Abstract
Background
The photolithography process in the semiconductor industry uses various chemicals with little information on their constitution. This study aimed to identify the chemical constituents of photoresist (PR) products and their by-products and to compare these constituents with material safety data sheets (MSDSs) and analytical results.
Methods
A total of 51 PRs with 48 MSDSs were collected. Analysis consisted of two parts: First, the constituents of the chemical products were identified and analyzed using MSDS data; second, for verification of the by-products of PR, volatile organic compounds were analyzed. The chemical constituents were categorized according to hazards.
Results
Forty-five of 48 products contained trade secrets in amounts ranging from 1 to 65%. A total of 238 ingredients with multiple counting (35 ingredients without multiple counting) were identified in the MSDS data, and 48.7% of ingredients were labeled as trade secrets under the Korea Occupational Safety and Health Act. The concordance rate between the MSDS data and the analytical result was 41.7%. The by-product analysis identified 129 chemicals classified according to Chemical Abstracts Service No., with 17 chemicals that are carcinogenic, mutagenic, and reprotoxic substances. Formaldehyde was found to be released from 12 of 21 products that use novolak resin.
Conclusion
We confirmed that several PRs contain carcinogens, and some were not specified in the toxicological information in the MSDS. Hazardous chemicals, including benzene and formaldehyde, are released from PRs products as by-products. Therefore, it is necessary to establish a systematic management system for chemical compounds and the working environment.
Keywords: By-product, Material safety data sheet, Photoresist, Semiconductor, Trade secret
1. Introduction
The main processes in the semiconductor industry consist of wafer manufacturing, fabrication (fab), and packaging. Wafer manufacturing uses silicon (Si) extracted from silicon dioxide (SiO2). Fab adjusts the circuit pattern on the wafer through specific processes. The packaging process assembles chips by cutting wafers. Fab processes, which use numerous chemicals, are divided into several sequences: oxidation, photolithography, etching, and stripping [1], [2], [3], [4], [5].
Although studies related to occupational disease in the semiconductor industry have been conducted, logical bases that can confirm the correlation between task and disease have been insufficient. It is difficult to identify the hazards that employees may be exposed to if the constituents of chemical compounds are not sufficiently identified [2].
The greatest number of chemicals in the fab process is used during photolithography. Photoresists (PRs), the main set of chemicals used in photolithography, consist of a polymer, solvent, sensitizer, and additives. A PR can be classified as positive or negative depending on its response to light, and most chemical constituents are specified as trade secrets [1], [5], [6], [7]. In addition, by-products can be released by ultraviolet (UV) light and heat during the development process [8], [9].
Detailed chemical information is difficult to obtain due to lack of sufficient data, trade secrecy, and fast-changing technology. However, it is necessary to evaluate past exposure information, such as transitions in chemicals, process technologies, and management techniques, for the investigation of exposure to hazards [3], [7], [10].
The general chemical constituents used in photolithography are known, but information regarding specific and real chemical use is insufficient [5]. For example, xylene, ethylene glycol monoethyl ether acetate (EGMEA), toluene, hexamethyldisilazane, and propylene glycol ether (PGE) have been used as coating agents in PR chemical products [1], [7]. Because of its reproductive toxicity, EGMEA was replaced with PGE in the 1990s [11].
It is thus necessary to investigate chemical constituents and toxicological information through material safety data sheets (MSDSs). Subsequently, comparisons between MSDS information and product analyses that detail the chemical composition of products is required [12], [13].
Carcinogenic risk in the semiconductor industry is widely known; however, no studies identifying chemicals used in semiconductor industry processes have been conducted. It is therefore necessary to investigate the chemical constituents of chemical products for the protection of employee health, establishment of a systematic management system for chemical products, and further study of the correlation between chemical exposure and disease.
The aim of this study was to identify the chemical constituents of PR products and their by-products by analytical result and to compare this information with MSDS data.
2. Materials and methods
2.1. Sample and MSDS collection
A total of 51 PR products used in the fab process and their associated MSDS were collected, through convenience sampling from three companies and one academic semiconductor research center in Korea. An MSDS was not available for three of the PR products (Table 1). After reviewing the MSDS to confirm basic information, organic solvents in PR products and their possible by-products were analyzed, and the results acquired from instrumental analyses were compared with the MSDS.
Table 1.
The number of photoresist (PR) products used in this study
| Company | Total no of products | MSDS not provided |
|---|---|---|
| A | 26 | 1 |
| B | 7 | 2 |
| C | 9 | 0 |
| D | 9 | 0 |
| Total | 51 | 3 |
2.2. Sample analysis
Organic solvents contained in PR products were analyzed after dilution with carbon disulfide (CS2; Kanto, Japan) and methanol (99.8%; Sigma Aldrich, USA). Qualitative analysis was first performed to identify organic solvents in 51 PR products, and further analysis was conducted for quantitation of the identified 20 chemicals. Diluted samples were sonicated for 30 min at room temperature, and viscous chemical samples were filtered through a nylon syringe filter (13 mm, 0.2 μm, Whatman, USA). Qualitative analysis was conducted by gas chromatography (GC, 7890A; Agilent Technology, USA)–mass spectrometry (MS, 5975C Series; Agilent Technology, USA) and auto sampler (Combi PAL, CTC analytics, Switzerland) in scan mode. A DB-5MS column (122-5532; Agilent Technology, USA) was used for analysis. Each mass spectrum was matched up with a GC-MS library (W10N11), and the chemical matching rate selected was higher than 80%.
Then, quantitative analysis was conducted by GC (6890N; Agilent Technology) with a flame ionization detector (FID) and auto sampler (7683B Series; Agilent Technology). The chemical used for quantitative analysis was selected from chemicals detected from the qualitative analysis listed in the MSDS, or if not listed in the MSDS, a chemical known to be toxic was selected. An EN-5 column (053139; SGE Analytical Science, Australia) was used for the analysis.
2.3. By-products of the PRs
Analysis was conducted on volatile organic compounds (VOCs) released from the PR as by-products, using headspace solid-phase microextraction (HS-SPME; Combi PAL, CTC analytics) with GC/MS in scan mode. A 100-mg portion of the bulk sample was sealed in a 20-mL amber headspace glass vial with an aluminum-coated polytetrafluoroethylene/silicone septum. A 75-μm carboxen/polydimethyl siloxane SPME fiber was used. The vial was placed into a heating block set to 110°C, which is the normal PR application temperature, for 3 min, and then the SPME fiber was inserted into the vial for adsorption. Afterward, the SPME fiber was transferred into the injector of the GC for thermal desorption at 250°C for 5 min. A DB-5MS column (122-5532; Agilent Technology) was used. Each mass spectrum was matched with a GC-MS library (W10N11), and the chemical matching rate selected was higher than 80%.
The formaldehyde emissions from novolak resin were also investigated by HS/SPME-GC/MS in the selected ion monitoring mode; a 500-mg portion of the bulk sample was sealed in a 20-mL amber vial, and a 65-μm polydimethylsiloxane/divinylbenzene SPME fiber was used. The heating block was set to 180°C for 3 min, and the injector was set at 200°C for 5 min for thermal desorption. A DB-WAX column (122-7032; Agilent Technology) was used for analysis.
2.4. Quality control
Quality control was conducted to evaluate the accuracy and precision of analysis. The accuracy and precision, measured as recovery of the spiked samples and standard deviations, respectively, were acquired by analyzing 3 μL of the spiked stock solution in 1 mL of CS2 10 times.
The stock solution consisted of 1 mL of each seven representative ingredient substance of PR including propylene glycol monomethyl ether (PGME) , 1,4-dioxane, ethylbenzene, propylene glycol monomethyl ether acetate (PGMEA) , 2-heptanone, cyclohexanone, and ethyl 3-ethoxypropionate (EEP). The mean accuracy and standard deviation of all ingredients was 100.81 ± 2.71% and ranged from 94.61 ± 2.06% (EEP) to 108.01 ± 1.99 % (2-heptanone).
3. Results
3.1. Review of the chemical components in the MSDS
According to the review of MSDS data, each product contained an average of five ingredients. It was found that 45 products contained trade secret materials in a range from 1 to 65%. Only three products had a full list of chemical constituents with Chemical Abstracts Service (CAS) numbers on the MSDS.
A total of 238 ingredients with multiple counting were identified in the MSDS data for 48 products, and 48.7% of ingredients were counted as trade secrets. When removing multiple counts, 35 chemical ingredients were identified. Top six ingredients among 35 ingredients are shown in Table 2, and the details of trade secret ingredients that were counted as one ingredient are shown in Table 3. Other 29 ingredients are listed in Supplementary table 1.
Table 2.
Chemical names frequently found in the material safety data sheets (MSDSs) of photoresist (PR) products
| No. | Constituent | CAS no. | Frequency of usage |
|---|---|---|---|
| 1 | Trade secret ingredients∗ | — | 116 |
| 2 | Propylene glycol monomethyl ether acetate | 108-65-6 | 33 |
| 3 | Cyclohexanone | 108-94-1 | 11 |
| 4 | Propylene glycol monomethyl ether | 107-98-2 | 7 |
| 5 | 2-Heptanone | 110-43-0 | 7 |
| 6 | Ethyl 3-ethoxypropionate | 763-69-9 | 7 |
| 7 | Other ingredients† | — | 57 |
| Total ingredients | 238 | ||
CAS, Chemical Abstracts Service.
Trade secret ingredients are shown in Table 3.
Other ingredients are shown in Supplementary table 1.
Table 3.
Trade secret ingredients according to Table 2 in the text
| No. | General name of trade secret constituent∗ | Frequency of usage |
|---|---|---|
| 1 | Resin | 36 |
| 2 | Sensitizer | 21 |
| 3 | Pigment | 16 |
| 4 | Additive | 13 |
| 5 | Monomer | 9 |
| 6 | Derivatives | 6 |
| 7 | Photoactive | 4 |
| 8 | Polymer | 3 |
| 9 | Cross-linker | 2 |
| 10 | Initiator | 2 |
| 11 | Trade secret | 2 |
| 12 | Generator | 1 |
| 13 | Others | 1 |
| Total trade secret ingredients | 116 | |
The ingredients that were having a not specified CAS No. were regarded as trade secret ingredients.
PGMEA was the most commonly used ingredient (33 of 48 products), followed by cyclohexanone (11 of 48 products), PGME, 2-heptanone, and EEP (in 7 products) (Table 2). Thirteen constituents were trade secret ingredients without a CAS number, and these are listed in Table 3.
At least one of four carcinogens was specified in the MSDS data of 16 products (33.3%) as shown in Table 4, but some of the corresponding hazardous identification and toxicological information were inappropriately specified in the MSDS. Eight products accurately specified hazardous identification and toxicological information in the MSDS, whereas other eight products that include cyclohexanone, ethylbenzene, pyridine, and 1,4-dioxane have incorrect toxicological information. Six products among them only specified international toxicological information from the International Agency for Research on Cancer, the American Conference of Governmental Industrial Hygienists, or the Occupational Safety and Health Administration, and domestic information from the Ministry of Employment and Labor (MOEL) was not specified. In addition, other two products do not have any toxicological information.
Table 4.
Information on carcinogens specified in the material safety data sheets (MSDSs) for photoresist (PR) products
| Constituent∗ | CAS No.∗ | No. of products | Content (%)∗ | Korea MOEL† | IARC‡ | ACGIH§ | NTP‖ | EU CLP¶ |
|---|---|---|---|---|---|---|---|---|
| Cyclohexanone | 108-94-1 | 11 | 3–40 | Car. 2 | Group 3 | A3 | ||
| Ethylbenzene | 100-41-4 | 1 | 0.1–1.0 | Car. 2 | Group 2B | A3 | ||
| Pyridine | 110-86-1 | 1 | 0.1–1.0 | Car. 2 | Group 3 | A3 | ||
| 1,4-Dioxane | 123-91-1 | 4 | <1.0 | Car. 2 | Group 2B | A3 | R | Car. 2 |
ACGIH, American Conference of Governmental Industrial Hygienists; CAS, Chemical Abstracts Service; IARC, International Agency for Research on Cancer; MOEL, Ministry of Employment and Labor.
The name of constituent, CAS number, and content (%) are only specified in the MSDS, and the toxic information of each institute was accessed as follows: Korea MOEL (2018.03.20), ACGIH (2018 booklet), IARC (2018.04.18), NTP (14th report), and EU CLP (Annex vi table 2018).
Ministry of Employment and Labor, Carcinogen classifications – Carcinogen 1A: Sufficient evidence of carcinogenicity to human, Carcinogen 1B: Sufficient evidence of carcinogenicity to animals or limited evidence of carcinogenicity to human and animals, Carcinogen 2: Insufficient evidence of carcinogenicity to humans and animals.
International Agency for Research on Cancer, Carcinogen classifications – Group 1: Carcinogenic to humans, Group 2A: Probably carcinogenic to humans, Group 2B: Possibly carcinogenic to humans, Group 3: Not classifiable as to its carcinogenicity to humans, Group 4: Probably not carcinogenic to humans.
American Conference of Governmental Industrial Hygienists, Carcinogen classifications – A1: Confirmed human carcinogen, A2: Suspected human carcinogen, A3: Confirmed animal carcinogen with unknown relevance to humans, A4: Not classifiable as a human carcinogen, A5: Not suspected as a human carcinogen.
National Toxicology Program, Carcinogen classifications – K: Known to be human carcinogens, R: Reasonably anticipated to be human carcinogens.
Classification, Labeling, Packing of substances and mixture, Carcinogen classifications – Carcinogen 1A: Known to have carcinogenic potential for humans, Carcinogen 1B: May cause cancer, Carcinogen 2: Suspected of causing cancer.
Cyclohexanone was contained in 11 products, with contents varying from 3 to 40%. All products containing cyclohexanone also contained PGMEA and acrylic resin. In four products, 1,4-dioxane was contained as an impurity, with contents less than 1%, and ethylbenzene and pyridine were used in one product, with contents varying from 0.1 to 1%.
3.2. Comparison of analytical results with MSDS
According to the Korea Occupational Safety and Health Act (KOSHAct) of the Korea MOEL, carcinogenic, mutagenic, and reprotoxic (CMR) substances and hazardous substances requiring management specified by the KOSHAct and toxic chemicals specified by the Chemical Management Act of the Ministry of Environment should not be designated as trade secret substance in MSDS, and its chemical name, content, and hazardous information should be presented.
As a result of the qualitative analysis, nine ingredients that should not be designated as trade secrets by the KOSHAct were identified, with no information in the MSDS (Table 5). Five of the nine ingredients did not have toxicological information specified in the MSDS, although this is required. These contained CMR substances (ethylbenzene, 1,4-dioxane, styrene, and 2-butoxyethanol); toxic chemicals (p-cresol, 1,3-dimethylbenzene, 2,3-dimethylphenol, and 3,4-dimethylphenol) defined by the Chemical Management Act; and hazardous substances requiring management by the KOSHAct (ethylbenzene, p-cresol, 1,3-dimethylbenzene, 1,4-dioxane, styrene, and 2-butoxyethanol).
Table 5.
Chemical constituents identified which should not be listed as trade secrets by the Korea Occupational Safety and Health Act (KOSHAct), but for which there is no information in the material safety data sheets (MSDSs)
| Compound | CAS No. | Korea MOEL∗ | KOSHA† | IARC‡ | ACGIH§ | NCIS‖ | NTP¶ | EU CLP# |
|---|---|---|---|---|---|---|---|---|
| Ethylbenzene | 100-41-4 | Car. 2 | Hazardous substances requiring management | Group 2B | A3 | |||
| p-Cresol | 106-44-5 | Hazardous substances requiring management | A4 | Toxic chemical | ||||
| 1,3-Dimethylbenzene | 108-38-3 | Hazardous substances requiring management | A4 | Toxic chemical | ||||
| 1,4-Dioxane | 123-91-1 | Car. 2 | Hazardous substances requiring management | Group 2B | A3 | R | Car. 2 | |
| 2,3-Dimethylphenol | 526-75-0 | Toxic chemical | ||||||
| 3,4-Dimethylphenol | 95-65-8 | Toxic chemical | ||||||
| Styrene | 100-42-5 | Car. 2, Repr. 2 | Hazardous substances requiring management | Group 2A | A4 | R | Repr. 2 | |
| 2-Butoxyethanol | 111-76-2 | Car. 2 | Hazardous substances requiring management | Group 3 | A3 | |||
| 2-Heptanone | 110-43-0 | Hazardous substances requiring management |
ACGIH, American Conference of Governmental Industrial Hygienists; CAS, Chemical Abstracts Service; IARC, International Agency for Research on Cancer; KOSHA, Korean Occupational Safety and Health Administration; MOEL, Ministry of Employment and Labor.
*Toxicological information for these ingredients was not specified in the MSDS.
The toxic information of each institute was accessed as follows; Korea MOEL (2018.03.20), ACGIH (2018 booklet), IARC (2018.04.18), NTP (14th report), and EU CLP (Annex vi table 2018).
Ministry Of Employment and Labor, Carcinogen classifications – Carcinogen 1A: Sufficient evidence of carcinogenicity to human, Carcinogen 1B: Sufficient evidence of carcinogenicity to animals or limited evidence of carcinogenicity to human and animals, Carcinogen 2: Insufficient evidence of carcinogenicity to humans and animals.
Korea Occupational Safety and Health Agency – Hazardous substances requiring management should not be designated as trade secrets by KOSHAct.
International Agency for Research on Cancer, Carcinogen classifications – Group 1: Carcinogenic to humans, Group 2A: Probably carcinogenic to humans, Group 2B: Possibly carcinogenic to humans, Group 3: Not classifiable as to its carcinogenicity to humans, Group 4: Probably not carcinogenic to humans.
American Conference of Governmental Industrial Hygienists, Carcinogen classifications – A1: Confirmed human carcinogen, A2: Suspected human carcinogen, A3: Confirmed animal carcinogen with unknown relevance to humans, A4: Not classifiable as a human carcinogen, A5: Not suspected as a human carcinogen.
National Chemicals Information System in Korea– Toxic chemical should not be designated as the trade secret by KOSHAct.
National Toxicology Program, Carcinogen classifications – K: Known to be human carcinogens, R: Reasonably anticipated to be human carcinogens.
Classification, Labeling, Packing of substances and mixture, Carcinogen classifications – Carcinogen 1A: Known to have carcinogenic potential humans, Carcinogen 1B: May causes cancer, Carcinogen 2: Suspected of causing cancer.
According to the MSDS data, 1,4-dioxane was listed as an impurity in four products but was detected in only one product. In addition, 1,4-dioxane was detected in seven products, in which no information on 1,4-dioxane was specified in the MSDS. Those seven products included both 2-heptanone and novolak resin. We detected 2-butoxyethanol in three products and styrene in one product with no information specified in the MSDS (Table 5).
Quantitative evaluation was conducted for 20 chemicals. Target chemicals were selected based on two criteria: first, VOCs specified in the MSDS, and second, VOCs identified by qualitative analysis, which should not be designated as trade secrets by the KOSHAct.
According to the KOSHAct, the contents specified in MSDS should be stated as fixed number or range. When expressed with range, the lowest and highest contents must fall within ±5% of the real content. The quantitative analysis results were compared with the MSDS data. A total of 89 ingredients with multiple counting were in accordance with the MSDS. The quantitated content of these constituents in 20 of 48 products (41.7%) was in the appropriate range of ±5% of the MSDS. Thirteen and 15 of 48 products (27.1% and 31.3%, respectively) include at least one ingredient outranging ± 5–10% or more than ± 10% of the contents written in MSDS. (Table 6). Table 7 shows an example of chemical constituents and content comparison between MSDS data and the quantitated result.
Table 6.
Concurrence rates between the material safety data sheet (MSDS) and quantitated results*
| The result of comparison | No. of product |
|---|---|
| Within the range of ±5% | 20 (41.7%) |
| Within the range of ±5–10% | 13 (27.1%) |
| Over the range of ±10% | 15 (31.3%) |
| Total | 48 (100.0%) |
*According to the KOSHAct, the contents specified in the chemical composition information should be stated in range from the lowest to the highest, and the lowest and highest contents must fall within ± 5% of the real content. Also, if the content is lower than 5%, the lowest content over 1% must be specified. Carcinogens and mutagen must be specified over 0.1%, and reproductive toxicants must be specified over 0.3%.
Table 7.
An example of chemical content comparison between MSDS and the analytical result
| No. | Compound | CAS no. | Content in MSDS (%) | Analytical content (%) |
|
|---|---|---|---|---|---|
| Diluted with CS2 | Diluted with methanol | ||||
| 1 | 2-Heptanon | 110-43-0 | 40–50 | 33.49 | 44.14 |
| Ethyl-(s)-lactate | 687-47-8 | 10–20 | 10.48 | 12.74 | |
| p-Cresol∗ | 106-44-5 | 2.20 | 2.40 | ||
| 1,4-Dioxane∗ | 123-91-1 | 0.25 | 0.27 | ||
| 2 | 3-Methoxybutyl acetate | 4435-53-4 | 45–55 | 72.28 | 70.55 |
| Propylene glycol monomethyl ether acetate | 108-65-6 | 10–20 | 12.90 | 13.47 | |
| Cyclohexanone | 108-94-1 | 10–20 | 11.95 | 14.12 | |
| n-Butyl acetate | 123-86-4 | <5 | 1.52 | 1.38 | |
| 3 | 2-Heptanone | 110-43-0 | 77–83 | 65.66 | 64.54 |
| 1,4-Dioxane∗ | 123-91-1 | 0.41 | 0.34 | ||
| p-Cresol∗ | 106-44-5 | 1.11 | 0.80 | ||
| Gamma-butyrolactone∗ | 96-48-0 | 3.01 | 2.86 | ||
| 4 | Propylene glycol monomethyl ether acetate | 108-65-6 | 60–70 | 55.84 | 59.01 |
| Ethyl 3-ethoxypropionate (EEP) | 763-69-9 | 10–20 | 14.52 | 14.48 | |
| Propylene glycol monomethyl ether | 107-98-2 | 1–5 | 1.63 | 1.20 | |
| 2-Butoxyethanol∗ | 111-76-2 | 0.70 | 0.53 | ||
p-Cresol, 1,4-dioxane, gamma-butyrolactone, and 2-butoxyethanol are not specified in MSDS.
Among VOCs that should not be designated as trade secrets by the KOSHAct, 2-butoxyethanol were detected over 1% and 1,4-dioxane, styrene, ethylbenzene, PGME, and PGMEA were detected at less than 1%.
According to the KOSHAct, the contents specified in the chemical composition information should be stated in a range from the lowest to the highest, and the lowest and highest contents must fall within ±5% of the real content. Also, if the content is lower than 5%, the lowest content above 1% must be specified. Carcinogens and mutagens must be specified over 0.1%, and reproductive toxicants must be specified over 0.3%.
3.3. The VOCs emitted from the PR after heat treatment
A total of 51 products were analyzed by SPME-GC/MS to determine the by-products resulting from heat treatment. The analysis confirmed the presence of 129 chemicals classified according to CAS No., none of which were specified in the MSDS. Toluene was the most frequently released chemical (29 of 51 products), followed by p-cresol (23 products), PGME (22 products), and acetone (18 products).
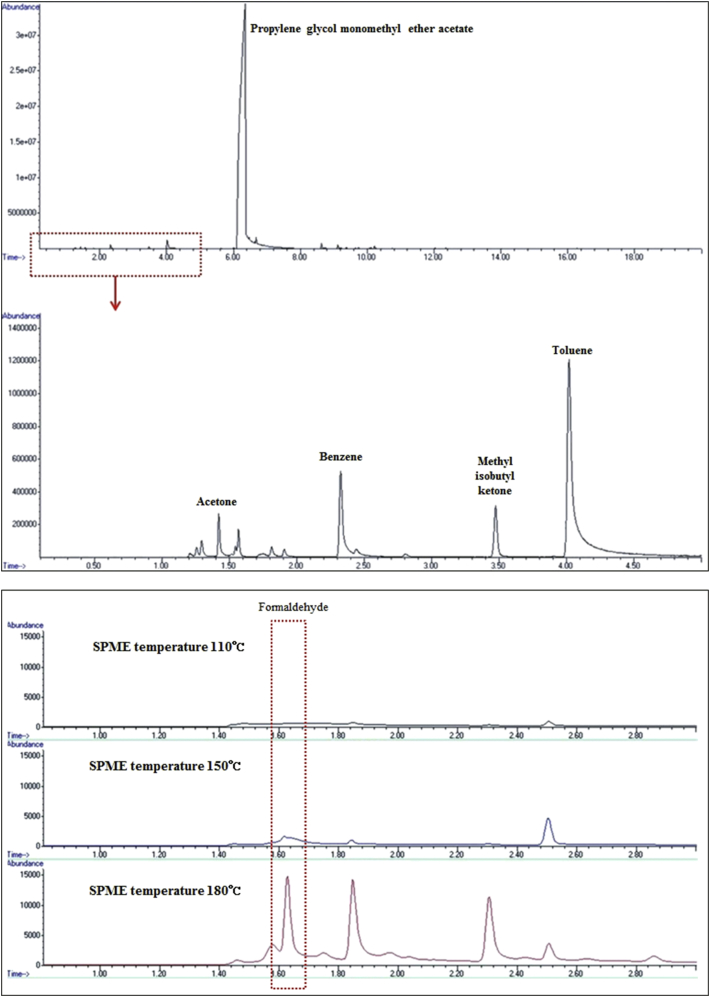
The analytical results showed that 17 of 129 chemicals (13.2%) were CMR substances and that 13 of 17 CMR substances (72.2%) were classified as carcinogen 2 by the MOEL (Table 8). Toluene was detected in 29 of 51 products (56.9%), and methyl isobutyl ketone and 1,4-dioxane were released from 13 products (25.5%). Benzene was detected in nine products (17.6%). Aromatic compounds such as styrene, ethylbenzene, and chlorobenzene were also detected. The upper part in Fig. 1 shows a typical example of an HS/SPME-GC/MS chromatogram. Hazardous chemicals such as benzene, methyl isobutyl ketone, and toluene were released from PR products.
Table 8.
Information on carcinogenic, mutagenic, and reprotoxic (CMR) substances detected using headspace solid-phase microextraction (HS-SPME) with gas chromatography/mass spectrometry (GC/MS)
| Compound | CAS no. | No. of products | Korea MOEL∗ |
Carcinogenicity |
||||
|---|---|---|---|---|---|---|---|---|
| TWA (STEL), ppm | CMR | IARC† | ACGIH‡ | NTP§ | EU CLP‖ | |||
| Toluene | 108-88-3 | 29 (56.9%) | 50 (150) | Repr. 2 | Group 3 | A4 | ||
| Methyl isobutyl ketone | 108-10-1 | 13 (25.5%) | 50 (75) | Car. 2 | Group 2B | A3 | ||
| 1,4-Dioxane | 123-91-1 | 13 (25.5%) | 20 | Car. 2 | Group 2B | A3 | R | Car. 2 |
| Benzene | 71-43-2 | 9 (17.6%) | 1 (5) | Car. 1A, Mut. 1B | Group 1 | A1 | K | Car. 1A |
| Styrene | 100-42-5 | 5 (9.8%) | 20 (40) | Car. 2 | Group 2B | A4 | R | |
| Chlorobenzene | 108-90-7 | 4 (7.8%) | 10 (20) | Car. 2 | A3 | |||
| Ethylbenzene | 100-41-4 | 3 (5.9%) | 100 (125) | Car. 2 | Group 2B | A3 | ||
| Cyclohexanone | 108-94-1 | 3 (5.9%) | 25 (50) | Car. 2 | Group 3 | A3 | ||
| 2-Butoxyethanol | 111-76-2 | 3 (5.9%) | 20 | Car. 2 | Group 3 | |||
| Chloroform | 67-66-3 | 3 (5.9%) | 10 | Car. 2 | Group 2B | A3 | R | Car. 2 |
| 2-Butoxyethyl acetate | 112-07-2 | 2 (3.9%) | 20 | Car. 2 | A3 | |||
| Naphthalene | 91-20-3 | 2 (3.9%) | 10 (15) | Car. 2 | Group 2B | A3 | R | Car. 2 |
| 1,4-Dichlorobenzene | 106-46-7 | 1 (2.0%) | 10 (20) | Car. 2 | Group 2B | A3 | R | Car. 2 |
| Phenol | 108-95-2 | 1 (2.0%) | 5 | Mut. 2 | Group 3 | |||
| Hexane | 110-54-3 | 1 (2.0%) | 50 | Rep. 2 | ||||
| Dichloromethane | 75-09-2 | 1 (2.0%) | 50 | Car. 2 | Group 2B | A3 | R | Car. 2 |
| Cumene | 98-82-8 | 1 (2.0%) | 50 | Car. 2 | Group 2B | |||
ACGIH, American Conference of Governmental Industrial Hygienists; CAS, Chemical Abstracts Service; CMR, carcinogenic, mutagenic, and reprotoxic; IARC, International Agency for Research on Cancer; MOEL, Ministry of Employment and Labor; TWA, Time Weighted Average; STEL, Short Term Exposure Limit.
Ministry Of Employment and Labor, Carcinogen classifications – Carcinogen 1A: Sufficient evidence of carcinogenicity to human, Carcinogen 1B: Sufficient evidence of carcinogenicity to animals or limited evidence of carcinogenicity to human and animals, Carcinogen 2: Insufficient evidence of carcinogenicity to humans and animals.
International Agency for Research on Cancer, Carcinogen classifications – Group 1: Carcinogenic to humans, Group 2A: Probably carcinogenic to humans, Group 2B: Possibly carcinogenic to humans, Group 3: Not classifiable as to its carcinogenicity to humans, Group 4: Probably not carcinogenic to humans.
American Conference of Governmental Industrial Hygienists, Carcinogen classifications – A1: Confirmed human carcinogen, A2: Suspected human carcinogen, A3: Confirmed animal carcinogen with unknown relevance to humans, A4: Not classifiable as a human carcinogen, A5: Not suspected as a human carcinogen.
National Toxicology Program, Carcinogen classifications – K: Known to be human carcinogens, R: Reasonably anticipated to be human carcinogens.
Classification, Labeling, Packing of substances and mixture, Carcinogen classifications – Carcinogen 1A: Known to have carcinogenic potential humans, Carcinogen 1B: May causes cancer, Carcinogen 2: Suspected of causing cancer.
Fig. 1.
An example of the volatile organic compounds (upper) and formaldehyde emission (lower) after heating by headspace solid-phase microextraction (HS-SPME) with gas chromatography/mass spectrometry (GC/MS).
According to the MSDS, phenolic resin (novolak) was the most used resin (41.2%), followed by acrylic resin (27.5%), and polystyrene resin (3.9%). The resin type was unknown in 27.5% of the products. Twenty-one products including novolak resin were analyzed to determine formaldehyde emissions in the actual working temperature. First, analysis was conducted at the same temperature as the VOC method (110°C); however, no formaldehyde peak was detected. Thus, further analysis was conducted at 150 and 180°C with the same product. At 150°C, the formaldehyde peak increased slightly but was too small to quantify. At 180°C, formaldehyde was detected in 12 of 21 products (57.1%) (Fig. 1). In addition, p-cresol was released from 90% of products including novolak resin. We also analyzed 14 products including acrylic resin for comparison, but no formaldehyde was found to be released from acrylic resin.
4. Discussion
We identified the chemical constituents in the PRs and their possible by-products during process and then compared the analytical result with the MSDS data in this study. We found that not only some PR products have contained toxic chemicals but also their by-products could be formed in the conditions of actual process condition. Even some constituents were not specified in MSDS comparing with the analytical results.
First, we reviewed the MSDS data to acquire the basic information. A total of 238 ingredients with multiple counting (35 ingredients removing multiple counting) were used, and half of them were listed as a trade secret. Table 3 shows that trade secret ingredients were specified in various terms. Furthermore, some ingredients consisted of similar components. For example, the high-molecular-weight compound used for determining mechanical properties was specified using a number of terms such as a resin, polymer, and monomer. Also, ingredients that control the photochemical reaction during exposure to light were denoted with various names, including sensitizer, photoactive, initiator, and generator [7], [14] Thus, unification of the names of individual ingredients is required for systematic management. Also, we suggest that resin type should be informed in MSDS at least because a previous study [9] has shown that novolak resin could release aromatic compounds such as benzene, toluene, phenol, and cresol through thermal energy. In this study, eight products informed the CAS No. of resin, and 37 products informed the type of resin.
PGME and PGMEA were used instead of ethylene glycol ether derivatives [ethylene glycol methyl ether (EGME), EGMEA, ethylene glycol ethyl ether (EGEE), and ethylene glycol ethyl ether acetate (EGEEA)] and were identified in 33 and 7 products, respectively (Table 2). It is known that PGME and PGMEA have been substituted for EGME, EGMEA, EGEE, and EGEEA, which seem to correlate with reproductive toxicants [1], [6]. Although PGE derivatives (PGME and PGMEA) have a lower toxicity than ethylene glycol ether derivatives (EGME, EGMEA, EGEE, and EGEEA) and seem to have few deleterious effects, there were not enough references to substantiate that PGME and PGMEA are entirely safe, and thus they should be handled with caution [15].
According to the review of MSDS data, four carcinogens were included in PR products: cyclohexanone, ethylbenzene, pyridine and 1,4-dioxane. However, toxicological information is not included in their MSDS; thus, it is difficult to make a decision about their harmfulness using the MSDS. Furthermore, four products including cyclohexanone did not have a hazardous identification specified in the MSDS. According to the MOEL, if the content of a carcinogen 2 in the products is more than 1%, the product is considered to be a carcinogen 2. Cyclohexanone is classified as a carcinogen 2 by the MOEL, and it occurred in contents ranging from 3 to 40% in 11 products (Table 4); thus, it is recommended that the MOEL standard should be specified in the MSDS for these products.
As shown in Table 5, we confirmed that hazardous chemicals were included in PR products. For example, 1,4-dioxane is classified as a carcinogen 2 by the MOEL and EU CLP, and group 2B carcinogens, based on the IARC classification, were detected in eight products, while one product contained it as an impurity. Seven products did not specify whether or not 1,4-dioxane was present. We assumed that there is a risk of 1,4-dioxane exposure from the use of products including 2-heptanone and novolak resin. From the MSDS review, most products did not specify information on impurities, although toxicological information should be indicated for these constituents. In addition, 2-butoxyethanol [ethylene glycol monobutyl ether (EGBE)], classified as a carcinogen 2 by the MOEL, was detected in three products, but no information was specified in the MSDS. Previous studies have shown that EGBE has hemolytic and fetotoxic effects and also has tumor-forming capabilities [16], [17]. According to the KOSHAct, 1,4-dioxane and EGBE should not be listed as a trade secret due to their toxicity because they are classified as hazardous substances requiring management by the KOSHAct; thus, their toxicity should be specified in the MSDS. p-Cresol, 2,3-dimethylphenol, and 3,4-dimethylphenol are categorized as toxic chemicals by the Korea National Chemicals Information System (NCIS), and information for those chemicals should be specified in the MSDS. According to the KOSHAct, chemicals that are classified as toxic by the NCIS should not be designated as trade secrets. Also, we assume that p-cresol, 2,3-dimethylphenol, and 3,4-dimethylphenol are in the form of novolak resin. As mentioned previously, the resin type should be specified in the MSDS due to the possibility that novolak resin may contain hazardous chemicals [9].
After qualitative analysis, quantitative analysis was performed for CMR substances and toxic materials. Table 7 shows differing analytical results according to the dilution solvent; this arises because each chemical has a different solubility in each solvent. Accordingly, when analyzing the constituents of an unknown sample, crossover analysis should be performed using solvents with different characteristics.
On the basis of the qualitative evaluation, we compared the chemical contents between the MSDS and the GC/FID results. The analytical result shows that only 41.7% of total products correspond with MSDS, and 58.3% of products do not correspond with MSDS (Table 6). Similarly, previous studies have also shown limited agreement between MSDS data and analytical results [18], [19], [20], [21].
We performed the analysis of by-products along with the analysis of the diluted sample. According to a US patent, volatile compounds, such as benzene and phenyl sulfide, can be released from PR products as by-products. In addition, analytical results from a thermal decomposition experiment detected aromatic compounds such as benzene, toluene, and cresol; however, the temperature conditions in that experiment were higher than the operating temperature [8], [9]. Hence, analysis was performed at 110°C in accordance with the operating temperature for the photolithography process: soft baking between 70 and 90°C and hard baking between 120 and 135°C [5], [7]. Table 8 shows that various chemical compounds were released as the by-product at actual operative temperature. In this study, benzene, which is known to induce leukemia, was detected in nine products. Toluene was detected in more than 50% of the total products, which is known as a reproductive toxicant. Furthermore, 1,4-dioxane as an impurity was detected in 14 products, whereas only four products specified this impurity in the MSDS.
A previous study found that formaldehyde, which is classified as Group 1 and is a known human carcinogen, could be released from novolak resin by high temperature or pressing [9]. For this reason, analysis was conducted to determine formaldehyde emissions from novolak resin (21 products). Formaldehyde peak was not found at 110°C and appeared small at 150°C but appeared clearly at 180°C in most novolak resin containing PR. The temperature could be raised up to 180°C at the other process called molding in packaging process in which the epoxy molding compound that might contain novolak resin was used. Two products in which the highest peaks were detected included 2-heptanone and gamma-butyrolactone, and both products were provided by one supplier from Korea. In addition, p-cresol was released from 90% of products including novolak resin. We also analyzed 14 products including acrylic resin for comparison, but no formaldehyde was found to be released from acrylic resin. We verified that PR products generate hazardous by-products at the operating temperature. Because legal regulations associated with by-products are not yet established, by-products are not under regulatory control in Korea. However, because there is a potential emission of hazardous chemicals in the fab process, consideration should be given to managing these emissions.
The limitations of this study were that it was difficult to collect MSDS data for all products, and only VOCs were analyzed. Moreover, the results differed with different dilution solvents (Table 7), so we assume that there is a need for selection of different solvents for each target chemical or to perform crossover analysis using solvents with different characteristics. It was also difficult to adjust for the effects of UV light during by-product analysis. Therefore, further studies require the analysis of macromolecules in PR products, considering the dilution solvent and UV light.
This study provides useful information for the management of chemicals used in the fab process by identifying the constituents of PR products and their by-products. The results of the comparison between MSDS data and analysis results show that MSDS data need to be reexamined. Also, diverse management regimes suitable for each process are necessary because exposure rates can vary with process characteristics.
In this study, we identified a total of 51 PR products and their by-products. Samples were collected with MSDS data. The MSDS data were evaluated and chemical constituents were identified through qualitative and quantitative methods. Finally, a qualitative analysis of possible by-products was performed at the operating temperature. Our main findings are as follows: First, PR products contained various chemicals, and some were harmful to humans. Chemical information was not correctly specified in the MSDS; Second, from the analysis results, hazardous chemicals were detected which were not specified in the MSDS. Furthermore, chemical constituents were not matched between MSDS data and analytical results; Third, CMR substances including benzene were released as by-products at the operating temperature, and formaldehyde and p-cresol were also released from some products containing novolak resin. Therefore, systematic management, and reexamination of chemicals used in fabrication processes and their MSDS data are required. In addition, the risk of exposure to possible by-products should be recognized in the management of the working environment.
Conflicts of interest
The authors state that there is no conflict of interest.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) (No. 2011-0002926) and BK21 Plus project (No. 5280-20140100) of National Research Foundation of Korea (NRF).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.shaw.2018.08.001.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Chelton C.F., Glowatz M., Mosovsky J.A. Chemical hazards in the semiconductor industry. IEEE Trans Educ. 1991;34:269–288. [Google Scholar]
- 2.Marano D.E., Boice J.D., Jr., Munro H.M., Chadda B.K., Wiliams M.E., McCarthy C.M. Exposure assessment among US workers employed in semiconductor wafer fabrication. J Occup Environ Med. 2010;52:1075–1081. doi: 10.1097/JOM.0b013e3181f6ee1d. [DOI] [PubMed] [Google Scholar]
- 3.Park D.U. Retrospective exposure assessment of wafer fabrication workers in the semiconductor industry. J Korean Environ Health Sci. 2011;37:12–21. [in Korean] [Google Scholar]
- 4.Quirk M., Serda J. Prentice Hall Upper; Saddle River, NJ: 2001. Semiconductor manufacturing technology. [Google Scholar]
- 5.Wald P.H., Jones J.R. Semiconductor manufacturing: an introduction to processes and hazards. Am J Ind Med. 1987;11:203–221. doi: 10.1002/ajim.4700110209. [DOI] [PubMed] [Google Scholar]
- 6.Hallock M.F., Hammond S.K., Hines C.J., Woskie S.R., Schenker M.B. Patterns of chemical use and exposure control in the semiconductor health study. Am J Ind Med. 1995;28:681–697. doi: 10.1002/ajim.4700280605. [DOI] [PubMed] [Google Scholar]
- 7.Park D.U., Byun H.J., Choi S.J., Jeong J.Y., Yoon C.S., Kim C.N. Review on potential risk factors in wafer fabrication process of semiconductor industry. J Kor Occup Environ Med. 2011;23(3):333–342. [Google Scholar]
- 8.Goodner MD. UV-activated dielectric layer. Intel Corporation, assignee. United States Patent US7358597, 2008.
- 9.Park S.H., Shin J.A., Park H.H. Exposure to volatile organic compounds and possibility of exposure to by-product volatile organic compounds in photolithography processes in semiconductor manufacturing factories. Saf Health Work. 2011;2:210–217. doi: 10.5491/SHAW.2011.2.3.210. [in Korean] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoon C.S. Much concern but little research on semiconductor occupational health issues. J Kor Med Sci. 2012;27:461–464. doi: 10.3346/jkms.2012.27.5.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim Y.H., Kim K.H., Szulejko J.E., Bae M.S., Broun R.J. Experimental validation of an effective carbon number-based approach for the gas chromatography–mass spectrometry quantification of ‘compounds lacking authentic standards or surrogates’. Anal Chim Acta. 2014;830:32–41. doi: 10.1016/j.aca.2014.04.052. [DOI] [PubMed] [Google Scholar]
- 12.Welsh M.S., Lamesse M., Karpinski E. The verification of hazardous ingredients disclosures in selected material safety data sheets. Appl Occup Environ Hyg. 2000;15:409–420. doi: 10.1080/104732200301368. [DOI] [PubMed] [Google Scholar]
- 13.Bernstein J.A. Material safety data sheets: are they reliable in identifying human hazards? J Allergy Clin Immunol. 2002;110:35–38. doi: 10.1067/mai.2002.124891. [DOI] [PubMed] [Google Scholar]
- 14.Zant P.V. 5th ed. McGraw-Hill; NY: 2000. Microchip fabrication: a practical guide to semiconductor processing. [Google Scholar]
- 15.Multigner L., Catala M., Cordier S., Delaforge M., Fenaux P., Garnier R. The INSERM expert review on glycol ethers: findings and recommendations. Toxicol Lett. 2005;156:29–37. doi: 10.1016/j.toxlet.2003.12.077. [DOI] [PubMed] [Google Scholar]
- 16.Cherry N., Moore H., McNamee R., Pacey A., Burgess G., Clyma J.A. Occupation and male infertility: glycol ethers and other exposure. Occup Environ Med. 2008;65:708–714. doi: 10.1136/oem.2007.035824. [DOI] [PubMed] [Google Scholar]
- 17.Hammond S.K., Hines C.J., Hallock M.F., Woskie S.R., Kenyou E.M., Schenker M.B. Exposures to glycol ethers in the semiconductor industry. Ann Occup Hyg. 1996;2:355–366. [Google Scholar]
- 18.Hong M.K., Song S.W., Lee K.S., Choi S.B., Lee J.H. A study of MSDS reliability evaluation in chemicals including formaldehyde. J Kor Soc Occup Environ Hyg. 2014;23(3):287–298. [in Korean] [Google Scholar]
- 19.Lee K.S., Choi J.H., Jo J.H., Choi S.B., Lee J.H., Yang J.S. MSDSs reliability evaluation in workplaces manufacturing aromatic hydrocarbon. J Kor Soc Occup Environ Hyg. 2009;19(4):370–380. [in Korean] [Google Scholar]
- 20.Lee K.S., Han I.S., Han J.H., Park D.U., Lee D.W., Hwang H.S. A study on the chemical composition and MSDS reliability of powder coatings. J Kor Soc Occup Environ Hyg. 2004;14(3):221–232. [in Korean] [Google Scholar]
- 21.Lee K.S., Kwon H.W., Han I.S., Yu I.J., Lee Y.M. A study on the reliability of material safety data sheets (MSDS) for paint thinner. J Kor Soc Occup Environ Hyg. 2003;13(3):261–272. [in Korean] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.