Abstract
MMP28 belongs to the matrix metalloproteinases (MMPs) family and functions in tissue homeostasis and development. Although many other MMPs have been reported to regulate tumor progression, the roles of MMP28 in cancer remain largely elusive. In this study, we investigated the potential roles of MMP28 in hepatocellular carcinoma (HCC). The upregulation of MMP28 was first determined by the analysis on different public datasets. Further quantitative real-time PCR (qPCR) analysis, western blot (WB) assay and immunohistochemistry (IHC) assay on tumor and tumor-adjacent samples from HCC patients confirmed the aberrant elevation of MMP28 in HCC. Pathological analysis showed that increased MMP28 was associated with tumor size, vascular invasion, TNM stage and overall survival in HCC patients. Meanwhile, upregulated MMP28 was identified as an independent prognosis factor in multivariate analysis, and the incorporation of MMP28 expression with TNM staging system established a novel model to improve the accuracy of the predictions. In vivo and in vitro data revealed that MMP28 promoted migration and invasion of HCC cells, and enhanced epithelial-mesenchymal transition (EMT) via elevating zinc finger E-box binding homeobox (ZEB) homologues levels. Furthermore, we determined that Notch3 signaling was critical for the functions of MMP28 in HCC. In conclusion, upregulated MMP28 in HCC promoted migration and invasion and predicted poor prognosis for HCC patients, and the effects of MMP28 depended on Notch3 signaling.
Keywords: MMP28, Notch3, hepatocellular carcinoma, prognosis
Introduction
Hepatocellular carcinoma (HCC) is a primary malignant tumor originated from hepatocytes and the most common liver cancer. HCC ranks sixth in neoplasm prevalence and third in cancer-related mortality worldwide 1. The incidence of HCC in East Asia and sub-Saharan Africa ranks first (over 80%), and the number of the patients in China account for 54% of the world 2. And the risk of developing HCC in individuals with cirrhosis increases by about 3% every year 3. Despite the development of diagnosis and treatment, the mortality of HCC patients remains very high due to the incidence of invasion and metastasis. Meanwhile, although some markers like alpha-fetoprotein (AFP) has been widely used in clinic nowadays, their diagnostic accuracy and sensitivity on early and metastatic tumors is still limited4. Hence, to cut the cost for cancer survivors and improve their life quality, it is urgent to understand the molecular basis and apply new biomarkers for the precise diagnosis.
In recent decades, the scientific attention on matrix metalloproteinases (MMPs) gains overwhelming growth because of their participation in numerous physiological and pathological processes. MMPs are different zinc-dependent endopeptidases with high heterogeneity 5. After synthesised as inactive prozymogens, MMPs are activated in the process of extracellular secretion. The activation is regulated by many proteases, such as furins, plasmin, meprin and even other MMPs 6. Activated MMPs can degrade almost all extracellular matrix (ECM) components, and modulate several signaling pathways via activating or inactivating substrates such as proteases, cytokines, cell-surface receptors 7. A slight imbalance in MMPs level or MMPs activity leads to pathological conditions for instance vascular diseases 8, autoimmunity disorders 9, and cancer 10. In cancer, many MMPs are upregulated, which regulates ECM remodeling thus to promote tumor invasion, and eventually promote tumor progression 11. Among these MMPs, MMP2, MMP9, and MMP13 are overexpressed in HCC, and have been used for HCC diagnosis 12. MMP28, also named epilysin, is the newest identified MMP 13. Like other MMPs, MMP28 is cleaved in the Golgi apparatus and released as an active hydrolase. MMP28 have been detected in some normal tissues 14, closely associated with cell proliferation during epithelial repair 15. The aberrant upregulation of MM28 has been detected in different cancers, including gastric carcinoma 16, grade I squamous cell carcinoma (SCC)15, and invasive ductal cell carcinoma17. However, the expression and functions of MMP28 in HCC remain unclear.
Notch signaling pathway is crucial for tissue development, and is transduced by the transmembrane ligands of the Jagged (Jagged1 and 2) and Delta-like (DLL1, 3, and 4) proteins expressed on the neighboring cells 18. Four Notch receptors, known as Notch1, 2, 3 and 4, have been identified, and all of them have 3 domains including an extracellular fragment, a transmembrane subunit and an intracellular domain. Ligand binding to the extracellular segment triggers cleavage events on the Notch intracellular domain (NICD), and then NICD is released into the cytoplasm 19. Afterwards, NICD translocates into the nucleus and binds to the transcription factor CBF1/RBP-Jk to form the transcriptional activation complex that ultimately activates the target genes 20. Notch receptors and their ligands have been detected in the human mature liver, and the dysregulation on their expression is closely associated with liver diseases including HCC 21. However, although perturbed Notch signaling or aberrant expression of Notch proteins have been observed in HCC, their effects on HCC progression remain controversial. For example, although Notch1 is upregulated in HCC 22, and Notch1 signaling promotes the hepatocarcinogenesis in animal models 23, Notch1 was reported to suppress HCC in some cases 24, and overexpressing active Notch1 triggers cell cycle arrest and apoptosis 25. Similar controversial effects have been observed in Notch3 26, 27 and its abnormal accumulation in HCC 28. In the present study, we determined that MMP28 functions via Notch3 signaling in HCC, and analyzed the potential mechanism how MMP28 and Notch3 regulates HCC progression.
Materials and Methods
Patients and specimens
A total of 117 pairs of tumor and adjacent normal tissue specimens were enrolled in the present study, and these patients had undergone hepatectomy from February 2010 to May 2011 or from December 2014 to August 2015, at Department of General Surgery, First Affiliated Hospital of Wenzhou Medical University (Zhejiang, China). All participants were selected if they were pathologically diagnosed with HCC. The adjacent normal tissue specimens were taken at a distance of 3 cm from the tumor tissues during surgery. Among them, 30 pairs of surgical sections were cryopreserved at -80˚C for western blot analysis, and another 87 pairs were formalin-fixed and paraffin embedded for Immunohistochemistry analysis. Each pair of specimens has its own hospital number, the clinicopathological characteristics of the patients including age, gender, tumor size, lymph node invasion, distant metastasis, and tumor stage were recorded in detail. Tumor stages were histologically classified according to the 7th Edition of the American Joint Committee on Cancer TNM classification. All methods were approved by the research medical ethics committee of Wenzhou Medical University (Zhejiang, China) and were carried out in accordance with the approved guidelines. The written consent conforming to the ethical guidelines of the Helsinki Declaration were obtained from all patients before the start of the experiment.
Animal study
Five to six-week-old male Balb/c nude mice were purchased from Shanghai Laboratory Animal Center of Chinese Academy Sciences (Shanghai, China) and were housed in a separate pathogen-free room. All of the animal experiments were approved by the research medical ethics committee of Wenzhou Medical University and carried out in accordance with the approved guidelines. All of the mice were randomly grouped (n = 6 in each group). For the lung metastasis model, Huh7-luc cells transfected with MMP28 expression vector were resuspended in ice-cold PBS (1 × 106 cells/mouse in 100 μl PBS) and were injected into mice through the tail vein. Four weeks later, all the mice were monitored for bioluminescence every week with IVIS200 (Xenogen, Caliper, CA) after intraperitoneal administration of 200 μl luciferin (at 15 mg/ml; Promega, WI, USA). The luciferase signals intensity was calculated by ROI analysis.
Western blot analysis
HCC tissues were homogenized with RIPA buffer (Beyotime, Institute of Biotechnology, Jiangsu, China). After boiled, samples were loaded in SDS-polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride membranes (PVDF; Millipore, Bedford, MA, USA), and incubated with primary antibodies. Antibodies against MMP28, Notch3 and GAPDH were used. The membranes were further incubated with IgG-HRP secondary antibodies (Santa Cruz Biotechnology) and visualized using an enhanced chemiluminescence kit (Tiangen Biotech, Beijing, China) in the image analyzer ImageQuant LAS 4000 (GE Healthcare, Abingdon, UK).
Quantitative real-time PCR
Total RNA was extracted from HCC tissues using TRIzol (Ambion and life technologies, CA, USA) according to the manufacturer's instructions. Reversed-transcriptional PCR (RT-PCR) was performed using a PrimeScript™ RT reagent Kit (TaKaRa, Dalian, China), and the resulting cDNA was analyzed by quantitative real-time PCR using SYBR® Premix Ex Taq™ (TaKaRa). GAPDH was controlled as a reference gene. The primers used were as follows:
Tissue microarray and Immunohistochemistry
Tissue microarrays (TMAs) were constructed from surgical specimens that are formalin-fixed and paraffin embedded. Chips of 1mm diameter were punched from cancer tissues and paracarcinoma tissues respectively. Immunohistochemistry analysis was applied on tissue microarray using Dako REAL EnVision Detection System (Dako, Denmark) according to the manufacture instruction. Primary antibodies against MMP28, ZEB-1, ZEB-2, E-cadherin, N-cadherin, and Notch3 were used to quantify the relative expression level. Hematoxylin was used for counterstaining. The staining intensity was scored as 0 for negative; 1 for weak; 2 for moderate weak; 3 for moderate strong and 4 for strong. The score for the stained area was set as 1 for 0%-33%; 2, 33%-66%; and 3, 66%-100%. The final staining score was obtained by multiplying staining intensity score with staining area score, and the results are a series number ranging from 0 to 12.
Cell lines and reagents
All the human HCC cell lines including BEL-7402 and Huh7 were obtained from Cell Bank of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). Cells are cultured in Dulbecco's modified Eagle medium (Sigma-Aldrich, St Louis, MO, USA) replenished with 10% fetal bovine serum (Gibco, Grand Island, NY, USA) at 37 °C in a humidified incubator containing 5% CO2. Antibodies against MMP28, Notch3, Flag-tag and GAPDH were purchased from Proteintech (Chicago, IN, USA). Antibodies against ZEB-1, ZEB-2, Snail, E-cadherin, N-cadherin, Vimentin, β-catenin, Notch4, Akt, phospho-Akt (Ser473/Thr308), PKG-I, VASP and phospho-VASP (Ser239) were purchased from Cell Signaling Technology (Beverly, MA, USA).
Plasmid construction, siRNA and transfection
The cDNA encoding human MMP28 was obtained by PCR and cloned into the pCMV-Flag vector (Sigma, St. Louis, MO, USA) to generate pCMV-Flag-MMP28 construct. The siRNA targeting MMP28 was purchased from Proteintech (Chicago, IN, USA). Transient transfections were performed with Lipofectamine 3000 (Invitrogen) according to the manufacturer's instructions.
CRISPR/Cas9 system construction
The sgRNAs were designed on E-CRISPR to disrupt the target DNA region of Notch3, and the reverse sequences were completed. The sequence for sgRNA was GGGTGAGCGGTGTCAGCTGG. The oligos was flanked on the 3' end referred to the target site sequence (20bp). After annealing, DNA oligos was inserted into PX459 plasmid vector according to the manufacture instruction. Stable cell lines were generated with puromycin (1μg/ml) in the medium.
Transwell assay
Transwell migration and invasion assays were carried out with 12-well transwell plates containing 8-μm pore filters (Corning, New York, NY, USA). For invasion assays, the bottom of transwell chamber was coated with BD Matrigel Basement Membrane Matrix (BD Biosciences, San Diego, CA, USA). 1× 105 cells were seeded into the upper chamber in 500 μl serum-free basic medium, and the lower chamber was filled with 1500 μl complete culture medium. The migration and invasion systems were incubated for 24 h and 48 h at 37 °C respectively. Cells on the upper side of the chambers were removed by scrubbing, and cells on the lower surface of the membrane were fixed with 4% paraformaldehyde for 15 minutes. Then it was stained with 0.1% crystal violet (Beyotime Institute of Biotechnology, Jiangsu, China) for 30 minutes. The infiltrating cells were viewed and counted in five randomly selected fields under a microscope.
GEO and TCGA datasets
Data involved in this study are available from the Cancer Genome Atlas (TCGA) and the GEO datasets (accession number: GSE36376, GSE25097, GSE39791). The number of tumor specimens in each sub-database is equal to the number of paracancerous specimens. For TCGA dataset, mRNA levels were measured by RNA sequencing V2 RSEM. For GEO dataset, mRNA levels were achieved from Oncomine database.
Statistical analysis
IBM SPSS Statistics 22.0 (SPSS Inc, Chicago, IL), Prism 6.01 software (GraphPad, La Jolla, CA) and R software 3.4.1 with the “rms” package (R Foundation for Statistical Computing, Vienna, Austria) were used for all data analyses. ROC curves were conducted to determine the cut-off value of the Immunohistochemistry staining score to divide the specimens into two groups and compare the prognostic sensitive and specificity of combination model. To evaluate the correlations the between MMP28 expression and clinicopathologic variables, independent sample t-test was used to analyze continuous variables, and chi-squared test was applied for categorical variables. Kaplan-Meier method was used to establish survival curves and the significance was calculated by Log-rank test The Cox proportional hazard regression analysis was employed to perform multivariate analyses. Nomogram was displayed by R software with the “rms” package and the C-index and AIC were calculated to measure the prognostic accuracy. The gray value of the western blot band was quantified by ImageJ 1.8.0 (National Institutes of Health, USA). Differences between groups were determined with Student's t test. Statistical significance was set at two-tails P < 0.05.
Results
MMP28 is overexpressed in Hepatocellular Carcinoma
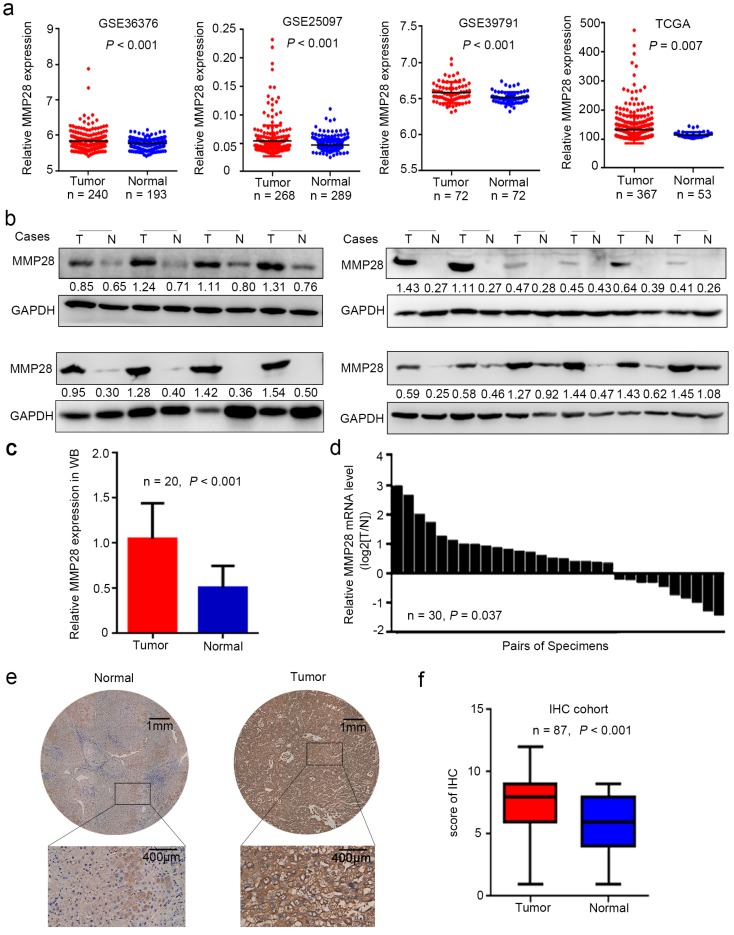
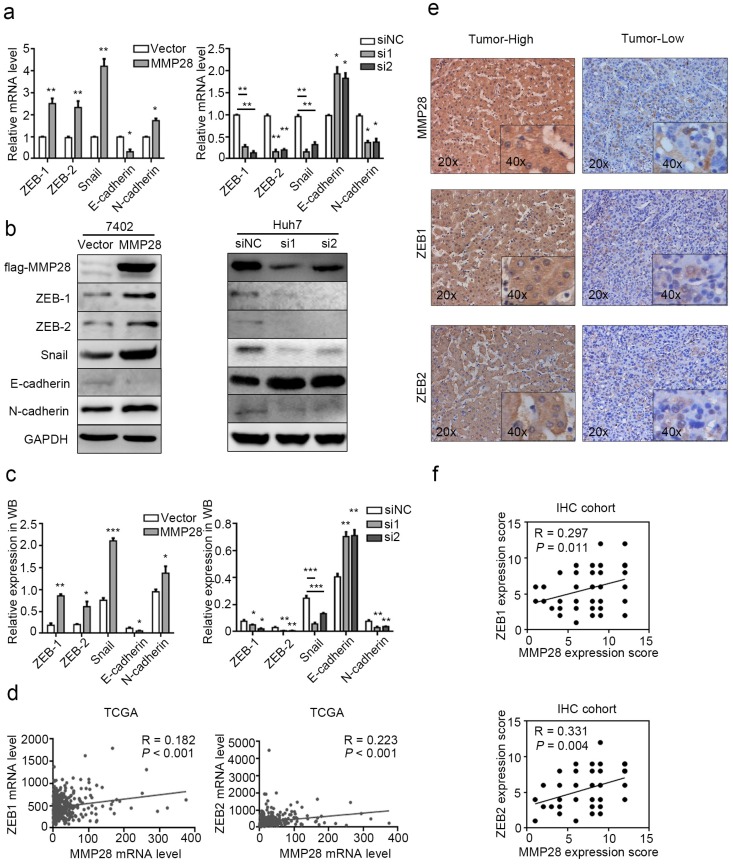
To determine whether MMP28 is involved in HCC progression, we first examined its mRNA levels in different public datasets from Gene Expression Omnibus (GEO) and The Cancer Genome Atlas (TCGA) database. Data revealed that MMP28 levels were significantly increased in tumor tissues in GSE36376 29 (P < 0.001), GSE25097 30 (P < 0.001), GSE39791 31 (P < 0.001), and TCGA 32 datasets (P = 0.007) (Fig. 1a). To verify the upregulation of MMP28 in HCC, we next examined MMP28 levels in 30 paired HCC tissues and adjacent normal tissues. Both the western blot and quantitative real-time PCR (qPCR) analysis revealed that 66.7% (20/30) of primary tumor tissues expressed more MMP28 compared with matched paracancerous tissues, and statistical analysis verified that MMP28 was significantly upregulated in both mRNA and protein levels (P < 0.001 in western blot and P = 0.037 in qPCR analysis) (Fig. 1b-d). We further applied immunohistochemistry (IHC) assay on a tissue microarray including other 87 pairs of HCC samples, and confirmed the significant upregulation of MMP28 in HCC tumor tissues (P < 0.001) (Fig. 1e, f). Our IHC results also revealed that MMP28 was mainly expressed in cytoplasm and extracellular matrix (Fig. 1e).
Figure 1.
MMP28 was upregulated in hepatocellular carcinoma. (a) Relative expression of MMP28 mRNA in HCC tissues and normal paracancerous tissues in GSE36376, GSE25097, GSE39791, TCGA datasets. (b-d) The expression of MMP28 was detected by western blot (b, c) and real-time PCR (d). (e) Representative IHC images of MMP28 protein staining in tissue sections. Regional magnification images were shown below. (f) The score of MMP28 expression in 87 paired HCC tissue sections determined by IHC assay.
Correlations between MMP28 expression and clinicopathologic characteristics of HCC patients
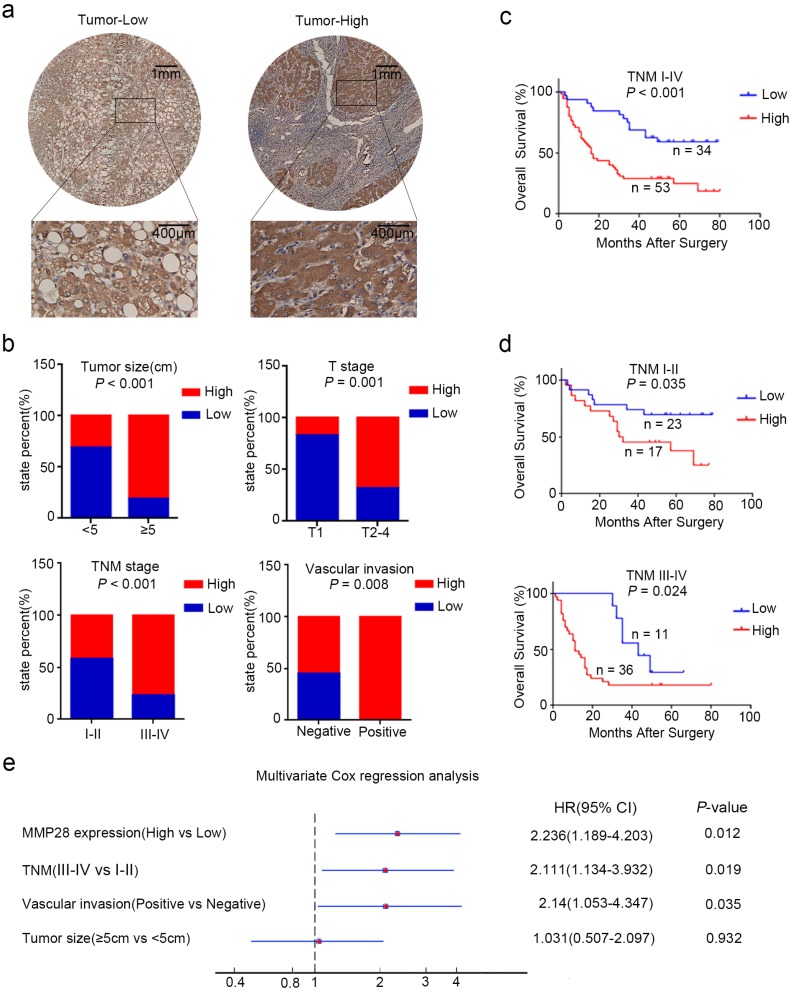
To explore the clinicopathologic significance of MMP28 in HCC, we further analyzed the IHC data. The receiver operating characteristic (ROC) curve was established and the patients were eventually divided into two groups according to the cut-off value of IHC score 6. Among 87 cancer specimens, 53 (60.9%) conferred high expression of MMP28. The representative IHC staining was showed (Fig. 2a). The correlations between MMP28 expression and clinicopathologic characteristics were analyzed by chi-square test (Table. 1). And the results showed that increased MMP28 in HCC was positively correlated with tumor size (P < 0.001), tumor (T) stage (P = 0.001), tumor node metastasis (TNM) stage (P < 0.001), vascular invasion (P = 0.008) (Fig. 2b). These data indicated that upregulated MMP28 had a diagnostic significance for patients with HCC at advanced stage.
Figure 2.
MMP28 was correlated with the poor prognosis of HCC patient in IHC cohort. (a) Low and high expression of MMP28 protein in HCC tissue sections determined by IHC. Representative images were shown. (b) The correlations between MMP28 expression and the clinicopathological variables in IHC cohort. (c, d) Kaplan-Meier survival curves for the overall survival of the delaminated HCC patients from IHC cohort. (e) Multivariate Cox regression analysis showing the independent prognostic factors for overall survival.
Table 1.
Correlations between MMP28 expression and clinicopathological variables of HCC patients
| Variables | Number | MMP28 expression | P-value | |
|---|---|---|---|---|
| Low (%) | High (%) | |||
| Gender | 0.054 | |||
| Male | 53 | 25 (28.7) | 28 (32.2) | |
| Female | 34 | 9 (10.3) | 25 (28.7) | |
| Age (years) | 0.426 | |||
| <60 | 63 | 23 (26.4) | 40 (46.0) | |
| ≥60 | 24 | 11 (12.6) | 13 (14.9) | |
| Tumor size (cm) | <0.001 | |||
| <5 | 35 | 24 (27.6) | 11 (12.6) | |
| ≥5 | 52 | 10 (11.5) | 42 (48.3) | |
| Tumor number | 0.915 | |||
| Single | 48 | 19 (21.8) | 29 (33.3) | |
| Multiple | 39 | 15 (17.2) | 24 (27.6) | |
| Pathological stage | 0.12 | |||
| I-II | 58 | 26 (29.9) | 32 (36.8) | |
| III-IV | 29 | 8 (9.2) | 21 (24.1) | |
| T stage | 0.001 | |||
| T1 | 12 | 10 (11.5) | 2 (2.3) | |
| T2-T4 | 75 | 24 (27.6) | 51 (58.6) | |
| TNM stage | <0.001 | |||
| I-II | 40 | 23 (26.4) | 17 (19.5) | |
| III-IV | 47 | 11 (12.6) | 36 (41.4) | |
| Vascular invasion | 0.008 | |||
| Negative | 75 | 34(39.1) | 41(47.1) | |
| Positive | 12 | 0(0) | 12(13.8) | |
Abbreviations: P < 0.05 is considered to have statistical significance.
We next used Kaplan-Meier analysis to evaluate the relationship between MMP28 levels and the overall survival (OS) of HCC patients. The results indicated that overexpressed MMP28 was significantly associated with poorer overall survival (P < 0.001) (Fig. 2c). To further explore whether MMP28 expression could be a stratifying factor in HCC patients within different TNM stages, we dichotomized these subjects into two groups: early stage (TNM I-II) and advanced stage (TNM III-IV). The survivorship curves showed significant value to predict the prognosis of HCC patient in both early and advanced stages (Fig. 2d) (P = 0.035 in early stage and P = 0.024 in advanced stage).
MMP28 can be used as an independent prognosis factor in patients with HCC
We also used univariate analysis to evaluate the prognostic factors for overall survival in HCC patients. TNM stage (HR, 2.871; 95% CI, 1.648-5.003; P < 0.001), MMP28 expression (HR, 2.778; 95% CI, 1.505-5.129; P < 0.001), tumor size (HR, 1.992; 95% CI, 1.133-3.503; P = 0.017), and vascular invasion (HR, 3.577; 95% CI, 1.866-6.855; P < 0.001) were identified as significant risk factors that could affect the survival in patients with HCC (Table. 2). Further multivariate Cox analysis revealed some independent predict factors for overall survival of HCC patients including MMP28 (HR, 2.236; 95% CI, 1.189-4.203; P = 0.012), TNM stage (HR, 2.111; 95% CI, 1.134-3.932; P = 0.019) and vascular invasion (HR, 2.14; 95% CI, 1.053-4.347; P = 0.035) (Fig. 2e). These results indicated that MMP28 could be used as an independent prognostic variable in HCC patients.
Table 2.
Univariate Cox regression analysis of clinicopathological characteristics influencing the overall survival of HCC patients
| Variables | Univariate | ||
|---|---|---|---|
| HR | 95% CI | P-value | |
| Gender | |||
| Male vs Female | 1.453 | 0.633-3.067 | 0.416 |
| Age (years) | |||
| ≥60 vs <60 | 0.621 | 0.37-1.113 | 0.133 |
| Tumor size (cm) | |||
| ≥5 vs <5 | 1.992 | 1.133-3.503 | 0.017 |
| Vascular invasion | |||
| Positive vs Negative | 3.577 | 1.866-6.855 | <0.001 |
| Pathological stage | |||
| III-IV vs I-II | 1.257 | 0.739-2.204 | 0.388 |
| T stage | |||
| T2-4 vs T1 | 2.247 | 0.894-5.649 | 0.085 |
| TNM stage | |||
| III-IV vs I-II | 2.871 | 1.648-5.003 | <0.001 |
| MMP28 expression | |||
| High vs Low | 2.778 | 1.505-5.129 | <0.001 |
Abbreviations: HR= hazard ratio; 95% CI= 95% confidence interval; P < 0.05 is considered to have statistical significance.
Predictive nomogram for survival of patients with HCC
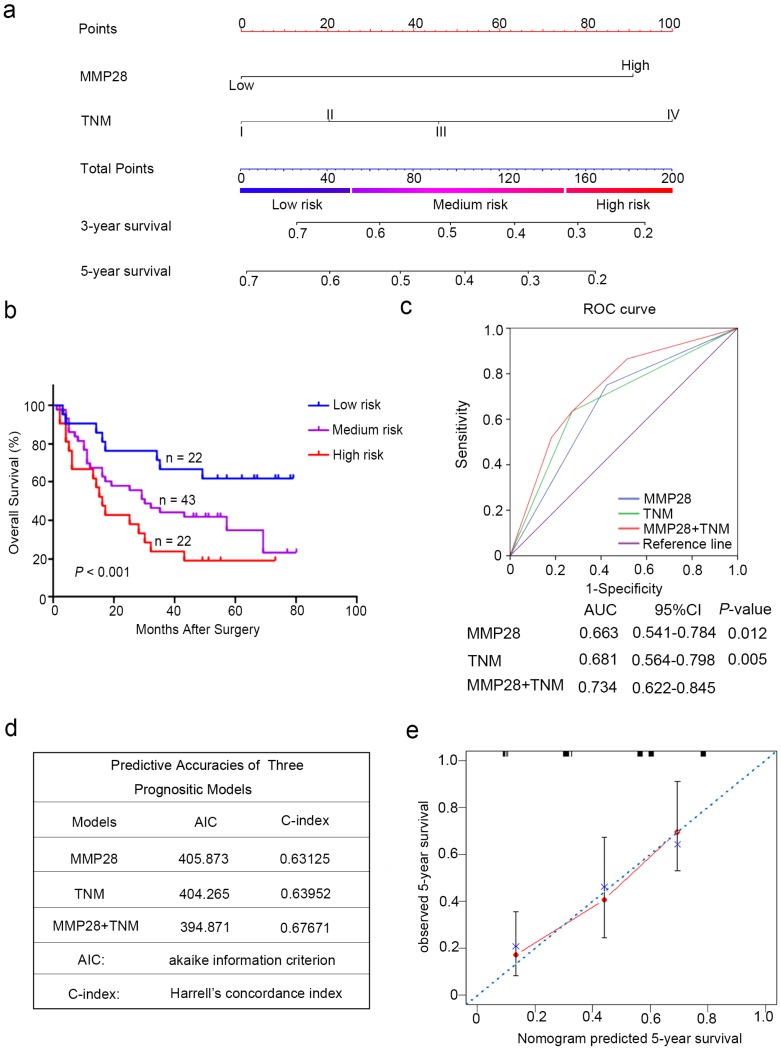
According to all these independent prognostic factors selected above, we constructed prognostic nomogram for predicting 5-year survival rate in patients with HCC. Each independent prognostic factor had a risk score and the total point was raised by adding the score of MMP28 expression (0 for “Low”, 91 for “High”), the TNM stage (0 for “I”, 20 for “II”, 46 for “III”, 100 for “IV”). Higher total point reflected a lower survival rate (Fig. 3a). Then the patients were divided into three subgroup based on the total risk score: subgroups I, low risk score (< 25%); subgroup II, medium risk score (25%-75%); and subgroup III, high risk score (> 75%). Kaplan-Meier curves showed that scoring with MMP28-based nomogram remarkably distinguished the risk of postoperative survival of HCC patients (P < 0.001) (Fig. 3b).
Figure 3.
Combination of MMP28 expression with TNM stage established a better predictive model to predict the survival rate of HCC patients. (a) Nomogram integrated MMP28 expression and TNM stage. (b) Kaplan-Meier survival analysis of overall survival according to the risk score calculated by the nomogram. (c) ROC curve analysis of the specificity and sensitivity of the predictive value of the MMP28 model, TNM stage model and the combined model. (d) AIC and Harrell's C-index analysis of the predictive accuracies of the three models. (e) The calibration plots for predicted nomogram and actual 5-year overall survival. The dash line was regarded as an ideal model.
TNM staging prognostic model for HCC patients is improved by the combination of MMP28 expression
To establish a more reasonable model to predict the outcomes of HCC patients, TNM staging system was combined with MMP28 expression. ROC curve analysis was employed to show the prognostic sensitive and specificity of MMP28 expression, TNM staging system and the combined system. Our results indicated that the combined system revealed a significantly better prognostic value (AUC [95% CI], 0.734 [0.622-0.845]) than MMP28 expression alone (AUC [95% CI], 0.663 [0.541-0.784]) or TNM staging system alone (AUC [95% CI], 0.681 [0.564-0.798]) (Fig. 3c). In addition, the Akaike information criterion (AIC) for the combination of TNM stage and MMP28 was 394.87, lower than that for TNM stage (404.27), whereas the Harrell's concordance index (C-index) of combination model was increased to 0.677 compared with that of TNM stage (0.640) alone (Fig. 3d). For internal validation, the calibration curves revealed that the nomogram predicting 5-year overall survival rate performed well with the ideal prediction model (Fig. 3e). These date demonstrated that the novel system could generate a more accurate predictive model for the prognosis of HCC patients.
MMP28 promotes EMT, migration and invasion of HCC cells
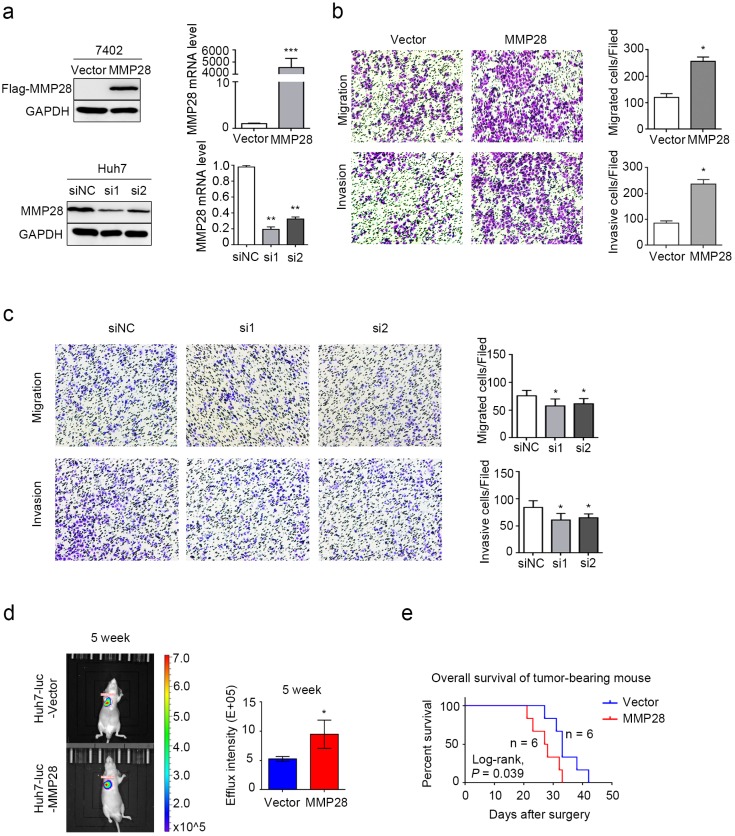
We next evaluated effect of MMP28 on abilities of both migration and invasion of HCC cells in vitro. Our data revealed that MMP28 was differently expressed in different HCC cell lines (Supplementary Fig S1). BEL-7402 cells with low basal expression were transfected with MMP28 overexpression plasmids, and Huh7 cells with high basal expression were transfected with MMP28 small-interfering RNA (siRNA). The overexpressing and interfering efficiency was determined by WB and qPCR (Fig. 4a). The transwell migration and invasion assays were employed to confirm the aggressive ability of the HCC cells, and the results revealed that both the migrated and invasive cells were significantly increased in MMP28- overexpressing group, and significantly decreased in MMP28-knock-down group, compared with control ones (Fig. 4b, c). Consistent with the results, we found that mice with high level MMP28 expression appeared more frequent metastasis (Fig. 4d), and MMP28 upregulation significantly decreased the survival of tumor-bearing mice (P = 0.039) (Fig. 4e). These data indicated that MMP28 could promote migration and invasion in vivo and in vitro.
Figure 4.
MMP28 promoted migratory and invasive abilities of HCC cells in vivo and in vitro. (a) The overexpression and knockdown efficiencies of MMP28 in cells were detected by western blot and qPCR. (b) Transwell assays were performed to determine the influence of MMP28 overexpressing on the migratory and invasive abilities in 7402 cells. (c) Transwell assays were performed to determine the influence of MMP28 silencing on the migratory and invasive abilities in Huh7 cells. (d) In vivo effect of MMP28 in lung metastasis model 5 weeks after Huh7-luc cells injected. Representative images of metastasis models were presented on the left, and statistical data was presented on the right. (e) Overall survival of grouped mice. *P < 0.05; **P < 0.01; ***P < 0.001. Data were represented as Means ± SD.
Since EMT is considered to be the first step in metastasis, the involvement of molecules in this process varies among different cancer types33. Thus we determined the effect of MMP28 on the EMT-related hallmarks. Compared with the control, we found the EMT-inducing transcription factors ZEB-1, ZEB-2, Snail and mesenchymal marker N-cadherin were significantly upregulated in MMP28-overexpressing cells and downregulated in MMP28-knock-down cells respectively, while the epithelial marker E-cadherin was repressed in MMP28-overexpressing group and elevated in MMP28-knock-down group respectively (Fig. 5a). WB analysis showed the corresponding effects on the protein level of these factors (Fig. 5b, c). However, in parallel IHC analysis, it suggested the expression level of MMP28 was positively correlated only with ZEB-1 and ZEB-2 (R = 0.297, P = 0.011 for ZEB-1 and R = 0.331, P = 0.004 for ZEB-2) (Fig. 5e, f), which was similar to the data from TCGA dataset (R = 0.182, P < 0.001 for ZEB-1 and R = 0.223, P < 0.001 for ZEB-2) (Fig. 5d).
Figure 5.
MMP28 promoted EMT of HCC cells. (a-c) The effect of MMP28 overexpression and knockdown on the expression of EMT-related factors in 7402 and Huh7 cells were determined by real-time PCR (a) and western blot (b, c). (d) The correlations of MMP28 with ZEB-1 and ZEB-2 in TCGA dataset. (e) IHC assays of MMP28, ZEB-1 and ZEB-2. Representative IHC images were shown. (f) The correlations of MMP28 with ZEB-1 and ZEB-2 in IHC cohort. *P < 0.05; **P < 0.01; ***P < 0.001. Data were represented as Means ± SD.
MMP28 promotes EMT via activating Notch3 signaling
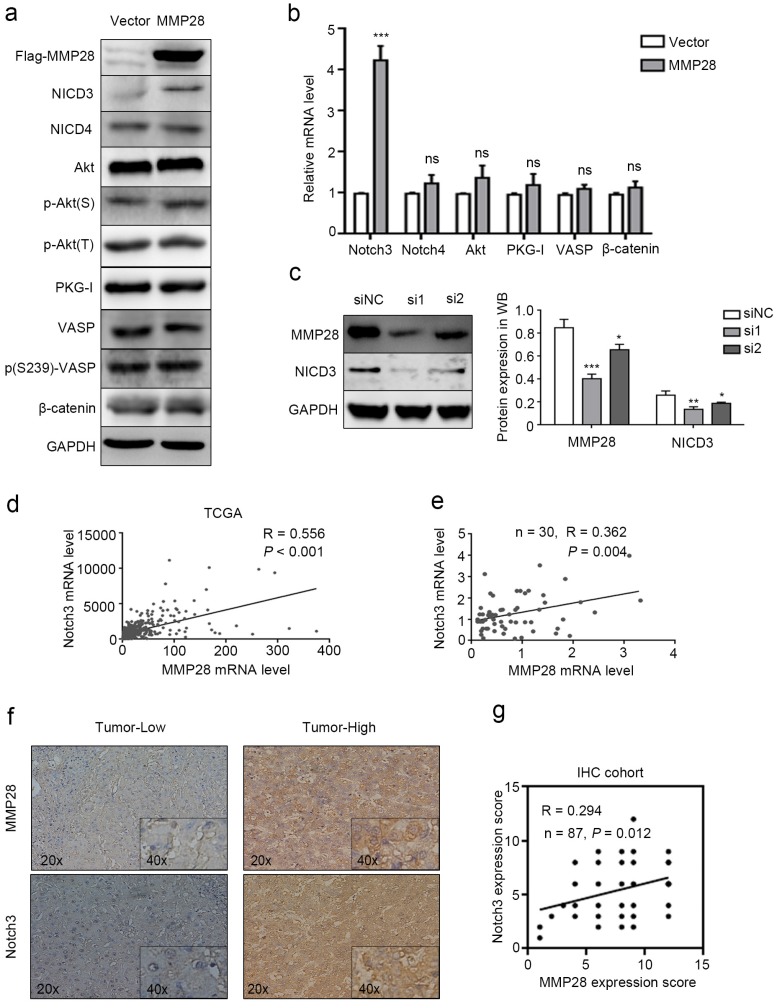
To further investigate how MMP28 upregulates the aggressive abilities of HCC cells, the genes strongly correlated with MMP28 from TCGA database were all taken into account (Pearson |R| ≥ 0.3 and Spearman |R| ≥ 0.3). We sorted out 837 genes that strongly correlated with MMP28 (Supplementary Fig S2). A considerable amount of the genes were significantly correlated with environmental information processing in functional enrichments of the KEGG pathway (Supplementary Fig S3 and Supplementary Table S1). We selected several feasible signaling pathways that are relatively significant to search the molecules involved, including Notch signaling, AKT signaling, and PKG signaling, and performed western blot analysis to examine the expression of relevant molecules. Phosphorylated- Ser239- VASP was used to reflect the activity of PKG-I34. Our results showed that among these signals, NICD3 was elevated in MMP28-overexpressing cells (Fig. 6a, b). And in MMP28-knockdowned cells, NICD3 was also significantly downregulated (Fig. 6c). Analysis on TCGA dataset also revealed the positive correlation between MMP28 and Notch3 (R = 0.556, P < 0.001) (Fig. 6d), which was confirmed in qPCR analysis (R = 0.362, P =0.004) and IHC analysis (R = 0.294, P = 0.012) on our HCC samples (Fig. 6e-g). And the positive staining area of NICD3 in cytoplasm was increased in MMP28-high tumor tissues (Fig. 6f). In conclusion, MMP28 is associated with Notch3 activation.
Figure 6.
MMP28 promoted EMT, migration and invasion of HCC cells via activating Notch3 signals. (a) Levels of NICD3, NICD4, Akt, p-Akt(S), p-Akt(T), PKG-I, VASP, p(S239)-VASP and β-catenin in MMP28-overexpressing 7402 cells were determined by western blot. (b) The mRNA levels of Notch3, Notch4, Akt, PKG1, VASP and β-catenin in MMP28-overexpressing 7402 cells was determined by real-time PCR. (c) The expression of MMP28 and NICD3 in MMP28-knockdowned Huh7 cells were determined by western blot. (d) Expression correlation between MMP28 and Notch3 from TCGA dataset. (e) Correlation of MMP28 mRNA levels with Notch3 in HCC tissues was determined by real-time PCR. (f-g) Correlation of MMP28 mRNA levels with Notch3 in IHC cohort was determined by IHC analysis. Representative images of MMP28 and Notch3 in HCC tissues were shown. *P < 0.05; **P < 0.01; ***P < 0.001. Data are represented as Means ± SD.
The effects of MMP28 on HCC progression depend on Notch3 activation
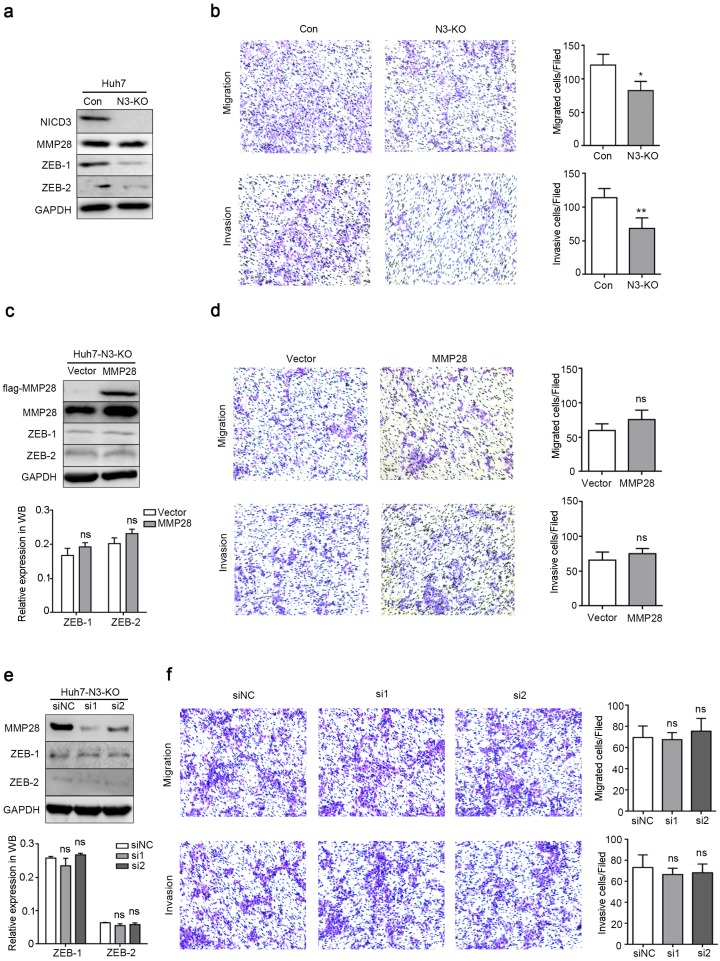
To further examine whether Notch3 signaling is involved in the functions of MMP28, we generated a stable Huh7 cell line with Notch3 knockout (N3-KO) using CRISPR/Cas9 system. The knockout efficacy was determined by western blot (Fig. 7a). In N3-KO cells, the expression of ZEB-1 and ZEB-2 was downregulated (Fig. 7a). And transwell assays showed that N3-KO cells exhibited less migratory and invasive potentials than parental cells (Fig. 7b). Moreover, neither overexpression nor knockdown of MMP28 in N3-KO cells conferred significant effects on migration or invasion of N3-KO cells (Fig. 7d, f). ZEBs levels were also not changed at the same time (Fig. 7c, e). Collectively, these data indicated that Notch3 is required for the promotion of MMP28 on EMT, migration and invasion of HCC cells.
Figure 7.
Notch3 knockout dispelled the metastasis-promoting ability of MMP28. (a) Levels of NICD3, MMP28, ZEB-1 and ZEB-2 in Notch3-knockout Huh7 stable cells (N3-KO) were determined by western blot. (b) Transwell assays were performed to determine the influence of Notch3 knockout on the migratory and invasive abilities of Huh7 stable cells. (c) The expression of ZEB-1 and ZEB-2 in N3-KO cells transfected with MMP28 was determined by western blot. (d) Transwell assays were performed to determine the influence of MMP28 overexpressing on the migratory and invasive abilities in N3-KO cells. (e) The expression of ZEB-1 and ZEB-2 in N3-KO cells transfected with MMP28 siRNA was determined by western blot. (f) Transwell assays were performed to determine the influence of MMP28 silencing on the migratory and invasive abilities in N3-KO cells. *P < 0.05; **P < 0.01. Data are represented as Means ± SD.
Discussion
In this study, our data revealed that MMP28 is upregulated in HCC tumor tissues, which is associated with elevated vascular invasion, higher TNM stage and worse overall survival. Upregulated MMP28 was also identified as an independent prognosis factor to predict the poor outcomes of patients with HCC. Furthermore, since TNM staging system does not take the heterogeneity of HCC into consideration and thus is not sufficiently accuracy, we developed a novel model combining MMP28 expression and TNM staging system to improve the accuracy of the predictions. All these results indicate that MMP28 could be used as a potential biomarker.
Pathologically, the expression and the activation of MMPs are abnormal in human cancers, which are involved in several critical processes including tumor proliferation, differentiation, migration, invasion, angiogenesis, and apoptosis 35. MMPs are often aberrantly upregulated in cancers, which leads to unsatisfied results. For example, MMP13 secreted from fibroblasts promotes tumor angiogenesis 36, and MMP2 and MMP9 degrades ECM components to promote metastasis 37. MMP28 is also benefit for tumor progression. MMP28 is initially is found in the basal keratinocytes and fetal tissues, and functions in tissue homeostasis and development 38. Further evidence suggests that MMP28 expression is induced by tumor necrosis factor α (TNF-α), so as to accelerate wound healing 15. Similar with its epithelial repair ability in normal tissues, MMP28 promotes proliferation of tumor cells in oral squamous cell carcinomas (OSCCs) and esophageal carcinomas 39. And a recent research demonstrated that high levels of MMP28 predict unsatisfactory prognosis in gastric carcinoma, and are correlate with the gastric tumor invasion and metastasis 40. Here, our data proved that MMP28 is also upregulated in HCC, and promote metastasis.
In epithelial cells, upregulated MMP28 induces EMT via activating TGF-β and decreasing a major mediator of cell-cell adhesion, E-cadherin 41. The transient activity of MMP28 in A549 lung adenocarcinoma cells causes TGF-β-dependent EMT as well, which leads to the atypical hyperplasia and invasive tendency of cancer cells 42. Meanwhile, one of the substrates of MMP28, casein, has been identified as a novel inhibitor of tumor progression 43, 44. Additionally, several TIMPs that inhibit the activity of MMP28 suppress the progression of breast carcinoma 45, and colorectal cancer 46, and have been used in treating cancers 47. Considering the clinicopathologic roles of MMP28 in HCC, targeting MMP28 might also be a promising strategy in clinical practice for HCC patients.
EMT is a histopathological process that occurs in abnormal cells, which destroys the links between tumor cells and the ECM, and enhances the ability of aggressiveness for tumor cells. Notch signaling pathway, that regulates local cell-cell communication and cell differentiation 48, is related to the EMT process 49. Notch3 promoted EMT and tumor initiation in human squamous cell carcinoma 50, and the mutations on Notch3 disrupted the expansion of EMT-related cells in non-small cell lung cancer 51. Notch3 also induces the formation of metastasis lesions in the pancreatic ductal adenocarcinoma cells via MMP2 and MMP9 52. Although the overexpression of Notch3 has been reported in HCC 27, little is known about the functions of Notch3 in HCC. Here, we proved that activated Notch3 could regulate the expression of ZEB homologues in HCC tissues, thus to promote EMT. And Notch3 was involved in MMP28-triggered tumor cell infiltration. However, although in vitro results showed that the upregulation of MMP28 led to the expression changes of Snail, E-cadherin and N-cadherin, we failed to find similar results in IHC data of HCC patients. These data suggested that despite the direct promotion of MMP28 and Notch3 on migration and invasion of HCC tumor cells, the effects of MMP28 and Notch3 on tumors might be changed by some other factors in microenvironment, which requires further investigation.
In conclusion, this study explored the expression pattern of MMP28 in HCC, and identified the upregulation of MMP28 as an independent prognostic factor in HCC patients. Meanwhile, a more sensitive predict model for HCC patients combining MMP28 expression with TNM system was established. Moreover, our study revealed the effects of MMP28 on metastasis depend on Notch3 signals. Future studies are required to investigate the precise molecular mechanisms underlying the association between activated Notch3 and MMP28.
Supplementary Material
Supplementary figures and table.
Table A.
Primer Sequences
| Gene | Primer Sequence |
|---|---|
| MMP28 | forward, TCCCACCTCCACTCGATTCAG |
| reverse, GCCGCATAACTGTTGGTATCT | |
| ZEB1 | forward, GATGATGAATGCGAGTCAGATGC |
| reverse, ACAGCAGTGTCTTGTTGTTGT | |
| ZEB2 | forward, CAAGAGGCGCAAACAAGCC |
| reverse, GGTTGGCAATACCGTCATCC | |
| Snail | forward, TCGGAAGCCTAACTACAGCGA |
| reverse, AGATGAGCATTGGCAGCGAG | |
| E-cadherin | forward, CGAGAGCTACACGTTCACGG |
| reverse, GGGTGTCGAGGGAAAAATAGG | |
| N-cadherin | forward, TCAGGCGTCTGTAGAGGCTT |
| reverse, ATGCACATCCTTCGATAAGACTG | |
| Vimentin | forward, GACGCCATCAACACCGAGTT |
| reverse, CTTTGTCGTTGGTTAGCTGGT | |
| β-catenin | forward, AAAGCGGCTGTTAGTCACTGG |
| reverse, CGAGTCATTGCATACTGTCCAT | |
| Notch3 | forward, TGGCGACCTCACTTACGACT |
| reverse, CACTGGCAGTTATAGGTGTTGAC | |
| Notch4 | forward, TGTGAACGTGATGTCAACGAG |
| reverse, ACAGTCTGGGCCTATGAAACC | |
| Akt | forward, AGCGACGTGGCTATTGTGAAG |
| reverse, GCCATCATTCTTGAGGAGGAAGT | |
| PKG-I | forward, CTTGGAGCTGTCGCAGATCC |
| reverse, TCTTTGATGATGCAACTGTCCTT | |
| VASP | forward, ATGGCAACAAGCGATGGCT |
| reverse, CGATGGCACAGTTGATGACCA | |
| GAPDH | forward, GAGTCAACGGATTTGGTCGT |
| reverse, TTGATTTTGGAGGGATCTCG |
Acknowledgments
This work was supported by grants from the National Natural Science Foundation For Young Scientists of China (31300676), Foundation of Science Technology Department of Zhejiang Province (2013C33098), Foundation of Wenzhou Science &Technology Bureau (Y20140110).
References
- 1.Forner A, Bruix J. Hepatocellular carcinoma - Authors' reply. Lancet. 2012;380:470–1. [Google Scholar]
- 2.Gong Y, Wu X, Wang T, Zhao J, Liu X, Yao Z. et al. Targeting PEPT1: a novel strategy to improve the antitumor efficacy of Doxorubicin in human Hepatocellular carcinoma therapy. Oncotarget. 2017;8:40454–68. doi: 10.18632/oncotarget.17117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor EJ, Jones RL, Guthrie JA, Rowe IA. Modelling the benefits and harms of surveillance for hepatocellular carcinoma: information to support informed choices. Hepatology. 2017;66:1546. doi: 10.1002/hep.29315. [DOI] [PubMed] [Google Scholar]
- 4.Malaguarnera G, Giordano M, Paladina I, Berretta M, Cappellani A, Malaguarnera M. Serum markers of hepatocellular carcinoma. Digestive diseases and sciences. 2010;55:2744–55. doi: 10.1007/s10620-010-1184-7. [DOI] [PubMed] [Google Scholar]
- 5.Zhang X, Huang S, Guo J, Zhou L, You L, Zhang T, Insights into the distinct roles of MMP-11 in tumor biology and future therapeutics (Review) International Journal of Oncology; 2016. p. 48. [DOI] [PubMed] [Google Scholar]
- 6.Boon L, Ugarteberzal E, Vandooren J, Opdenakker G. Glycosylation of matrix metalloproteases and tissue inhibitors: present state, challenges and opportunities. Biochemical Journal. 2016;473:1471–82. doi: 10.1042/BJ20151154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pardo A, Selman M. Role of matrix metaloproteases in idiopathic pulmonary fibrosis. Fibrogenesis Tissue Repair. 2012;5:1–5. doi: 10.1186/1755-1536-5-S1-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu J, Steen PEVD, Sang QXA, Opdenakker G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nature Reviews Drug Discovery. 2007;6:480. doi: 10.1038/nrd2308. [DOI] [PubMed] [Google Scholar]
- 9.Milner JM, Cawston TE. Matrix metalloproteinase knockout studies and the potential use of matrix metalloproteinase inhibitors in the rheumatic diseases. Current Drug Targets-Inflammation & Allergy. 2005;4:363–75. doi: 10.2174/1568010054022141. [DOI] [PubMed] [Google Scholar]
- 10.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wieczorek E, Jablonska E, Wasowicz W, Reszka E. Matrix metalloproteinases and genetic mouse models in cancer research: a mini-review. Tumour Biology the Journal of the International Society for Oncodevelopmental Biology & Medicine. 2015;36:163–75. doi: 10.1007/s13277-014-2747-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu J, Hao ZW, Zhao YX, Yang XM, Tang H, Zhang X. et al. Full-length soluble CD147 promotes MMP-2 expression and is a potential serological marker in detection of hepatocellular carcinoma. Journal of Translational Medicine. 2014;12:190. doi: 10.1186/1479-5876-12-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Illman SA, Lohi J, Keski-Oja J. Epilysin (MMP-28)-structure, expression and potential functions. Experimental dermatology. 2008;17:897–907. doi: 10.1111/j.1600-0625.2008.00782.x. [DOI] [PubMed] [Google Scholar]
- 14.Marchenko GN, Strongin AY. MMP-28, a new human matrix metalloproteinase with an unusual cysteine-switch sequence is widely expressed in tumors. Gene. 2001;265:87–93. doi: 10.1016/s0378-1119(01)00360-2. [DOI] [PubMed] [Google Scholar]
- 15.Saarialho-Kere U, Kerkelä E, Jahkola T, Suomela S, Keski-Oja J, Lohi J. Epilysin (MMP-28) expression is associated with cell proliferation during epithelial repair. Journal of Investigative Dermatology. 2002;119:14–21. doi: 10.1046/j.1523-1747.2002.01790.x. [DOI] [PubMed] [Google Scholar]
- 16.Jian P, Yanfang T, Zhuan Z, Jian W, Xueming Z, Jian N. MMP28 (epilysin) as a novel promoter of invasion and metastasis in gastric cancer. Bmc Cancer. 2011;11:1–8. doi: 10.1186/1471-2407-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Overall CM, Tam EM, Kappelhoff R, Connor A, Ewart T, Morrison CJ. et al. Protease degradomics: mass spectrometry discovery of protease substrates and the CLIP-CHIP, a dedicated DNA microarray of all human proteases and inhibitors. Biological Chemistry. 2004;385:493–504. doi: 10.1515/BC.2004.058. [DOI] [PubMed] [Google Scholar]
- 18.Capaccione KM, Pine SR. The Notch signaling pathway as a mediator of tumor survival. Carcinogenesis. 2013;34:1420–30. doi: 10.1093/carcin/bgt127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andersson ER, Lendahl U. Therapeutic modulation of Notch signalling |[mdash]| are we there yet? Nature Reviews Drug Discovery. 2014;13:357–78. doi: 10.1038/nrd4252. [DOI] [PubMed] [Google Scholar]
- 20.Castel D, Mourikis P, Bartels SJJ, Brinkman AB, Tajbakhsh S, Stunnenberg HG. Dynamic binding of RBPJ is determined by Notch signaling status. Genes & Development. 2013;27:1059–71. doi: 10.1101/gad.211912.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nijjar SS, Wallace L, Crosby HA, Hubscher SG, Strain AJ. Altered Notch Ligand Expression in Human Liver Disease: Further Evidence for a Role of the Notch Signaling Pathway in Hepatic Neovascularization and Biliary Ductular Defects. American Journal of Pathology. 2002;160:1695–703. doi: 10.1016/S0002-9440(10)61116-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ning L, Wentworth L, Chen H, Weber SM. Down-regulation of Notch1 signaling inhibits tumor growth in human hepatocellular carcinoma. American Journal of Translational Research. 2009;1:358. [PMC free article] [PubMed] [Google Scholar]
- 23.Villanueva A, Alsinet C, Yanger K, Hoshida Y, Zong Y, Toffanin S. et al. Notch signaling is activated in human hepatocellular carcinoma and induces tumor formation in mice. Gastroenterology. 2012;143:1660. doi: 10.1053/j.gastro.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roy M, Pear WS, Aster JC. The multifaceted role of Notch in cancer. Current Opinion in Genetics & Development. 2007;17:52–9. doi: 10.1016/j.gde.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Qi R, An H, Yu Y, Zhang M, Liu S, Xu H. et al. Notch1 signaling inhibits growth of human hepatocellular carcinoma through induction of cell cycle arrest and apoptosis. Cancer research. 2003;63:8323. [PubMed] [Google Scholar]
- 26.Hu L, Xue F, Shao M, Deng A, Wei G. Aberrant expression of Notch3 predicts poor survival for hepatocellular carcinomas. Bioscience Trends. 2013;7:152. [PubMed] [Google Scholar]
- 27.Gramantieri L, Giovannini C, Lanzi A, Chieco P, Ravaioli M, Venturi A. et al. Aberrant Notch3 and Notch4 expression in human hepatocellular carcinoma. Liver International. 2007;27:997–1007. doi: 10.1111/j.1478-3231.2007.01544.x. [DOI] [PubMed] [Google Scholar]
- 28.Gao J, Song Z, Chen Y, Xia L, Wang J, Fan R. et al. Deregulated expression of Notch receptors in human hepatocellular carcinoma. Dig Liver Dis. 2008;40:114–21. doi: 10.1016/j.dld.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Lim HY, Sohn I, Deng S, Lee J, Jung SH, Mao M. et al. Prediction of disease-free survival in hepatocellular carcinoma by gene expression profiling. Annals of surgical oncology. 2013;20:3747–53. doi: 10.1245/s10434-013-3070-y. [DOI] [PubMed] [Google Scholar]
- 30.Tung EK, Mak CK, Fatima S, Lo RC, Zhao H, Zhang C. et al. Clinicopathological and prognostic significance of serum and tissue Dickkopf-1 levels in human hepatocellular carcinoma. Liver International. 2011;31:1494–504. doi: 10.1111/j.1478-3231.2011.02597.x. [DOI] [PubMed] [Google Scholar]
- 31.Hoon KJ, Hwa SB, Hyun-Sung L, Sang-Bae K, Eun YJ, Yun-Yong P. et al. Genomic Predictors for Recurrence Patterns of Hepatocellular Carcinoma: Model Derivation and Validation. PLoS medicine. 2014;11:e1001770. doi: 10.1371/journal.pmed.1001770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomczak K, Czerwińska P, Wiznerowicz M. The Cancer Genome Atlas (TCGA): an immeasurable source of knowledge. Contemporary Oncology. 2015;19:68–77. doi: 10.5114/wo.2014.47136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. Journal of Clinical Investigation. 2009;119:1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang S, Lincoln TM, Murphyullrich JE. Glucose downregulation of PKG-I protein mediates increased thrombospondin1-dependent TGF-{beta} activity in vascular smooth muscle cells. American Journal of Physiology Cell Physiology. 2010;298:C1188. doi: 10.1152/ajpcell.00330.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masson V. [Roles of serine proteases and matrix metalloproteinases in tumor invasion and angiogenesis] Bull Mem Acad R Med Belg. 2006;161:320–6. [PubMed] [Google Scholar]
- 36.Kudo Y, Iizuka S, Yoshida M, Tsunematsu T, Kondo T, Subarnbhesaj A. et al. Matrix Metalloproteinase-13 (MMP-13) Directly and Indirectly Promotes Tumor Angiogenesis. Journal of Biological Chemistry. 2012;287:38716–28. doi: 10.1074/jbc.M112.373159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shipley JM, Doyle GA, Fliszar CJ, Ye Q-Z, Johnson LL, Shapiro SD. et al. The Structural Basis for the Elastolytic Activity of the 92-kDa and 72-kDa Gelatinases ROLE OF THE FIBRONECTIN TYPE II-LIKE REPEATS. Journal of Biological Chemistry. 1996;271:4335–41. doi: 10.1074/jbc.271.8.4335. [DOI] [PubMed] [Google Scholar]
- 38.Lohi J, Wilson CL, Roby JD, Parks WC. Epilysin, a novel human matrix metalloproteinase (MMP-28) expressed in testis and keratinocytes and in response to injury. Journal of Biological Chemistry. 2001;276:10134–44. doi: 10.1074/jbc.M001599200. [DOI] [PubMed] [Google Scholar]
- 39.Lin MH, Liu SY, Su HJ, Liu YC. Functional role of matrix metalloproteinase-28 in the oral squamous cell carcinoma. Oral Oncology. 2006;42:907–13. doi: 10.1016/j.oraloncology.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 40.Jian P, Yanfang T, Zhuan Z, Jian W, Xueming Z, Jian N. MMP28 (epilysin) as a novel promoter of invasion and metastasis in gastric cancer. BMC cancer. 2011;11:200. doi: 10.1186/1471-2407-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Illman SA, Lehti K, Keski-Oja J, Lohi J. Epilysin (MMP-28) induces TGF-beta mediated epithelial to mesenchymal transition in lung carcinoma cells. Journal of Cell Science. 2006;119:3856–65. doi: 10.1242/jcs.03157. [DOI] [PubMed] [Google Scholar]
- 42.Illman SA, Lehti K, Keskioja J, Lohi J. Epilysin (MMP-28) induces TGF-beta mediated epithelial to mesenchymal transition in lung carcinoma cells. Journal of Cell Science. 2006;119:3856. doi: 10.1242/jcs.03157. [DOI] [PubMed] [Google Scholar]
- 43.Bonuccelli G, Castello-Cros R, Capozza F, Martinez-Outschoorn UE, Lin Z, Tsirigos A. et al. The milk protein α-casein functions as a tumor suppressor via activation of STAT1 signaling, effectively preventing breast cancer tumor growth and metastasis. Cell cycle. 2012;11:3972–82. doi: 10.4161/cc.22227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le Parc A, Houeto EH, Pigat N, Chat S, Leonil J, Chanat E. The membrane-associated form of αs1-casein interacts with cholesterol-rich detergent-resistant microdomains. PloS one. 2014;9:e115903. doi: 10.1371/journal.pone.0115903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ławicki S, Zajkowska M, Głażewska EK, Będkowska GE, Szmitkowski M. Plasma levels and diagnostic utility of VEGF, MMP-2 and TIMP-2 in the diagnostics of breast cancer patients. Biomarkers. 2017;22:157–64. doi: 10.1080/1354750X.2016.1252955. [DOI] [PubMed] [Google Scholar]
- 46.Lorenc Z, Waniczek D, Lorenc-Podgórska K, Krawczyk W, Domagała M, Majewski M. et al. Profile of Expression of Genes Encoding Matrix Metallopeptidase 9 (MMP9), Matrix Metallopeptidase 28 (MMP28) and TIMP Metallopeptidase Inhibitor 1 (TIMP1) in Colorectal Cancer: Assessment of the Role in Diagnosis and Prognostication. Medical Science Monitor. 2017;23:1305–11. doi: 10.12659/MSM.901593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Folgueras AR, Pendás AM, Sánchez LM, Lópezotín C. Matrix metalloproteinases in cancer: from new functions to improved inhibition strategies. International Journal of Developmental Biology. 2004;48:411–24. doi: 10.1387/ijdb.041811af. [DOI] [PubMed] [Google Scholar]
- 48.Artavanistsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 49.Illam SP, Narayanankutty A, Mathew SE, Valsalakumari R, Jacob RM, Raghavamenon AC. Epithelial Mesenchymal Transition in Cancer Progression: Preventive Phytochemicals. Recent Pat Anticancer Drug Discov. 2017;12:1–1. doi: 10.2174/1574892812666170424150407. [DOI] [PubMed] [Google Scholar]
- 50.Natsuizaka M, Whelan KA, Kagawa S, Tanaka K, Giroux V, Chandramouleeswaran PM. et al. Interplay between Notch1 and Notch3 promotes EMT and tumor initiation in squamous cell carcinoma. Nature Communications. 2017;8:1758. doi: 10.1038/s41467-017-01500-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li C, Song G, Zhang S, Wang E, Cui Z. Wnt3a increases the metastatic potential of non-small cell lung cancer cells in vitro in part via its upregulation of Notch3. Oncology Reports. 2015;33:1207. doi: 10.3892/or.2014.3700. [DOI] [PubMed] [Google Scholar]
- 52.Zhou JX, Zhou L, Li QJ, Feng W, Wang PM, Li EF, Association between high levels of Notch3 expression and high invasion and poor overall survival rates in pancreatic ductal adenocarcinoma. Oncology Reports; 2016. p. 36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures and table.