Abstract
Alzheimer’s disease (AD) is a progressive neurodegenerative disease. Although it has been studied for years, the pathogenesis of AD is still controversial. Genetic factors may play an important role in pathogenesis, with the apolipoprotein E (APOE) gene among the greatest risk factors for AD. In this review, we focus on the influence of genetic factors, including the APOE gene, the interaction between APOE and other genes, and the polygenic risk factors for cognitive function and dementia. The presence of the APOE ε4 allele is associated with increased AD risk and reduced age of AD onset. Accelerated cognitive decline and abnormal internal environment, structure, and function of the brain were also found in ε4 carriers. The effect of the APOE promoter on cognition and the brain was confirmed by some studies, but further investigation is still needed. We also describe the effects of the associations between APOE and other genetic risk factors on cognition and the brain that exhibit a complex gene–gene interaction, and we consider the importance of using a polygenic risk score to investigate the association between genetic variance and phenotype.
Keywords: Alzheimer’s disease, APOE, cognition, brain structure, brain function, polygenic risk score
1. Introduction
1.1. Alzheimer’s Disease and Genetics
Alzheimer’s disease (AD) is a chronic progressive neurodegenerative disease characterized by memory loss and deficits in other cognitive abilities and is the most common form of dementia. With the increase in life expectancy, the prevalence of dementia rose gradually in recent years. The World Alzheimer Report 2018 estimated that, around the world, there will be one new case of dementia every three seconds; 50 million people worldwide are living with dementia in 2018, and that number will be more than 152 million by 2050. The total estimated worldwide cost of dementia in 2018 was one trillion United states dollars (US$), and this figure will rise to two trillion US$ by 2030 [1]. Based on the age of onset of the disease, AD can be divided into early-onset AD (EOAD) and late-onset AD (LOAD). EOAD, also known as familial AD, is an autosomal dominant disorder with onset before the age of 65 years, and it accounts for approximately 5% of all AD cases [2,3]. Most cases of EOAD are caused by mutations in the amyloid precursor protein (APP), presenilin 1 (PSEN1), and presenilin 2 (PSEN2) genes [4,5,6]. LOAD, also known as sporadic AD, accounts for most AD cases. Evidence from a twin study showed that the heritability for AD was 60–80% [7]. Genome-wide association studies (GWAS) identified susceptibility loci for LOAD, including the apolipoprotein E (APOE) gene, as well as the clusterin (CLU), phosphatidylinositol-binding clathrin assembly protein (PICALM), complement receptor 1 (CR1), bridging integrator 1 (BIN1), sortilin-related receptor L (SORL1), and translocase of outer mitochondrial membrane 40 (TOMM40) genes [8,9,10,11]. These genes may affect the risk of LOAD through different pathways, such as cholesterol metabolism, immune system function, and endocytic processes [12]. Among them, the APOE gene is the strongest risk factor for LOAD.
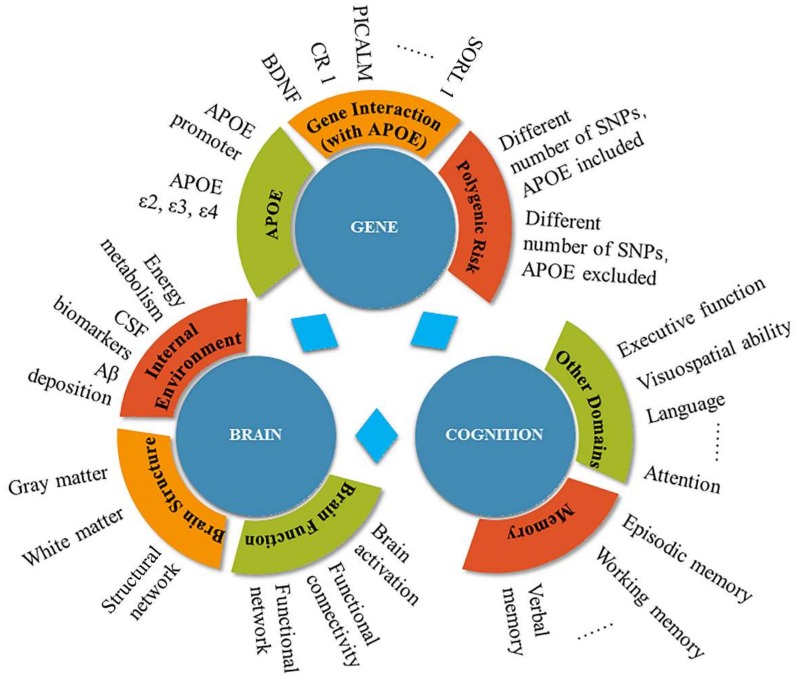
Based on the APOE gene-related research over the last decade, we conducted a brief review focused on the influence of genetic factors, including the APOE gene, the interaction between the APOE gene and other genes, and polygenic risk factors for AD development, cognition, brain structure, and function (Figure 1).
Figure 1.
An overview of the gene, brain, and cognition facets of the current review.
1.2. APOE: Risk Factor for AD
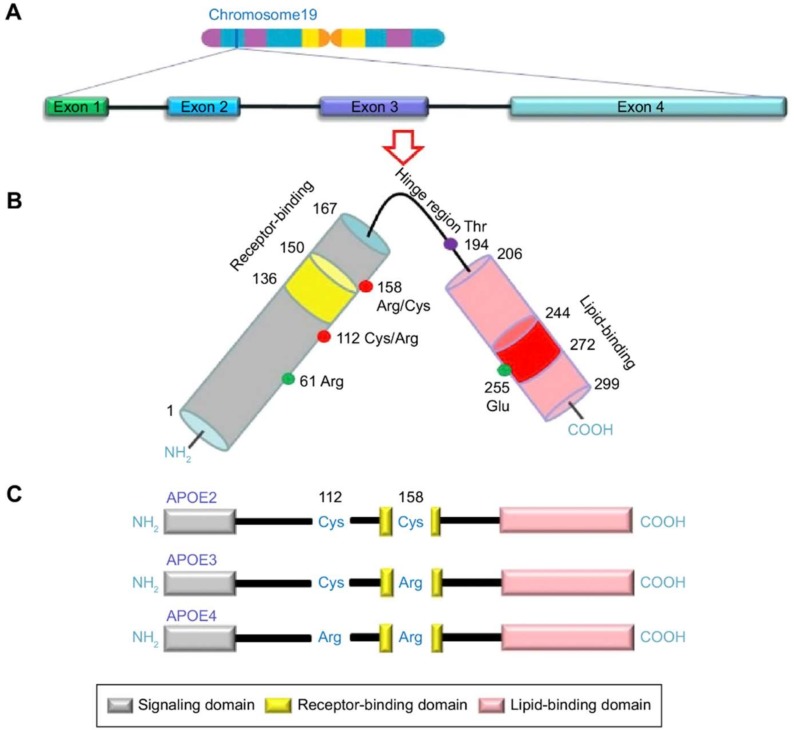
The APOE gene is located on chromosome 19q13.2 with a total of 3597 bases with four exons and three introns, and the gene has three major alleles (ε2, ε3, and ε4). The ε3 allele is the most frequently occurring allele, constituting 60–90% of the allelic variation, while the frequencies of ε2 and ε4 are 0–20% and 10–20%, respectively [13,14,15]. Genetic variation at the APOE locus induces three common isoforms: APOE2 (Cys112, Cys158), APOE3 (Cys112, Arg158), and APOE4 (Arg112, Arg158) which are coded by the ε2, ε3, and ε4 alleles, respectively (Figure 2) [14,16]. APOE ε4 is an acknowledged genetic risk for AD [12]. A meta-analysis demonstrated that, for APOE ε4 carriers, prevalence of AD was 48.7%, and homozygote prevalence was 9.6%. There are also differences across regions/ethnic groups, with the lowest ε4 carrier prevalence observed in Asia (41.9%) and southern Europe (40.9%), while northern Europe has the highest prevalence of ε4 carriers (61.3%) [17]. Each copy of the ε4 allele increases the risk of AD by approximately threefold, and two copies increase the risk of AD by 8–14-fold compared to the ε3/ε3 genotype. Conversely, the APOE ε2 allele has a protective effect. The risk of AD of ε2 allele carriers is only 0.6 that of the ε3/ε3 genotype [18,19,20,21]. Additionally, the age of onset of AD is also influenced by the number of APOE ε4 alleles and decreases by approximately 3–4 years for every ε4 allele carried [18,19]. Mild cognitive impairment (MCI) is a transitional zone between normal aging and AD, and the annual rate of progression from MCI to AD is more rapid than the progression to AD of normal subjects [22]. Meta-analysis also revealed that the APOE ε4 allele was associated with more than double the risk for progressing from MCI to AD across studies compared to ε4 non-carriers [23,24]. Thus, APOE ε4 is associated with developing AD by increasing the relative risk of AD and by lowering the age of onset of the disease. Other rare APOE variations in addition to the three common alleles were also reported [25,26]. For example, the ε7 allele, with two lysine residues replacing glutamic acid at positions 244 and 245 in the carboxyl terminus, is associated with hyperlipidemia and atherosclerosis [27]. Furthermore, since the ε7 mutant (like ε4) associates preferentially with very low-density lipoproteins, it is also presumed to be related to AD risk. Youn and colleagues investigated the association between APOE ε7 expression and cognitive impairment, with the results suggesting that ε7 could serve as a risk factor for cognitive impairment and is particularly associated with vascular disease [28]. However, related research is still limited, and further studies are needed to investigate the role of ε7 in cognitive impairment and AD.
Figure 2.
Schematic illustration of structural and functional regions of apolipoprotein E (APOE). (A) Location and structure of the APOE gene on chromosome 19. (B) APOE protein. (C) Three major APOE isoforms. (adapted from Reference [16]).
In addition to polymorphisms within the coding region, the polymorphisms within the APOE promoter region are also related to AD risk [29]. These polymorphisms are proposed to modulate the transcriptional activity of the APOE coding region [30]. Three single-nucleotide polymorphisms (SNPs) (491A/T, rs449647; 427T/C, rs769446; 219T/G, rs405509) within this region were identified [31]. Xin and colleagues conducted a meta-analysis of 40 studies with 9662 cases and 9696 controls, and revealed that the rs449647 polymorphism and rs405509 polymorphism showed a modest but significant association with AD susceptibility, identifying rs449647 AA and rs405509 TT as risk factors. However, the association between the rs769446 polymorphism and the risk of AD is not consistent, as a significant association was not identified in this meta-analysis [32]. Nonetheless, a recent meta-analysis of 23 publications that included 5703 AD patients and 5692 controls revealed that the C allele of rs769446 was significantly associated with an increase in the risk of AD, while there was no association between the rs449647 or rs405509 polymorphisms and the risk of AD [33]. The two meta-analyses included 13 overlapping studies. The reasons for these heterogeneous conclusions may include differences among study designs, including the studied populations, sample selection (e.g., age of onset, diagnosis criteria), sample size, methods, and interactions with other risk factors, especially APOE ε4 status. Thus, the relationship between APOE promoter polymorphisms and the risk for AD may differ across studies, and represents a complex pattern with the APOE genotype. Future studies including large samples and different ethnicities should be conducted.
2. The Effects of the APOE Gene on Cognitive Function and Dementia
2.1. APOE ε4 Allele
Negative effects associated with this gene on cognition are also found in APOE ε4 carriers. Compared to non-carriers, APOE ε4 carriers exhibit worse cognitive performance and accelerated cognitive decline in healthy, MCI, and AD subjects [34,35,36].
As memory deficits are considered the major cognitive impairment in AD [37], the relationship between memory decline and the presence of the APOE ε4 allele in AD was examined a lot. Van der Vlies and colleagues investigated the relationship between the APOE ε4 allele and cognitive function in 229 AD patients, and the results indicated that memory was more impaired in ε4 carriers than in non-carriers, suggesting that the APOE genotype may modify the clinical phenotype of AD [38]. Similar results were also found in a study including 523 AD patients that evaluated memory performance based on standard tests and daily function, and found a strong relationship between amnestic presentation and the parameters of increased age of onset, family history, and the presence of the APOE ε4 allele [39]. Wolk and Dickerson investigated a broad range of cognitive functions, including verbal memory, working memory, executive function, and verbal ability, and they found that the ε4 carriers exhibited decreased verbal memory and increased medial temporal lobe (MTL) atrophy compared with the non-carriers who exhibited poor working memory, executive function, and verbal ability, as well as increased frontoparietal atrophy [40]. Similar results were also found by Kim and colleagues when examining the impact of the APOE ε4 allele in AD patients of different ages. AD patients under 75 years of age who did not carry the APOE ε4 allele or who were heterozygotic showed poor performance in language, visuospatial, and frontal functional tests, while homozygotes older than 75 exhibited worse memory. The cortical atrophy pattern was also different across genotypes and age groups [41]. A longitudinal study also indicated that the ε4 allele predicted a faster cognitive decline than other alleles, with gene dose-dependent effects observed in mild AD [42] and more evident in younger AD patients than in older AD patients [43]. Taken together, these results suggest that AD patients who are ε4 allele carriers and non-carriers may suffer from different patterns of cognitive dysfunction, in which age may play an important role.
Farlow and colleagues investigated the impact of the APOE genotype on MCI in 494 participants, and they found that the presence of the APOE ε4 allele decreased global cognition and memory performance, seemingly resembling the cognition and memory of patients in the early stages of AD [34]. Another study also found an association between the APOE ε4 allele and impaired memory function in both middle-aged and elderly MCI subjects [44]. Furthermore, longitudinal studies revealed that the APOE ε4 genotype is predictive of an increased decline in general cognition in MCI, as the ε4 carriers had a significantly more rapid decline [45] in addition to a higher conversion rate to AD, as mentioned above [23,24].
Similar to MCI or AD, the negative effects associated with this gene are also found in cognitively healthy APOE ε4 carriers. Although some studies indicated null negative effects [46,47], many studies indicated that, compared to non-ε4 carriers, ε4 carriers exhibit worse performance in a range of cognitive functions [36,48] at old age, especially in episodic memory [49,50,51,52], executive function [50,52,53,54], and global cognition [52,55]. In addition to cognitive status, APOE ε4 is also related to accelerated cognitive decline in old adults, particularly in memory [36]. Caselli and colleagues investigated the APOE ε4 effect on memory decline in cognitively normal subjects aged between 21 and 97 years. After approximately five years of follow-up, they found that the ε4 carriers exhibited memory decline beginning before the sixth decade of life and that memory declined more rapidly than in non-carriers [56]. Homozygous subjects had the most rapid memory decline, indicating that the APOE ε4 effect on cognitive decline may be dependent on gene dose [56,57]. Similar results were found in studies with different follow-up times and subjects of different ages [58,59]. Additionally, studies across ethnic groups and regions also confirmed the negative effect of the ε4 allele. Barnes and colleagues found that APOE ε4 is related to a more rapid rate of decline in episodic memory in blacks, similar to the effect of this allele in whites [60]. Another study also found a significant ε4 effect on the incidence of AD and on the cognitive decline in Yoruba and African Americans in a large longitudinal comparative study [61]. Lipnicki et al. conducted a longitudinal study of 14 cohorts from 12 countries to investigate the relationship between cognitive decline and the APOE gene, with a follow-up duration of 2–15 years. They found that ε4 allele carriers exhibited a slightly more rapid decline in cognitive function, including memory, processing speed, and language than non-carriers [62].
Although some studies did not find significant results, the detrimental effects of the APOE ε4 allele on cognitive function were confirmed. In addition, memory is commonly affected by the ε4 allele with gene dose-dependent effects in AD, MCI, and cognitively healthy subjects. The inconsistent null pattern in some studies may derive from methodological issues, including differences in age, cognitive measures, sample size, and other cognitive risk factors [36,48]. It is noteworthy that the differential effects of APOE ε4 on cognition during different life stages represents an example of antagonistic pleiotropy [63]: a reduced or null negative effect is observed in middle age [50,64]; a reversed positive effect is observed in young adults [49]; and the effect size in the elderly is also affected by age [48]. Thus, any APOE-related study must take into account the interaction between age and the APOE gene.
2.2. Promoter Polymorphisms of the APOE Gene
In addition to the risk for AD, polymorphisms in the APOE gene promoter also affect cognition in the aged. Shu and her colleagues included 837 non-dementia elderly subjects living in the community and found that participants with the rs405509 TT genotype showed worse general cognitive function, attention, and executive function than the G allele carriers, regardless of APOE ε4 state [65]. Similarly, Chang et al. also confirmed that the rs405509 TT genotype is significantly associated with poor general cognitive function, episodic memory, and executive function. After controlling for the APOE ε4 genotype, the TT genotype also had a significant age-related decline in global cognition, memory, processing speed, and executive function [66]. Poor language performance in the rs405509 TT genotype was also found in elderly subjects without dementia, and the TT genotype exhibited a more rapid rate of decline in global cognition [67]. Ma and colleagues investigated the interaction effect of the rs405509 T allele and the APOE ε4 allele on cognitive ability in Chinese participants, and found significant interaction effects between rs405509 and APOE on general cognition, memory, and attention. The double homozygous genotype (rs405509 TT/ε4ε4) exhibited a significant reduction in general cognition, memory, and attention [68]. However, in a recent study that included elderly men from Finland, researchers found that the rs405509 TT genotype was significantly associated with improved general cognition, language, arithmetic, and visual spatial ability, independent of the APOE major isoforms [69]. This likely indicates that the effects of rs405509 are different among different ethnicities. In addition, the researchers found that subjects with the CC genotype of rs440446 showed better visual spatial ability than subjects with the GG genotype. These studies suggested that the promoter of APOE can significantly affect cognitive function, but only a few studies were performed, and most of these studies focused on rs405509. Further studies including multiple promoters may increase the understanding of the effect of APOE on cognition.
2.3. Genetic Association with the APOE Gene
Although the APOE gene explains a part of the genetic risks associated with AD, other genes may still modify the APOE ε4 effect [70]. Previous APOE-related studies also investigated the effects of associations between APOE and other gene polymorphisms on cognition and dementia (Table 1).
Table 1.
Studies of genetic association with the apolipoprotein E (APOE) gene.
| Study | Participants | Genes | Interaction Impact on Disease | Possible Mechanisms Described by the Authors |
|---|---|---|---|---|
| Martinez et al., 2009 [70] | 223 MCI patients, 345 AD and 253 HC | COMT | COMT (Val158 Met) polymorphism is not an independent risk factor for AD or MCI, but shows a synergistic effect with APOE ε4 allele that proves greater in women with AD. | Lowering the estrogen levels of brain. |
| Wang et al., 2005 [75] | 66 AD and 86 HC | COMT | The COMT high-activity genotypes and APOEε4 allele had a synergistic effect on the risk of AD. | A high metabolism of estrogen by COMT may have reduced the protective effect of estrogen in AD. |
| Sapkota et al., 2017 [76] | 634 non-demented older adults | COMT BDNF | APOE ε4+ carriers with BDNF Met/Met genotype and increasing allelic risk in the COMT + BDNF risk panel had poorer executive function performance. | − |
| Ward et al., 2014 [71] | 433 older adults (50–79 years) | BDNF | In BDNF Val homozygotes, the cognitive consequences of APOE polymorphisms were minimal. However, in BDNF Met carriers, the hypothesized beneficial/detrimental effects of APOE polymorphisms were found. | Firstly, there is a biological interaction related to the systems or aging-related roles of the encoded proteins. Secondly, the additive effects of the polymorphisms caused the analyses to reach statistical significance. |
| Gomar et al., 2016 [72] | 175 healthy subjects and 222 with prodromal and established AD | BDNF | BDNF Met and APOE ε4 carriers had thinner posterior cingulate and precuneus cortices in healthy subjects, and longitudinal decline in entorhinal thickness in MCI and AD. | − |
| Persson et al., 2013 [73] | 888 non-demented adults (35–85 years) | BDNF | A joint effect on memory decline in BDNF × APOE × age, with the subjects carrying the Met allele, as well as at least one copy of the APOE ε4 allele showing magnified effect sizes with increasing age on memory decline, while the homozygote Val subjects carrying the ε4 allele showed a decreased slope. | − |
| Yu et al., 2007 [77] | 193 late-onset AD, 232 subjects with no cognitive impairment, and 125 individuals with other neurodegenerative disorders | TOMM40 | It showed intriguing linkage disequilibrium with the ε4 allele and was strongly associated with the risk for developing late onset AD. | − |
| Roses et al., 2009 [78] | 191 AD and 131 HC (mean age: about 75 years) | TOMM40 | Individuals with long poly-T repeats linked to APOE ε3 develop late onset AD on an average of 7 years earlier than individuals with shorter poly-T repeats linked to APOE ε3. | It is possible that the rs10524523 polymorphism, alone or in conjunction with other single-nucleotide polymorphisms in TOMM40, acts at a distance to affect transcription of APOE. |
| Johnson et al., 2011 [80] | 117 healthy APOE ε3 homozygous adults (mean age: about 55 years) | TOMM40 | Those who were homozygous for very long poly-T lengths had poorer memory than those who were homozygous for short poly-T length in APOE ε3/3. | − |
| Yu et al., 2017 [81] | 1151 old people (mean age: about 78.5 years) | TOMM40 | It revealed an association of APOE ε3/3-TOMM40′523 haplotypes with cognitive decline in community-based older persons such that the S/S poly-T genotype is related to faster cognitive decline, primarily in the domains of episodic and semantic memory. | The TOMM40 variant is implicated in affecting the level of neurofilament light proteins in cerebrospinal fluid. |
| Louwersheimer et al., 2017 [82] | A family with 9 AD patients spanning 4 generations, with an inheritance pattern suggestive of autosomal dominant | SORL1 | All four affected family members carried a rare variant in the vacuolar protein sorting domain 10 domain of the SORL1 gene, associated with Aβ protein precursor processing and AD risk. | A combination of homozygous or heterozygous APOEε4 and dysfunctional SORL1 may lead to abnormal increases in extracellular Aβ loads. |
| Barral et al., 2012 [83] | 1365 subjects in the National Institute on Aging Late-Onset Alzheimer’s Disease Family Study | CR1, BIN1, CLU, PICALM | Several genotype patterns influenced episodic memory performance. | − |
| Gharesouran et al., 2014 [84] | 160 patients with late-onset AD and in 163 HC | PICALM, BIN1 | The associations with PICALM and BIN1 were only significant among subjects without the APOE ε4 allele. | − |
| Keenan et al., 2012 [85] | 1709 subjects (697 deceased) from the Religious Orders Study and the Rush Memory and Aging Project | CR1 | A significant interaction between our candidate functional variant rs4844609 and the presence or absence of APOE ε4 on episodic memory decline. | − |
| Liao et al., 2014 [86] | 536 AD cases and 307 cognitive-intact elder controls | ABCA7 | The influence of ABCA7 was only evident in individuals without APOE ε4 alleles but absent in ε4 carriers. | − |
| Casati et al., 2018 [87] | 57 MCI, 50 AD, and 42 non-demented healthy subjects (mean age: about 78.5 years) | TREM2 | Higher TREM2 levels in allele ε4 of apolipoprotein E carriers than non-carriers in MCI and particularly in MCI-AD. | The upregulation of TREM2 could be a mechanism to counteract neuroinflammatory processes in MCI patients who progress to AD. |
| Espeseth et al., 2006 [157] | 230 healthy middle-aged (53–64 years) and older (65–75 years) adults | CHRNA4 | APOE-ε4 carriers who were also CHRNA4 TT homozygotes showed disproportionately slowed reaction time (RT) following invalid location cues. There was also a trend for individuals with combined APOE-ε4/CHRNA4 TT genotypes to show both lower white-matter volume and slower overall RT on the attention task. | It remains for further research to determine which of several underlying mechanisms—acetylcholine synthesis, cholinergic neuronal metabolism, synaptic availability of acetylcholine, the affinity of cholinergic receptors, or other factors—are responsible for the interactive effects of APOE and CHRNA4 on attention. |
| Morgen et al., 2014 [158] | 165 patients with early AD dementia | PICALM | There was a synergistic adverse effect of homozygosity for the PICALM risk allele G in rs3851179 and APOE ε4 on volume in prefrontal and performance on the Trail Making Test. | The APOE and PICALM risk genotypes may contribute to Aβ accumulation through different mechanisms, ultimately leading to synaptic dysfunction and loss. |
| Thambisetty et al., 2013 [159] | 57 non-demented older individuals (mean age: about 78.5 years) and 22 cognitively normal older individuals (mean age: about 77.1 years) | CR1 | Carrying a risk allele of the CR1 rs3818361 results in a reduced brain amyloid burden compared to non-carriers, but only in ε4 non-carriers. | The CR1 risk allele might modify the relationship between APOE genotype and brain amyloid deposition. |
| Liu et al., 2018 [160] | 710 individuals (mean age: about 65 years) | SPON1 | Significant SPON1 × APOE genotype interactions in working memory and executive function performances. The effects of ε4 allele on activation of right inferior frontal gyrus, triangular part were modulated by rs2618516 in a working memory task. | − |
| Shen et al., 2017 [161] | 287 healthy, young, right-handed subjects (mean age: 22.7 ± 2.4 years, ranging from 18 to 29 years) | SORL1 | Significant SORL1 × APOE non-additive interaction was found in negative resting state functional connectivity (rsFC) between the hippocampus and inferior frontal gyrus. Compared with subjects with TT genotype, SORL1 G-allele carriers had a stronger negative rsFC in APOE ε4 carriers, but a weaker negative rsFC in APOE non-ε4 carriers. | − |
| Zhang et al., 2017 [162] | 267 healthy young adults (mean age: about 22.8 years) | KIBRA | Epistatic effects showed APOE × KIBRA interaction in the functional connectivity density (FCD) of the dorsolateral prefrontal cortex (DLPFC). The FCD of the DLPFC showed APOE risk-allele-dependent reduction (ε2 > ε3 > ε4) in KIBRA TT homozygotes, but APOE risk-allele-dependent increase in KIBRA C-carriers. | One candidate explanation for the complex APOE–KIBRA interactions on brain FCD may be the differential effects of genetic variations in APOE and KIBRA on the long-term potentiation of memory-related brain regions. |
| Porter et al., 2018 [163] | 602 CN adults | KIBRA | In comparison to APOE ε4- individuals carrying the rs17070145-T allele, significantly faster rates of cognitive decline, and hippocampal atrophy were observed in individuals who were APOE ε4+ and did not carry the rs17070145-T allele. | Synaptic plasticity, which is altered in AD, is modulated by dendrin, which in turn binds to the protein that KIBRA encodes. |
HC, healthy control; CN, cognitive normal; MCI, mild cognitive impairment; AD, Alzheimer’s disease.
Ward and colleagues compared cognitive function in 433 older adults to determine the association between APOE and brain-derived neurotrophic factor (BDNF Val66Met) polymorphisms. They found a significant APOE × BDNF interaction in episodic memory, with APOE ε2 carriers displaying episodic memory superior only in BDNF Met carriers [71]. Episodic memory was also found to be impaired in a sample of APOE ε4-carrying MCI/AD subjects who were also carriers of the Met mutation of BDNF compared to those who Val/Val homozygotes for BDNF [72]. When exploring the synergistic effects of BDNF, APOE, and HbA1c (glycated hemoglobin) on cognitive decline over 10 years in adults without dementia, Persson et al. found joint effects on memory decline in BDNF × APOE × age, with the subjects carrying the Met allele, as well as at least one copy of the APOE ε4 allele, showing magnified effect sizes with increasing age on memory decline, while the homozygote Val subjects carrying the ε4 allele showed a decreased slope [73]. APOE and BDNF may impact cognition together through their interactional effect, but this effect is impacted by education because neither APOE nor BDNF modify the beneficial effects of a university-based educational intervention on cognitive function [74].
Martinez and colleagues examined the synergistic effects of the catechol-O-methyltransferase (COMT rs4680) gene and APOE in AD and MCI subjects. Although neither COMT alleles nor genotypes were independent risk factors for AD or MCI, the high-activity genotypes increased the risk of AD in ε4 carriers and exhibited a synergistic interaction with the APOE allele. COMT heterozygotes who carry the ε4 allele exhibited a higher risk for MCI [70]. This study is in accordance with findings by Wang et al., in which the subjects with the COMT high-activity genotypes had an increased risk of developing AD with the presence of at least one ε4 allele [75]. Additionally, the combined COMT and BDNF risk is also associated with poor executive function in ε4 carriers without dementia [76].
The TOMM40 gene shows a linkage disequilibrium pattern with APOE and is associated with the development of AD [77]. Longer poly-T tracts at TOMM40 rs10524523 (‘523) are significantly correlated with earlier age of onset of LOAD in APOE ε3 carriers [78,79]. Johnson and colleagues investigated the relationship between TOMM40’523 and cognitive function in 117 APOE ε3 homozygous adults, and found that those who were homozygous for very long (VL) poly-T lengths had poorer memory than those who were homozygous for short (S) poly-T length [80]. However, another study also found that the S/S poly-T genotype with APOE e3/3 homozygosity was related to more rapid declines in global cognition and memory in community-based older persons [81]. Thus, the effect of TOMM40 on cognition and whether there are additive effects between APOE and TOMM40 require further investigation.
The effect of the APOE gene on cognition and dementia is also modified by other genetic factors, including the SORL1 [82], PICALM [83,84], CR1 [83,85], ABCA7 [86], TREM2 [87,88], and BIN1 [83,84], showing complex gene-gene effects. Thus, combining multiple genes and investigating their joint effects is a logical experimental design and may help clarify these complex genetic processes.
2.4. Polygenic Risk Factors for Cognitive Decline
As cognition and AD are highly heritable polygenic traits in humans, investigating polygenic effects on phenotypes is also very important. Therefore, researchers tend to integrate different genetic loci to construct polygenic risk scores (PGS). Studies confirmed that polygenic genetic risk increases the incidence of AD [89], increases the conversion rate of MCI to AD [90], and increases the risk of cognitive decline [91,92,93].
A study of older adults in Belgium showed that weighted PGSs consisting of 22 SNPs (including APOE ε4) increased the incidence of AD 2.32-fold, and the age of onset of AD decreased by 2.39 years per unit increase in risk score [89]. Another study in the Han Chinese population also found PGSs based on three SNPs were associated with AD risk independent of APOE genotype, and PGSs based on AD risk-associated SNPs may supplement APOE for better assessing individual risk for AD [94]. Marden and colleagues also found that the PGSs based on ten polymorphisms confirmed to predict AD can predict dementia risk among both non-Hispanic whites and blacks [92]. Lee and colleagues generated AD risk prediction models using a combination of the top-ranked SNPs associated with AD in a Korean elderly sample; when considering age effect, their models were able to predict the onset of AD in an independent Japanese AD sample, suggesting the potential practical clinical use of combining age and polygenic risk score in predicting AD [95]. Therefore, the association of polygenic risk score and the incidence of AD was confirmed in different ethnic groups. In addition, increased polygenic risk score can also increase the conversion rate of MCI to AD. A study that included eight risk SNPs (APOE excluded) found that, when an individual carried six or more risk alleles, the conversion rate of MCI to AD increased twofold [90]. Another European multicenter study found that weighted PGSs constructed with nine AD risk SNPs were significantly associated with the conversion of MCI to AD in APOE ε4 allele carriers [96]. A study of MCI patients also found that weighted PGSs calculated with 18 non-APOE AD risk variants were significantly associated with a decline in general cognition [97].
Verhaaren and colleagues included 5171 middle-aged and older people without dementia to investigate the association of weighted PGSs constructed with 12 AD risk SNPs (including APOE ε4) with general cognition, memory, and possessing speed. A significant correlation between PGS and memory was found, and this association was attenuated when the APOE ε4 allele was excluded [91]. Similar results were also found in an Australian study on non-demented elderly, and the study found that the weighted PGSs using 12 risk SNPs (including APOE) were related to episodic memory [98]. Marden and colleagues included non-Hispanic white and black participants and examined the efficacy of weighted PGSs constructed by 10 AD risk SNPs (including APOE) in predicting AD and memory performance. The study found that PGSs were associated with a risk of dementia among whites and blacks, whereas the association between PGS and poor memory performance was only found in whites [92]. Similar results were also found in a longitudinal study. In 2016, researchers included a total of 8253 non-Hispanic white and black participants and examined the interaction of weighted PGSs constructed by 22 AD risk SNPs (including APOE) and age to predict memory decline. The study found that PGSs with the APOE ε4 allele and PGSs without the APOE ε4 allele in whites both can predict a more rapid decline in memory, whereas only PGSs with the APOE ε4 allele in blacks can predict a faster decline in memory, indicating the genetic efficacy of APOE in different ethnic groups [93]. Carrasquillo et al. also found that PGSs including the APOE ε4 allele were associated with memory decline and the progression to MCI/AD in a Caucasian cohort [99]. PGSs are an appropriate method to integrate multiple genetic risk factors. As PGS is an emerging method, the number of studies is also limited. Future related studies may help deepen our understanding of genetic effects on cognitive function and dementia.
3. The Effects of the APOE Gene on Brain Function
3.1. APOE ε4 Allele
The β-amyloid (Aβ) hypothesis is the most accepted pathological theory of AD, and it is believed that Aβ deposition causes a series of subsequent pathological changes, including the emergence of p-tau and the increase in neurofibrillary tangles in the brain. A number of previous reviews summarized a significant correlation between APOE ε4 and amyloid load in the brain [2,100,101]. We overviewed the articles investigating the influence of APOE ε4 on brain amyloid deposition published in the last decade, and this conclusion is well proven. Pittsburgh Compound-B (PiB) uptake in the temporoparietal and frontal cortices of patients with AD is positively correlated with ε4, and both the distribution and the annual increase in Aβ deposition exhibited a gene dose-dependent effect [102,103]. The same relationship is observed in patients with MCI and in cognitively healthy elderly subjects; those who carry ε4 have more deposition than non-carriers [104,105]. In fact, a meta-analysis summarized by Jansen and colleagues revealed Aβ deposition onset early in the fourth decade of life of ε4 carriers, and they had Aβ deposition levels that were two to three times higher than that of non-carriers [101]. APOE ε4 also reduced the age that Aβ was detected; Aβ positivity occurs in normal subjects at approximately 56 years of age and in non-carriers at approximately 76 years of age [106]. A significant APOE × age interaction was also observed by Gonneaud and colleagues. Aβ deposition increased nonlinearly with age in APOE ε4 carriers but not in non-carriers in their study [107]. Moreover, it seems that the association between Aβ deposition and cognitive performance is modified by APOE status. One study reported that higher amyloid content was predictive of a longitudinal decline in executive function and memory tests in ε4 non-carriers [108]; however, another study reported severe cognitive impairment in addition to higher Aβ deposition in ε4 carriers [109]. This discrepancy may result from the difference of age in the two groups (62 years old in the first study and 80 years old in the latter one). Furthermore, there was also a report that increased cognitive activity over a lifetime can diminish cortical PiB retention in ε4 carriers [110], which makes the relationship among APOE, cognition, and Aβ deposition quite interesting and worthy of further study.
Corresponding to the influence of ε4 on Aβ deposition, a study also showed that the decrease in Aβ42 and the increase in tau/p-tau in the cerebrospinal fluid (CSF) were related to ε4. Liu and colleagues observed 1718 participants from Alzheimer’s Disease Neuroimaging Initiative (ADNI) and found significantly decreased Aβ42 and increased tau/p-tau levels in the CSF in the ε4 carriers, which appeared earlier than other biomarkers of AD [111]. This effect is gene dose-dependent; homozygosity for the APOE ε4 allele results in a worse phenotype than both heterozygosity and lack of the APOE ε4 allele. The influence of ε4 on CSF biomarkers was even stronger than the clinical status [112,113]. However, the association between tau and the APOE genotype is not observed in people without Aβ [114,115]. Considering the negative correlation between Aβ deposition in the brain and Aβ42 in the CSF, as well as the influence of Aβ deposition on the change in tau/p-tau, this relationship between ε4 and the CSF biomarkers is understandable. However, there are negative results that should arouse our attention at the same time. For example, a study including samples from Sweden, Finland, and Germany found that the change in CSF Aβ42 was independent of the APOE genotype in some of their sub-cohort. They also found that ε4 may not have a direct effect on CSF levels of Aβ42, but did demonstrate APOE ε4-associated preclinical pathology in the elderly [116]. For this reason, the effect of ε4 on CSF Aβ42 may not exist in some younger sample populations because they do not have amyloid.
As one of the earliest biomarkers of AD, glucose hypometabolism is also affected by the APOE genotype. Dozens of studies observed that preclinical ε4 carriers have glucose hypometabolism in regions similar to the change in glucose patterns in AD [117], including regions in the parietal, temporal, and prefrontal areas, with the posterior cingulate cortex (PCC) representing the most significant region [118]. This association could be observed in cognitively normal individuals as early as 30 years of age [119]. As a risk factor, an increase in the number of ε4 alleles will result in worse glucose metabolism with increasing age, and this gene dose effect may be more severe in women [120]. A few researchers reviewed articles on APOE ε4 and metabolism, suggesting that the dysfunction of many brain parameters related to metabolism may reflect an inherent dysregulation of glucose metabolism in ε4 carriers [121]. For instance, Zhao et al. reported that ε4 may impair cerebral insulin signaling, which could partly explain the way ε4 affects glucose metabolism [122]. Moreover, the association between ε4 and the reduced mitochondrial cytochrome oxidase activity observed in the PCC may also contribute to this abnormal energy metabolism in the brain [123].
APOE can also influence brain structure to a certain extent, including the atrophy of the hippocampus, amygdala, entorhinal cortex, and total brain volume. Additionally, increases in white-matter hyperintensity volumes are often reported in many studies [124]. Among the gray-matter atrophy associated with ε4, the hippocampal region was the most frequently mentioned. A meta-analysis conducted by Liu and colleagues found a statistically significant association between the ε4 allele and hippocampal atrophy in six cross-sectional studies that included both healthy elderly subjects and MCI/AD subjects [100]. They further confirmed this association between ε4 and hippocampal atrophy in late MCI and AD patients, with a gene dose effect even shown in the late MCI patients [111]. Other studies also reported that increasing ε4 gene dose caused hippocampal atrophy [125,126]. Atrophy is not restricted to the hippocampus. A previous study also found an association of the ε4 allele with increased susceptibility of the temporal cortex and decreased vulnerability in the frontoparietal neocortical regions in AD patients [127,128,129]. However, at the same time, many studies showed that ε4 and gray-matter atrophy were not always closely related. Several studies compared the gray-matter volume of ε4 carriers and non-carriers and found no difference [130,131], especially in young children and elderly with advanced age [132,133]. This phenomenon makes researchers suggest that the effect of ε4 may not be detectable when the subjects are young, and, in old age, the shrinkage caused by age may mask the effect of ε4 [124]. The effect of ε4 on brain structure may also be an example of antagonistic pleiotropy. However, considering that reports also showed negative results in middle-aged adults [134], another suggestion is that the atrophy influenced by ε4 may only occur more proximal to the onset of clinical symptoms of dementia [133], which is in agreement with the close relationship between ε4 and AD. The study of white matter is very similar to that of gray matter, and both positive and negative results were reported. The area impaired by ε4 was mainly centered in regions around the medial temporal lobe, with decreased white-matter fractional anisotropy (FA) in the left parahippocampal gyrus [135], limbic, and medial temporal regions [136], decreased mean diffusivity (MD) in the corona radiate and corpus callosum, and decreased axial diffusivity (AD) in the genu of corpus callosum [137]. Since APOE has effects on the brain vascular system [121], it may be one of the ε4-mediated pathways that impairs white matter, and some cerebrovascular diseases could accompany these effects [138].
Functional MRI (fMRI) can reflect brain activity both in a resting state and while performing a task. The first fMRI study that investigated gene effects on brain function was conducted by Smith and colleagues in 1999, who reported decreased brain activation during a visual task in ε4 carriers [139]. Trachtenberg and colleagues reviewed 27 fMRI studies before 2012 and concluded that ε4 carriers can present both decreased and increased brain activation compared to non-carriers, and the location of change was inconsistent as well. They thought the reasons for these inconsistent findings regarding the direction and location of activation among studies included the different tasks used in the studies, the different family history of AD of the participants, and the age difference [140]. The ε4 carriers exhibited damaged connectivity between the precuneus and several regions during a memory encoding task [141], but recruited more regions in low load and displayed fewer increases in activation in high load in an n-back working task [142]. Old age was associated with increased activity in ε4 carriers in a face–name task [143]. Brain activity is susceptible to many factors during task fMRI; it is quite difficult to identify a consistent pattern; however, with an increasing number of resting state studies in recent years, the functional connectivity (FC) related to certain areas is more consistently affected. The default mode network (DMN) was the most affected network; both decreased FC inside the DMN [144] and decreased FC between the DMN and other networks [145] were found in different studies. FC alterations between the hippocampus or parahippocampal region and other brain regions were also frequently reported [146,147,148,149]. At the same time, there are still other areas with both increased and decreased FC characteristics reported in the literature [150,151,152]. Although we excluded the effect of task paradigm in resting fMRI, the differences in age, disease stage, and sex were still easily unified in different research samples.
3.2. Promoter Polymorphisms of the APOE Gene
In addition to the impacts on cognitive function and risk for AD mentioned above, polymorphisms in the promoter region of the APOE gene influence brain structure and function. Lambert and colleagues measured the Aβ load in Brodman areas 8 and 9 in 74 AD patients with different promoter polymorphisms. Both rs449647 AA carriers and rs405509 TT carriers showed a significant increase in the Aβ load of APOE ε4 non-carriers compared to that of carriers [153]. Another study in elderly people without dementia also found that the rs449647 AA carriers had significantly increased Aβ deposition [154]. However, at the same time, it is noteworthy that there is study a reporting conflicting results, which showed that the severity of cerebral amyloid angiopathy was not affected by rs449647 and rs405509 genotype [155]. Our recent work systematically studied the impact of rs405509 on brain structure and function. We observed that there was a significant interaction between rs405509 and APOE on general mental status in a sample of 836 community-based elderly people, and a significant interaction between rs405509 and APOE on the right inferior temporal gyrus and right fusiform gyrus in 102 people who had an MRI scan. The carriers of both ε4 and rs405509 T had the smallest gray-matter volume [68]. The interaction between rs405509 and age was also demonstrated in 120 people without dementia. The carriers of the rs405509 TT genotype showed a steeper decline with aging than the G carriers, and the cortical thickness covariance between several brain regions was also modulated by the interaction of the rs405509 genotype and age [67]. The same interaction effect was observed in the white-matter network; the rs405509 TT carriers had reduced global and local efficiency, mainly in the left anterior and posterior cingulate cortices [65], and decreased network betweenness centrality in the left inferior frontal gyrus pars opercularis, the left posterior cingulate cortex, the right inferior occipital gyrus, and the left angular gyrus [66]. Additionally, resting-state fMRI revealed that rs405509 also significantly interacted with APOE in the anterior cingulate gyrus, medial frontal region, and precuneus in the anterior and posterior DMN, with both TT and ε4 carriers mostly impairing the DMN [156]. The results listed above suggest that the promoter of APOE can significantly affect the structure and function of the brain. However, only a few studies related to APOE were performed, which limits our understanding of its importance.
3.3. Genetic Association with the APOE Gene
Similar to the cognitive function we mentioned above, the interaction between other genes and APOE can also be demonstrated with neuroimaging (Table 1). Elderly people who carry both CHRNA4 (a nicotinic receptor subunit gene) TT and APOE ε4 showed slower reaction time and lower white-matter volume in a visuospatial attention task [157]. The risk allele of the PICALM gene in rs3851179 may also interact with APOE ε4 to cause gray-matter volume impairment of the prefrontal cortex [158]. Carrying a risk allele of the CR1 rs3818361 results in a reduced brain amyloid burden compared to non-carriers, but only in ε4 non-carriers; APOE ε4 individuals show a significantly increased brain amyloid burden [159]. Liu and colleagues found that the effects of APOE ε4 on the activation of the triangular part of the right inferior frontal gyrus were modulated by rs2618516 in the spondin 1 (SPON1) gene in a working memory task [160]. Another study reported alterations of FC between the hippocampus and inferior frontal gyrus in a resting-state network in people who carry both the G allele of the SORL1 gene and the ε4 allele of APOE [161]. In addition, significant APOE risk-allele-dependent reduction in the brain FC density in the dorsolateral prefrontal cortex can be observed in carriers of the TT allele of the kidney and brain expressed protein (KIBRA) gene, but a significant APOE risk-allele-dependent increase in the brain FC density was observed in carriers of the C allele of KIBRA [162]. At the same time, people with both APOE ε4 and KIBRA C were observed to have significant hippocampal atrophy [163]. In addition to the genes we described above, there are still many other genes that have significant interactions with APOE, and their effects on neuroimaging are also worth further exploring. All of these results suggest that different AD risk genes may interact with each other, and this interaction can cause many observable effects on neuroimaging. It is important to consider the interaction between genes when we discuss the influence of innate factors on AD pathology, and the underlying mechanism still needs to be further studied.
3.4. Polygenic Risk for the Brain
In recent years, polygenic risk was frequently applied to AD studies because it maximizes the impact of all genetic factors. An increasing number of studies proved that polygenic risk can cause significant changes in brain structure and function.
Sleegers and colleagues found that PGS consisting of 22 SNPs was negatively correlated with the density of Aβ42 in the CSF in a sample of elderly people in Belgium [89]. The same negative correlation was also found in a Dutch study with 22 SNPs, coupled with a significant correlation between PGS and the density of tau in the CSF [164]. Louwersheimer and colleagues reported that the density of tau and p-tau in CSF was significantly correlated with PGS consisting of 18 SNPs, not including APOE, in a group of MCI patients [97]. In another study, Voyle and colleagues used case control PGS (APOE excluded) to predict CSF tau and Aβ, and they found it to be more predictive of Aβ and tau pathologies than the predictors that consisted of age, sex, and APOE genotype, although marginally [165]. This correlation was much stronger in a study of cognitively normal elderly subjects and MCI patients; the PGS consisted of 31 SNPs (APOE excluded) that were strongly associated with CSF Aβ, CSF tau, Aβ deposition load, neurofibrillary tangles, and rapid longitudinal clinical progression [166]. However, not all of the results showed a significant correlation. Darst and colleagues found that pathway-specific PGS consisting of genes involved in Aβ clearance, cholesterol metabolism, and immune response cannot provide increased predictive power for CSF Aβ, neurodegeneration, and tau pathology after excluding APOE [167]. The inconsistent results among different studies may be because of the different SNPs used in each study and the different sample characteristics, which should be addressed in further studies.
Structural MRI and functional MRI research provided more evidence about the influence of polygenic risk on the brain. Sabuncu and colleagues used PGS consisting of 31 SNPs (including APOE) to investigate the relationship of polygenic risk with cortical thickness in seven AD-specific regions. They found that PGS was significantly correlated with the thickness of these regions, including the entorhinal area and part of the temporoparietal area [168]. Additionally, PGSs consisting of nine SNPs related to inflammation or immunity were reported to be significantly correlated with the thickness of the regional cortex in a group of healthy older people [169]. In some specific regions, an association between PGS and precuneus volume was found in a recent study [170]; however, the most frequently reported structural regions that were affected by PGS were the entorhinal cortex and hippocampus. Harrison and colleagues calculated PGS that represented APOE, CLU, PICALM, and family history, and found that both weighted and unweighted PGSs were strongly related to the change in the thickness of the hippocampus and entorhinal cortex [171]. The volume loss in these two regions was also reported in an ADNI study, and the PGS consisted of 31 SNPs in this study [172]. Lupton and colleagues once again highlighted the sensitivity of the hippocampus. They examined the association of the hippocampus and amygdaloid volumes, and the PGS consisted of 19 SNPs that did not include APOE. They found a significant correlation between hippocampus volume and PGS in healthy old people and in MCI patients, but not in healthy young people [173]. However, the effect of PGS on the hippocampal function of healthy young people was found in an fMRI study [174]. The influence of PGS is also reflected with white-matter disruption. Foley and colleagues not only reported lower hippocampal volume with higher PGS, even when excluding the effect of APOE, but also reported that the FA of the right cingulum was inversely correlated with PGS [175]. As of now, the number of neuroimaging studies on PGS risk is still insufficient, especially studies examining white matter and brain functional activity. We expect these future studies to provide a deeper understanding of the pathology of PGS through neuroimaging.
4. Discussion
In this review, we examined the effects of genetic factors on AD risk, cognition, and the brain. The ε4 allele of APOE not only increases the risk of AD, but also reduces the age of onset of AD. Behavioral studies showed that the ε4 allele is significantly associated with decreased cognitive performance (especially memory) and with cognitive decline. Imaging studies also showed that ε4 is associated with changes in the brain internal environment (Aβ deposition, CSF biomarkers, and glucose metabolism), gray-matter atrophy, white-matter damage, brain activation, and brain connectivity. The detrimental effect of the APOE ε4 allele in the aged was confirmed. In addition to the ε4 allele, polymorphisms within the APOE promoter region are also associated with the risk of AD, cognition, and brain changes. However, there are few related studies, and the promoter region also has a complex effect on the APOE genotype; therefore, further research is needed. In addition, some other genetic factors also interact with the APOE gene and produce a complex gene–gene effect. Both AD and individual cognitive function are affected by multiple genes. Considering the complex effects of different genes, PGS seems to be an appropriate method to comprehensively investigate genetic factors. This method was also applied to cognitive and brain research by increasing numbers of researchers. Next, we conduct a simple discussion based on past research and make recommendations for future research.
4.1. Study Sample
4.1.1. Age
Age is one of the most important factors that can significantly influence the effect of the expression of APOE and other risk genes. The negative influence of APOE ε4 on cognition increases with age [176]. There is evidence demonstrating that APOE has no association with cognition or AD risk in the young [177] or the oldest old people [178,179]. The effects of genetics on neuroimaging are more likely to be observed at specific ages [124]. This may be because the effect of APOE ε4 may not be detectable when the subjects are young, and, in old age, the impairment caused by age may mask the effect of APOE ε4. However, there were also studies reporting a protective effect of APOE ε4 on cognition in children and young adults, suggesting that APOE ε4 carriers may have superior cognitive performance than non-carriers [180]. An “antagonistic pleiotropy” theory was used to explain this phenomenon, which represents a positive effect of a gene in early life but a negative effect of the gene later in life [181]. Superior cognitive performance in the oldest old were also reported, which makes the APOE ε4 allele seem protective [182]. However, researchers believe that this is due to “selective survivors”, and survivors may have a more positive effect in the absence of other AD risk factors or the existence of other protective factors [183]. In addition, the cognitive domain that was significantly affected by APOE ε4 differed in age. Kim and colleagues found that language, visuospatial, and frontal function were affected by APOE4 in subjects younger than 75 years, but memory was the most affected function in people older than 75. Additionally, a genotype × age interaction was also found in the same study [41]. After controlling for the status of the APOE genotype, the APOE promoter caused a decline in global cognition, memory, processing speed, and executive function, which were effects that were also age-related [66]. The gene dose effect of APOE improved with increasing age as well [120]. Regarding the causes of this series of phenomena, we believe aging is one of the unavoidable interfering factors. Since aging causes similar cognition and brain impairments, it is important for researchers to distinguish the effects of aging and AD genetic risk factors. Only in this way can the effect of APOE and other risk genes on the pathology of AD be accurately described.
4.1.2. Family History
In the process of summarizing these studies, we found that the differences in family history may also influence the role of the APOE gene in the pathological development of AD. Bloss and colleagues found that, in children, carrying APOE ε4 had no effect on cognition; however, those children with both APOE ε4 and a family history had significantly poorer performance on cognitive tests [184]. Increased activation in the hippocampus, posterior cingulate, and temporoparietal regions was also found in older people with both APOE ε4 and family history [185]. However, the effect was sometimes reversed. In an episodic memory task, APOE ε4 carriers without a family history of AD showed increased MTL activation, but those with a family history of AD showed the least activation in the same region [186]. A family history is always strongly linked with genes. The interaction between the APOE gene and family history may actually be an interaction between genes. Due to the development of polygenic testing, we observed an increasing number of genes that interacted with the APOE gene, such as BDNF [71], COMT [70], and the APOE promoter [68]. However, improved models are still needed to shed more light on the relationship between family history and these risk factors.
4.1.3. Other Diseases
The APOE gene is not the only genetic risk factor for AD; also, it is not the only gene associated with AD. Many other neurological disorders were confirmed to be affected by APOE. Compared to non-carriers, APOE ε4 carriers with traumatic brain injury had significantly increased Aβ deposition [187], and they may also suffer from poor neurological outcomes [188]. People with both APOE ε4 and type 2 diabetes had a significantly higher risk of AD than non-carriers [189]. However, at the same time, diabetes was associated with the non-APOE genotype in an AD patient group [190]. The coexistence of APOE ε4 and hypertension was associated with worse cognitive function compared to those with neither or either alone [191]. As exhibited by stroke, vascular dementia, multiple sclerosis, Parkinson’s disease, dementia with Lewy bodies, and so on, there are many other neurological disorders that are affected by the APOE gene or that can interact with the APOE gene [138]. It is more complicated with polygenes, and these genes may all be closely related to different diseases. Those risk genes involved in cholesterol metabolism, such as CLU, ATP-binding cassette subfamily A member 7 (ABCA7), and SORL1, may also be associated with hyperlipidemia; those risk genes involved in immune response, such as CR1, CD33, MS4A, TREM2, and CLU, may also be associated with diseases related to the immune system [192]. Therefore, the approach to identifying pathways related to the pathogenesis of AD from complex gene effects is particularly important in AD research. However, the pathological theory of AD is still controversial, and it is difficult to completely separate some diseases from AD.
4.2. Methodological Issue
4.2.1. Uniformity
The very large discrepancy between study sample characteristics makes it particularly difficult to compare the results of different studies. The inclusion and exclusion criteria of participants, the ethnic composition of the research sample, the family history of AD of each subject, and even the sex ratio can make a difference in the results. The neuropsychological tests used in studies were sometimes different; for example, different memory tests could focus on different types of memory or different components of memory, and the reliability and validity may also differ between these tests. These factors make comparisons between studies inconclusive, although they all measure memory function. Another large difference between studies is examining polygenic risk. As we can see from Table 2, the number of SNPs used to estimate the PGSs is quite different; the number could be as low as three in one study [94] to as high as 31 in another study [172]. The criteria for obtaining SNPs are also diverse from each other; they could be defined as specific SNPs [166] or they could be determined by different thresholds [169]. Some genes such as APOE, BIN1, CLU, ABCA7, CR1, PICALM, MS4A, CD33, TMM40, and CD2AP were included in the PGSs frequently, but the pathways via which they affect AD deserve further investigation. There was also a difference in whether the APOE gene was included, which could make a large difference in the results. It is difficult to resolve all the differences among studies, but we at least need to be aware of these differences when we attempt to compare the results from different studies.
Table 2.
Studies of polygenic risk on cognition and brain.
| Study | Participants | Study Design | SNP | APOE | Conversion Risk | Cognitive Impact | Neuroimaging Impact |
|---|---|---|---|---|---|---|---|
| Sabuncu et al., 2012 [168] | 104 CN (75.9 ± 5.1) and 100 AD (75.1 ± 7.8) | Cross-sectional study | 26 | N | The PGS was significantly associated with CDR-SB, MMSE, and AD diagnosis. | AD-specific cortical thickness was correlated with the PGS, even after controlling for APOE genotype and CSF levels of Aβ42. The association remained significant in CN subjects with levels of CSF Aβ42 in the normal range and in APOE ε3 homozygotes. | |
| Rodriguez-Rodriguez et al., 2013 [90] | 228 MCI | Longitudinal study (26.3 months) | 8 | N | PGS was not associated with risk of conversion from MCI to AD. MCI-converters to AD harboring six or more risk alleles progressed twofold more rapidly to AD when compared with those with less than six risk alleles. | ||
| Verhaaren et al., 2013 [91] | Non-demented 5171 (age range 45–99) | Cross-sectional study | 12 | Y | PGS was primarily associated with memory. | ||
| Marden et al., 2014 [92] | 10401 (memory score sample), 7690 (AD probability scores) non-Hispanic white and black | Cross-sectional study | 10 | Y | Each 0.10 unit change in PGS was associated with larger relative effects on dementia among aged 65+. | Each 0.10 unit change in the PGS was associated with a −0.07 standard deviation difference in memory score among aged 50+. | |
| Carrasquillo et al., 2015 [99] | CN 2674 | Longitudinal study | 10 | Y | PGS was associated with progression to MCI/LOAD. | PGS was associated with worse memory. | |
| Martiskainen et al., 2015 [164] | 890 AD (69.8 ± 8.2) and 701 CN (69.1 ± 6.2) | Cross-sectional study | 22 | Y/N | PGS associated with CSF Aβ42 levels in the clinical cohort, and with soluble Aβ42 levels and γ-secretase activity in the neuropathological cohort. The γ-secretase effect was independent of APOE. | ||
| Xiao et al., 2015 [94] | 459 AD (71.2 ± 9.6), 751 CN (72.7 ± 5.9) Chinese | Cross-sectional study | 3 | N | PGS significantly associated with AD risk. | ||
| Sleegers et al., 2015 [89] | 1162 AD (74.4 ± 8.9) and 1019 CN (76.2 ± 8.5) | Cross-sectional study | 22 | Y | Risk of AD increased with PGS; onset age decreased with increasing PGS. | CSF Aβ42 decreased with increasing PGS. | |
| Andrews et al., 2016 [98] | Non-demented 1689 (62.54 ± 1.51) | Longitudinal study | 12 | Y | PGS was associated with worse performance on episodic memory. | ||
| Harrison et al., 2016 [171] | 66 baseline participants (63.0 ± 10.4) and 45 follow-up participants (63.2±7.8) | Longitudinal study (2 years) | 21 | Y | Both unweighted risk score and weighted risk score correlated strongly with the percentage change in thickness across the whole hippocampal complex, driven by a strong relationship to entorhinal cortex thinning. By contrast, at baseline, the risk scores showed no relationship to thickness in any hippocampal complex subregion. | ||
| Louwersheimer et al., 2016 [97] | 1730 MCI from 4 independent datasets | Longitudinal study | 18 | N | PGS was modestly associated with cognitive decline over time. | PGS was modestly associated with CSF levels of tau and p-tau. | |
| Lupton et al., 2016 [173] | 1674 older (aged >53 years; 17% AD, 39% MCI) and 467 young (16–30 years) adults | Cross-sectional study | Different thresholds | N | PGS associated with reduced hippocampal volume in older CN and MCI. No associations were found in young adults. | ||
| Marden et al., 2016 [93] | 8253 non-Hispanic whites and blacks | Longitudinal study | 22 | Y/N | PGS can predict a more rapid decline in memory in whites and blacks; PGS without APOE ε4 only can predict memory decline in whites. | ||
| Darst et al., 2017 [167] | 1200 at baseline (53.6 ± 6.6) | Longitudinal study | 21 | Y | Non-significant for associations between the PGS and cognitive outcomes. | These additional variants did not add much predictive power over APOE alone on biomarkers of Aβ deposition, neurodegeneration and tau pathology. | |
| Desikan et al., 2017 [172] | More than 80,000 people from two projects | Longitudinal study | 31 | N | ADGC Phase 1: highest PGS quartile, lower age onset and the highest yearly AD incidence rate. APOE ε3/3 individuals: PGS modified expected age of AD onset by more than 10 years between the lowest and highest deciles. Independent cohorts: PGS strongly predicted empirical age of AD onset and longitudinal progression. | PGS was associated with neuropathology (Braak stage of neurofibrillary tangles and Consortium to Establish a Registry for Alzheimer’s Disease score for neurotic plaques) and in vivo markers of AD neurodegeneration (volume loss within the entorhinal cortex and hippocampus) | |
| Foley et al., 2017 [175] | 272 T1 (24.8 ± 6.9), 197 DTI (23.9 ± 5.1), 87 Hopkins Verbal Learning Task (23.9 ± 4.4) | Cross-sectional study | 7 thresholds | Y/N | A significant association between PGS and left hippocampal volume; this effect remained when the APOE gene was excluded. The fractional anisotropy of the right cingulum was inversely correlated with PGS. | ||
| Lacour et al., 2017 [96] | 4 MCI groups 853/812/1245/306 | Longitudinal study | 9 | N | PGS predicted a small effect on the risk of MCI to AD progression in APOE ε4 carriers. | ||
| Voyle et al., 2017 [165] | About 250 people with normal and abnormal CSF Aβ from ADNI | Cross-sectional study | − | N | A case/control PGS is marginally more predictive of Aβ and tau pathology than the basic models (with age, gender and APOE genotype). | ||
| Xiao et al., 2017 [174] | 231 CN (age range 19–55) | Cross-sectional study | 6 thresholds | N | Almost no significant association of PGS with cognition. | There was a significant negative relationship between PGS and hippocampal function. | |
| Ge et al., 2018 [104] | 702 participants (221 CN, 367 MCI, and 114 AD) and a subset of 669 participants | Longitudinal study | Different thresholds | N | Only weak associations between PGS and baseline Aβ were present. PGSs were associated with hippocampal atrophy in Aβ− and weakly associated with baseline hippocampal volume in Aβ+. | ||
| Kauppi et al., 2018 [193] | 336 MCI (baseline age range 55–89) | Longitudinal study (3 year) | 31 | Y | PGS significantly predicted time to progression from MCI to AD over 120 months, and PGS was significantly more predictive than APOE alone. | PGS improved the prediction of change in the CDR-SB score and MMSE over 36 months in MCI at baseline, beyond both APOE and baseline levels of brain atrophy. | |
| Li et al., 2018 [170] | 360 CN (19.4 ± 1.1) in discovery dataset and 323 CN (22.7 ± 2.5) in replication dataset | Cross-sectional study | − | Y/N | No correlation between PGS and any cognitive measure in either sample. | In both cohorts, an elevated PGS was associated with a smaller precuneal volume, and the effect remained after excluding the APOE genotype. | |
| Lin et al., 2019 [194] | 2907 stroke-free individuals (76.73 ± 5.83) | Cross-sectional study | 3 thresholds | Y/N | PGSs were associated with lobar cerebral microbleeds, white-matter lesion load, and coronary artery calcification, mostly explained by single-nucleotide polymorphism in the APOE region. The effect of PGS on cognition was partially but significantly mediated by cerebral microbleeds, white-matter lesions, and coronary artery calcification. | ||
| Tan et al., 2018 [166] | 347 CN (baseline age range 59.7–90.1), 599 MCI (baseline age range 54.4–91.4), and 485 (age at death range = 71.3–108.3) in another cohort | Longitudinal study | 31 | N | Even after accounting for APOE ε4 effects, PGS may be useful in MCI and preclinical AD therapeutic trials to enrich for biomarker-positive individuals at highest risk for short-term clinical progression. |
CN, cognitive normal; MCI, mild cognitive impairment; AD, Alzheimer’s disease; PGS: polygenic risk score; Y, APOE included in PGS; N, APOE not included in PGS; Y/N, Both situations of APOE included and not in PGS; CDR-SB, Clinical Dementia Rating Sum of Boxes; MMSE, Mini-Mental State Examination; CSF, cerebrospinal fluid; ADGC, Alzheimer’s Disease Genetics Consortium.
4.2.2. Study Design
Longitudinal studies are needed. The effect of APOE ε4 is different depending on disease stage of AD [111], and longitudinal tracking of the same sample can more accurately describe the changes in the influence of the APOE gene on various biomarkers, establishing a better pathological change model. Functional MRI studies with improved designs are needed and would increase the interpretability of risk genes on specific cognitive abilities. However, there are currently few of these research designs, especially in the study of the APOE promoter, gene interaction, and polygenic risk factors. Some new techniques may also lead to interesting discoveries in this field, such as machine learning, virtual reality, and multimodal synchronous imaging. At the same time, researchers should be thinking about exploring the effect of non-risk alleles on risk genes, including the protective effect of APOE ε2 and its mechanism [195]. Some researchers found that drug interventions had better effects in people with APOE ε4 [196]. The mechanism of this effect and whether it also happens with other risk genes should be addressed by further intervention studies.
5. Conclusions
Although the precise biological changes that cause AD are still not fully revealed, genetics remains a non-negligible factor in pathogenesis. Many genes, including the APOE gene, are related to AD risk. Thus, integrating different genetic loci and investigating polygenic risk is reasonable. Although some researchers considered the association between polygenic risk, cognition, and the brain, the studies are limited. The influence and mechanism of polygenic risk on cognition and the brain are still undefined. The interaction between polygenic risk and other AD risk factors (e.g., age, cardiovascular disease risk factors, education, and social and cognitive engagement) also warrants further study. Continued investigations integrating polygenic risk, the brain, and cognition will move the field closer to revealing the mechanism of AD pathogenesis.
Author Contributions
Conceptualization, J.F., W.T., and Y.C.; paper selection, J.F., W.T., X.L., H.L., J.Z., and D.W.; writing—original draft preparation, J.F. and W.T.; writing—review and editing, X.L., H.L., J.Z., and D.W.; supervision, Y.C. and Z.Z.; funding acquisition, Z.Z. All authors read and approved the final manuscript.
Funding
This research was supported by the National Science Fund for Distinguished Young Scholars (grant number 81625025), the Funds for International Cooperation and Exchange of the National Natural Science Foundation of China (grant number 81820108034), the National Key Research and Development Project of China (grant number 2018YFC1315200), the State Key Program of National Natural Science of China (grant number 81430100), the Beijing Municipal Science & Technology Commission (grant number Z161100000216135), the National Natural Science Foundation of China (grant number 31700997 and 31500925), and the Fundamental Research Funds for the Central Universities (grant number 2017XTCX04).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Patterson C. World Alzheimer Report 2018—The State of the Art of Dementia Research: New Frontiers. Alzheimer’s Disease International (ADI); London, UK: 2018. [Google Scholar]
- 2.Liu C.C., Liu C.C., Kanekiyo T., Xu H., Bu G. Apolipoprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nat. Rev. Neurol. 2013;9:106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campion D., Dumanchin C., Hannequin D., Dubois B., Belliard S., Puel M., Thomas-Anterion C., Michon A., Martin C., Charbonnier F. Early-Onset Autosomal Dominant Alzheimer Disease: Prevalence, Genetic Heterogeneity, and Mutation Spectrum. Am. J. Hum. Genet. 1999;65:664–670. doi: 10.1086/302553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goate A., Chartier-Harlin M.C., Mullan M., Brown J., Crawford F., Fidani L., Giuffra L., Haynes A., Irving N., James L., et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature. 1991;349:704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- 5.Sherrington R., Rogaev E.I., Liang Y., Rogaeva E.A., Levesque G., Ikeda M., Chi H., Lin C., Li G., Holman K., et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature. 1995;375:754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- 6.Levy-Lahad E., Wasco W., Poorkaj P., Romano D.M., Oshima J., Pettingell W.H., Yu C.E., Jondro P.D., Schmidt S.D., Wang K., et al. Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science. 1995;269:973–977. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- 7.Gatz M., Reynolds C.A., Fratiglioni L., Johansson B., Mortimer J.A., Berg S., Fiske A., Pedersen N.L. Role of genes and environments for explaining Alzheimer disease. Arch. Gen. Psychiatry. 2006;63:168–174. doi: 10.1001/archpsyc.63.2.168. [DOI] [PubMed] [Google Scholar]
- 8.Kamboh M.I. Molecular genetics of late-onset Alzheimer’s disease. Ann. Hum. Genet. 2012;68:381–404. doi: 10.1046/j.1529-8817.2004.00110.x. [DOI] [PubMed] [Google Scholar]
- 9.Lambert J.C. Meta-analysis in more than 74,000 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Alzheimers Dement. 2013;9:1452–1458. doi: 10.1016/j.jalz.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avila J., Gomez-Ramos A., Bolos M. AD genetic risk factors and tau spreading. Front. Aging Neurosci. 2015;7:99. doi: 10.3389/fnagi.2015.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harold D., Abraham R., Hollingworth P., Sims R., Gerrish A., Hamshere M.L., Pahwa J.S., Moskvina V., Dowzell K., Williams A. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat. Genet. 2009;41:1088. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karch C.M., Goate A.M. Alzheimer’s Disease Risk Genes and Mechanisms of Disease Pathogenesis. Biol. Psychiatry. 2015;77:43–51. doi: 10.1016/j.biopsych.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zannis V.I., Just P.W., Breslow J.L. Human apolipoprotein E isoprotein subclasses are genetically determined. Am. J. Hum. Genet. 1981;33:11. [PMC free article] [PubMed] [Google Scholar]
- 14.Singh P.P., Singh M., Mastana S.S. APOE distribution in world populations with new data from India and the UK. Ann. Hum. Biol. 2006;33:279–308. doi: 10.1080/03014460600594513. [DOI] [PubMed] [Google Scholar]
- 15.Gerdes L.U., Gerdes C., Hansen P.S., Klausen I.C., Faergeman O., Dyerberg J. The apolipoprotein E polymorphism in Greenland Inuit in its global perspective. Hum. Genet. 1996;98:546–550. doi: 10.1007/s004390050257. [DOI] [PubMed] [Google Scholar]
- 16.Giau V.V., Bagyinszky E., An S.S., Kim S.Y. Role of apolipoprotein E in neurodegenerative diseases. Neuropsychiatr. Dis. Treat. 2015;11:1723–1737. doi: 10.2147/NDT.S84266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ward A., Crean S., Mercaldi C.J., Collins J.M., Boyd D., Cook M.N., Arrighi H.M. Prevalence of apolipoprotein E4 genotype and homozygotes (APOE e4/4) among patients diagnosed with Alzheimer’s disease: A systematic review and meta-analysis. Neuroepidemiology. 2012;38:1–17. doi: 10.1159/000334607. [DOI] [PubMed] [Google Scholar]
- 18.Kwon O.D., Khaleeq A., Chan W., Pavlik V.N., Doody R.S. Apolipoprotein E polymorphism and age at onset of Alzheimer’s disease in a quadriethnic sample. Dement. Geriatr. Cogn. Disord. 2010;30:486–491. doi: 10.1159/000322368. [DOI] [PubMed] [Google Scholar]
- 19.Sando S.B., Melquist S., Cannon A., Hutton M.L., Sletvold O., Saltvedt I., White L.R., Lydersen S., Aasly J.O. APOE epsilon 4 lowers age at onset and is a high risk factor for Alzheimer’s disease; a case control study from central Norway. BMC Neurol. 2008;8:9. doi: 10.1186/1471-2377-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corder E.H., Saunders A.M., Strittmatter W.J., Schmechel D.E., Gaskell P.C., Small G.W., Roses A.D., Haines J.L., Pericak-Vance M.A. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 21.Farrer L.A., Cupples L.A., Haines J.L., Hyman B., Kukull W.A., Mayeux R., Myers R.H., PericakVance M.A., Risch N., van Duijn C.M. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease—A meta-analysis. JAMA. 1997;278:1349–1356. doi: 10.1001/jama.1997.03550160069041. [DOI] [PubMed] [Google Scholar]
- 22.Petersen R.C. Mild cognitive impairment: Transition between aging and Alzheimer’s disease. Neurologia. 2000;15:93–101. doi: 10.1016/S0197-4580(00)82678-0. [DOI] [PubMed] [Google Scholar]
- 23.Ma F., Wang J. Apolipoprotein ε4-allele as a significant risk factor for conversion from mild cognitive impairment to Alzheimer’s disease: A meta-analysis of prospective studies. J. Mol. Neurosci. 2013;50:257–263. doi: 10.1007/s12031-012-9934-y. [DOI] [PubMed] [Google Scholar]
- 24.Elias-Sonnenschein L.S., Wolfgang V., Ramakers I.H.G.B., Verhey F.R.J., Pieter Jelle V. Predictive value of APOE-ε4 allele for progression from MCI to AD-type dementia: A meta-analysis. J. Neurol. Neurosurg. Psychiatry. 2011;82:1149–1156. doi: 10.1136/jnnp.2010.231555. [DOI] [PubMed] [Google Scholar]
- 25.Nickerson D.A., Taylor S.L., Fullerton S.M., Weiss K.M., Clark A.G., Stengard J.H., Salomaa V., Boerwinkle E., Sing C.F. Sequence diversity and large-scale typing of SNPs in the human apolipoprotein E gene. Genome Res. 2000;10:1532–1545. doi: 10.1101/gr.146900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richard P., Thomas G., de Zulueta M.P., De Gennes J.L., Thomas M., Cassaigne A., Bereziat G., Iron A. Common and rare genotypes of human apolipoprotein E determined by specific restriction profiles of polymerase chain reaction-amplified DNA. Clin. Chem. 1994;40:24–29. [PubMed] [Google Scholar]
- 27.Yamamura T., Dong L.M., Yamamoto A. Characterization of apolipoprotein E7 (Glu(244)-->Lys, Glu(245)--->Lys), a mutant apolipoprotein E associated with hyperlipidemia and atherosclerosis. J. Lipid Res. 1999;40:253–259. [PubMed] [Google Scholar]
- 28.Youn Y.C., Lim Y.K., Han S.-H., Giau V.V., Lee M.-K., Park K.-Y., Kim S., Bagyinszky E., An S.S.A., Kim H.R. Apolipoprotein ε7 allele in memory complaints: Insights through protein structure prediction. Clin. Interv. Aging. 2017;12:1095–1102. doi: 10.2147/CIA.S131172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bizzarro A., Seripa D., Acciarri A., Matera M.G., Pilotto A., Tiziano F.D., Brahe C., Masullo C. The complex interaction between APOE promoter and AD: An Italian case-control study. Eur. J. Hum. Genet. 2009;17:938–945. doi: 10.1038/ejhg.2008.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laws S.M., Hone E., Gandy S., Martins R.N. Expanding the association between the APOE gene and the risk of Alzheimer’s disease: Possible roles for APOE promoter polymorphisms and alterations in APOE transcription. J. Neurochem. 2003;84:1215–1236. doi: 10.1046/j.1471-4159.2003.01615.x. [DOI] [PubMed] [Google Scholar]
- 31.Artiga M.J., Bullido M.J., Sastre I., Recuero M., Garcia M.A., Aldudo J., Vazquez J., Valdivieso F. Allelic polymorphisms in the transcriptional regulatory region of apolipoprotein E gene. FEBS Lett. 1998;421:105–108. doi: 10.1016/S0014-5793(97)01543-3. [DOI] [PubMed] [Google Scholar]
- 32.Xin X.Y., Ding J.Q., Chen S.D. Apolipoprotein E promoter polymorphisms and risk of Alzheimer’s disease: Evidence from meta-analysis. J. Alzheimers Dis. 2010;19:1283–1294. doi: 10.3233/JAD-2010-1329. [DOI] [PubMed] [Google Scholar]
- 33.Xiao H., Gao Y., Liu L., Li Y. Association between polymorphisms in the promoter region of the apolipoprotein E (APOE) gene and Alzheimer’s disease: A meta-analysis. EXCLI J. 2017;16:921–938. doi: 10.17179/excli2017-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farlow M.R., He Y., Tekin S., Xu J., Xu J., Charles H.C. Impact of APOE in mild cognitive impairment. Neurology. 2004;63:1898–1901. doi: 10.1212/01.WNL.0000144279.21502.B7. [DOI] [PubMed] [Google Scholar]
- 35.El Haj M., Antoine P., Amouyel P., Lambert J.C., Pasquier F., Kapogiannis D. Apolipoprotein E (APOE) epsilon4 and episodic memory decline in Alzheimer’s disease: A review. Ageing Res. Rev. 2016;27:15–22. doi: 10.1016/j.arr.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Donoghue M.C., Murphy S.E., Zamboni G., Nobre A.C., Mackay C.E. APOE genotype and cognition in healthy individuals at risk of Alzheimer’s disease: A review. Cortex. 2018;104:103–123. doi: 10.1016/j.cortex.2018.03.025. [DOI] [PubMed] [Google Scholar]
- 37.McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Kawas C.H., Klunk W.E., Koroshetz W.J., Manly J.J., Mayeux R., et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van der Vlies A.E., Pijnenburg Y.A., Koene T., Klein M., Kok A., Scheltens P., van der Flier W.M. Cognitive impairment in Alzheimer’s disease is modified by APOE genotype. Dement. Geriatr. Cogn. Disord. 2007;24:98–103. doi: 10.1159/000104467. [DOI] [PubMed] [Google Scholar]
- 39.Snowden J.S., Stopford C.L., Julien C.L., Thompson J.C., Davidson Y., Gibbons L., Pritchard A., Lendon C.L., Richardson A.M., Varma A. Cognitive Phenotypes in Alzheimer’s Disease and Genetic Risk. Cortex. 2007;43:835–845. doi: 10.1016/S0010-9452(08)70683-X. [DOI] [PubMed] [Google Scholar]
- 40.Wolk D.A., Dickerson B.C. Apolipoprotein E (APOE) genotype has dissociable effects on memory and attentional-executive network function in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2010;107:10256–10261. doi: 10.1073/pnas.1001412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim J., Park S., Yoo H., Jang H., Kim Y., Kim K.W., Jang Y.K., Lee J.S., Kim S.T., Kim S., et al. The Impact of APOE varepsilon4 in Alzheimer’s Disease Differs According to Age. J. Alzheimers Dis. 2018;61:1377–1385. doi: 10.3233/JAD-170556. [DOI] [PubMed] [Google Scholar]
- 42.Cosentino S., Scarmeas N., Helzner E., Glymour M.M., Brandt J., Albert M., Blacker D., Stern Y. APOE epsilon 4 allele predicts faster cognitive decline in mild Alzheimer disease. Neurology. 2008;70:1842–1849. doi: 10.1212/01.wnl.0000304038.37421.cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang Y.L., Fennema-Notestine C., Holland D., McEvoy L.K., Stricker N.H., Salmon D.P., Dale A.M., Bondi M.W., Alzheimer’s Disease Neuroimaging Initiative APOE interacts with age to modify rate of decline in cognitive and brain changes in Alzheimer’s disease. Alzheimers Dement. 2014;10:336–348. doi: 10.1016/j.jalz.2013.05.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramakers I.H., Visser P.J., Aalten P., Bekers O., Sleegers K., van Broeckhoven C.L., Jolles J., Verhey F.R. The association between APOE genotype and memory dysfunction in subjects with mild cognitive impairment is related to age and Alzheimer pathology. Dement. Geriatr. Cogn. Disord. 2008;26:101–108. doi: 10.1159/000144072. [DOI] [PubMed] [Google Scholar]
- 45.Whitehair D.C., Sherzai A., Emond J., Raman R., Aisen P.S., Petersen R.C., Fleisher A.S., Alzheimer’s Disease Neuroimaging Initiative Influence of apolipoprotein E varepsilon4 on rates of cognitive and functional decline in mild cognitive impairment. Alzheimers Dement. 2010;6:412–419. doi: 10.1016/j.jalz.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Foster J.K., Albrecht M.A., Greg S., Lautenschlager N.T., Ellis K.A., Paul M., Cassandra S., Kevin T., Ralph M., Masters C.L. Lack of reliable evidence for a distinctive ε4-related cognitive phenotype that is independent from clinical diagnostic status: Findings from the Australian Imaging, Biomarkers and Lifestyle Study. Brain. 2013;136:2201–2216. doi: 10.1093/brain/awt127. [DOI] [PubMed] [Google Scholar]
- 47.Lyall D.M., Ward J., Ritchie S.J., Davies G., Cullen B., Celis C., Bailey M.E., Anderson J., Evans J., McKay D.F., et al. Alzheimer disease genetic risk factor APOE e4 and cognitive abilities in 111,739 UK Biobank participants. Age Ageing. 2016;45:511–517. doi: 10.1093/ageing/afw068. [DOI] [PubMed] [Google Scholar]
- 48.Wisdom N.M., Callahan J.L., Hawkins K.A. The effects of apolipoprotein E on non-impaired cognitive functioning: A meta-analysis. Neurobiol. Aging. 2011;32:63–74. doi: 10.1016/j.neurobiolaging.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 49.Schultz M.R., Lyons M.J., Franz C.E., Grant M.D., Boake C., Jacobson K.C., Xian H., Schellenberg G.D., Eisen S.A., Kremen W.S. Apolipoprotein E genotype and memory in the sixth decade of life. Neurology. 2008;70:1771–1777. doi: 10.1212/01.wnl.0000286941.74372.cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu F., Pardo L.M., Schuur M., Sanchez-Juan P., Isaacs A., Sleegers K., de Koning I., Zorkoltseva I.V., Axenovich T.I., Witteman J.C., et al. The apolipoprotein E gene and its age-specific effects on cognitive function. Neurobiol. Aging. 2010;31:1831–1833. doi: 10.1016/j.neurobiolaging.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 51.Kerchner G.A., Berdnik D., Shen J.C., Bernstein J.D., Fenesy M.C., Deutsch G.K., Wyss-Coray T., Rutt B.K. APOE epsilon4 worsens hippocampal CA1 apical neuropil atrophy and episodic memory. Neurology. 2014;82:691–697. doi: 10.1212/WNL.0000000000000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Espeseth T., Westlye L.T., Fjell A.M., Walhovd K.B., Rootwelt H., Reinvang I. Accelerated age-related cortical thinning in healthy carriers of apolipoprotein E ε4. Neurobiol. Aging. 2008;29:329–340. doi: 10.1016/j.neurobiolaging.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 53.Luciano M., Gow A.J., Harris S.E., Hayward C., Allerhand M., Starr J.M., Visscher P.M., Deary I.J. Cognitive ability at age 11 and 70 years, information processing speed, and APOE variation: The Lothian Birth Cohort 1936 study. Psychol. Aging. 2009;24:129. doi: 10.1037/a0014780. [DOI] [PubMed] [Google Scholar]
- 54.Izaks G.J., Gansevoort R.T., van der Knaap A.M., Navis G., Dullaart R.P.F., Slaets J.P.J. The association of APOE genotype with cognitive function in persons aged 35 years or older. PLoS ONE. 2011;6:e27415. doi: 10.1371/journal.pone.0027415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Quintino-Santos S., Diniz B.S., Firmo J.O., Moriguchi E.H., Lima-Costa M.F., Castro-Costa E. APOE epsilon4 allele is associated with worse performance in memory dimensions of the mini-mental state examination: The Bambui Cohort Study of Aging. Int. J. Geriatr. Psychiatry. 2015;30:573–579. doi: 10.1002/gps.4186. [DOI] [PubMed] [Google Scholar]
- 56.Caselli R.J., Dueck A.C., Osborne D., Sabbagh M.N., Connor D.J., Ahern G.L., Baxter L.C., Rapcsak S.Z., Shi J., Woodruff B.K., et al. Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N. Engl. J. Med. 2009;361:255–263. doi: 10.1056/NEJMoa0809437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caselli R.J., Dueck A.C., Locke D.E., Hoffman-Snyder C.R., Woodruff B.K., Rapcsak S.Z., Reiman E.M. Longitudinal modeling of frontal cognition in APOE epsilon4 homozygotes, heterozygotes, and noncarriers. Neurology. 2011;76:1383–1388. doi: 10.1212/WNL.0b013e3182167147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schiepers O.J., Harris S.E., Gow A.J., Pattie A., Brett C.E., Starr J.M., Deary I.J. APOE E4 status predicts age-related cognitive decline in the ninth decade: Longitudinal follow-up of the Lothian Birth Cohort 1921. Mol. Psychiatry. 2012;17:315–324. doi: 10.1038/mp.2010.137. [DOI] [PubMed] [Google Scholar]
- 59.Rawle M.J., Davis D., Bendayan R., Wong A., Kuh D., Richards M. Apolipoprotein-E (Apoe) epsilon4 and cognitive decline over the adult life course. Transl. Psychiatry. 2018;8:18. doi: 10.1038/s41398-017-0064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barnes L.L., Arvanitakis Z., Yu L., Kelly J., De Jager P.L., Bennett D.A. Apolipoprotein E and change in episodic memory in blacks and whites. Neuroepidemiology. 2013;40:211–219. doi: 10.1159/000342778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hendrie H.C., Murrell J., Baiyewu O., Lane K.A., Purnell C., Ogunniyi A., Unverzagt F.W., Hall K., Callahan C.M., Saykin A.J., et al. APOE epsilon4 and the risk for Alzheimer disease and cognitive decline in African Americans and Yoruba. Int. Psychogeriatr. 2014;26:977–985. doi: 10.1017/S1041610214000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lipnicki D.M., Crawford J.D., Dutta R., Thalamuthu A., Kochan N.A., Andrews G., Lima-Costa M.F., Castro-Costa E., Brayne C., Matthews F.E., et al. Age-related cognitive decline and associations with sex, education and apolipoprotein E genotype across ethnocultural groups and geographic regions: A collaborative cohort study. PLoS Med. 2017;14:e1002261. doi: 10.1371/journal.pmed.1002261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tuminello E.R., Han S.D. The apolipoprotein e antagonistic pleiotropy hypothesis: Review and recommendations. Int. J. Alzheimers Dis. 2011;2011:726197. doi: 10.4061/2011/726197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lancaster C., Tabet N., Rusted J. The APOE paradox: Do attentional control differences in mid-adulthood reflect risk of late-life cognitive decline. Neurobiol. Aging. 2016;48:114–121. doi: 10.1016/j.neurobiolaging.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 65.Shu N., Li X., Ma C., Zhang J., Chen K., Liang Y., Chen Y., Zhang Z. Effects of APOE promoter polymorphism on the topological organization of brain structural connectome in nondemented elderly. Hum. Brain Mapp. 2015;36:4847–4858. doi: 10.1002/hbm.22954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chang P., Li X., Ma C., Zhang S., Liu Z., Chen K., Ai L., Chang J., Zhang Z. The Effects of an APOE Promoter Polymorphism on Human White Matter Connectivity during Non-Demented Aging. J. Alzheimers Dis. 2017;55:77–87. doi: 10.3233/JAD-160447. [DOI] [PubMed] [Google Scholar]
- 67.Chen Y., Li P., Gu B., Liu Z., Li X., Evans A.C., Gong G., Zhang Z. The effects of an APOE promoter polymorphism on human cortical morphology during nondemented aging. J. Neurosci. 2015;35:1423–1431. doi: 10.1523/JNEUROSCI.1946-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ma C., Zhang Y., Li X., Zhang J., Chen K., Liang Y., Chen Y., Liu Z., Zhang Z. Is there a significant interaction effect between apolipoprotein E rs405509 T/T and epsilon4 genotypes on cognitive impairment and gray matter volume? Eur. J. Neurol. 2016;23:1415–1425. doi: 10.1111/ene.13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rantalainen V., Lahti J., Henriksson M., Kajantie E., Tienari P., Eriksson J.G., Raikkonen K. APOE and aging-related cognitive change in a longitudinal cohort of men. Neurobiol. Aging. 2016;44:151–158. doi: 10.1016/j.neurobiolaging.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 70.Martinez M.F., Martin X.E., Alcelay L.G., Flores J.C., Valiente J.M., Juanbeltz B.I., Beldarrain M.A., Lopez J.M., Gonzalez-Fernandez M.C., Salazar A.M., et al. The COMT Val158 Met polymorphism as an associated risk factor for Alzheimer disease and mild cognitive impairment in APOE 4 carriers. BMC Neurosci. 2009;10:125. doi: 10.1186/1471-2202-10-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ward D.D., Summers M.J., Saunders N.L., Janssen P., Stuart K.E., Vickers J.C. APOE and BDNF Val66Met polymorphisms combine to influence episodic memory function in older adults. Behav. Brain Res. 2014;271:309–315. doi: 10.1016/j.bbr.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 72.Gomar J.J., Conejero-Goldberg C., Huey E.D., Davies P., Goldberg T.E., Alzheimer’s Disease Neuroimaging Initiative Lack of neural compensatory mechanisms of BDNF val66met met carriers and APOE E4 carriers in healthy aging, mild cognitive impairment, and Alzheimer’s disease. Neurobiol. Aging. 2016;39:165–173. doi: 10.1016/j.neurobiolaging.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Persson N., Lavebratt C., Wahlin A. Synergy effects of HbA1c and variants of APOE and BDNFVal66Met explains individual differences in memory performance. Neurobiol. Learn. Mem. 2013;106:274–282. doi: 10.1016/j.nlm.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 74.Thow M.E., Summers M.J., Summers J.J., Saunders N.L., Vickers J.C. Variations in the APOE allele or BDNF Val66Met polymorphism are not associated with changes in cognitive function following a tertiary education intervention in older adults: The Tasmanian Healthy Brain Project. Neurobiol. Aging. 2017;55:175–176. doi: 10.1016/j.neurobiolaging.2017.03.028. [DOI] [PubMed] [Google Scholar]
- 75.Wang P.N., Liu H.C., Liu T.Y., Chu A., Hong C.J., Lin K.N., Chi C.W. Estrogen-metabolizing gene COMT polymorphism synergistic APOE epsilon4 allele increases the risk of Alzheimer disease. Dement. Geriatr. Cogn. Disord. 2005;19:120–125. doi: 10.1159/000082663. [DOI] [PubMed] [Google Scholar]
- 76.Sapkota S., Backman L., Dixon R.A. Executive function performance and change in aging is predicted by apolipoprotein E, intensified by catechol-O-methyltransferase and brain-derived neurotrophic factor, and moderated by age and lifestyle. Neurobiol. Aging. 2017;52:81–89. doi: 10.1016/j.neurobiolaging.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yu C.-E., Seltman H., Peskind E.R., Galloway N., Zhou P.X., Rosenthal E., Wijsman E.M., Tsuang D.W., Devlin B., Schellenberg G.D. Comprehensive analysis of APOE and selected proximate markers for late-onset Alzheimer’s disease: Patterns of linkage disequilibrium and disease/marker association. Genomics. 2007;89:655–665. doi: 10.1016/j.ygeno.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roses A.D., Lutz M.W., Amrine-Madsen H., Saunders A.M., Crenshaw D.G., Sundseth S.S., Huentelman M.J., Welsh-Bohmer K.A., Reiman E.M. A TOMM40 variable-length polymorphism predicts the age of late-onset Alzheimer’s disease. Pharmacogenom. J. 2009;10:375. doi: 10.1038/tpj.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lutz M.W., Crenshaw D., Welsh-Bohmer K.A., Burns D.K., Roses A.D. New Genetic Approaches to AD: Lessons from APOE-TOMM40 Phylogenetics. Curr. Neurol. Neurosci. Rep. 2016;16:48. doi: 10.1007/s11910-016-0643-8. [DOI] [PubMed] [Google Scholar]
- 80.Johnson S.C., Rue A.L., Hermann B.P., Xu G., Koscik R.L., Jonaitis E.M., Bendlin B.B., Hogan K.J., Roses A.D., Saunders A.M. The effect of TOMM40 poly-T length on gray matter volume and cognition in middle-aged persons with APOE ε3/ε3 genotype. Alzheimers Dement. 2011;7:456–465. doi: 10.1016/j.jalz.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yu L., Lutz M.W., Wilson R.S., Burns D.K., Roses A.D., Saunders A.M., Gaiteri C., De Jager P.L., Barnes L.L., Bennett D.A. TOMM40’523 variant and cognitive decline in older persons with APOE epsilon3/3 genotype. Neurology. 2017;88:661–668. doi: 10.1212/WNL.0000000000003614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Louwersheimer E., Cohn-Hokke P.E., Pijnenburg Y.A., Weiss M.M., Sistermans E.A., Rozemuller A.J., Hulsman M., van Swieten J.C., van Duijn C.M., Barkhof F., et al. Rare Genetic Variant in SORL1 May Increase Penetrance of Alzheimer’s Disease in a Family with Several Generations of APOE-varepsilon4 Homozygosity. J. Alzheimers Dis. 2017;56:63–74. doi: 10.3233/JAD-160091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Barral S., Bird T., Goate A., Farlow M.R., Diaz-Arrastia R., Bennett D.A., Graff-Radford N., Boeve B.F., Sweet R.A., Stern Y., et al. Genotype patterns at PICALM, CR1, BIN1, CLU, and APOE genes are associated with episodic memory. Neurology. 2012;78:1464–1471. doi: 10.1212/WNL.0b013e3182553c48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gharesouran J., Rezazadeh M., Khorrami A., Ghojazadeh M., Talebi M. Genetic evidence for the involvement of variants at APOE, BIN1, CR1, and PICALM loci in risk of late-onset Alzheimer’s disease and evaluation for interactions with APOE genotypes. J. Mol. Neurosci. 2014;54:780–786. doi: 10.1007/s12031-014-0377-5. [DOI] [PubMed] [Google Scholar]
- 85.Keenan B.T., Shulman J.M., Chibnik L.B., Raj T., Tran D., Sabuncu M.R., Alzheimer’s Disease Neuroimaging Initiative. Allen A.N., Corneveaux J.J., Hardy J.A., et al. A coding variant in CR1 interacts with APOE-epsilon4 to influence cognitive decline. Hum. Mol. Genet. 2012;21:2377–2388. doi: 10.1093/hmg/dds054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liao Y.C., Lee W.J., Hwang J.P., Wang Y.F., Tsai C.F., Wang P.N., Wang S.J., Fuh J.L. ABCA7 gene and the risk of Alzheimer’s disease in Han Chinese in Taiwan. Neurobiol. Aging. 2014;35:2423. doi: 10.1016/j.neurobiolaging.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 87.Casati M., Ferri E., Gussago C., Mazzola P., Abbate C., Bellelli G., Mari D., Cesari M., Arosio B. Increased expression of TREM2 in peripheral cells from mild cognitive impairment patients who progress into Alzheimer’s disease. Eur. J. Neurol. 2018;25:805–810. doi: 10.1111/ene.13583. [DOI] [PubMed] [Google Scholar]
- 88.Jendresen C., Arskog V., Daws M.R., Nilsson L.N.G. The Alzheimer’s disease risk factors apolipoprotein E and TREM2 are linked in a receptor signaling pathway. J. Neuroinflamm. 2017;14:59. doi: 10.1186/s12974-017-0835-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sleegers K., Bettens K., De Roeck A., Van Cauwenberghe C., Cuyvers E., Verheijen J., Struyfs H., Van Dongen J., Vermeulen S., Engelborghs S., et al. A 22-single nucleotide polymorphism Alzheimer’s disease risk score correlates with family history, onset age, and cerebrospinal fluid Abeta42. Alzheimers Dement. 2015;11:1452–1460. doi: 10.1016/j.jalz.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 90.Rodríguez-Rodríguez E., Sánchez-Juan P., Vázquez-Higuera J.L., Mateo I., Pozueta A., Berciano J., Cervantes S., Alcolea D., Martinez-Lage P., Clarimón J. Genetic risk score predicting accelerated progression from mild cognitive impairment to Alzheimer’s disease. J. Neural Transm. 2013;120:807–812. doi: 10.1007/s00702-012-0920-x. [DOI] [PubMed] [Google Scholar]
- 91.Verhaaren B.F.J., Vernooij M.W., Koudstaal P.J., Uitterlinden A.G., Duijn C.M.V., Hofman A., Breteler M.M.B., Ikram M.A. Alzheimer’s Disease Genes and Cognition in the Nondemented General Population. Biol. Psychiatry. 2013;73:429–434. doi: 10.1016/j.biopsych.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 92.Marden J.R., Walter S., Tchetgen Tchetgen E.J., Kawachi I., Glymour M.M. Validation of a polygenic risk score for dementia in black and white individuals. Brain Behav. 2014;4:687–697. doi: 10.1002/brb3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Marden J.R., Mayeda E.R., Walter S., Vivot A., Tchetgen E.J.T., Kawachi I., Glymour M.M. Using an Alzheimer Disease Polygenic Risk Score to Predict Memory Decline in Black and White Americans Over 14 Years of Follow-up. Alzheimer Dis. Assoc. Disord. 2016;30:195–202. doi: 10.1097/WAD.0000000000000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Qianyi X., Zhi-Jun L., Sha T., Yi-Min S., Deke J., Hong-Lei L., Haitao C., Xu L., Brittany L., Chi-Hsiung W. Risk prediction for sporadic Alzheimer’s disease using genetic risk score in the Han Chinese population. Oncotarget. 2015;6:36955–36964. doi: 10.18632/oncotarget.6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee J.J., Kang S., Lee W., Gunasekaran T.I., Gim J., Wi S.O., Kang D.O., Choo I., Kim B.C., Kim H., et al. Risk prediction of alzheimer’s disease with age stratification using polygenic risk scores. Alzheimers Dement. 2018;14:P339–P340. doi: 10.1016/j.jalz.2018.06.166. [DOI] [Google Scholar]
- 96.Lacour A., Espinosa A., Louwersheimer E., Heilmann S., Hernández I., Wolfsgruber S., Fernández V., Wagner H., Rosende-Roca M., Mauleón A., et al. Genome-wide significant risk factors for Alzheimer’s disease: Role in progression to dementia due to Alzheimer’s disease among subjects with mild cognitive impairment. Mol. Psychiatry. 2017;22:153–160. doi: 10.1038/mp.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Louwersheimer E., Wolfsgruber S., Espinosa A., Lacour A., Heilmann-Heimbach S., Alegret M., Hernandez I., Rosende-Roca M., Tarraga L., Boada M., et al. Alzheimer’s disease risk variants modulate endophenotypes in mild cognitive impairment. Alzheimers Dement. 2016;12:872–881. doi: 10.1016/j.jalz.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 98.Andrews S.J., Das D., Cherbuin N., Anstey K.J., Easteal S. Association of genetic risk factors with cognitive decline: The PATH through life project. Neurobiol. Aging. 2016;41:150–158. doi: 10.1016/j.neurobiolaging.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 99.Carrasquillo M.M., Crook J.E., Pedraza O., Thomas C.S., Pankratz V.S., Allen M., Nguyen T., Malphrus K.G., Ma L., Bisceglio G.D. Late-onset Alzheimer’s risk variants in memory decline, incident mild cognitive impairment, and Alzheimer’s disease. Neurobiol. Aging. 2015;36:60–67. doi: 10.1016/j.neurobiolaging.2014.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu Y., Yu J.T., Wang H.F., Han P.R., Tan C.C., Wang C., Meng X.F., Risacher S.L., Saykin A.J., Tan L. APOE genotype and neuroimaging markers of Alzheimer’s disease: Systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry. 2015;86:127–134. doi: 10.1136/jnnp-2014-307719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jansen W.J., Rik O., Knol D.L., Tijms B.M., Philip S., Verhey F.R.J., Pieter Jelle V., Pauline A., Dag A., Daniel A. Prevalence of cerebral amyloid pathology in persons without dementia: A meta-analysis. JAMA. 2015;313:1924–1938. doi: 10.1001/jama.2015.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Drzezga A., Grimmer T., Henriksen G., Muhlau M., Perneczky R., Miederer I., Praus C., Sorg C., Wohlschlager A., Riemenschneider M., et al. Effect of APOE genotype on amyloid plaque load and gray matter volume in Alzheimer disease. Neurology. 2009;72:1487–1494. doi: 10.1212/WNL.0b013e3181a2e8d0. [DOI] [PubMed] [Google Scholar]
- 103.Grimmer T., Tholen S., Yousefi B.H., Alexopoulos P., Forschler A., Forstl H., Henriksen G., Klunk W.E., Mathis C.A., Perneczky R., et al. Progression of cerebral amyloid load is associated with the apolipoprotein E epsilon4 genotype in Alzheimer’s disease. Biol. Psychiatry. 2010;68:879–884. doi: 10.1016/j.biopsych.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ge T., Sabuncu M.R., Smoller J.W., Sperling R.A., Mormino E.C., Alzheimer’s Disease Neuroimaging Initiative Dissociable influences of APOE epsilon4 and polygenic risk of AD dementia on amyloid and cognition. Neurology. 2018;90:e1605–e1612. doi: 10.1212/WNL.0000000000005415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Scheinin N.M., Wikman K., Jula A., Perola M., Vahlberg T., Rokka J., Nagren K., Viitanen M., Rinne J.O. Cortical (1)(1)C-PIB uptake is associated with age, APOE genotype, and gender in “healthy aging”. J. Alzheimers Dis. 2014;41:193–202. doi: 10.3233/JAD-132783. [DOI] [PubMed] [Google Scholar]
- 106.Fleisher A.S., Kewei C., Xiaofen L., Napatkamon A., Auttawut R., Pradeep T., Hillary P., Joshi A.D., Marwan S., Sadowsky C.H. Apolipoprotein E ε4 and age effects on florbetapir positron emission tomography in healthy aging and Alzheimer disease. Neurobiol. Aging. 2013;34:1–12. doi: 10.1016/j.neurobiolaging.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 107.Gonneaud J., Arenaza-Urquijo E.M., Fouquet M., Perrotin A., Fradin S., de La Sayette V., Eustache F., Chetelat G. Relative effect of APOE epsilon4 on neuroimaging biomarker changes across the lifespan. Neurology. 2016;87:1696–1703. doi: 10.1212/WNL.0000000000003234. [DOI] [PubMed] [Google Scholar]
- 108.Mielke M.M., Machulda M.M., Hagen C.E., Christianson T.J., Roberts R.O., Knopman D.S., Vemuri P., Lowe V.J., Kremers W.K., Jack C.R., Jr., et al. Influence of amyloid and APOE on cognitive performance in a late middle-aged cohort. Alzheimers Dement. 2016;12:281–291. doi: 10.1016/j.jalz.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kantarci K., Lowe V., Przybelski S.A., Weigand S.D., Senjem M.L., Ivnik R.J., Preboske G.M., Roberts R., Geda Y.E., Boeve B.F., et al. APOE modifies the association between Abeta load and cognition in cognitively normal older adults. Neurology. 2012;78:232–240. doi: 10.1212/WNL.0b013e31824365ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wirth M., Villeneuve S., La Joie R., Marks S.M., Jagust W.J. Gene-environment interactions: Lifetime cognitive activity, APOE genotype, and beta-amyloid burden. J. Neurosci. 2014;34:8612–8617. doi: 10.1523/JNEUROSCI.4612-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu Y., Tan L., Wang H.F., Liu Y., Hao X.K., Tan C.C., Jiang T., Liu B., Zhang D.Q., Yu J.T., et al. Multiple Effect of APOE Genotype on Clinical and Neuroimaging Biomarkers Across Alzheimer’s Disease Spectrum. Mol. Neurobiol. 2016;53:4539–4547. doi: 10.1007/s12035-015-9388-7. [DOI] [PubMed] [Google Scholar]
- 112.Vemuri P., Wiste H.J., Weigand S.D., Knopman D.S., Shaw L.M., Trojanowski J.Q., Aisen P.S., Weiner M., Petersen R.C., Jack C.R., Jr., et al. Effect of apolipoprotein E on biomarkers of amyloid load and neuronal pathology in Alzheimer disease. Ann. Neurol. 2010;67:308–316. doi: 10.1002/ana.21953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Risacher S.L., Kim S., Shen L., Nho K., Foroud T., Green R.C., Petersen R.C., Jack C.R., Jr., Aisen P.S., Koeppe R.A., et al. The role of apolipoprotein E (APOE) genotype in early mild cognitive impairment (E-MCI) Front. Aging Neurosci. 2013;5:11. doi: 10.3389/fnagi.2013.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Crary J.F., Trojanowski J.Q., Schneider J.A., Abisambra J.F., Abner E.L., Irina A., Arnold S.E., Johannes A., Beach T.G., Bigio E.H. Primary age-related tauopathy (PART): A common pathology associated with human aging. Acta Neuropathol. 2014;128:755–766. doi: 10.1007/s00401-014-1349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Farfel J.M., Yu L., De Jager P.L., Schneider J.A., Bennett D.A. Association of APOE with tau-tangle pathology with and without β-amyloid. Neurobiol. Aging. 2015;37:19. doi: 10.1016/j.neurobiolaging.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lautner R., Palmqvist S., Mattsson N., Andreasson U., Wallin A., Palsson E., Jakobsson J., Herukka S.K., Owenius R., Olsson B., et al. Apolipoprotein E genotype and the diagnostic accuracy of cerebrospinal fluid biomarkers for Alzheimer disease. JAMA Psychiatry. 2014;71:1183–1191. doi: 10.1001/jamapsychiatry.2014.1060. [DOI] [PubMed] [Google Scholar]
- 117.Wolf A.B., Caselli R.J., Reiman E.M., Jon V. APOE and neuroenergetics: An emerging paradigm in Alzheimer’s disease. Neurobiol. Aging. 2013;34:1007–1017. doi: 10.1016/j.neurobiolaging.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Reiman E.M., Caselli R.J., Yun L.S., Chen K., Bandy D., Minoshima S., Thibodeau S.N., Osborne D. Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N. Engl. J. Med. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 119.Reiman E.M., Kewei C., Alexander G.E., Caselli R.J., Daniel B., David O., Saunders A.M., John H. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc. Natl. Acad. Sci. USA. 2004;101:284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nielsen H.M., Chen K., Lee W., Chen Y., Bauer R.J., Reiman E., Caselli R., Bu G. Peripheral apoE isoform levels in cognitively normal APOE ε3/ε4 individuals are associated with regional gray matter volume and cerebral glucose metabolism. Alzheimers Res. Ther. 2017;9:5. doi: 10.1186/s13195-016-0231-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brandon J.A., Farmer B.C., Williams H.C., Johnson L.A. APOE and Alzheimer’s Disease: Neuroimaging of Metabolic and Cerebrovascular Dysfunction. Front. Aging Neurosci. 2018;10:180. doi: 10.3389/fnagi.2018.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhao N., Liu C.C., Ingelgom A.J.V., Martens Y.A., Linares C., Knight J.A., Painter M.M., Sullivan P.M., Bu G. Apolipoprotein E4 Impairs Neuronal Insulin Signaling by Trapping Insulin Receptor in the Endosomes. Neuron. 2017;96:115–129. doi: 10.1016/j.neuron.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Valla J., Yaari R., Wolf A.B., Kusne Y., Beach T.G., Roher A.E., Corneveaux J.J., Huentelman M.J., Caselli R.J., Reiman E.M. Reduced posterior cingulate mitochondrial activity in expired young adult carriers of the APOE epsilon4 allele, the major late-onset Alzheimer’s susceptibility gene. J. Alzheimers Dis. 2010;22:307–313. doi: 10.3233/JAD-2010-100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cherbuin N., Leach L.S., Christensen H., Anstey K.J. Neuroimaging and APOE genotype: A systematic qualitative review. Dement. Geriatr. Cogn. Disord. 2007;24:348–362. doi: 10.1159/000109150. [DOI] [PubMed] [Google Scholar]
- 125.Saeed U., Mirza S.S., MacIntosh B.J., Herrmann N., Keith J., Ramirez J., Nestor S.M., Yu Q., Knight J., Swardfager W., et al. APOE-epsilon4 associates with hippocampal volume, learning, and memory across the spectrum of Alzheimer’s disease and dementia with Lewy bodies. Alzheimers Dement. 2018;14:1137–1147. doi: 10.1016/j.jalz.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 126.Gutierrez-Galve L., Lehmann M., Hobbs N.Z., Clarkson M.J., Ridgway G.R., Crutch S., Ourselin S., Schott J.M., Fox N.C., Barnes J. Patterns of cortical thickness according to APOE genotype in Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2009;28:476–485. doi: 10.1159/000258100. [DOI] [PubMed] [Google Scholar]
- 127.Pievani M., Rasser P.E., Galluzzi S., Benussi L., Ghidoni R., Sabattoli F., Bonetti M., Binetti G., Thompson P.M., Frisoni G.B. Mapping the effect of APOE epsilon4 on gray matter loss in Alzheimer’s disease in vivo. Neuroimage. 2009;45:1090–1098. doi: 10.1016/j.neuroimage.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Donix M., Burggren A.C., Suthana N.A., Siddarth P., Ekstrom A.D., Krupa A.K., Jones M., Rao A., Martin-Harris L., Ercoli L.M., et al. Longitudinal changes in medial temporal cortical thickness in normal subjects with the APOE-4 polymorphism. Neuroimage. 2010;53:37–43. doi: 10.1016/j.neuroimage.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mishra S., Blazey T.M., Holtzman D.M., Cruchaga C., Su Y., Morris J.C., Benzinger T.L.S., Gordon B.A. Longitudinal brain imaging in preclinical Alzheimer disease: Impact of APOE epsilon4 genotype. Brain. 2018;141:1828–1839. doi: 10.1093/brain/awy103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Adamson M.M., Landy K.M., Duong S., Fox-Bosetti S., Ashford J.W., Murphy G.M., Weiner M., Taylor J.L. Apolipoprotein E epsilon4 influences on episodic recall and brain structures in aging pilots. Neurobiol. Aging. 2010;31:1059–1063. doi: 10.1016/j.neurobiolaging.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lim Y.Y., Williamson R., Laws S.M., Villemagne V.L., Bourgeat P., Fowler C., Rainey-Smith S., Salvado O., Martins R.N., Rowe C.C., et al. Effect of APOE Genotype on Amyloid Deposition, Brain Volume, and Memory in Cognitively Normal Older Individuals. J. Alzheimers Dis. 2017;58:1293–1302. doi: 10.3233/JAD-170072. [DOI] [PubMed] [Google Scholar]
- 132.Khan W., Giampietro V., Banaschewski T., Barker G.J., Bokde A.L., Buchel C., Conrod P., Flor H., Frouin V., Garavan H., et al. A Multi-Cohort Study of ApoE varepsilon4 and Amyloid-beta Effects on the Hippocampus in Alzheimer’s Disease. J. Alzheimers Dis. 2017;56:1159–1174. doi: 10.3233/JAD-161097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Habes M., Toledo J.B., Resnick S.M., Doshi J., Van der Auwera S., Erus G., Janowitz D., Hegenscheid K., Homuth G., Volzke H., et al. Relationship between APOE Genotype and Structural MRI Measures throughout Adulthood in the Study of Health in Pomerania Population-Based Cohort. AJNR Am. J. Neuroradiol. 2016;37:1636–1642. doi: 10.3174/ajnr.A4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bunce D., Anstey K.J., Cherbuin N., Gautam P., Sachdev P., Easteal S. APOE genotype and entorhinal cortex volume in non-demented community-dwelling adults in midlife and early old age. J. Alzheimers Dis. 2012;30:935–942. doi: 10.3233/JAD-2012-112126. [DOI] [PubMed] [Google Scholar]
- 135.Honea R.A., Vidoni E., Harsha A., Burns J.M. Impact of APOE on the healthy aging brain: A voxel-based MRI and DTI study. J. Alzheimers Dis. 2009;18:553–564. doi: 10.3233/JAD-2009-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bagepally B.S., Halahalli H.N., John J.P., Kota L., Purushottam M., Mukherjee O., Sivakumar P.T., Bharath S., Jain S., Varghese M. Apolipoprotein E4 and brain white matter integrity in Alzheimer’s disease: Tract-based spatial statistics study under 3-Tesla MRI. Neurodegener. Dis. 2012;10:145–148. doi: 10.1159/000334761. [DOI] [PubMed] [Google Scholar]
- 137.Cai S., Jiang Y., Wang Y., Wu X., Ren J., Lee M.S., Lee S., Huang L. Modulation on brain gray matter activity and white matter integrity by APOE epsilon4 risk gene in cognitively intact elderly: A multimodal neuroimaging study. Behav. Brain Res. 2017;322:100–109. doi: 10.1016/j.bbr.2017.01.027. [DOI] [PubMed] [Google Scholar]
- 138.Verghese P.B., Castellano J.M., Holtzman D.M. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol. 2011;10:241–252. doi: 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Smith C.D., Andersen A.H., Kryscio R.J., Schmitt F.A., Kindy M.S., Blonder L.X., Avison M.J. Altered brain activation in cognitively intact individuals at high risk for Alzheimer’s disease. Neurology. 1999;53:1391. doi: 10.1212/WNL.53.7.1391. [DOI] [PubMed] [Google Scholar]
- 140.Trachtenberg A.J., Filippini N., Mackay C.E. The effects of APOE-ε4 on the BOLD response. Neurobiol. Aging. 2012;33:323–334. doi: 10.1016/j.neurobiolaging.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 141.Chen Y., Liu Z., Zhang J., Chen K., Yao L., Li X., Gong G., Wang J., Zhang Z. Precuneus degeneration in nondemented elderly individuals with APOE varepsilon4: Evidence from structural and functional MRI analyses. Hum. Brain Mapp. 2017;38:271–282. doi: 10.1002/hbm.23359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chen C.J., Chen C.C., Wu D., Chi N.F., Chen P.C., Liao Y.P., Chiu H.W., Hu C.J. Effects of the apolipoprotein E epsilon4 allele on functional MRI during n-back working memory tasks in healthy middle-aged adults. AJNR Am. J. Neuroradiol. 2013;34:1197–1202. doi: 10.3174/ajnr.A3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Matura S., Prvulovic D., Hartmann D., Scheibe M., Sepanski B., Butz M., Oertel-Knochel V., Knochel C., Karakaya T., Fusser F., et al. Age-Related Effects of the Apolipoprotein E Gene on Brain Function. J. Alzheimers Dis. 2016;52:317–331. doi: 10.3233/JAD-150990. [DOI] [PubMed] [Google Scholar]
- 144.Shu H., Shi Y., Chen G., Wang Z., Liu D., Yue C., Ward B.D., Li W., Xu Z., Chen G., et al. Opposite Neural Trajectories of Apolipoprotein E 4 and 2 Alleles with Aging Associated with Different Risks of Alzheimer’s Disease. Cereb. Cortex. 2016;26:1421–1429. doi: 10.1093/cercor/bhu237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Goveas J.S., Xie C., Chen G., Li W., Ward B.D., Franczak M.B., Jones J.L., Antuono P.G., Li S.J. Functional network endophenotypes unravel the effects of apolipoprotein E epsilon 4 in middle-aged adults. PLoS ONE. 2013;8:e55902. doi: 10.1371/journal.pone.0055902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Heise V., Filippini N., Trachtenberg A.J., Suri S., Ebmeier K.P., Mackay C.E. Apolipoprotein E genotype, gender and age modulate connectivity of the hippocampus in healthy adults. Neuroimage. 2014;98:23–30. doi: 10.1016/j.neuroimage.2014.04.081. [DOI] [PubMed] [Google Scholar]
- 147.Shu H., Yuan Y., Xie C., Bai F., You J., Li L., Li S.J., Zhang Z. Imbalanced hippocampal functional networks associated with remitted geriatric depression and apolipoprotein E epsilon4 allele in nondemented elderly: A preliminary study. J. Affect. Disord. 2014;164:5–13. doi: 10.1016/j.jad.2014.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Risacher S.L., Kim S., Nho K., Foroud T., Shen L., Petersen R.C., Jack C.R., Jr., Beckett L.A., Aisen P.S., Koeppe R.A., et al. APOE effect on Alzheimer’s disease biomarkers in older adults with significant memory concern. Alzheimers Dement. 2015;11:1417–1429. doi: 10.1016/j.jalz.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Song H., Long H., Zuo X., Yu C., Liu B., Wang Z., Wang Q., Wang F., Han Y., Jia J. APOE Effects on Default Mode Network in Chinese Cognitive Normal Elderly: Relationship with Clinical Cognitive Performance. PLoS ONE. 2015;10:e0133179. doi: 10.1371/journal.pone.0133179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yuan B., Xie C., Shu H., Liao W., Wang Z., Liu D., Zhang Z. Differential Effects of APOE Genotypes on the Anterior and Posterior Subnetworks of Default Mode Network in Amnestic Mild Cognitive Impairment. J. Alzheimers Dis. 2016;54:1409–1423. doi: 10.3233/JAD-160353. [DOI] [PubMed] [Google Scholar]
- 151.Luo X., Qiu T., Jia Y., Huang P., Xu X., Yu X., Shen Z., Jiaerken Y., Guan X., Zhou J., et al. Intrinsic functional connectivity alterations in cognitively intact elderly APOE epsilon4 carriers measured by eigenvector centrality mapping are related to cognition and CSF biomarkers: A preliminary study. Brain Imaging Behav. 2017;11:1290–1301. doi: 10.1007/s11682-016-9600-z. [DOI] [PubMed] [Google Scholar]
- 152.Luo X., Li K., Jia Y.L., Zeng Q., Jiaerken Y., Qiu T., Huang P., Xu X., Shen Z., Guan X., et al. Altered effective connectivity anchored in the posterior cingulate cortex and the medial prefrontal cortex in cognitively intact elderly APOE epsilon4 carriers: A preliminary study. Brain Imaging Behav. 2018 doi: 10.1007/s11682-018-9857-5. [DOI] [PubMed] [Google Scholar]
- 153.Lambert J.C., Mann D., Goumidi L., Harris J., Amouyel P., Iwatsubo T., Lendon C., Chartier-Harlin M.C. Effect of the APOE promoter polymorphisms on cerebral amyloid peptide deposition in Alzheimer’s disease. Lancet. 2001;357:608–609. doi: 10.1016/S0140-6736(00)04063-0. [DOI] [PubMed] [Google Scholar]
- 154.Pahnke J., Walker L.C., Schroeder E., Vogelgesang S., Stausske D., Walther R., Warzok R.W. Cerebral beta-amyloid deposition is augmented by the -491AA promoter polymorphism in non-demented elderly individuals bearing the apolipoprotein E epsilon4 allele. Acta Neuropathol. 2003;105:25–29. doi: 10.1007/s00401-002-0602-0. [DOI] [PubMed] [Google Scholar]
- 155.Chalmers K.A., Culpan D., Kehoe P.G., Wilcock G.K., Hughes A., Love S. APOE promoter, ACE1 and CYP46 polymorphisms and beta-amyloid in Alzheimer’s disease. Neuroreport. 2004;15:95–98. doi: 10.1097/00001756-200401190-00019. [DOI] [PubMed] [Google Scholar]
- 156.Ma C., Zhang Y., Li X., Chen Y., Zhang J., Liu Z., Chen K., Zhang Z. The TT allele of rs405509 synergizes with APOE epsilon4 in the impairment of cognition and its underlying default mode network in non-demented elderly. Curr. Alzheimer Res. 2016;13:708–717. doi: 10.2174/1567205013666160129100350. [DOI] [PubMed] [Google Scholar]
- 157.Espeseth T., Greenwood P.M., Reinvang I., Fjell A.M., Walhovd K.B., Westlye L.T., Wehling E., Lundervold A., Rootwelt H., Parasuraman R. Interactive effects of APOE and CHRNA4 on attention and white matter volume in healthy middle-aged and older adults. Cogn. Affect. Behav. Neurosci. 2006;6:31–43. doi: 10.3758/CABN.6.1.31. [DOI] [PubMed] [Google Scholar]
- 158.Morgen K., Ramirez A., Frolich L., Tost H., Plichta M.M., Kolsch H., Rakebrandt F., Rienhoff O., Jessen F., Peters O., et al. Genetic interaction of PICALM and APOE is associated with brain atrophy and cognitive impairment in Alzheimer’s disease. Alzheimers Dement. 2014;10:S269–S276. doi: 10.1016/j.jalz.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 159.Thambisetty M., An Y., Nalls M., Sojkova J., Swaminathan S., Zhou Y., Singleton A.B., Wong D.F., Ferrucci L., Saykin A.J., et al. Effect of complement CR1 on brain amyloid burden during aging and its modification by APOE genotype. Biol. Psychiatry. 2013;73:422–428. doi: 10.1016/j.biopsych.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Liu Z., Dai X., Tao W., Liu H., Li H., Yang C., Zhang J., Li X., Chen Y., Ma C., et al. APOE influences working memory in non-demented elderly through an interaction with SPON1 rs2618516. Hum. Brain Mapp. 2018;39:2859–2867. doi: 10.1002/hbm.24045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shen J., Qin W., Xu Q., Xu L., Xu J., Zhang P., Liu H., Liu B., Jiang T., Yu C. Modulation of APOE and SORL1 genes on hippocampal functional connectivity in healthy young adults. Brain Struct. Funct. 2017;222:2877–2889. doi: 10.1007/s00429-017-1377-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang N., Liu H., Qin W., Liu B., Jiang T., Yu C. APOE and KIBRA Interactions on Brain Functional Connectivity in Healthy Young Adults. Cereb. Cortex. 2017;27:4797–4805. doi: 10.1093/cercor/bhw276. [DOI] [PubMed] [Google Scholar]
- 163.Porter T., Burnham S.C., Dore V., Savage G., Bourgeat P., Begemann K., Milicic L., Ames D., Bush A.I., Maruff P., et al. KIBRA is associated with accelerated cognitive decline and hippocampal atrophy in APOE epsilon4-positive cognitively normal adults with high Abeta-amyloid burden. Sci. Rep. 2018;8:2034. doi: 10.1038/s41598-018-20513-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Martiskainen H., Helisalmi S., Viswanathan J., Kurki M., Hall A., Herukka S.K., Sarajarvi T., Natunen T., Kurkinen K.M., Huovinen J., et al. Effects of Alzheimer’s disease-associated risk loci on cerebrospinal fluid biomarkers and disease progression: A polygenic risk score approach. J. Alzheimers Dis. 2015;43:565–573. doi: 10.3233/JAD-140777. [DOI] [PubMed] [Google Scholar]
- 165.Voyle N., Patel H., Folarin A., Newhouse S., Johnston C., Visser P.J., Dobson R.J., Kiddle S.J. Genetic Risk as a Marker of Amyloid-beta and Tau Burden in Cerebrospinal Fluid. J. Alzheimers Dis. 2017;55:1417–1427. doi: 10.3233/JAD-160707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tan C.H., Fan C.C., Mormino E.C., Sugrue L.P., Broce I.J., Hess C.P., Dillon W.P., Bonham L.W., Yokoyama J.S., Karch C.M., et al. Polygenic hazard score: An enrichment marker for Alzheimer’s associated amyloid and tau deposition. Acta Neuropathol. 2018;135:85–93. doi: 10.1007/s00401-017-1789-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Darst B.F., Koscik R.L., Racine A.M., Oh J.M., Krause R.A., Carlsson C.M., Zetterberg H., Blennow K., Christian B.T., Bendlin B.B., et al. Pathway-Specific Polygenic Risk Scores as Predictors of Amyloid-beta Deposition and Cognitive Function in a Sample at Increased Risk for Alzheimer’s Disease. J. Alzheimers Dis. 2017;55:473–484. doi: 10.3233/JAD-160195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sabuncu M.R., Buckner R.L., Smoller J.W., Lee P.H., Fischl B., Sperling R.A., Alzheimer’s Disease Neuroimaging Initiative The association between a polygenic Alzheimer score and cortical thickness in clinically normal subjects. Cereb. Cortex. 2012;22:2653–2661. doi: 10.1093/cercor/bhr348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Corlier F., Hafzalla G., Faskowitz J., Kuller L.H., Becker J.T., Lopez O.L., Thompson P.M., Braskie M.N. Systemic inflammation as a predictor of brain aging: Contributions of physical activity, metabolic risk, and genetic risk. Neuroimage. 2018;172:118–129. doi: 10.1016/j.neuroimage.2017.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Li J., Zhang X., Li A., Liu S., Qin W., Yu C., Liu Y., Liu B., Jiang T. Polygenic risk for Alzheimer’s disease influences precuneal volume in two independent general populations. Neurobiol. Aging. 2018;64:116–122. doi: 10.1016/j.neurobiolaging.2017.12.022. [DOI] [PubMed] [Google Scholar]
- 171.Harrison T.M., Mahmood Z., Lau E.P., Karacozoff A.M., Burggren A.C., Small G.W., Bookheimer S.Y. An Alzheimer’s Disease Genetic Risk Score Predicts Longitudinal Thinning of Hippocampal Complex Subregions in Healthy Older Adults. eNeuro. 2016;3 doi: 10.1523/ENEURO.0098-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Desikan R.S., Fan C.C., Wang Y., Schork A.J., Cabral H.J., Cupples L.A., Thompson W.K., Besser L., Kukull W.A., Holland D., et al. Genetic assessment of age-associated Alzheimer disease risk: Development and validation of a polygenic hazard score. PLoS Med. 2017;14:e1002258. doi: 10.1371/journal.pmed.1002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lupton M.K., Strike L., Hansell N.K., Wen W., Mather K.A., Armstrong N.J., Thalamuthu A., McMahon K.L., de Zubicaray G.I., Assareh A.A., et al. The effect of increased genetic risk for Alzheimer’s disease on hippocampal and amygdala volume. Neurobiol. Aging. 2016;40:68–77. doi: 10.1016/j.neurobiolaging.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Xiao E., Chen Q., Goldman A.L., Tan H.Y., Healy K., Zoltick B., Das S., Kolachana B., Callicott J.H., Dickinson D., et al. Late-Onset Alzheimer’s Disease Polygenic Risk Profile Score Predicts Hippocampal Function. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2017;2:673–679. doi: 10.1016/j.bpsc.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 175.Foley S.F., Tansey K.E., Caseras X., Lancaster T., Bracht T., Parker G., Hall J., Williams J., Linden D.E. Multimodal Brain Imaging Reveals Structural Differences in Alzheimer’s Disease Polygenic Risk Carriers: A Study in Healthy Young Adults. Biol. Psychiatry. 2017;81:154–161. doi: 10.1016/j.biopsych.2016.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Laukka E.J., Lövdén M., Herlitz A., Karlsson S., Ferencz B., Pantzar A., Keller L., Graff C., Fratiglioni L., Bäckman L. Genetic effects on old-age cognitive functioning: A population-based study. Psychol. Aging. 2013;28:262–274. doi: 10.1037/a0030829. [DOI] [PubMed] [Google Scholar]
- 177.Ihle A., Bunce D., Kliegel M. APOE ε4 and Cognitive Function in Early Life: A meta-analysis. Neuropsychology. 2012;26:267–277. doi: 10.1037/a0026769. [DOI] [PubMed] [Google Scholar]
- 178.Bathum L., Christiansen L.B., Vaupel J., Mcgue M., Christensen K. Apolipoprotein e genotypes: Relationship to cognitive functioning, cognitive decline, and survival in nonagenarians. J. Am. Geriatr. Soc. 2010;54:654–658. doi: 10.1111/j.1532-5415.2005.53554.x. [DOI] [PubMed] [Google Scholar]
- 179.Østbye T. Neuropsychological performance in advanced age: Influences of Demographic factors and Apolipoprotein E: Findings from the Cache County Memory Study. Clin. Neuropsychol. 2009;23:77–99. doi: 10.1080/13854040801894730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bunce D., Bielak A.A., Anstey K.J., Cherbuin N., Batterham P.J., Easteal S. APOE genotype and cognitive change in young, middle-aged, and older adults living in the community. J. Gerontol. A Biol. Sci. Med. Sci. 2014;69:379–386. doi: 10.1093/gerona/glt103. [DOI] [PubMed] [Google Scholar]
- 181.Han S.D., Bondi M.W. Revision of the apolipoprotein E compensatory mechanism recruitment hypothesis. Alzheimers Dement. 2008;4:251–254. doi: 10.1016/j.jalz.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 182.Carrion-Baralt J.R., Melendez-Cabrero J., Rodriguez-Ubinas H., Schmeidler J., Beeri M.S., Angelo G., Sano M., Silverman J.M. Impact of APOE epsilon4 on the cognitive performance of a sample of non-demented Puerto Rican nonagenarians. J. Alzheimers Dis. 2009;18:533–540. doi: 10.3233/JAD-2009-1160. [DOI] [PubMed] [Google Scholar]
- 183.Duchek J.M., Balota D.A., Cortese M. Prospective memory and apolipoprotein E in healthy aging and early stage Alzheimer’s disease. Neuropsychology. 2006;20:633–644. doi: 10.1037/0894-4105.20.6.633. [DOI] [PubMed] [Google Scholar]
- 184.Bloss C.S., Delis D.C., Salmon D.P., Bondi M.W. Decreased cognition in children with risk factors for Alzheimer’s disease. Biol. Psychiatry. 2008;64:904–906. doi: 10.1016/j.biopsych.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Woodard J.L., Seidenberg M., Nielson K.A., Antuono P., Guidotti L., Durgerian S., Zhang Q., Lancaster M., Hantke N., Butts A. Semantic memory activation in amnestic mild cognitive impairment. Brain. 2009;132:2068. doi: 10.1093/brain/awp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Johnson S.C., Schmitz T.W., Trivedi M.A., Ries M.L., Torgerson B.M., Carlsson C.M., Asthana S., Hermann B.P., Sager M.A. The influence of Alzheimer disease family history and apolipoprotein E epsilon4 on mesial temporal lobe activation. J. Neurosci. 2006;26:6069–6076. doi: 10.1523/JNEUROSCI.0959-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Roberts G.W., Gentleman S.M., Lynch A., Graham D.I. beta A4 amyloid protein deposition in brain after head trauma. Lancet. 1991;338:1422–1423. doi: 10.1016/0140-6736(91)92724-G. [DOI] [PubMed] [Google Scholar]
- 188.Teasdale G.M., Nicoll J.A., Murray G., Fiddes M. Association of apolipoprotein E polymorphism with outcome after head injury. Lancet. 1997;350:1069–1071. doi: 10.1016/S0140-6736(97)04318-3. [DOI] [PubMed] [Google Scholar]
- 189.Irie F., Fitzpatrick A., Kuller O.L., Peila R., Newman A., Launer L. Enhanced risk for Alzheimer disease in persons with type 2 diabetes and APOE epsilon4: The Cardiovascular Health Study Cognition Study. Arch. Neurol. 2008;65:89–93. doi: 10.1001/archneurol.2007.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Profenno L.A., Faraone S.V. Diabetes and overweight associate with non-APOE4 genotype in an Alzheimer’s disease population. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2010;147B:822–829. doi: 10.1002/ajmg.b.30694. [DOI] [PubMed] [Google Scholar]
- 191.Ihab H., Farzaneh S., Lipsitz L.A. Apolipoprotein e, carbon dioxide vasoreactivity, and cognition in older adults: Effect of hypertension. J. Am. Geriatr. Soc. 2015;63:276–281. doi: 10.1111/jgs.13235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Giri M., Zhang M., Yang L. Genes associated with Alzheimer’s disease: An overview and current status. Clin. Interv. Aging. 2016;11:665–681. doi: 10.2147/CIA.S105769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kauppi K., Fan C.C., McEvoy L.K., Holland D., Tan C.H., Chen C.H., Andreassen O.A., Desikan R.S., Dale A.M., Alzheimer’s Disease Neuroimaging Initiative Combining Polygenic Hazard Score With Volumetric MRI and Cognitive Measures Improves Prediction of Progression From Mild Cognitive Impairment to Alzheimer’s Disease. Front. Neurosci. 2018;12:260. doi: 10.3389/fnins.2018.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lin Y.F., Smith A.V., Aspelund T., Betensky R.A., Smoller J.W., Gudnason V., Launer L.J., Blacker D. Genetic overlap between vascular pathologies and Alzheimer’s dementia and potential causal mechanisms. Alzheimers Dement. 2019;15:65–75. doi: 10.1016/j.jalz.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Suri S., Heise V., Trachtenberg A.J., Mackay C.E. The forgotten APOE allele: A review of the evidence and suggested mechanisms for the protective effect of APOE ε2. Neurosci. Biobehav. Rev. 2013;37:2878–2886. doi: 10.1016/j.neubiorev.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 196.Prins N.D., van der Flier W.A., Knol D.L., Fox N.C., Brashear H.R., Nye J.S., Barkhof F., Scheltens P. The effect of galantamine on brain atrophy rate in subjects with mild cognitive impairment is modified by apolipoprotein E genotype: Post-hoc analysis of data from a randomized controlled trial. Alzheimers Res. Ther. 2014;6:47. doi: 10.1186/alzrt275. [DOI] [PMC free article] [PubMed] [Google Scholar]