Abstract
Background
In both developed and developing countries, noise is regarded as the most common occupational hazard in various industries. The present study aimed to examine the effect of sound pressure level (SPL) on serum cortisol concentration in three different times during the night shift.
Methods
This case–control study was conducted among 75 workers of an industrial and mining firm in 2017. The participants were assigned to one of the three groups (one control and two case groups), with an equal number of workers (25 participants) in each group. Following the ISO 9612 standard, dosimetry was adopted to evaluate equivalent SPL using a TES-1345 dosimeter. The influence of SPL on serum cortisol concentration was measured during the night shift. The serum cortisol concentration was measured using a radioimmunoassay (RIA) test in the laboratory. Repeated measure analysis of variance and linear mixed models were used with α = 0.05.
Results
The results indicated a downward trend in the serum cortisol concentration of the three groups during the night shift. Both SPL and exposure time significantly affected cortisol concentration (p < 0.0001, p < 0.0001). Conversely, age and body mass index had no significant influence on cortisol concentration (p = 0.360, p = 0.62).
Conclusion
Based on the obtained results, increasing SPL will lead to enhancement of serum cortisol concentration. Given that cortisol concentration varies while workers are exposed to different SPLs, this hormone can be used as a biomarker to study the effect of noise-induced stress.
Keywords: Cortisol, Noise, Shift work, Sound pressure level
1. Introduction
Noise refers to every unwanted sound generated by natural phenomena (e.g., wind, volcanic eruption, oceans, etc.) or human-based sources (e.g., automobiles, machines, explosions, etc.) [1]. It is regarded as the most typical harmful industrial factor in both developed and developing countries [2].
Research shows that every day in Europe, about 450 million individuals are exposed to noise levels of at least 55 dBA, 113 million people experience a minimum noise level of 64 dBA, and 9.7 million persons are exposed to noise levels of 75 dBA or more [3]. Exposure to high levels of occupational noise is still a big challenge in all corners of the world. Considering the USA, for instance, more than 30 million workers are exposed to hazardous noise [4]. Similarly, in Germany, 4–5 million people (which constitute 12–15% of the workforce) experience hazardous noise levels, as defined by the World Health Organization (WHO) [5]. As an occupational health hazard, exposure to excessive noise may lead to a wide array of social and physiological (e.g., anatomical, nonauditory, and auditory) problems [6], [7], [8]. Shifts in hearing threshold and speech perception deterioration, which generally entail noise-induced hearing loss (NIHL), are common consequences of contact with excessive noise. In addition to such auditory impacts, noise exposure can also have nonauditory consequences, which cause damage to the autonomic nervous system and eventually lead to heightened skin temperature and pulse rate, high blood pressure, constriction of blood vessels, abnormal hormone secretion, and muscle tenseness [9], [10].
As the final product of the hypothalamus–pituitary–adrenal (HPA) axis in humans, cortisol is the main glucocorticoid produced in the adrenal cortex [11]. It is a steroid hormone and is regarded as the major indicator of physiological alterations stemming from stressful stimuli [12], [13]. HPA activation and cortisol release are natural responses to physiological stress in humans [14]. Some studies have used salivary and/or serum cortisol to gauge stress hormone [15], [16]. Cortisol is normally produced after a circadian rhythm; in fact, the highest level of cortisol is generated early in the morning, whereas the minimum level produced is usually observed at night [17], [18]. If cortisol concentration goes up irrespective of the circadian rhythm, it is indicative of a response to stress [19]. Recently, attempts have been made to estimate stress in workers who are exposed to excessive noise by measuring cortisol concentration [20], [21], [22]. However, few studies have examined the impact of sound pressure level (SPL) on cortisol concentration in night-shift workers. As a result, the present article reports on a study which aimed to
-
1.
compare the average serum cortisol concentration of three exposure groups in three different times during the night shift,
-
2.
assess the possibility of using serum cortisol as a biomarker of noise-induced stress in night shifts.
2. Material and methods
2.1. Industry selection
The study was conducted in a mining and industrial firm located in southeastern Iran. Because it was feasible to form control and experimental groups among workers in this firm, the researchers decided to carry out the study there.
2.2. Participants
In total, 75 firm workers took part in the study (which was conducted in 2017). Before data collection, the objectives of the study were explained to the participants, and written consent was obtained from them. They provided demographic information on the experiment day. Before conducting the study, workers' health condition was screened by examining their medical history, and individuals who did not have any record of physical problems were selected as the target group.
2.3. Sampling procedure
This case–control study included a control group and two case groups. Thus, sampling was performed in a way that an equal number of participants were assigned to each group. Based on previous studies, at least 25 people should exist in each group to achieve a power level of 80% and avoid Type I error (Alpha = 0.05). Therefore, 75 people were selected for the three groups.
2.4. Study design
In this study, the participants were divided into three groups of 25 (one control and two case groups). To study the effect of SPLs on serum cortisol concentration, participants in the control group were selected from individuals who had office jobs. On the other hand, participants of the two case groups (which were exposed to noise) were selected among workers in two factories of the firm. The metabolism of participants in the three groups was defined in accordance with ISO 8996 [23]. The studied participants followed a 3–3–3–3 work shift pattern (3 mornings, 3 evenings, 3 nights, and 3 days off). To study the impact of work shift on serum cortisol concentration, cortisol level was measured during the night shift. Hence, the effect of SPL on serum cortisol concentration was assessed for the night shift. In particular, serum cortisol concentration was gauged in three different occasions during the night shift: at the beginning of the shift (11–11:30 PM), 3 hours into the shift (2–2:30 AM), and 6 hours into the shift (5–5:30 AM). Furthermore, in an attempt to study the role of intervening environmental factors, heat and lighting were assessed in the work environment in these three measurement occasions.
2.5. Measurement
2.5.1. Equivalent SPL
In line with the ISO 9612 standard, dosimetry was adopted to assess equivalent SPL using a TES-1345 (Sunlight Electronic Technology Co. Ltd., China) dosimeter. Before using it, the machine was calibrated by a CEL-110/2 calibrator (CASELLA, USA) [24].
2.5.2. Heat
As a reliable index of environmental heat, wet-bulb globe temperature (WBGT) was measured using a machine, manufactured by Casella (a UK-based company). The machine was calibrated to gauge dry temperature, natural wet temperature, glowing temperature, and relative humidity. The ISO 7243 standard was followed to calculate the WBGT index, whereas the ISO 8996 standard was adopted to estimate the metabolism rate.
Atmospheric conditions in the work environment may differ in various work shifts. Thus, WBGT should be measured in various occasions during a work shift, followed by calculating its time-weighted average by the use of the following formula [23], [25]:
| (1) |
2.5.3. Light
The intensity of general lighting was assessed following the procedure proposed by the Illuminating Engineering Society of North America (IESNA). A calibrated luxmeter (Lutron Lx, model 102, Taiwan) was used to measure lighting in the work environments in three occasions during the night shift [26].
2.5.4. Serum cortisol
To assess the concentration of serum cortisol, 5 mL of the blood sample was obtained from the workers in the three data collection occasions during the night shift. While taking blood samples, all the participants were sitting. The blood samples were transferred into numbered tubes that contained anticoagulant ethylenediaminetetraacetic acid (EDTA). The tubes were immediately taken to an authentic medical diagnostic laboratory under controlled condition (ice box). In the laboratory, serum cortisol concentration was measured using a radioimmunoassay (Diagnostic Products Corporation, Los Angeles, USA) [27].
2.6. Statistical analysis
The collected data were fed into SPSS 18 (SPSS, Inc., Chicago, Illinois, USA). Descriptive methods (mean, standard deviation, and frequency) were then used to summarize the data, followed by using the Shapiro–Wilk test to examine normality of data distribution. Because the data were assessed in different occasions, repeated measure analysis of variance (ANOVA) and linear mixed models were carried out as inferential statistics. All the preassumptions of these two procedures were tested, and the significance level was set at p = 0.05.
2.7. Ethical considerations
The ethical principles proposed by Kerman University of Medical Sciences (ID: IR.KMU.REC.1396.2298) were strictly followed in this study. More specifically, written informed consent was obtained from the participants before data collection. They were further ensured that the provided data would remain confidential and would be used only for research purposes. The participants could also withdraw from the study at any point without being punished.
3. Results
3.1. Demographic features
Table 1 displays the mean age, body mass index, and work experience of the three studied groups.
Table 1.
Demographic characteristics the participants (n = 75)
| Variables | Control group exposed to noise level 67 dBA (Mean ± SD) | Case group exposed to noise level 80 dBA (Mean ± SD) | Case group exposed to noise level 92 dBA (Mean ± SD) |
|---|---|---|---|
| Age (years) | 29.14 ± 2.14 | 29.46 ± 2.80 | 30.40 ± 2.93 |
| BMI (kg/m2) | 25.20 ± 2.10 | 25.38 ± 2.91 | 25.81 ± 2.48 |
| Work experiences (months) | 29 ± 5 | 30 ± 7 | 32 ± 6 |
BMI, body mass index; SD, standard deviation.
3.2. Equivalent SPL
The results of dosimetry indicated that the workers in the control group were exposed to an equivalent SPL of 67 ± 3 dBA, whereas the participants in the case groups were in contact with equivalent SPLs of 80 ± 4 and 92 ± 4 dBA.
3.3. WBGTTWA in the three groups
Measuring WBGTTWA in the three groups demonstrated values of 21.1°C for participants in the control group and 24.6°C and 25.5°C for participants in the two case groups. Therefore, in the light of ISO 7243, the members of none of the groups were exposed to heat stress.
3.4. Lighting
The results of lighting measurement revealed that the average lighting intensity in the control and case groups were 350 lux, 300 lux, and 370 lux, in that order. Thus, the lighting intensity was rather similar in the three groups.
3.5. Average serum cortisol concentration
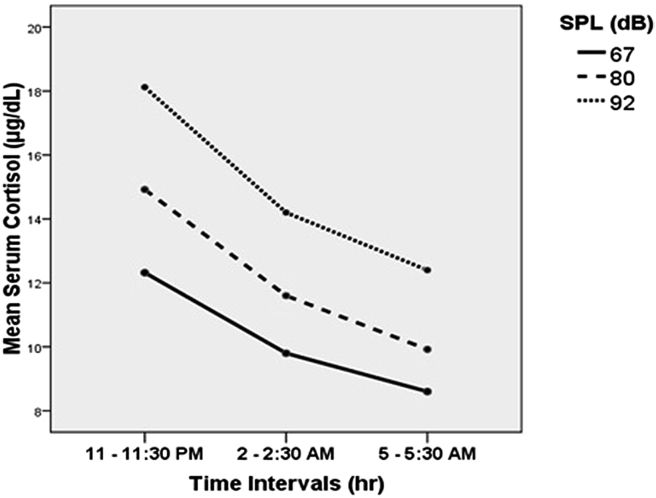
Fig. 1 illustrates the average serum cortisol concentration of the three groups measured in three different occasions (at the beginning, in the middle, and at the end of the work shift). It is observed that in all the groups, the average value of serum cortisol concentration was higher at the beginning of the night shift (11–11:30 PM) than at the end of this shift (5–5:30 AM). Thus, a declining trend is observed during the shift.
Fig. 1.
Changes in the average serum cortisol concentration of the three groups in the three occasions.
3.6. The effect of SPL on cortisol concentration
Repeated measure ANOVA was used to compare the cortisol concentration across the three data collection occasions, with the results being illustrated in Table 2. Accordingly, in the first data collection time (11–11:30 PM), significant differences were observed in the cortisol concentrations of participants of the three exposure groups (p < 0.05). Considering the second data collection time (2–2:30 AM), however, no statistically measurable discrepancy was detected between the participants in the control group and those in the case group exposed to 80 dBA SPL (p = 0.10). In this time, significant differences were recorded in the cortisol concentrations of participants who were exposed to an SPL of 92 dBA, on the one hand, and those who were exposed to an SPL of 80 dBA and those in the control group, on the other hand (p < 0.05). Furthermore, in the third data collection time (5–5:30 AM), no considerable difference was observed between the control group and the case group exposed to 80 dBA SPL in terms of their cortisol concentrations (p = 0.06). Conversely, significant discrepancies were registered between the case group exposed to 92 dBA SPL and the other two groups (p < 0.0001).
Table 2.
The effect of various SPLs on participants' cortisol concentrations in the three data collection times
| Time intervals | Group | Group | Mean difference | Std. error | p |
|---|---|---|---|---|---|
| 11–11:30 PM | SPL1 = 67 dBA | SPL2 = 80 dBA | −2.60 | .91 | 0.01 |
| SPL3 = 92 dBA | −5.80 | .91 | <0.0001 | ||
| SPL2 = 80 dBA | SPL3 = 92 dBA | −3.20 | .91 | 0.002 | |
| 2–2:30 AM | SPL1 = 67 dBA | SPL2 = 80 dBA | −1.80 | .83 | 0.10 |
| SPL3 = 92 dBA | −4.40 | .83 | <0.0001 | ||
| SPL2 = 80 dBA | SPL3 = 92 dBA | −2.60 | .83 | 0.008 | |
| 5–5:30 AM | SPL1 = 67 dBA | SPL2 = 80 dBA | −1.32 | .54 | 0.06 |
| SPL3 = 92 dBA | −3.80 | .54 | <0.0001 | ||
| SPL2 = 80 dBA | SPL3 = 92 dBA | −2.48 | .54 | <0.0001 |
SPL, sound pressure level.
3.7. The effect of various data collection times on cortisol concentrations
Repeated measure ANOVA was carried out to examine within-group differences with respect to cortisol concentrations in the three data collection times (Table 3). Considering the control group, no significant difference was observed in the participants' cortisol concentrations between the second (2–2:30 AM) and third (5–5:30 AM) data collection occasions (p = 0.18). However, measurable differences were detected between the first and second times as well as the first and third ones (p < 0.05). In both the case groups (SPL2 and SPL3), significant differences were observed within all data collection times (p < 0.05). It is noteworthy that cortisol concentrations dwindled in all the three groups in the course of time.
Table 3.
Within-group comparison of cortisol concentrations in the light of the three data collection times
| Group | Time intervals | Time intervals | Mean difference | Std. error | p |
|---|---|---|---|---|---|
| SPL1 = 67 dBA | 11–11:30 PM | 2–2:30 AM | 2.52 | .86 | 0.01 |
| 5–5:30 AM | 3.72 | .74 | <0.0001 | ||
| 2–2:30 AM | 5–5:30 AM | 1.20 | .63 | 0.18 | |
| SPL2 = 80 dBA | 11–11:30 PM | 2–2:30 AM | 3.32 | .86 | 0.001 |
| 5–5:30 AM | 5 | .74 | <0.0001 | ||
| 2–2:30 AM | 5–5:30 AM | 1.68 | .63 | 0.02 | |
| SPL3 = 92 dBA | 11–11:30 PM | 2–2:30 AM | 3.92 | .86 | <0.0001 |
| 5–5:30 AM | 5.72 | .74 | <0.0001 | ||
| 2–2:30 AM | 5–5:30 AM | 1.80 | .63 | 0.01 |
SPL, sound pressure level.
4. Discussion
This study aimed at investigating the effect of SPL on serum cortisol concentration among workers in a mining and industrial firm during various exposure times.
Haratian et al examined the role of age and gender in cortisol secretion, demonstrating a significant negative relationship between the two variables [28]. The results of the present study showed no significant difference between the three groups in terms of their age and body mass index (p > 0.05). In addition, age and body mass index had no significant effect on cortisol concentration (F = 0.84, p = 0.360, F = 0.23, p = 0.62).
The overwhelming majority of previous studies have used the measurement of adrenaline catecholamines, noradrenaline, and cortisol to examine noise-induced stress [29], [30]. In the same vein, the present study aimed to investigate the effect of noise on serum cortisol concentration. Although cortisol secretion follows the circadian rhythm, it may also be influenced by environmental factors [31]. In addition, the effect of two environmental factors (i.e., heat and lightning) on cortisol concentration was examined in the three data collection times during the night shift. The results indicated similar patterns among the three groups with regard to heat stress and lighting intensity.
On the other hand, the results of comparing cortisol concentrations during the shift revealed a considerable reduction (from the beginning to the end of the shift) in all the three groups. Moreover, rise in SPL leads to increase of cortisol concentration, meaning that cortisol concentration was significantly higher in the 92-dBA SPL case group than in the other two groups exposed to 80-dBA SPL and 67-dBA SPL (the control group) (Fig. 1).
Based on the developed statistical model, SPL and exposure time had a significant impact on cortisol concentration (p < 0.0001, p < 0.0001). Therefore, rises in both SPL and exposure time lead to greater cortisol concentrations.
Examining the impact of SPL on cortisol concentration, Tafalla et al discovered no significant increase in cortisol concentration as a result of extending the exposure time. Thus, their findings are in conflict with our results [30].
Ising et al have demonstrated that exposure to maximal noise pressure levels above 92 dBA may stimulate the sympathetic nervous system and enhance adrenaline and noradrenaline release. Noise levels above 120 dBA raise cortisol in humans and animals [32]. Furthermore, exposure to low frequency noise with Lmax <50 dBA for a long time during nights leads to chronic increase of excretion of free cortisol in the first half of the night [33]. These findings are in alignment with our results.
Zumanian et al, examining the impact of excessive noise on cortisol concentration, concluded that high SPLs significantly affect cortisol concentration, a finding that further confirms the results obtained in the present study [34].
Melamed and Bruhis investigated the effect of chronic exposure to industrial noise on urinary cortisol concentration among 35 industrial workers who were exposed to SPLs above 85 dBA and did not use any ear protector. They measured urinary cortisol concentration in three different occasions during the day shift (6:30 AM, 10:30 AM, and 1:30 PM). The results revealed that the concentration of serum cortisol was higher at the end of the shift than that at the beginning of the work shift [21]. In this study, serum cortisol concentration was measured in the case and control groups in three different times (11–11:30 PM, 2–2:30 AM, and 5–5:30 AM) during the night shift. The results showed a constant decline in cortisol concentration during the night shift.
Brandenberger et al demonstrated that participants in the case groups who were exposed to 85–105 dBA SPLs did not significantly differ from their counterparts in the control group in terms of their cortisol concentration [35]. Hence, their findings are in line with the results of the present study. Because blood sampling is an aggressive method of data collection, some of the workers refrained from participating in the study, an issue which is a limitation of this research.
A limitation of the study was the problems that the researchers encountered to convince the stakeholders in the industry to participate in the study. Particularly, some of the workers were reluctant to donate blood samples in various times during their shift.
5. Conclusion
During the night shift, SPL and exposure time have significant impacts on cortisol concentration. More specifically, rise in SPL leads to significant increase in cortisol concentration, whereas extension of exposure time reduces it. Given that cortisol concentration varies while workers are exposed to different SPLs, this hormone can be used as a biomarker to study the effect of noise-induced stress.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
This article was extracted from a research project (code: 96000865), which was sponsored by the University Students' Research Committee at Kerman University of Medical Sciences. The authors express their gratitude to the committee and the research deputy of Kerman University of Medical Sciences for the kind support.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.shaw.2018.07.002.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Gadhave P.K., Gadhave K.C., Dighe U.P. vol. 1. 2014. Cardiovascular parameters as an appraisal to the stress caused due to clamorous work-place environments in metal machining units; pp. 615–617. (Proceedings of the world congress on engineering). [Google Scholar]
- 2.Zare S., Nassiri P., Monazzam M.R., Pourbakht A., Azam K., Golmohammadi T. Evaluation of Distortion Product Otoacoustic Emissions (DPOAEs) among workers at an Industrial Company exposed to different industrial noise levels in 2014. Electron Physician. 2015 Jul;7(3):1126–1134. doi: 10.14661/2015.1126-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO . World Health Organization; Geneva: 1999. Overview of the environment and health in Europe in the 1990s. Background Document. [Google Scholar]
- 4.NIOSH . National Institute for Occupational Safety and Health; Cincinnati, OH: 1998. Criteria for a recommended standard: occupational noise exposure. Revised criteria 1998. [Google Scholar]
- 5.WHO . World Health Organization; Geneva: 2001. Occupational and community noise (Fact Sheet No. 258) [Google Scholar]
- 6.Nassiri P., Zare S., Monazzam M.R., Pourbakht A., Azam K., Golmohammadi T. Evaluation of the effects of various sound pressure levels on the level of serum aldosterone concentration in rats. Noise Health. 2017 Jul;19(89):200–206. doi: 10.4103/nah.NAH_64_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nassiri P., Zare S., Monazzam M.R., Pourbakht A., Azam K., Golmohammadi T. Modeling signal-to-noise ratio of otoacoustic emissions in workers exposed to different industrial noise levels. Noise Health. 2016;18:391–398. doi: 10.4103/1463-1741.195808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Safari Variani A., Ahmadi S., Zare S., Ghorbanideh M. Water pump noise control using designed acoustic curtains in a residential building of Qazvin city. Iran Occup Health. 2018 May 15;15(1):126–135. [Google Scholar]
- 9.Fouladi D.B., Nassiri P., Monazzam E.M., Farahani S., Hassanzadeh G., Hoseini M. Industrial noise exposure and salivary cortisol in blue collar industrial workers. Noise Health. 2012;14:184–189. doi: 10.4103/1463-1741.99894. [DOI] [PubMed] [Google Scholar]
- 10.Zare S., Nassiri P., Monazzam M.R., Pourbakht A., Azam K., Golmohammadi T. Evaluation of the effects of occupational noise exposure on serum aldosterone and potassium among industrial workers. Noise Health. 2016 Jan;18(80):1–6. doi: 10.4103/1463-1741.174358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fries E., Dettenborn L., Kirschbaum C. The cortisol awakening response (CAR): facts and future directions. Int J Psychophysiol. 2009 Apr 30;72(1):67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 12.Kirschbaum C., Hellhammer D.H. Salivary cortisol in psychobiological research: an overview. Neuropsychobiology. 1989;22(3):150–169. doi: 10.1159/000118611. [DOI] [PubMed] [Google Scholar]
- 13.Kirschbaum C., Hellhammer D.H. Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinology. 1994 Dec 31;19(4):313–333. doi: 10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 14.Ockenfels M.C., Porter L., Smyth J., Kirschbaum C., Hellhammer D.H., Stone A.A. Effect of chronic stress associated with unemployment on salivary cortisol: overall cortisol levels, diurnal rhythm, and acute stress reactivity. Psychosom Med. 1995;57:460–467. doi: 10.1097/00006842-199509000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Selander J., Bluhm G., Theorell T., Pershagen G., Babisch W., Seiffert I., Houthuijs D., Breugelmans O., Vigna-Taglianti F., Antoniotti M.C., Velonakis E. Saliva cortisol and exposure to aircraft noise in six European countries. Environ Health Perspect. 2009;117:1713–1717. doi: 10.1289/ehp.0900933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bigert C., Bluhm G., Theorell T. Saliva cortisol - a new approach in noise research to study stress effects. Int J Hyg Environ Health. 2005;208:227–230. doi: 10.1016/j.ijheh.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Hucklebridge F., Hussain T., Evans P., Clow A. The diurnal patterns of the adrenal steroids cortisol and dehydroepiandrosterone (DHEA) in relation to awakening. Psychoneuroendocrinology. 2005;30:51–57. doi: 10.1016/j.psyneuen.2004.04.007. Back to cited text no. 17. [DOI] [PubMed] [Google Scholar]
- 18.Knutsson U., Dahlgren J., Marcus C., Rosberg S., Brönnegård M., Stierna P., Albertsson-Wikland K. Circadian cortisol rhythms in healthy boys and girls: relationship with age, growth, body composition, and pubertal development. J Clin Endocrinol Metab. 1997;82:536–540. doi: 10.1210/jcem.82.2.3769. Back to cited text no. 18. [DOI] [PubMed] [Google Scholar]
- 19.Miller G.E., Chen E., Zhou E.S. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007 Jan;133(1):25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- 20.Monsefi M., Bahoddini A., Nazemi S., Dehghani G.A. Effects of noise exposure on the volume of adrenal gland and serum levels of cortisol in rat. Iran J Med Sci. 2006;31(1):5–8. [Google Scholar]
- 21.Melamed S., Bruhis S. The effects of chronic industrial noise exposure on urinary cortisol, fatigue and irritability: a controlled field experiment. J Occup Environ Med. 1996;38(3):252–256. doi: 10.1097/00043764-199603000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Smyth J., Ockenfels M.C., Porter L., Kirschbaum C., Hellhammer D.H., Stone A.A. Stressors and mood measured on a momentory basis are associated with salivary cortisol secretion. Psychoneuroendocrinology. 1998;23:353–370. doi: 10.1016/s0306-4530(98)00008-0. [DOI] [PubMed] [Google Scholar]
- 23.ISO . 1990. ISO 8996: ergonomics-determination of metabolic heat production. [Google Scholar]
- 24.ISO . 2009. ISO 9612: acoustics-determination of occupational noise exposure-engineering method. [Google Scholar]
- 25.International Organization Standardization. ISO 7243: hot environments: estimation of the heat stress on working man.
- 26.Rea M.S. Illuminating Engineering Society of North America; 2000. IESNA lighting handbook. [Google Scholar]
- 27.Yalow R.S., Berson S.A. Immunoassay of endogenous plasma insulin in man. Obesity. 1996 Nov 1;4(6):583–600. doi: 10.1002/j.1550-8528.1996.tb00274.x. [DOI] [PubMed] [Google Scholar]
- 28.Haratian M., Rajabian R., Ayatollahi H. Evaluation of the level of salivary and serum cortisol. Med J Mashad Univ Med Sci. 2008;51(1):13–18. [Google Scholar]
- 29.Ising H., Kruppa B. Health effects caused by noise: evidence in the literature from the past 25 years. Noise Health. 2004;6(22):5–13. [PubMed] [Google Scholar]
- 30.Tafalla R.J., Evans G.W. Noise, physiology, and human performance: the potential role of effort. J Occup Health Psychol. 1997 Apr;2(2):148–155. doi: 10.1037//1076-8998.2.2.148. PubMed PMID: 9552287. [DOI] [PubMed] [Google Scholar]
- 31.Abedi K., Pour Ebadiyan S., Habibi E., Zare M. Assessment of blood cortisol changes in shift workers and its relationship to personal characteristics, and compliance with the shiftworking. J Shaheed Sadoughi Univ Med Sci. 2008;16(1):48–56. [Google Scholar]
- 32.Ising H., Babisch W., Kruppa B. Noise-induced endocrine effects and cardiovascular risk. Noise Health. 1999;1(4):37–48. [PubMed] [Google Scholar]
- 33.Ising H., Ising M. Chronic cortisol increases in the first half of the night caused by road traffic noise. Noise Health. 2002;4(16):13–21. [PubMed] [Google Scholar]
- 34.Zamanian Z., Rostami R., Nikeghbal K. The effects of occupational noise exposure on serum cortisol level and some blood parameters in steel industry workers. J Health Sci Surveill Syst. 2015 Jan 3;3(1):45–49. [Google Scholar]
- 35.Brandenberger G., Follenius M., Muzet A. Interactions between spontaneous and provoked cortisol secretory episodes in man. J Clin Endocrinol Metab. 1984;59(3):406–411. doi: 10.1210/jcem-59-3-406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.