Abstract
Background and Objectives
The association of susceptibility loci for atrial fibrillation (AF) with AF recurrence after ablation has been reported, although with controversial results. In this prospective cohort analysis, we aimed to investigate whether a genetic risk score (GRS) can predict the rhythm outcomes after catheter ablation of AF.
Methods
We determined the association between 20 AF-susceptible single nucleotide polymorphisms (SNPs) and AF recurrence after catheter ablation in 746 patients (74% males; age, 59±11 years; 56% paroxysmal AF). A GRS was calculated by summing the unweighted numbers of risk alleles of selected SNPs. A Cox proportional hazard model was used to identify the association between the GRS and risk of AF recurrence after catheter ablation.
Results
AF recurrences after catheter ablation occurred in 168 (22.5%) subjects with a median follow-up of 23 months. The GRS was calculated using 5 SNPs (rs1448818, rs2200733, rs6843082, rs6838973 at chromosome 4q25 [PITX2] and rs2106261 at chromosome 16q22 [ZFHX3]), which showed modest associations with AF recurrence. The GRS was significantly associated with AF recurrence (hazard ratio [HR] per each score, 1.13; 95% confidence interval [CI], 1.03–1.24). Patients with intermediate (GRS 4–6) and high risks (GRS 7–10) showed HRs of 2.00 (95% CI, 0.99–4.04) and 2.66 (95% CI, 1.32–5.37), respectively, compared to patients with low risk (GRS 0–3).
Conclusions
Our novel GRS using 5 AF-susceptible SNPs was strongly associated with AF recurrence after catheter ablation in Korean population, beyond clinical risk factors. Further efforts are warranted to construct a generalizable, robust genetic prediction model which can guide the optimal treatment strategies.
Keywords: Atrial fibrillation, Genetics, Catheter ablation, Recurrence
INTRODUCTION
Atrial fibrillation (AF) is the most common cardiac arrhythmia in clinical practice, affecting 1–2% of the general population worldwide.1) AF is associated with increased cardiovascular morbidity and mortality, thus imposing a substantial and increasing economic burden on the healthcare system.2) Since the landmark study by Haïssaguerre et al.3) which revealed the main triggering foci of AF within the pulmonary veins (PVs), advances in catheter ablation of AF have revolutionized the management of this common arrhythmia. The current guidelines recommend that catheter ablation should be considered for patients with symptomatic, antiarrhythmic drug-refractory AF,4) and catheter ablation is now a widely accepted treatment option in clinical practice.
The reported efficacy of catheter ablation varies depending on the patient characteristics, ablation strategies, and intensity of surveillance. Successful catheter ablation of AF leads to a reduction of the AF burden and improvement in symptoms, but more than 40% of patients experience recurrence during the long-term follow-up, and 20–40% of patients require repeated ablation procedures.5),6) Considering the inherent risk of procedure-related adverse events and high cost of the ablation procedure, appropriate selection of candidate patients is as important as technical proficiency to ensure the success of this invasive strategy. Previous studies have reported variable risk factors associated with the clinical response to AF ablation, including patient characteristics, biomarkers, medications, and presence of structural heart diseases.7),8),9) However, our understanding on the mechanism of recurrence and precise prediction of clinical response remains incomplete.
In addition to traditional clinical risk factors, genetic predisposition plays a substantial role in the development and progression of AF. During the past decade, researchers have investigated the genetic basis of AF, and a number of common genetic variants that increase the susceptibility to AF have been discovered.10),11),12),13),14),15) To date, however, studies on the utility of such genetic markers to improve the prediction of outcome after AF ablation have shown controversial results, even for the most promising markers at the 4q25 locus.16),17),18) Furthermore, many of the candidate AF-related genetic markers have not been studied for their association with the clinical response to catheter ablation. Accordingly, in this study, we sought to perform a comprehensive analysis on the relationship between genetics and outcome of AF ablation by constructing a genetic risk score (GRS) based on common genetic markers known to be associated with AF incidence.
METHODS
Study population
This study was performed in 2 centers, Seoul National University Hospital and Korea University Guro Hospital, using an AF ablation cohort comprising patients with available genomic DNA data. Consecutive patients who were admitted and underwent de novo or repeat radiofrequency catheter ablation (RFCA) for symptomatic paroxysmal or persistent AF between June 2008 and March 2015 were enrolled. A detailed medical and personal history of each participant was obtained at the time of admission. Transesophageal and transthoracic echocardiography were performed prior to catheter ablation to exclude the presence of atrial thrombi and to measure the cardiac chamber size, wall thickness, and left ventricular systolic function with standard methods. After the index catheter ablation procedure, the prescription of antiarrhythmic drugs during the blanking period was left to the attending clinician's judgement. Follow-up information was obtained from regular outpatient visits at 1, 3, 6, and 12 months and every 3 to 6 months thereafter, as clinically indicated. Electrocardiograms were performed at every visit, and 24-hour Holter monitoring was performed at 3 and 12 months after the ablation. Supplementary 24-hour Holter monitoring was obtained when recurrence was suspected on the basis of the patient's symptoms.
The primary endpoint was the recurrence of atrial tachyarrhythmia after AF ablation. Recurrence was defined as any documented episode of AF, atrial flutter (AFL), or atrial tachycardia (AT) lasting more than 30 seconds after a 3-month blanking period.19) The study adhered to the tenets of the Declaration of Helsinki and was exempt from review by the Institutional Review Board of Seoul National University Hospital Biomedical Research Institute (No. 1408-106-605). All subjects provided written informed consent.
Mapping and catheter ablation procedure
Ablation was guided by 3-dimensional electroanatomical mapping using CARTO (CARTO XP or CARTO-3; Biosense Webster Inc., Diamond Bar, CA, USA) or EnSite (EnSite NavX Classic or EnSite Velocity; Abbott Laboratories, North Chicago, IL, USA) mapping system. A duo-decapolar Lasso circular mapping catheter was used to guide and map the PV. Ablation was performed using open irrigation catheters (Celsius or Navistar Thermocool SF; Biosense Webster Inc., Cool Flex; Abbott Laboratories). All patients underwent circumferential PV isolation. Exit and entrance blocks were confirmed after PV isolation. For patients with persistent AF, additional ablation was performed at the roof line, posterior inferior line, anterior line, mitral isthmus line, cavotricuspid line, or regions of complex fractionated electrograms, at the operator's discretion.
Single nucleotide polymorphism selection and genotyping
Through a comprehensive screening of previous reports, we selected 20 single nucleotide polymorphisms (SNPs) from genome-wide association studies in which the robust association between SNPs and incident AF were identified (p<5×10−8).12),14),15),16),17) The selected SNPs are listed in Supplementary Table 1, along with the risk allele frequencies in our cohort and published effect sizes for incident AF. All SNPs have a reported minor allele frequency of >0.01.
Genomic DNA extraction was performed by standard methods with whole blood samples collected during admission for index catheter ablation. The selected 20 SNPs were genotyped using validated TaqMan SNP genotyping assays (Applied Biosystems, Foster City, CA, USA) on the ABI PRISM 7900HT Real-time polymerase chain reaction system according to the supplier's recommendations, and allelic discrimination was calculated with SDS v2.3 software (Applied Biosystems) using the same system. The laboratory personnel were blinded to the subjects' clinical characteristics and outcomes of the ablation procedure. All SNPs had a call rate of >99%, and agreement of genotype frequencies with Hardy-Weinberg equilibrium expectations was tested by using a χ2 goodness-of-fit test.
Genetic risk score construction
To estimate genetic risk of AF recurrence using a minimal set of genetic variants, candidate SNPs were selected before the GRS construction. The 20 SNPs were separately examined for independent cross-sectional association with AF recurrence using a logistic regression analysis, and SNPs showing at least borderline significant associations (p<0.1) with AF recurrence were included in the final GRS model. Because the associations between each AF susceptibility-associated SNP and clinical response to catheter ablation were not previously reported in most cases, the magnitude of genetic effect size of each SNP could not be estimated. Therefore, we applied an additive unweighted model, and the total number of risk alleles each subject carries was summed to calculate the GRS. Previous research has shown that unweighted GRS model gives unbiased prediction of the associations, when there is a lack of relevant data on the individual effects of each genetic variant.20) Subjects with missing genotype data of the target SNPs included in the final GRS model were excluded from the analysis.
Statistical analysis
Baseline characteristics are presented as mean±standard deviation for continuous variables and as number and percent for categorical variables. A Cox multivariable proportional hazard model was used to identify the association between the GRS and risk of AF recurrence after catheter ablation. Baseline characteristics including age, sex, body mass index (BMI), persistent (vs. paroxysmal) AF, hypertension, diabetes mellitus (DM), heart failure, previous history of stroke, left atrium (LA) diameter, and left ventricular ejection fraction (LVEF) were used to adjust the endpoint. Among the confounders included in the multivariate analysis, age, LA diameter, and LVEF were used as continuous variables, while others were applied as categorical variables. We divided the subjects into 3 subgroups by GRS risk, and the differences in the cumulative AF recurrence rate between the subgroups were calculated by the Kaplan-Meier method and compared by the log-rank test. Comparisons of baseline characteristics of each subgroup were made with independent-samples t-tests for continuous variables and χ2 tests for categorical variables. The risks of AF recurrence in the intermediate and high GRS groups were compared with the low GRS group using a Cox multivariable proportional hazard model, adjusted for the same risk factors. All analyses were performed using SPSS Statistics 20.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Study population and AF recurrence
A total of 746 subjects were included. The characteristics of the study population are summarized in Table 1. The median follow-up time was 684 days (interquartile range, 324–1,205 days). During the follow-up period, 168 subjects (22.5%) experienced recurrence of atrial tachyarrhythmia. AF accounted for 57.1% of all recurrence events, and AFL or AT accounted for the remainder. The 6- and 12-month recurrence rates were 8.2% and 14.7%, respectively (Table 1). The overall recurrence patterns throughout the study period are presented in Supplementary Figure 1.
Table 1. Baseline characteristics and clinical outcomes after catheter ablation of AF.
| Total cohort (n=746) | |||
|---|---|---|---|
| Baseline characteristics | |||
| Age (years) | 59.4±10.6 | ||
| Sex (Male) | 548 (73.5) | ||
| BMI (kg/m2) | 24.8±2.8 | ||
| Paroxysmal AF | 420 (56.3) | ||
| Hypertension | 358 (48.1) | ||
| DM | 115 (15.4) | ||
| Heart failure | 91 (12.2) | ||
| History of stroke | 38 (5.2) | ||
| Echocardiography | |||
| LA dimension (mm) | 42.5±6.4 | ||
| LA volume index (mL/m2) | 42.5±15.9 | ||
| LVEDD (mm) | 49.3±6.2 | ||
| IVSd (mm) | 9.4±1.9 | ||
| LVEF | 62.6±8.0 | ||
| Clinical outcomes | |||
| Median follow-up, days (interquartile range) | 684 (324–1,205) | ||
| Recurrence during blanking period* | 21.4 | ||
| 6-month recurrence | 8.2 | ||
| 12-month recurrence | 14.7 | ||
| Type of recurrence | |||
| AF | 57.1 | ||
| AFL or AT | 42.9 | ||
Data are shown as mean±standard deviation or number (%) not otherwise specified.
AF = atrial fibrillation; AFL = atrial flutter; AT = atrial tachycardia; BMI = body mass index; DM = diabetes mellitus; IVSd = interventricular septal thickness at diastole; LA = left atrium; LVEDD = left ventricular end-diastolic dimension; LVEF = left ventricular ejection fraction.
*The blanking period is within 90 days after catheter ablation.
Target single nucleotide polymorphism identification for genetic risk score modeling
All of the 20 selected SNPs were in accordance with Hardy-Weinberg expectations (p>0.001). We evaluated each SNP separately to assess the cross-sectional association between each of the 20 SNPs and recurrence of atrial tachyarrhythmia, and to identify an essential set of SNPs for joint analysis by a GRS. We assumed that each copy of the risk alleles adds an equal risk effect, and an unweighted additive model was applied in the logistic regression analysis.
Table 2 presents the associations of the individual genetic variants with recurrent AF after catheter ablation. Overall, 6/20 SNPs showed at least borderline association (p<0.1) with recurrence: 5 SNPs at the 4q25 locus (rs1448818, rs6817105, rs2200733, rs6843082, and rs6838973) and 1 SNP at the 16q22 locus (rs2106261). Although modest trends were noted, none of these selected SNPs remained significantly associated with recurrence in the multivariable Cox proportional hazard model adjusted for traditional risk factors (Supplementary Table 2). Of these 6 SNPs, rs6817105 was excluded from the final GRS model because of its strong linkage disequilibrium (r2=1.0) with rs2200733 (Supplementary Table 3), which has been reported as an independent predictor of treatment response after catheter ablation of AF in different populations.16),17)
Table 2. Cross-sectional association between the 20 AF-susceptibility SNPs and AF recurrence after catheter ablation.
| SNP | Loci | Nearest gene | AF associated risk allele | Recurrence | Inclusion in the GRS model | |
|---|---|---|---|---|---|---|
| OR (95% CI) | p value | |||||
| rs6666258 | 1q21 | KCNN3 | C | 2.579 (0.786–8.465) | 0.118 | - |
| rs13376333 | 1q21 | KCNN3 | T | 0.385 (0.115–1.289) | 0.122 | - |
| rs3903239 | 1q24 | PRRX1 | G | 1.077 (0.841–1.380) | 0.555 | - |
| rs4642101* | 3p25 | CAND2 | G | 0.747 (0.554–1.009) | 0.057 | - |
| rs1448818 | 4q25 | PITX2 | C | 1.240 (0.971–1.583) | 0.085 | Yes |
| rs6817105† | 4q25 | PITX2 | C | 1.405 (1.075–1.837) | 0.013 | - |
| rs2200733 | 4q25 | PITX2 | T | 1.430 (1.093–1.871) | 0.009 | Yes |
| rs4400058 | 4q25 | PITX2 | A | 0.816 (0.576–1.155) | 0.251 | - |
| rs6843082 | 4q25 | PITX2 | G | 1.438 (0.988–2.092) | 0.058 | Yes |
| rs6838973 | 4q25 | PITX2 | C | 1.269 (0.978–1.648) | 0.073 | Yes |
| rs13216675 | 6q22 | GJA1 | T | 1.010 (0.782–1.305) | 0.939 | - |
| rs3807989 | 7q31 | CAV1 | G | 1.010 (0.773–1.318) | 0.944 | - |
| rs10821415 | 9q22 | C9orf3 | A | 0.946 (0.720–1.244) | 0.691 | - |
| rs10824026 | 10q22 | SYNPO2L | A | 0.917 (0.723–1.164) | 0.477 | - |
| rs12415501 | 10q24 | NEURL | T | 1.173 (0.859–1.602) | 0.314 | - |
| rs6490029 | 12q24 | CUX2 | A | 0.818 (0.626–1.070) | 0.142 | - |
| rs10507248 | 12q24 | TBX5 | T | 0.956 (0.754–1.213) | 0.713 | - |
| rs1152591 | 14q23 | SYNE2 | A | 0.934 (0.726–1.200) | 0.593 | - |
| rs7164883 | 15q24 | HCN4 | G | 1.231 (0.856–1.772) | 0.263 | - |
| rs2106261 | 16q22 | ZFHX3 | T | 1.289 (1.007–1.652) | 0.044 | Yes |
The associations were tested with a univariable logistic regression analysis. Additive genetic modeling was used for all SNPs.
AF = atrial fibrillation; CI = confidence interval; GRS = genetic risk score; OR = odds ratio; SNP = single nucleotide polymorphism.
*The rs4642101 was excluded from the GRS model because the known risk allele showed a negative association with recurrence; †The rs6817105 was excluded from the GRS model because of its strong linkage disequilibrium with rs2200733.
Association between the genetic risk score and atrial fibrillation recurrence
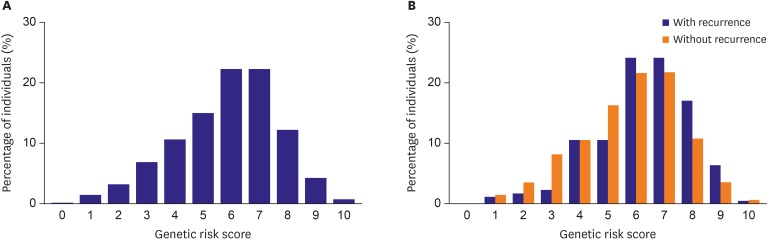
The primary endpoint of the study was AF recurrence, defined as any episode of atrial arrhythmia including AF, AT, or AFL.19) To assess the cumulative effects of genetic variants on AF recurrence, we performed a joint analysis by generating a GRS with the 5 selected SNPs. As we adopted an unweighted additive model, the GRS was calculated by summing the number of risk alleles for each of the 5 selected SNPs, which resulted in a score ranging from 0 to 10. The overall distribution of the GRS is shown in Figure 1. Although the distribution of the GRS between those who experienced recurrence during follow-up and those who remained in sinus rhythm tended to overlap, the mean values of each group significantly differed. The mean GRSs were 6.3±1.7 and 5.7±1.8 in the AF-recurrence and no-recurrence groups, respectively (p<0.001).
Figure 1. Distribution of the GRS among (A) the total cohort population and (B) subjects who experienced recurrence after AF ablation (blue bars) and who remained in sinus rhythm (orange bars).
AF = atrial fibrillation; GRS = genetic risk score.
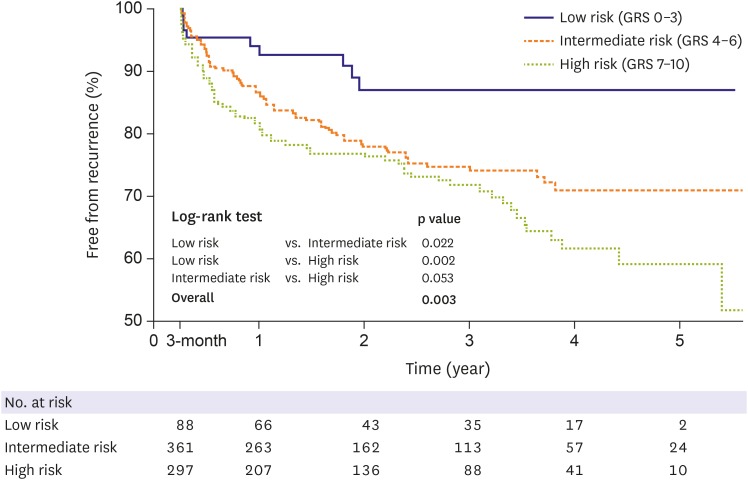
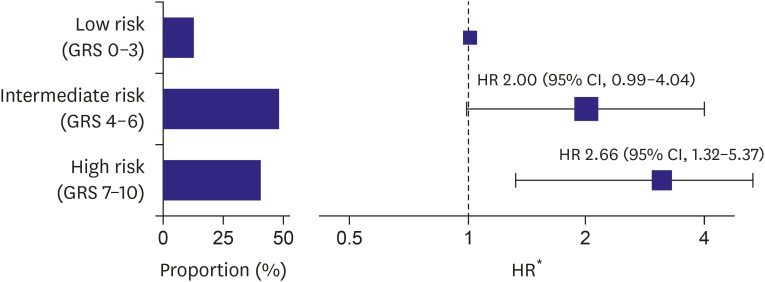
Cox regression analysis demonstrated a significant association between the GRS and AF recurrence after adjusting for baseline characteristics, including age, sex, BMI, type of AF, hypertension, DM, heart failure, previous history of stroke, LA diameter, and LVEF. The addition of 1 risk allele was associated with a 13% increased risk of recurrence (hazard ratio [HR], 1.13; 95% confidence interval [CI], 1.03–1.24; p=0.007) (Table 3). To illustrate this association further, the subjects were partitioned into 3 groups according to the GRS: the low-risk reference group (GRS 0–3), intermediate-risk group (GRS 4–6), and high-risk group (GRS 7–10). The epidemiologic and echocardiographic data of each group are presented in Supplementary Table 4. There were statistically significant differences in the type of AF and LA size among the groups, with subjects with higher GRS tending to have a higher prevalence of persistent AF and larger LA. Figure 2 shows Kaplan-Meier curves of the proportions of subjects remaining in sinus rhythm in the 3 groups, which revealed that there was a significant trend toward an increased risk of AF recurrence in the group with higher GRS (log-rank test; p=0.003). Compared with the low-risk reference group (GRS 0–3), subjects with intermediate-risk (GRS 4–6) tended to be associated with a higher risk of recurrence, with an adjusted HR of 2.00 (95% CI, 0.99–4.04; p=0.055). Subjects at high-risk (GRS 7–10) showed further increased risk of recurrence, with an adjusted HR of 2.66 (95% CI, 1.32–5.37; p=0.007) (Figure 3). When we put a narrow definition on the AF recurrence by censoring AFL or AT events at the time of the first episode, the GRS maintained the performance of the recurrence prediction after AF ablation: Cox regression analysis demonstrated a significant association between the GRS and AF recurrence after multivariable adjustment (HR, 1.49; 95% CI, 1.07–2.08; p=0.019), and each GRS subgroup showed different recur profile (log-rank test; p=0.014).
Table 3. Multivariable association of risk factors including GRS with AF recurrence after catheter ablation.
| Variable | Recurrence | |||
|---|---|---|---|---|
| Unadjusted univariable HR (95% CI) | p value | Adjusted HR* (95% CI) | p value | |
| GRS | 1.166 (1.066–1.275) | 0.001 | 1.132 (1.034–1.240) | 0.007 |
| Age | 0.995 (0.981–1.009) | 0.513 | 0.996 (0.980–1.012) | 0.589 |
| Sex (male) | 1.406 (0.969–2.041) | 0.073 | 1.182 (0.792–1.764) | 0.413 |
| BMI | 0.978 (0.924–1.034) | 0.430 | 0.909 (0.851–0.971) | 0.005 |
| Persistent AF | 1.549 (1.144–2.098) | 0.005 | 1.062 (0.754–1.496) | 0.730 |
| Hypertension | 0.843 (0.620–1.145) | 0.274 | 0.896 (0.644–1.247) | 0.515 |
| DM | 0.867 (0.558–1.346) | 0.525 | 0.966 (0.615–1.517) | 0.881 |
| Heart failure | 1.572 (1.053–2.345) | 0.027 | 1.160 (0.759–1.773) | 0.492 |
| Previous stroke | 1.606 (0.912–2.830) | 0.101 | 1.680 (0.946–2.984) | 0.077 |
| LA diameter | 1.063 (1.041–1.086) | <0.001 | 1.060 (1.034–1.087) | <0.001 |
| LVEF | 0.951 (0.936–0.967) | <0.001 | 0.967 (0.949–0.984) | <0.001 |
AF = atrial fibrillation; BMI = body mass index; CI = confidence interval; DM = diabetes mellitus, GRS = genetic risk score; HR = hazard ratio; LA = left atrium; LVEF = left ventricular ejection fraction; SNP = single nucleotide polymorphism.
*The associations were tested with a Cox proportional hazards model that included all the variables listed in the table. Additive genetic modeling was used for all SNPs. Risk for GRS is per allele, age per year, LA size per mm, LVEF per % increase. All other risks are per risk category.
Figure 2. Kaplan-Meier curves depicting the proportion of subjects without recurrence after AF ablation according to risk groups stratified by the GRS.
AF = atrial fibrillation; GRS = genetic risk score.
Figure 3. Graded HRs of recurrence after catheter ablation according to the risk groups stratified by the GRS. Bar graphs indicate the proportions of subjects in each risk group.
CI = confidence interval; GRS = genetic risk score; HR = hazard ratio.
*Compared with the low-risk group (GRS 0–3).
In the current study, we enrolled consecutive patients who underwent catheter ablation for symptomatic AF, and study subjects constitute a heterogeneous group with variable epidemiologic and procedural characteristics. In the procedural aspect, both de novo (696/746, 93.3%) and redo (50/746, 6.7%) ablation procedures were performed, which have different procedural strategies and clinical outcomes. We performed a subgroup analysis including only subjects underwent de novo AF catheter ablation with the same outcome variables. The results were substantially similar to the analysis of overall population. After adjusting for the clinical risk factors, the GRS was significantly associated with AF recurrence (HR, 1.15; 95% CI, 1.04–1.27; Supplementary Table 5), and graded risk of recurrence was demonstrated according to the GRS tertiles (Supplementary Figure 2). On the other hand, statistically significant predictor of AF recurrence was not found in the patients who underwent redo ablation.
DISCUSSION
In the current study of AF patients of Korean ancestry who had undergone catheter ablation, we sought to demonstrate the influence of 20 well-known AF susceptibility genetic variants on AF recurrence. We found that our GRS, generated from 5 SNPs, was associated with the risk of recurrence of AF after catheter ablation after adjusting for baseline characteristics. Especially, patients carrying several risk alleles (high-risk group) were found to have a 2.66-fold increased risk of recurrence when compared with those in the lowest risk group.
Since the initial era of familial linkage analysis, numerous genetic variants contributing to the risk of AF have been identified. Recently, genome-wide association studies have revealed multiple loci robustly associated with AF,10),11),12),13),14) and several investigators have tried to incorporate these genetic data by proposing cumulative GRSs and providing predictive models for reliable assessment of AF risk. Lubitz et al.14) reported that a GRS comprising 12 SNPs was associated with AF in not only European but also Japanese populations. Another GRS constructed with the top 12 AF-associated SNPs was examined in the Women's Health Study cohort, and the addition of this GRS to a clinical AF risk model improved discrimination and category-less reclassification.21) A similar GRS constructed from 12 SNPs was significantly associated with incident AF and ischemic stroke in a Swedish population, and modestly, but significantly, improved risk discrimination and reclassification were reported.22) Recently, another multi-center prospective study showed that a GRS was able to predict incident AF independent of established clinical risk factors.23) However, there is currently no study reporting the association between a GRS and AF recurrence after catheter ablation.
In contrast to the previous studies showing the impact of genetic predisposition to AF development, there have been relatively few studies evaluating the correlations of genetic variations and response to AF treatment. Several studies have examined limited number of AF-associated common variants, which were revealed as independent predictors of clinical response to electrical cardioversion and anti-arrhythmic drug therapy.24),25) However, the previous evidence of associations between individual genetic variations and the rhythm outcome after catheter ablation of AF is conflicting. Studies conducted in European populations reported positive independent associations between SNPs on chromosome 4q25, one of the most significant AF-associated genetic loci, and increased risk of AF recurrence after catheter ablation.16),17),26) In contrast, we did not find any correlation between AF-susceptibility SNPs and AF recurrence after catheter ablation in our previous study in an Asian population.18) In the current study, the lack of association between the individual AF-risk SNPs and AF recurrence after catheter ablation was consistent with our previous findings. Nonetheless, our GRS constructed using 5 SNPs was associated with AF recurrence independent of clinical risk factors. The predictive power of individual risk alleles appears small, but combining these risk alleles increased the predictive power, which showed significant association with clinical outcomes. Therefore, our study supports and extends the findings of previous studies that genetic polymorphisms related to AF risk are associated with AF recurrence after catheter ablation.
The clinical implication of our GRS includes the identification of proper candidates of invasive procedure in AF management by improving the prediction of clinical response to catheter ablation. One recent study reported that the rs2106216 polymorphism (ZFHX3) was independently associated with a good response to RFCA for longstanding persistent AF.27) The GRS may provide more comprehensive information which can discriminate high from low-risk patients with AF recurrence, and assist in improving patient care by avoiding ineffective invasive procedures and suggesting individualized treatment plans. Moreover, the GRS may guide the effective catheter ablation strategies in technical aspect. PV antrum isolation is the cornerstone of AF catheter ablation, but some patients recur due to non-PV foci. The Dyspnoea, Eosinopenia, Consolidation, Acidaemia and atrial Fibrillation study reported associations of genetic polymorphisms and increased risks of LA scars and non-PV triggers in AF patients.28) Although we did not analyze the relationship between the GRS and treatment strategy of AF, future analyses are warranted to determine whether such tailored ablation strategies based on genetic information will show better outcomes compared to the conventional strategies.
There are several possible mechanisms explaining the role of genetic variants on the recurrence after radiofrequency ablation of AF. In this study, 5 SNPs which showed modest association with AF recurrence were related to PITX2 at chromosome 4q25 and ZFHX3 at chromosome 16q22. PITX2 has an important role in the development of pulmonary myocardial cells,29) which suggests that chromosome 4q25 variant carriers could be different in their PV phenotype, also could influence on the results of catheter ablation of the PVs. Although the function of ZFHX3 was not clearly demonstrated, it has been known to relate to Janus kinase/signal transducers and activators of transcription signaling cascade, a regulator of paracrine function and inflammation,11) and also regulates myogenic and neuronal differentiation.30) Therefore, structural remodeling of atrium by inflammation could be affected by the variant of ZFHX3, which also could affect the outcomes of catheter ablation in persistent AF. However, estimating the mechanism of recurrence with GRS model has innate limitation, it is difficult to suggest specific mechanism of AF recurrence based on current data.
In the current work, we present the first attempt to study the association between a GRS and risk of AF recurrence after ablation; however, our study has some potential limitations. Our study consists of Korean patients, and hence the current results cannot be generalized to other populations. Further, our panel of genetic variants may be incomplete. As previously described, we adopted common genetic variants obtained from previous genetic association studies that increase the susceptibility to AF, not the risk of recurrence after ablation. Thus far, the relative scarcity of unraveled genetic variants associated with AF recurrence prevents the development of a robust predictive genetic model. Finally, the cost of genetic testing would be the major concern for the patients, and cost-effectiveness analysis should be conducted to rationalize this genetic assay-guided approach in AF treatment.
In conclusion, to our knowledge, this is the first study to evaluate whether a cumulative GRS can predict the outcome of AF treatment. Our GRS comprising 5 known AF-susceptibility SNPs was associated with recurrent AF after catheter ablation in a Korean population. Although this observation may not apply to all populations, it suggests the potential of genetic screening for decision-making in AF management by providing additional information over classic predictors of treatment outcome. Further studies with a complete set of genetic loci associated with AF recurrence in the general population are needed.
Footnotes
Funding: This study was supported by grant No. 2520140060 from the Seoul National University Hospital Research Fund, a Korea National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2014R1A1A2A16055218). The funding source has no involvement in the current study.
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Choi EK, Oh S, Lim HE.
- Data curation: Choi EK, Kang JH, Shin SY, Lim HE.
- Formal analysis: Choi EK, Choe WS.
- Funding acquisition: Choi EK.
- Investigation: Choi EK, Choe WS, Kang JH, Shin SY, Lim HE.
- Methodology: Choi EK, Choe WS, Kang JH, Shin SY, Ellinor PT, Oh S, Lim HE.
- Resources: Choi EK, Kang JH, Shin SY, Lim HE.
- Supervision: Choi EK, Lubitz SA, Ellinor PT, Oh S, Lim HE.
- Writing - original draft: Choe WS.
- Writing - review & editing: Choi EK, Lubitz SA, Ellinor PT, Oh S, Lim HE.
SUPPLEMENTARY MATERIALS
Profiles of the 20 candidate AF-susceptibility SNPs
Multivariable analysis of the associations between the individual SNPs included in the GRS and AF recurrence
Linkage disequilibrium (r2) between identified SNPs at chromosome 4q25 which showed a borderline association with AF recurrence
Baseline characteristics of the study population according to the GRS groups
Multivariable association of risk factors AF recurrence in subjects underwent de-novo or redo AF catheter ablation
Kaplan-Meier curve for recurrence after AF ablation: total study population.
Kaplan-Meier curves for recurrence after AF ablation in patients who underwent (A) de novo and (B) redo procedure.
References
- 1.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Kim MH, Johnston SS, Chu BC, Dalal MR, Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4:313–320. doi: 10.1161/CIRCOUTCOMES.110.958165. [DOI] [PubMed] [Google Scholar]
- 3.Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 4.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the heart rhythm society. J Am Coll Cardiol. 2014;64:e1–e76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 5.Sawhney N, Anousheh R, Chen WC, Narayan S, Feld GK. Five-year outcomes after segmental pulmonary vein isolation for paroxysmal atrial fibrillation. Am J Cardiol. 2009;104:366–372. doi: 10.1016/j.amjcard.2009.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ouyang F, Tilz R, Chun J, et al. Long-term results of catheter ablation in paroxysmal atrial fibrillation: lessons from a 5-year follow-up. Circulation. 2010;122:2368–2377. doi: 10.1161/CIRCULATIONAHA.110.946806. [DOI] [PubMed] [Google Scholar]
- 7.Berruezo A, Tamborero D, Mont L, et al. Pre-procedural predictors of atrial fibrillation recurrence after circumferential pulmonary vein ablation. Eur Heart J. 2007;28:836–841. doi: 10.1093/eurheartj/ehm027. [DOI] [PubMed] [Google Scholar]
- 8.Park JH, Oh YS, Kim JH, et al. Effect of angiotensin converting enzyme inhibitors and angiotensin receptor blockers on patients following ablation of atrial fibrillation. Korean Circ J. 2009;39:185–189. doi: 10.4070/kcj.2009.39.5.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hussein AA, Saliba WI, Martin DO, et al. Plasma B-type natriuretic peptide levels and recurrent arrhythmia after successful ablation of lone atrial fibrillation. Circulation. 2011;123:2077–2082. doi: 10.1161/CIRCULATIONAHA.110.007252. [DOI] [PubMed] [Google Scholar]
- 10.Gudbjartsson DF, Arnar DO, Helgadottir A, et al. Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature. 2007;448:353–357. doi: 10.1038/nature06007. [DOI] [PubMed] [Google Scholar]
- 11.Benjamin EJ, Rice KM, Arking DE, et al. Variants in ZFHX3 are associated with atrial fibrillation in individuals of European ancestry. Nat Genet. 2009;41:879–881. doi: 10.1038/ng.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellinor PT, Lunetta KL, Glazer NL, et al. Common variants in KCNN3 are associated with lone atrial fibrillation. Nat Genet. 2010;42:240–244. doi: 10.1038/ng.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellinor PT, Lunetta KL, Albert CM, et al. Meta-analysis identifies six new susceptibility loci for atrial fibrillation. Nat Genet. 2012;44:670–675. doi: 10.1038/ng.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lubitz SA, Lunetta KL, Lin H, et al. Novel genetic markers associate with atrial fibrillation risk in Europeans and Japanese. J Am Coll Cardiol. 2014;63:1200–1210. doi: 10.1016/j.jacc.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sinner MF, Tucker NR, Lunetta KL, et al. Integrating genetic, transcriptional, and functional analyses to identify 5 novel genes for atrial fibrillation. Circulation. 2014;130:1225–1235. doi: 10.1161/CIRCULATIONAHA.114.009892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Husser D, Adams V, Piorkowski C, Hindricks G, Bollmann A. Chromosome 4q25 variants and atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol. 2010;55:747–753. doi: 10.1016/j.jacc.2009.11.041. [DOI] [PubMed] [Google Scholar]
- 17.Benjamin Shoemaker M, Muhammad R, Parvez B, et al. Common atrial fibrillation risk alleles at 4q25 predict recurrence after catheter-based atrial fibrillation ablation. Heart Rhythm. 2013;10:394–400. doi: 10.1016/j.hrthm.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi EK, Park JH, Lee JY, et al. Korean atrial fibrillation (AF) network: genetic variants for AF do not predict ablation success. J Am Heart Assoc. 2015;4:e002046. doi: 10.1161/JAHA.115.002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace. 2018;20:e1–e160. doi: 10.1093/europace/eux274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burgess S, Thompson SG. Use of allele scores as instrumental variables for Mendelian randomization. Int J Epidemiol. 2013;42:1134–1144. doi: 10.1093/ije/dyt093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Everett BM, Cook NR, Conen D, Chasman DI, Ridker PM, Albert CM. Novel genetic markers improve measures of atrial fibrillation risk prediction. Eur Heart J. 2013;34:2243–2251. doi: 10.1093/eurheartj/eht033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tada H, Shiffman D, Smith JG, et al. Twelve-single nucleotide polymorphism genetic risk score identifies individuals at increased risk for future atrial fibrillation and stroke. Stroke. 2014;45:2856–2862. doi: 10.1161/STROKEAHA.114.006072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muse ED, Wineinger NE, Spencer EG, et al. Validation of a genetic risk score for atrial fibrillation: a prospective multicenter cohort study. PLoS Med. 2018;15:e1002525. doi: 10.1371/journal.pmed.1002525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parvez B, Shoemaker MB, Muhammad R, et al. Common genetic polymorphism at 4q25 locus predicts atrial fibrillation recurrence after successful cardioversion. Heart Rhythm. 2013;10:849–855. doi: 10.1016/j.hrthm.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parvez B, Vaglio J, Rowan S, et al. Symptomatic response to antiarrhythmic drug therapy is modulated by a common single nucleotide polymorphism in atrial fibrillation. J Am Coll Cardiol. 2012;60:539–545. doi: 10.1016/j.jacc.2012.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shoemaker MB, Bollmann A, Lubitz SA, et al. Common genetic variants and response to atrial fibrillation ablation. Circ Arrhythm Electrophysiol. 2015;8:296–302. doi: 10.1161/CIRCEP.114.001909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park JK, Lee JY, Yang PS, et al. Good responders to catheter ablation for long-standing persistent atrial fibrillation: clinical and genetic characteristics. J Cardiol. 2017;69:584–590. doi: 10.1016/j.jjcc.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 28.Mohanty S, Hall AW, Mohanty P, et al. Novel association of polymorphic genetic variants with predictors of outcome of catheter ablation in atrial fibrillation: new directions from a prospective study (DECAF) J Interv Card Electrophysiol. 2016;45:7–17. doi: 10.1007/s10840-015-0069-2. [DOI] [PubMed] [Google Scholar]
- 29.Mommersteeg MT, Brown NA, Prall OW, et al. Pitx2c and Nkx2-5 are required for the formation and identity of the pulmonary myocardium. Circ Res. 2007;101:902–909. doi: 10.1161/CIRCRESAHA.107.161182. [DOI] [PubMed] [Google Scholar]
- 30.Nojiri S, Joh T, Miura Y, et al. ATBF1 enhances the suppression of STAT3 signaling by interaction with PIAS3. Biochem Biophys Res Commun. 2004;314:97–103. doi: 10.1016/j.bbrc.2003.12.054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Profiles of the 20 candidate AF-susceptibility SNPs
Multivariable analysis of the associations between the individual SNPs included in the GRS and AF recurrence
Linkage disequilibrium (r2) between identified SNPs at chromosome 4q25 which showed a borderline association with AF recurrence
Baseline characteristics of the study population according to the GRS groups
Multivariable association of risk factors AF recurrence in subjects underwent de-novo or redo AF catheter ablation
Kaplan-Meier curve for recurrence after AF ablation: total study population.
Kaplan-Meier curves for recurrence after AF ablation in patients who underwent (A) de novo and (B) redo procedure.