Highlights
-
•
Bone metastases negatively impact on patients’ quality of life (QoL).
-
•
Skeletal related events have a detrimental effect on both QoL and survival.
-
•
Both local and systemic treatments are often required to manage bone metastases.
-
•
Bone turnover modulators reduce the risk of skeletal complications and improve pain.
-
•
Novel agents may deserve further investigation for the management of bone metastases.
Keywords: Bone metastases, Osteotropic tumors, Skeletal related events, Bone targeting agents
Abbreviations: ActRIIA, activin-A type IIA receptor; BC, breast cancer; BM, bone metastases; BMD, bone mineral density; BMPs, bone morphogenetic proteins; BMSC, bone marrow stromal cells; BPs, bisphosphonates; BTA, bone targeting agents; BTM, bone turnover markers; CCR, chemokine-receptor; CRPC, castration-resistant PC; CXCL-12, C–X–C motif chemokine-ligand-12; CXCR-4, chemokine-receptor-4; DFS, disease-free survival; DKK1, dickkopf1; EBC, early BC; ECM, extracellular matrix; ET-1, endothelin-1; FDA, food and drug administration; FGF, fibroblast growth factor; GAS6, growth-arrest specific-6; GFs, growth factors; GnRH, gonadotropin-releasing hormone; HER-2, human epidermal growth factor receptor 2; HR, hormone receptor; IL, interleukin; LC, lung cancer; MAPK, mitogen-activated protein kinase; MCSF, macrophage colony-stimulating factor; MCSFR, MCSF receptor; MIP-1α, macrophage inflammatory protein-1 alpha; MM, multiple myeloma; MPC, malignant plasma cells; mTOR, mammalian target of rapamycin; N-BPs, nitrogen-containing BPs; NF-κB, nuclear factor-κB; non-N-BPs, non-nitrogen containing BPs; ONJ, osteonecrosis of the jaw; OS, overall survival; PC, prostate cancer; PDGF, platelet-derived growth factor; PFS, progression-free survival; PIs, proteasome inhibitors; PSA, prostate specific antigen; PTH, parathyroid hormone; PTH-rP, PTH related protein; QoL, quality of life; RANK-L, receptor activator of NF-κB ligand; RT, radiation therapy; SREs, skeletal-related events; SSEs, symptomatic skeletal events; TGF-β, transforming growth factor β; TK, tyrosine kinase; TKIs, TK inhibitors; TNF, tumornecrosis factor; v-ATPase, vacuolar-type H+ ATPase; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor
Abstract
Bone metastases (BM) are a common complication of cancer, whose management often requires a multidisciplinary approach. Despite the recent therapeutic advances, patients with BM may still experience skeletal-related events and symptomatic skeletal events, with detrimental impact on quality of life and survival.
A deeper knowledge of the mechanisms underlying the onset of lytic and sclerotic BM has been acquired in the last decades, leading to the development of bone-targeting agents (BTA), mainly represented by anti-resorptive drugs and bone-seeking radiopharmaceuticals. Recent pre-clinical and clinical studies have showed promising effects of novel agents, whose safety and efficacy need to be confirmed by prospective clinical trials.
Among BTA, adjuvant bisphosphonates have also been shown to reduce the risk of BM in selected breast cancer patients, but failed to reduce the incidence of BM from lung and prostate cancer. Moreover, adjuvant denosumab did not improve BM free survival in patients with breast cancer, suggesting the need for further investigation to clarify BTA role in early-stage malignancies.
The aim of this review is to describe BM pathogenesis and current treatment options in different clinical settings, as well as to explore the mechanism of action of novel potential therapeutic agents for which further investigation is needed.
1. Introduction
Bone metastases (BM) represent a common complication of cancer, whose incidence reaches 70–95% in multiple myeloma (MM) [1], up to 65–90% in prostate cancer (PC) and about 65–75% in breast cancer (BC). Skeletal involvement is less frequent in other malignancies, ranging from approximately 10% in colorectal tumors to 17–64% in lung cancer (LC) [2].
Among epithelial malignancies, PC and BC are associated with the longest median survival after BM diagnosis (12–53 and 19–25 months, respectively) [3], reflecting the therapeutic advances of the last decades. Similarly, MM patients diagnosed after 2010 experienced improved clinical outcomes, the 2-year survival rate having increased from 69.9% in 2006 to 87.1% in 2012 [4].
Patients with BM may experience skeletal complications, such as pathological fractures, hypercalcaemia, spinal cord injury and uncontrolled pain requiring bone surgery and/or radiotherapy, which are collectively called skeletal-related events (SREs) [5]. In particular, those events inducing an exacerbation of cancer-related pain have been recently defined “symptomatic skeletal events” (SSEs) and evaluated in clinical trials to monitor patients’ quality of life (QoL) [6]. The major treatment options for BM aim at symptom palliation and SRE prevention, while specific anti-cancer therapies are concurrently delivered to reduce the tumor burden at both skeletal and extra-skeletal sites [7].
Bone-targeting treatments include loco-regional and systemic approaches. The former, mainly represented by orthopedic surgery and radiotherapy, are usually performed to control bone pain and prevent pathological fractures. The latter include inhibitors of bone resorption, anabolic agents and radiopharmaceuticals, whose activity is aimed at restoring physiological bone turnover, badly impaired in bone-metastatic patients [7]. Recently, novel therapeutic approaches have been developed, including the bone-targeting radiopharmaceutical Radium-223 dichloride, that has been licensed for the treatment of BM associated with castration-resistant PC (CRPC) [6], and is under investigation in other settings.
Further studies showed the potential effectiveness of bisphosphonates in preventing BM from high-risk early BC (EBC) in women with established menopause at diagnosis or receiving gonadotropin-releasing hormone (GnRH) analogues [8], [9], although no benefit was observed in patients with other solid malignancies [10], [11].
This review focuses on BM pathogenesis in solid tumors and MM; current treatment options will be discussed, together with promising and potentially novel approaches to be further investigated.
2. Methods
We conducted an extensive research among international literature included in the PubMed database and published between 2008 and 2018, by using key words such as “bone metastases”, “skeletal related events” and “bone targeting agents”, in association with “multiple myeloma”, “breast cancer”, “prostate cancer” or “lung cancer”.
Titles and abstracts of the articles were first screened to identify the most relevant papers; selected articles and their bibliography were then further examined for relevancy. Articles written in languages other than English were not taken into consideration.
Conference abstracts from the websites of relevant international oncology meetings were also screened and included if deemed appropriate.
3. Pathogenesis of BM
3.1. Physiological bone turnover
Bone is made up of an extracellular matrix (ECM) surrounding osteoclasts, osteoblasts, osteocytes and bone marrow stromal cells (BMSC). The ECM contains both an organic component, formed by type I collagen, proteoglycans and glycoproteins, and inorganic ions (calcium and phosphate) organized in hydroxyapatite crystals [12].
Osteoclasts derive from monocyte-macrophages and are deputed to bone resorption. Their activation is promoted by systemic and local factors; the former include 1,25-dihydroxyvitaminD3 and parathyroid hormone (PTH) while the latter comprise interleukin-1 (IL-1), IL-6, macrophage colony-stimulating factor (MCSF) and PTH related protein (PTH-rP). Anti-osteoclastogenic factors (e.g. calcitonin, IL-4, IL-18, interferon-β) prevent excessive bone resorption [13].
The receptor activator of nuclear factor-κB (NF-κB) ligand (RANK-L)/RANK/osteoprotegerin axis plays a major role in both osteoclastogenesis and osteoclast activation. RANK-L is a member of the tumor necrosis factor (TNF) family, produced by osteoblasts, stromal and active T-cells in response to pro-osteoclastogenic stimuli. Once RANK-L interacts with its receptor (RANK) expressed by osteoclast precursors, these are activated via NF-kB and Jun N-terminal kinase pathways. Osteoprotegerin, a soluble decoy receptor for RANK-L, prevents osteoclast hyper-activation [3].
Osteoblasts originate from mesenchymal stem cells and are deputed to osteogenesis. Their differentiation is promoted by endothelin-1 (ET-1), platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), bone morphogenetic proteins (BMPs) and transforming growth factor β (TGF-β) which in turn activate the transcription factor Runx-2 [13]. Some osteoblasts are embedded in the bone matrix and become osteocytes, cells with dendritic protrusions acting as mechano-transducers [14].
3.2. Onset of BM from solid tumors
Primary malignancies can drive the metastatic process at early stages, stimulating BMSC to prepare the pre-metastatic niches [15]. Meanwhile, epithelial tumor cells may undergo a morphological and functional remodeling, losing epithelial markers (i.e. E-cadherin and cytokeratins) and features, such as polarity and intercellular junctions, in favor of mesenchymal-like shape and markers (i.e. N-cadherin, fibronectin and vimentin). This process, termed the “epithelial-to-mesenchymal transition”, enhances cancer cell migration and invasiveness, which are necessary for metastasis onset [16].
In 1889, Paget [17] postulated that cancer cells (seeds) metastasize towards a favorable microenvironment (soil), and recent studies have provided a molecular explanation of his theory. Bone-homing tumor cells overexpress chemokine receptors, such as C–X–C motif chemokine-receptor-4 (CXCR-4), whose ligand C–X–C motif chemokine-ligand-12 (CXCL-12) is secreted by stromal cells, including BMSC. Other chemokine axes, namely CXCR-6/CXCL-16 and CXCR-3/CXCL-10, are involved in this process [18], [19], while the calcium sensing receptor is implicated in BC cell migration towards calcium-rich sites [20]. From these metastatic niches, cancer cells may spread to other organs; in the meantime, they enter a state of dormancy, promoted by BMPs and growth-arrest specific-6 (GAS6) protein, secreted by mesenchymal cells [21], [22]. This quiescent state, together with the acquisition of osteoblast and/or osteoclast markers (the so-called “osteomimicry”) permit tumor cell escape from anti-cancer drugs and immune response [5].
Once the surrounding environment becomes suitable for cancer cell proliferation, lytic or sclerotic BM may arise. The former (Fig. 1) underlie the establishment of a vicious circle in which tumor cells secrete pro-osteoclastogenic cytokines, increasing bone resorption. Growth factors (GFs) physiologically stored in bone (e.g. TGF-β, PDGF, etc.) are released during this process, and stimulate cancer cell proliferation [23].
Fig. 1.
The vicious circle of lytic BM in solid malignancies. The onset of lytic BM from solid tumors (e.g. BC) is due to the establishment of a self-propagating vicious circle, based on the cross-talk between cancer cells and the bone microenvironment. Tumor cells secrete pro-osteoclastogenic cytokines that, either directly or indirectly (via osteoblasts), stimulate osteoclast differentiation and activity. This leads to an enhanced bone resorption, and consequent release of matrix-embedded growth factors (e.g. TGF-β, PDGF and insulin-like growth factor) which in turn promote cancer cell proliferation.
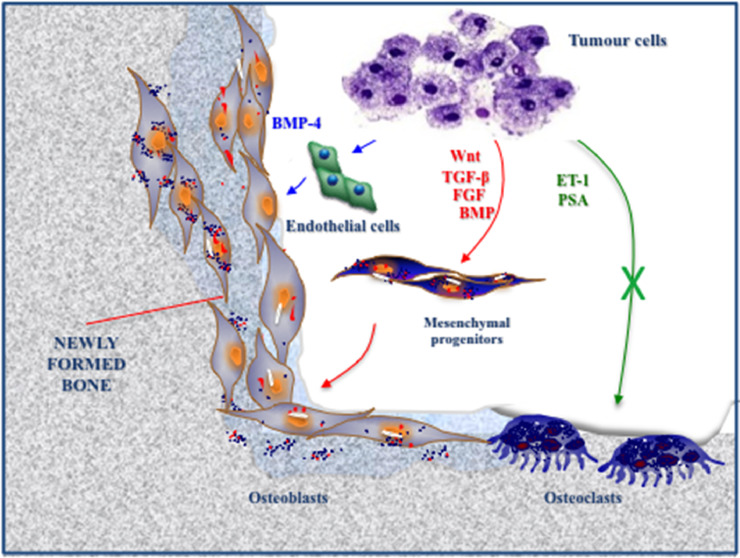
The mechanisms leading to sclerotic BM are less clear (Fig. 2). A number of tumor-derived GFs (e.g. TGF-β, BMPs, FGF and Wnt) may enhance osteoblast differentiation and activity, while ET-1 inhibits osteoclasts [24]. In PC, prostate specific antigen (PSA) is capable of cleaving PTHrP, shifting bone turnover towards osteogenesis. Moreover, PC cells secrete BMP-4, that promotes an endothelial-to-osteoblast conversion in the bone marrow [25].
Fig. 2.
Mechanisms of sclerotic BM formation: major hypotheses. The mechanisms leading to the onset of sclerotic BM have not been completely elucidated. Tumor cells secrete a number of growth factors (e.g. TGF-β, BMP, FGF and Wnt) which enhance the differentiation of mesenchymal progenitors into osteoblasts (red). In PC, tumor-derived ET-1 and PSA are capable of inhibiting bone resorption, shifting the balance of bone turnover towards osteogenesis (green). More recently, BMP-4 has emerged as a novel factor promoting osteogenesis, through the induction of endothelial cell conversion to osteoblasts (blue). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
In most patients with solid malignancies, lytic and sclerotic BM coexist, suggesting a partial overlap of such mechanisms [3].
3.3. Mechanisms of BM development in MM
The pathogenesis of myeloma bone disease relies on reciprocal interactions between malignant plasma cells (MPC) and bone-residing cells, leading to the prevalence of bone resorption over osteogenesis (Fig. 3).
Fig. 3.
Mechanisms of BM onset in MM. In MM, reciprocal interactions between MPC and bone-residing cells lead both to suppressed osteogenesis and increased bone resorption.
MPC interfere with osteoblast differentiation (green) through the secretion of sclerostin and DKK1, and the inhibition of the transcription factor Runx-2 in osteoblast precursors.
In addition, MPC promote the apoptosis of osteocytes (red), whose number and viability are reduced in MM patients, compared to healthy controls.
Finally, the cross-talk between tumor cells and bone microenvironment induces the release of pro-osteoclastogenic factors, including RANK-L, IL-6, Activin A, MCSF and MIP-1α, with a consequent increase of osteoclast maturation and activity (blue). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
MPC inhibit osteoblast differentiation through the secretion of sclerostin and dickkopf1 (DKK1) that dysregulate Wnt signaling, which is essential for osteoblastogenesis; moreover, MPC inhibit the transcription factor Runx-2 in osteoblast precursors, further impairing their maturation [12].
In addition, osteocyte number and viability are reduced in MM patients due to abnormal apoptosis, first activated (via Notch signaling) by the interaction with MPC, then sustained by tumor-derived TNF-α [26].
The cross-talk between MPC and bone microenvironment induces the release of pro-osteoclastogenic factors, including RANK-L, IL-6, Activin-A and MCSF [1], [12]. Moreover, macrophage inflammatory protein-1 alpha (MIP-1α) is secreted by MPC and binds both chemokine-receptor type 1 (CCR1) and 5 (CCR5) expressed by osteoclasts, increasing their activation. MIP-1α also stimulates MPC proliferation and survival, through the autocrine activation of the mitogen-activated protein kinase (MAPK) pathway [27], [28].
Several studies suggested that MPC could also directly participate in bone destruction, undergoing functional trans-differentiation into bone resorbing cells [29], [30], [31], [32]. In this regard, MPC may generate osteoclast-like polykarions in vitro [30], and acquire the expression of myeloid and osteoclast markers (e.g. RANK; TRAP; MCSF receptor, MCSFR; vacuolar-type H + ATPase, v-ATPase), while forming erosive pits on calcium/phosphate slices, under appropriate stimuli [30], [31], [32].
4. Therapeutic approaches to BM
4.1. Loco-regional treatments
Loco-regional approaches to BM include radiation therapy (RT) and orthopedic surgery, whose major purposes are pain relief and management of SREs, such as pathological fractures and spinal cord compression.
With respect to RT, ossification of lytic BM usually begins 3–6 weeks after treatment delivery, reaching its highest degree within 6 months [33]. Pain relief is achieved almost completely in 50% of patients, and generally occurs within the first 2 weeks of treatment [7]. RT doses, techniques and schedules vary according to patients’ features and preferences, as well as primary tumor histology and clinicians’ judgment. In the United States, prolonged fractionated schedules are preferred over a short-course treatment, which is more commonly delivered in Europe and Canada [7], [33].
Several randomized trials compared different fractionation schedules and showed that fractionated and single-fraction treatments were equally effective for pain control [34], [35], [36]. Re-irradiation of the same anatomical site may be considered in case of inadequate pain relief, or to manage pain relapse after initial clinical benefit. Single-fraction RT is often preferred by patients and caregivers because of the shorter duration, but it is associated with higher re-treatment rate. On the other hand, long-course schedules are more frequently complicated by acute toxicities than short course ones [33].
Orthopedic surgery is generally performed in case of pathological fractures or to stabilize high-risk lesions. Treatment modality depends upon life expectancy and metastasis site; in particular, spinal BM may cause severe instability with consequently uncontrolled pain and high risk of spinal cord injury [3].
Besides traditional surgery, less invasive procedures such as percutaneous vertebroplasty and kyphoplasty can be considered for selected patients [37]. These options aim at the stabilization of high-risk spinal BM by the injection of bone cement into the vertebral body, to restore its physiological height and prevent neurological complications [38]. In case of spinal lesions, stereotactic radio-surgery could be also performed to avoid both open surgery and the bone marrow toxicity induced by standard RT. However, results from randomized clinical trials are awaited to establish its effectiveness and safety, compared to standard irradiation [3].
Other loco-regional treatment options include radiofrequency ablation and cryoablation which induce tissue heating or freezing, respectively, to reduce the tumor burden in bone [3].
4.2. Systemic treatments
4.2.1. Inhibitors of bone resorption
Systemic approaches to BM include specific anti-tumor treatments, necessary to control disease progression at both skeletal and extra-skeletal sites, and BTA (Fig. 4), among which anti-resorptive drugs represent the mainstay of BM management. Several agents belong to this category (Table 1), including approved drugs (bisphosphonates and denosumab) and not licensed molecules (cathepsin-K inhibitors, inhibitors of c-Src). Among systemic anti-tumor treatments, several agents (inhibitors of mammalian target of rapamycin, proteasome inhibitors, androgen modulators) have been shown to actively modulate osteoclastogenesis and will be also discussed in this section for their contribution to BM management.
Fig. 4.
Mechanisms of action of common and potential therapeutic agents for BM management. The image shows the mechanisms of action of common BTA, such as denosumab and BPs, as well as potentially novel therapeutic options which warrant further investigation. On one hand, denosumab interacts with RANK-L, thus interfering with its binding to RANK on osteoclasts (OC); on the other hand, BPs directly act on the latter, compromising their survival and/or bone-resorbing activity. Moreover, BPs have been shown to exert a direct anti-tumor activity (in vitro and in vivo), and to stimulate an anti-cancer immune response. Other agents (e.g. Src-inhibitors, mTOR inhibitors) inhibit fundamental signaling pathways in OC, while mTOR inhibitors also exert an anti-cancer effect. Inhibitors of cathepsin-K, a lysosomal enzyme involved in bone matrix degradation, have also been developed, although routine use is limited by their toxicity. Due to their ability to interfere with osteoblast (OB) differentiation and activity, both sclerostin and DKK-1 are under investigation as therapeutic targets for BM management.
Table 1.
Inhibitors of bone resorption for the management of BM.
| Drug class | Mechanism of action | Experimental phase | Indication for BM treatment | References |
|---|---|---|---|---|
| BPs |
N-BPs: ↓ mevalonate pathway, essential for osteoclast activity and survival; Non—N-BPs: ↑ osteoclast apoptosis |
Phase III | Treatment of BM and SRE prevention in MM, BC, CRPC and other solid tumors (if clinically indicated) | [2, 46–55] |
| Denosumab | Anti-RANK-L mAb: ↓ osteoclast differentiation and activity |
Phase III | Treatment of BM and SRE prevention in BC, CRPC and other solid tumors (if clinically indicated). Recently approved by FDA in MM setting. | [2, [46], [56]–60] |
| Cathepsin-K inhibitors | ↓ bone matrix degradation by osteoclasts | Discontinued | No indications | [[28], [62]–64] |
| c-Src inhibitors | ↓ RANK-L-induced osteoclast differentiation | Phase I/II | No indications | [[28], [67]–71] |
| mTOR inhibitors | ↓ osteoclast differentiation and activity; ↑ osteoclast apoptosis | Phase III in BC Phase II in other solid tumors Phase I in MM |
Everolimus approved in association with exemestane in advanced HR + HER2-BC with bone-prevalent disease; BPs or Denosumab to be associated | [2, 74–82] |
| Proteasome inhibitors | ↓ osteoclastogenesis; ↑ osteoblast differentiation; ↑ synthesis of collagen and BMP |
Phase III in MM | Bortezomib and Carfilzomib + BPs (in association, or not, with cht, IMiDs and steroids) approved in MM | [83–87] |
| Abiraterone acetate | ↓ osteoclastogenesis and osteoclast activity; ↑ osteoblast differentiation; ↑ bone matrix deposition; anti-tumor effect |
Phase III in CRPC | Treatment of BM and SRE prevention in CRPC | [88], [89], [92], [93] |
Acronyms: BM: bone metastases; BPs: bisphosphonates; N-BPs: nitrogen-containing BPs; non-N-BPs: non-nitrogen-containing BPs; MM: multiple myeloma; BC: breast cancer; CRPC: castration-resistant prostate cancer; receptor activator of nuclear factor-κB ligand; mAb: monoclonal antibody; SRE: skeletal related events; FDA: food and drug administration; mTOR: mammalian target of rapamycin; cht: chemotherapy; IMiDs: immunomodulatory drugs.
Approved BTA
Bisphosphonates (BPs): BPs are pyrophosphate analogues, whose chemical structure includes a P-C-P central domain binding to bone matrix, and a variable R’ chain [39]. According to the presence, or not, of a nitrogen atom in R’, BPs are defined as “nitrogen-containing” (N-BPs: zoledronate, ibandronate, etc.) or “non-nitrogen containing” (non-N-BPs: clodronate, etidronate, etc.). The former inhibit farnesyl pyrophosphate synthase, which is essential for osteoclast survival and activity; the latter are metabolized to cytotoxic adenosine triphosphate analogues that induce osteoclast apoptosis [2].
BPs have been shown to target several cell types including immune cells, osteoblasts and endothelial cells [40], [41], [42], while a direct anti-tumor activity has also been described for N-BPs, both in vitro and in vivo [43]. BPs stimulate innate anti-cancer immune response by up-regulating γδT-cells [44]. Moreover, zoledronate is able to generate tumor-suppressive BMSC in murine models of BC [45].
During the late 1990s, BPs became the standard of care for BM treatment in both solid tumors and MM, as well as the major therapeutic option for SRE prevention [46].
Several clinical trials demonstrated that, among N-BPs, zoledronate was the most effective for SRE prevention in both MM and solid tumors, while a significant improvement of survival outcomes was reached only in MM setting [47], [48], [49], [50]. With respect to PC, no significant benefit was observed in bone-metastatic patients with castration-sensitive disease, in terms of both median time to first SRE (31.9 months with zoledronate vs 29.8 months with placebo, P = 0.39) and overall survival (OS) (P = 0.29) [51]; on the other hand, in the CR setting zoledronate reduced the risk of SREs by 36% compared to placebo (P = 0.002), while significantly delaying the time to first SRE (488 days vs 321 days, P = 0.002) [52].
According to current guidelines, BPs represent a valuable treatment option for patients with skeletal metastases. In particular, among intravenous agents, zoledronate is approved for BM management in both solid tumors and MM, while pamidronate can be administered to BC and MM patients. Ibandronate is effective in BC patients in both intravenous and oral formulations. Oral clodronate is another therapeutic option for the management of lytic BM [46].
In MM and BC, BPs should be prescribed from the first radiological confirmation of BM, regardless of the presence of symptoms; in the PC setting this treatment should be restricted to CR patients, due to the low risk of SREs in men with hormone-sensitive disease. In patients with BM from other malignancies, BPs should be considered in the presence of bone symptoms, skeletal-dominant disease, and/or complications. For instance, among BPs zoledronate is the most effective in reducing serum calcium levels in patients with hypercalcaemia, which is a serious and potentially life-threatening complication of lytic BM [46].
It is recommended to administer BPs whilst clinical benefit remains evident, but close monitoring of the patients is mandatory, due to the risk of adverse events (AEs) such as osteonecrosis of the jaw (ONJ), kidney failure and hypocalcemia [46].
In patients with BM zoledronate is usually administered every 3–4 weeks, but several studies have recently shown the non-inferiority of a less intensive schedule (every 12 weeks), at least in BC, PC and MM [53], [54], [55]. Such observations suggest that a 3-monthly schedule could be considered to reduce the risk of AEs, without affecting treatment outcome.
Denosumab: This agent is a fully human anti-RANK-L IgG2 antibody that inhibits the interaction between RANK-L and RANK, to reduce osteoclast maturation and activity [2].
A number of phase III clinical trials compared 4-weekly subcutaneous 120 mg denosumab to 4-weekly intravenous 4 mg zoledronate, showing superiority of the former, in terms of time to first and subsequent SREs (P < 0.05 in all instances), in patients with BM from BC and PC [56], [57], [58], [59]. In other bone-metastatic solid malignancies and in MM, denosumab was not inferior to zoledronate in terms of time to first SRE [57], [60].
General guidelines for denosumab use in BM are almost identical to BPs' [46]. In MM, the Food and Drug Administration (FDA) has recently approved this agent on the basis of a multicenter randomized phase III clinical trial, which demonstrated non-inferiority to zoledronate in delaying SREs (P = 0.01), and superiority of denosumab in terms of progression-free survival (PFS) (46.1 vs 35.4 months, P = 0.036) [60]. Moreover, in contrast to BPs, denosumab is not nephrotoxic, providing a valid treatment alternative in patients with kidney failure. Similarly to BPs, denosumab is generally well tolerated, with hypocalcaemia and ONJ being the most common AEs [46].
At present, there is no evidence supporting less intensive schedules of denosumab treatment; unlike BPs, the antibody does not accumulate in bone and its suspension, even for a few months, could impair treatment efficacy. Indeed, osteoporotic patients experienced a rebound increase of both bone resorption and vertebral fractures after denosumab discontinuation [61].
Non-approved agents
Cathepsin-K inhibitors: Cathepsin-K is a lysosomal proteinase produced by osteoclasts, involved in bone matrix degradation and collagen cleavage [28].
Several antagonists of cathepsin-K have been developed, including irreversible and reversible inhibitors of its catalytic site. The latter (e.g. odanacatib, dutacatib and balicatib) underwent pre-clinical and clinical investigation for the management of osteoporosis, osteoarthritis and BC-related BM [62], [63], [64]. In particular, odanacatib was shown to inhibit bone resorption in post-menopausal osteoporotic women [63] and reduce bone turnover markers (BTM) in patients with BM from BC [62]. Unfortunately, odanacatib administration was associated with the onset of atrial fibrillation and stroke, leading to discontinuation of drug development and clinical trial withdrawal [2].
Inhibitors of c-Src: c-Src proto-oncogene encodes a non-receptor tyrosine kinase (TK) involved in tumor cell migration, invasiveness, and RANK-L induced-osteoclastogenesis. c-Src knockout correlates with osteopetrosis and defective dentition in mice [28], suggesting that pharmacological inhibition of this kinase could suppress bone resorption in lytic BM.
Pre-clinical studies with c-Src inhibitors showed their efficacy in preventing both bone and visceral metastases in animal models of BC, with a favorable impact on survival [65]. Dasatinib, a dual Src/Abl inhibitor, reduced tumor growth in murine models of MM, in synergism with anti-myeloma agents [66].
In bone-metastatic patients with BC and CRPC, phase I/II clinical trials demonstrated safety and tolerability of c-Src inhibitors, with promising results in terms of bone turnover modulation [67], [68], [69], [70]. However, the SWOG S0622 clinical trial investigated the efficacy of two different schedules of dasatinib in metastatic BC patients with bone-predominant disease; the study showed no significant efficacy of this single agent in terms of both bone resorption inhibition and PFS improvement, suggesting the need for better patient selection and/or use in combination with other therapeutic agents [71].
Moreover, due to c-Src expression by neurons, these agents have been investigated for the management of cancer-related neuropathic pain, eliciting promising results in vivo [72] that led to clinical experimentation (ClinicalTrials.gov Identifier: NCT02085603).
Anti-cancer agents modulating bone resorption
Inhibitors of mammalian target of rapamycin (mTOR): mTOR exerts both anti-apoptotic and pro-differentiative activities in osteoclasts [2]. In pre-clinical studies, mTOR inhibition by rapamycin analogues reduced the number of lytic BM while increasing bone mass in tumor-bearing mice [73]. Such observations led to several clinical trials in bone-metastatic malignancies.
In particular, BOLERO-1 and BOLERO-2 trials evaluated the association of everolimus with chemotherapy or exemestane, respectively, in advanced BC. BOLERO-2 showed improved PFS in the exemestane + everolimus arm compared to exemestane alone (P < 0.001), leading to the combination approval for patients with hormone receptor (HR)-positive, human epidermal growth factor receptor (HER)-2-negative advanced BC, previously treated with a non-steroidal aromatase inhibitor [74], [75], [76]. Interestingly, everolimus exerted a beneficial effect on bone turnover and skeletal disease, regardless of BP administration [77].
Other clinical trials showed the efficacy and safety of everolimus, in association with BPs, in bone-metastatic LC, kidney malignancies and PC [78], [79], [80]. In MM, phase I clinical trials described anti-myeloma activity of everolimus either alone or in combination with lenalidomide [81], [82].
Proteasome inhibitors (PIs): These agents reduce RANK-L-mediated osteoclastogenesis via inhibition of NF-kB; they also exert anabolic effects on bone, by stimulating osteoblast differentiation and promoting type I collagen and BMP synthesis [83].
The first PI licensed for MM treatment was bortezomib, thanks to its combined anti-myeloma and anti-osteoclast activity which improved PFS, OS and response rate [84], [85]. However, mechanisms of resistance to bortezomib have been described in MM, including drug extrusion from tumor cells and proteasome up-regulation [83]. Several clinical trials investigating the association of bortezomib with other treatments have been developed, including the SWOG S0777 study which showed a significant benefit derived from bortezomib addition to lenalidomide + dexamethasone treatment in patients with newly diagnosed MM, in terms of median PFS (43 months vs 30 months of control, P = 0.0018) and median OS (75 months vs 64 months of control, P = 0.0025) [84]. Second generation inhibitors have been also developed, to overcome bortezomib limitations [86], [87].
Androgen modulators: At its earliest stages, PC is an androgen-dependent disease which benefits from androgen-deprivation therapies. Even in the CR setting, modulation of androgen signaling represents the mainstay of PC treatment, and the introduction of novel agents (i.e. abiraterone acetate and enzalutamide) in the clinical practice led to significant improvements of OS and PFS in both chemotherapy-naïve and docetaxel-treated patients [88], [89], [90], [91].
Abiraterone acetate is a cytochrome P17 irreversible inhibitor which blocks androgen biosynthesis [89], while enzalutamide selectively inhibits the androgen receptor [90]. Both these agents have been shown to improve bone pain and delay the onset of SREs [92], although the mechanisms underlying these bone-specific effects have not been completely elucidated. Iuliani et al. described that non-cytotoxic concentrations of abiraterone significantly inhibited osteoclast maturation and activity, while stimulating osteoblast differentiation and bone matrix deposition in vitro [93]. On the other hand, the effects of enzalutamide on bone seem to correlate with its anti-cancer effects rather than modulation of bone turnover [92].
4.2.2. Osteoblast modulators
Impaired bone formation contributes to the onset of lytic BM; on the other hand, sclerotic BM occur as a consequence of excessive osteogenesis. Thus, researchers explored also the development of osteoblast modulators for BM management (Table 2). At present, none of the agents belonging to this group has been licensed for the prevention of SREs. Among anti-cancer agents, TK inhibitors (TKIs) may contribute to restore the physiological osteogenesis and showed efficacy in terms of bone-related outcomes, so will be discussed in this section.
Table 2.
Modulators of osteoblast activity for the management of BM.
| Drug class | Mechanism of action | Experimental phase | Indication for BM treatment | References |
|---|---|---|---|---|
| PTH | ↑ Wnt pathway | Pre-clinical | No indications | [94–98] |
| ↑ osteoblast differentiation | ||||
| ↓sclerostin and DKK-1 | ||||
| ↓ tumor cell migration | ||||
| towards bone | ||||
| Anti-sclerostin antibodies | Sclerostin inhibition: | Pre-clinical | No indications | [99–105] |
| ↑ Wnt pathway | ||||
| ↑ osteoblast differentiation | ||||
| DKK-1 inhibitors | DKK-1 inhibition: | Phase I/II | No indications | [106–109] |
| ↑ Wnt pathway | ||||
| ↑ osteoblast differentiation | ||||
| Inhibitors of activin-A | ↓ osteoclastogenesis | Phase I/II | No indications | [28], [111], [117] |
| ↑ osteoblast differentiation | ||||
| ↓ tumor cell migration | ||||
| towards bone | ||||
| ET-1 antagonists | ↓ osteoblast inhibition of sclerotic BM | Phase II/III | No indications | [24], [118], [119] |
| Cabozantinib | TKI; | Phase III | Metastatic renal cell carcinoma (with/without BM) | [121–124] |
| Inhibition of VEGF/VEGFR pathway |
Acronyms: BM: bone metastases; PTH: parathyroid hormone; DKK-1: dickkopf1; ET: endothelin; TKI: tyrosine kinase inhibitor; VEGF: vascular endothelial growth factor; VEGFR: VEGF receptor.
PTH: PTH can exert anabolic effects on bone by up-regulating genes involved in the Wnt pathway, while down-regulating DKK-1 and sclerostin, in osteoblasts [94]. Interestingly, PTH improved bone mineral density (BMD) in murine models of MM and BC by increasing osteoblast differentiation [95] and reducing tumor cell migration towards bone [96].
However, clinical data were less encouraging, since serum PTH ≥68.3 pg/mL at diagnosis correlated with unfavorable outcome in MM patients [97]. Moreover, a PTH analogue (teriparatide) approved for osteoporosis treatment was shown to induce osteosarcoma in mice [98], arousing safety concerns about PTH administration to cancer patients.
Anti-sclerostin antibodies: as a Wnt-inhibitor, sclerostin operates a potent brake on osteoblast differentiation, whose production has been attributed to osteocytes, MPC and BC cells [99].
Terpos et al. [100] found higher serum levels of this protein in MM patients with fractures, as compared to those without SREs at diagnosis (P < 0.01), while a significant correlation between high sclerostin levels and poor survival (P = 0.031) was also described.
Pre-clinical studies analyzed the effectiveness of anti-sclerostin antibodies in skeletal diseases, showing bone anabolic activity in ovariectomized mice and murine models of MM, together with anti-cancer properties [101], [102]. McDonald and colleagues have recently described that treatment with an anti-sclerostin antibody may prevent the onset of MM-bone disease while increasing resistance to fractures in mice, especially when administered in combination with zoledronate [103].
Anti-sclerostin antibodies (e.g. romosozumab, blosozumab and BPS804) have been investigated in clinical trials of benign bone diseases [104], [105]; however, due to its cardiotoxicity, romosozumab was not approved by the FDA for osteoporosis treatment.
DKK-1 inhibitors: DKK-1 is another Wnt inhibitor produced by several tumors including MM, PC and BC. Alongside sclerostin, DKK-1 reduces β-catenin levels, leading to impaired osteoblastogenesis [2], [106], [107].
Pre-clinical studies on DKK-1 inhibitors showed increased bone formation and reduced osteolysis in MM-bearing mice, associated with reduced secretion of IL-6 by BMSC [107].
Promising results came also from a phase IB clinical trial investigating the safety and efficacy of a DKK-1 inhibitor (BHQ880) in MM, in combination with zoledronate and anti-myeloma treatment [108].
An open-label multicenter phase II study evaluated the effect of BHQ880 in smoldering MM at high risk of progression; preliminary results confirmed the bone anabolic activity of this agent, evaluated in terms of bone strength, but showed no anti-tumor effect [109].
Another potential treatment option, currently under preclinical evaluation, is a bi-specific antibody against sclerostin and DKK-1 [110], that should theoretically overcome resistance to single-target inhibitors.
Inhibitors of activin-A: activin-A is a cytokine secreted by BMSC, osteoblasts and osteoclasts which stimulates osteoclastogenesis in synergism with RANK-L, while inhibiting osteoblast differentiation through the activation of SMAD-2 [28].
Increased levels of activin-A have been found in the sera of patients with BM from MM and solid malignancies [111], [112], as well as in primary PC of men with BM [113].
Activin-A effects are mediated by its transmembrane type II serine/threonine kinase receptor (ActRIIA), whose recombinant analogues have been investigated in pre-clinical and clinical studies. Treatment with these agents inhibited the onset of lytic BM, while increasing BMD, in murine models of MM and BC [114]. A phase II clinical trial showed improved BMD and bone pain in MM patients receiving sotatercept (a recombinant ActRIIA ligand), in association with anti-myeloma treatment and BPs [115]. Interestingly, a dose-dependent increase in haemoglobin levels was observed after sotatercept administration [116], suggesting a potential role of this drug for the management of chemotherapy-induced anemia, that has been further investigated in phase II trials (ClinicalTrials.gov Identifiers: NCT01190644 and NCT01284348).
The immunomodulatory drug lenalidomide, licensed for MM treatment, was shown to promote activin-A production by BMSC [117]; hence, a phase I clinical trial is currently investigating safety and efficacy of sotatercept in refractory MM, in combination with lenalidomide/pomalidomide and dexamethasone (ClinicalTrials.gov Identifier: NCT01562405).
ET-1 antagonists: ET-1 is involved in the pathogenesis of sclerotic BM; it is produced by PC and stimulates osteoblasts while acting as an anti-osteoclastogenic factor [24]. A significantly increased plasma concentration of ET-1 was found in patients with CRPC and BM, as compared to those with localized tumors (13.2 ± 1.1 pg/ml vs 5.7 ± 0.3 pg/ml, P < 0.0001) [118].
ET-1 also stimulates cancer cell proliferation by interacting with a G-protein coupled receptor (ET-A) that activates intracellular signaling pathways, such as protein kinase C and MAPK ones [24].
A meta-analysis published by Qiao et al. included nine clinical trials investigating the effectiveness of ET-A antagonists (Zibotentan and Atrasentan) in CRPC. Although none of the agents improved OS and PFS, compared to placebo, Atrasentan significantly delayed the increase of PSA and BTM (P < 0.05 in both instances), while improving BM-related pain [119]; such observations deserve further investigation on Atrasentan to establish its role in BM management.
TKIs: osteoblast hyperactivation in PC is also stimulated by the interaction between vascular endothelial growth factor (VEGF) and its receptor (VEGFR). Moreover, the expression of VEGFR by bone marrow precursors of both endothelial and hematopoietic cells has been correlated with the development of pre-metastatic niches [120]. Thus, VEGFR targeting by TKIs has been attempted, although preliminary studies with sunitinib and sorafenib were unsatisfactory [2].
Cabozantinib, a more selective TKI which preferentially targets VEGFR2 and the hepatocyte GF receptor, was shown to inhibit BM in PC animal models [121], prompting the activation of several clinical trials.
In particular, a phase III study investigated the efficacy of cabozantinib versus prednisone in heavily pre-treated CRPC patients with BM. Despite not improving OS (11.0 months vs 9.8 months, P = 0.213), the agent significantly ameliorated bone scan response, PFS and time to first SRE (P < 0.001 in all instances) [122].
Interestingly, in the randomized open-label phase III METEOR trial involving 658 patients with advanced renal cell carcinoma, cabozantinib improved both OS and PFS, as compared to everolimus (P = 0.00026 and P < 0.0001, respectively) [123]. Moreover, in patients with BM at baseline, cabozantinib was associated with less SREs (23 % vs 29%), longer median time to first SRE (3.7 months vs 2.5 months), improved bone scan response and greater changes in BTM levels (P < 0.05 for bone-specific alkaline phosphatase, C-terminal telopeptide of type I collagen and pro-collagen type 1 amino-terminal pro-peptide after 5 weeks of treatment; P < 0.05 for bone-specific alkaline phosphatase and C-terminal telopeptide of type I collagen after 9 weeks), as compared to the mTOR inhibitor [124].
5. Bone-targeting radiopharmaceuticals
The therapeutic role of radiopharmaceuticals has been widely investigated in this setting, since radioactive-labeled tracers can be selectively delivered towards bone, sparing healthy organs from irradiation. Once target cells have been reached, radionucleotides induce DNA damage and apoptosis [3].
β-emitter radionucleotides, such as the calcium-mimetic Strontium-89 and Samarium-153 (which is also a γ-emitter), showed their efficacy in improving BM-related pain, but induced significant myelotoxicity [125]. The α-emitter Radium-223 dichloride localizes in bone and creates complexes with hydroxyapatite, thanks to its similarity to calcium. Interestingly, Radium-223 emits short range (<100 µm) high-energy particles that exert a highly selective anti-tumor effect, with low toxicity [125].
In the placebo-controlled ALSYMPCA trial, Radium-223 improved median OS in CRPC patients with symptomatic BM, compared to control (14.9 vs 11.3 months, P < 0.001), and prolonged the time to first SRE (15.6 vs 9.8 months with placebo, HR 0.66, P < 0.001). No safety issues emerged from the trial, even when Radium-223 was given alongside BPs [6], [126]. These results led to its fast-track approval by the FDA in this setting; clinical trials are investigating Radium-223 effectiveness in other solid malignancies and MM, in combination with standard anti-cancer treatments (ClinicalTrials.gov Identifiers: NCT02390934, NCT02406521, NCT02258464, NCT02258451, NCT02928029, etc.).
6. Towards BM prevention: state of the art
The evidence that BTA may interfere with different steps of BM development led researchers to hypothesize their potential use in early-stage tumors, in order to prevent skeletal colonization by osteotropic cancer cells.
In this regard, positive results have been achieved in BC, especially by ABCSG-12 and AZURE trials. The former showed that the addition of zoledronate to standard adjuvant treatment improved disease-free survival (DFS) in pre-menopausal women receiving GnRH analogues (88.4 % vs 85%, P < 0.05) [9], while the latter demonstrated improved invasive DFS in women with established menopause at diagnosis [8]. These observations raised the hypothesis that adjuvant BPs could exert their activity in women with low circulating levels of reproductive hormones. In agreement with these data, in the NSABP-B34 trial clodronate added to standard adjuvant treatment improved both skeletal and extra-skeletal metastasis-free survival (P < 0.05 in both instances) in women >50 years at study entry [127]. Moreover, a large meta-analysis involving 22,982 patients from 36 clinical trials found that adjuvant BPs significantly reduced distant recurrence in post-menopausal women, compared with controls (18.4 % vs 21.9%). Such effects were independent of primary tumor features, type of BPs, and treatment schedules [8], [128].
At present, both North American and European BC experts recommend adjuvant BPs (zoledronate, clodronate or ibandronate) alongside vitamin D and calcium supplementation in post-menopausal women with EBC at intermediate-high risk of recurrence, as well as in pre-menopausal patients undergoing ovarian suppression [129], [130].
As to denosumab, preliminary data suggested its potential ability to reduce BC recurrence in bone [131]. However, results from D-CARE trial after a median follow-up of 67 months showed no benefit deriving from the adjuvant administration of this agent, in terms of BM-free survival (HR 0.97, 95%CI 0.82–1.14, P = 0.70), DFS (HR 1.04, 95%CI 0.91–1.19, P = 0.57) and OS (HR 1.03, 95%CI 0.85–1.25) [132].
In LC, exploratory studies on BTA showed no benefits from zoledronate in terms of median PFS (9.0 months vs 11.3 months of control, P = 0.096), median OS (30.3 months vs 29.3 months of control) and BM onset (15 events in zoledronate arm vs 19 events in control group) [10], while studies with denosumab are still ongoing.
In PC, zoledronate exhibited no impact on survival in men starting androgen deprivation therapy [133] and other studies with BPs failed to show any benefit in terms of BM prevention [11]. Interestingly, a placebo-controlled trial investigated the effects of denosumab in high-risk non-metastatic CRPC patients, finding an improved BM-free survival in denosumab arm (P = 0.028) [134]. However, due to the relatively high incidence of ONJ (4% at 3 years) [134] denosumab did not receive regulatory approval in this setting.
7. Conclusion and future perspectives
BM management poses one of the greatest challenges for oncologists, due not only to safety issues, but also to the significant impairment of patients’ QoL and survival. A multidisciplinary approach to BM is essential, to ensure a proper integration of local and systemic therapies.
In the last decades, a deeper knowledge of the mechanisms underlying BM onset has been acquired, leading to the development of effective therapeutic agents, such as anti-resorptive drugs and bone-seeking radiopharmaceuticals. Further clinical investigation is needed to confirm pre-clinical evidence about potentially novel agents. These and other observations will hopefully contribute to further improvements of the clinical approach to osteotropic tumors, with the most ambitious goals being the early identification of high-risk patients and the prevention of skeletal metastases.
Fundings
This work was supported by a grant from the Italian Association for Cancer Research (AIRC) [grant number: IG17536], and from the Apulia Region [Oncogenomic Project, 2015; no grant number applicable].
Disclosures
SD and FS: no conflicts of interest. RC: advisory board fees and travel support from Amgen. JB: advisory boards and speaker bureaux for Amgen, Novartis and Bayer. SD: designed the study and wrote the manuscript. RC, JB, FS: critically revised the manuscript for intellectual content. All authors have read and approved the final version of the manuscript.
References
- 1.Silvestris F, Lombardi L, De Matteo M, Bruno A, Dammacco F. Myeloma bone disease: pathogenetic mechanisms and clinical assessment. Leuk. Res. 2007;31:129–138. doi: 10.1016/j.leukres.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Sousa S, Clézardin P. Bone-targeted therapies in cancer-induced bone disease. Calcif. Tissue Int. 2018;102:227–250. doi: 10.1007/s00223-017-0353-5. [DOI] [PubMed] [Google Scholar]
- 3.Macedo F, Ladeira K, Pinho F, Saraiva N, Bonito N, Pinto L. Bone metastases: an overview. Oncol. Rev. 2017;11(321):43–49. doi: 10.4081/oncol.2017.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fonseca R, Abouzaid S, Bonafede M, Cai Q, Parikh K, Cosler L. Trends in overall survival and costs of multiple myeloma, 2000–2014. Leukemia. 2017;31:1915–1921. doi: 10.1038/leu.2016.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kan C, Vargas G, Pape FL, Clézardin P. Cancer Cell colonisation in the bone microenvironment. Int. J. Mol. Sci. 2016;17(10):1–16. doi: 10.3390/ijms17101674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parker C, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, Fosså SD. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013;369:213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 7.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 2006;12(20 Suppl.):6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 8.Coleman R, Cameron D, Dodwell D, Bell R, Wilson C, Rathbone E. Adjuvant zoledronic acid in patients with early breast cancer: final efficacy analysis of the AZURE (BIG 01/04) randomised open-label phase 3 trial. Lancet Oncol. 2014;15:997–1006. doi: 10.1016/S1470-2045(14)70302-X. [DOI] [PubMed] [Google Scholar]
- 9.Gnant M, Mlineritsch B, Stoeger H, Luschin-Ebengreuth G, Knauer M, Moik M. Zoledronic acid combined with adjuvant endocrine therapy of tamoxifen versus anastrozol plus ovarian function suppression in premenopausal early breast cancer: final analysis of the Austrian Breast and Colorectal Cancer Study Group Trial 12. Ann Oncol. 2015;26:313–320. doi: 10.1093/annonc/mdu544. [DOI] [PubMed] [Google Scholar]
- 10.Scagliotti GV, Kosmidis P, de Marinis F, Schreurs AJ, Albert I, Engel-Riedel W. Zoledronic acid in patients with stage IIIA/B NSCLC: results of a randomized, phase III study. Ann. Oncol. 2012;23(8):2082–2087. doi: 10.1093/annonc/mds128. [DOI] [PubMed] [Google Scholar]
- 11.Wirth M, Tammela T, Cicalese V, Gomez Veiga F, Delaere K, Miller K. Prevention of bone metastases in patients with high-risk nonmetastatic prostate cancer treated with zoledronic acid: efficacy and safety results of the Zometa European Study (ZEUS) Eur. Urol. 2015;67(3):482–491. doi: 10.1016/j.eururo.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Terpos E, Ntanasis-Stathopoulos I, Gavriatopoulou M, Dimopoulos MA. Pathogenesis of bone disease in multiple myeloma: from bench to bedside. Blood Cancer J. 2018;8:7. doi: 10.1038/s41408-017-0037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D'Oronzo S, Brown J, Coleman R. The role of biomarkers in the management of bone-homing malignancies. J. Bone Oncol. 2017;9:1–9. doi: 10.1016/j.jbo.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonewald LF. The amazing osteocyte. J. Bone Min. Res. Off. J. Am. Soc. Bone Min. Res. 2011;26:229–238. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sceneay J, Smyth MJ, Möller A. The pre-metastatic niche: finding common ground. Cancer Metastasis Rev. 2013;32:449–464. doi: 10.1007/s10555-013-9420-1. [DOI] [PubMed] [Google Scholar]
- 16.Meng F, Wu G. The rejuvenated scenario of epithelial–mesenchymal transition (EMT) and cancer metastasis. Cancer Metastasis Rev. 2012;31:455–467. doi: 10.1007/s10555-012-9379-3. [DOI] [PubMed] [Google Scholar]
- 17.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;1:571–573. [PubMed] [Google Scholar]
- 18.Ha HK, Lee W, Park HJ, Lee SD, Lee JZ, Chung MK. Clinical significance of CXCL16/CXCR6 expression in patients with prostate cancer. Mol. Med. Rep. 2011;4:419–424. doi: 10.3892/mmr.2011.446. [DOI] [PubMed] [Google Scholar]
- 19.Lee JH, Kim HN, Kim KO, Jin WJ, Lee S, Kim HH. CXCL10 promotes osteolytic bone metastasis by enhancing cancer outgrowth and osteoclastogenesis. Cancer Res. 2012;72:3175–3186. doi: 10.1158/0008-5472.CAN-12-0481. [DOI] [PubMed] [Google Scholar]
- 20.Saidak Z, Boudot C, Abdoune R, Petit L, Brazier M, Mentaverri R. Extracellular calcium promotes the migration of breast cancer cells through the activation of the calcium sensing receptor. Exp. Cell Res. 2009;315:2072–2080. doi: 10.1016/j.yexcr.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Shiozawa Y, Pedersen EA, Patel LR, Ziegler AM, Havens AM, Jung Y. GAS6/AXL axis regulates prostate cancer invasion, proliferation, and survival in the bone marrow niche. Neoplasia. 2010;12:116–127. doi: 10.1593/neo.91384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi A, Okuda H, Xing F, Pandey PR, Watabe M, Hirota S. Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in bone. J. Exp. Med. 2011;208:2641–2655. doi: 10.1084/jem.20110840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D'Oronzo S, Brown J, Coleman R. The value of biomarkers in bone metastasis. Eur. J. Cancer Care. 2017;26(6) doi: 10.1111/ecc.12725. [DOI] [PubMed] [Google Scholar]
- 24.Chiao JW, Moonga BS, Yang YM, Kancherla R, Mittelman A, Wu-Wong JR. Endothelin-1 from prostate cancer cells is enhanced by bone contact which blocks osteoclastic bone resorption. Br. J. Cancer. 2000;83(3):360–365. doi: 10.1054/bjoc.2000.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin SC, Lee YC, Yu G, Cheng CJ, Zhou X, Chu K. Endothelial-to-osteoblast conversion generates osteoblastic metastasis of prostate cancer. Dev. Cell. 2017;41:467–480. doi: 10.1016/j.devcel.2017.05.005. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delgado-Calle J, Anderson J, Cregor MD, Hiasa M, Chirgwin JM, Carlesso N. Bidirectional notch signaling and osteocyte-derived factors in the bone marrow microenvironment promote tumor cell proliferation and bone destruction in multiple myeloma. Cancer Res. 2016;76(5):1089–1100. doi: 10.1158/0008-5472.CAN-15-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang XT, He YC, Zhou SY, Jiang JZ, Huang YM, Liang YZ. Bone marrow plasma macrophage inflammatory protein protein-1 alpha (MIP-1 alpha) and sclerostin in multiple myeloma: relationship with bone disease and clinical characteristics. Leuk. Res. 2014;38:525–531. doi: 10.1016/j.leukres.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 28.Longo V, Brunetti O, D'Oronzo S, Dammacco F, Silvestris F. Therapeutic approaches to myeloma bone disease: an evolving story. Cancer Treat. Rev. 2012;38:787–797. doi: 10.1016/j.ctrv.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 29.McDonald DF, Schofield BH, Prezioso EM, Adams VL, Frondoza CA, Trivedi SM. Direct bone resorbing activity of murine myeloma cells. Cancer Lett. 1983;19:119–124. doi: 10.1016/0304-3835(83)90145-3. [DOI] [PubMed] [Google Scholar]
- 30.Silvestris F, Ciavarella S, De Matteo M, Tucci M, Dammacco F. Bone-resorbing cells in multiple myeloma: osteoclasts, myeloma cell polykaryons, or both? Oncologist. 2009;14:264–275. doi: 10.1634/theoncologist.2008-0087. [DOI] [PubMed] [Google Scholar]
- 31.Tucci M, Stucci S, Savonarola A, Ciavarella S, Cafforio P, Dammacco F. Immature dendritic cells in multiple myeloma are prone to osteoclast-like differentiation through interleukin-17A stimulation. Br. J. Haematol. 2013;161:6821–6831. doi: 10.1111/bjh.12333. [DOI] [PubMed] [Google Scholar]
- 32.Cafforio P*, D'Oronzo S*, Felici C, Sigala S, Fragni M, Silvestris F. 1,25(OH)2 vitamin D(3) contributes to osteoclast-like trans-differentiation of malignant plasma cells. Exp. Cell Res. 2017;358:260–268. doi: 10.1016/j.yexcr.2017.06.023. *Equally contributing authors. [DOI] [PubMed] [Google Scholar]
- 33.De Felice F, Piccioli A, Musio D, Tombolini V. The role of radiation therapy in bone metastases management. Oncotarget. 2017;8(15):25691–25699. doi: 10.18632/oncotarget.14823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartsell WF, Scott CB, Bruner DW, Scarantino CW, Ivker RA, Roach M., 3rd Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J. Natl. Cancer Inst. 2005;97(11):798–804. doi: 10.1093/jnci/dji139. [DOI] [PubMed] [Google Scholar]
- 35.Foro Arnalot P, Fontanals AV, Galcerán JC, Lynd F, Latiesas XS, de Dios NR. Randomized clinical trial with two palliative radiotherapy regimens in painful bone metastases: 30 Gy in 10 fractions compared with 8 Gy in single fraction. Radiother. Oncol. 2008;89(2):150–155. doi: 10.1016/j.radonc.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 36.Steenland E, Leer JW, van Houwelingen H, Post WJ, van den Hout WB, Kievit J. The effect of a single fraction compared to multiple fractions on painful bone metastases: a global analysis of the Dutch Bone Metastasis Study. Radiother. Oncol. 1999;52(2):101–109. doi: 10.1016/s0167-8140(99)00110-3. [DOI] [PubMed] [Google Scholar]
- 37.Manabe J, Kawaguchi N, Matsumoto S, Tanizawa T. Surgical treatment of bone metastasis: indications and outcomes. Int. J. Clin. Oncol. 2005;10:103–111. doi: 10.1007/s10147-005-0478-9. [DOI] [PubMed] [Google Scholar]
- 38.Gdowski AS, Ranjan A, Vishwanatha JK. Current concepts in bone metastasis, contemporary therapeutic strategies and ongoing clinical trials. J. Exp. Clin. Cancer Res. 2017;36:108. doi: 10.1186/s13046-017-0578-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roelofs AJ, Thompson K, Ebetino H, Rogers MJ, Coxon FP. Bisphosphonates: molecular mechanisms of action and effects on bone cells, monocytes and macrophages. Curr. Pharm. Des. 2010;16:2950–2960. doi: 10.2174/138161210793563635. [DOI] [PubMed] [Google Scholar]
- 40.Junankar S, Shay G, Jurczyluk J, Ali N, Down J, Pocock N, Parker A, Nguyen A, Sun S, Kashemirov B, McKenna CE, Croucher PI, Swarbrick A, Weilbaecher K, Phan TG, Rogers MJ. Real-time intravital imaging establishes tumor-associated macrophages as the extraskeletal target of bisphosphonate action in cancer. Cancer Discov. 2015;5(1):35–42. doi: 10.1158/2159-8290.CD-14-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cabillic F, Toutirais O, Lavoue V, de La Pintière CT, Daniel P, Rioux-Leclere N. Aminobisphosphonate-pretreated dendritic cells trigger successful Vc9Vd2 T cell amplification for immunotherapy in advanced cancer patients. Cancer Immunol. Immunother. 2010;59:1611–1619. doi: 10.1007/s00262-010-0887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalyan S, Chandrasekaran V, Quabius ES, Lindhorst TK, Kabelitz D. Neutrophil uptake of nitrogen-bisphosphonates leads to the suppression of human peripheral blood cd T cells. Cell Mol. Life Sci. 2014;71:2335–2346. doi: 10.1007/s00018-013-1495-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dedes P, Gialeli C, Tsonis A, Kanakis I, Theocharis AD, Kletsas D. Expression of matrix macromolecules and functional properties of breast cancer cells are modulated by the bisphosphonate zoledronic acid. Biochim. Biophys. Acta. 2012;1820:1926–1939. doi: 10.1016/j.bbagen.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 44.Naoe M, Ogawa Y, Takeshita K, Morita J, Shichijo T, Fuji K. Zoledronate stimulates gamma delta T cells in prostate cancer patients. Oncol. Res. 2010;18(10):493–501. doi: 10.3727/096504010x12671222663638. [DOI] [PubMed] [Google Scholar]
- 45.Ubellacker JM, Haider MT, De Cristo MJ, Allocca G, Brown NJ, Silver DP. Zoledronic acid alters hematopoiesis and generates breast tumor-suppressive bone marrow cells. Breast Cancer Res. 2017;19:23. doi: 10.1186/s13058-017-0815-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coleman R, Body JJ, Aapro M, Hadji P, Herrstedt J. Bone health in cancer patients: ESMO clinical practice guidelines. Ann. Oncol. 2014;25(Suppl 3:iii):124–137. doi: 10.1093/annonc/mdu103. https://doi:10.1093/annonc/mdu103. [DOI] [PubMed] [Google Scholar]
- 47.Morgan GJ, Davies FE, Gregory WM, Bell SE, Szubert AJ, Cook G. Long-term follow-up of MRC myeloma IX trial: survival outcomes with bisphosphonate and thalidomide treatment. Clin. Cancer Res. 2013;19:6030–6038. doi: 10.1158/1078-0432.CCR-12-3211. [DOI] [PubMed] [Google Scholar]
- 48.Rosen LS, Gordon D, Kaminski M, Howell A, Belch A, Mackey J. Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase III, double-blind, comparative trial. Cancer J. 2001;7(5):377–387. [PubMed] [Google Scholar]
- 49.Rosen LS, Gordon D, Kaminski M, Howell A, Belch A, Mackey J. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in treatment of skeletal complications in patients with advanced multiple myeloma or breast cancer: a randomized, double-blind, multicenter, comparative trial. Cancer. 2003;98:1735–1744. doi: 10.1002/cncr.11701. [DOI] [PubMed] [Google Scholar]
- 50.Rosen LS, Gordon D, Tchekmedyian N, Yanagihara R, Hirsh V, Krzakowski M. Long term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with non small cell lung carcinoma and other solid tumors: a randomized, phase III, double blind, placebo-controlled trial. Cancer. 2004;100:2613–2621. doi: 10.1002/cncr.20308. [DOI] [PubMed] [Google Scholar]
- 51.Smith MR, Halabi S, Ryan CJ, Hussain A, Vogelzang N, Stadler W. Randomized controlled trial of early zoledronic acid in men with castration-sensitive prostate cancer and bone metastases: results of CALGB 90202 (Alliance) J. Clin. Oncol. 2014;32:1143–1150. doi: 10.1200/JCO.2013.51.6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with advanced prostate cancer and bone metastasis. J. Natl. Cancer Inst. 2004;96:879–882. doi: 10.1093/jnci/djh141. [DOI] [PubMed] [Google Scholar]
- 53.Hutton B, Addison CL, Campbell K, Fergusson D, Mazarello S, Clemons M. A systematic review of dosing frequency with bone-targeted agents for patients with bone metastases from breast cancer. J. Bone Oncol. 2013;2(3):123–131. doi: 10.1016/j.jbo.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hortobagy GN, Van Poznak C, Harker WG, Gradishar WJ, Chew H, Dakhil SR. Continued treatment effect of zoledronic acid dosing every 12 vs 4 weeks in women with breast cancer metastatic to bone: the OPTIMIZE-2 randomized clinical trial. JAMA Oncol. 2017;3(7):906–912. doi: 10.1001/jamaoncol.2016.6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Himelstein AL, Foster JC, Khatcheressian JL, Roberts JD, Seisler DK, Novotny PJ. Effect of longer-interval vs standard dosing of zoledronic acid on skeletal events in patients with bone metastases: a randomized clinical trial. JAMA. 2017;317(1):48–58. doi: 10.1001/jama.2016.19425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fizazi K, Carducci M, Smith M, Damião R, Brown J, Karsh L. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lipton A, Fizazi K, Stopeck AT, Henry DH, Smith MR, Shore N. Effect of denosumab versus zoledronic acid in preventing skeletal-related events in patients with bone metastases by baseline characteristics. Eur. J. Cancer. 2016;53:75–83. doi: 10.1016/j.ejca.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 58.Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, de Boer RH. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J. Clin. Oncol. 2010;28:5132–5139. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 59.Stopeck AT, Fizazi K, Body JJ, Brown JE, Carducci M, Diel I. Safety of long-term denosumab therapy: results from the open label extension phase of two phase 3 studies in patients with metastatic breast and prostate cancer. Support Care Cancer. 2016;24:447–455. doi: 10.1007/s00520-015-2904-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raje N, Terpos E, Willenbacher W, Shimizu K, Garcia-Sanz R, Durie B. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: an international, double-blind, double-dummy, randomised, controlled, phase 3 study. Lancet Oncol. 2018;19(3):370–381. doi: 10.1016/S1470-2045(18)30072-X. [DOI] [PubMed] [Google Scholar]
- 61.Tsourdi E, Langdahl B, Cohen-Solal M, Aubry-Rozier B, Eriksen EF, Guañabens N. Discontinuation of Denosumab therapy for osteoporosis: a systematic review and position statement by ECTS. Bone. 2017;105:11–17. doi: 10.1016/j.bone.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 62.Jensen AB, Wynne C, Ramirez G, He W, Song Y, Berd Y. The cathepsin K inhibitor odanacatib suppresses bone resorption in women with breast cancer and established bone metastases: results of a 4-week, double-blind, randomized, controlled trial. Clin. Breast Cancer. 2010;10:452–458. doi: 10.3816/CBC.2010.n.059. [DOI] [PubMed] [Google Scholar]
- 63.Bone HG, Dempster DW, Eisman JA, Greenspan SL, McClung MR, Nakamura T. Odanacatib for the treatment of postmenopausal osteoporosis: development history and design and participant characteristics of LOFT, the long-term odanacatib fracture trial. Osteoporos Int. 2015;26:699–712. doi: 10.1007/s00198-014-2944-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Le Gall C, Bellahcène A, Bonnelye E, Gasser JA, Castronovo V, Green J. A cathepsin K inhibitor reduces breast cancer-induced osteolysis and skeletal tumor burden. Cancer Res. 2007;67:9894–9902. doi: 10.1158/0008-5472.CAN-06-3940. [DOI] [PubMed] [Google Scholar]
- 65.Rucci N, Recchia I, Angelucci A, Alamanou M, Del Fattore A, Fortunati D. Inhibition of protein kinase c-Src reduces the incidence of breast cancer metastases and increases survival in mice: implications for therapy. J. Pharmacol. Exp. Ther. 2006;318(1):161–172. doi: 10.1124/jpet.106.102004. [DOI] [PubMed] [Google Scholar]
- 66.Coluccia AM, Cirulli T, Neri P, Mangieri D, Colanardi MC, Gnoni A. Validation of PDGFR beta and c-Src tyrosine kinases as tumor/vessel targets in patients with multiple myeloma: preclinical efficacy of the novel, orally available inhibitor dasatinib. Blood. 2008;112(4):1346–1356. doi: 10.1182/blood-2007-10-116590. [DOI] [PubMed] [Google Scholar]
- 67.Campone M, Bondarenko I, Brincat S, Hotko Y, Munster PN, Chmielowska E. Phase II study of single-agent bosutinib, a Src/Abl tyrosine kinase inhibitor, in patients with locally advanced or metastatic breast cancer pretreated with chemotherapy. Ann. Oncol. 2012;23:610–617. doi: 10.1093/annonc/mdr261. [DOI] [PubMed] [Google Scholar]
- 68.Antonarakis ES, Heath EI, Posadas EM, Yu EY, Harrison MR, Bruce JY. A phase 2 study of KX2-391, an oral inhibitor of Src kinase and tubulin polymerization, in men with bone-metastatic castration-resistant prostate cancer. Cancer Chemother. Pharmacol. 2013;71:883–892. doi: 10.1007/s00280-013-2079-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mitri Z, Nanda R, Blackwell K, Costelloe CM, Hood I, Wei C. TBCRC-010: phase I/II study of dasatinib in combination with zoledronic acid for the treatment of breast cancer bone metastasis. Clin. Cancer Res. 2016;22:5706–5712. doi: 10.1158/1078-0432.CCR-15-2845. [DOI] [PubMed] [Google Scholar]
- 70.Yu EY, Massard C, Gross ME, Carducci MA, Culine S, Hudes G. Once-daily dasatinib: expansion of phase II study evaluating safety and efficacy of dasatinib in patients with metastatic castration-resistant prostate cancer. Urology. 2011;77:1166–1171. doi: 10.1016/j.urology.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schott AF, Barlow WE, Van Poznak CH, Hayes DF, Moinpour CM, Lew DL. Phase II studies of two different schedules of dasatinib in bone-metastasis predominant metastatic breast cancer: SWOG S0622. Breast Cancer Res. Treat. 2016;159(1):87–95. doi: 10.1007/s10549-016-3911-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Felice M, Lambert D, Holen I, Escott KJ, Andrew D. Effects of Src-kinase inhibition in cancer-induced bone pain. Mol. Pain. 2016;12 doi: 10.1177/1744806916643725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hussein O, Tiedemann K, Murshed M, Komarova SV. Rapamycin inhibits osteolysis and improves survival in a model of experimental bone metastases. Cancer Lett. 2012;314:176–184. doi: 10.1016/j.canlet.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 74.Hortobagyi GN. Everolimus plus exemestane for the treatment of advanced breast cancer: a review of subanalyses from BOLERO-2. Neoplasia. 2015;17:279–288. doi: 10.1016/j.neo.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hurvitz SA, Andre F, Jiang Z, Shao Z, Mano MS, Neciosup SP. Combination of everolimus with trastuzumab plus paclitaxel as first-line treatment for patients with HER2-positive advanced breast cancer (BOLERO-1): a phase 3, randomised, double-blind, multicentre trial. Lancet Oncol. 2015;16:816–829. doi: 10.1016/S1470-2045(15)00051-0. [DOI] [PubMed] [Google Scholar]
- 76.Yardley DA, Noguchi S, Pritchard KI, Burris HA, 3rd, Baselga J, Gnant M. Everolimus plus exemestane in postmenopausal patients with HR(+) breast cancer: BOLERO-2 final progression-free survival analysis. Adv. Ther. 2013;30:870–884. doi: 10.1007/s12325-013-0060-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gnant M, Baselga J, Rugo HS, Noguchi S, Burris HA, Piccart M. Effect of everolimus on bone marker levels and progressive disease in bone in BOLERO-2. J. Natl. Cancer Inst. 2013;105:654–663. doi: 10.1093/jnci/djt026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yu Y, Song Z, Yang S, Yang X, Zhang J, Lu S. Everolimus and zoledronic acid-a potential synergistic treatment for lung adenocarcinoma bone metastasis. Acta Biochim. Biophys. Sin. 2014;46:792–801. doi: 10.1093/abbs/gmu069. [DOI] [PubMed] [Google Scholar]
- 79.Broom RJ, Hinder V, Sharples K, Proctor J, Duffey S, Pollard S. Everolimus and zoledronic acid in patients with renal cell carcinoma with bone metastases: a randomized first-line phase II trial. Clin. Genitourin. Cancer. 2015;13:50–58. doi: 10.1016/j.clgc.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 80.Vaishampayan U, Shevrin D, Stein M, Heilbrun L, Land S, Stark K. Phase II trial of carboplatin, everolimus, and prednisone in metastatic castration-resistant prostate cancer pretreated with docetaxel chemotherapy: a prostate cancer clinical trial consortium study. Urology. 2015;86:1206–1211. doi: 10.1016/j.urology.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Günther A, Baumann P, Burger R, Kellner C, Klapper W, Schmidmaier R. Activity of everolimus (RAD001) in relapsed and/or refractory multiple myeloma: a phase I study. Haematologica. 2015;100:541–547. doi: 10.3324/haematol.2014.116269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yee AJ, Hari P, Marcheselli R, Mahindra AK, Cirstea DD, Scullen TA. Outcomes in patients with relapsed or refractory multiple myeloma in a phase I study of everolimus in combination with lenalidomide. Br. J. Haematol. 2014;166:401–409. doi: 10.1111/bjh.12909. [DOI] [PubMed] [Google Scholar]
- 83.Accardi F, Toscani D, Bolzoni M, Dalla Palma B, Aversa F, Giuliani N. Mechanism of action of bortezomib and the new proteasome inhibitors on myeloma cells and the bone microenvironment: impact on myeloma-induced alterations of bone remodeling. Biomed. Res. Int. 2015 doi: 10.1155/2015/172458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Durie BGM, Hoering A, Abidi MH, Rajkumar SV, Epstein J, Kahanic SP. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet. 2017;389:519–527. doi: 10.1016/S0140-6736(16)31594-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sezer O, Beksac M, Hajek R, Sucak G, Cagirgan S, Linkesch W. Effects of single-agent bortezomib as post-transplant consolidation therapy on multiple myeloma-related bone disease: a randomized phase II study. Br. J. Haematol. 2017;178(1):61–71. doi: 10.1111/bjh.14637. [DOI] [PubMed] [Google Scholar]
- 86.Dimopoulos MA, Moreau P, Palumbo A, Joshua D, Pour L, Hájek R. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open label, multicentre study. Lancet Oncol. 2016;17:27–38. doi: 10.1016/S1470-2045(15)00464-7. [DOI] [PubMed] [Google Scholar]
- 87.Garcia-Gomez A, Quwaider D, Canavese M, Ocio EM, Tian Z, Blanco JF. Preclinical activity of the oral proteasome inhibitor MLN9708 in Myeloma bone disease. Clin. Cancer Res. 2014;20:1542–1554. doi: 10.1158/1078-0432.CCR-13-1657. [DOI] [PubMed] [Google Scholar]
- 88.Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P. Randomized phase 3 trial of abiraterone acetate in men with metastatic castration-resistant prostate cancer and no prior chemotherapy. N. Engl. J. Med. 2013;368(2):138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 91.Beer TM, Armstrong AJ, Rathkopf D, Loriot Y, Sternberg CN, Higano CS. Enzalutamide in men with chemotherapy-naïve metastatic castration-resistant prostate cancer: extended analysis of the phase 3 PREVAIL study. Eur. Urol. 2017;71(2):151–154. doi: 10.1016/j.eururo.2016.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rizzo S, Galvano A, Pantano F, Iuliani M, Vincenzi B, Passiglia F. The effects of enzalutamide and abiraterone on skeletal related events and bone radiological progression free survival in castration resistant prostate cancer patients: An indirect comparison of randomized controlled trials. Crit. Rev. Oncol./Hematol. 2017;120:227–233. doi: 10.1016/j.critrevonc.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 93.Iuliani M, Pantano F, Buttigliero C, Fioramonti M, Bertaglia V, Vincenzi B. Biological and clinical effects of abiraterone on anti-resorptive and anabolic activity in bone microenvironment. Oncotarget. 2015;6(14):12520–12528. doi: 10.18632/oncotarget.3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bellido T, Ali AA, Gubrij I, Plotkin LI, Fu Q, O'Brien CA. Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology. 2005;146(11):4577–4583. doi: 10.1210/en.2005-0239. [DOI] [PubMed] [Google Scholar]
- 95.Pennisi A, Ling W, Li X, Khan S, Wang Y, Barlogie B. Consequences of daily administered parathyroid hormone on myeloma growth, bone disease, and molecular profiling of whole myelomatous bone. PLoS One. 2010;5(12):e15233. doi: 10.1371/journal.pone.0015233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Swami S, Johnson J, Bettinson LA, Kimura T, Zhu H, Albertelli MA. Prevention of breast cancer skeletal metastases with parathyroid hormone. JCI Insight. 2017;2(17):e90874. doi: 10.1172/jci.insight.90874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kang MG, Won EJ, Choi HW, Kim HR, Choi HJ, Park HR. Serum parathyroid hormone is a new potential risk factor in multiple myeloma. Biomed. Res. Int. 2014;2014 doi: 10.1155/2014/804182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vahle JL, Long GG, Sandusky G, Westmore M, Ma YL, Sato M. Bone neoplasms in F344 rats given teriparatide [rhPTH(1–34)] are dependent on duration of treatment and dose. Toxicol. Pathol. 2004;32(4):426–438. doi: 10.1080/01926230490462138. [DOI] [PubMed] [Google Scholar]
- 99.Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat. Med. 2013;19:179–192. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- 100.Terpos E, Christoulas D, Katodritou E, Bratengeier C, Gkotzamanidou M, Michalis E. Elevated circulating sclerostin correlates with advanced disease features and abnormal bone remodeling in symptomatic myeloma: reduction post-bortezomib monotherapy. Int. J. Cancer. 2012;131:1466–1471. doi: 10.1002/ijc.27342. [DOI] [PubMed] [Google Scholar]
- 101.Taylor S, Ominsky MS, Hu R, Pacheco E, He YD, Brown DL. Time-dependent cellular and transcriptional changes in the osteoblast lineage associated with sclerostin antibody treatment in ovariectomized rats. Bone. 2016;84:148–159. doi: 10.1016/j.bone.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 102.Reagan MR, McDonald M, Terry R, Pettitt J, Le L, Mohanty S. Anti-sclerostin treatment prevents multiple myeloma induced bone loss and reduces tumor burden. Blood. 2015;126:119. [Google Scholar]
- 103.McDonald MM, Reagan MR, Youlten SE, Mohanty ST, Seckinger A, Terry RL. Inhibiting the osteocyte-specific protein sclerostin increases bone mass and fracture resistance in multiple myeloma. Blood. 2017;129(26):3452–3464. doi: 10.1182/blood-2017-03-773341. https://doi:10.1182/blood-2017-03-773341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Recker RR, Benson CT, Matsumoto T, Bolognese MA, Robins DA, Alam J. A randomized, double-blind phase 2 clinical trial of blosozumab, a sclerostin antibody, in postmenopausal women with low bone mineral density. J. Bone Miner. Res. 2015;30:216–224. doi: 10.1002/jbmr.2351. [DOI] [PubMed] [Google Scholar]
- 105.Cosman F, Crittenden DB, Adachi JD, Binkley N, Czerwinski E, Ferrari S. Romosozumab treatment in postmenopausal women with osteoporosis. N. Engl. J. Med. 2016;375:1532–1543. doi: 10.1056/NEJMoa1607948. [DOI] [PubMed] [Google Scholar]
- 106.Voorzanger-Rousselot N, Goehrig D, Journe F, Doriath V, Body JJ, Clézardin P. Increased Dickkopf-1expression in breast cancer bone metastases. Br. J. Cancer. 2007;97:964–970. doi: 10.1038/sj.bjc.6603959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Eda H, Santo L, Wein MN, Hu DZ, Cirstea DD, Nemani N. Regulation of sclerostin expression in multiple myeloma by Dkk-1: a potential therapeutic strategy for myeloma bone disease. J. Bone Miner Res. 2016;31:1225–1234. doi: 10.1002/jbmr.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ier SP, Beck JT, Stewart AK, Shah J, Kelly KR, Isaacs R. A phase IB multicentre dose determination study of BHQ880 in combination with antimyeloma therapy and zoledronic acid in patients with relapsed or refractory multiple myeloma and prior skeletal-related events. Br. J. Haematol. 2014;167:366–375. doi: 10.1111/bjh.13056. [DOI] [PubMed] [Google Scholar]
- 109.Munshi NC, Abonour R, Beck JT, Bensinger W, Facon T, Keith S.-G. Early evidence of anabolic bone activity of BHQ880, a fully human anti-DKK1 neutralizing antibody: results of a phase 2 study in previously untreated patients with smoldering multiple myeloma at risk for progression. Blood. 2012;120:331. [Google Scholar]
- 110.Florio M, Gunasekaran K, Stolina M, Li X, Liu L, Tipton B. A bispecific antibody targeting sclerostin and DKK-1 promotes bone mass accrual and fracture repair. Nat. Commun. 2016;7:11505. doi: 10.1038/ncomms11505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Terpos E, Kastritis E, Christoulas D, Gkotzamanidou M, Eleutherakis-Papaiakovou E, Kanellias N. Circulating activin-A is elevated in patients with advanced multiple myeloma and correlates with extensive bone involvement and inferior survival; no alterations post-lenalidomide and dexamethasone therapy. Ann. Oncol. 2012;23(10):2681–2686. doi: 10.1093/annonc/mds068. [DOI] [PubMed] [Google Scholar]
- 112.Leto G, Incorvaia L, Badalamenti G, Tumminello FM, Gebbia N, Flandina C. Activin A circulating levels in patients with bone metastasis from breast or prostate cancer. Clin. Exp. Metastasis. 2006;23(2):117–122. doi: 10.1007/s10585-006-9010-5. [DOI] [PubMed] [Google Scholar]
- 113.Kang HY, Huang HY, Hsieh CY, Li CF, Shyr CR, Tsai MY. Activin A enhances prostate cancer cell migration through activation of androgen receptor and is overexpressed in metastatic prostate cancer. J. Bone Miner. Res. 2009;24(7):1180–1193. doi: 10.1359/jbmr.090219. [DOI] [PubMed] [Google Scholar]
- 114.Chantry AD, Heath D, Mulivor AW, Pearsall S, Baud'huin M, Coulton L. Inhibiting activin-A signaling stimulates bone formation and prevents cancer-induced bone destruction in vivo. J. Bone Miner. Res. 2010;25(12):2633–2646. doi: 10.1002/jbmr.142. [DOI] [PubMed] [Google Scholar]
- 115.Abdulkadyrov KM, Salogub GN, Khuazheva NK, Sherman ML, Laadem A, Barger R. Sotatercept in patients with osteolytic lesions of multiple myeloma. Br. J. Haematol. 2014;165(6):814–823. doi: 10.1111/bjh.12835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Raftopoulos H, Laadem A, Hesketh PJ, Goldschmidt J, Gabrail N, Osborne C. Sotatercept (ACE-011) for the treatment of chemotherapy-induced anemia in patients with metastatic breast cancer or advanced or metastatic solid tumors treated with platinum-based chemotherapeutic regimens: results from two phase 2 studies. Support Care Cancer. 2016;24:1517–1525. doi: 10.1007/s00520-015-2929-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Scullen T, Santo L, Vallet S, Fulciniti M, Eda H, Cirstea D. Lenalidomide in combination with an activin A-neutralizing antibody: preclinical rationale for a novel anti-myeloma strategy. Leukemia. 2013;27:1715–1721. doi: 10.1038/leu.2013.50. [DOI] [PubMed] [Google Scholar]
- 118.Nelson JB, Hedican SP, George DJ, Reddi AH, Piantadosi S, Eisenberger MA. Identification of endothelin-1 in the pathophysiology of metastatic adenocarcinoma of the prostate. Nat. Med. 1995;1:944–949. doi: 10.1038/nm0995-944. [DOI] [PubMed] [Google Scholar]
- 119.Qiao L, Liang Y, Li N, Hu X, Luo D, Gu J. Endothelin-A receptor antagonists in prostate cancer treatment-a meta-analysis. Int. J. Clin. Exp. Med. 2015;8(3):3465–3473. [PMC free article] [PubMed] [Google Scholar]
- 120.Kitagawa Y, Dai J, Zhang J, Keller JM, Nor J, Yao Z. Vascular endothelial growth factor contributes to prostate cancer-mediated osteoblastic activity. Cancer Res. 2005;65:10921–10929. doi: 10.1158/0008-5472.CAN-05-1809. [DOI] [PubMed] [Google Scholar]
- 121.Nguyen HM, Ruppender N, Zhang X, Brown LG, Gross TS, Morrissey C. Cabozantinib inhibits growth of androgen-sensitive and castration-resistant prostate cancer and affects bone remodeling. PLoS One. 2013;8:e78881. doi: 10.1371/journal.pone.0078881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Smith M, De Bono J, Sternberg C, Le Moulec S, Oudard S, De Giorgi U. Phase III study of cabozantinib in previously treated metastatic castration-resistant prostate cancer: COMET-1. J. Clin. Oncol. 2016;34:3005–3013. doi: 10.1200/JCO.2015.65.5597. [DOI] [PubMed] [Google Scholar]
- 123.Choueiri TK, Escudier B, Powles T, Tannir NM, Mainwaring PN, Rini BI. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17(7):917–927. doi: 10.1016/S1470-2045(16)30107-3. [DOI] [PubMed] [Google Scholar]
- 124.Escudier B, Powles T, Motzer RJ, Olencki T, Arén Frontera O, Oudard S. Cabozantinib, a new standard of care for patients with advanced renal cell carcinoma and bone metastases? Subgroup analysis of the METEOR trial. J. Clin. Oncol. 2018;36(8):765–772. doi: 10.1200/JCO.2017.74.7352. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Turner PG, O'Sullivan JM. (223)Ra and other bone-targeting radiopharmaceuticals-the translation of radiation biology into clinical practice. Br. J. Radiol. 2015;88 doi: 10.1259/bjr.20140752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Parker CC, Coleman RE, Sartor O, Vogelzang NJ, Bottomley D, Heinrich D. Three-year safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases from phase 3 randomized alpharadin in symptomatic prostate cancer trial. Eur. Urol. 2017 doi: 10.1016/j.eururo.2017.06.021. pii: S0302-2838(17)30516-X. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 127.Paterson AH, Anderson SJ, Lembersky BC, Fehrenbacher L, Falkson CI, King KM. Oral clodronate for adjuvant treatment of operable breast cancer (National Surgical Adjuvant Breast and Bowel Project protocol B-34): a multicentre, placebo-controlled, randomised trial. Lancet Oncol. 2012;13:734–742. doi: 10.1016/S1470-2045(12)70226-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Coleman R, Powles T, Paterson A. Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet. 2015;386(10001):1353–1361. doi: 10.1016/S0140-6736(15)60908-4. [DOI] [PubMed] [Google Scholar]
- 129.Dhesy-Thind S, Fletcher GG, Blanchette PS, Clemons MJ, Dillmon MS, Frank ES. Use of adjuvant bisphosphonates and other bone-modifying agents in breast cancer: a cancer care ontario and american society of clinical oncology clinical practice guideline. J. Clin. Oncol. 2017;35(18):2062–2081. doi: 10.1200/JCO.2016.70.7257. [DOI] [PubMed] [Google Scholar]
- 130.Hadji P, Coleman RE, Wilson C, Powles TJ, Clézardin P, Aapro M. Adjuvant bisphosphonates in early breast cancer: consensus guidance for clinical practice from a European panel. Ann. Oncol. 2016;27(3):379–390. doi: 10.1093/annonc/mdv617. [DOI] [PubMed] [Google Scholar]
- 131.Gnant M, Pfeiler G, Dubsky PC, Hubalek M, Greil R, Jakesz R. The impact of adjuvant denosumab on disease-free survival: results form 3,425 postmenopausal patients of the ABCSG-18 trial. Cancer Res. 2016;76(4 Suppl) San Antonio Breast Cancer Symposium (SABCS) 2015: Abstract S2-02. Abstract nr S2-02. [Google Scholar]
- 132.Coleman RE, Finkelstein D, Barrios CH, Martin M, Iwata H, Glaspy JA. Adjuvant denosumab in early breast cancer: first results from the international multicenter randomized phase III placebo controlled D-CARE study. J. Clin. Oncol. 2018;36(15_suppl):501. [Google Scholar]
- 133.James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163–1177. doi: 10.1016/S0140-6736(15)01037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Smith MR, Saad F, Coleman R, Shore N, Fizazi K, Tombal B. Denosumab and bone metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomized, placebo-controlled trial. Lancet. 2012;379:39–46. doi: 10.1016/S0140-6736(11)61226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]