Abstract
Background and Objectives
Udenafil, a new phosphodiesterase-5 inhibitor (PDE5i), has been used to treat erectile dysfunction. Given the proven benefit of PDE5i in pulmonary arterial hypertension (PAH), we evaluated serial hemodynamic changes after single udenafil administration to determine the appropriate therapeutic dose.
Methods
Eighteen patients were randomly allocated into one of 3 groups: placebo, udenafil 50 mg (U50), and udenafil 100 mg (U100). Diagnosis for inclusion was idiopathic PAH or PAH associated with connective tissue disease. Patients with any contraindication to PDE5i, and/or PDE5i treatment in the past 1 month were excluded. Continuous hemodynamic monitoring was performed by placing a Swan-Ganz catheter. Information on cardiac index (CI), mean pulmonary arterial pressure (mPAP), mean systemic arterial pressure (mSAP), pulmonary arterial wedge pressure (PAWP), and pulmonary vascular resistance index (PVRI) was obtained for 4 hours after drug administration.
Results
The mPAP significantly decreased in both the U50 and U100 (−11 mmHg and −8 mmHg from baseline, respectively, p<0.1). The mSAP also decreased in both U50 and U100; however, the decrease was greater in the U100 (Δ=−8.5 mmHg and Δ=−14.0 mmHg). CI increased in the U50, but decreased in the U100. Although PVRI decreased in both, statistical significance was only achieved in the U50 compared to placebo. PAWP was stable during monitoring. U50 had at least 4 hour-effect after administration. Only 2 patients with U100 experienced mild adverse events.
Conclusions
This is the first demonstration of the acute hemodynamic changes induced by udenafil. U50 is considered an optimal dose for treating PAH with more than 4-hour treatment effect.
Trial Registration
ClinicalTrials.gov Identifier: NCT01553721.
Keywords: Hypertension, Pulmonary; Phosphodiesterase 5 inhibitors; Hemodynamic monitoring
INTRODUCTION
Udenafil (Zydena®; Dong-A ST, Seoul, Korea) is a drug within the phosphodiesterase-5 inhibitor (PDE5i) class. Similar to other PDE5i drugs, udenafil has been developed for erectile dysfunction and is approved in Asia with single dosages of 50, 100, and 200 mg. There have been personal reports of off-label use of udenafil for pulmonary arterial hypertension (PAH), and udenafil has a theoretical background of pulmonary vasodilator effect as a member of the PDE5i class. Furthermore, udenafil has a longer half-life (10–13 hours) than sildenafil and has a rapid onset of action (peak plasma concentration 1–2 hours after administration); thus, drug usage twice per day is possible. However, acute hemodynamic response and optimal dosage for patients with PAH has not yet been evaluated. We investigated the acute hemodynamic impact of udenafil on pulmonary and systemic circulation and determine the optimal dosage for a phase IIb study.
METHODS
Patient population
Patients (age ≥18 years) who were diagnosed with idiopathic PAH or PAH associated with connective tissue disease with symptoms (New York Heart Association [NYHA] functional class II–IV) were included. At baseline right heart catheterization, all patients met the following hemodynamic criteria for inclusion; mean pulmonary arterial pressure (mPAP) ≥25 mmHg, PVR ≥400 dyne/sec/cm−5, and pulmonary artery wedge pressure (PAWP) or left ventricular end diastolic pressure ≤15 mmHg.
All patients should have maintained their medication for the last 30 days and endothelin receptor antagonist (ERA) dosage should not be changed for 12 weeks before the screening test.
Exclusion criteria was as follows: 1) expected survival less than 6 months, 2) body mass index less than 18.5 kg/m2, 3) HIV infection, 4) recent myocardial infarction, cerebral infarction, and life threatening arrhythmia within 6 months before screening, 5) history of atrial septostomy or angina within 12 weeks of screening, 6) left ventricular ejection fraction less than 45% or any left ventricular outflow tract obstruction or aortic stenosis at screening echocardiography, 7) abnormal liver function test (3 times higher aspartate aminotransferase/alanine aminotransferase level than normal range), 8) hypotension less than 90/50 mmHg or uncontrolled hypertension greater than 170/100 mmHg, 9) severe lung disease (total lung capacity less than 70%), 10) severe renal or moderate to severe liver disease, 11) history of non-arteritic anterior ischemic optic neuropathy, 12) pregnancy, peripartum, or breast-feeding woman, 13) Corrected QT prolongation on electrocardiogram, 14) history of hypersensitivity to PDE5i, 15) drugs or diet that can affect udenafil metabolism such as antibacterials, antifungals, antiviral agent, cimetidine, and grapefruit juice.
The Institutional Review Board of each institute (1105-039-361 at Seoul National University Hospital; 2011-05-037 at Samsung Medical Center; 4-2011-0343 at Yonsei Severance Hospital) and regulatory authorities approved the study protocol, and written informed consent was obtained from all participants. The Ministry of Food and Drug Safety in Korea approved the use of udenafil in patients with PAH for this study.
Study protocol
After screening, study subjects (n=18) were randomly allocated to udenafil 50 mg (U50), udenafil 100 mg (U100), or placebo groups. There were 6 subjects in each group. Swan-Ganz catheters were inserted through the internal jugular vein, and baseline hemodynamics were evaluated. After initial assessment, undenafil or placebo was administrated to all patients. Hemodynamic monitoring was maintained for 4 hours and hemodynamics were repeatedly assessed at 30, 60, 90, 120, 180, and 240 minutes after drug administration.
Hemodynamic evaluation
Major hemodynamic parameters for drug response were evaluated as follows: pulmonary vascular resistance index (PVRI), pulmonary vascular resistance (PVR), cardiac index (CI), and cardiac output (CO). Standard thermodilution technique was used to estimate CO, and CI was calculated by multiplying CO by heart rate (HR). Additional hemodynamic parameters were acquired from continuous monitoring from Swan-Ganz catheters and a-line monitoring as follows: mPAP, systolic pulmonary arterial pressure, diastolic pulmonary arterial pressure, systemic vascular resistance index, systemic vascular resistance, mean systemic arterial pressure (mSAP), systolic systemic arterial pressure, diastolic systemic arterial pressure, PAWP, right atrial pressure, central venous pressure, HR, mixed venous oxygen saturation, partial pressure of arterial oxygen, partial pressure of carbon dioxide, and oxygen saturation.
Adverse events, adverse drug reactions, and serious adverse events were monitored throughout the study by the attending physician.
Data analysis
This was an exploratory clinical trial to determine the dose of udenafil for PAH, and formal sample size calculations were not conducted. Considering a drop-out rate of 15%, a total of 18 patients (or 6 patients in each group) was the target number of subjects.
Demographics, baseline characteristics, hemodynamic variables, and gas exchange variables were summarized by treatment group and all subjects. Demographics and baseline characteristics were summarized with descriptive statistics as mean±standard deviation for continuous variables and frequency for categorical variables. Also, hemodynamic parameters were described with median and quartiles as (Q1, Q3).
This study enrolled fewer subjects and nonparametric methods were used. Wilcoxon signed rank test was conducted at a significance level of 0.1 to compare the maximal decrease from baseline within each treatment group. In addition, Page's trend test was conducted to confirm increasing the tendency for change from baseline CI in the U50 group.
All statistical analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, NC, USA) and R software version 3.2.3 (R Foundation, Vienna, Austria).
RESULTS
Eighteen patients (6 patients per group) were finally included and analyzed. Characteristics of study subjects and baseline hemodynamic parameters are described in Table 1. Mean age was 40, and 78% of all patients were female. According to the NYHA functional classification, 56% of patients were in functional class II and 44% of patients were in functional class III. ERA medication was already given in 72% of the patients. The etiology of PAH was idiopathic in 61% of patients and the rest were PAH associated with connective tissues disease. The mPAP was markedly elevated (52.0 mmHg; 38.0, 58.0), and mSAP was normal. CI was in the lower normal range (2.95 L/min/m2) with a correspondingly increased PVRI (PVRI, 1,182 dyne/sec/cm−5/m−2; 679, 2,150).
Table 1. Baseline hemodynamic and gas exchange variables.
| All (n=18) | U50 group (n=6) | U100 group (n=6) | Placebo group (n=6) | ||
|---|---|---|---|---|---|
| Age (years) | 40±14 | 36±18 | 49.00±13.61 | 36.17±6.11 | |
| Gender (women) | 14 (78) | 5 (83) | 6 (100) | 3 (50) | |
| PAH cause | |||||
| IPAH | 11 (61.1) | 5 (83.3) | 2 (33.3) | 4 (66.7) | |
| CTD | 7 (38.9) | 1 (16.7) | 4 (66.7) | 2 (33.3) | |
| NYHA functional class | |||||
| Class II | 10 (56) | 1 (17) | 3 (50) | 6 (100) | |
| Class III | 8 (44) | 5 (83) | 3 (50) | 0 (0) | |
| ERA medication | 13 (72) | 4 (67) | 4 (67) | 5 (83) | |
| HR (beats/min) | 74 (62, 82) | 83 (58, 94) | 65 (62, 74) | 74 (67, 76) | |
| mSAP (mmHg) | 84.5 (78.0, 88.0) | 84.0 (84.0, 88.0) | 81.5 (74.0, 86.0) | 87.0 (82.0, 96.0) | |
| mPAP (mmHg) | 52.0 (38.0, 58.0) | 50.5 (43.0, 72.0) | 47.0 (30.0, 57.0) | 55.0 (49.0, 66.0) | |
| CI (L/min/m2) | 2.95 (1.79, 3.50) | 2.21 (1.48, 3.03) | 3.24 (2.71, 3.50) | 3.18 (1.79, 3.65) | |
| SVRI (dyne/sec/cm−5/m−2) | 1,944 (1,733, 3,069) | 2,968 (1,819, 3,070) | 1,743 (1,606, 3,131) | 1,945 (1,733, 2,959) | |
| PVRI (dyne/sec/cm−5/m−2) | 1,182 (679, 2,150) | 1,521 (738, 3,237) | 887 (575, 1,187) | 1,213 (1,098, 2,150) | |
| MVO2 | 78.6 (68.2, 83.1) | 77.9 (71.0, 85.3) | 67.2 (65.0, 79.8) | 82.3 (77.4, 88.0) | |
| PaO2 (mmHg) | 73.8 (68.7, 91.6) | 81.8 (72.0, 91.6) | 72.9 (60.0, 79.0) | 70.0 (67.1, 94.6) | |
Data are presented as mean±standard deviation or number (%), or median value with quartiles (Q1, Q3).
CI = cardiac index; CTD = connective tissue disease; ERA = endothelin-receptor antagonist; HR = heart rate; IPAH = idiopathic pulmonary arterial hypertension; mPAP = mean pulmonary arterial pressure; mSAP = mean systemic arterial pressure; MVO2 = mixed venous oxygen saturation; NYHA = New York Heart Association; PAH = pulmonary arterial hypertension; PaO2 = partial pressure of arterial oxygen; PVRI = pulmonary vascular resistance index; SVRI = systemic vascular resistance index; U50 = udenafil 50 mg; U100 = udenafil 100 mg.
Hemodynamic changes after udenafil administration
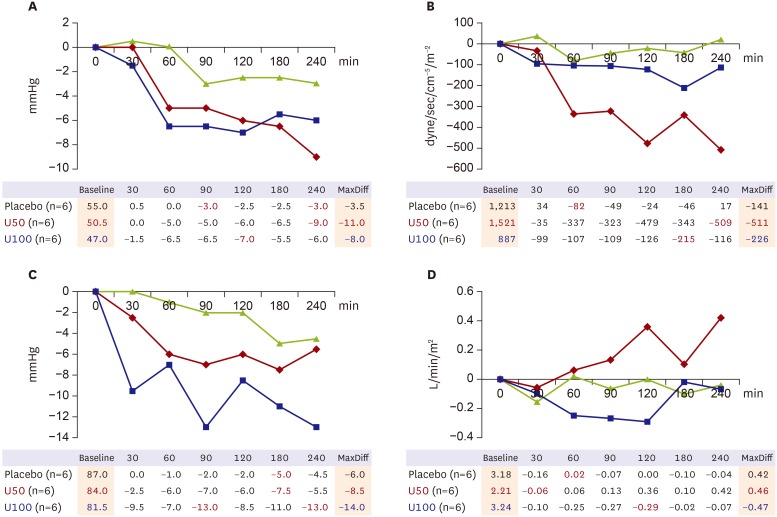
Hemodynamic monitoring showed decreased mPAP after udenafil administration with a single dose of both 50 and 100 mg (Figure 1A). Maximal mPAP reduction during 4 hours of monitoring was 11.0 mmHg in the U50 group and 8.0 mmHg in the U100 group. However, PVRI decreased only in the U50 group (MaxDiff=−511 dyne/sec/cm−5/m−2) and the U100 and placebo groups did not show a significant change (MaxDiff=226 and −141 dyne/sec/cm−5/m−2) (Figure 1B). Change in mSAP was significant in the U100 group, with a maximal difference of 14 mmHg, which is significantly lower than the baseline value. However, this was not true in the U50 group (Figure 1C). CI tended to increase in the U50 group (p<0.01) and there was no change in the placebo and U100 groups (p>0.05, both) (Figure 1D).
Figure 1. Changes in major hemodynamic parameters according to time. (A) Change in mPAP from baseline. (B) Change in PVRI from baseline. (C) Change in mSAP from baseline. (D) Change in CI from baseline.
CI = cardiac index; mPAP = mean pulmonary arterial pressure; mSAP = mean systemic arterial pressure; PVRI = pulmonary vascular resistance index; U50 = udenafil 50 mg; U100 = udenafil 100 mg.
There were no serious adverse events during the study. Adverse events were observed in 4 patients in the placebo group, no patients in the U50 group, and 2 patients in the U100 group. Adverse events and adverse drug reactions are shown in Table 2.
Table 2. Adverse event and adverse drug reaction.
| Placebo | U50 | U100 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of subjects | No. of events | No. of subjects | No. of events | No. of subjects | No. of events | ||||
| Adverse event | 4 | Mild | 8 | 0 | Mild | 0 | 2 | Mild | 2 |
| Moderate | 1 | Moderate | 0 | Moderate | 2 | ||||
| Severe | 0 | Severe | 0 | Severe | 0 | ||||
| Adverse drug reaction | 0 | Mild | 0 | 0 | Mild | 0 | 1 | Mild | 2 |
| Moderate | 0 | Moderate | 0 | Moderate | 0 | ||||
| Severe | 0 | Severe | 0 | Severe | 0 | ||||
| Serious adverse event | 0 | 0 | 0 | 0 | 0 | 0 | |||
U50 = udenafil 50 mg; U100 = udenafil 100 mg.
Of note, there were no adverse events or adverse drug reactions in the U50 group. Adverse events in the placebo group were procedure-related, such as puncture site pain. Adverse events in the U100 group were exclusively puncture-related, and 1 patient experienced the adverse drug reaction of mild fever, which was possibly related to udenafil.
DISCUSSION
Udenafil is a newly developed long-acting PDE5i with efficacy and safety profiles comparable with those of other PDE5i. Similar to other PDE5i, udenafil was originally developed for the treatment of erectile dysfunction.1) Udenafil has a longer half-life than sildenafil and shows persistent vasodilator effects for 12 hours after administration.2),3) Like other PDE5i, udenafil has been used for a variety of clinical applications for important disease entities, including left ventricular hypertrophy,4) heart failure,5) and portal hypertension.6)
Because sildenafil7) and tadalafil8) are already approved for PAH-specific treatment, the class effect of udenafil in this rare but clinically important disease has been anticipated. Because of delayed approval of sildenafil and tadalafil for PAH treatment by the Korean Food & Drug Administration, there have been off-label uses of udenafil for patients with PAH. Clinical experiences for PAH in Korea have been shared in a clinical working group. This study was designed to determine optimal dosage and administration intervals of udenafil in patients with PAH by demonstrating continuous hemodynamic monitoring data, which can be used for efficacy study of udenafil in PAH. Udenafil tablets are already available as 3 different types, i.e., 50, 100, and 200 mg, in clinical practice. Among the 3 different doses, the 50 and 100 mg tablets were adopted for this study to investigate the basis of pharmacokinetics in patients with PAH, based on the earlier study performed in patients with heart failure.5)
In the current study, we found that mPAP was significantly and similarly decreased in both the U50 and U100 groups compared to the placebo group. This salutary effect started 1 hour after udenafil administration and persisted for at least 4 hours. Of interest, PVRI was only decreased in the U50 group up to 511 dyne/sec/cm−5/m−2 compared to the baseline value, but a reduction in PVRI was not observed in U100, which only demonstrated a small decrease in PVRI. This observation should be considered together with serial changes in mSAP and CI; although mSAP decreased in both U50 and U100, the amount of reduction in the U100 group was greater than the corresponding value observed in the U50 group. More importantly, change of mSAP was even greater than that of mPAP in the U100 group. Of note, CI was significantly increased in U50, suggesting an immediate benefit in PAH hemodynamics. However, CI significantly decreased in U100 for 2 hours after administration of U100. This finding implies that 50 mg udenafil should be considered the optimal dose in PAH management rather than 100 mg as a single dosage. It is conceivable that mSAP was significantly decreased in U100 for the first 90 minutes after udenafil administration, possibly resulting in preload reduction in the right ventricle, and finally leading to decreased CO even though PVRI was decreased.
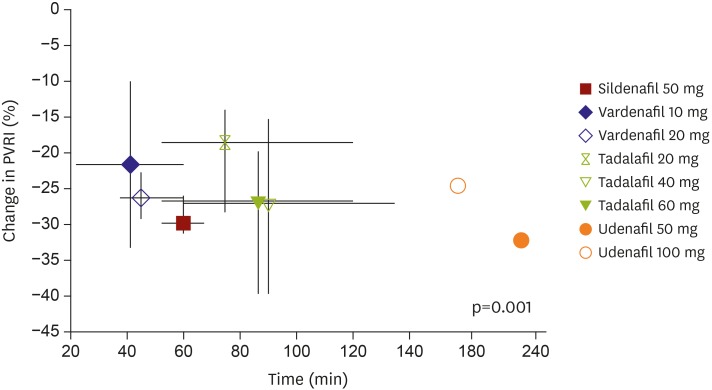
PDE5i is a potent vasodilator, and its selectivity for pulmonary circulation is an essential component when a new PDE5i is considered for PAH treatment. The acute hemodynamic effects of sildenafil, tadalafil, and vardenafil on pulmonary circulation in earlier studies9) were variable. In addition, there were variable effects in their kinetics of pulmonary vasorelaxation (most rapid effect by vardenafil), selectivity for pulmonary circulation (sildenafil and tadalafil, but not vardenafil), and impact on arterial oxygenation (improvement with sildenafil only). Currently, sildenafil and tadalafil have been approved for PAH treatment based on clinical evidence. Our study shows good selectivity of udenafil for pulmonary circulation at a dosage of 50 mg, resulting in pulmonary vasodilation without significant compromise of systemic circulation. Hemodynamic response to udenafil started an hour after udenafil administration and persisted for at least 4 hours. When we compared the study results with previous data published by using sildenafil, tadalafil, and vardenafil,9) a change in maximal PVRI was greatest with U50. This result suggests that udenafil has the highest selectivity for pulmonary circulation, although udenafil is similar to sildenafil in molecular structures and is comparable in terms of PDE5 selectivity10) (Figure 2). Considering the consistent and continuing reduction in mPAP and PVRI in the U50 group and the long half-life of udenafil,11),12) a 12-hour administration interval of U50 is possible and clinically relevant.
Figure 2. Plotting of maximal PVRI change in udenafil on data from previous study by Ghofrani et al.9) Although plotted on different study subsets, change in maximal PVRI was greatest with U50, suggesting the highest selectivity of udenafil for pulmonary circulation, although udenafil is similar to sildenafil in molecular structure and is comparable in terms of PDE5 selectivity.
PDE5 = phosphodiesterase-5; PVRI = pulmonary vascular resistance index; U50 = udenafil 50 mg; U100 = udenafil 100 mg.
In this study, despite a small number of patients enrolled, we found only minor adverse drug reactions in the U100 group. Given a long half-life and only 2 administrations per day, patient compliance would be augmented with udenafil compared to sildenafil because daily medication frequency is generally accepted to exert a significant effect on patient compliance and potentially on prognosis.13),14)
In conclusion, for the first time, we demonstrated the acute hemodynamic changes induced by udenafil in patients with PAH. PVRI reduction was greater in a 50 mg-dose of udenafil as compared to that in a 100 mg-dose of udenafil. CI did not decrease at most points and safety was excellent in the U50 group, suggesting that 50 mg udenafil is adequate for the phase progress of PAH clinical trials. This study lays the foundation for clinical trials with udenafil for PAH treatment.
Footnotes
Funding: This study was supported by Dong-A ST, Seoul, Korea.
Conflict of Interest: This study was supported by Dong-A ST, but the sponsor had no role in the design of the study, the collection, and analysis of the data, or the preparation of the manuscript. The authors have no financial conflicts of interest.
- Conceptualization: Kim DK.
- Data curation: Chang SA, Kim HK, Chang HJ, Kim DK.
- Formal analysis: Chang SA, Kim HK, Chang HJ, Kim DK.
- Funding acquisition: Kim DK.
- Investigation: Kim HK, Kim DK.
- Methodology: Chang SA, Kim HK, Chang HJ, Kim DK.
- Project administration: Kim DK.
- Resources: Chang SA, Kim HK.
- Supervision: Kim DK.
- Validation: Chang SA, Kim HK, Kim DK.
- Visualization: Chang SA, Kim HK.
- Writing - original draft: Chang SA, Kim HK.
- Writing - review & editing: Chang SA, Kim HK, Kim DK.
References
- 1.Zhao C, Kim SW, Yang DY, et al. Efficacy and safety of once-daily dosing of udenafil in the treatment of erectile dysfunction: results of a multicenter, randomized, double-blind, placebo-controlled trial. Eur Urol. 2011;60:380–387. doi: 10.1016/j.eururo.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 2.Park HJ, Park JK, Park K, Min K, Park NC. Efficacy of udenafil for the treatment of erectile dysfunction up to 12 hours after dosing: a randomized placebo-controlled trial. J Sex Med. 2010;7:2209–2216. doi: 10.1111/j.1743-6109.2010.01817.x. [DOI] [PubMed] [Google Scholar]
- 3.Kim BH, Lim HS, Chung JY, et al. Safety, tolerability and pharmacokinetics of udenafil, a novel PDE-5 inhibitor, in healthy young Korean subjects. Br J Clin Pharmacol. 2008;65:848–854. doi: 10.1111/j.1365-2125.2008.03107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim HL, Kim YJ, Kim KH, et al. Therapeutic effects of udenafil on pressure-overload cardiac hypertrophy. Hypertens Res. 2015;38:597–604. doi: 10.1038/hr.2015.46. [DOI] [PubMed] [Google Scholar]
- 5.Kim KH, Kim HK, Hwang IC, et al. PDE 5 inhibition with udenafil improves left ventricular systolic/diastolic functions and exercise capacity in patients with chronic heart failure with reduced ejection fraction; a 12-week, randomized, double-blind, placebo-controlled trial. Am Heart J. 2015;169:813–822.e3. doi: 10.1016/j.ahj.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 6.Kreisel W, Deibert P, Kupcinskas L, et al. The phosphodiesterase-5-inhibitor udenafil lowers portal pressure in compensated preascitic liver cirrhosis. A dose-finding phase-II-study. Dig Liver Dis. 2015;47:144–150. doi: 10.1016/j.dld.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 7.Galiè N, Ghofrani HA, Torbicki A, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 8.Galiè N, Brundage BH, Ghofrani HA, et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009;119:2894–2903. doi: 10.1161/CIRCULATIONAHA.108.839274. [DOI] [PubMed] [Google Scholar]
- 9.Ghofrani HA, Voswinckel R, Reichenberger F, et al. Differences in hemodynamic and oxygenation responses to three different phosphodiesterase-5 inhibitors in patients with pulmonary arterial hypertension: a randomized prospective study. J Am Coll Cardiol. 2004;44:1488–1496. doi: 10.1016/j.jacc.2004.06.060. [DOI] [PubMed] [Google Scholar]
- 10.Paick JS, Kim SW, Yang DY, et al. The efficacy and safety of udenafil, a new selective phosphodiesterase type 5 inhibitor, in patients with erectile dysfunction. J Sex Med. 2008;5:946–953. doi: 10.1111/j.1743-6109.2007.00723.x. [DOI] [PubMed] [Google Scholar]
- 11.Salem EA, Kendirci M, Hellstrom WJ. Udenafil, a long-acting PDE5 inhibitor for erectile dysfunction. Curr Opin Investig Drugs. 2006;7:661–669. [PubMed] [Google Scholar]
- 12.Kim TE, Kim BH, Kim JR, et al. Effect of food on the pharmacokinetics of the oral phosphodiesterase 5 inhibitor udenafil for the treatment of erectile dysfunction. Br J Clin Pharmacol. 2009;68:43–46. doi: 10.1111/j.1365-2125.2009.03404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fitzgerald AA, Powers JD, Ho PM, et al. Impact of medication nonadherence on hospitalizations and mortality in heart failure. J Card Fail. 2011;17:664–669. doi: 10.1016/j.cardfail.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Frishman WH. Importance of medication adherence in cardiovascular disease and the value of once-daily treatment regimens. Cardiol Rev. 2007;15:257–263. doi: 10.1097/CRD.0b013e3180cabbe7. [DOI] [PubMed] [Google Scholar]