophysiological responses to basic auditory stimuli in infants at high risk for LLI, reporting overall: (a) atypical discrimination processes as indexed by reduced amplitude of the MisMatch Response (MMR) (Cantiani et al., 2016; Choudhury and Benasich, 2011; van Leeuwen et al., 2006; van Zuijen et al., 2013, 2012) (b) delayed latency of the main ERP components (Cantiani et al., 2016; Choudhury and Benasich, 2011; Friedrich et al., 2004; Riva et al., 2018b; van Herten et al., 2008; van Zuijen et al., 2012) and/or (c) atypical brain lateralization of the ERP responses (Cantiani et al., 2016; Choudhury and Benasich, 2011; Friedrich et al., 2009; Guttorm et al., 2005; Leppänen et al., 2010, 2002, 1999; van Herten et al., 2008). Although results involving reduced amplitude and/or delayed latency of the primary emerging ERP peaks seem to be quite established, the atypical brain lateralization observed in at-risk infants has been controversial. For instance, while some studies have reported attenuated ERP responses in left hemisphere (Leppänen et al., 2010, 2002; van Herten et al., 2008; van Leeuwen et al., 2007) other studies have reported atypically enhanced responses in the right hemisphere (Friedrich et al., 2009; Guttorm et al., 2010, 2005, 2001; Leppänen et al., 1999) or a combination of the two patterns (Choudhury and Benasich, 2011). In a recent study from our group, analyzing 18 electrodes localized in the left and right fronto-central areas, equal left and right amplitudes were found for the control group, whereas reduced right as compared to left hemisphere amplitude was seen in the at-risk group (Cantiani et al., 2016). One of the reasons for these controversial results might be in the different reference electrode(s) used in EEG measurement/analysis that might strongly affect the waveforms at the scalp level. These findings require further investigation using a fine-grained analysis and more appropriate analytic strategies that can closely examine the neural substrates that support RAP (e.g., Musacchia et al., 2013). Specifically, the use of source localization might explicate the issue relative to the choice of the reference electrode(s), since the underlying potential distribution (the potential map) is independent of the reference used and thus solely determined by the underlying brain processes.
1.2. Source localization of ERP generators during auditory processing
In order to better characterize the mechanisms underlying typical and atypical RAP in infancy, it is essential to reliably determine brain areas that might sub-serve the ERP responses. Although functional magnetic resonance imaging (fMRI) is a gold standard technique that provides spatial information about brain areas involved in cognitive tasks, excellent estimation of activity location can achieved within the EEG/ERP domain, using source localization techniques that allow identification of the loci of the neural activation measured at the scalp surface (Slotnick, 2004). Despite source localization being widely used in adults, only a few studies have specifically investigated the cortical sources of the MMR in childhood and infancy. Albrecht et al. (2000) explored the potential of dipole source analysis for studying localization of auditory processing over development in healthy children, from pre-school age to adolescence. Dehaene-Lambertz and Baillet (1998) used a continuous stream of syllables to examine a mismatch-like response in 3-to-4 month infants and reported sources located in left and right temporal lobes. EEG-derived source localization in the first year of life has also been studied using tone pairs differing in fundamental frequency (Hämäläinen et al., 2011; Musacchia et al., 2017, 2013) as well as consonant–vowel syllables differing in voice onset time (Ortiz-Mantilla et al., 2019, 2016, 2013, 2012). Bilateral activation in auditory cortex has been reported in all the studies, with an additional activation near anterior cingulate cortex reported in Hämäläinen et al. (2011) and Ortiz-Mantilla et al., 2012, Ortiz-Mantilla et al., 2013, Ortiz-Mantilla et al., 2016. For the first time, Piazza et al. (2016) applied independent component analysis (ICA) decomposition to 6-month-old infant ERP data collected in response to non-speech tones. This method allowed identification of several important contributors to the ERP response from bilateral auditory cortex and multiple extra-auditory cortical areas, including mid-cingulate cortex.
To our knowledge, no studies have reported source localization of ERP generators during auditory processing in infants at risk for LLI. The use of this technique in developmental clinical populations has shown potential for clarifying controversial findings. For example, source analyses on ERP responses to shortening of pseudo-words were helpful in identifying a sub-group of dyslexic children characterized by atypically enhanced brain responses originating from a more posterior area of the right temporal cortex, as compared to the responses of control children and children at-risk but without reading disorder (Lohvansuu et al., 2014).
1.3. Spectrotemporal brain dynamics related to auditory processing
In addition to evoked information related to amplitude, latency and location of neural generators, measurement of changes in the event-related spectrum over time yields information that is not apparent in the averaged ERPs (Makeig, 1993). Specifically, spectrotemporal analysis of event-related brain responses captures phase-resetting and amplitude shifts in neuronal oscillations, which are defined as the periodic and rhythmic shifting of an ensemble of neurons between high and low excitability states (Bishop, 1933; Schroeder et al., 2008). In humans, peaks of oscillatory activity usually group into the following bands: delta (1–3 Hz), theta (4–8 Hz), alpha (9–12 Hz), beta (13–30 Hz), and gamma (>30 Hz), although the boundaries of corresponding bands appear to be lower in infants and children (see Saby and Marshall, 2012 for a review). The examination of neural oscillatory activity provides two kinds of information: (1) a measure of amplitude strength, reflecting the amount of energy (or power) in a given frequency range, and (2) a measure of phase-locking across trials, reflecting the temporal stability of the oscillatory phase across trials (Tallon-Baudry et al., 1996; Tallon-Baudry and Bertrand, 1999). Over and above traditional averaged ERPs, trial-by-trial analysis of neural oscillations characterizes the stability of the phase-locked response, perturbations of the ongoing EEG and induced neural activity that is not phase-locked to stimulus onset (Nunez and Srinivasan, 2006). Recent studies have established that examination of spectrotemporal brain dynamics in infants is of great value in understanding the basic neural mechanisms that relate to auditory discrimination and early language acquisition (Bosseler et al., 2013; Isler et al., 2012; Musacchia et al., 2017, 2015, 2013; Ortiz-Mantilla et al., 2019, 2016, 2013).
It has been proposed that changes in oscillatory activity play a role in cognitive and information processing (for a review, see Başar et al., 2001). During brain development, synchrony of oscillations within and across nuclei facilitates maturation of cortical networks (for a review, see Uhlhaas et al., 2010), including auditory processing networks in subcortical and cortical structures (Buzsaki and Draguhn, 2004; Kaas and Hackett, 2000). Accordingly, neural synchrony has been proposed to play a crucial mechanistic role in the development of RAP (Musacchia et al., 2013) and in language acquisition (Tallal, 2004; Tallal and Gaab, 2006).
In particular, neural oscillations in both the theta and gamma range have been related to rapid auditory processing (i.e. RAP) and speech perception. Shifts in theta and gamma amplitude have been shown to index the temporal dynamics of speech at different time scales. The oscillatory activity in the gamma band is more directly associated to RAP, since it has been shown to correlate with temporal sampling at the level of short-duration cues typically associated with the phonemic scale, such as formant transitions (for example,/ba/versus/da/) or voicing (for example,/ba/versus/pa/). Conversely, the oscillatory activity in the theta band correlates more closely with temporal sampling at slower modulation rates, corresponding to the syllabic scale (Poeppel, 2003). Importantly, theta and gamma generators that are weakly coupled at rest become more strongly coupled and nested in response to a more complex acoustic stream including information supporting acoustic characteristics of different timescales (e.g., formant transitions or syllables) (Giraud and Poeppel, 2012). The suggestion from Poeppel and colleagues is that theta band oscillations may function to “package” rapid acoustic changes that exceed the theta rate, enabling the construction of an information-bearing representation on a multi-dimensional timescale.
In typically developing infants, an overall increase in theta power and in theta phase synchronization has been reported in response to auditory discrimination (Isler et al., 2012; Musacchia et al., 2017, 2013; Ortiz-Mantilla et al., 2016, 2013; Piazza et al., 2014), with greater enhancement of left auditory activity for speech stimuli (Ortiz-Mantilla et al., 2013) as well as rapid-rate presentation of non-verbal stimuli (Musacchia et al., 2013). To our knowledge, the study from Musacchia and colleagues was the first to show that theta oscillations encode fast acoustic changes in infants.
In another series of studies, theta synchronization has been shown to index change detection with greater synchronization seen in adults as compared to children (Bishop et al., 2011). In comparison with age-matched controls, children with LLI showed typical synchronization of theta oscillatory activity in the initial detection or discrimination of sound differences, as reflected in even greater levels of intertrial coherence in the time window corresponding to the MMR. However, they also showed a reduction of low frequency event-related desynchronization (i.e., a less prolonged decrease in power) in a later time window, corresponding to the late discriminative negativity (LDN), reflecting differences in the late-stage auditory processing (Bishop et al., 2010; Halliday et al., 2014).
Auditory-evoked gamma activity, which most closely relates to neuronal firing (Fries et al., 2002) indexes learning and experience in the auditory system in adults (Heim and Keil, 2006). Selective enhancement of induced gamma oscillations was also found in response to specific native-contrast discrimination at 6 months of age (Ortiz-Mantilla et al., 2013). Interestingly, the oscillatory synchronization in both the theta and gamma frequency bands has been shown to index a more automatized and efficient processing of native language from 6 to 12 months-of-age (Ortiz-Mantilla et al., 2016). Specifically, compared with 6-month-olds, 12-month-olds' responses to native phonemes showed a general decrease in the magnitude of power elicited in the theta range, and a corresponding increase in high-gamma power in both frontal and left auditory sources, supporting the gradual shift from lower- to higher-frequency bands characterizing development (Ortiz-Mantilla et al., 2016).
In response to rapidly successive tones, Nagarajan et al. (1999) found that adults with poor reading abilities exhibited weaker cross-scalp coherence in the gamma frequency range compared to controls. Similarly, brain responses of school-age children with LLI, to the second of two fast-rate complex tones, showed oscillations in the gamma frequency range that were characterized by reduced power and attenuated phase-locking with respect to control children (Heim et al., 2011). In a longitudinal follow-up study, the same fast-rate complex tones were presented to the children after they participated in a specific computerized intervention program that targeted RAP skills (Heim et al., 2013). The RAP-focused intervention was found to boost gamma power in the immediate post-training period, but did not impact phase locking (Heim et al., 2013). Taken together, these studies highlight the role of gamma oscillations during auditory discrimination and suggest reduction in gamma power as the neural mechanism subserving deficits in RAP seen in LLI. But, since no studies of oscillatory dynamics have been conducted in pre-verbal infants at risk for LLI it is still unknown if the same gamma patterns could be seen at such an early age.
1.4. Hemispheric specialization for auditory processing
The debate over the nature of hemispheric specialization for auditory processing has moved from the classical views positing left lateralization for speech vs. right for non-speech (Lenneberg, 1966), to the hypothesis that left hemisphere specialization reflects processing of rapid elements characterizing both speech and non-speech signals whereas right hemisphere specialization is engaged for spectral processing, i.e., required for discrimination of slow pitch changes (e.g., Abrams et al., 2006; Belin et al., 1998; Jamison et al., 2006; Minagawa-Kawai et al., 2011a; Poeppel, 2003; Zaehle et al., 2004). Similar hemispheric asymmetries have already been identified in childhood and infancy (Dehaene-Lambertz, 2017; Minagawa-Kawai et al., 2011a; Thompson et al., 2016). Whereas the speech (left) vs. non-speech (right) asymmetry has been extensively replicated in infants from the fetal period onward (Dehaene-Lambertz et al., 2002; Homae et al., 2011; Mahmoudzadeh et al., 2013; Minagawa-Kawai et al., 2011c; Peña et al., 2003), the temporal (left) vs. spectral (right) asymmetry is still quite controversial and thus merits further investigation (Minagawa-Kawai et al., 2011b; Telkemeyer et al., 2009). Recently, Musacchia et al. (2013) explored this hypothesis by examining the neural substrates of various temporal modulations of complex acoustic stimuli in 4-month-old infants. Specifically, combining source localization and time-frequency analyses of event-related oscillations, an overall right-hemisphere dominance for tone processing with an additional left-hemisphere recruitment specific to rapid frequency change was demonstrated. Additionally, a more mature left-to-right asymmetry for rapid frequency changes was reported in 7-month-olds who received early auditory training consisting of an interactive acoustic experience with temporally modulated non-speech stimuli (Musacchia et al., 2017).
Several lines of research support the hypothesis that asymmetric routing between cerebral hemispheres represents an important mechanism for auditory encoding in the developing human auditory system (see Bishop, 2013 for a review). The use of Near Infrared Spectroscopy (NIRS), fMRI and source localization of dense-array EEG/ERPs techniques have recently made it possible to study normal and abnormal development of lateralized function in the developing brain, confirming links with LLI. In particular, with respect to the left-hemisphere specialization observed in response to rapid vs. slow transitions, children with developmental dyslexia as well as pre-reading children with a familial risk for dyslexia showed neuronal disruption of left prefrontal brain regions during rapid spectrotemporal processing of nonlinguistic stimuli (Gaab et al., 2007; Raschle et al., 2013). A different study, using paired tones characterized by short within-pair intervals, reported that both typically developing and at-risk children without dyslexia showed larger responses in the source waveforms in the left than right hemisphere, whereas at-risk children with dyslexia showed equal amplitudes over both hemispheres and thus diminished hemispheric asymmetry (Khan et al., 2011). Higher order sequelae of auditory processing, such as reading skill, have also been shown to vary with degree of auditory hemispheric asymmetry (Abrams et al., 2009).
1.5. Aims of the study
In the present study, using the same cohort reported in Cantiani et al. (2016), we examine; (1) the sources of the neural activity elicited by rapid-rate presentation of stimuli changing in two different auditory attributes: frequency and duration, and (2) the oscillatory underpinnings of these responses at the source level. Whereas responses to frequency changes have been more often investigated in the framework of RAP (Benasich et al., 2002; Choudhury and Benasich, 2011; Hämäläinen et al., 2011; Heim et al., 2011; Musacchia et al., 2017, 2013), the introduction of changes in duration was driven by the critical role that this acoustical feature may have for phonemic discrimination in Italian. Specifically, sound duration in Italian is an essential cue for a specific phenomenon known as “consonant germination” (Esposito and Di Benedetto, 1999), where the duration of the intervocalic consonant is a discriminative feature indicating differences in meaning (e.g., the word “note” [no:te] music notes vs. the word “notte” [not:e] night).
Based on previous studies from our group (Cantiani et al., 2016; Choudhury and Benasich, 2011), we focus our analyses on the large positivity at about 300 ms from deviant stimulus onset. In the infant literature, this component is thought to reflect a neural change detection process (e.g., Kushnerenko et al., 2002). In previous studies, conducted on similar populations and using similar paradigms, it has been labeled the MisMatch Response (MMR), specifically in reference to the component of the calculated difference waveform (e.g. DEV-STD, Choudhury and Benasich, 2011) or P3 component, to reflect its polarity and average time of onset when considering standard and deviant waveforms (Cantiani et al., 2016). It is important to note that ERP components in infants are not necessarily the same as those in older children and adults, and that, in particular, the underlying neural substrates may differ (e.g., Lippé et al., 2009; Morr et al., 2002). For this reason, the component analyzed here cannot be directly interpreted as the typical mismatch negativity elicited within similar paradigms in older children and adults, nor as the P3 historically described in the adult EEG literature (Friedman et al., 2001). For this reason, in the present study this component will be labeled as the “positive response”.
Based on the literature on typical infants (Bosseler et al., 2013; Hämäläinen et al., 2011; Isler et al., 2012; Musacchia et al., 2017, 2015, 2013; Ortiz-Mantilla et al., 2013), we hypothesize that we will observe bilateral activation in the auditory cortex, particularly in the left hemisphere, and theta/gamma enhancement in response to the auditory input and discrimination processes. Based on the literature on LLI in children and adults (Bishop et al., 2010; Halliday et al., 2014; Heim et al., 2013, 2011), we also expect reduced strength in theta/gamma oscillatory mechanisms in the group of infants at high risk for LLI.
Moreover, we expect the present study to provide more clarity on the controversial evidence regarding hemispheric anomalies in infants at risk for LLI. The studies that applied neuroimaging techniques supporting good spatial resolution (fMRI and/or source localization of dense-array EEG/ERPs) on typical infants (Musacchia et al., 2017, 2013) and on LLI children and adults (Gaab et al., 2007; Khan et al., 2011; Raschle et al., 2013) have shown variations in the pattern of hemispheric activation in infants with and without familial risk for LLI in response to non-speech stimuli characterized by rapid changes. In our sample we expect the group of typically developing infants to be characterized by greater enhancement of left auditory activity as compared to the group of infants at familial risk.
Finally, the association between these measures of neural activation at the source level at 6 months and early linguistic outcome will be explored. Following Cantiani et al. (2016), correlations will be performed with a measure of expressive vocabulary at 20 months that is highly correlated with concurrent and later measures of language development (Rescorla and Alley, 2001). Significant correlations are expected based on the wide literature on the predictive role of early ERP measures on later language development (Benasich et al., 2002; Cantiani et al., 2016; Choudhury and Benasich, 2011; Leppänen et al., 2010; Lohvansuu et al., 2018; Riva et al., 2018a; van Zuijen et al., 2012). Piazza et al. (2016) confirmed these correlations when taking into account ERP measures at the source level (derived by independent component analysis decomposition) and suggested that measures obtained from source analysis might be even more predictive of later outcome than those measures provided by traditional ERP analysis (see Piazza, 2016 for further discussion [Unpublished PhD dissertation]).
2. Method
2.1. Participants
Two groups of infants were included in this study: typically-developing infants with no known family history of LLI (Family History Negative, FH−, n = 32) and infants at high familial risk for LLI (Family History Positive, FH+, n = 24). In the present study we retained all infants previously reported (Cantiani et al., 2016). EEG/ERPs during a non-speech double oddball paradigm were recorded at 6-months-of-age and a follow-up session to monitor expressive language development was scheduled at 20-months-of-age.
Families were recruited by local advertisement and/or physician referral from three hospitals within the Lecco and Monza-Brianza area (Northern Italy). Written informed consent was obtained from all parents prior to testing. Ethical and Scientific Committees of all participant institutes approved the study protocol.
Infants were included in the study if: (1) both parents were native-Italian speakers and infants reside in monolingual households, (2) gestational age was ≥37 weeks, (3) birth-weight ≥ 2500 g, (4) APGAR scores at birth at 1′ and 5′ were ≥ 9 and (5) the Bayley Cognitive Score ≥ 7, (6) infants did not have a history of hearing impairments and passed the hearing screening at birth.
Infants were assigned to FH+ if at least one first-degree relative (sibling or parent): (1) had a certified (clinical) diagnosis of LLI and/or (2) performed at least two standard deviations (SD) below the population mean on at least one reading task from a selected battery of tests standardized on the Italian population including a text-reading test (Judica and De Luca, 2005) and a single word- and a pseudoword-reading test (Sartori et al., 1995) and (3) had no certified diagnosis of intellectual deficiency, attention-deficit disorder, sensorial and neurological disorders or autism. This yielded 24 infants (13 Males and 11 Females), of which 7 had a positive family history for language impairment, 14 for reading difficulties and 3 for both.
Infants were assigned to the FH− group if all first-degree relatives (1) had an absence of a certified diagnosis of LLI and (2) a performance above 0.5 SD below the population mean on all reading tasks. This yielded 32 infants (17 Males and 15 Females). For a more complete description of the recruitment procedure see Cantiani et al., 2016.
The two groups of infants did not differ on demographic (e.g., socioeconomic status, parents' age and educational level) or clinical (gestational age, Bayley cognitive subscale at 6 months, expressive vocabulary at 20 months) characteristics (all ps > 0.05). A complete description of the sample can be found in Cantiani et al. (2016).
2.2. General testing procedure
Families were contacted two weeks prior to the child's 6-month birthday: a first visit to the laboratory was scheduled at 6 months, 15 days ± two weeks. During the visit, the EEG/ERP was recorded (see Section 2.3) and the cognitive subscale of the Bayley Scales of Infant Development (Bayley, 2006) administered. Before the child's 20-month birthday, caregivers were mailed packets containing the Language Development Survey (LDS) to fill-in at home. The LDS is a 310-word parental-report screening tool that provides an expressive vocabulary score and has been shown to significantly correlate with the MacArthur Communicative Development Inventories (CDI) words and sentences (Fenson et al., 1993; Rescorla et al., 2005). The CDI has recently been standardized on an Italian population (Rescorla et al., 2014) and norms (including percentile ranks and age-equivalent scores) are available from ages 18–35 months (Rescorla and Alley, 2001). Parents were asked to bring the forms to a scheduled laboratory visit at 20 months, 15 days ± two weeks.
2.3. Double oddball paradigm
2.3.1. Stimuli
Electro-cortical activity related to Rapid Auditory Processing (RAP) was assessed by means of a non-speech multi-feature paradigm, in which pairs of complex tones with an ISI of 70 ms were presented. The first tone in the pair always had a fundamental frequency of 100 Hz with 15 harmonics (6 dB roll-off per octave) and 70 ms (5 ms rise time and 5 ms fall time) duration. For standard tone-pairs (STD) the same tone was repeated twice (i.e., 100–100 Hz). Two deviant tone-pairs differing with respect to the second tone were presented: for the frequency deviant (DEVF) the second tone had a fundamental frequency of 300 Hz and 70 ms duration; for the duration deviant (DEVD) the second tone had a duration of 200 ms and a fundamental frequency of 100 Hz.
The stimuli were presented in a passive oddball paradigm where 1200 stimuli (80% STD, 10% DEVF, 10% DEVD) were pseudo-randomized, so that at least three standard tone-pairs were presented before each deviant pair. The intertrial interval (offset-to-onset, ITI) randomly varied from 700 to 900 ms. All stimuli were presented at an intensity of 75 dB via speakers located on either side of and equidistant (95 cm) from the subject (for a more complete description of the stimuli see Cantiani et al., 2016).
2.3.2. EEG data acquisition and preprocessing
During EEG recording, children were seated on their caregiver's lap in a sound-attenuated and electrically-shielded room, and watched silent movies or were entertained with quiet toys. EEG was recorded from 60 scalp sites using a dense-array EGI recording system (Electric Geodesic, Inc., Eugene, Oregon) with vertex as the online reference. Sampling rate was 250 Hz with 0.1–100 Hz online bandpass filter.
After recording, EEG data were processed offline following the procedure described in Cantiani et al., 2016, in order to extract individual and group ERPs. Data were exported to a MATLAB (Mathworks, Natick, MA) compatible format and processed using EEGLAB (Delorme and Makeig, 2004) and ERPLAB (Lopez-Calderon and Luck, 2014). An offline bandpass filter of 0.5–30 Hz was used. Noisy channels were interpolated with a spherical spline (never >12 of the 60 channels). The signals were then re-referenced to an average reference and the 13 outermost channels were removed due to significant movement-related artifacts and a high rate of interpolation. The remaining 48 channels were considered for analyses. The continuous EEG was segmented according to stimulus type (pre-deviant STD, DEVF and DEVD) with 100 ms pre-stimulus time (used for baseline correction) and 800 ms post-stimulus time. Bad EEG epochs (including epochs contaminated by blink artifacts and/or artifacts due to infants' movements) were identified and rejected using both automatic criteria and visual inspection (for further information of ERP data processing see Cantiani et al., 2016). A minimum of 60 artifact-free trials was used for averaging ERPs. The obtained ERPLAB files containing trigger and sensor information were finally imported to BESA Research 5.3 in order to estimate sources.
2.3.3. Source localization of ERP generators
For this paper, we used the ERPs reported in Cantiani et al. (2016) to compute cortical sources and investigate where in the brain the ERP responses were generated. For source localization, ERPs were mapped onto a 6-month infant MRI brain template, using BESA Research 5.3 and Brain Voyager QX (Scherg et al., 2010) software, and age-appropriate estimates of scalp, skull bone, and subarchnoid thickness applied following previous source localization infant studies (see Hämäläinen et al., 2011; Ortiz-Mantilla et al., 2012). Specifically, the MRI brain template for 6-month-olds was created by affine transform of MRI images collected from 19 sleeping 6–7-month-old infants (see Raschle et al., 2012 for further details about MRI acquisition techniques) into the MRI space of an infant with the median age of the sample and combined into an average image. The average brain template was aligned and transformed into Talairach space. The parameters used for scalp and skull thickness, and subarachnoid width were as follows: skull thickness = 1.5 mm; average subarachnoid space = 1.7 mm; average scalp thickness = 2.5 mm, estimated bone conductivity = 0.0581.
To localize the generators of the ERP responses, we utilized an established technique of auditory source localization and analysis in the infant brain (Hämäläinen et al., 2011; Musacchia et al., 2013, Musacchia et al., 2017, 2015; Ortiz-Mantilla et al., 2012, Ortiz-Mantilla et al., 2013, Ortiz-Mantilla et al., 2016, Ortiz-Mantilla et al., 2019). This technique is based on a two-step procedure. First, we localized the generators of the grand average ERP file, comprised of the individual files of all infants by group. In that way we increased the signal to noise ratio and obtain an accurate source localization by group. Second, we localized the generators at the individual level. In order to do so, we first visually inspected the localization of the dipoles after fitting the model to the data to assure they were located as close as possible to the grand average localization; and secondly, we looked at the residual variance (RV) to determine how well the dipole-model explained the variance in the data (RV below 20%). Only infants that had both reliable dipole location and RV below the cut-off were included in the statistical analyses (in the analyses related to DEVF, 28 FH− infants and 20 FH+ were included, whereas in the analyses related to DEVD, 24 FH− infants and 17 FH+ were included).
Since the main aim of the present work was characterization of the auditory discrimination process, the analyses focused on the positive response, specifically targeting the neural change detection process. The longitudinal studies conducted to date (Cantiani et al., 2016; Choudhury and Benasich, 2011) have also identified a negative peak defined as the N2* peak, which is developmentally considered the “precursor” of the emerging mismatch negativity. This peak is not analyzed here due to methodological constraints: in our experience we have found it quite difficult to source localize small, just emerging peaks at the individual level. Thus, we report here only those peaks for which we found reliable source localization that explains most of the variance. In order to preserve the cleanest possible signal we use response to the pre-deviant standard and response to each deviant (which represents the discrimination response), instead of using the more traditional mathematical subtraction to conduct temporo-spectral analyses.
As expected, the positive response was larger, and detectable in individual averaged responses to the deviant stimuli (DEVF and DEVD), compared to the STD responses. For this reason, for the deviant stimuli the peak was identified and source localized both in the grand average and in individual ERP waveforms, whereas the response of the standard stimulus was identified and source localized in the grand average only. A dipole source model (Scherg and Von Cramon, 1985) using a 4-shell ellipsoidal head model was fitted to the data. A time window of ±20 ms around each individual's max peak was used for dipole fitting. Following the dipole fit, source peaks were identified and measures of latency, amplitude and x (medial–lateral), y (posterior-anterior) and z (inferior-superior) source coordinates extracted for each individual's dipoles in both deviant conditions and submitted to statistical analyses. In order to confirm the sources of activation identified by the dipole source model addition, we used a distributed source model calculated using the Classic LORETA Recursively Applied (CLARA) method (Hoechstetter et al., 2010). We selected this method because, as compared to other distributed source models, it identifies more focal distributed source images by applying them iteratively.
2.3.4. Time-frequency analysis
2.3.4.1. Temporal spectral evolution (TSE)
Spectrotemporal changes during processing of non-speech stimuli were subsequently examined in source space. For this part of the analysis, we included only participants for which source localization was possible at the individual level (see paragraph 2.3.3) and used for each child the dipole model created during source analysis in the individual averaged ERP data. The dipole model works as a virtual source montage (Scherg and Ebersole, 1994) to transform the raw, unfiltered 48-channel EEG recording into source space. For each stimulus type (STD, DEVF and DEVD), the ongoing EEG was then transformed into a 2-channel (i.e., left auditory cortex [LAC] and right auditory cortex [RAC]) source space). For DEVF and DEVD, the dipole model created for the corresponding condition was used. Since source localization at the individual level was not possible for the STD stimulus, the dipole model created for DEVF was used in this condition. This choice maximized the sample size for these analyses (as reported in Paragraph 2.3.3., source localization at the individual level was possible for 48 infants for DEVF and 41 infants for DEVD). Next, single trials were transformed into the time-frequency domain using complex demodulation with 1 Hz wide frequency bins and 50 ms time resolution, from −300 to 800 ms in the range of 2–80 Hz, (for detailed information of this methodology, see Scherg et al., 2010). TSE was used to examine percent change (relative to the baseline) of evoked (phase-locked) and induced (non-phase-locked) amplitude of oscillatory activity related to stimulus presentation at a particular time–frequency sampling point (Hari et al., 1997; Tallon-Baudry et al., 1996; Tallon-Baudry and Bertrand, 1999). Following the previous literature on auditory-evoked infant oscillations (Isler et al., 2012; Musacchia et al., 2013, Musacchia et al., 2017; Ortiz-Mantilla et al., 2013), TSE was examined only in the delta/theta frequency band (2–12 Hz) and in the gamma frequency band (30–80 Hz).
2.3.4.2. Inter-trial phase locking (ITPL)
In addition to TSE, the inter-trial phase locking (ITPL) value, which relates only to evoked activity, was used to measure how consistently the phase at different frequency bands locks to stimulation presented across trials. ITPL has a value that ranges from 0 to 1, with 0 indicating random phase across trials, and a value of 1 corresponding to perfect inter-trial phase alignment (Tallon-Baudry et al., 1996; Tallon-Baudry and Bertrand, 1999). Based on the above-mentioned infant literature on auditory oscillations, ITPL was examined only in lower frequency range (2–12 Hz).
TSE and ITPL measurements were obtained at the two sources previously identified (i.e., LAC and RAC), for each stimulus type (i.e., STD, DEVF, DEVD) for each FH− and FH+ participant and exported to MATLAB for plotting graphics across subjects.
2.4. Analytic strategy
2.4.1. Source analyses
Statistical source analyses were carried out using SPSS Statistics 21 software. Effects of Stimulus Type (DEVF vs. DEVD), source (LAC vs. RAC) and Group (FH− vs. FH+) were examined using 2×2×2 repeated measures ANOVAs for source amplitude, latency and dipole coordinates (x: medial– lateral; y: posterior-anterior; and z: inferior-superior). When necessary to clarify interactions, post -hoc analyses (t-tests between relevant conditions) were conducted.
2.4.2. Time-frequency analysis
For time-frequency, statistical analysis on magnitude of spectral amplitude change (TSE) and phase-coherence (ITPL) over Left and Right auditory cortices were conducted through data clustering, in combination with permutation testing, using BESA Statistics 2.0 (BESA). The statistical method used is parameter-free permutation testing on the basis of Student's t-test (Maris and Oostenveld, 2007). In particular, it automatically identifies clusters in time and frequency where two groups or conditions are significantly different. Results are considered corrected for multiple comparisons as only clusters that have higher values than 95% of all clusters derived by 1000 random permutations of data are identified (for a more detailed description of this method see the on-line BESA Statistics manual: http://www.besa.de/products/besa-statistics/brochures/). In this way, we identified clusters where significant differences in the time-frequency domain were found either between the conditions (paired t-tests) or between the groups (unpaired t-tests). The first set of analyses is aimed to explore for each group the oscillatory patterns in the different frequency bands that are related to discrimination, whereas the second set of analyses directly focuses on differences between the groups. The p-values reported here are corrected for multiple comparisons through data clustering in combination with permutation testing. Only the effects that survived to permutations are reported.
2.4.3. Correlations of ERP/EEG measures with the linguistic outcome
Pearson's product moment correlations were conducted to assess associations between infant electrophysiological measures and 20-month language abilities (expressive vocabulary percentile scores). First, in order to examine the association of source components and linguistic outcome, measures of latency and amplitude of the source peaks separated by stimulus type and source location were entered in the correlation analysis. Second, in order to assess the association of magnitude of spectral power (TSE) and phase coherence (ITPL) and linguistic outcome, cluster permutation testing, based on the correlation analysis was applied in BESA Statistics v2.0. Specifically, the presence of spatially contiguous clusters of coherent r values was identified within the previously identified clusters of activity differentiating STD from DEV stimuli.
3. Results
3.1. Source localization
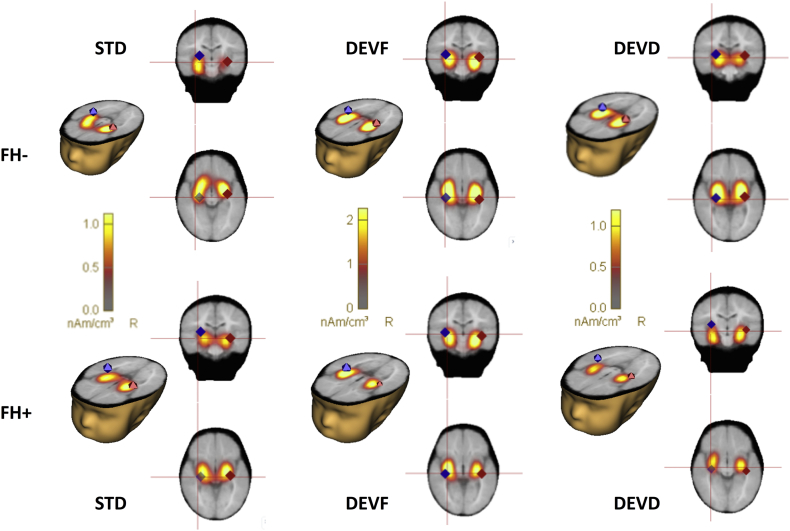
For both groups, we found that a 2-dipole model explained about 98% of the variance (residual variance in the grand average for STD, FH−: 1.7%, FH+: 2.6%; DFVF, FH−: 1.3%, FH+: 1.8%; and DEVD: FH−: 1.2%, FH+: 1.3%) with 2 dipoles located at LAC and RAC. A distributed source model (CLARA) confirmed sources of activation (Fig. 1).
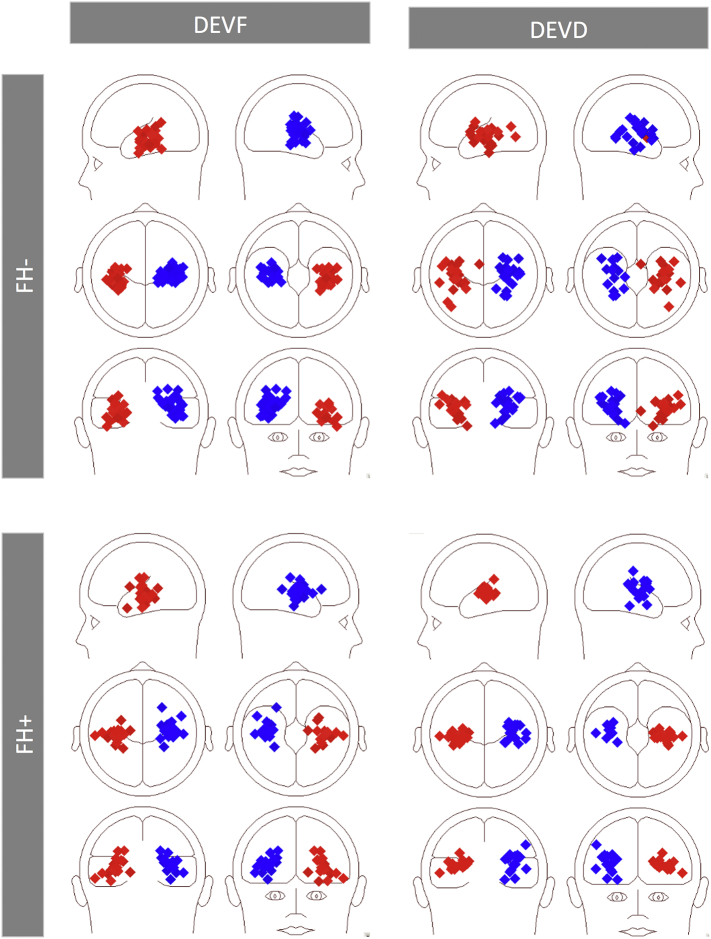
Fig. 1.
Left and Right Auditory Cortex (LAC, RAC) source localizations for FH− and FH+ infants. The two-dipole best fit source models are overlaid on distributed source activity (CLARA) over the infant template for 6-month-old infants. Plots are separated for groups (FH− and FH+ infants) and stimulus type (Standard, STD; frequency deviant, DEVF; duration deviant, DEVD).
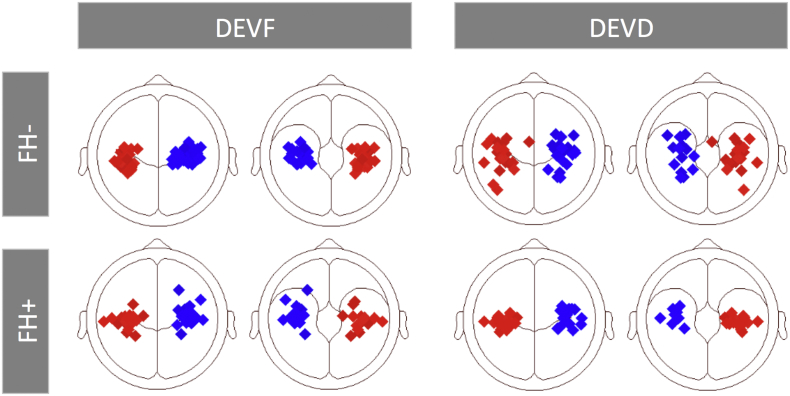
At the individual level the positive response for DEVF could be reliably modeled in 28 out of 32 (87%) FH− infants (mean of individual fit intervals: 420.6–460.6 [SD = 26.6], min: 348–388, max: 488–528) and in 20 out of 24 (71%) FH+ infants (mean of individual fit intervals: 422–462 [SD = 33.3], min: 352–392, max: 472–512), whereas the response for DEVD was reliably modeled in 24 out of 32 (75%) FH− and 17 out of 24 (70%) FH+ infants (mean of individual fit intervals: 491.2–531.2 [SD = 31.0], min: 444–484, max: 564–604; and 487.3–527.3 [SD = 28.3], min: 432–472, max: 540–580, respectively). Individual source locations are displayed in Fig. 2. The 2-dipole model explained ~95% of the variance for FH− infants (residual variance for DEVF: 4.47 [SD = 3.38] and DEVD: 5.59 [SD = 4.17]) and ~93% for the FH+ group (residual variance for DEVF: 5.61 [SD = 2.31] and DEVD: 7.09 [SD = 3.77]). No significant group differences were found between start and end time used for fitting or in the residual variance for each condition (all ps > 0.05).
Fig. 2.
Individual source locations superimposed on the schematic head (left and right transverse views). Plots are separated for groups (FH− and FH+ infants) and stimulus type (frequency deviant, DEVF; duration deviant, DEVD).
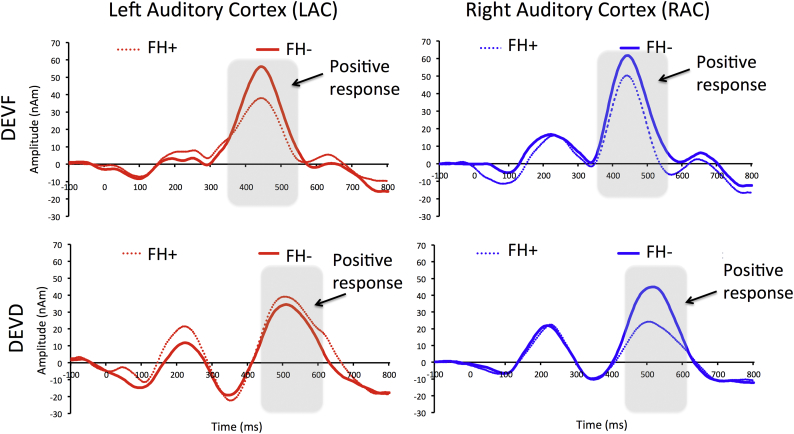
The source waveforms are displayed in Fig. 3. In both groups, a large positive deflection emerged, occurring with different timing for each stimulus type and resembling the positive (P3) component of the original ERP waveforms (Cantiani et al., 2016). Means and SD of amplitude and latency of the positive source peaks based on individual source analyses can be found in Table 1.
Fig. 3.
Grand average source waveforms for FH− and FH+ infants. Plots are separated for source (Left and Right Auditory Cortex) and stimulus type (frequency deviant, DEVF; duration deviant, DEVD). Waveforms relative to FH− infants (continuous lines) are plotted against the waveforms relative to FH+ infants (dashed lines). The grey squares highlight the time-windows in which the positive response has been analyzed for the two stimulus types, corresponding to the range including all individual fit intervals. Positivity is plotted up; amplitude is given in nanoampere meters (nAm) and latency in milliseconds (ms). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 1.
Latency and amplitude of the positive response peak to DEVF and DEVD stimuli based on individual source analyses. Latency is given in milliseconds (ms) and amplitude in nanoampere meters (nAm).
| FH− (n = 24) | FH+ (n = 16) | t(df) | p | Cohen's d | ||
|---|---|---|---|---|---|---|
| Latency | DEVF LAC | 444.3 (32.5) | 439.0 (34.9) | 0.494 (38) | 0.624 | 0.157 |
| DEVF RAC | 441.3 (27.7) | 433.2 (36.0) | 0.801(38) | 0.428 | 0.252 | |
| DEVD LAC | 507.2 (34.0) | 511.5 (38.4) | −0.375 (38) | 0.710 | 0.118 | |
| DEVD RAC | 510.3 (35.0) | 504.5 (26.8) | 0.564 (38) | 0.576 | 0.186 | |
| Amplitude | DEVF LAC | 72.93 (26.06) | 60.30 (22.34) | 1.586 (38) | 0.121 | 0.520 |
| DEVF RAC | 72.56 (28.55) | 69.86 (26.67) | 0.301 (38) | 0.765 | 0.520 | |
| DEVD LAC | 51.21 (30.72) | 43.09 (13.10) | 1.148 (38) | 0.259 | 0.343 | |
| DEVD RAC | 55.93 (35.47) | 35.47 (20.43) | 2.360 (38) | 0.024 | 0.706 | |
The 2x2x2 (Source [LAC vs. RAC] x Stimulus Type [DEVF vs. DEVD] x Group [FH− vs. FH+]) repeated-measures ANOVAs were conducted separately on the source latency and amplitude. The ANOVA for peak latency revealed a main effect of stimulus type (F(1,38) = 98.756, p < .001, η2 = 0.722) in which the positive response to the DEVF stimulus had shorter latency than the response to DEVD. Statistical analysis on the source amplitude demonstrated a Condition x Hemisphere x Group interaction (F(1,38) = 4.639, p = .038, η2 = 0.109). Post-hoc t-tests conducted to further investigate this interaction revealed that as compared to FH+ the FH− group had higher amplitude for the DEVD stimulus in the RAC source (t(1,38) = 2.36, p = .024). In addition, a main effect of stimulus type, (F(1,38) = 37.146, p < .001, η2 = 0.494) was also found, in which peak amplitude for the DEVD stimulus was smaller than for DEVF.
Differences in source localization across groups were examined individually by 2x2x2 (Source [LAC vs. RAC] x Stimulus Type [DEVF vs. DEVD] x Group [FH− vs. FH+]) ANOVAs for the x, y, and z coordinates. A significant Source x Group interaction emerged for the x coordinate, F(1,38) = 4.288, p = .045, η2 = 0.101. However, no significant difference emerged between groups, neither in the RAC source (FH−: M = 0.40, SD = 0.10; FH+: M = 0.45, SD = 0.07) nor in the LAC source (FH−: M = −0.43; SD = 0.08; FH+: M = −0.41, SD = 0.08). A main effect of source (F(1,38) = 4.692, p = .037, η2 = 0.110) and a main effect of Group (F(1,38) = 4.278, p = .045, η2 = 0.101) were found for the z coordinate. Sources in LAC (M = 0.05, SD = 0.10) were more inferior compared with the RAC sources (M = 0.09, SD = 0.10) and sources in the FH− group (M = 0.05, SD = 0.07) were more inferior compared with the sources in the FH+ group (M = 0.10, SD = 0.07). No significant differences were found for the y coordinate.
3.2. Time-frequency analyses
3.2.1. Temporal spectral evolution (TSE) measures
3.2.1.1. Paired comparisons within Group
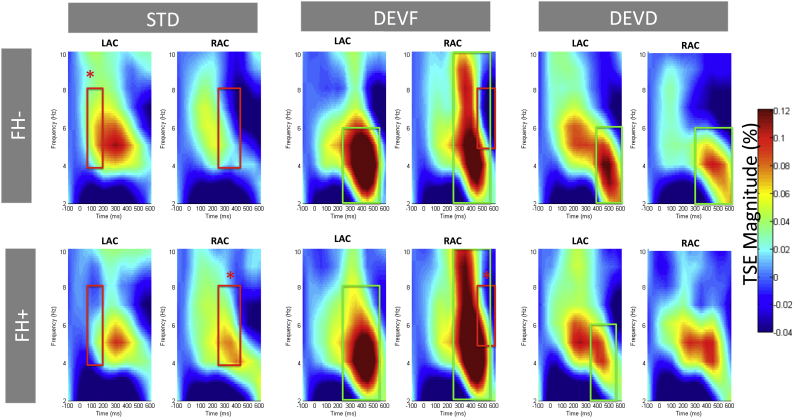
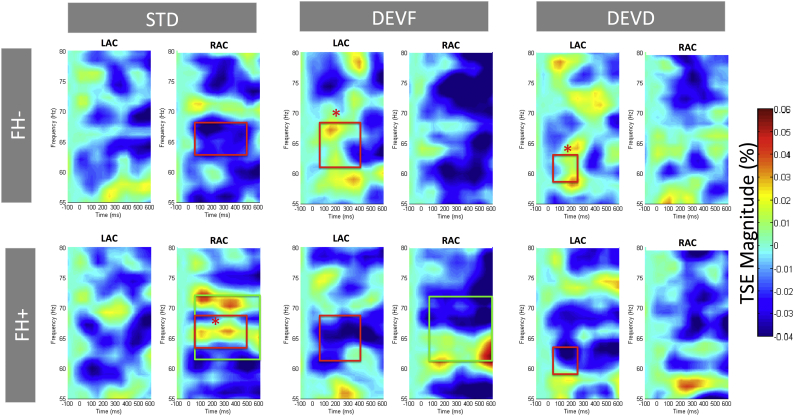
Differences in spectral power between each deviant (DEVF and DEVD) and the STD were estimated through paired comparisons within each group (FH− and FH+) to assure that both groups discriminated the stimuli. We confirmed that infants in both FH− and FH+ groups generated significantly more theta power for the deviant than for the STD in both auditory cortices. The only exception to this pattern was the absence of differences between DEVD and STD in RAC for the FH+ group. In addition, this group showed more power in the gamma frequency range for STD than DEVF in RAC (means, p values and cluster definitions where differences were found are presented in Table 2). See Fig. 4 for theta and Fig. 5 for gamma power representation.
Table 2.
Power differences between conditions in the FH− and FH+ groups: condition comparison using cluster identification and permutation testing.
| Stimulus comparison | Time (ms) | Frequency band | Frequency (Hz) | Source | Direction | p-value | Mean STD | Mean DEV |
|---|---|---|---|---|---|---|---|---|
| FH− group | ||||||||
| STD vs. DEVF | 250–550 | θ | 2–10 | RIGHT | F > STD | <0.001 | −0.016 | 0.048 |
| STD vs. DEVF | 250–550 | θ | 2–6 | LEFT | F > STD | 0.002 | 0.020 | 0.079 |
| STD vs. DEVD | 300–600 | θ | 2–6 | RIGHT | D > STD | 0.003 | −0.017 | 0.040 |
| STD vs. DEVD | 400–600 | θ | 2–6 | LEFT | D > STD | 0.033 | 0.020 | 0.061 |
| FH+ group | ||||||||
| STD vs. DEVF | 250–550 | θ | 2–10 | RIGHT | F > STD | 0.002 | 0.027 | 0.090 |
| STD vs. DEVF | 250–550 | θ | 2–8 | LEFT | F > STD | 0.002 | 0.013 | 0.071 |
| STD vs. DEVF | 50–600 | ɣ | 62–72 | RIGHT | STD > F | <0.001 | 0.019 | −0.047 |
| STD vs. DEVD | 350–550 | θ | 2–6 | LEFT | D > STD | 0.031 | −0.018 | 0.051 |
Fig. 4.
Temporal Spectral Evolution (TSE) grand average plots in the theta band (2–10 Hz) of the source waveforms at left auditory cortex (LAC) and right auditory cortex (RAC) sources. Plots are separated for group (FH− infants, first row; FH+ infants, second row) and stimulus type (standard, STD; frequency deviant, DEVF; duration deviant, DEVD). Time (from left to right) is presented in milliseconds (ms) and frequency (from bottom to top) in hertz (Hz). Green squares mark the within-group effects (DEVF and DEVD with respect to STD; as reported in Table 2). Red squares mark between-group effects, with * highlighting the group in which power values are significantly higher (as reported in Table 3). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 5.
Temporal Spectral Evolution (TSE) grand average plots in the gamma band (55–80 Hz) of the source waveforms at left auditory cortex (LAC) and right auditory cortex (RAC) sources. Plots are separated for group (FH− infants, first row; FH+ infants, second row) and stimulus type (standard, STD; frequency deviant, DEVF; duration deviant, DEVD). Time (from left to right) is presented in milliseconds (ms) and frequency (from bottom to top) in hertz (Hz). Green squares mark the within-group effects (DEVF vs. STD; as reported in Table 2). Red squares mark between-group effects, with * highlighting the group in which power values are significantly higher (as reported in Table 3). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2.1.2. Comparison between Groups
As a second step, power differences between the groups (FH− vs. FH+) were estimated for each stimulus type (STD, DEVF and DEVD) (Table 3). No significant differences in spectral power between the groups were found in a pre-stimulus period examined from −300 to 0 ms. Differences in spectral power for each condition revealed a distinctive pattern of oscillatory activity for each group: while FH− group displayed more power for all three stimuli in LAC (STD in theta and both deviants in gamma range) at earlier time frames, the FH+ presented more power in RAC (STD in theta and gamma and DEVF in theta) at later periods of processing (means, p values and cluster definitions where differences were found are presented in Table 3).
Table 3.
Power differences between groups: comparison between FH− and FH+ groups using cluster identification and permutation testing.
| Stimulus | Time (ms) | Frequency band | Frequency range (Hz) | Source | Direction | p-value | Mean FH− | Mean FH+ |
|---|---|---|---|---|---|---|---|---|
| STD | 50–200 | θ | 4–8 | LEFT | FH− > FH+ | <0.001 | 0.061 | 0.005 |
| STD | 250–450 | θ | 4–8 | RIGHT | FH+ > FH− | 0.032 | −0.002 | 0.059 |
| STD | 50–500 | ɣ | 63–68 | RIGHT | FH+ > FH− | <0.001 | −0.034 | 0.025 |
| DEVF | 450–600 | θ | 5–8 | RIGHT | FH+ > FH− | 0.031 | −0.004 | 0.078 |
| DEVF | 50–400 | ɣ | 61–68 | LEFT | FH− > FH+ | 0.001 | 0.012 | −0.048 |
| DEVD | 50–250 | ɣ | 59–63 | LEFT | FH− > FH+ | <0.001 | 0.012 | −0.051 |
3.2.2. Inter-trial phase locking (ITPL) measures
3.2.2.1. Paired comparisons within group
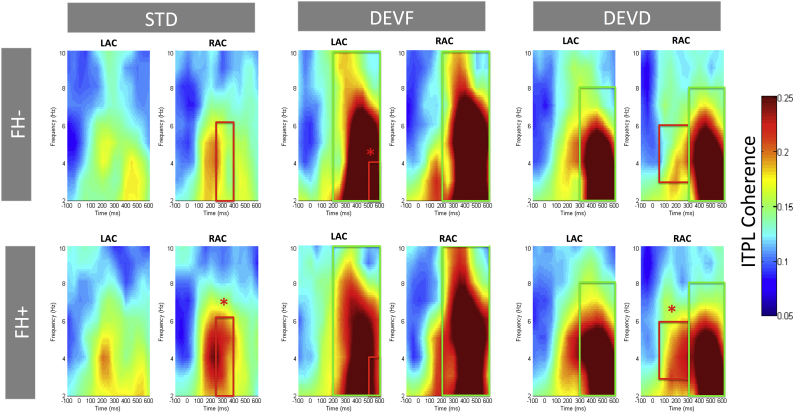
Differences in phase-coherence between both deviant stimuli (DEVF and DEVD) and the STD were estimated in the theta frequency range through paired comparisons within each group (FH− and FH+). In general, for both groups phase synchrony was higher for both deviant stimuli than the STD in both left and right auditory sources (ps <= 0.001, Table 4; Fig. 6)
Table 4.
Differences in phase-coherence (ITPL) between conditions in the FH− and FH+ group: Condition comparison using cluster identification and permutation testing.
| Stimulus comparison | Time (ms) | Frequency band | Frequency (Hz) | Source | Direction | p-value | Mean STD | Mean DEV |
|---|---|---|---|---|---|---|---|---|
| FH− group | ||||||||
| STD vs. DEVF | 200–600 | θ | 2–10 | RIGHT | F > STD | 0.001 | 0.142 | 0.243 |
| STD vs. DEVF | 200–600 | θ | 2–10 | LEFT | F > STD | <0.001 | 0.136 | 0.300 |
| STD vs. DEVD | 300–600 | θ | 2–8 | RIGHT | D > STD | 0.001 | 0.144 | 0.207 |
| STD vs. DEVD | 300–600 | θ | 2–8 | LEFT | D > STD | <0.001 | 0.147 | 0.213 |
| FH+ group | ||||||||
| STD vs. DEVF | 200–600 | θ | 2–10 | RIGHT | F > STD | <0.001 | 0.154 | 0.262 |
| STD vs. DEVF | 200–600 | θ | 2–10 | LEFT | F > STD | 0.001 | 0.141 | 0.227 |
| STD vs. DEVD | 300–600 | θ | 2–8 | RIGHT | D > STD | 0.001 | 0.169 | 0.197 |
| STD vs. DEVD | 300–600 | θ | 2–8 | LEFT | D > STD | <0.001 | 0.155 | 0.222 |
Fig. 6.
Inter-trial Phase Locking (ITPL) in the theta band (2–10 Hz) of the source waveforms at left (LAC) and right (RAC) auditory cortex sources. Plots are separated for group (FH− infants, first row; FH+ infants, second row) and stimulus type (standard, STD; frequency deviant, DEVF; duration deviant, DEVD). Time (from left to right) is presented in milliseconds (ms) and frequency (from bottom to top) in hertz (Hz). Green squares mark the within-group effects (DEVF and DEVD with respect to STD; as reported in Table 4). Red squares mark between-group effects, with * highlighting the group in which phase coherence values are significantly higher (as reported in Table 5). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2.2.2. Comparison between groups
Differences in phase coherence (ITPL) between the groups (FH− vs. FH+) were estimated in the theta frequency range for each stimulus type (STD, DEVF and DEVD) (Table 5). No significant differences between the groups were found in the pre-stimulus period (from −300 to 0 ms). Similar to what was found for power differences, the FH− group was characterized by higher ITPL values in the left auditory area, whereas the FH+ presented higher ITPL values in the right auditory areas for both the STD and the DEVD stimulus (means, p values and cluster definitions where differences were found are presented in Table 5.
Table 5.
Differences in phase-coherence (ITPL) between groups: Comparison between FH− and FH+ groups using cluster identification and permutation testing.
| Stimulus | Time (ms) | Frequency band | Frequency (Hz) | Source | Direction | p-value | Mean FH− | Mean FH− |
|---|---|---|---|---|---|---|---|---|
| STD | 250–400 | θ | 2–6 | RIGHT | FH+ > FH− | 0.033 | 0.163 | 0.207 |
| DEVF | 500–600 | θ | 2–4 | LEFT | FH− > FH+ | 0.049 | 0.327 | 0.268 |
| DEVD | 50–300 | θ | 3–6 | RIGHT | FH+ > FH− | 0.034 | 0.199 | 0.156 |
3.3. Associations between electrophysiological measures at 6 months and language abilities at 20 months of age
3.3.1. Language abilities at 20 months of age
Follow-up data were available for 49 subjects, 88% of the larger sample. The percentile score of the Language Development Survey (LDS) (Rescorla et al., 2014) relative to expressive vocabulary was calculated based on gender-specific norms. Since no differences emerged between groups in the expressive vocabulary percentile score (FH−, n = 26, M = 44.04, SD = 28.71; FH+, n = 23, M = 32.39, SD = 26.15), correlations were computed using the combined groups. Correlations within groups are mentioned in the text and also shown in the scatterplots for descriptive purposes.
3.3.2. Associations between amplitude and latency of the positive response at source level and language abilities
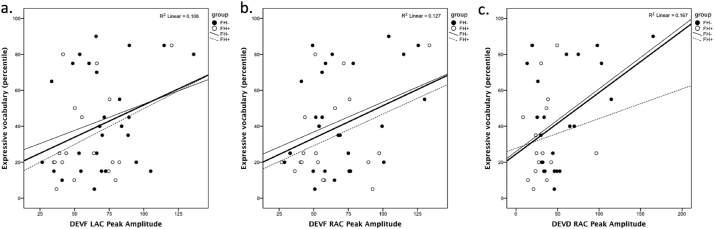
The measure of expressive vocabulary was correlated by means of Pearson's product moment correlations with measures of latency and amplitude of the peaks of the positive response separated by stimulus type and source (LAC, RAC). Only peak amplitude was significantly correlated with the expressive vocabulary outcome (Fig. 7). Specifically, higher amplitude of the positive response to DEVF stimulus, in both LAC and RAC sources, was associated with better expressive vocabulary at 20 months-of-age (LAC: r(45) =0.325, p = .029; RAC: r(45) =0.357, p = .016). In response to DEVD stimulus peak amplitude was positively associated with vocabulary in the RAC source (r(38) =0.409, p = .011). As seen in the scatterplots (Fig. 7), correlations within groups showed similar directions, with the exception of the correlation between peak amplitude for DEVD in RAC that correlated significantly with vocabulary only for FH− children (r(23) =0.441, p = .035).
Fig. 7.
Person product moment correlations between 6-month source ERP measures and 20-month expressive language (percentile score in the Language Development Survey) for FH− (black dots) and FH+ (white dots) infants. Correlations include amplitude of the peak of the positive response for (a) DEVF in the left auditory cortex (LAC) source, (b) DEVF in the right auditory cortex (RAC) source, and (c) DEVD in the RAC source. Bold regression lines represent the combined group, whereas thin continuous lines represent the FH− group and thin dashed lines represent the FH+ group.
3.3.3. Associations between TSE and ITPL measures and language abilities
Cluster permutation testing based on correlation analysis was applied in BESA Statistics v2.0 in order to test the association between expressive vocabulary at 20 months and the magnitude of TSE and ITPL measures. Specifically, the presence of spatially contiguous clusters of coherent r values was identified within the previously identified clusters of activity differentiating STD from DEV stimuli (see Table 2, Table 4). TSE and ITPL relative to the difference between responses evoked by deviant versus standard stimuli (DEVF-STD and DEVD-STD) were submitted to the analyses. The results of the correlations presented here were corrected for multiple comparisons of frequency and time points using permutation statistics.
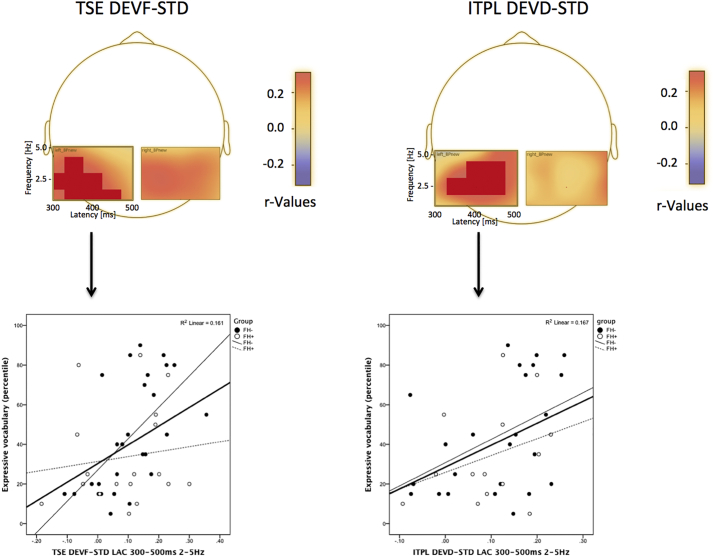
In the theta range, amount of spectral power in LAC during frequency change and of phase coherence in LAC during duration change were positively related to language abilities. Fig. 8 shows the emerging significant clusters associated with expressive vocabulary. The first cluster (Fig. 8a, p = .030) included TSE in the DEVF-STD condition (300–500 ms; 2–5 Hz – LAC source) whereas the second cluster (Fig. 8b, p = .048) included ITPL in the DEVD-STD condition (300–500 ms; 2–5 Hz – LAC source). As can be observed from the scatterplots (Fig. 8), correlations within groups resembled those seen in the combined groups. However, the first cluster correlated significantly with vocabulary only for children in the FH− group (p = .017).
Fig. 8.
Person product moment correlations between 6-month amount of spectral power in the left auditory cortex source during frequency (DEVF) and duration (DEVD) changes and 20-month expressive language (percentile score in the Language Development Survey). The above panel shows the emerging significant clusters associated with expressive vocabulary, including (a) Temporal Spectral Evolution (TSE) in LAC during frequency changes (DEVF-STD condition; 300–500 ms; 2–5 Hz) and (b) Inter-trial Phase Locking (ITPL) in LAC during duration change (DEVD-STD condition; 300–500 ms; 2–5 Hz).The below panel shows the relative scatterplots for FH− (black dots) and FH+ (white dots) infants. Bold regression lines represent the combined group, whereas thin continuous lines represent the FH− group and thin dashed lines represent the FH+ group.
4. Discussion
In this study, we used the same cohort of infants with and without familial risk for LLI reported in Cantiani et al. (2016) to examine more closely the neural mechanisms that support typical and atypical Rapid Auditory Processing (RAP) skills in infancy. Specifically, we examined the sources of the neural activity and the oscillatory dynamics characterizing the response to rapid presentation of stimuli changing in frequency and duration.
In both groups, the waveforms at the source level in response to the two deviant stimulus types were characterized by a large positive response peaking at approximately 440 ms for DEVF and at about 510 ms for DEVD. In the infant literature, this component has been traditionally referred to as the MisMatch Response, specifically when considering the peak in the difference waveform (e.g. DEV-STD) and thus is usually interpreted as reflecting a neural change detection process (e.g., Kushnerenko et al., 2002). In addition to this classical interpretation, this component might very well reflect the involuntary switching of attention that occurs whenever there is a sound change in the environment (e.g., Escera et al., 1998; Friedman et al., 2001; Escera and Corral, 2007). It should also be kept in mind that although the oddball paradigm in this study was presented in a passive fashion, at this time in development infants are recognizing and establishing cortical representations of their native speech sounds. Therefore, infants are more attentive to any acoustic change and may be well be scanning the auditory environment for even subtle changes that “could be language”.
We found that both groups of infants showed bilateral activation in auditory cortices, as suggested by a two-dipole model explaining around 98% of the variance and confirmed by a distributed source model (CLARA). At the level of neural oscillations, the acoustic discrimination process was also clearly reflected in both groups by the predicted enhancement of theta and gamma power and the increase in theta phase synchronization in response to deviants. Most importantly however, our results demonstrate that even at this early preverbal stage, left gamma power is already reduced in infants at risk for LLI and this reduction could provide an earlier biomarker for detection of RAP deficits in infancy.
4.1. Lateralization differences between groups
Although the overall neural pattern observed was similar in the two groups of infants, a number of significant differences emerged in the processing of both sound representation (standard stimulus) and discrimination (deviant stimuli). One of the most striking differences between groups was related to the lateralization of the oscillatory responses. When directly comparing the groups, FH− infants demonstrated a more left-lateralized pattern of oscillatory activity in auditory cortex when processing the rapidly presented non-speech auditory stimuli, whereas FH+ infants exhibited a more right-lateralized pattern. More specifically, when looking at the percent change of both evoked and induced amplitude of oscillatory activity related to stimulus presentation (TSE) (Hari et al., 1997; Tallon-Baudry et al., 1996; Tallon-Baudry and Bertrand, 1999), the FH− group generated greater left-lateralized theta power than the FH+ group in response to the STD stimuli and greater left-lateralized gamma power in response to both deviant stimuli. Conversely, the FH+ group generated greater right-lateralized theta power than the FH− group in response to the STD and DEVF stimuli and greater right-lateralized gamma power in response to STD stimulus. A similar lateralization pattern emerged when looking at inter-trial phase alignment (ITPL) in the theta frequency band (Tallon-Baudry et al., 1996; Tallon-Baudry and Bertrand, 1999). Whereas the FH− group displayed greater left-lateralized phase locking in response to DEVF stimulus, the FH+ group had greater right-lateralized phase locking in response to STD and DEVD stimuli.
To date, brain lateralization during auditory processing in infants at familial risk for LLI has been investigated by a number of ERP studies with dissimilar outcomes, mainly but not exclusively, showing reduced ERP responses in the left hemisphere and/or enhanced responses in the right hemisphere (Cantiani et al., 2016; Choudhury and Benasich, 2011; Friedrich et al., 2009; Guttorm et al., 2005; Leppänen et al., 2010; 2002, 1999; van Herten et al., 2008; van Leeuwen et al., 2007). A caveat to ERP studies is that responses are typically analyzed using low-pass filtered data that greatly reduces high gamma frequency content. Our results using time-frequency analysis and source localization techniques not only support the above-mentioned ERP results but also provide three aspects of information not available with standard ERP analyses: (1) measures of phase synchrony, (2) oscillatory power in both low and high frequency bands, and (3) a more accurate localization of the signal sources. In addition, by means of source localization we alleviate issues related to the choice of the reference electrode(s) that determines the recorded signals and might strongly affect the waveforms seen at the scalp level. By performing time-frequency analyses in source space, we should have eliminated the effect of the specific reference selected (in our case the average of all electrodes) as the 2-dipole montage used to covert the raw scalp data to source space works independently of the reference used for collection of the ERPs.
Our findings of a left-lateralized pattern of oscillatory activity in auditory cortex in FH− infants vs. a right-lateralized pattern in FH+ infants are consistent with studies showing that older children with LLI have a disrupted pattern of left-sided brain lateralization for language (see Bishop, 2013 for a review). While our study does not examine language processing per se, we examined non-speech stimuli characterized by rapidly changing successive acoustic cues, thus reflecting the spectrotemporal characteristics of speech. Processing of such spectrotemporally modulated non-speech stimuli have been shown to robustly predict later language outcomes (Benasich et al., 2006; Choudhury and Benasich, 2011). Based on the recent views proposing early left hemisphere specialization for temporal processing vs. right hemisphere specialization for spectral processing (Abrams et al., 2006; Dehaene-Lambertz, 2017; Minagawa-Kawai et al., 2011a), we expected to see enhanced processing for our stimuli in left auditory areas. With similar stimuli, studies using fMRI and source localization of EEG/ERPs techniques have revealed functional alterations in the left hemisphere in children with developmental dyslexia and/or in pre-reading children with a familial risk for dyslexia (Gaab et al., 2007; Khan et al., 2011; Raschle et al., 2013). Although results from neuroimaging studies of participants with specific language impairment are inconsistent (Dibbets et al., 2006; Hugdahl et al., 2004; Weismer et al., 2005), a recent fMRI study (de Guibert et al., 2011) examined a specific subgroup of children with LI and reported several significant findings, which included absence of left lateralization across all core language regions and a right hyperactivation in the anterior insula and the adjacent inferior frontal gyrus, suggesting a compensatory mechanism for altered structural function. The present study adds to the literature by demonstrating at the oscillatory level that compared to controls, 6-month-old infants at risk for LLI already show lateralization differences when processing auditory signals, including reduced activation in left auditory cortex and larger activation in right auditory cortex.
The left>right asymmetry for processing rapid frequency changes characterizing FH− but not FH+ infants, appears to reflect a more typical pattern of processing rapidly presented sounds. For example, evidence to support our claim comes from structural studies in neonates showing that, similar to adults (Geschwind and Levitsky, 1968), the left planum temporale is larger than the right (Witelson and Pallie, 1973). However, the functional maturation of lateralization patterns seems to be driven not only by anatomical differences and developmental epochs, but also by the auditory environment (Minagawa-Kawai et al., 2011a). For instance, a recent study found evidence that early interactive acoustic experience with temporally modulated non-speech stimuli can promote enhanced auditory processing efficiency (Benasich et al., 2014), as reflected in larger and faster P2 peaks, faster change discrimination peak (N2*) and overall more complex waveforms (more often seen in typically developing older children). Interestingly, a recent follow-up study hypothesized that these changes might be supported by more mature left-lateralized patterns of oscillatory activity for tones (Musacchia et al., 2017) and speech (Ortiz-Mantilla et al., 2019). In the study by Musacchia et al. (2017) the shift toward left-lateralized processing was particularly evident in the gamma band, in accordance with studies reporting enhanced oscillatory activity in this frequency range linked to perceptual specialization for native phonemes at 6 months-of-age (Ortiz-Mantilla et al., 2013), with a more robust effect seen at 12 months-of-age in the left hemisphere (Ortiz-Mantilla et al., 2016).
In the present study, the hemispheric differences between FH− and FH+ groups were not limited to the gamma range but involved also the theta range. However, it is important to note that although stimulus discrimination has been demonstrated to involve changes in oscillatory dynamics in both theta (Ko et al., 2012) and gamma ranges, the fast oscillatory rate characteristic of gamma oscillations makes gamma more suited for processing acoustic information that varies in the tens of milliseconds range including discrimination of the segmental sublexical information contained in phonemes (Giraud and Poeppel, 2012; Poeppel et al., 2008). The left auditory cortex in particular, has been more often related to rapid temporal processing (Zatorre, 2001) and discrete phonological representations (Phillips et al., 2000). Our results showing that compared to controls, FH+ infants generate lower amounts of gamma power in left auditory cortex, clearly suggest difficulties in processing rapidly presented information.
4.2. Source localization of ERP generators
The presence of between-group differences both in the amount of spectral power and phase synchrony seen here suggests that oscillatory dynamics in FH+ infants may be disrupted in ways that do not simply reflect a developmental delay. Although not specifically investigated in the present study, differences between FH+ and FH− infants in the oscillatory dynamics could be due to anatomical differences that diverge from a typical developmental timeline. Our results showing group differences in the source localization of ERP generators seem to point in this direction.
These differences specifically concern the fact that both RAC and LAC sources were more superior for FH+ than for FH− infants. Although we recognize that the exact source locations must be regarded cautiously given the relatively poor spatial resolution of EEG, this is the first evidence of spatial differences in the neural sources activated during RAP in infants at high-risk for LLI. Previous studies revealed that the brain responses of a sub-group of dyslexic children originated from a more posterior area of the right temporal cortex as compared to the responses of the other participants (Lohvansuu et al., 2014). Anatomical studies have provided evidence of cortical anomalies in the brains of dyslexic individuals, characterized by the presence of neuronal ectopias, micropolygyria and architectonic dysplasias, located mainly but not exclusively, in the left hemisphere, and of differences in symmetry of the planum temporale, which deviates from the standard asymmetric pattern observed in the planum (Galaburda et al., 1985). If present, these structural differences might account for group differences in dipole location seen in the present study. In addition to differences between groups, source analyses revealed that the right source dipole was more superior than the left dipole, similar to previously reported responses to non-speech stimuli in 4- and 6-month-old infants (Hämäläinen et al., 2011; Musacchia et al., 2013) and in response to syllables in 12-month-olds (Ortiz-Mantilla et al., 2016). As mentioned, asymmetry in dipole location has been consistently observed over infancy and aligns with reported anatomical differences between left and right temporal speech-related areas (Geschwind and Levitsky, 1968; Witelson and Pallie, 1973).
4.3. Associations of ERP/EEG measures with the linguistic outcome
A further discussion topic concerns the associations observed among source components, oscillatory activity and linguistic outcome (expressive vocabulary) at 20 months. Consistent correlational results were found for the amplitude of the positive response at the source level and the magnitude of TSE and ITPL measures. Overall, infants with higher peak amplitude and higher amounts of spectral power and phase coherence at 6 months demonstrated richer expressive vocabulary at 20 months-of-age. These results support previous research showing that infant ERPs are predictive of linguistic skills at pre-school ages (Benasich et al., 2006; Cantiani et al., 2016; Choudhury and Benasich, 2011; Guttorm et al., 2005; Riva et al., 2018a; van Zuijen et al., 2012). To our knowledge, the only studies that have investigated the impact of cortical measures other than the traditional scalp ERP on language development were conducted by Piazza and collaborators (Piazza, 2016; Piazza et al., 2016) using techniques including independent component analysis (ICA) and Adaptive Autoregressive identification with spectral power decomposition. In these studies, statistically robust correlational results were reported for the amplitude of the positive response, at both scalp (channel F6) and cortical levels (mid-cingulate source) (rs range 0.51–0.68 and 0.58–0.77, respectively), whereas weaker correlations were found between language ability at 20 months and the delta/theta power peak difference at the scalp (rs = 0.49) (Piazza et al., 2014). These findings suggest that the oscillatory mechanisms thought to be involved in ERP generation (Klimesch et al., 2007; Makeig, 2002), might be correlated only indirectly with language outcomes. When all the results were combined in a regression model, Piazza et al. (2016) found that the greatest amount of variance in 20-month linguistic outcome was explained by ERP measures computed at the source level, suggesting that these parameters are more sensitive than other EEG measures in the prediction of language abilities.
The present study provides further support for the idea that analyses of neural activity at the source level (even with different methods of quantification) may be more highly related to clinical measures than the mixture of brain and non-brain signals that usually converge in standard scalp EEG recordings (Lenartowicz et al., 2014; Loo et al., 2015; Rissling et al., 2014). In our previous report with the same sample, we found that at the scalp level the amplitude of the positive response to DEVF in the right hemisphere (see Cantiani et al., 2016) explained 17% of variance of expressive vocabulary at 20 months (adjusted r2). Accordingly, the same percentage of variance (17%, adjusted r2) was explained when including in the model the amplitude of the source peak (see paragraph 3.3.2), whereas a higher percentage of the variance (29% adjusted r2) was explained by TSE and ITPL measures (see paragraph 3.3.3). Finally, when all the variables significantly correlated with the linguistic outcome (amplitude of the positive response at both the scalp and the source level and TSE/ITPL measures) were combined in a regression model, 33% of variance in 20-month linguistic outcome was explained. Taken together, these results provide further evidence that at least in our sample, time-frequency measures computed at the source level may produce better correlations to language outcome than the scalp EEG channel measures alone.
4.4. The processing of duration in the right auditory cortex
The processing of DEVD seems to differ in a number of ways from the processing of the STD and DEVF stimuli. In particular, three convergent pieces of evidence point toward the specificity of DEVD processing occurring primarily in right auditory cortex (RAC). The first evidence concerns the oscillatory pattern in FH+ infants. Although processing of DEVD generated more power (TSE) than the STD in LAC, no power differences emerged in RAC when DEVD was compared to STD stimulus in the FH+ group (Table 2). Second, the amplitude of the RAC source peak was significantly higher in the FH− group compared to the FH+ group (Table 1). Third, the correlational pattern between the amplitude of the source peak and later language skills shows that whereas the amplitude of the positive response to DEVF stimulus in both LAC and RAC correlates with expressive language, in response to DEVD only the amplitude in RAC correlates with language (see Section 3.3.2). Changes in sound duration, and specifically a change consisting of a longer-than-expected sound (200 ms instead of 70 ms) could be interpreted as a slow spectral modulation, that is expected to predominantly activate the right hemisphere (Poeppel et al., 2008; Telkemeyer et al., 2011). In particular, as already highlighted in our previous work (Cantiani et al., 2016), changes in sound duration seem to produce a strong irregularity of the rhythm of sound presentation. It might be hypothesized that this rhythmatic irregularity of the presentation requires a more substantial activation of the right hemisphere, and that could be significantly more challenging for the high-risk FH+ group.
5. Conclusion
In the present study, data from a cohort of infants with and without familiar risk for LLI were analyzed to examine the sources of neural activity and the oscillatory dynamics supporting typical and atypical Rapid Auditory Processing (RAP) skills in infancy. Overall, the present study has demonstrated that FH+ infants show significantly less activation in left auditory cortex and greater activation in right auditory cortex, as compared to FH− controls. In addition to the previous ERP results, our study explores oscillatory measures of neural synchrony that have been proposed to play a mechanistic role in RAP skills. FH− infants generated higher amounts of early left gamma power than their FH+ controls during discrimination of both deviants. Since oscillations in the gamma band are known to be critical during sensory/perceptual processing, reduced power in this specific frequency band might underlie RAP difficulties and therefore provide a potential biomarker for early identification of infants at high-risk for LLI deficits. Furthermore, measures of amplitude at the source level as well as the magnitude of power and phase synchrony were found to be associated with expressive vocabulary at 20 months, thus supporting the predictive role of early RAP skills on later language development and importantly showing that combining these measures increases prediction.
A note of caution should be raised concerning the relative heterogeneity of our sample of at-risk infants, defined as “at-risk for LLI” and thus including infants with a first-degree relative with either language impairment, reading difficulties or both disorders. Although it is clear that language and reading (dis)abilities are not etiologically distinct (Plomin and Kovas, 2005; Hohnen and Stevenson, 1999; Bishop, 2001), and similarities can be assumed at both the behavioral and the neural level, it should be noted that to date the specific electrophysiological patterns characterizing infants at-risk for language impairment versus infants at-risk for reading disorders have never been directly investigated. Further studies are needed to address this issue in detail in order to delineate potential specific and perhaps alternate profiles. Steps in this direction will allow more accurate identification of infants at highest risk for LLI, and facilitate the design of targeted strategies to implement early interventions and rehabilitation techniques that enable development of more efficient RAP skills in order to prevent and/or ameliorate LLI.
The following are the supplementary data related to this article.
Supplementary Fig. 1.
Individual source locations superimposed on the schematic head (sagittal, transverse and coronal views). Plots are separated for groups (FH− and FH+ infants) and stimulus type (frequency deviant, DEVF; duration deviant, DEVD).
Acknowledgement
The authors wish to thank the nursing and clinical staff of the Department of Gynecology & Obstetrics of the Manzoni Hospital of Lecco and of the Hospital of Desio and Vimercate (branch of Carate Brianza). We are also grateful to Giulia Melesi and Giulia Mornati for their help in data collection and analyses. Finally, special thanks go to all infants and their parents participating in this study. This study was supported by the Italian Ministry of Health (Ricerca Corrente "2016, 2017, 2018, 2019" to dr "Chiara Cantiani"; Ricerca Finalizzata "NET-2013-02355263-2" and by the “Fondazione Banca del Monte di Lombardia” [Progetto Professionalità Ivano Becchi].
References
- Abrams D.A., Nicol T., Zecker S.G., Kraus N. Auditory brainstem timing predicts cerebral asymmetry for speech. J. Neurosci. 2006;26:11131–11137. doi: 10.1523/JNEUROSCI.2744-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrams D.A., Nicol T., Zecker S., Kraus N. Abnormal cortical processing of the syllable rate of speech in poor readers. J. Neurosci. 2009;29:7686–7693. doi: 10.1523/JNEUROSCI.5242-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht R., Suchodoletz W., Uwer R. The development of auditory evoked dipole source activity from childhood to adulthood. Clin. Neurophysiol. 2000;111:2268–2276. doi: 10.1016/s1388-2457(00)00464-8. [DOI] [PubMed] [Google Scholar]
- Aslin R.N. Discrimination of frequency transitions by human infants. J. Acoust. Soc. Am. 1989;86:582–590. doi: 10.1121/1.398237. [DOI] [PubMed] [Google Scholar]
- Banai K., Nicol T., Zecker S.G., Kraus N. Brainstem timing: implications for cortical processing and literacy. J. Neurosci. 2005;25:9850–9857. doi: 10.1523/JNEUROSCI.2373-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Başar E., Başar-Eroglu C., Karakaş S., Schürmann M. Gamma, alpha, delta, and theta oscillations govern cognitive processes. Int. J. Psychophysiol. 2001;39:241–248. doi: 10.1016/s0167-8760(00)00145-8. [DOI] [PubMed] [Google Scholar]
- Bayley N. 3rd ed. Giunti O.S; Firenze: 2006. Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III) [Google Scholar]
- Belin P., Zilbovicius M., Crozier S., Thivard L., Fontaine A., Masure M.C., Samson Y. Lateralization of speech and auditory temporal processing. J. Cogn. Neurosci. 1998;10:536–540. doi: 10.1162/089892998562834. [DOI] [PubMed] [Google Scholar]
- Benasich A.A., Tallal P. Infant discrimination of rapid auditory cues predicts later language impairment. Behav. Brain Res. 2002;136:31–49. doi: 10.1016/s0166-4328(02)00098-0. [DOI] [PubMed] [Google Scholar]
- Benasich A.A., Thomas J.J., Choudhury N.A., Leppänen P.H.T. The importance of rapid auditory processing abilities to early language development: evidence from converging methodologies. Dev. Psychobiol. 2002;40:278–292. doi: 10.1002/dev.10032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benasich A.A., Choudhury N., Friedman J.T., Realpe-Bonilla T., Chojnowska C., Gou Z. The infant as a prelinguistic model for language learning impairments: predicting from event-related potentials to behavior. Neuropsychologia. 2006;44:396–411. doi: 10.1016/j.neuropsychologia.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benasich A.A., Choudhury N.A., Realpe-Bonilla T., Roesler C.P. Plasticity in developing brain: active auditory exposure impacts prelinguistic acoustic mapping. J. Neurosci. 2014;34:13349–13363. doi: 10.1523/JNEUROSCI.0972-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop G.H. Cyclic changes in excitability of the optic pathway of the rabbit. Am. J. Phys. 1933;103:2013–2224. [Google Scholar]
- Bishop D.V. Genetic influences on language impairment and literacy problems in children: same or different? J. Child Psychol. Psychiatry. 2001;42(2):189–198. [PubMed] [Google Scholar]
- Bishop D.V. Cerebral asymmetry and language development: cause, correlate, or consequence? Science. 2013;340:1230531. doi: 10.1126/science.1230531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D.V.M., Snowling M.J. Developmental dyslexia and specific language impairment: same or different? Psychol. Bull. 2004;130:858–886. doi: 10.1037/0033-2909.130.6.858. [DOI] [PubMed] [Google Scholar]
- Bishop D.V., Hardiman M.J., Barry J.G. Lower-frequency event-related desynchronization: a signature of late mismatch responses to sounds, which is reduced or absent in children with specific language impairment. J. Neurosci. 2010;30:15578–15584. doi: 10.1523/JNEUROSCI.2217-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D.V., Hardiman M.J., Barry J.G. Is auditory discrimination mature by middle childhood? A study using time-frequency analysis of mismatch responses from 7 years to adulthood. Dev. Sci. 2011;14:402–416. doi: 10.1111/j.1467-7687.2010.00990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosseler A.N., Taulu S., Pihko E., Mäkelä J.P., Imada T., Ahonen A., Kuhl P.K. Theta brain rhythms index perceptual narrowing in infant speech perception. Front. Psychol. 2013;4:690. doi: 10.3389/fpsyg.2013.00690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G., Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Cantiani C., Lorusso M.L., Valnegri C., Molteni M. Perception of non-verbal auditory stimuli in Italian dyslexic children. Dev. Neuropsychol. 2010;35:115–123. doi: 10.1080/87565640903335955. [DOI] [PubMed] [Google Scholar]
- Cantiani C., Riva V., Piazza C., Bettoni R., Molteni M., Choudhury N., Marino C., Benasich A.A. Auditory discrimination predicts linguistic outcome in Italian infants with and without familial risk for language learning impairment. Dev. Cogn. Neurosci. 2016;20:23–34. doi: 10.1016/j.dcn.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury N., Benasich A.A. Maturation of auditory evoked potentials from 6 to 48 months: prediction to 3 and 4 year language and cognitive abilities. Clin. Neurophysiol. 2011;122:320–338. doi: 10.1016/j.clinph.2010.05.035. [DOI] [PubMed] [Google Scholar]
- Choudhury N., Leppanen P.H.T., Leevers H.J., Benasich A.A. Infant information processing and family history of specific language impairment: converging evidence for RAP deficits from two paradigms. Dev. Sci. 2007;10:213–236. doi: 10.1111/j.1467-7687.2007.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guibert C., Maumet C., Jannin P., Ferré J.-C., Tréguier C., Barillot C., Le Rumeur E., Allaire C., Biraben A. Abnormal functional lateralization and activity of language brain areas in typical specific language impairment (developmental dysphasia) Brain. 2011;134:3044–3058. doi: 10.1093/brain/awr141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene-Lambertz G. The human infant brain: a neural architecture able to learn language. Psychon. Bull. Rev. 2017;24:48–55. doi: 10.3758/s13423-016-1156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene-Lambertz G., Baillet S. A phonological representation in the infant brain. Neuroreport. 1998;9:1885–1888. doi: 10.1097/00001756-199806010-00040. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G., Dehaene S., Hertz-Pannier L. Functional neuroimaging of speech perception in infants. Science. 2002;298:2013–2015. doi: 10.1126/science.1077066. [DOI] [PubMed] [Google Scholar]
- Delorme A., Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Dibbets P., Bakker K., Jolles J. Functional MRI of task switching in children with specific language impairment (SLI) Neurocase. 2006;12:71–79. doi: 10.1080/13554790500507032. [DOI] [PubMed] [Google Scholar]
- Escera C., Corral M.J. Role of mismatch negativity and novelty-P3 in involuntary auditory attention. J. Psychophysiol. 2007;21:251–264. [Google Scholar]
- Escera C., Alho K., Winkler I., Näätänen R. Neural mechanisms of involuntary attention to acoustic novelty and change. J. Cogn. Neurosci. 1998;10:590–604. doi: 10.1162/089892998562997. [DOI] [PubMed] [Google Scholar]
- Esposito A., Di Benedetto M.G. Acoustical and perceptual study of gemination in Italian stops. J. Acoust. Soc. Am. 1999;106:2051–2062. doi: 10.1121/1.428056. [DOI] [PubMed] [Google Scholar]
- Fenson L., Dale P.S., Reznick J.S., Thal D., Bates E., Hartung J.P., Pethick S., Reilly J.S. Paul H. Brokes Publishing Co; Baltimore: 1993. The MacArthur Communicative Development Inventories: User's Guide and Technical Manual. [Google Scholar]
- Friedman D., Cycowicz Y.M., Gaeta H. The novelty P3: an event-related potential (ERP) sign of the brain's evaluating of novelty. Neurosci. Biobehav. Rev. 2001;25:355–373. doi: 10.1016/s0149-7634(01)00019-7. [DOI] [PubMed] [Google Scholar]
- Friedrich M., Weber C., Friederici A.D. Electrophysiological evidence for delayed mismatch response in infants at-risk for specific language impairment. Psychophysiology. 2004;41:772–782. doi: 10.1111/j.1469-8986.2004.00202.x. [DOI] [PubMed] [Google Scholar]
- Friedrich M., Herold B., Friederici A.D. ERP correlates of processing native and non-native language word stress in infants with different language outcomes. Cortex. 2009;45:662–676. doi: 10.1016/j.cortex.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Fries P., Schröder J.-H., Roelfsema P.R., Singer W., Engel A.K. Oscillatory neuronal synchronization in primary visual cortex as a correlate of stimulus selection. J. Neurosci. 2002;22:3739–3754. doi: 10.1523/JNEUROSCI.22-09-03739.2002. https://doi.org/20026318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaab N., Gabrieli J.D., Deutsch G.K., Tallal P., Temple E. Neural correlates of rapid auditory processing are disrupted in children with developmental dyslexia and ameliorated with training: an fMRI study. Restor. Neurol. Neurosci. 2007;25:295–310. [PubMed] [Google Scholar]
- Galaburda A.M., Sherman G.F., Rosen G.D., Aboitiz F., Geschwind N. Developmental dyslexia: four consecutive patients with cortical anomalies. Ann. Neurol. 1985;18:222–233. doi: 10.1002/ana.410180210. [DOI] [PubMed] [Google Scholar]
- Geschwind N., Levitsky W. Human brain: left-right asymmetries in temporal speech region. Science. 1968;161:186–187. doi: 10.1126/science.161.3837.186. [DOI] [PubMed] [Google Scholar]
- Giraud A.L., Poeppel D. Cortical oscillations and speech processing: emerging computational principles and operations. Nat. Neurosci. 2012;15:511–517. doi: 10.1038/nn.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttorm T.K., Leppänen P.H., Richardson U., Lyytinen H. Event-related potentials and consonant differentiation in newborns with familial risk for dyslexia. J. Learn. Disabil. 2001;34:534–544. doi: 10.1177/002221940103400606. [DOI] [PubMed] [Google Scholar]
- Guttorm T.K., Leppänen P.H.T., Poikkeus A.M., Eklund K.M., Lyytinen P., Lyytinen H. Brain event-related potentials (ERPs) measured at birth predict later language development in children with and without familial risk for dyslexia. Cortex. 2005;41:291–303. doi: 10.1016/s0010-9452(08)70267-3. [DOI] [PubMed] [Google Scholar]
- Guttorm T.K., Leppänen P.H.T., Hämäläinen J.A., Eklund K.M., Lyytinen H.J. Newborn event-related potentials predict poorer pre-reading skills in children at risk for dyslexia. J. Learn. Disabil. 2010;43:391–401. doi: 10.1177/0022219409345005. [DOI] [PubMed] [Google Scholar]
- Halliday L.F., Barry J.G., Hardiman M.J., Bishop D.V. Late, not early mismatch responses to changes in frequency are reduced or deviant in children with dyslexia: an event-related potential study. J. Neurodev. Disord. 2014;6:21. doi: 10.1186/1866-1955-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämäläinen J.A., Ortiz-Mantilla S., Benasich A.A. Source localization of event-related potentials to pitch change mapped onto age-appropriate MRIs at 6 months of age. Neuroimage. 2011;54:1910–1918. doi: 10.1016/j.neuroimage.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Hämäläinen J.A., Salminen H.K., Leppänen P.H.T. Basic auditory processing deficits in dyslexia: systematic review of the behavioral and event-related potential/field evidence. J. Learn. Disabil. 2013;46:413–427. doi: 10.1177/0022219411436213. [DOI] [PubMed] [Google Scholar]
- Hari R., Salmelin R., Mäkelä J.P., Salenius S., Helle M. Magnetoencephalographic cortical rhythms. Int. J. Psychophysiol. 1997;26:51–62. doi: 10.1016/s0167-8760(97)00755-1. [DOI] [PubMed] [Google Scholar]
- Hayiou-Thomas M.E. Genetic and environmental influences on early speech, language and literacy development. J. Commun. Disord. 2008;41:397–408. doi: 10.1016/j.jcomdis.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim S., Keil A. Effects of classical conditioning on identification and cortical processing of speech syllables. Exp. Brain Res. 2006;175:411–424. doi: 10.1007/s00221-006-0560-1. [DOI] [PubMed] [Google Scholar]
- Heim S., Friedman J.T., Keil A., Benasich A.A. Reduced sensory oscillatory activity during rapid auditory processing as a correlate of language-learning impairment. J. Neurolinguistics. 2011;24:539–555. doi: 10.1016/j.jneuroling.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim S., Keil A., Choudhury N., Friedman J.T., Benasich A.A. Early gamma oscillations during rapid auditory processing in children with a language-learning impairment: changes in neural mass activity after training. Neuropsychologia. 2013;51:990–1001. doi: 10.1016/j.neuropsychologia.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoechstetter K., Berg P., Scherg M. 2010. BESA Research Tutorial 4: Distributed Source Imaging. BESA Res. Tutor. 1–29. [Google Scholar]
- Hohnen B., Stevenson J. The structure of genetic influences on general cognitive, language, phonological, and reading abilities. Dev. Psychol. 1999;35(2):590–603. doi: 10.1037//0012-1649.35.2.590. [DOI] [PubMed] [Google Scholar]
- Homae F., Watanabe H., Nakano T., Taga G. Large-scale brain networks underlying language acquisition in early infancy. Front. Psychol. 2011;2:93. doi: 10.3389/fpsyg.2011.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugdahl K., Gundersen H., Brekke C., Thomsen T., Rimol L.M., Ersland L., Niemi J. FMRI brain activation in a finnish family with specific language impairment compared with a normal control group. J. Speech. Lang. Hear. Res. 2004;47:162–172. doi: 10.1044/1092-4388(2004/014). [DOI] [PubMed] [Google Scholar]
- Isler J.R., Tarullo A.R., Grieve P.G., Housman E., Kaku M., Stark R.I., Fifer W.P. Toward an electrocortical biomarker of cognition for newborn infants. Dev. Sci. 2012;15:260–271. doi: 10.1111/j.1467-7687.2011.01122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamison H.L., Watkins K.E., Bishop D.V., Matthews P.M. Hemispheric specialization for processing auditory nonspeech stimuli. Cereb. Cortex. 2006;16:1266–1275. doi: 10.1093/cercor/bhj068. 10.1093/cercor/bhj068. [DOI] [PubMed] [Google Scholar]
- Judica A., De Luca M. IRCCS Fondazione Santa Lucia; Roma: 2005. Prova di velocità di lettura di brani per la scuola media superiore. [Google Scholar]
- Kaas J.H., Hackett T.A. How the visual projection map instructs the auditory computational map. J. Comp. Neurol. 2000;421:143–145. doi: 10.1002/(sici)1096-9861(20000529)421:2<143::aid-cne1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Khan A., Hämäläinen J.A., Leppänen P.H.T., Lyytinen H. Auditory event-related potentials show altered hemispheric responses in dyslexia. Neurosci. Lett. 2011;498:127–132. doi: 10.1016/j.neulet.2011.04.074. [DOI] [PubMed] [Google Scholar]
- Klimesch W., Sauseng P., Hanslmayr S., Gruber W., Freunberger R. Event-related phase reorganization may explain evoked neural dynamics. Neurosci. Biobehav. Rev. 2007;31:1003–1016. doi: 10.1016/j.neubiorev.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Ko D., Kwon S., Lee G., Im C.H., Kim K.H., Jung K.-Y. Theta oscillation related to the auditory discrimination process in mismatch negativity: oddball versus control paradigm. J. Clin. Neurol. 2012;8:35. doi: 10.3988/jcn.2012.8.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnerenko E., Ceponiene R., Balan P., Fellman V., Huotilaine M., Näätänen R. Maturation of the auditory event-related potentials during the first year of life. Neuroreport. 2002;13:47–51. doi: 10.1097/00001756-200201210-00014. [DOI] [PubMed] [Google Scholar]
- Lenartowicz A., Delorme A., Walshaw P.D., Cho A.L., Bilder R.M., McGough J.J., McCracken J.T., Makeig S., Loo S.K. Electroencephalography correlates of spatial working memory deficits in attention-deficit/hyperactivity disorder: vigilance, encoding, and maintenance. J. Neurosci. 2014;34:1171–1182. doi: 10.1523/JNEUROSCI.1765-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenneberg E.H. MIT Press; Cambridge: 1966. New Directions in the Study of Language. [Google Scholar]
- Leppänen P.H.T., Pihko P., Eklund K.M., Lyytinen H. Cortical responses of infants with and without a genetic risk for dyslexia: II. Group effects. Neuroreport. 1999;10:969–973. doi: 10.1097/00001756-199904060-00014. [DOI] [PubMed] [Google Scholar]
- Leppänen P.H.T., Richardson U., Pihko E., Eklund K.M., Guttorm T.K., Aro M., Lyytinen H. Brain responses to changes in speech sound durations differ between infants with and without familial risk for dyslexia. Dev. Neuropsychol. 2002;22:407–422. doi: 10.1207/S15326942dn2201_4. [DOI] [PubMed] [Google Scholar]
- Leppänen P.H.T., Hämäläinen J.A., Salminen H.K., Eklund K.M., Guttorm T.K., Lohvansuu K., Puolakanaho A., Lyytinen H. Newborn brain event-related potentials revealing atypical processing of sound frequency and the subsequent association with later literacy skills in children with familial dyslexia. Cortex. 2010;46:1362–1376. doi: 10.1016/j.cortex.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Lippé S., Martinez-montes E., Arcand C., Lassonde M. Electrophysiological study of auditory development. Neuroscience. 2009;164:1108–1118. doi: 10.1016/j.neuroscience.2009.07.066. [DOI] [PubMed] [Google Scholar]
- Lohvansuu K., Hämäläinen J.A., Tanskanen A., Ervast L., Heikkinen E., Lyytinen H., Leppänen P.H.T. Enhancement of brain event-related potentials to speech sounds is associated with compensated reading skills in dyslexic children with familial risk for dyslexia. Int. J. Psychophysiol. 2014;94:298–310. doi: 10.1016/j.ijpsycho.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Lohvansuu K., Hämäläinen J.A., Ervast L., Lyytinen H., Leppänen P.H.T. Longitudinal interactions between brain and cognitive measures on reading development from 6 months to 14 years. Neuropsychologia. 2018;108:6–12. doi: 10.1016/j.neuropsychologia.2017.11.018. [DOI] [PubMed] [Google Scholar]
- Loo S.K., Lenartowicz A., Makeig S. Research review: use of EEG biomarkers in child psychiatry research - current state and future directions. J. Child Psychol. Psychiatry. 2015 doi: 10.1111/jcpp.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Calderon J., Luck S.J. ERPLAB: an open-source toolbox for the analysis of event-related potentials. Front. Hum. Neurosci. 2014;8:213. doi: 10.3389/fnhum.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudzadeh M., Dehaene-Lambertz G., Fournier M., Kongolo G., Goudjil S., Dubois J., Grebe R., Wallois F. Syllabic discrimination in premature human infants prior to complete formation of cortical layers. Proc. Natl. Acad. Sci. U. S. A. 2013;110:4846–4851. doi: 10.1073/pnas.1212220110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeig S. Auditory event-related dynamics of the EEG spectrum and effects of exposure to tones. Electroencephalogr. Clin. Neurophysiol. 1993;86:283–293. doi: 10.1016/0013-4694(93)90110-h. [DOI] [PubMed] [Google Scholar]
- Makeig S. Response: event-related brain dynamics -- unifying brain electrophysiology. Trends Neurosci. 2002;25:390. doi: 10.1016/s0166-2236(02)02198-7. S0166-2236(02)02198-7. [DOI] [PubMed] [Google Scholar]
- Maris E., Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Minagawa-Kawai Y., Cristià A., Dupoux E. Cerebral lateralization and early speech acquisition: a developmental scenario. Dev. Cogn. Neurosci. 2011;1:217–232. doi: 10.1016/j.dcn.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa-Kawai Y., Cristià A., Vendelin I., Cabrol D., Dupoux E. Assessing signal-driven mechanisms in neonates: brain responses to temporally and spectrally different sounds. Front. Psychol. 2011;2:135. doi: 10.3389/fpsyg.2011.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa-Kawai Y., van der Lely H., Ramus F., Sato Y., Mazuka R., Dupoux E. Optical brain imaging reveals general auditory and language-specific processing in early infant development. Cereb. Cortex. 2011;21:254–261. doi: 10.1093/cercor/bhq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molfese V.J., Molfese D.L., Modgline A.A. Newborn and preschool predictors of second-grade reading scores: an evaluation of categorical and continuous scores. J. Learn. Disabil. 2001;34:545–554. doi: 10.1177/002221940103400607. [DOI] [PubMed] [Google Scholar]
- Morr M.L., Shafer V.L., Kreuzer J.A., Kurtzberg D. Maturation of mismatch negativity in typically developing infants and preschool children. Ear Hear. 2002;23:118–136. doi: 10.1097/00003446-200204000-00005. [DOI] [PubMed] [Google Scholar]
- Musacchia G., Choudhury N.A., Ortiz-Mantilla S., Realpe-Bonilla T., Roesler C.P., Benasich A.A. Oscillatory support for rapid frequency change processing in infants. Neuropsychologia. 2013;51:2812–2824. doi: 10.1016/j.neuropsychologia.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Musacchia G., Ortiz-Mantilla S., Realpe-Bonilla T., Roesler C.P., Benasich A.A. Infant auditory processing and event-related brain oscillations. J. Vis. Exp. 2015 doi: 10.3791/52420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchia G., Ortiz-Mantilla S., Choudhury N., Realpe-Bonilla T., Roesler C., Benasich A.A. Active auditory experience in infancy promotes brain plasticity in Theta and Gamma oscillations. Dev. Cogn. Neurosci. 2017;26:9–19. doi: 10.1016/j.dcn.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan S., Mahncke H., Salz T., Tallal P., Roberts T., Merzenich M.M. Cortical auditory signal processing in poor readers. Proc. Natl. Acad. Sci. USA. 1999;96:6483–6488. doi: 10.1073/pnas.96.11.6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez P.L., Srinivasan R. A theoretical basis for standing and traveling brain waves measured with human EEG with implications for an integrated consciousness. Clin. Neurophysiol. 2006;117:2424–2435. doi: 10.1016/j.clinph.2006.06.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Mantilla S., Hämäläinen J.A., Benasich A.A. Time course of ERP generators to syllables in infants: a source localization study using age-appropriate brain templates. Neuroimage. 2012;59:3275–3287. doi: 10.1016/j.neuroimage.2011.11.048. [DOI] [PubMed] [Google Scholar]
- Ortiz-Mantilla S., Hämäläinen J.A., Musacchia G., Benasich A.A. Enhancement of gamma oscillations indicates preferential processing of native over foreign phonemic contrasts in infants. J. Neurosci. 2013;33:18746–18754. doi: 10.1523/JNEUROSCI.3260-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Mantilla S., Hämäläinen J.A., Realpe-Bonilla T., Benasich A.A. Oscillatory dynamics underlying perceptual narrowing of native phoneme mapping from 6 to 12 months of age. J. Neurosci. 2016;36:12095–12105. doi: 10.1523/JNEUROSCI.1162-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Mantilla S., Realpe-Bonilla T., Benasich A.A. Early interactive acoustic experience with non-speech generalizes to speech and confers a syllabic processing advantage at 9 months. Cereb. Cortex. 2019;29:1789–1801. doi: 10.1093/cercor/bhz001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña M., Maki A., Kovacić D., Dehaene-Lambertz G., Koizumi H., Bouquet F., Mehler J. Sounds and silence: an optical topography study of language recognition at birth. Proc. Natl. Acad. Sci. U. S. A. 2003;100:11702–11705. doi: 10.1073/pnas.1934290100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips C., Pellathy T., Marantz A., Yellin E., Wexler K., Poeppel D., McGinnis M., Roberts T. Auditory cortex accesses phonological categories: an MEG mismatch study. J. Cogn. Neurosci. 2000;12:1038–1055. doi: 10.1162/08989290051137567. [DOI] [PubMed] [Google Scholar]
- Piazza C. Politecnico di Milano; Milano, Italy: 2016. Advanced EEG/ERP Signal Analysis for the Investigation of Early Sensory Processing and Cognitive Development. [Google Scholar]
- Piazza C., Cantiani C., Tacchino G., Molteni M., Reni G., Bianchi A.M. ERP and adaptive autoregressive identification with spectral power decomposition to study rapid auditory processing in infants. Conf. Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. IEEE Eng. Med. Biol. Soc. Annu. Conf. 2014;2014:4591–4594. doi: 10.1109/EMBC.2014.6944646. [DOI] [PubMed] [Google Scholar]
- Piazza C., Cantiani C., Akalin-Acar Z., Miyakoshi M., Benasich A.A., Reni G., Bianchi A.M., Makeig S. ICA-derived cortical responses indexing rapid multi-feature auditory processing in six-month-old infants. Neuroimage. 2016;133:75–87. doi: 10.1016/j.neuroimage.2016.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomin R., Kovas Y. Generalist genes and learning disabilities. Psychol. Bull. 2005;131(4):592–617. doi: 10.1037/0033-2909.131.4.592. [DOI] [PubMed] [Google Scholar]
- Poeppel D. The analysis of speech in different temporal integration windows: cerebral lateralization as ‘asymmetric sampling in time. Speech Commun. 2003;41:245–255. [Google Scholar]
- Poeppel D., Idsardi W.J., van Wassenhove V. Speech perception at the interface of neurobiology and linguistics. Philos. Trans. R. Soc. London. B, Biol. Sci. 2008;363:1071–1086. doi: 10.1098/rstb.2007.2160. https://doi.org/TM425571U1117682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle N.M., Zuk J., Ortiz-Mantilla S., Sliva D.D., Franceschi A., Grant P.E., Benasich A.A., Gaab N. Pediatric neuroimaging in early childhood and infancy: challenges and practical guidelines. Ann. N. Y. Acad. Sci. 2012;1252:43–50. doi: 10.1111/j.1749-6632.2012.06457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle N.M., Stering P.L., Meissner S.N., Gaab N. Altered neuronal response during rapid auditory processing and its relation to phonological processing in Prereading children at familial risk for dyslexia. Cereb. Cortex. 2013;24:2489–2501. doi: 10.1093/cercor/bht104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla L., Alley A. Validation of the language development survey (LDS): a parent report tool for identifying language delay in toddlers. J. Speech. Lang. Hear. Res. 2001;44:434–445. doi: 10.1044/1092-4388(2001/035). [DOI] [PubMed] [Google Scholar]
- Rescorla L., Ratner N.B., Jusczyk P., Jusczyk A.M. Concurrent validity of the language development survey: associations with the MacArthur-Bates communicative development inventories: words and sentences. Am. J. Speech. Lang. Pathol. 2005;14:156–163. doi: 10.1044/1058-0360(2005/016). [DOI] [PubMed] [Google Scholar]
- Rescorla L., Frigerio A., Sali M.E., Spataro P., Longobardi E. Typical and delayed lexical development in italian. J. Speech. Lang. Hear. Res. 2014;57:1792–1803. doi: 10.1044/2014_JSLHR-L-13-0242. [DOI] [PubMed] [Google Scholar]
- Rissling A.J., Miyakoshi M., Sugar C.A., Braff D.L., Makeig S., Light G.A. Cortical substrates and functional correlates of auditory deviance processing deficits in schizophrenia. NeuroImage. Clin. 2014;6:424–437. doi: 10.1016/j.nicl.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva V., Cantiani C., Benasich A.A., Molteni M., Piazza C., Giorda R., Dionne G., Marino C. From CNTNAP2 to early expressive language in infancy: the mediation role of rapid auditory processing. Cereb. Cortex. 2018;28:2100–2108. doi: 10.1093/cercor/bhx115. [DOI] [PubMed] [Google Scholar]
- Riva V., Cantiani C., Mornati G., Gallo M., Villa L., Mani E., Saviozzi I., Marino C., Molteni M. Distinct ERP profiles for auditory processing in infants at-risk for autism and language impairment. Sci. Rep. 2018;8:715. doi: 10.1038/s41598-017-19009-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saby J.N., Marshall P.J. The utility of EEG band power analysis in the study of infancy and early childhood. Dev. Neuropsychol. 2012;37:253–273. doi: 10.1080/87565641.2011.614663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartori G., Job R., Tressoldi P.E. OS; Firenze: 1995. Batteria per la valutazione della dislessia e della disortografia evolutiva in età evolutiva. [Google Scholar]
- Scherg M., Ebersole J.S. Brain source imaging of focal and multifocal epileptiform EEG activity. Neurophysiol. Clin. 1994;24:51–60. doi: 10.1016/s0987-7053(05)80405-8. [DOI] [PubMed] [Google Scholar]
- Scherg M., Von Cramon D. Two bilateral sources of the late AEP as identified by a spatio-temporal dipole model. Electroencephalogr. Clin. Neurophysiol. 1985;62:32–44. doi: 10.1016/0168-5597(85)90033-4. [DOI] [PubMed] [Google Scholar]
- Scherg M., Berg P., Hoechstetter K. 2010. BESA Research Tutorial 2: EEG-fMRI Coregistration, Preprocessing. (ERP and source analysis) [Google Scholar]
- Schroeder C.E., Lakatos P., Kajikawa Y., Partan S., Puce A. Neuronal oscillations and visual amplification of speech. Trends Cogn. Sci. 2008;12:106–113. doi: 10.1016/j.tics.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick S.D. Source localization of ERP generators. In: Handy T.C., editor. Event-Related Potentials: A Methods Handbook. MIT Press; Cambridge: 2004. pp. 149–166. [Google Scholar]
- Tallal P. Auditory temporal perception, phonics, and reading disabilities in children. Brain Lang. 1980;9:182–198. doi: 10.1016/0093-934x(80)90139-x. [DOI] [PubMed] [Google Scholar]
- Tallal P. Improving language and literacy is a matter of time. Nat. Rev. 2004;5:721–728. doi: 10.1038/nrn1499. [DOI] [PubMed] [Google Scholar]
- Tallal P., Gaab N. Dynamic auditory processing, musical experience and language development. Trends Neurosci. 2006;29:382–390. doi: 10.1016/j.tins.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C., Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends Cogn. Sci. 1999;3:151–162. doi: 10.1016/s1364-6613(99)01299-1. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C., Bertrand O., Delpuech C., Pernier J. Stimulus specificity of phase-locked and non-phase-locked 40 Hz visual responses in human. J. Neurosci. 1996;16:4240–4249. doi: 10.1523/JNEUROSCI.16-13-04240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telkemeyer S., Rossi S., Koch S.P., Nierhaus T., Steinbrink J., Poeppel D., Obrig H., Wartenburger I. Sensitivity of newborn auditory cortex to the temporal structure of sounds. J. Neurosci. 2009;29:14726–14733. doi: 10.1523/JNEUROSCI.1246-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telkemeyer S., Rossi S., Nierhaus T., Steinbrink J., Obrig H., Wartenburger I. Acoustic processing of temporally modulated sounds in infants: evidence from a combined near-infrared spectroscopy and EEG study. Front. Psychol. 2011;1:62. doi: 10.3389/fpsyg.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson E.C., Woodruff Carr K., White-Schwoch T., Tierney A., Nicol T., Kraus N. Hemispheric asymmetry of endogenous neural oscillations in young children: implications for hearing speech in noise. Sci. Rep. 2016;6:19737. doi: 10.1038/srep19737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomblin J.B., Buckwalter P.R. Heritability of poor language achievement among twins. J. Speech. Lang. Hear. Res. 1998;41:188–199. doi: 10.1044/jslhr.4101.188. [DOI] [PubMed] [Google Scholar]
- Uhlhaas P.J., Roux F., Rodriguez E., Rotarska-Jagiela A., Singer W. Neural synchrony and the development of cortical networks. Trends Cogn. Sci. 2010;14:72–80. doi: 10.1016/j.tics.2009.12.002. [DOI] [PubMed] [Google Scholar]
- van Herten M., Pasman J., van Leeuwen T.H., Been P.H., van der Leij A., Zwarts F., Maassen B. Differences in AERP responses and atypical hemispheric specialization in 17-month-old children at risk of dyslexia. Brain Res. 2008;1201:100–105. doi: 10.1016/j.brainres.2008.01.060. [DOI] [PubMed] [Google Scholar]
- van Leeuwen T., Been P., Kuijpers C., Zwarts F., Maassen B., van der Leij A. Mismatch response is absent in 2-month-old infants at risk for dyslexia. Neuroreport. 2006;17:351–355. doi: 10.1097/01.wnr.0000203624.02082.2d. [DOI] [PubMed] [Google Scholar]
- van Leeuwen T., Been P., van Herten M., Zwarts F., Maassen B., van der Leij A. Cortical categorization failure in 2-month-old infants at risk for dyslexia. Neuroreport. 2007;18:857–861. doi: 10.1097/WNR.0b013e3280c1e2bf. [DOI] [PubMed] [Google Scholar]
- van Zuijen T.L., Plakas A., Maassen B.A.M., Been P., Maurits N.M., Krikhaar E., van Driel J., van der Leij A. Temporal auditory processing at 17 months of age is associated with preliterate language comprehension and later word reading fluency: an ERP study. Neurosci. Lett. 2012;528:31–35. doi: 10.1016/j.neulet.2012.08.058. [DOI] [PubMed] [Google Scholar]
- van Zuijen T.L., Plakas A., Maassen B.A., Maurits N.M., van der Leij A. Infant ERPs separate children at risk of dyslexia who become good readers from those who become poor readers. Dev. Sci. 2013;16:554–563. doi: 10.1111/desc.12049. [DOI] [PubMed] [Google Scholar]
- Weismer S.E., Plante E., Jones M., Tomblin J.B. A functional magnetic resonance imaging investigation of verbal working memory in adolescents with specific language impairment. J. Speech. Lang. Hear. Res. 2005;48:405–425. doi: 10.1044/1092-4388(2005/028). [DOI] [PubMed] [Google Scholar]
- Witelson S.F., Pallie W. Left hemisphere specialization for language in the newborn. Neuroanatomical evidence of asymmetry. Brain. 1973;96:641–646. doi: 10.1093/brain/96.3.641. [DOI] [PubMed] [Google Scholar]
- Zaehle T., Wüstenberg T., Meyer M., Jäncke L. Evidence for rapid auditory perception as the foundation of speech processing: a sparse temporal sampling fMRI study. Eur. J. Neurosci. 2004;20:2447–2456. doi: 10.1111/j.1460-9568.2004.03687.x. [DOI] [PubMed] [Google Scholar]
- Zatorre R.J. Spectral and temporal processing in human auditory cortex. Cereb. Cortex. 2001;11:946–953. doi: 10.1093/cercor/11.10.946. [DOI] [PubMed] [Google Scholar]