Abstract
The in vitro maturation of oocytes is frequently used as an assisted reproductive technique (ART), and has been successfully established in humans and rodents. To overcome the limitations of ART, novel procedures for the in vitro maturation of early follicles are emerging. During the follicle isolation procedure, the unintended rupture of each follicle leads to a release of extra oocytes. Such oocytes, which are obtained during follicle isolation from marmosets, can be used for early maturation studies. Marmoset (Callithrix jacchus), which is classified as a new-world monkey, is a novel model that has been employed in reproductive biomedical research, as its reproductive physiology is similar to that of humans in several aspects. The ovaries of female marmosets were collected, and the excess oocytes present during follicle isolation were retrieved without pre-gonadotropin induction. Each oocyte was matured in vitro for 48 h in the presence of various concentrations of human chorionic gonadotropin (hCG) and epidermal growth factor (EGF), and the maturity of oocytes and optimal maturation conditions were evaluated. Each oocyte was individually reverse-transcribed, and the expression of mRNAs and microRNAs (miRs) were analyzed. Concentrations of hCG significantly affected the maturation rate of oocytes [the number of metaphase II (MII) oocytes]. The expression of BMP15 and ZP1 was highest when the oocytes were matured using 100 IU/L of hCG without pre-treatment with gonadotropins, and that of Cja-mir-27a was highest when cultured with follicle stimulating hormone. These results suggest that these up-regulated miRs affect the maturation of oocytes. Interactions with other protein networks were analyzed, and a strong association of BMP15 and ZP1 with sperm binding receptor (ACR), anti-Müllerian hormone (AMH), and AMH receptor was demonstrated, which is related to the proliferation of granulosa cells. Collectively, on the basis of these results, the authors propose optimal maturation conditions of excess oocytes of marmoset without in vivo gonadotropin treatment, and demonstrated the roles of miRs in early oocyte maturation at the single-cell level in marmosets.
Keywords: oocyte, marmoset, in vitro maturation, microRNA
1. Introduction
Oocyte in vitro maturation (IVM) is a popular technology in assisted reproductive technology (ART). The process is associated with the developmental competency of oocyte; therefore, the precise optimization of conditions is necessary [1]. Factors, environmental and otherwise, affecting the developmental competency of oocytes have been studied using many species, such as mice and domestic animals; however, such animals have different reproductive characteristics than those of humans [2,3,4,5,6,7]. These animals have estrous cycles and multi-fetal pregnancies, while humans undergo a menstrual cycle and mostly have a single-fetal pregnancy. Therefore, because of these differences in reproductive physiology, there is a need for other animal experimental models that are physiologically close to humans, such as non-human primates (NHP).
The common marmoset (Callithrix jacchus) is an emerging NHP model for reproductive biology with the advantage of a short gestation period and a reproductive physiology similar to that of humans [8]. Therefore, ART-related studies using the marmoset model are increasing. Furthermore, many of them use a similar protocol as that of human ovarian stimulation [9]. In addition, limited access to using marmoset has diminished ART-related studies, such as the IVM of oocytes or in vitro follicular maturation (IVFM). The IVFM of early follicles is an alternate option for women showing a poor response to gonadotropin treatment, women in early menopause who barely have gonadotropin-responsive follicles, and cancer survivors with extremely decreased fertility due to loss of ovarian function, in order to achieve pregnancy. Therefore, during the isolation of follicles for IVFM, the use of oocytes from the unintended rupture of early follicles could be another strategy that could be studied and developed using marmoset as a reproductive study model.
The microRNAs (miRNAs, miRs), which are small RNA families of ~25 bases, regulate gene expression at the post-transcriptional level [10,11] in many developmental processes such as embryonic development and maternal-to-embryonic transition, and have been well-studied [12,13,14]. Furthermore, their association with fertility [15,16] and the developmental competence of oocytes during IVM or IVFM are well-known [17,18,19,20,21]. However, their roles in the reproduction processes in marmoset are only the focus of phylogenetic studies that have focused on the roles of microRNAs, and their roles regarding reproduction in marmoset have been merely studied [22,23,24]. The regulatory roles of miRs in oocyte maturation have been demonstrated in the mouse and bovine models, as well as in humans; however, they have been rarely investigated in marmosets. Due to the increasing use of marmoset as a biomedical research model, generations of knockout marmoset are being actively studied; therefore, multiple ways to use extra oocytes released during unintended follicular rupture could be beneficial to studies in the related fields.
In this study, the authors sought to establish optimal IVM conditions for marmoset oocytes retrieved from the ovary, without treatment with gonadotropins, and analyze the differential expressions of miRs according to IVM conditions to elucidate their roles in oocyte maturation.
2. Results
2.1. In Vitro Maturation of Marmoset Oocytes at Various Concentrations of Factors
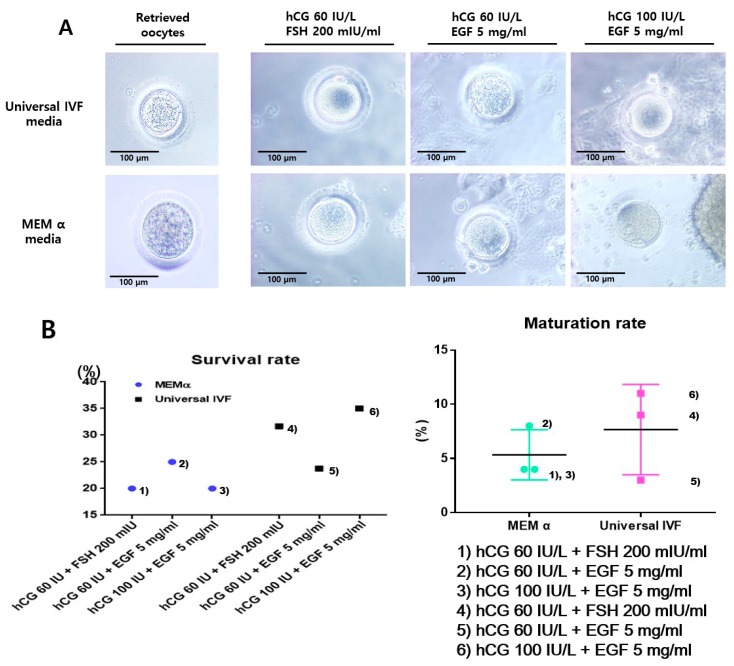
The experimental scheme of this study is presented in Figure 1. Retrieved oocytes were matured in vitro with any one of the following conditions: (1) human chorionic gonadotropin (hCG) 60 IU/L + FSH 200 mIU/mL in MEM α; (2) hCG 60 IU/L + epidermal growth factor (EGF) 5 mg/mL in MEM α; (3) hCG 100 IU/L + EGF 5 mg/mL in MEM α; (4) hCG 60 IU/L + FSH 200 mIU/mL in Universal IVF media; (5) hCG 60 IU/L + EGF 5 mg/mL in Universal IVF media; and (6) hCG 100 IU/L + EGF 5 mg/mL in Universal IVF media. All of the matured oocytes showed polar bodies and clear morphology (Figure 2A).
Figure 1.
Experimental flow of oocyte retrieval and in vitro maturation. Oocytes not pre-treated with gonadotropins were retrieved during follicle isolation. Immediate in vitro maturation of ruptured and retrieved oocytes was conducted in six different conditions; (1) human chorionic gonadotropin (hCG) 60 IU/L + FSH 200 mIU/mL in MEM α, (2) hCG 60 IU/L + epidermal growth factor (EGF) 5 mg/mL in MEM α, (3) hCG 100 IU/L + EGF 5 mg/mL in MEM α, (4) hCG 60 IU/L + FSH 200 mIU/mL in Universal IVF media, (5) hCG 60 IU/L + EGF 5 mg/mL in Universal IVF media, and (6) hCG 100 IU/L + EGF 5 mg/mL in Universal IVF media. Additionally, the collection of sperm by ejaculation using a vibrator from male marmoset is shown (left), and the collection of female ovaries by surgical operation under anesthesia is represented (right).
Figure 2.
In vitro maturation of marmoset oocytes using different conditions. (A) Morphological observation of oocytes matured in various conditions. The survival rate was calculated based on the health status of oocytes, and the degenerated oocytes during the maturation process were considered as dead. After 36 h, oocytes with polar bodies were ascertained as matured oocytes. (B) Survival and maturation rates of in vitro matured oocytes. Among the maturation conditions, ‘condition (6)’ as mentioned in the methods section was the most efficacious in regard to the survival and maturation of oocytes.
Among the conditions employed, the addition of hCG 100 IU/L + EGF 5 mg/mL in Universal IVF media (condition 6) was most effective condition for maturation of the retrieved oocytes. In contrast, in conditions using MEM α (e.g., condition 3), arrested or shrunken oocytes were also observed, as well as mature oocytes. Using these in vitro mature oocytes, a fertilization process was attempted and interestingly, the formation of embryos was successfully achieved (Figure 2B). Thereafter, the survival and maturation rate were calculated.
2.2. Fertilization of in vitro Matured Oocytes
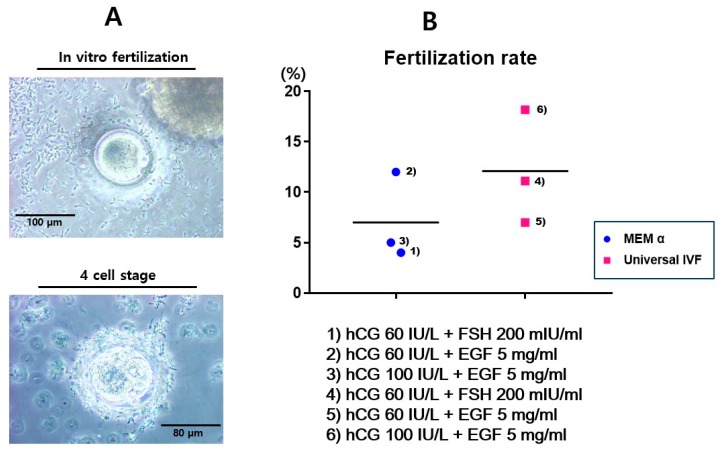
The fertilization of in vitro matured oocytes was achieved using live sperm injection (Figure 3A). Sperms were collected from marmosets by the induction of ejaculation. It was observed that the quality of sperm varied in individual marmosets due to the age factor. Therefore, a pre-screening of sperm quality, based on normal morphology and swimming activity, was performed. Three-years-old marmosets showed good sperm quality, whereas the sperm of male marmosets aged under 30 months demonstrated weak activity and immature morphology.
Figure 3.
In vitro fertilization of marmoset oocytes using different conditions. (A) Fertilization and development into the four-cell-stage. In vitro fertilization (IVF) was achieved using viable sperm, and fertilization was confirmed by observation of 2-pronuclei (2PN). In vitro development into the further stage was confirmed after 48 h. (B) Fertilization rate using each condition of hormone combinations. The rates were calculated based on the appearance of 2PN after IVF.
The rate of fertilization was the highest in the condition using hCG 100 IU/L + EGF 5 mg/mL in Universal IVF medium (condition 6). The accomplished fertilization was confirmed by the presence of two polar nuclei (2PN) and the development of embryos into the four-cell stage was confirmed (Figure 3B). Development into the four-cell stage was better achieved using condition 6; however, 10% of the fertilized eggs were arrested at the 2PN stage. Development beyond eight cells was achieved after three days of fertilization, and the embryos were cryopreserved for further manipulation.
2.3. Localization of Specific Protein in in vitro Matured Oocytes
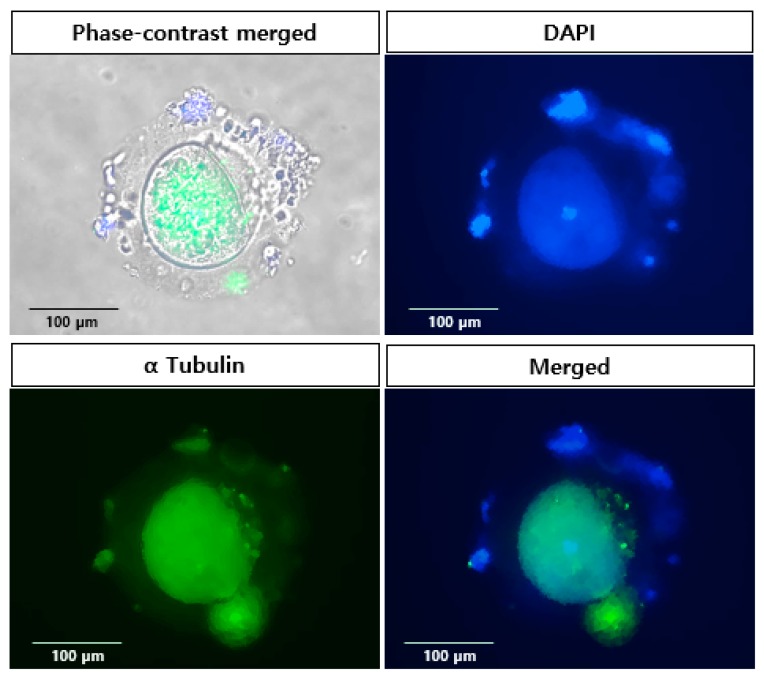
The expression of alpha tubulin was confirmed in matured oocytes (Figure 4). The zona pellucida (ZP) of marmoset oocyte is thicker than that of mouse; however, it does not interfere in the fluorescence imaging. Tubulin was consistently expressed in oocytes, and the even distribution of the protein was observed. The expression of the protein co-localized with nucleus inside the ZP was demonstrated by merged images using phase-contrast. The expression clearly showed the developmental competency of in vitro matured oocytes.
Figure 4.
Localization of specific protein in in vitro matured marmoset oocytes. Immunofluorescence demonstrates the expression of alpha tubulin. The localization was confirmed using merged images of phase-contrast and fluorescence images. Magnification: ×400.
2.4. Differential Expression of miRNA and mRNA in Matured Oocytes
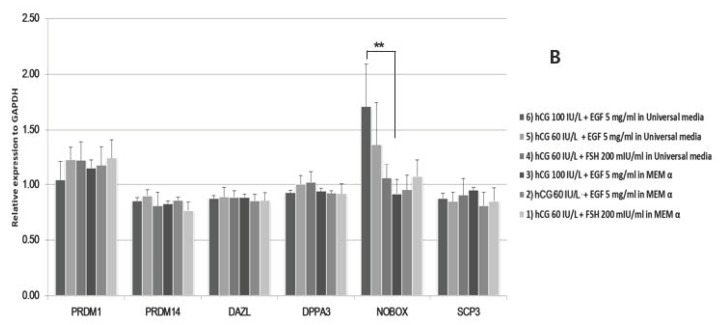
Early development-specific gene expression in the matured oocytes was also analyzed. The expression of ZP1 was up-regulated in condition 6) group (Figure 5A). The expression of BMP15 was also up-regulated when matured using hCG 100 IU/L. The expression of Oct4 showed differences among the different conditions. NOBOX genes were also up-regulated in the condition 6 group (Figure 5B).
Figure 5.
MicroRNA (mRNA) expression of marmoset single oocytes from different in vitro maturational conditions. Developmental gene expression of oocytes (A) and of primordial germ cells (B) was evaluated using qPCR. BMP15 and ZP1 were highly up-regulated in the ‘condition 6′ group, and Novox was also up-regulated. ** p < 0.05.
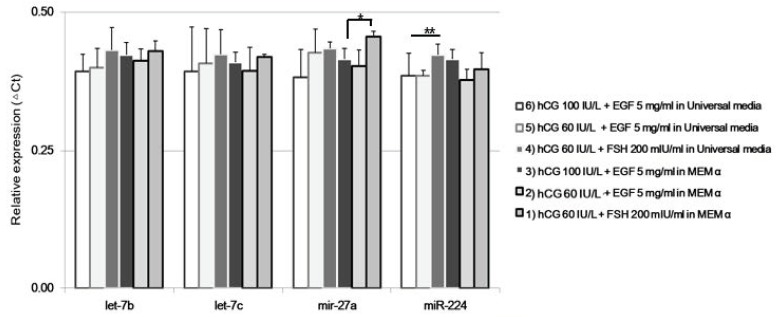
The expression of Cja-miRs was up-regulated in oocytes matured using Universal IVF medium (conditions 1 and 4). The conditions using Universal IVF medium demonstrated a trend of lower expression compared to MEM α. Cja-miR-27a was up-regulated in oocytes matured in the condition 1 group (Figure 6).
Figure 6.
miRNAs expression of single marmoset oocytes from different in vitro maturation conditions. The expression of specific miRNAs, Cja-let-7b, Cja-let-7c, Cja-miR-27a, and Cja-miR-224 was analyzed at the single-cell level. * p < 0.05; ** p < 0.05.
2.5. Annotation of miRNAs Using Database Set
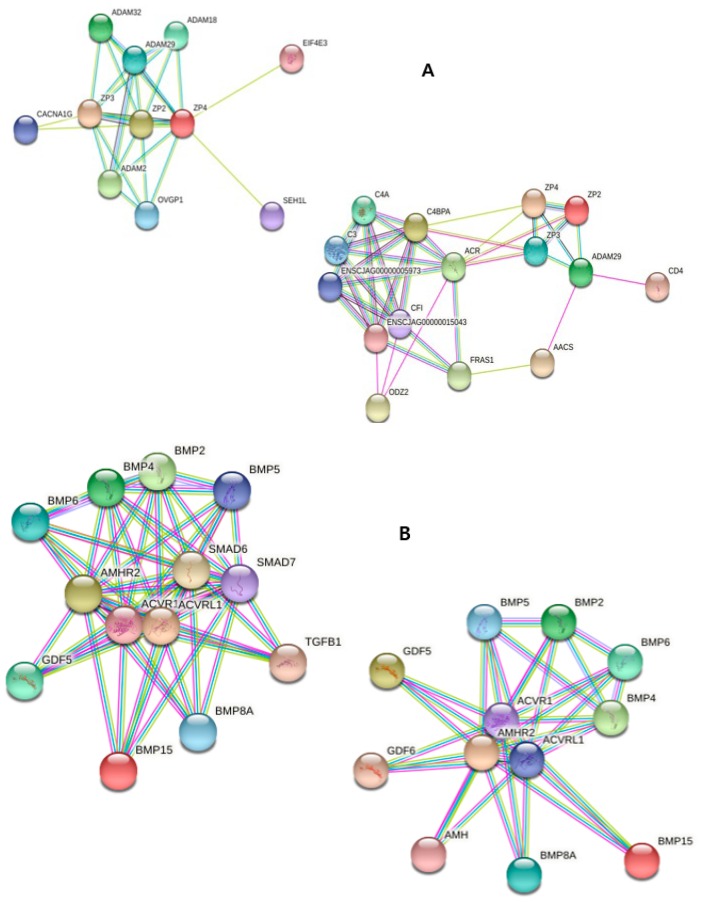
The correlation of protein interaction was analyzed using KEGG pathway and STRING database (Figure 7). The target genes of up-regulated miRNA were those related to oocyte maturation and estrogen pathways. The up-regulated gene, ZP, had a strong correlation with genes such as acrosome reaction regulator (ACR) and calcium channel regulator, CACNA1G. The correlation between genes was similar to that of mouse. BMP15 was closely related to growth/differentiation factor (GDF) families, BMP families, anti-Müllerian hormone (AMH), and AMH receptor II (AMHR2), which are related to persistent Müllerian duct syndrome, and those proteins were related to the TGF-beta signaling pathway (KEGG).
Figure 7.
Predictive interaction of specific proteins in marmoset oocytes. The interaction of the up-regulated genes with other genes at protein level was analyzed mainly using the STRING database. Comparison within monkey species was conducted; however, the annotation is not demonstrated due to the limitation of marmoset resources.
3. Discussion
The developmental competence of oocytes is one of the most important requirements for ART. Numerous studies focused on the maturation conditions and factors regulating or enhancing developmental competence for a higher yield of in vitro maturation and transition to early stage embryos, e.g., vitamin D [25,26] and inositol [27,28]. Although many of them were conducted using a murine model, the necessity of using an NHP model for pre-clinical evaluation nonetheless remains, due to the difference of reproductive physiology in humans as compared to the mouse model, such as menstrual cycle, the structure of the uterus, implantation process, gestation period, and the number of offspring.
Non-human primates can be classified into old-world and new-world monkeys [29]; the marmoset belongs to the class of new-world monkeys. Their features and application to various biomedical research studies have been mainly conducted in Japan, and recently, the wider usage of marmoset as a model is emerging [23,30,31,32,33,34]. From the perspective of reproductive physiology, non-human primates are closely related to human models; however, due to the high cost of the supply and maintenance of marmosets, a limited number of researchers are using this NHP model. Although ART studies using the marmoset model are reported, studies have been focused on the IVM of oocytes using a human IVF protocol, mainly consisting of inducing maturation using gonadotropins [9,35,36,37,38]. Nevertheless, using NHP as the model for a wider range of female reproductive diseases has arisen [39].
In vitro follicular maturation (IVFM) processes are newly emerging concepts in reproductive medicine due to their roles for women who do not achieve pregnancy; however, their clinical applications have been limited to date. Hence, the evaluation of this technology in NHP should be for wider usage in the future. Our group has previously reported the IVFG process in mouse [17,18,40] and autopsied rhesus monkey models [41]. However, the marmoset is a different strain of monkey, and its ovaries are morphologically similar to those of the rat. Therefore, in this study, the authors envisaged the use of extra oocytes of marmosets from unintended rupture during the follicle isolation procedure. The isolated oocytes were at varied developmental stages due to non-induction using gonadotropins prior to isolation. Ideally, oocytes at various stages of maturation may not be optimal for immediate fertilization; however, a limited supply of marmosets for biomedical research compelled us to undertake the immediate maturation procedures of such oocytes.
The maturation of marmoset oocytes was achieved with various combinations and concentrations of hormones. The progress to maturation was slower in marmosets, as the zona pellucida (ZP) was thicker than that of mice. Although low concentrations of hCG led to the maturation of the oocytes, the most effective condition for the maturation of such non-induced oocytes was a combination of hCG (100 IU/L) with EGF (5 mg/mL) in Universal IVF medium. The concentration ranges of these hormones were relatively higher than those used in mice [42,43]. Some studies have revealed that the concentrations used for marmosets in pre-gonadotropin-induced IVM ranged from 1 to 10 IU with or without a combination of other factors, such as FSH and estradiol [38,44,45,46]. Our results indicated that the immediate IVM of marmoset oocytes from non-gonadotropin-induced ovaries needs a higher concentration of hCG.
ZP is a translucent glycoprotein matrix jelly-like layer of oocytes, which has numerous roles in fertilization, including oocyte activation and acting as receptors for sperms [47,48,49,50]. The ZP genes are divided into three classes in mouse and human [51], and their existence is confirmed in the old world monkeys, bonnet monkeys (Macaca radiata), [52,53], and new-world monkeys, such as marmosets [54,55]. The expression of ZP1 was demonstrated to occur at a lower incidence, while the other two genes, ZP2 and ZP3, were expressed according to the different stages of folliculogenesis [54,56]. In this study, the IVM of marmoset oocytes was demonstrated; however, excess oocytes during follicle isolation were not focused as per this experiment. The up-regulation of BMP15 and ZP1 indicate that the maturation condition was optimal for marmoset oocytes and that the early maturation of oocytes obtained without ovulation may correlate to the expression of ZP1. In addition, the other developmental regulatory genes, PRDM1 and NOBOX, were also up-regulated. Taken together, the authors demonstrated the correlation of ZP1 to IVM of marmoset oocytes without gonadotropin pre-treatment.
The expression patterns of miRNAs correlated with the maturation rate, which were different from those of mRNA expression. MicroRNAs are well-known regulators of biological phenomena [10], especially in the oocytes of vertebrates [57]. A majority of previous studies were conducted using the murine model, which included oocyte–somatic cell communication [58], the prevention of oocyte apoptosis [59], and the stimulation or inhibition of maturation [60,61,62]. In this study, the authors analyzed the expressions of Cja-let-7b, Cja-let-7c, Cja-miR-27a, and Cja-miR-224. These miR and let-7 families are related to oocyte maturation [60], development [63,64], and hormone balance in granulosa cells [65,66].
The evolution and expression features of miRNAs in marmosets have been demonstrated and compared with other monkeys [22,23]. Their roles in male reproduction, such as the morphogenesis of epithelium and tube development, have been studied [24]; however, the distinctive roles of these miRNAs in the female reproductive processes are barely known. The marmoset genome has common and unique features compared to other monkeys, which include rapid changes in the miRNAs expressed in the placenta. The non-synonymous changes of genes involved in reproductive physiology are GDF9 and BMP15. The changes of genes targeted by let-7 families were distinctive in marmoset, and the roles of miR-let-7 families in reproduction have been demonstrated in mouse and bovine species [17,63]. In our study, the expression of let-7b and let-7c was up-regulated in those oocytes cultured using high-concentration hormones, while Cja-mir-224 expression was decreased. These results were in contrast with previous studies [60], which may be due to the loss of cumulus cells during follicle rupture and using denuded oocytes for immediate IVM.
In conclusion, the authors proposed optimal conditions for the in vitro maturation of marmoset oocytes without pre-induction using gonadotropins, and analyzed the correlation of mRNA and miRNAs related to oocyte development. The maturation needs a higher concentration of hCG, and the miRNAs may regulate the early maturation of marmoset oocytes, as observed in other animal models.
4. Materials and Methods
4.1. Ethics and Animal Anesthesia
All of the experimental procedures were reviewed and authorized by the Institutional Animal Care and Use Committee (IACUC) of Seoul National University Hospital (SNUH-IACUC No: 15-0032-C1A1(1), 14 January 2016).
Marmosets were imported from Central Institute for Experimental Animals (CIEA) of Japan and maintained in cages in a temperature and light-controlled room (23 ± 3 °C, 40–60% humidity, and 12 h light/dark cycle). They were under veterinary supervision to maintain a healthy status. Animals were sacrificed by intravenous injection of 10 mL of saturated KCl after anesthesia with intravenous ketamine (10 mg/kg), medetomidine (0.04 mg/kg), and vecuronium (0.2 mg/kg).
4.2. Isolation of Ovaries and in vitro Maturation of Oocytes
The ovaries (n = 4) of female marmosets were removed by laparoscopy. The excised ovaries were transferred in a transport media containing MEM α, 20% fetal bovine serum (FBS), and penicillin/streptomycin (purchased from Invitrogen, Grand Island, NY, USA). Extra oocytes were retrieved by the unintended rupture of follicles during the dissection of the ovaries. Oocytes at the metaphase I stage were selected and matured in vitro for 36 to 48 h in different conditions.
The in vitro maturation media mainly consisted of either MEM α (Invitrogen, Grand Island, NY, USA) or Universal IVF medium (Orgio, Måløv, Denmark), supplemented with different concentrations of human chorionic gonadotropin (hCG, Merck-Serono, Darmstadt, Germany) and epidermal growth factor (EGF, Invitrogen). Maturated oocytes were either utilized for fertilization or subjected to further analyses. The in vitro maturation conditions are summarized in Table 1.
Table 1.
Comparison of maturation rate according to media conditions for in vitro maturation (IVM) of marmoset oocytes. MII: metaphase II.
| Media Composition | Number of Matured (MII) Oocytes | |
|---|---|---|
| MEM α | (1) hCG 60 IU/L + FSH 200 mIU/mL | 4 |
| (2) hCG 60 IU/L + EGF 5 mg/mL | 8 | |
| (3) hCG 100 IU/L + EGF 5 mg/mL | 4 | |
| Universal IVF | (4) hCG 60 IU/L + FSH 200 mIU/mL | 9 |
| (5) hCG 60 IU/L + EGF 5 mg/mL | 3 | |
| (6) hCG 100 IU/L + EGF 5 mg/mL | 11 |
4.3. Sperm Collection
Ejaculated viable sperms of marmosets were collected using a 20-mV vibrator applied to the penis of a 3.5-year-old male marmoset. The collected sperms were washed with human tubal fluid (HTF) medium (Orgio) and incubated for 1 h for capacitation. Actively swimming sperms were injected into the prepared, matured oocytes.
4.4. Fertilization of in vitro Matured Oocytes
Matured MII oocytes were transferred to a fertilization dish containing SAGE-1 step media (Orgio), and sperms were injected into each drop. The dish was incubated for 1 day, and the formation of two pronucleus (PN) was judged as an occurrence of fertilization, and the development of the zygote into further stages was studied.
4.5. Single Cell Reverse Transcription of Oocyte
Each collected oocyte was washed with HBSS (Invitrogen) and mixed with RNA lysis buffer from the Single Cell-to-CT™ kit (Ambion, Waltham, MA, USA) and stored at −20 °C until use for qRT-PCR reaction. Reverse transcription of the single oocyte from marmoset was performed using a Single Cell-to-CT™ kit according to the manufacturer’s instructions. Briefly, the oocyte was mixed with the lysis solution with gentle pipetting and incubated for 5 min at 24 °C. Following the lysis, stop solution and RT mix were added, and the oocytes were incubated using the following conditions: 10 min at 25 °C, 60 min at 42 °C, and 5 min at 85 °C.
4.6. Evaluation of miRNAs Using qPCR
The synthesized cDNA were further used as templates for miRNA qPCR or target gene qRT-PCR. Briefly, miRNA PCR was performed using specific primers, and cDNAs were mixed with NCode™ Express SYBR® GreenER™ miRNA qPCR premix (Invitrogen) and amplified using the following conditions: initial incubation for 2 min at 50 °C, followed by 2 min at 95 °C, and then, 40 cycles of 15 s at 95 °C and 60 s at 60 °C. All of the reactions were performed in triplicate, and the Ct value was calculated based on the U6 expression. The specific primers used for qRPCR are shown in Table 2.
Table 2.
miRNAs specific primer sequences for single-cell profiling of marmoset oocytes.
| microRNA | Mature Sequence | Accession ID |
|---|---|---|
| Cja-let-7b | ugagguaguagguugugugguu | MIMAT0039325 |
| Cja-let-7c | ugagguaguagguuguaugguu | MIMAT0049314 |
| Cja-mir-27a | uucacaguggcuaaguuccgc | MIMAT0039518 |
| Cja-miR-224 | caagucacuagugguuccauuu | MIMAT0039393 |
4.7. qRT-PCR to Evaluate the Levels of Candidate Gene Expression
Synthesized cDNA were mixed with QuantiTect SYBR green PCR premix (Qiagen, Germantown, MD, USA) and specific primers. The amplification conditions were as follows: initial incubation at 95 °C for 15 min, followed by 45 cycles of denaturation at 95 °C for 15 s, annealing at 58 °C for 20 s, and extension at 72 °C for 30 s. All of the reactions were run in triplicate, and the relative gene expression was normalized using the corresponding GAPDH expression. The specific primers used for qRT-PCR are shown in Table 3.
Table 3.
Primer sequences for target gene qRT-PCR.
| Genes | Forward | Reverse |
|---|---|---|
| Cja_GAPDH | TGCTGGCGCTGAGTATGTG | AGCCCCAGCCTTCTCCAT |
| Cja_OCT4 | GGAACAAAACACGGAGGAGTC | CAGGGTGATCCTCTTCTGCTTC |
| Cja_BMP15 | CATTCACTGCGGTACATGCT | TAGTTGGAGATGATGGCGGT |
| Cja_VASA | TGGACATGATGCACCACCAGCA | TGGGCCAAAATTGGCAGGAGAAA |
| Cja_ZP1 | CACAGAACAGACCCCCACCTAG | CGCTGGTGGTGTGAGGGAAATG |
| Cja_ZP2 | ACTCCCCTCTGTGTTCTGTG | CTGCCTCCTCCCTTGTTT |
| Cja_ZP3 | TGTGGCACTCCAAGCCATGC | AGGGCGAGCCACAGGAACCAATG |
| Cja_SALL4 | AAGGCAACTTGAAGGTTCACTACA | GATGGCCAGCTTCCTTCCA |
| Cja_LIN28A | GACGTCTTTGTGCACCAGAGTAA | CGGCCTCACCTTCCTTCAA |
| Cja_PRDM1 | ATGAAGTTGCCTCCCAGCAA | TTCCTACAGGCACCCTGACT |
| Cja_PRDM14 | CGGGGAGAAGCCCTTCAAAT | CTCCTTGTGTGAACGTCGGA |
| Cja_DAZL | GAAGAAGTCGGGCAGTGCTT | AACGAGCAACTTCCCATGAA |
| Cja_DPPA3 | GCGGATGGGATCCTTCTGAG | GAGTAGCTTTCTCGGTCTGCT |
| Cja_NOBOX | GAAGACCACTATCCTGACAGTG | TCAGAAGTCAGCAGCATGGGG |
| Cja_SCP3 | TGGAAAACACAACAAGATCA | GCTATCTCTTGCTGCTGAGT |
4.8. Immunostaining of Oocytes
The oocytes were individually fixed with 4% paraformaldehyde (Sigma-Aldrich, Saint Louis, MO, USA) for 20 min at RT. After washing with phosphate-buffered saline (PBS, Sigma-Aldrich), the oocytes were incubated with 3% bovine serum albumin (BSA, Sigma-Aldrich) solution for 12 h to block the non-specific reaction. The oocytes were incubated with primary antibody (mouse alpha tubulin (SantaCruz Biotechnology, SantaCruz, CA, USA)) for 12 h at 4 °C and washed twice with PBS containing 0.05% Tween 20 (PBST). They were further incubated with a secondary antibody (Alexa Fluor 488-labeled donkey anti-mouse IgG (Molecular Probes, Calsbad, CA, USA)) for 1 h at RT and washed twice with PBST. DAPI solution (10 µM, Invitrogen) was added for the staining of the nucleus, and the images were taken using an EVOS-FL microscope (Thermo Scientific, Waltham, MA, USA).
4.9. Database Analysis
For analyses of microRNA and target mRNA correlations, Follicle Online (http://mcg.ustc.edu.cn/bsc/follicle), TargetScan (http://www.targetscan.org), miRTarBase (http://mirtarbase.mbc.nctu.edu.tw), STRING (http://string-db.org), DAVID Bioinformatics Resources 6.8 (https://david.ncifcrf.gov), and KEGG pathway enrichment databases were used.
4.10. Statistical Analysis
Statistical analysis was performed using SPSS version 21.0 (SPSS Inc., Chicago, IL, USA). Data were expressed as the mean ± standard errors and were analyzed by Student t-test. A p value of less than 0.05 was considered to be statistically significant.
Acknowledgments
The authors would like to express sincere thanks to the assistance of Sang Young Park, Sa Hoon Kim, Jae Bum Ahn, Kyung Min Noh, Bo Bin Choi, and Ji Won Lim.
Author Contributions
Y.Y.K. Investigation, Methodology; B.-C.K. Validation, Resources; J.W.Y. Resources; J.H.A. Methodology; Y.J.K. Data curation; H.K. Methodology; Z.R. Supervision; S.Y.K. Funding acquisition, Investigation, Resources, Supervision.
Funding
This study was supported by the grants of the Ministry of Science and Technology, Republic of Korea (2016R1E1A1A01943455).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Pfeffer P.L., Sisco B., Donnison M., Somers J., Smith C. Isolation of genes associated with developmental competency of bovine oocytes. Theriogenology. 2007;68:S84–S90. doi: 10.1016/j.theriogenology.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 2.Espey L.L. The distribution of collagenous connective tissue in rat ovarian follicles. Biol. Reprod. 1976;14:502–506. doi: 10.1095/biolreprod14.4.502. [DOI] [PubMed] [Google Scholar]
- 3.Espey L.L., Coons P.J. Factors which influence ovulatory regradation of rabbit ovarian follicles. Biol. Reprod. 1976;14:233–245. doi: 10.1095/biolreprod14.3.233. [DOI] [PubMed] [Google Scholar]
- 4.Kim Y.J., Park K.E., Kim Y.Y., Kim H., Ku S.Y., Suh C.S., Kim S.H., Choi Y.M. Effects of Estradiol on the Paracrine Regulator Expression of In Vitro Maturated Murine Ovarian Follicles. Tissue Eng. Regen. Med. 2017;14:31–38. doi: 10.1007/s13770-016-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park K.E., Ku S.Y., Jung K.C., Liu H.C., Kim Y.Y., Kim Y.J., Kim S.H., Choi Y.M., Kim J.G., Moon S.Y. Effects of urinary and recombinant gonadotropins on in vitro maturation outcomes of mouse preantral follicles. Reprod. Sci. 2013;20:909–916. doi: 10.1177/1933719112468948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas F.H., Leask R., Srsen V., Riley S.C., Spears N., Telfer E.E. Effect of ascorbic acid on health and morphology of bovine preantral follicles during long-term culture. Reproduction. 2001;122:487–495. doi: 10.1530/rep.0.1220487. [DOI] [PubMed] [Google Scholar]
- 7.Kim Y.Y., Min H., Kim H., Choi Y.M., Liu H.C., Ku S.Y. Differential MicroRNA Expression Profile of Human Embryonic Stem Cell-Derived Cardiac Lineage Cells. Tissue Eng. Regen. Med. 2017;14:163–169. doi: 10.1007/s13770-017-0051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwatsuki-Horimoto K., Nakajima N., Kiso M., Takahashi K., Ito M., Inoue T., Horiuchi M., Okahara N., Sasaki E., Hasegawa H., et al. The Marmoset as an Animal Model of Influenza: Infection With A(H1N1)pdm09 and Highly Pathogenic A(H5N1) Viruses via the Conventional or Tracheal Spray Route. Front. Microbiol. 2018;9:844. doi: 10.3389/fmicb.2018.00844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanazawa K., Mueller T., Becker T., Heistermann M., Behr R., Sasaki E. Minimally invasive transabdominal collection of preimplantation embryos from the common marmoset monkey (Callithrix jacchus) Theriogenology. 2012;78:811–816. doi: 10.1016/j.theriogenology.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 10.Ambros V. MicroRNA pathways in flies and worms: Growth, death, fat, stress, and timing. Cell. 2003;113:673–676. doi: 10.1016/S0092-8674(03)00428-8. [DOI] [PubMed] [Google Scholar]
- 11.Lai E.C. microRNAs: Runts of the genome assert themselves. Curr. Biol. 2003;13:R925–R936. doi: 10.1016/j.cub.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 12.Tay Y., Zhang J., Thomson A.M., Lim B., Rigoutsos I. MicroRNAs to Nanog, Oct4 and Sox2 coding regions modulate embryonic stem cell differentiation. Nature. 2008;455:1124–1128. doi: 10.1038/nature07299. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y., Baskerville S., Shenoy A., Babiarz J.E., Baehner L., Blelloch R. Embryonic stem cell-specific microRNAs regulate the G1-S transition and promote rapid proliferation. Nat. Genet. 2008;40:1478–1483. doi: 10.1038/ng.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mondou E., Dufort I., Gohin M., Fournier E., Sirard M.A. Analysis of microRNAs and their precursors in bovine early embryonic development. Mol. Hum. Reprod. 2012;18:425–434. doi: 10.1093/molehr/gas015. [DOI] [PubMed] [Google Scholar]
- 15.Chakrabarty A., Tranguch S., Daikoku T., Jensen K., Furneaux H., Dey S.K. MicroRNA regulation of cyclooxygenase-2 during embryo implantation. Proc. Natl. Acad. Sci. USA. 2007;104:15144–15149. doi: 10.1073/pnas.0705917104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCallie B., Schoolcraft W.B., Katz-Jaffe M.G. Aberration of blastocyst microRNA expression is associated with human infertility. Fertil. Steril. 2010;93:2374–2382. doi: 10.1016/j.fertnstert.2009.01.069. [DOI] [PubMed] [Google Scholar]
- 17.Kim Y.J., Ku S.Y., Kim Y.Y., Liu H.C., Chi S.W., Kim S.H., Choi Y.M., Kim J.G., Moon S.Y. MicroRNAs transfected into granulosa cells may regulate oocyte meiotic competence during in vitro maturation of mouse follicles. Hum. Reprod. 2013;28:3050–3061. doi: 10.1093/humrep/det338. [DOI] [PubMed] [Google Scholar]
- 18.Kim Y.J., Ku S.Y., Rosenwaks Z., Liu H.C., Chi S.W., Kang J.S., Lee W.J., Jung K.C., Kim S.H., Choi Y.M., et al. MicroRNA expression profiles are altered by gonadotropins and vitamin C status during in vitro follicular growth. Reprod. Sci. 2010;17:1081–1089. doi: 10.1177/1933719110377663. [DOI] [PubMed] [Google Scholar]
- 19.Kim Y.Y., Kim Y.J., Cho K.M., Kim S.H., Park K.E., Kang B.C., Jung K.C., Kim M.S., Ku S.Y. The expression profile of angiotensin system on thawed murine ovaries. Tissue Eng. Regen. Med. 2016;13:724–731. doi: 10.1007/s13770-016-0009-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim Y.Y., Tamadon A., Ku S.Y. Potential Use of Antiapoptotic Proteins and Noncoding RNAs for Efficient In Vitro Follicular Maturation and Ovarian Bioengineering. Tissue Eng. Part B Rev. 2017;23:142–158. doi: 10.1089/ten.teb.2016.0156. [DOI] [PubMed] [Google Scholar]
- 21.Amanai M., Brahmajosyula M., Perry A.C. A restricted role for sperm-borne microRNAs in mammalian fertilization. Biol. Reprod. 2006;75:877–884. doi: 10.1095/biolreprod.106.056499. [DOI] [PubMed] [Google Scholar]
- 22.Kishi N., Sato K., Sasaki E., Okano H. Common marmoset as a new model animal for neuroscience research and genome editing technology. Dev. Growth Differ. 2014;56:53–62. doi: 10.1111/dgd.12109. [DOI] [PubMed] [Google Scholar]
- 23.Sato K., Kuroki Y., Kumita W., Fujiyama A., Toyoda A., Kawai J., Iriki A., Sasaki E., Okano H., Sakakibara Y. Resequencing of the common marmoset genome improves genome assemblies and gene-coding sequence analysis. Sci. Rep. 2015;5:16894. doi: 10.1038/srep16894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J., Liu Y., Dong D., Zhang Z. Evolution of an X-linked primate-specific micro RNA cluster. Mol. Biol. Evol. 2010;27:671–683. doi: 10.1093/molbev/msp284. [DOI] [PubMed] [Google Scholar]
- 25.Lagana A.S., Vitale S.G., Ban Frangez H., Vrtacnik-Bokal E., D’Anna R. Vitamin D in human reproduction: The more, the better? An evidence-based critical appraisal. Eur. Rev. Med. Pharmacol. Sci. 2017;21:4243–4251. [PubMed] [Google Scholar]
- 26.Nandi A., Sinha N., Ong E., Sonmez H., Poretsky L. Is there a role for vitamin D in human reproduction? Horm. Mol. Biol. Clin. Investig. 2016;25:15–28. doi: 10.1515/hmbci-2015-0051. [DOI] [PubMed] [Google Scholar]
- 27.Vitale S.G., Rossetti P., Corrado F., Rapisarda A.M., La Vignera S., Condorelli R.A., Valenti G., Sapia F., Lagana A.S., Buscema M. How to Achieve High-Quality Oocytes? The Key Role of Myo-Inositol and Melatonin. Int. J. Endocrinol. 2016;2016:4987436. doi: 10.1155/2016/4987436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reyes-Munoz E., Sathyapalan T., Rossetti P., Shah M., Long M., Buscema M., Valenti G., La Rosa V.L., Cianci S., Vitale S.G. Polycystic Ovary Syndrome: Implication for Drug Metabolism on Assisted Reproductive Techniques-A Literature Review. Adv. Ther. 2018;35:1805–1815. doi: 10.1007/s12325-018-0810-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu M., Fazleabas A.T., Shikanov A., Jackson E., Barrett S.L., Hirshfeld-Cytron J., Kiesewetter S.E., Shea L.D., Woodruff T.K. In vitro oocyte maturation and preantral follicle culture from the luteal-phase baboon ovary produce mature oocytes. Biol. Reprod. 2011;84:689–697. doi: 10.1095/biolreprod.110.088674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uehara S., Uno Y., Yuki Y., Inoue T., Sasaki E., Yamazaki H. A New Marmoset P450 4F12 Enzyme Expressed in Small Intestines and Livers Efficiently Metabolizes Antihistaminic Drug Ebastine. Drug Metab. Dispos. 2016;44:833–841. doi: 10.1124/dmd.116.070367. [DOI] [PubMed] [Google Scholar]
- 31.Shimada S., Nunomura S., Mori S., Suemizu H., Itoh T., Takabayashi S., Okada Y., Yahata T., Shiina T., Katoh H., et al. Common marmoset CD117+ hematopoietic cells possess multipotency. Int. Immunol. 2015;27:567–577. doi: 10.1093/intimm/dxv031. [DOI] [PubMed] [Google Scholar]
- 32.Sadakane O., Watakabe A., Ohtsuka M., Takaji M., Sasaki T., Kasai M., Isa T., Kato G., Nabekura J., Mizukami H., et al. In Vivo Two-Photon Imaging of Dendritic Spines in Marmoset Neocortex. eNeuro. 2015;2 doi: 10.1523/ENEURO.0019-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin Z.Y., Hikabe O., Suzuki S., Hirano T., Siomi H., Sasaki E., Imamura M., Okano H. Sphere-formation culture of testicular germ cells in the common marmoset, a small New World monkey. Primates. 2016;57:129–135. doi: 10.1007/s10329-015-0500-4. [DOI] [PubMed] [Google Scholar]
- 34.Debowski K., Drummer C., Lentes J., Cors M., Dressel R., Lingner T., Salinas-Riester G., Fuchs S., Sasaki E., Behr R. The transcriptomes of novel marmoset monkey embryonic stem cell lines reflect distinct genomic features. Sci. Rep. 2016;6:29122. doi: 10.1038/srep29122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogonuki N., Inoue H., Matoba S., Kurotaki Y.K., Kassai H., Abe Y., Sasaki E., Aiba A., Ogura A. Oocyte-activating capacity of fresh and frozen-thawed spermatids in the common marmoset (Callithrix jacchus) Mol. Reprod. Dev. 2018;85:376–386. doi: 10.1002/mrd.22971. [DOI] [PubMed] [Google Scholar]
- 36.Gilchrist R.B., Nayudu P.L., Nowshari M.A., Hodges J.K. Meiotic competence of marmoset monkey oocytes is related to follicle size and oocyte-somatic cell associations. Biol. Reprod. 1995;52:1234–1243. doi: 10.1095/biolreprod52.6.1234. [DOI] [PubMed] [Google Scholar]
- 37.Grupen C.G., Gilchrist R.B., Nayudu P.L., Barry M.F., Schulz S.J., Ritter L.J., Armstrong D.T. Effects of ovarian stimulation, with and without human chorionic gonadotrophin, on oocyte meiotic and developmental competence in the marmoset monkey (Callithrix jacchus) Theriogenology. 2007;68:861–872. doi: 10.1016/j.theriogenology.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 38.Tkachenko O.Y., Delimitreva S., Heistermann M., Scheerer-Bernhard J.U., Wedi E., Nayudu P.L. Critical estradiol dose optimization for oocyte in vitro maturation in the common marmoset. Theriogenology. 2015;83:1254–1263. doi: 10.1016/j.theriogenology.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 39.Yun J.W., Kim Y.Y., Ahn J.H., Kang B.C., Ku S.Y. Use of nonhuman primates for the development of bioengineered female reproductive organs. Tissue Eng. Regen. Med. 2016;13:323–334. doi: 10.1007/s13770-016-9091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim Y.J., Kim Y.Y., Kang B.C., Kim M.S., Ko I.K., Liu H.C., Rosenwaks Z., Ku S.Y. Induction of multiple ovulation via modulation of angiotensin II receptors in in vitro ovarian follicle culture models. J. Tissue Eng. Regen. Med. 2017;11:3100–3110. doi: 10.1002/term.2214. [DOI] [PubMed] [Google Scholar]
- 41.Kim Y.Y., Yun J.W., Kim J.M., Park C.G., Rosenwaks Z., Liu H.C., Kang B.C., Ku S.Y. Gonadotropin ratio affects the in vitro growth of rhesus ovarian preantral follicles. J. Investig. Med. 2016;64:888–893. doi: 10.1136/jim-2015-000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kovacovicova K., Awadova T., Mikel P., Anger M. In Vitro Maturation of Mouse Oocytes Increases the Level of Kif11/Eg5 on Meiosis II Spindles. Biol. Reprod. 2016;95:18. doi: 10.1095/biolreprod.115.133900. [DOI] [PubMed] [Google Scholar]
- 43.Vanhoutte L., Nogueira D., Dumortier F., De Sutter P. Assessment of a new in vitro maturation system for mouse and human cumulus-enclosed oocytes: Three-dimensional prematuration culture in the presence of a phosphodiesterase 3-inhibitor. Hum. Reprod. 2009;24:1946–1959. doi: 10.1093/humrep/dep104. [DOI] [PubMed] [Google Scholar]
- 44.Tkachenko O.Y., Delimitreva S., Isachenko E., Valle R.R., Michelmann H.W., Berenson A., Nayudu P.L. Epidermal growth factor effects on marmoset monkey (Callithrix jacchus) oocyte in vitro maturation, IVF and embryo development are altered by gonadotrophin concentration during oocyte maturation. Hum. Reprod. 2010;25:2047–2058. doi: 10.1093/humrep/deq148. [DOI] [PubMed] [Google Scholar]
- 45.Delimitreva S., Zhivkova R., Isachenko E., Umland N., Nayudu P.L. Meiotic abnormalities in in vitro-matured marmoset monkey (Callithrix jacchus) oocytes: Development of a non-human primate model to investigate causal factors. Hum. Reprod. 2006;21:240–247. doi: 10.1093/humrep/dei283. [DOI] [PubMed] [Google Scholar]
- 46.Kanda A., Nobukiyo A., Yoshioka M., Hatakeyama T., Sotomaru Y. Quality of common marmoset (Callithrix jacchus) oocytes collected after ovarian stimulation. Theriogenology. 2018;106:221–226. doi: 10.1016/j.theriogenology.2017.10.023. [DOI] [PubMed] [Google Scholar]
- 47.Ringuette M.J., Sobieski D.A., Chamow S.M., Dean J. Oocyte-specific gene expression: Molecular characterization of a cDNA coding for ZP-3, the sperm receptor of the mouse zona pellucida. Proc. Natl. Acad. Sci. USA. 1986;83:4341–4345. doi: 10.1073/pnas.83.12.4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Philpott C.C., Ringuette M.J., Dean J. Oocyte-specific expression and developmental regulation of ZP3, the sperm receptor of the mouse zona pellucida. Dev. Biol. 1987;121:568–575. doi: 10.1016/0012-1606(87)90192-8. [DOI] [PubMed] [Google Scholar]
- 49.Murayama Y., Mizuno J., Kamakura H., Fueta Y., Nakamura H., Akaishi K., Anzai K., Watanabe A., Inui H., Omata S. Mouse zona pellucida dynamically changes its elasticity during oocyte maturation, fertilization and early embryo development. Hum. Cell. 2006;19:119–125. doi: 10.1111/j.1749-0774.2006.00019.x. [DOI] [PubMed] [Google Scholar]
- 50.Newport A., Carroll J. Structure and composition of the zona pellucida of the mouse oocyte. Biochem. Soc. Trans. 1976;4:896–897. doi: 10.1042/bst0040896. [DOI] [PubMed] [Google Scholar]
- 51.Topper E.K., Kruijt L., Calvete J., Mann K., Topfer-Petersen E., Woelders H. Identification of bovine zona pellucida glycoproteins. Mol. Reprod. Dev. 1997;46:344–350. doi: 10.1002/(SICI)1098-2795(199703)46:3<344::AID-MRD13>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 52.Kolluri S.K., Kaul R., Banerjee K., Gupta S.K. Nucleotide sequence of cDNA encoding bonnet monkey (Macaca radiata) zona pellucida glycoprotein-ZP3. Reprod. Fertil. Dev. 1995;7:1209–1212. doi: 10.1071/RD9951209. [DOI] [PubMed] [Google Scholar]
- 53.Gupta S.K., Sharma M., Behera A.K., Bisht R., Kaul R. Sequence of complementary deoxyribonucleic acid encoding bonnet monkey (Macaca radiata) zona pellucida glycoprotein-ZP1 and its high-level expression in Escherichia coli. Biol. Reprod. 1997;57:532–538. doi: 10.1095/biolreprod57.3.532. [DOI] [PubMed] [Google Scholar]
- 54.Bogner K., Hinsch K.D., Nayudu P., Konrad L., Cassara C., Hinsch E. Localization and synthesis of zona pellucida proteins in the marmoset monkey (Callithrix jacchus) ovary. Mol. Hum. Reprod. 2004;10:481–488. doi: 10.1093/molehr/gah074. [DOI] [PubMed] [Google Scholar]
- 55.Martinez M.L., Fontenot G.K., Harris J.D. The expression and localization of zona pellucida glycoproteins and mRNA in cynomolgus monkeys (Macaca fascicularis) J. Reprod. Fertil. Suppl. 1996;50:35–41. [PubMed] [Google Scholar]
- 56.Konrad L., Kuhnert S., Nayudu P.L., Einspanier R., Hinsch K.D., Hinsch E. Quantification of ZP1, ZP2 and ZP3 mRNA of marmoset monkey (Callithrix jacchus) oocytes from periantral and antral follicles. Andrologia. 2012;44:349–353. doi: 10.1111/j.1439-0272.2011.01188.x. [DOI] [PubMed] [Google Scholar]
- 57.Lim L.P., Glasner M.E., Yekta S., Burge C.B., Bartel D.P. Vertebrate microRNA genes. Science. 2003;299:1540. doi: 10.1126/science.1080372. [DOI] [PubMed] [Google Scholar]
- 58.Hawkins S.M., Matzuk M.M. Oocyte-somatic cell communication and microRNA function in the ovary. Ann. Endocrinol. 2010;71:144–148. doi: 10.1016/j.ando.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Han X., Xue R., Yuan H.J., Wang T.Y., Lin J., Zhang J., Liang B., Tan J.H. MicroRNA-21 plays a pivotal role in the oocyte-secreted factor-induced suppression of cumulus cell apoptosis. Biol. Reprod. 2017;96:1167–1180. doi: 10.1093/biolre/iox044. [DOI] [PubMed] [Google Scholar]
- 60.Li X., Wang H., Sheng Y., Wang Z. MicroRNA-224 delays oocyte maturation through targeting Ptx3 in cumulus cells. Mech. Dev. 2017;143:20–25. doi: 10.1016/j.mod.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 61.Pan B., Toms D., Li J. MicroRNA-574 suppresses oocyte maturation via targeting hyaluronan synthase 2 in porcine cumulus cells. Am. J. Physiol. Cell Physiol. 2018;314:C268–C277. doi: 10.1152/ajpcell.00065.2017. [DOI] [PubMed] [Google Scholar]
- 62.Pan B., Toms D., Shen W., Li J. MicroRNA-378 regulates oocyte maturation via the suppression of aromatase in porcine cumulus cells. Am. J. Physiol. Endocrinol. Metab. 2015;308:E525–E534. doi: 10.1152/ajpendo.00480.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miles J.R., McDaneld T.G., Wiedmann R.T., Cushman R.A., Echternkamp S.E., Vallet J.L., Smith T.P. MicroRNA expression profile in bovine cumulus-oocyte complexes: Possible role of let-7 and miR-106a in the development of bovine oocytes. Anim. Reprod. Sci. 2012;130:16–26. doi: 10.1016/j.anireprosci.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 64.Cao R., Wu W.J., Zhou X.L., Xiao P., Wang Y., Liu H.L. Expression and preliminary functional profiling of the let-7 family during porcine ovary follicle atresia. Mol. Cells. 2015;38:304–311. doi: 10.14348/molcells.2015.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang M., Liu M., Sun J., Jia L., Ma S., Gao J., Xu Y., Zhang H., Tsang S.Y., Li X. MicroRNA-27a-3p affects estradiol and androgen imbalance by targeting Creb1 in the granulosa cells in mouse polycytic ovary syndrome model. Reprod. Biol. 2017;17:295–304. doi: 10.1016/j.repbio.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 66.Wang M., Sun J., Xu B., Chrusciel M., Gao J., Bazert M., Stelmaszewska J., Xu Y., Zhang H., Pawelczyk L., et al. Functional Characterization of MicroRNA-27a-3p Expression in Human Polycystic Ovary Syndrome. Endocrinology. 2018;159:297–309. doi: 10.1210/en.2017-00219. [DOI] [PubMed] [Google Scholar]