Abstract
With the aim to discuss the similarities and differences of phytochemicals in Moringa oleifera leaves collected from China (CML) and India (IML) in mind, comparative ultra-performance liquid chromatography coupled with quadrupole-time-of-flight mass spectrometry (UPLC-QTOF-MS) analysis was performed in this study. A screening analysis based on a UNIFI platform was first carried out to discuss the similarities. Next, untargeted metabolomic analysis based on multivariate statistical analysis was performed to discover the differences. As a result, a total of 122 components, containing 118 shared constituents, were characterized from CML and IML. The structure types included flavonoids, alkaloids, glyosides, organic acids and organic acid esters, iridoids, lignans, and steroids, etc. For CML, 121 compounds were characterized; among these, 18 potential biomarkers with higher contents enabled differentiation from IML. For IML, 119 compounds were characterized; among these, 12 potential biomarkers with higher contents enabled differentiation from CML. It could be concluded that both CML and IML are rich in phytochemicals and that CML is similar to IML in the kinds of the compounds it contains, except for the significant differences in the contents of some compounds. This comprehensive phytochemical profile study provides a basis for explaining the effect of different growth environments on secondary metabolites and exists as a reference for further research into or applications of CML in China.
Keywords: Moringa oleifera leaves, analysis, UPLC-QTOF-MS, China, India
1. Introduction
Moringa oleifera, a herb native to India [1] which is also known as “miracle tree” or “the diamond in the plant”, has been widely cultivated throughout the world for its multiple uses such as its being a source of nutrients and a medical herb [2]. Most studies have focused on the leaves of the plant grown in India, Africa, or Madagascar [3,4]. The Moringa oleifera leaf (ML) have been proven to have antioxidant [5,6], anti-inflammatory [7,8], anticancer [9,10], anti-hypertensive [11], hypolipidemic [12], hypoglycemic [13,14], antimicrobial [15,16], and hepatoprotective [10,17] pharmacological activities. It has also been reported that ML contains many phytoconstituents such as flavonoids, alkaloids, steroids, saponins, glucosinolates, tannis, phenolic acids, and terpenes, etc. [18]. Certainly, its numerous pharmacological effects are due to the diversity of the phytochemicals in ML [19].
In China, as a complement to medicinal plant resources, Moringa oleifera was introduced from India in the 1960s and had been cultivated on a large scale in Guangdong Province, Yunnan Province, and other areas since then [20]. Additionally, ML was approved as a new food resource by the Chinese government in 2012 [21]. In China, relative research on extraction, preparation, and activity evaluation has been carried out recently, and there have been some achievements [22,23]. However, there has been a lack of profound research on the comprehensive screening and identification of the chemical constituents of ML grown in China. Furthermore, just as with other natural plants, M. oleifera ecotypes/cultivars differ from each other and can show many differences in leaf-mass production, growth performance, and secondary plant metabolite contents [24,25]. Therefore, with an aim to evaluate the similarities and the differences between the chemical constituents of Chinese Moringa oleifera leaf (CML) and Indian Moringa oleifera leaf (IML), a comparative analysis of the phytochemical composition of these two kinds of ML was performed in this study. On one hand, a comprehensive screening analysis of chemical components may be conducted to evaluate the similarity of CML to IML. During this section, a combination of ultra-high-performance liquid chromatography (UPLC) separation, quadrupole time-of-flight tandem mass spectrometry (QTOF-MS) detection and a UNIFI platform automated data process would be applied [26,27,28,29,30,31]. The accurate and specific mass could be provided by HR-MS when the coeluting constituents possess different m/z values. UNIFI might efficiently integrate data acquisition or mining and search libraries, and could generate reports using its comprehensive, simple, high throughput platform. The shared constituents of the Chinese and India Moringa oleifera leaves could be evaluated. On the other hand, with an aim to reveal the diversity of the metabolites, the untargeted metabolomics might be used to profile diverse classes of metabolites and compare the overall small-molecule metabolites of two kinds of samples [32]. This means a combination of UPLC separation, QTOF-MS detection, and multivariate statistical analyses, such as principal component analysis (PCA) and orthogonal projections to latent structures discriminant analysis (OPLS-DA), would be used to profile these two leaves.
The study in this paper comparatively analyzes the chemical constituents of Moringa oleifera leaves in China and India for the first time and determines the similarities and differences between these two items. Our data might support further research and the exploration of potential applications in China.
2. Materials and Methods
2.1. Materials and Reagents
CML and IML were collected from their respective cultivation areas or purchased from herbal markets in China or India (Table 1). The identity of the Moringa oleifera leaf was confirmed by the authors and the corresponding voucher specimens were deposited in the Research Center of Natural Drug, School of Pharmaceutical Sciences, Jilin University, China.
Table 1.
The list of the tested samples from China and India. Legend: CML, Chinese Moringa oleifera leaf; IML, Indian Moringa oleifera leaf.
| Species | Sample No. | Source | Collection Time |
|---|---|---|---|
| CML | 1 | Pu‘er City, Yunnan Province, China; market | November 2017 |
| 2 | Xishuangbanna City, Yunnan Province, China; field | March 2018 | |
| 3 | Shaoguan City, Guangdong Province, China; market | January 2017 | |
| 4 | Guangzhou City, Guangdong Province, China; field | December 2017 | |
| 5 | Danzhou City, Hainan Province, China; market | January 2018 | |
| 6 | Changjiang City, Hainan Province, China; market | March 2017 | |
| IML | 1 | Howrah, India; market | December 2017 |
| 2 | Howrah, India; market | November 2017 | |
| 3 | Tamil Nadu, India; market | February 2018 | |
| 4 | Tamil Nadu, India; market | March 2018 | |
| 5 | Maharastra, India; market | January 2018 | |
| 6 | Maharastra, India; market | January 2017 |
Methanol and acetonitrile (Fisher Chemical Company, USA) were used as they were suitable for UPLC-MS. Deionized water was purified using a Millipore water purification system (Millipore, Billerica, MA, USA). Formic acid for UPLC was purchased from the Sigma-Aldrich Company. All other chemicals were of analytical grade.
Standard compounds α-maltose, adenosine, catechin, chlorogenic acid, rutin, quercetin, kaempferol, caffeic acid, oleic acid, epicatechin, hyperoside, kaempferol-3-O-rutinoside, isorhamnetin, isorhamnetin-3-O-rutinoside, luteolin, scutellarein, methyl palmitate, ricinoleic acid, linolenic acid, dibutyl sebacate, eugenol, azelaic acid, (−)-epiafzelechin, methyl myristate, and 2′-hydroxygenistein were purchased from the National Institutes for Food and Drug Control (Beijing, China). Other reference compounds including parinaric acid, quinic acid, and 1,3-dicaffeoylquinic acid were purchased from Beijing Zhongke Quality Inspection Biotechnology Co., Ltd. (Beijing, China).
2.2. Sample Preparation and Extraction
Stalks were removed and the leaves air-dried, grinded, and sieved (Chinese National Standard Sieve No. 3, R40/3 series) to obtain a homogeneous powder. Then, the powder (1.0 g) was extracted with 80% methanol (1.0 L) at 80 °C thrice (for 3 h each time). After being filtered, the extraction solution was combined, concentrated, and evaporated to dryness. The desiccated extractions (all approximately 15 mg) were finally dissolved and diluted with 80% methanol 10.0 mL. The solution was filtered with a syringe filter (0.22 μm) and then injected into the UPLC system. Additionally, to ensure the suitability and stability consistency of MS analysis, a quality control (QC) sample was prepared by pooling the same volume (50 μL) from every sample. Through the whole worklist, 3 QC injections were performed randomly. The volume injected for the samples and QC was 2 μL for each run.
2.3. UPLC-QTOF-MSE
UPLC-QTOF-MSE analysis was performed on a Waters Xevo G2-XS QTOF mass spectrometer (Waters Co., Milford, MA, USA) equipped with a UPLC system through an electrospray ionization (ESI) interface. Chromatographic separation was performed on an ACQUITY UPLC BEH C18 (100 mm × 2.1 mm, 1.7 μm) column provided by Waters Corporation. The mobile phases were composed of eluent A (0.1% formic acid in water, v/v) and eluent B (0.1% formic acid in acetonitrile, v/v) with flow rate of 0.4 mL/min. The elution conditions applied were: 0–2 min, 10% B; 2–26 min, 10–100% B; 26–29 min, 100% B; 29–29.1 min, 100–10% B; 29.1–32 min, 10% B. Mixtures of 90/10 and 10/90 water/acetonitrile were used as the weak wash solvent and the strong wash solvent, respectively. The temperatures of the column and autosampler were 30°C and 15 °C, respectively. The mass spectrum was acquired from 100 to 1500 Da in MSE mode. The positive mode conditions were as follows: capillary voltage, 2.6 kV; source temperature, 150 °C; cone voltage, 40 V; cone gas flow, 50 L/h; desolvation temperature, 400 °C; desolvation gas flow, 800 L/h. Negative mode conditions were identical to the positive mode conditions except for the capillary voltage (2.2 kV). During a single LC run, data acquisition was performed via the mass spectrometer by rapidly switching from a low collision energy (CE) scan to a high-CE scan in MSE mode. The collision energy of low energy function was set to 6 V while the ramp collision energy of high energy function was set to 20~40 V. Leucine enkephalin (LE) (m/z 554.2615 in ESI− mode and 556.2771 in ESI+ mode), the external reference of Lock Spray™, was infused at a constant flow of 10 μL/min. During acquisition, data were collected in continuum mode for the screening analysis and in centroid mode for the metabolomics analysis. Masslynx™ V4.1 workstation (Waters, Manchester, UK) was used to record the data.
2.4. Screening Analysis of Components of CML and IML by UNIFI Platform
To quickly identify the chemical compounds, the MS raw data, compressed with Waters Compression and Archival Tool v1.10, was automatedly screened and identified using the streamlined workflow of UNIFI 1.7.0 software (Waters, Manchester, UK) [30,31,32,33]. The parameters were as follows: for 2D peak detection, 200 was set as the minimum peak area; for 3D peak detection, the peak intensities of low energy and high energy were set as over 1000 and over 200 counts, respectively; mass error in the range of ±5 ppm was set for identified compounds; retention time in the range of ±0.1 min was allowed to match the reference substance. Generated predicted fragments from the structure were identified as the matching compounds. Negative adducts containing +COOH and -H and positive adducts containing +H and +Na were selected in the analysis. Leucine enkaplin was selected as the reference compound, and [M − H]− 554.2620 was used for the negative ion and [M + H]+ 556.2766 for the positive ion. Components were further verified by comparing reference substances with retention time and by comparing characteristic MS fragmentation patterns in the literature. The chemical information database used for the components was as follows: besides the in-house Traditional Medicine Library in the Waters UNIFI platform, the investigation of chemical constituents was conducted systematically. A self-built database of compounds that were reported in ML was established by searching online databases or internet search engines such as PubMed, Full-Text Database (CNKI), ChemSpider, Web of Science, and Medline. Chemical information including the component name, structures of the components, and molecular formula were available from the database.
2.5. Metabonomics Analysis of CML and IML
The raw data were processed for alignment, deconvolution, and data reduction, etc., with MarkerLynx XS V4.1 software (Waters, Milford, CT, USA) [34]. A Markerlynx processing method was first created, and its main parameters included: retention time (RT) range 0~26 min, minimum intensity 5%, mass range 100~1500 Da, mass tolerance 0.10, mass window 0.10, marker intensity threshold 2000 counts, retention time window 0.20, and noise elimination level 6. After processing the data, the results were able to be shown in Extended Statistics (XS) Viewer. m/z-RT pairs with corresponding intensities for all the detected peaks from each data file were listed. The same values of RT and m/z in different batches of samples were regarded as the same component. Furthermore, multivariate statistical analysis was performed. Firstly, PCA was used to show the pattern recognition and maximum variation aiming to obtain the overview and classification. Secondly, OPLS-DA in ESI+ and ESI− modes was performed in order to get the maximum separation between the CML and IML groups and to explore the potential chemical markers that contribute to the differences. Then, S-plots were created to provide visualization of the OPLS-DA predictive component loading to facilitate model interpretation. Meanwhile, the use of variable importance for the projection (VIP) was helpful in screening the different components, and metabolites with VIP value > 1.0 and p-value below 0.05 were considered as potential markers [32]. In addition, permutation testing was performed to provide reference distributions of the R2/Q2 values that could indicate statistical significance [35,36]. Simca 15.0 software (Umetrics, Malmö, Sweden) was used to show the analysis results [33,35].
3. Results
3.1. Identification of Components from CML and IML Based on the UNIFI Platform
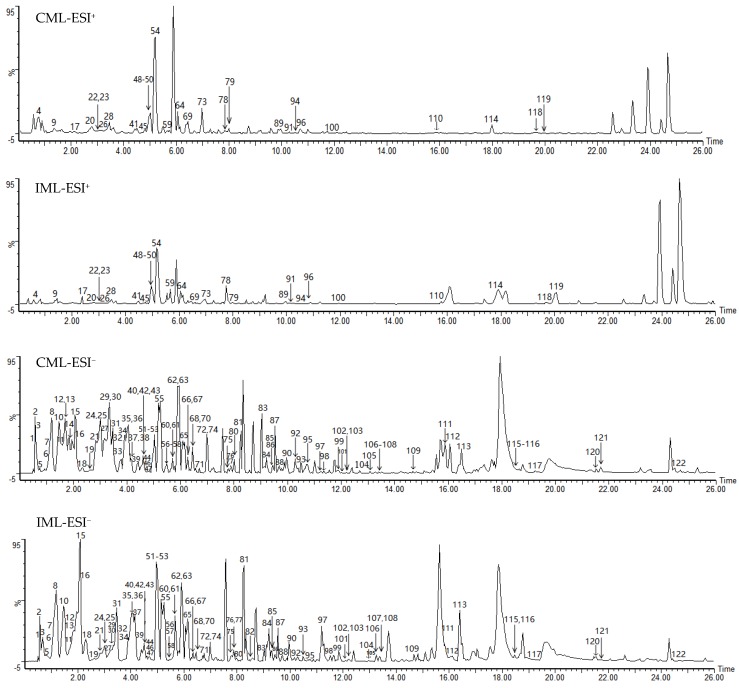
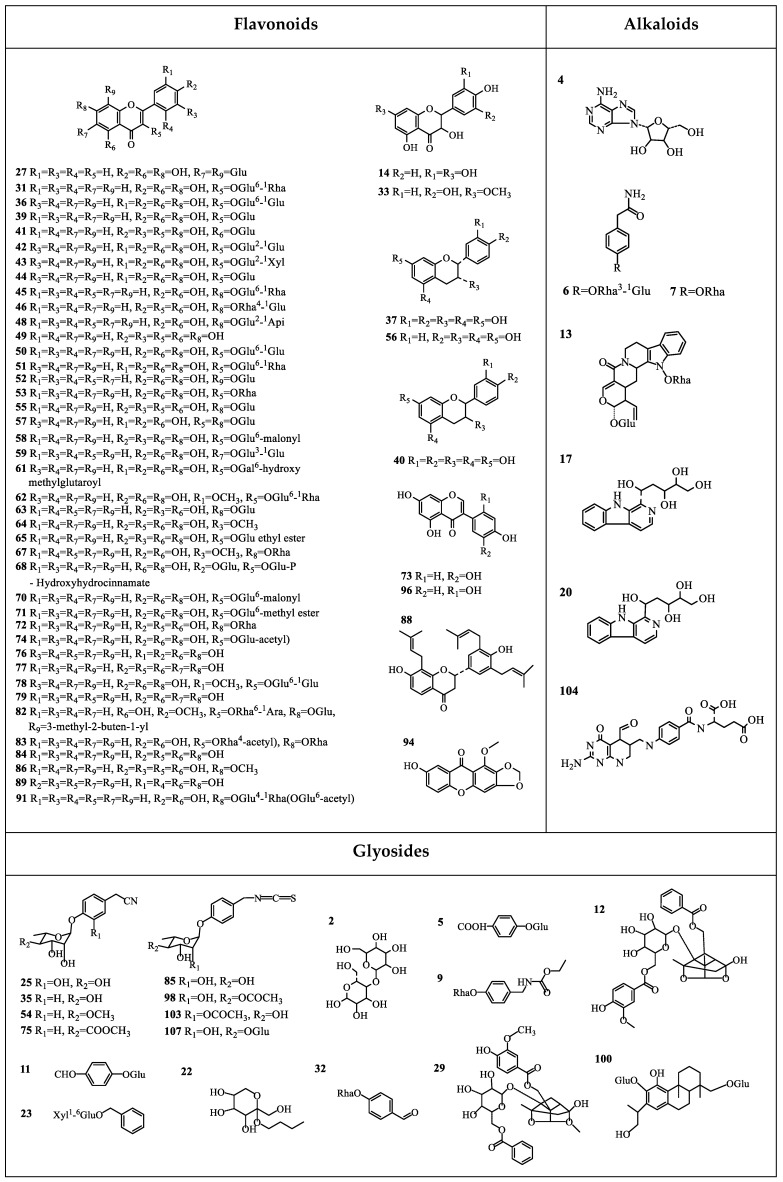
As a result of screening analysis, a total of 122 compounds were identified or tentatively characterized in both ESI+ and ESI− mode from CML and IML. There were 118 shared constituents identified in CML and IML. More specifically, 121 and 119 compounds were characterized from CML and IML, respectively (Table 2). Both of the two types of Moringa oleifera leaves are rich in natural components with various structural patterns, including flavonoids, alkaloids, glyosides, organic acids and organic acid esters, iridoids, lignans, and steroids, etc. Base peak intensity (BPI) chromatograms marked with the number of compounds are shown in Figure 1. The chemical structures of the compounds are summarized in Figure 2.
Table 2.
Compounds identified from CML and IML by ultra-high-performance liquid chromatography quadrupole time-of-flight tandem mass spectrometry UPLC-QTOF-MSE.
| No. | Retention Time (RT) (min) | Formula | Calculated Mass (Da) | Theoretical Mass (Da) | Mass Error (ppm) | MSE Fragmentation | Identification | Sources | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.59 | C7H12O6 | 192.0629 | 192.0634 | −2.6 | 191.0542[M − H]−, 173.0432[M-H-H2O]−, 145.0516[M-H-HCOOH]−, 137.0232[M-H-3H2O]−, 127.0401[M-H-H2O-HCOOH]− | Quinic acid | CML, IML | s |
| 2 | 0.60 | C12H22O11 | 342.1161 | 342.1162 | −0.3 | 387.1143[M + HCOO]−, 179.0554[M-H-Glu]− | α-Maltose | CML, IML | s |
| 3 | 0.62 | C16H18O9 | 354.0968 | 354.0951 | 4.8 | 353.0895[M − H]−, 335.0896[M-H-H2O]−, 190.0544[M-H-C9H7O3]−, 190.0391[M-H-3H2O-C6H5O2]−, 143.0346[M-H-HCOOH-C9H8O3]− | Cryptochlorogenic acid | CML, IML | [37] |
| 4 | 0.72 | C10H13N5O4 | 267.0968 | 267.0968 | 0.0 | 268.1041[M + H]+, 187.0620[M + H-C3H3N3]+, 161.0744[M + H-C4H3N4]+, 136.0612[M + H-Rib]+ | Adenosine | CML, IML | s |
| 5 | 0.80 | C13H16O8 | 300.0836 | 300.0845 | −2.9 | 299.0764[M − H]−, 178.0632[M-H-C7H5O2]−, 135.0231[M-H-Glu]−, 89.0347[M-H-Glu-HCOOH]− | Benzoic acid 4-O-β-glucoside | CML, IML | [18] |
| 6 | 1.03 | C20H29NO11 | 459.1739 | 459.1741 | −0.2 | 504.1721[M + HCOO]−, 427.1487[M-H-NH2-CH3]−, 307.0995[M-H-C8H9NO2]−, 279.1081[M-H-Glu]−, 150.0546[M-H-2Glu]− | 3 ′′-O-β-d-glucopyranosyl derivatives (marumoside B) | CML, IML | [38] |
| 7 | 1.04 | C14H19NO6 | 297.1210 | 297.1212 | −0.7 | 342.1192[M + HCOO]−, 262.0758[M-H-H2O-NH2]−, 149.0546[M-H-Rha]−, 105.0430[M-H-Rha-CONH2]− | 4 ′-Hydroxyphenylethanamide-α-l-rhamnopyranoside (marumoside A) | CML, IML | [38] |
| 8 | 1.18 | C16H18O9 | 354.0942 | 354.0951 | −2.4 | 353.0869[M − H]−, 281.1169[M-H-4H2O]−, 190.0546[M-H-C9H7O3]−, 161.0285[M-H-C7H12O6]−, 134.0436[M-H-C8H11O7]− | Neochlorogenic acid | CML, IML | [39] |
| 9 | 1.38 | C16H23NO7 | 341.1458 | 341.1475 | −4.7 | 342.1531[M + H]+, 261.1188[M + H-2H2O-C2H5O]+, 107.0492[M + H-Rha-C3H6NO2]+, 102.0550[M + H-Rha-C6H4]+ | O-Ethyl-4-[(α-l-rhamnosyloxy)-benzyl]carbamate | CML, IML | [18] |
| 10 | 1.47 | C7H6O4 | 154.0271 | 154.0266 | 3.2 | 153.0215[M − H]−, 135.0211[M-H-H2O]−, 89.0340[M-H-H2O-HCOOH]− | 3,4-Dihydroxy-benzoic acid | CML, IML | [40] |
| 11 | 1.51 | C13H16O7 | 300.0892 | 300.0896 | −1.5 | 299.0819[M − H]−, 160.0351[M-H-H2O-C7H5O2]−, 90.0343[M-H-Glu-HCOOH]− | Benzaldehyde 4-O-β-glucoside | CML, IML | [41] |
| 12 * | 1.70 | C31H34O14 | 630.1957 | 630.1949 | 1.3 | 675.1692[M + HCOO]−, 414.1127[M-H-Ph-CH3-C7H7O2]−, 353.0869[M-H-H2O-C7H6O-C8H8O3]−, 298.0797[M-H-CH3-C17H16O6]−, 222.0634[M-H-Ph-Glu-C8H7O3]− | Mudanpioside J | CML >> IML VIP: 2.73 p < 0.001 |
[42] |
| 13 | 1.71 | C32H40N2O13 | 660.2563 | 660.2530 | 4.6 | 705.2545[M + HCOO]−, 441.1367[M-H-Rha-C2H3-C2H4]−, 326.0797[M-H-Rha-C11H9N2]−, 263.0856[M-H-Rha-Glu-C4H5]−, 175.0444[M-H-Rha-Glu-C10H6N]− | N, α-l-Rhamnopyranosyl vincosamide | CML, IML | [43] |
| 14 * | 1.84 | C15H12O7 | 304.0573 | 304.0583 | −3.2 | 349.00618[M + HCOO]−, 285.0418[M-H-H2O]−, 162.0364[M-H-C6H5O4]−, 152.9691[M-H-CH3-C8H8O2]−, 132.0231[M-H-H2O-OCH3-C7H6O2]−, 130.0235[M-H-C11H9O2]− | Dihydroquercetin | CML VIP: 8.20 p < 0.001 |
[44] |
| 15 | 2.07 | C16H18O9 | 354.0951 | 354.0951 | 0.1 | 353.0878[M − H]−, 253.1035[M-H-3H2O-HCOOH]−, 190.0182[M-H-3H2O-C6H5O2]−, 144.0302[M-H-H2O-C7H11O6]−, 125.0251[M-H-H2O-HCOOH-C9H8O3]− | Chlorogenic acid | CML, IML | s |
| 16 | 2.09 | C17H20O9 | 368.1102 | 368.1107 | −1.5 | 367.1029[M − H]−, 336.0902[M-H-OCH3]−, 295.1124[M-H-4H2O]−, 243.0591[M-H-CH3-C6H5O2]−, 189.0549[M-H-CH3-C9H7O3]−, 178.0346[M-H-C8H13O5]− | Methyl-3-caffeoylquinate | CML, IML | [45] |
| 17 | 2.34 | C16H18N2O4 | 302.1254 | 302.1267 | −4.1 | 303.1327[M + H]+, 285.1232[M + H-H2O]+, 212.0983[M + H-H2O-C2H5O2]+, 176.0893[M + H-2H2O-C3H7O3]+ | Tangutorid E | CML, IML | [45] |
| 18 | 2.35 | C19H28O12 | 448.1578 | 448.1581 | −0.7 | 447.1505[M − H]−,417.0973[M-H-2CH3]−, 267.1031[M-H-Glu]−, 245.1016[M-H-OCOCH3-C6H7O4]−, 167.0480[M-H-Glu-C4H4O3]− | 8-O-Acetylshanzhiside methyl ester | CML, IML | [46] |
| 19 | 2.53 | C9H8O4 | 180.0414 | 180.0423 | −4.5 | 179.0335[M − H]−, 143.0430[M-H-2H2O]−, 133.0433[M-H-HCOOH]−, 108.0265[M-H-C3H3O2]− | Caffeic acid | CML, IML | s |
| 20 | 2.62 | C16H18N2O4 | 302.1257 | 302.1267 | −3.1 | 303.1330[M + H]+, 285.1248[M + H-H2O]+, 194.0881[M + H-2H2O-C2H5O2]+, 194.0895[M + H-H2O-C3H7O2]+, 118.0799[M + H-H2O-C11H7N2]+ | Tangutorid F | CML, IML | [45] |
| 21 | 2.87 | C17H20O9 | 368.1104 | 368.1107 | −0.9 | 367.1031[M − H]−, 336.0931[M-H-OCH3]−, 203.0655[M-H-C9H8O3]−, 188.0545[M-H-CH3-C9H8O3]−, 151.0384[M-H-2H2O-C9H8O4]− | Methyl-4-caffeoylquinate | CML, IML | [45] |
| 22 | 2.96 | C10H20O6 | 236.1260 | 236.1261 | 0.3 | 259.1153[M + Na]+, 219.1322[M + H-H2O]+, 176.0465[M + H-H2O-C3H7]+, 164.0694[M + H-C4H9O]+ | n-Butyl-β-d-fructopyranoside | CML, IML | [47] |
| 23 | 3.00 | C18H26O10 | 402.1536 | 402.1526 | 2.3 | 425.1428[M + Na]+, 296.1001[M + H-C7H7O]+, 253.1061[M + H-Xyl]+, 146.0584[M + H-Xyl-C7H7O]+, 73.0491[M + H-Glu-Xyl]+ | Benzyl-O-β-d-xylopyranosyl-(1→6)-β-d-glucopyranoside | CML, IML | [18] |
| 24 | 3.01 | C19H28O12 | 448.1587 | 448.1581 | 1.5 | 447.1514[M − H]−,398.1453[M-H-H2O-OCH3]−, 378.1102[M-H-3H2O-CH3]−, 291.0974[M-H-C8H12O3]−, 267.1025[M-H-Glu]−, 193.0447[M-H-Glu-OCH3-COCH3]− | 6-O-acetylshanzhiside methyl ester | CML, IML | [46] |
| 25 | 3.04 | C14H17NO6 | 295.1051 | 295.1056 | −1.7 | 294.0978[M-H]−, 268.1025[M-H-CN]−, 162.0436[M-H-C8H6NO]−, 130.0390[M-H-Rha]−, 104.0286[M-H-Rha-CN]− | Niaziridin | CML, IML | [48] |
| 26 | 3.13 | C9H8O3 | 164.0474 | 164.0473 | 0.3 | 165.0544[M + H]+, 147.0444[M + H-H2O]+, 119.0483[M + H-HCOOH]+ | o-Coumaric acid | CML, IML | [49] |
| 27 | 3.23 | C27H30O15 | 594.1590 | 594.1585 | 0.8 | 593.1517[M − H]−, 575.1371[M-H-H2O]−, 529.0871[M-H-H2O-Glu]−, 394.1305[M-H-2H2O-C9H6O3]− | Vicenin-2 | CML, IML | [50] |
| 28 | 3.32 | C9H8O3 | 164.0471 | 164.0473 | −1.4 | 165.0544[M + H]+, 147.0442[M + H-H2O]+, 119.0482[M + H-HCOOH]+, 107.0495[M + H-C2H2O2]+ | ρ-Coumaric acid | CML, IML | [49] |
| 29 * | 3.34 | C31H34O14 | 630.1943 | 630.1949 | −0.8 | 675.1939[M + HCOO]−, 464.0735[M-H-2CH3-C8H7O2]−, 339.0923[M-H-H2O-C7H5O2-C8H7O3]−, 223.0599[M-H-C7H7O2-Glu benzoate]−, 163.0386[M-H-C9H9O4-Glu benzoate]− | 6′-O-Benzoyl-4″-hydroxy-3″-methoxypaeoniflorin | CML >> IML VIP: 2.12 p < 0.001 |
[51] |
| 30 * | 3.35 | C16H18O8 | 338.997 | 338.1002 | −1.5 | 337.0930[M − H]−, 265.0787[M-H-4H2O]−, 173.0442[M-H-C9H7O3]−, 162.0386[M-H-C7H11O5]−, 127.0704[M-H-HCOOH-C9H7O3]− | 3-p-Coumaroylquinic acid | CML >> IML VIP: 9.19 p < 0.001 |
[52] |
| 31 | 3.47 | C27H30O15 | 594.1589 | 594.1585 | 0.7 | 593.1516[M − H]−, 575.1396[M-H-H2O]−, 411.0869[M-H-H2O-Rha]−, 287.0536[M-H-H2O-Rha-C6H4O3]−, 125.0302[M-H-Rha-Glu-C6H4O3]− | Kaempferol-3-O-rutinoside | CML, IML | s |
| 32 | 3.54 | C13H16O6 | 268.0942 | 268.0947 | −1.6 | 313.0924[M + HCOO]−, 213.0760[M-H-3H2O]−, 184.0768[M-H-3H2O-CHO]−, 147.0540[M-H-CH3-C7H5O]−, 103.0284[M-H-Rha]− | Benzaldehyde-4-O-α-l-rhamnopyranoside | CML, IML | [45] |
| 33 * | 3.55 | C16H14O7 | 318.0746 | 318.0740 | 1.9 | 363.0747[M + HCOO]−, 208.0473[M-H-C6H5O2]−, 193.0273[M-H-CH3-C6H5O2]−, 133.0452[M-H-H2O-C8H6O4]−, 121.0284[M-H-C9H8O5]− | Padmatin | CML VIP: 3.75 p < 0.001 |
s |
| 34 | 3.89 | C17H20O9 | 368.1097 | 368.1107 | −2.7 | 367.1025[M − H]−, 298.0387[M-H-3H2O-CH3]−, 288.1015[M-H-H2O-CH3-HCOOH]−, 192.0488[M-H-C7H11O5]−, 191.0629[M-H-C10H8O3]− | 4-Feruloylquinic acid | CML, IML | a |
| 35 | 4.02 | C14H17NO5 | 279.1100 | 279.1107 | −2.1 | 324.1082[M + HCOO]−, 188.0725[M-H-C3H6O]−, 147.0545[M-H-CH3-C8H6N]−, 114.0433[M-H-Rha]−, 88.0545[M-H-Rha-CN]− | Niazirin | CML, IML | [45] |
| 36 | 4.05 | C27H30O17 | 626.1487 | 626.1483 | 0.7 | 625.1414[M − H]−, 445.0853[M-H-Glu]−, 318.0205[M-H-Glu-H2O-C6H5O2]−, 324.1075[M-H-C15H9O7]−, 265.0333[M-H-2Glu]−, 275.0708[M-H-Glu-H2O-C7H4O4]− | QuerQuercetin-3-gentiobioside | CML, IML | a |
| 37 | 4.14 | C15H14O6 | 290.0784 | 290.0790 | −1.8 | 335.0766[M + HCOO]−, 162.0243[M-H-H2O-C6H5O2]−, 138.0291[M-H-H2O-C7H6O3]−, 120.0283[M-H-C8H9O4]−, 79.0342[M-H-H2O-C10H8O4]− | Epicatechin | CML, IML | s |
| 38 * | 4.16 | C18H22O8 | 366.1324 | 366.1315 | 2.5 | 411.1641[M + HCOO]−, 335.0765[M-H-2CH3]−, 232.0622[M-C9H9O]−, 173.0459[M-H-CH3-C10H9O3]−, 161.0243[M-CH3-Rha ethyl ester]− | 3-O-acetyl-2-O-p-methoxycinnamoyl-α-l-rhamnopyranose | CML VIP: 2.69 p < 0.001 |
[53] |
| 39 | 4.22 | C21H20O11 | 448.0999 | 448.1006 | −1.4 | 447.0926[M − H]−, 429.0850[M-H-H2O]−, 267.0395[M-H-Glu]−, 143.0288[M-H-Glu-C6H4O3]− | Astragalin | CML, IML | [54] |
| 40 | 4.47 | C15H14O6 | 290.0793 | 297.0790 | 0.6 | 335.0775[M + HCOO]−, 147.0436[M-H2O-C6H4O3]−, 137.0224[M-H-C8H8O3]−, 133.0295[M-H-H2O-C7H6O3]−, 90.0342[M-H-H2O-C9H9O4]− | Catechin | CML, IML | s |
| 41 | 4.49 | C21H20O12 | 464.0955 | 464.0949 | −1.2 | 465.1022[M + H]+, 285.0485[M + H-Glu]+, 231.0678[M + H-Glu-3H2O]+, 149.0150[M + H-Glu-C7H4O3]+, 152.0154[M + H-Glu-C8H5O2]+ | Hyperoside | CML, IML | s |
| 42 | 4.50 | C27H30O17 | 626.1475 | 626.1483 | −1.3 | 625.1402[M − H]−, 516.1277[M-H-C6H5O2]−, 396.0689[M-H-Glu-H2O-CH2OH]−, 265.0264[M-H-2Glu]−, 132.9991[M-H-2Glu-C8H5O2]− | Quercetin-3-sophoroside | CML, IML | a |
| 43 | 4.52 | C26H28O16 | 596.1400 | 596.1377 | 3.8 | 595.1327[M − H]−, 265.0264[M-H-Glu-Xyl]−, 138.0156[M-H-Glu-Xyl-H2O-C6H5O2]−, 115.9991[M-H-Glu-Xyl-C8H6O3]−, 144.0485[M-H-Xyl-C15H9O7]− | Quercetin-3-O-β-d-xylopyranosyl-(1→2)-β-d-glucopyranoside | CML, IML | a |
| 44 | 4.67 | C21H20O12 | 464.0939 | 464.0955 | −3.4 | 463.0866[M − H]−, 318.0758[M-H-2H2O-C6H5O2]−, 178.0513[M-H-C15H9O6]−, 159.0379[M-H-Glu-C6H4O3]− | Isoquercetin | CML, IML | [54] |
| 45 | 4.68 | C27H30O14 | 578.1635 | 578.1636 | −0.2 | 579.1707[M + H]+, 543.1466[M + H-2H2O]+, 415.1130[M + H-Rha]+, 322.0748[M + H-Rha-C6H5O]+, 235.0580[M + H-Glu-Rha]+ | Apigenin-7-O-rutinoside | CML, IML | [39] |
| 46 | 4.71 | C27H30O15 | 594.1596 | 594.1585 | 2.0 | 593.1524[M − H]−, 413.0899[M-H-Glu]−, 338.0756[M-H-Glu-H2O-C2HO2]−, 247.0305[M-H-Rha-Glu]−, 160.0677[M-H-Rha-C15H9O5]− | Kaempferol-3-O-α-l-rhamnoside-(1→4)-β-d-glucoside | CML, IML | a |
| 47 | 4.82 | C26H34O11 | 522.2118 | 522.2101 | 3.0 | 567.2100[M + HCOO]−, 461.2005[M-H-C2H4O]−, 341.1509[M-H-Glu]−, 401.1193[M-H-C9H12]−, 200.0871[M-H-Glu-H2O-C7H7O2]−, 134.0427[M-H-Glu-C12H15O3]− | Ligan glycoside A | CML, IML | b |
| 48 | 4.85 | C26H28O14 | 564.1475 | 564.1479 | −0.7 | 565.1548[M + H]+, 418.1217[M + H-C9H6O2]+, 298.0909[M + H-Api-C8H6O]+, 180.0776[M + H-Api-C15H9O4]+, 147.0593[M + H-Glu-Api-C6H3O2]+ | Apiin | CML, IML | [55] |
| 49 | 4.95 | C15H10O7 | 302.0429 | 302.0427 | 0.7 | 303.0501[M + H]+, 153.0162[M + H-C8H6O3]+, 151.0210[M + H-C7H4O4]+, 122.0388[M + H-C9H6O4]+ | Quercetin | CML, IML | s |
| 50 # | 4.97 | C27H30O16 | 610.1537 | 610.1534 | 0.6 | 611.1652[M + H]+, 447.1016[M + H-Rha]+, 267.0509[M + H-Glu-Rha]+, 158.0289[M + H-Glu-Rha-C6H5O2]+, 131.0222[M + H-Glu-Rha-C7H4O3]+ | Rutin | CML << IML VIP: 8.51 p < 0.001 |
s |
| 51 # | 5.01 | C27H30O16 | 610.1532 | 610.1534 | −0.3 | 609.1472[M − H]−, 427.0974[M-H-Rha-H2O]−, 336.0683[M-H-Rha-C6H5O2]−, 265.0326[M-H-Glu-Rha]−, 132.0015[M-H-Glu-Rha-C8H5O2]− | Quercetin-3-rutinoside | CML << IML VIP: 13.28 p < 0.001 |
[56] |
| 52 | 5.02 | C21H20O10 | 432.1048 | 432.1056 | −2.0 | 431.0975[M − H]−, 395.0746[M-H-2H2O]−, 338.0683[M-H-C6H5O]−, 251.0447[M-H-Glu]−, 100.0326[M-H-Glu-C7H3O4]− | Apigenin-8-C-glucoside | CML, IML | [39] |
| 53 | 5.04 | C21H20O10 | 432.1069 | 432.1056 | 2.7 | 477.1051[M + HCOO]−, 267.0464[M-H-Rha]−, 163.0701[M-H-C15H8O5]−, 115.0438[M-H-Rha-C7H4O4]− | Kaempherol-3-O-α-rhamnoside | CML, IML | [41] |
| 54 | 5.08 | C15H19NO5 | 293.1265 | 293.1263 | 0.5 | 294.1337[M + H]+, 131.0526[M + H-OCH3-C8H6NO]+, 99.0646[M + H-Rha-OCH3]+ | 4-(4 ′-O-methyl-α-l-rhamnosyloxy)benzyl nitrile | CML, IML | [18] |
| 55 * | 5.22 | C21H20O12 | 464.0938 | 464.0955 | −3.5 | 463.0880[M − H]−, 283.0502[M-H-Glu]−, 174.0278[M-H-Glu-C6H5O2]−, 150.0174[M-H-Glu-C8H5O2]− | Quercetin 3-O-β-d-glucopyranoside | CML >> IML VIP: 7.30 p < 0.001 |
[56] |
| 56 | 5.48 | C15H14O5 | 274.0837 | 274.0841 | −1.2 | 319.0819[M + HCOO]−, 144.0281[M-H-2H2O-C6H5O]−, 137.0222[M-H-C8H8O2]−, 117.0329[M-H-H2O-C7H6O3]−, 92.0344[M-H-C9H9O4]− | (−)-Epiafzelechin | CML, IML | s |
| 57 | 5.55 | C27H30O17 | 626.1490 | 626.1483 | 1.1 | 625.1417[M − H]−, 571.1354[M-H-3H2O]−, 391.0807[M-H-Glu-3H2O]−, 303.0966[M-H-Glu-H2O-C6H3O2]−, 265.0399[M-H-2Glu]− | Quercetin-3,7-O-β-d-diglucopyranoside | CML, IML | a |
| 58 | 5.65 | C24H22O15 | 550.0957 | 550.0959 | −0.2 | 549.0885[M − H]−, 445.0780[M-H-malonyl]−, 300.0267[M-H-malonyl-Glu]−, 160.0133[M-H-malonyl-Glu-C6H4O3]− | Quercetin-3-O-(6″-malonyl) glucoside | CML, IML | [49] |
| 59 # | 5.67 | C27H30O15 | 594.1579 | 594.1585 | −1.0 | 595.1678[M + H]+, 448.1063[M + H-3H2O-C6H5O]+, 385.1335[M + H-Glu-C2HO]+, 304.0494[M + H-H2O-Glu-C6H5O]+, 142.0169[M + H-2Glu-C6H5O]+ | Isovitexin-3″-O-glucopyranoside | CML << IML VIP: 5.63 p < 0.001 |
[57] |
| 60 # | 5.69 | C30H26O13 | 594.1355 | 594.1373 | −3.1 | 593.1507[M − H]−, 484.1116[M-H-C6H5O2]−, 439.0848[M-H-C7H6O4]−, 286.0394[M-H-H2O-C15H13O6]−, 153.9989[M-H-C23H20O9]− | Procyanidins | CML << IML VIP: 7.04 p < 0.001 |
[58] |
| 61 | 5.72 | C27H28O16 | 608.1378 | 608.1377 | 0.0 | 607.1305[M − H]−, 543.1275[M-H-H2O-HCOOH]−, 504.0985[M-H-C4H7O3]−, 440.0889[M-H-H2O-C5H9O3]−, 462.0868[M-H-C6H9O4]−, 282.0267[M-H-Glu-C6H9O4]− | Quercetin-3-O-hydroxy methylglutaroyl galactoside | CML, IML | [59] |
| 62 # | 5.84 | C28H32O16 | 624.1703 | 624.1690 | 2.1 | 623.1611[M − H]−, 590.1383[M-H-H2O-CH3]−, 466.1438[M-H-2H2O-C7H7O2]−, 337.0986[M-H-Rha-C6H4O3]−, 281.0460[M-H-Glu-Rha]− | Isorhamnetin-3-O-rutinoside | CML << IML VIP: 2.90 p < 0.001 |
s |
| 63* | 5.92 | C21H20O11 | 448.1003 | 448.1006 | −0.7 | 447.0929[M − H]−, 267.0463[M-H-Glu]−, 227.0343[M-H-Glu-C2O]−, 134.0018[M-H-Glu-C8H5O2]− | Kaempferol-3-O-glucoside | CML >> IML VIP: 10.89 p < 0.001 |
[49] |
| 64 | 6.07 | C16H12O7 | 316.0581 | 316.0583 | −0.6 | 317.0654[M + H]+, 302.0412[M + H-CH3]+, 299.0533[M + H-H2O]+, 152.0169[M + H-C9H8O3]+, 125.0388[M + H-C10H8O4]+ | Isorhamnetin | CML, IML | s |
| 65 | 6.16 | C23H22O13 | 506.1068 | 506.1060 | 1.4 | 505.0995[M − H]−, 490.0815[M-H-CH3]−, 428.0988[M-H-H2O-OCOCH3]−, 317.0980[M-H-2H2O-C7H4O4]−, 283.0198[M-H-Glu ethyl ester]− | Quercetin-3-O-(6″-O-acetyl)-β-d-glucopyranoside | CML, IML | [49] |
| 66 | 6.24 | C9H16O4 | 188.1045 | 188.1049 | 2.1 | 187.0965[M − H]−, 141.1105[M-H-HCOOH]−, 123.0957[M-H-H2O-HCOOH]−, 112.0644[M-H-H2O-C3H5O]− | Azelaic acid | CML, IML | s |
| 67 | 6.28 | C22H22O9 | 430.1242 | 430.1264 | −4.7 | 475.1224[M + HCOO]−, 288.0536[M-H-H2O-C7H7O2]−, 244.0915[M-H-2H2O-C9H8O2]−, 143.0398[M-H-CH3-Rha-C6H3O2]−, 130.0289[M-H-Rha-C7H3O3]− | Chryseriol-7-O-rhamnoside | CML, IML | [39] |
| 68 | 6.43 | C36H36O18 | 756.1900 | 756.1902 | −0.3 | 755.1827[M − H]−, 737.1844[M-H-H2O]−, 575.1386[M-H-Glu]−, 427.0933[M-H-Glu-C9H7O2]−, 405.0904[M-H-Glu-H2O-C7H4O4]−, 247.0320[M-H-2Glu-C9H7O2]− | Allivictoside A | CML, IML | b |
| 69 * | 6.44 | C34H24O10 | 592.1387 | 592.1370 | 3.0 | 593.1639[M + H]+, 483.1521[M + H-H2O-C6H4O]+, 266.0696[M + H-C8H5O2-C10H10O2]+, 241.0502[M + H-C20H16O6]+, 134.0267[M + H-C26H19O8]+ | Mulberrofuran Q | CML >> IML VIP: 5.76 p < 0.001 |
[59] |
| 70 | 6.46 | C24H22O14 | 534.1014 | 534.1010 | 0.8 | 533.0941[M − H]−, 447.0920[M-H-malonyl]−, 323.0962[M-H-malonyl-C6H4O3]−, 284.0320[M-H-malonyl-Glu]− | Kaempferol-3-O-(6″-malonyl) glucoside | CML, IML | [49] |
| 71 | 6.74 | C22H20O12 | 476.0964 | 476.0955 | 1.8 | 475.0891[M − H]−, 444.0726[M-H-OCH3]−, 351.0892[M-H-C6H4O3]−, 283.0394[M-H-methyl glucuronate]−, 172.0452[M-H-H2O-C15H9O6]− | Kaempferol-3-O-β-d-glucuronide-6″-methyl ester | CML, IML | [60] |
| 72 | 6.98 | C21H20O10 | 432.1052 | 432.1056 | −1.1 | 431.0979[M − H]−, 267.0327[M-H-Rha]−, 249.0447[M-H-Rha-H2O]−, 157.9997[M-H-Rha-C6H6O2]− | Kaempferol-7-O-α-l-rhamnoside | CML, IML | [61] |
| 73 | 6.99 | C15H10O6 | 286.0484 | 286.0477 | 2.4 | 287.0557[M + H]+, 153.0167[M + H-C8H6O2]+, 135.0583[M + H-C7H4O4]+, 124.0385[M + H-C9H6O3]+ | Orobol | CML, IML | [62] |
| 74 * | 7.01 | C23H22O12 | 490.1108 | 490.1111 | −0.6 | 489.1039[M − H]−, 446.1001[M-H-COCH3]−, 267.0323[M-H-Glu ethyl ester]−, 143.0443[M-H-Glu ethyl ester-C6H4O3]− | 3-O-(6″-O-acetyl)-β-d-glucopyranside | CML >> IML VIP: 6.15 p < 0.001 |
[63] |
| 75 | 7.80 | C16H19NO6 | 321.1213 | 321.1212 | 0.2 | 366.1195[M + HCOO]−, 249.0617[M-H-OCH 3-C2H2N]−, 189.0517[M-H-CH3-C8H6N]−, 97.0370[M-H-Rha-C2H3O2]− | Niazirinin | CML, IML | [45] |
| 76 | 7.86 | C15H10O6 | 286.0477 | 286.0473 | −1.7 | 285.0400[M − H]−, 121.0377[M-H-C6H4O3]−, 183.0010[M-H-C8H6]−, 133.0415[M-H-C7H4O4]−, 108.0280[M-H-C9H5O4]− | Luteolin | CML, IML | s |
| 77 | 7.89 | C15H10O7 | 302.0408 | 302.0427 | −1.8 | 301.0336[M − H]−, 244.0329[M-H-C2HO2]−, 190.0130[M-H-H2O-C6H5O]−, 133.0269[M-H-C7H4O5]−, 92.0343[M-H-C9H5O6]− | 6-Hydroxykaempferol | CML, IML | [64] |
| 78 # | 7.89 | C28H32O17 | 640.1653 | 640.1639 | 2.1 | 663.3153[M + Na]+, 443.0906[M + H-Glu-H2O]+, 281.0480[M + H-2Glu]+, 266.0487[M + H-2Glu-CH3]+, 158.0315[M + H-2Glu-C7H7O2]+ | Isorhamnetin 3-O-β-gentiobioside | CML << IML VIP: 4.79 p < 0.001 |
a |
| 79 * | 8.01 | C15H10O6 | 286.0479 | 286.0477 | 0.6 | 287.0567[M + H]+, 163.0580[M + H-C6H4O3]+, 147.0435[M + H-C6H4O4]+, 124.0384[M + H-C9H6O3]+ | Scutellarein | CML >> IML VIP: 3.69 p < 0.001 |
[65] |
| 80 * | 8.02 | C25H24O12 | 516.1266 | 516.1268 | −0.3 | 515.1203[M − H]−, 451.1465[M-H-H2O-HCOOH]−, 326.0487[M-H-3H2O-C8H7O2]−, 219.0638[M-H-2C9H7O2]−, 143.0279[M-H-H2O-C16H18O9]− | 1,3-Dicaffeoylquinic acid | CML >> IML VIP: 4.25 p < 0.001 |
s |
| 81 # | 8.26 | C20H26O9 | 410.1573 | 410.1577 | −1.0 | 409.1504[M − H]−, 336.0817[M-H-C4H9O]−, 251.1394[M-H-2H2O-C7H6O2]−, 202.0639[M-H-C3H7-C9H7O3]−, 134.0437[M-H-C12H19O7]− | 5-O-Caffeoylquinic acid butyl ester | CML << IML VIP: 4.72 p < 0.001 |
a |
| 82 # | 8.52 | C38H48O19 | 808.2757 | 808.2790 | −3.9 | 853.2706[M + HCOO]−, 700.2173[M-H-C7H7O]−, 572.1546[M-H-C4H7-Glu]−, 438.1262[M-H-C4H7-Rha-Ara]−, 274.1182[M-H-Glu-Ara-C12H11O3]− | 7-(α-L-Galactopyranosyloxy)-5-hydroxy-2-(4-methoxyphenyl)-8-(3-methyl-2-buten-1-yl)-4-oxo-4H-chromen | IML VIP: 2.08 p < 0.001 |
b |
| 83 | 9.08 | C30H34O15 | 634.1872 | 634.1898 | −3.7 | 679.1854[M + HCOO]−, 600.1579[M-H-H2O-CH3]−, 454.10677[M-H-Rha-CH3]−, 411.0997[M-H-CH3-Rha ethyl ester]−, 334.0931[M-H-Rha-C7H3O3]−, 296.0665[M-H-C9H7O-Rha ethyl ester]− | Kaempferol-3-O-α-l-(4-O-acetyl)-rhamnosyl-7-O-α-l-rhamnoside | CML, IML | [41] |
| 84 * | 9.23 | C15H10O6 | 286.0473 | 286.0477 | −1.7 | 285.0435[M − H]−, 228.0285[M-H-C2HO2]−, 161.0377[M-H-C6H4O3]−, 151.0010[M-H-C8H6O2]− | Kaempferol | CML >> IML VIP: 4.99 p < 0.001 |
s |
| 85 | 9.31 | C14H17NO5S | 311.0821 | 311.0827 | −1.9 | 356.0803[M + HCOO]−, 252.0915[M-H-NCS]−, 162.0681[M-H-C8H6NS]−, 88.0495[M-H-Rha-NCS]− | 4-[(α-l-rhamnosyloxy) benzyl] Isothiocyanate | CML, IML | [66] |
| 86 | 9.47 | C16H12O7 | 316.0575 | 316.0583 | −2.4 | 315.0503[M − H]−, 300.0268[M-H-CH3]−, 282.0400[M-H-H2O-CH3]−, 191.0163[M-H-CH3-C6H5O2]−, 165.0069[M-H-C8H6O3]− | Rhamnetin | CML, IML | [18] |
| 87 | 9.54 | C12H16O4 | 224.1038 | 224.1049 | −4.8 | 223.0965[M − H]−, 205.1027[M-H-H2O]−, 135.0421[M-H-C4H8O2]−, 123.0964[M-H-C4H4O3]−, 87.0295[M-H-C8H8O2]− | 3-Butylidene-4,5,6,7-tetrahydro-6,7-dihydroxy-1(3H)-isobenzofuranone | CML, IML | [67] |
| 88 | 9.69 | C30H36O4 | 460.2612 | 460.2614 | −0.4 | 505.2629[M + HCOO]−, 444.2249[M-H-CH3]−, 372.1847[M-H-H2O-C5H9]−, 240.1718[M-H-CH3-C12H12O3]−, 139.0822[M-H-H2O-C4H7O-C14H15O3]− | Sophoranone | CML, IML | a |
| 89 | 9.94 | C15H10O6 | 286.0480 | 286.0477 | 1.1 | 287.0553[M + H]+, 153.0163[M + H-C8H6O2]+, 135.0449[M + H-C7H4O4]+, 124.0382[M + H-C9H6O3]+, 110.0281[M + H-C9H5O4]+ | 5,7,2′,5′-Tetrahydroxyflavone | CML, IML | b |
| 90 | 9.98 | C27H28O12 | 544.1583 | 544.1581 | 0.4 | 589.1565[M + HCOO]−, 375.1260[M-H-C6H5O2-C2H3O2]−, 328.0465[M-H-H2O-2OCH3-C8H7O2]−, 244.0508[M-H-C9H7O3-C8H7O2]−, 153.0016[M-H-2OCH3-2C9H7O3]− | 1-O-methyl-3,5-O-dicaffeoylquinic acid methyl ester | CML, IML | a |
| 91 | 10.10 | C29H32O15 | 620.1741 | 620.1758 | 2.6 | 621.1830[M + H]+, 507.1514[M + H-C9H6]+, 310.0986[M + H-Rha-C9H6O2]+, 147.0658[M + H-Glu ethyl ester-C15H9O4]+ | Apigenin-7-O-α-l-rhamnopyranosyl(1 → 4)-6″-O-acetyl-β-d-glucopyranoside | CML, IML | [68] |
| 92 | 10.30 | C18H34O5 | 330.2405 | 330.2406 | −0.4 | 329.2332[M − H]−, 293.2084[M-H-2H2O]−, 226.1434[M-H-H2O-C6H13]−, 212.1325[M-H-HCOOH-C5H11]−, 168.1004[M-H-H2O-C9H19O]−, 137.1117[M-H-H2O-C3H7-C6H11O3]− | Sanleng acid | CML, IML | [26] |
| 93 * | 10.39 | C10H12O2 | 164.0837 | 164.0831 | −2.8 | 209.1118[M + HCOO]−, 122.0453[M-H-C3H5]−, 105.0495[M-H-OCH3-C2H3]− | Eugenol | CML >> IML VIP: 2.57 p < 0.001 |
s |
| 94 | 10.52 | C15H10O6 | 286.0477 | 286.0478 | 0.1 | 287.0550[M + H]+, 256.0427[M + H-OCH3]+, 167.0167[M + H-C7H4O2]+, 137.0227[M + H-C8H6O3]+, 121.0434[M + H-C8H6O4]+ | 1,7-Dihydroxy-2,3-methylenedioxyxanthone | CML, IML | b |
| 95 | 10.67 | C15H30O2 | 242.2241 | 242.2246 | −1.6 | 287.2223[M + HCOO]−, 170.1211[M-H-C5H11]−, 153.1120[M-H-OCH3-C4H9]−, 97.0818[M-H-OCH3-C8H17]−, 69.0512[M-H-OCH3-C10H21]− | Methyl myristate | CML, IML | s |
| 96 | 10.73 | C15H10O6 | 286.0480 | 286.0477 | 0.8 | 287.0552[M + H]+, 153.0171[M + H-C8H6O2]+, 124.0390[M + H-C9H6O3]+, 110.0285[M + H-C9H5O4]+ | 2′-Hydroxygenistein | CML, IML | s |
| 97 # | 11.22 | C18H34O5 | 330.2393 | 330.2406 | −4.0 | 329.2320[M − H]−, 213.1323[M-H-C2H5-C4H7O2]−, 208.1038[M-H-2H2O-C6H13]−, 183.1403[M-H-H2O-C7H13O2]−, 170.1223[M-H-C8H15O3]− | Tianshic acid | CML << IML VIP: 1.52 p < 0.001 |
[69] |
| 98 | 11.35 | C16H19NO6S | 353.0930 | 353.0933 | −0.8 | 398.0912[M + HCOO]−, 262.1963[M-H-H2O-C2H2NS]−, 236.0926[M-H-CH3-COCH3-C2H2S]−, 150.0741[M-H-C2H2NS-C6H10O3]− | 4-[(4′-O-acetyl-α-l-rhamnosyloxy)benzyl]isothiocyanate | CML, IML | [66] |
| 99 | 11.86 | C12H14O2 | 190.0988 | 190.0994 | −2.4 | 235.0970[M + HCOO]−, 146.0415[M-H-C3H7]−, 132.0273[M-H-C4H9]−, 113.0743[M-H-C6H4]− | 3-n-Butylphthalide | CML, IML | a |
| 100 # | 11.95 | C32H50O14 | 658.3196 | 658.3201 | −0.8 | 681.2695[M + Na]+, 617.2802[M + H-C3H6]+, 448.2238[M + H-Glu-CH2OH]+, 397.2184[M + H-Glu-C6H10]+, 203.0848[M + H-2Glu-C7H12]+ | Ajugaside A | CML << IML VIP: 3.40 p < 0.001 |
[70] |
| 101 | 12.01 | C30H40O12 | 592.2544 | 592.2520 | 4.1 | 591.2471[M − H]−, 561.2176[M-H-2CH3]−, 365.1552[M-H-CH3-OCH3-Glu]−, 315.1166[M-H-C16H20O4]−, 211.1134[M-H-Glu-C11H12O3]− | Syringaresinolmono-β-d-glucoside | CML, IML | [71] |
| 102 | 12.21 | C12H14O4 | 222.0883 | 222.0892 | −3.9 | 221.0811[M − H]−, 160.0546[M-H-OC2H5]−, 119.0282[M-H-C3H5O2-C2H5]−, | Diethyl phthalate | CML, IML | [67] |
| 103 | 12.22 | C16H19NO6S | 353.0930 | 353.0933 | −0.8 | 398.0912[M + HCOO]−, 265.0805[M-H-COCH3-CS]−, 161.0338[M-H-COCH3-C8H6NS]−, 101.0359[M-H-Rha-COCH3-CS]− | 4-[(2′-O-acetyl-α-l-rhamnosyloxy) benzyl] Isothiocyanate | CML, IML | [49] |
| 104 # | 12.98 | C20H23N7O7 | 473.1668 | 473.1659 | 1.9 | 472.1665[M − H]−, 423.1223[M-H-H2O-NH2-NH]−, 383.1668[M-H-H2O-CHO-CH2N2]−, 351.0896[M-H-H2O-HCOOH-CH3N3]−, 164.0386[M-H-CHO-CH3N-C12H12NO5]− | Folinic acid | CML << IML VIP: 3.43 p < 0.001 |
a |
| 105 | 13.10 | C21H38O4 | 354.2757 | 354.2770 | −3.3 | 399.2739[M + HCOO]−, 324.2187[M-H-C2H5]−, 238.1479[M-H-H2O-C7H13]−, 202.1101[M-H-C11H19]−, 151.1152[M-H-C8H15-C3H7O3] | 2-Monolinolein | CML, IML | [26] |
| 106 | 13.29 | C35H52O14 | 696.3375 | 696.3357 | 2.4 | 741.3357[M + HCOO]−, 571.2800[M-H-C7H8O2]−, 433.2317[M-H-Glu-2H2O-HCOOH]−, 366.2232[M-H-Glu-Ribose]−, 303.2063[M-H-Glu-H2O-C11H14O3]− | Erysimosole | CML, IML | b |
| 107 | 13.40 | C21H31NO10S | 489.1682 | 489.1669 | 2.8 | 488.1610[M − H]−, 473.1683[M-H-CH3]−, 308.1280[M-H-Glu]−, 293.0912[M-H-CH3-Glu]−, 218.0984[M-H-Glu-H2O-C2H2NS]− | 4-[(β-d-glucopyranosyl-1-4-α-l-rhamnopyranosyloxy) benzyl] Isothiocyanate | CML, IML | a |
| 108 | 13.52 | C13H16O3 | 220.1095 | 220.1099 | −1.9 | 219.1023[M − H]−, 164.0378[M-H-C4H7]−, 145.0326[M-H-OCH3-C3H7]− | 4-(1-Oxopentyl)-methyl ester,Benzoic acid | CML, IML | a |
| 109 | 14.68 | C18H34O4 | 314.2443 | 314.2457 | −4.6 | 313.2370[M − H]−, 199.1107[M-H-2C4H9]−, 184.1370[M-H-C7H13O2]−, 155.1048[M-H-C9H17O2]−, 125.1126[M-H-C4H9O-C6H11O2]− | Dibutyl sebacate | CML, IML | s |
| 110 | 15.87 | C18H28O2 | 276.2099 | 276.2089 | 3.3 | 277.2171[M + H]+, 150.1315[M + H-C7H11O2]+, 136.1167[M + H-C8H13O2]+, 107.0704[M + H-C5H9-C5H9O2]+, 95.0708[M + H-C4H7-C7H11O2]+ | Parinaric acid | CML, IML | s |
| 111 | 15.93 | C15H22O4 | 266.1506 | 266.1518 | −3.8 | 265.1488[M − H]−, 247.1494[M-H-H2O]−, 211.1328[M-H-C3H2O]−, 180.1365[M-H-CH3-C3H2O2]−, 169.1007[M-H-C3H6-C3H2O]−, 133.1009[M-H-H2O-C5H6O3]− | 4α,6α-Dihydroxyeud-esman-8β,12-olide | CML, IML | a |
| 112 | 16.00 | C17H34O2 | 270.2556 | 270.2559 | −0.9 | 315.2538[M + HCOO]−, 254.2163[M-H-CH3]−, 139.1285[M-H-OCH3-C7H15]−, 125.1118[M-H-OCH3-C8H17]− | Methyl palmitate | CML, IML | s |
| 113 | 16.49 | C18H30O4 | 310.2142 | 310.2144 | −0.6 | 309.2069[M − H]−, 291.1964[M-H-H2O]−, 245.2069[M-H-H2O-HCOOH]−, 208.1397[M-H-C5H9O2]−, 198.1177[M-H-C7H11O]−, 135.0958[M-H-C9H17O3]− | 9,16-Dihydroxy-10,12,14-octadecatrienoic acid | CML, IML | b |
| 114 | 17.88 | C16H30O2 | 254.2259 | 254.2246 | 4.8 | 277.2151[M + Na]+, 237.2359[M + H-H2O]+, 97.1016[M + H-C2H5-C7H13O2]+, 88.0605[M + H-C12H23]+, 69.0716[M + H-C4H9-C7H13O2]+ | Palmitoleic acid | CML, IML | [32] |
| 115 * | 18.44 | C18H30O3 | 294.2198 | 294.2195 | 0.9 | 293.2087[M − H]−, 275.2009[M-H-H2O]−, 247.2242[M-H-HCOOH]−, 232.1683[M-H-H2O-C3H7]−, 152.1063[M-H-H2O-C9H15]− | (E,E)-9-Oxooctadeca-10,12-dienoic acid | CML >> IML VIP: 2.45 p < 0.001 |
[30] |
| 116 * | 18.56 | C18H34O3 | 298.2494 | 298.2508 | −4.6 | 297.2440[M − H]−, 279.2478[M-H-H2O]−, 224.1515[M-H-H2O-C4H7]−, 139.1260[M-H-C2H5-C7H13O2]−, 139.1113[M-H-C3H7-C6H11O2]− | Ricinoleic acid | CML >> IML VIP: 3.12 p < 0.001 |
s |
| 117 | 19.27 | C18H32O3 | 296.2339 | 296.2351 | −4.3 | 295.2266[M − H]−, 266.1996[M-H-C2H5]−, 249.2382[M-H-HCOOH]−, 184.1156[M-H-C8H15]−, 152.1412[M-H-HCOOH-C7H13]−, 124.0960[M-H-H2O-C10H17O]− | Coronaric acid | CML, IML | [26] |
| 118 | 19.71 | C18H34O2 | 282.2558 | 282.2559 | −0.4 | 283.2631[M + H]+, 97.1020[M + H-C5H11-C6H11O2]+, 86.1024[M + H-C12H21O2]+, 72.0876[M + H-C13H23O2]+ | Oleic acid | CML, IML | s |
| 119 # | 19.95 | C21H36O4 | 352.2620 | 352.2614 | 1.8 | 353.2701[M + H]+, 335.2693[M + H-H2O]+, 214.2202[M + H-C3H7O3]+, 150.1320[M + H-C10H19O4]+, 123.1012[M + H-C5H9-C7H13O4]+, 83.0715[M + H-C7H11-C8H15O4]+ | 1-Linolenoylglycerol | CML << IML VIP: 5.63 p < 0.001 |
a |
| 120 | 21.54 | C18H30O2 | 278.2237 | 278.2246 | −3.0 | 277.2165[M − H]−, 182.1234[M-H-C7H11]−, 168.1230[M-H-C8H13]−, 110.0795[M-H-C11H17-H2O]− | Linolenic acid | CML, IML | s |
| 121 * | 21.70 | C15H30O | 226.2300 | 226.2297 | 1.3 | 271.2274[M + HCOO]−, 164.1118[M-H-C6H13]−, 108.0499[M-H-C10H21]− | n-Pentadecanal | CML >> IML VIP: 4.02 p < 0.001 |
[32] |
| 122 * | 24.46 | C17H34O | 254.2616 | 254.2610 | 2.1 | 299.2594[M + HCOO]−, 248.2224[M-H-C2H5]−, 122.0654[M-H-C11H23]−, 94.0506[M-H-C13H27]− | n-Heptadecanal | CML >> IML VIP: 3.12 p < 0.001 |
[32] |
* Characteristic component in CML; # characteristic component in IML; s identified with standard; a compared with spectral data obtained from Wiley Subscription Services, Inc. (USA); b compared with NIST Chemistry WebBook.
Figure 1.
The representative base peak intensity (BPI) chromatograms of CML and IML in ESI+ and ESI− modes, where ESI is electrospray ionization.
Figure 2.
Chemical structures of compounds identified in CML and IML.
3.2. Diversity Evaluation of CML and IML Using Metabolomics Analysis
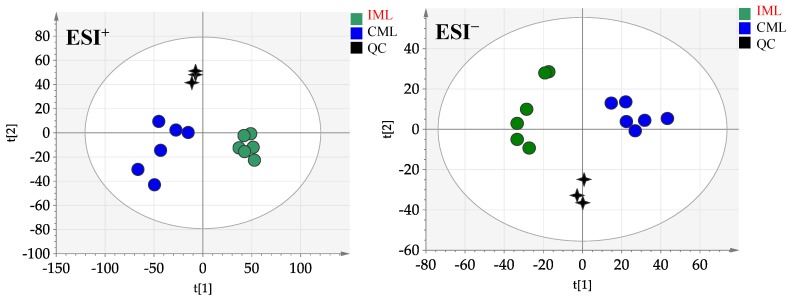
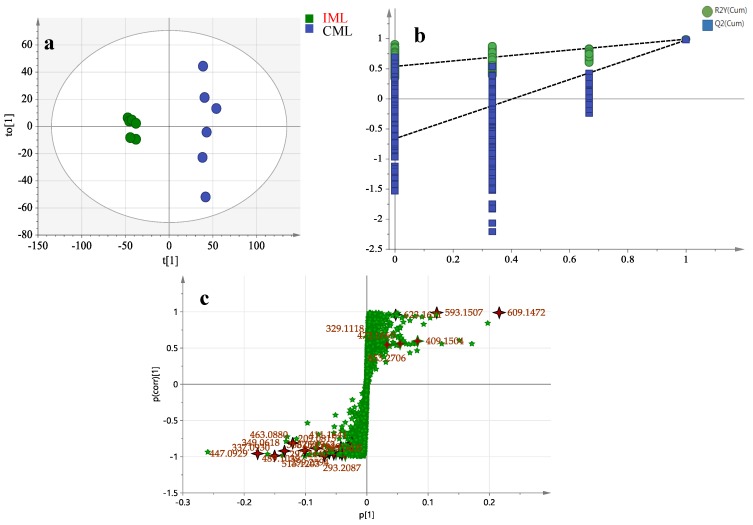
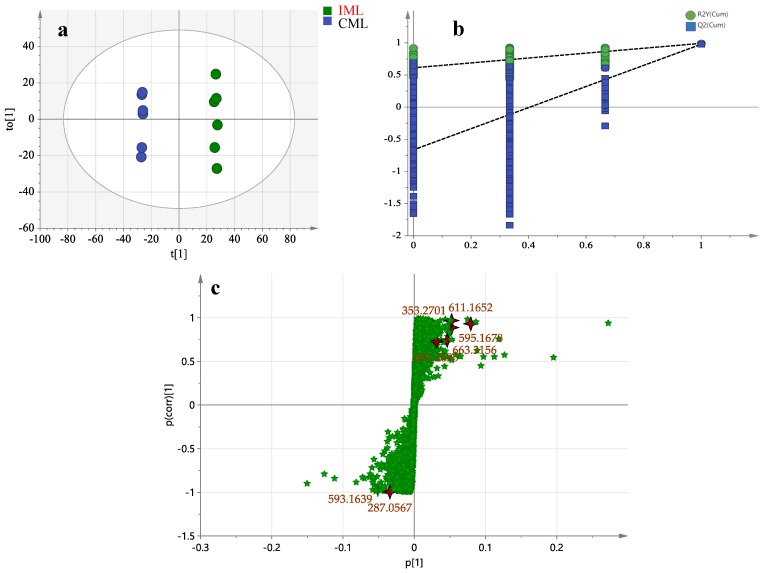
The QC injections were clustered tightly in PCA, indicating a satisfactory stability of the system. According to their common spectral characteristics, the PCA 2D plots of the samples from CML and IML groups were able to be easily classified within two clusters (Figure 3). The CML and IML samples were clearly separated, indicating that these two samples could be easily differentiated.
Figure 3.
The principal component analysis (PCA) of CML and IML in ESI+ mode and ESI− mode.
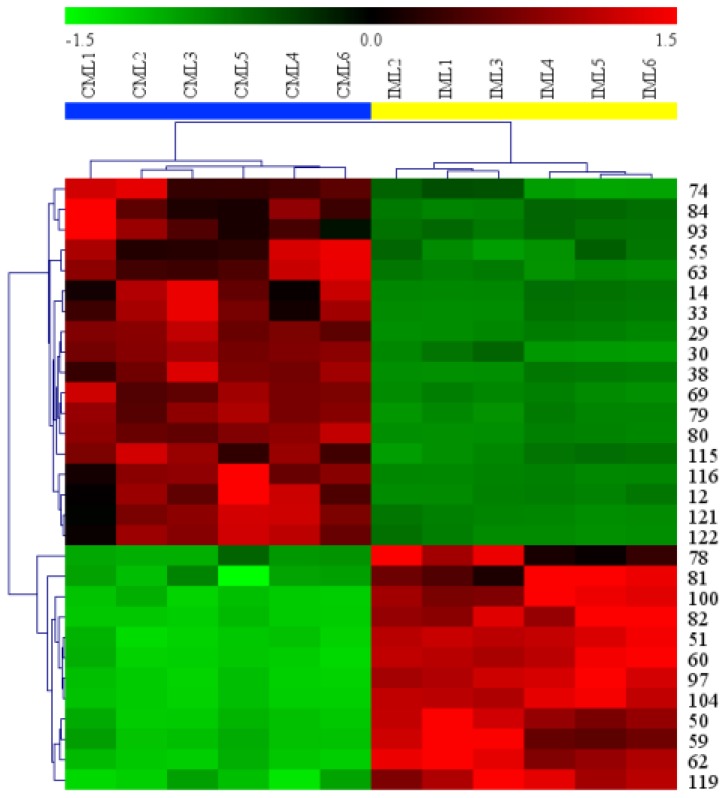
In order to evaluate the differences between the leaves in the two areas, OPLS-DA score plot, S-plot, permutation test, and variable importance in the projection values were obtained to understand which variables were responsible for this sample separation [72]. After OPLS-DA plots (Figure 4a and Figure 5a) in both ESI+ and ESI− modes were performed, the maximum separation between the CML and IML groups was available. With sufficient permutation testing, the lines of grouping samples were significantly located underneath the random sampling lines (Figure 4b and Figure 5b), which indicates a definite validity for the following characteristic metabolites biomarkers identification. S-plots were then created to explore the potential chemical markers that contributed to the differences. Based on p values (p < 0.05) and VIP values (VIP > 1) [26,30] from univariate statistical analysis, 30 robust known chemical markers enabling differentiation between CML and IML were marked and listed (Figure 4c and Figure 5c and Table 2). Additionally, a heatmap was generated from these chemical markers in order to systematically evaluate the markers (Figure 6), which visually showed the intensities of potential chemical markers between the two samples.
Figure 4.
Orthogonal partial least squares discriminant analysis (OPLS-DA) (a), permutation tests, (b) and S-plot (c) in ESI− mode.
Figure 5.
OPLS-DA (a), permutation tests, (b) and S-plot (c) in ESI+ mode.
Figure 6.
Heatmap visualizing the intensities of potential chemical markers.
4. Discussion
Via the screening analysis, 121 and 119 compounds were characterized in CML and IML, respectively. As the results show, 93 compounds were identified in negative mode and 29 compounds were identified in positive mode. From the BPI chromatograms, it seems that the negative ionization mode was better than the positive mode based on the quantity and the responses of the identified compounds. However, it was still necessary to have run the positive mode because some compounds showed better responses in this mode than in the negative mode. The results also showed that both these ML areas are rich in natural components. It has been reported that there is high flavonoid content (presenting in flavanol and glycoside forms) in M. oleifera leaves [4,18]. In this study, flavonoids were also the main chemical composition. Besides the most common flavonoids, 36 flavonoids, such as apigenin-8-C-glucoside, quercetin 3-O-β-d-glucopy-ranoside, kaempferol-7-O-α-l-rhamnoside, and 5, 7, 2′, 5′-tetrahydroxyflavone, were identified or tentatively characterized in M. oleifera leaves for the first time. Moreover, isothiocyanates have become a major topic of research interest regarding Moringa for their various biological activities [18]. In our study, there were 4 isothiocyanates which were found both in IML and CML. A total of 118 compounds were shared constituents in CML and IML, which means that they were similar in terms of the kinds of compound contained. This comprehensive phytochemical profile study has revealed the structural diversity of secondary metabolites and the similar patterns within CML and IML.
Furthermore, in nontargeted metabolomic analysis, when taking the contents of the constituents into account, it was found that there indeed existed differences between CML and IML. Thirty robust known biomarkers enabling this differentiation were discovered. These are able to illustrate the differences between CML and IML and provide a basis for explaining the effect of different growth environments on secondary metabolites. With CML, there are 18 potential biomarkers, including seven flavonoids (14, 33, 55, 63, 74, 79, and 84), five organic acids and organic acid esters (30, 38, 80, 115, and 116), two glyosides (12 and 29), and four others (69, 93, 121, and 122). Among these biomarkers, compounds 14, 33, and 38 were detected only in CML under experimental conditions, and the others’ contents in CML were greater than those in IML. Among these potential biomarkers, components 14, 33, 55, 74, 79, 30, and 80 were identified or tentatively characterized in M. oleifera leaves for the first time. It has been reported that M. oleifera leaves which originate from China have the maximum antioxidant activity when compared alongside those from Faisalabad, Multan, and India [73]. As is known, biological activity is caused by the high contents of phytochemicals. Correlation studies between potential markers and biological activities should be performed in the future. For IML, there are 12 potential biomarkers, including six flavonoids (50, 51, 59, 62, 78, and 82), three organic acids and organic acid esters (81, 97, and 119), one glyoside (100), one alkaloid (104), and one lignan (60). Among these, compound 82 was detected only in IML under experimental conditions, and the other 11 compounds’ contents were greater in IML than those in CML.
Based on the above results, it could be concluded that some of the secondary plant metabolite contents of CML and IML differ from each other. This is just as it is with other natural plants.
In summary, a total of 122 components, including 118 shared constituents, were characterized from CML and IML. For CML, 121 compounds were characterized, and among these, 18 potential biomarkers with higher contents enabled differentiation from IML. For IML, 119 compounds were characterized, and among these, 12 potential biomarkers with higher contents enabled differentiation from CML.
Even so, several unresolved issues still remain. For example, in the future, potential chemical markers’ and identified compounds’ pharmacological activities should be screened. In addition, there are still some unidentified components, despite 122 compounds being identified, as shown in the BPI chromatograms. Further research should be performed on these unknown components.
5. Conclusions
In this study, 121 and 119 chemical compounds, including 118 shared constituents, were respectively identified or tentatively characterized from CML and IML by combining UPLC-QTOF-MS and a UNIFI platform. Both CML and IML, which originate from two separate countries, are rich in phytochemicals and are similar in the kinds of compounds they contain. Moreover, a metabolomics study based on UPLC-QTOF-MS combined with multivariate statistical analysis has shown the significant differences in the contents of an amount of the compounds in these two accessions. A total of 30 robust known biomarkers enabling differentiation were discovered. For CML and IML, 18 and 12 potential biomarkers were identified, respectively. This study provides further data to make up for the deficient amount of study performed on the chemical constituents of Moringa oleifera leaves and can help with planning strategies focused on the proper utilization of this resource, as well as providing a reference for the further application of CML in China.
Author Contributions
Data curation, investigation, and writing—original draft, H.L.; methodology and software, H.Z.; formal analysis and writing—original draft, J.T.; formal analysis, writing, and editing, H.W.; conceptualization and methodology, Z.W.; funding acquisition, P.L.; data curation and methodology, C.Z.; supervision, J.L.
Funding
This research was supported by the Graduate Innovation Fund of Jilin University [Grant No. 101832018C085].
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Not available.
References
- 1.Makita C., Madala N.E., Cukrowska E., Abdelgadir H., Chimuka L., Steenkamp P., Ndhlala A.R. Variation in pharmacologically potent rutinoside-bearing flavonoids amongst twelve Moringa oleifera, Lam. cultivars. S. Afr. J. Bot. 2017;112:270–274. doi: 10.1016/j.sajb.2017.06.001. [DOI] [Google Scholar]
- 2.Khalafalla M.M., Abdellatef E., Dafalla H.M. Active principle from Moringa oleifera Lam leaves effective against two leukemias and a hepatocarcinoma. Afr. J. Biotechnol. 2010;9:8467–8471. [Google Scholar]
- 3.Mahmood K.T., Mugal T., Haq I.U. Moringa oleifera: A natural gift-a review. J. Pharm. Sci. Res. 2010;2:775–2781. [Google Scholar]
- 4.Vergara-Jimenez M., Almatrafi M.M., Fernandez M.L. Bioactive Components in Moringa oleifera Leaves Protect against Chronic Disease. Antioxidants. 2017;6:91. doi: 10.3390/antiox6040091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shih M.C., Chang C.M., Kang S.M., Tsai M.L. Effect of Different Parts (Leaf, Stem and Stalk) and Seasons (Summer and Winter) on the Chemical Compositions and Antioxidant Activity of Moringa oleifera. Int. J. Mol. Sci. 2011;12:6077–6088. doi: 10.3390/ijms12096077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iqbal S., Bhanger M.I. Effect of season and production location on antioxidant activity of Moringa oleifera leaves grown in Pakistan. J. Food Compos. Anal. 2006;19:544–551. doi: 10.1016/j.jfca.2005.05.001. [DOI] [Google Scholar]
- 7.Mahdi H.J., Khan N.A.K., Asmawi M.Z.B., Mahmud R., Murugauyah V.A. In vivo, anti-arthritic and anti-noceciptive effects of ethanol extract of Moringa oleifera, leaves on complete Freund’s adjuvant (CFA)-induced arthritis in rats. Integr. Med. Res. 2018;7:85–94. doi: 10.1016/j.imr.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheenpracha S., Park E.J., Yoshida W.Y., Barit C., Wall M., Pezzuto J.M., Chang L.C. Potential anti-inflammatory phenolic glycosides from the medicinal plant Moringa oleifera fruits. Bioorg. Med. Chem. 2010;18:6598–6602. doi: 10.1016/j.bmc.2010.03.057. [DOI] [PubMed] [Google Scholar]
- 9.Sreelatha S., Jeyachitra A., Padma P.R. Antiproliferation and induction of apoptosis by Moringa oleifera leaf extract on human cancer cells. Food Chem. Toxicol. 2011;49:1270–1275. doi: 10.1016/j.fct.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Tragulpakseerojn J., Yamaguchi N., Pamonsinlapatham P., Wetwitayaklung P., Yoneyama T., Ishikawa N., Ishibashi M., Apirakaramwong A. Anti-proliferative effect of Moringa oleifera Lam (Moringaceae) leaf extract on human colon cancer HCT116 cell line. Trop. J. Pharm. Res. 2017;16:371–378. doi: 10.4314/tjpr.v16i2.16. [DOI] [Google Scholar]
- 11.Kajihara R., Nakatsu S., Shiono T., Ishihara M., Sakamoto K., Muto N. Antihypertensive Effect of Water Extracts from Leaves of Moringa oleifera Lam. on Spontaneously Hypertensive Rats. Nippon Shokuhin Kagaku Kogaku Kaishi. 2008;55:183–185. doi: 10.3136/nskkk.55.183. [DOI] [Google Scholar]
- 12.Helmy S.A., Nfs M., Elaby S.M., Ghally M.A.A. Hypolipidemic Effect of Moringa oleifera Lam Leaf Powder and its Extract in Diet-Induced Hypercholesterolemic Rats. J. Med. Food. 2017;20:755–762. doi: 10.1089/jmf.2016.0155. [DOI] [PubMed] [Google Scholar]
- 13.Dharmendra S., Vrat A.P., Prakash A.V., Radhey S.G. Evaluation of Antioxidant and Hepatoprotective Activities of Moringa oleifera Lam. Leaves in Carbon Tetrachloride-Intoxicated Rats. Antioxidants. 2014;3:569–591. doi: 10.3390/antiox3030569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villarruel-López A., Mora L.L., Vázquez-Paulino O.D., Puebla-Mora A.G., Torres-Vitela M.R., Guerrero-Quiroz L.A., Nuño K. Effect of Moringa oleifera consumption on diabetic rats. BMC Complement. Altern. Med. 2018;18:127. doi: 10.1186/s12906-018-2180-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh R.S.G., Negi P.S., Radha C. Phenolic composition, antioxidant and antimicrobial activities of free and bound phenolic extracts of Moringa oleifera seed flour. J. Funct. Foods. 2013;5:1883–1891. doi: 10.1016/j.jff.2013.09.009. [DOI] [Google Scholar]
- 16.Pal S.K., Mukherjee P.K., Saha K., Pal M., Saha B.P. Antimicrobial action of the leaf extract of Moringa oleifera lam. Anc. Sci. Life. 1995;14:197–199. [PMC free article] [PubMed] [Google Scholar]
- 17.Selvakumar D., Natarajan P. Hepato-Protective activity of Moringa oleifera Lam Leaves in Carbon tetrachloride induced Hepato-Toxicity in Albino Rats. Pharmacogn. Mag. 2008;4:97–98. [Google Scholar]
- 18.Abd-Rani N.Z., Husain K., Kumolosasi E. Moringa Genus: A Review of Phytochemistry and Pharmacology. Front. Pharmacol. 2018;9:108. doi: 10.3389/fphar.2018.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kou X., Li B., Olayanju J.B., Drake J.M., Chen N. Nutraceutical or Pharmacological Potential of Moringa oleifera Lam. Nutrients. 2018;10:343. doi: 10.3390/nu10030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen R.J., Zhu B.F., Wang Y.Z., Liu Z. Extraction and hypolycemic effect of the total flavonoid from leaves of Moringa oleifera. J. Food Sci. Biotechnol. 2007;26:42–45. [Google Scholar]
- 21.The Minister of Health of the People’s Republic of China (MOHC) Announcement on the Approval of 4 New Resource Foods Such as Chlorella vulgaris (No. Nineteenth 2012). [EB/OL]. (12 November 2012) [(accessed on 17 December 2018)]; Available online: http://www.nhfpc.gov.cn/sps/s7891/201212/5d4c82e89a9e4713aba8f782eca51e09.shtml.
- 22.Rodríguez-Pérez C., Quirantes-Piné R., Fernández-Gutiérrez A., Segura-Carretero A. Optimization of extraction method to obtain a phenolic compounds-rich extract from Moringa oleifera Lam leaves. Ind. Crop. Prod. 2015;66:246–254. doi: 10.1016/j.indcrop.2015.01.002. [DOI] [Google Scholar]
- 23.Sheikh A., Yeasmin F., Agarwal S., Rahman M., Islam K., Hossain E., Hossain S., Karim M.D., Nikkon F., Saud Z.A., et al. Protective effects of Moringa oleifera Lam. leaves against arsenic-induced toxicity in mice. Asian Pac. J. Trop. Biomed. 2014;4:S353–S358. doi: 10.12980/APJTB.4.201414B44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ndhlala A.R., Mulaudzi R., Ncube B., Abdelgadir H.A., Plooy C.P., Van-Staden J. Antioxidant, antimicrobial and phytochemical variations in thirteen Moringa oleifera Lam. cultivars. Molecules. 2014;19:10480–10494. doi: 10.3390/molecules190710480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Förster N., Ulrichs C., Schreiner M., Arndt N., Schmidt R., Mewis I. Ecotype Variability in Growth and Secondary Metabolite Profile in Moringa oleifera: Impact of Sulfur and Water Availability. J. Agric. Food Chem. 2015;63:2852–2861. doi: 10.1021/jf506174v. [DOI] [PubMed] [Google Scholar]
- 26.Wang C.Z., Zhang N.Q., Wang Z.Z., Qi Z., Zheng B.Z., Li P.Y., Liu J.P. Rapid characterization of chemical constituents of Platycodon grandiflorum and its adulterant Adenophora stricta by UPLC-QTOF-MS/MS. J. Mass Spectrom. 2017;52:643–656. doi: 10.1002/jms.3967. [DOI] [PubMed] [Google Scholar]
- 27.Zhang F.X., Li M., Qiao L.R., Yao Z.H., Li C., Shen X.Y., Wang Y., Yu K., Yao X.S., Dai Y. Rapid characterization of Ziziphi Spinosae Semen by UPLC/Q-tof MS with novel informatics platform and its application in evaluation of two seeds from Ziziphus species. J. Pharm. Biomed. Anal. 2016;122:59–80. doi: 10.1016/j.jpba.2016.01.047. [DOI] [PubMed] [Google Scholar]
- 28.Deng L., Shi A.M., Liu H.Z., Meruva N., Liu L., Hu H., Yang Y., Huang C., Li P., Wang Q. Identification of chemical ingredients of peanut stems and leaves extracts using UPLC-QTOF-MS coupled with novel informatics UNIFI platform. J. Mass Spectrom. 2016;51:1157–1167. doi: 10.1002/jms.3887. [DOI] [PubMed] [Google Scholar]
- 29.Tang J.F., Li W.X., Tan X.J., Li P., Xiao X.H., Wang J.B., Zhu M.J., Li X.L., Meng F. A novel and improved UHPLC-QTOF/MS method for the rapid analysis of the chemical constituents of Danhong Injection. Anal. Methods. 2016;8:2904–2914. doi: 10.1039/C5AY03173G. [DOI] [Google Scholar]
- 30.Wang Y.R., Wang C.Z., Lin H.Q., Liu Y.H., Li Y.M., Zhao Y., Li P.Y., Liu J.P. Discovery of the Potential Biomarkers for Discrimination between Hedyotis diffusa and Hedyotis corymbosa by UPLC-QTOF/MS Metabolome Analysis. Molecules. 2018;23:1525. doi: 10.3390/molecules23071525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan J., Wang C.Z., Zhu H.L., Zhou B.S., Xiong L.X., Wang F., Li P.Y., Liu J.P. Comprehensive Metabolomics Analysis of Xueshuan Xinmaining Tablet in Blood Stasis Model Rats Using UPLC-Q/TOF-MS. Molecules. 2018;23:1650. doi: 10.3390/molecules23071650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang C.Z., Zhang N.Q., Wang Z.Z., Qi Z., Zhu H.L., Zheng B.Z., Li P.Y., Liu J.P. Nontargeted Metabolomic Analysis of Four Different Parts of Platycodon grandiflorum Grown in Northeast China. Molecules. 2017;22:1280. doi: 10.3390/molecules22081280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu H.L., Lin H.Q., Tan J., Wang H., Wu F.L., Dong Q.H., Liu Y.H., Li P.Y., Liu J.P. UPLC-QTOF/MS-Based Nontargeted Metabolomic Analysis of Mountain- and Garden-Cultivated Ginseng of Different Ages in Northeast China. Molecules. 2019;24:33. doi: 10.3390/molecules24010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Y.Y., Cheng X.L., Wei F., Xiao X.Y., Sun W.J., Zhang Y.M., Lin R.C. Serum metabonomics study of adenine-induced chronic renal failure in rats by ultra performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. Biomarkers. 2012;17:48–55. doi: 10.3109/1354750X.2011.637180. [DOI] [PubMed] [Google Scholar]
- 35.Pang Z.Q., Wang G.Q., Ran N., Lin H.Q., Wang Z.Y., Guan X.W., Yuan Y.Z., Fang K.Y., Liu J.P., Wang F. Inhibitory Effect of Methotrexate on Rheumatoid Arthritis Inflammation and Comprehensive Metabolomics Analysis Using Ultra-Performance Liquid Chromatography-Quadrupole Time of Flight-Mass Spectrometry (UPLC-Q/TOF-MS) Int. J. Mol. Sci. 2018;19:2894. doi: 10.3390/ijms19102894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuligowski J., Pérez-Guaita D., Escobar J., Guardia M.D., Vento M., Ferrer A., Quintáse Q. Evaluation of the effect of chance correlations on variable selection using Partial Least Squares-Discriminant Analysis. Talanta. 2013;116:835–840. doi: 10.1016/j.talanta.2013.07.048. [DOI] [PubMed] [Google Scholar]
- 37.Makita C., Chimuka L., Cukrowska E., Steenkamp P.A., Kandawa-Schutzd M., Ndhlalae A.R., Madala N.E. UPLC-qTOF-MS profiling of pharmacologically important chlorogenic acids and associated glycosides in Moringa ovalifolia, leaf extracts. S. Afr. J. Bot. 2017;108:193–199. doi: 10.1016/j.sajb.2016.10.016. [DOI] [Google Scholar]
- 38.Sahakitpichan P., Mahidol C., Disadee W., Ruchirawat S., Kanchanapoom T. Unusual glycosides of pyrrole alkaloid and 4′-hydroxyphenylethanamide from leaves of Moringa oleifera. Phytochemistry. 2011;72:791–795. doi: 10.1016/j.phytochem.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 39.Ezzat S., Hegazy A., Amer A.M., Kamel G.M. Isolation of biologically active constituents from Moringa peregrina (Forssk.) Fiori. (family: Moringaceae) growing in Egypt. Pharmacogn. Mag. 2011;7:109–115. doi: 10.4103/0973-1296.80667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo X.R., Chen X.H., Li L., Shen Z.D., Wang X.L., Zheng P., Duan F.X., Ma Y.F., Bi K.S. LC-MS determination and pharmacokinetic study of six phenolic components in rat plasma after taking traditional Chinese medicinal-preparation: Guanxinning lyophilized powder for injection. J. Chromatogr. B. 2008;873:51–58. doi: 10.1016/j.jchromb.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 41.Manguro L.O.A., Lemmen P. Phenolics of Moringa oleifera leaves. Nat. Prod. Res. 2007;21:56–68. doi: 10.1080/14786410601035811. [DOI] [PubMed] [Google Scholar]
- 42.Zhao W.J., Lin Y., Li P.F., Liu Y. Analysis of chemical constituents of Moutan cortex by HPLC-QTOF-MS. J. Pharm. Pract. 2014;32:261–265. [Google Scholar]
- 43.Panda S., Kar A., Sharma P., Sharma A. Cardioprotective potential of N,α-l-rhamnopyranosyl vincosamide, an indole alkaloid, isolated from the leaves of Moringa oleifera in isoproterenol induced cardiotoxic rats: In vivo and in vitro studies. Bioorg. Med. Chem. Lett. 2013;23:959–962. doi: 10.1016/j.bmcl.2012.12.060. [DOI] [PubMed] [Google Scholar]
- 44.Lee E.H., Kim H.J., Yun S.S., Jin C., Lee K.T., Cho J., Lee Y.S. Constituents of the stems and fruits of Opuntia ficus-indica var.saboten. Arch. Pharm. Res. 2003;26:1018–1023. doi: 10.1007/BF02994752. [DOI] [PubMed] [Google Scholar]
- 45.Li F.H., Wang H.Q., Su X.M., Li C.K., Li B.M., Chen R.Y., Kang J. Constituents isolated from n-butanol extract of leaves of Moringa oleifera. China J. Chin. Mater. Med. 2018;43:114–118. doi: 10.19540/j.cnki.cjcmm.20171027.009. [DOI] [PubMed] [Google Scholar]
- 46.Bianco A., Melchioni C., Ramunno A. Iridoid glucosides from Lamium garganicum flowers. Nat. Prod. Lett. 2003;17:225–227. doi: 10.1080/1057563021000040475. [DOI] [PubMed] [Google Scholar]
- 47.Lee S.Y., Choi S.U., Lee J.H. A new phenylpropane glycoside from the rhizome of Sparganium stoloniferum. Arch. Pharm. Res. 2010;33:515–521. doi: 10.1007/s12272-010-0404-1. [DOI] [PubMed] [Google Scholar]
- 48.Shanker K., Gupta M.M., Srivastava S.K., Bawankule D.U., Pal A., Khanuja S. Determination of bioactive nitrile glycoside(s) in drumstick (Moringa oleifera) by reverse phase HPLC. Food Chem. 2007;105:376–382. doi: 10.1016/j.foodchem.2006.12.034. [DOI] [Google Scholar]
- 49.Alessandro L., Giovanni F., Franca C., Stefano R., Laura S., Gelsomina F., Angela S., Alberto B., Alberto S., Federica P., et al. Nutritional Characterization and Phenolic Profiling of, Moringa oleifera Leaves Grown in Chad, Sahrawi Refugee Camps, and Haiti. Int. J. Mol. Sci. 2015;16:18923–18937. doi: 10.3390/ijms160818923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abubakar A.M., Sharida F., Palanisamy A., Pike S.C., Farida A., Sharida F. Evaluation of wound healing properties of bioactive aqueous fraction from Moringa oleifera Lam on experimentally induced diabetic animal model. Drug Des. Dev. Ther. 2016;10:1715–1730. doi: 10.2147/DDDT.S96968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang K., Zuo Y. GC-MS Determination of Flavonoids and Phenolic and Benzoic Acids in Human Plasma after Consumption of Cranberry Juice. J. Agric. Food Chem. 2004;52:222–227. doi: 10.1021/jf035073r. [DOI] [PubMed] [Google Scholar]
- 52.Ma C.M., Kully M., Khan J.K., Hattori M., Daneshtalab M. Synthesis of chlorogenic acid derivatives with promising antifungal activity. Bioorg. Med. Chem. 2007;15:6830–6833. doi: 10.1016/j.bmc.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 53.Nguyen A.T., Fontaine J., Malonne H., Claeys M., Luhmer M., Duez P. A sugar ester and an iridoid glycoside from Scrophularia ningpoensis. Phytochemistry. 2005;36:1186–1191. doi: 10.1016/j.phytochem.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 54.Vongsak B., Sithisarn P., Gritsanapan W. Simultaneous HPLC quantitative analysis of active compounds in leaves of Moringa oleifera Lam. J. Chromatogr. Sci. 2014;52:641–645. doi: 10.1093/chromsci/bmt093. [DOI] [PubMed] [Google Scholar]
- 55.Cuyckens F., Shahat A.A., Pieters L., Claeys M. Direct stereochemical assignment of hexose and pentose residues in flavonoid O-glycosides by fast atom bombardment and electrospray ionization mass spectrometry. J. Mass Spectrom. 2002;37:1272–1279. doi: 10.1002/jms.402. [DOI] [PubMed] [Google Scholar]
- 56.Zhang W.J., Xu M., Yu C.Q., Zhang G.F., Tang X. Simultaneous determination of vitexin-4″-O-glucoside, vitexin-2″-O-rhamnoside, rutin and vitexin from hawthorn leaves flavonoids in rat plasma by UPLC-ESI-MS/MS. J. Chromatogr. B. 2010;878:1837–1844. doi: 10.1016/j.jchromb.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 57.Deng X.Y., Gao G.H., Zheng S.N., Li F.M. Qualitative and quantitative analysis of flavonoids in the leaves of Isatis indigatica Fort. by ultra-performance liquid chromatography with PDA and electrospray ionization tandem mass spectrometry detection. J. Pharm. Biomed. Anal. 2008;48:562–567. doi: 10.1016/j.jpba.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 58.Atawodi S.E., Atawodi J.C., Idakwo G.A., Pfundstein B., Haubner R., Wurtele G., Bartsch H., Owen R.W. Evaluation of the Polyphenol Content and Antioxidant Properties of Methanol Extracts of the Leaves, Stem, and Root Barks of Moringa oleifera Lam. J. Med. Food. 2010;13:710–716. doi: 10.1089/jmf.2009.0057. [DOI] [PubMed] [Google Scholar]
- 59.Zhang L., Tu Z.C., Wang H., Fu Z.F., Wen Q.H., Chang H.X., Huang X.Q. Comparison of different methods for extracting polyphenols from Ipomoea batatas, leaves, and identification of antioxidant constituents by HPLC-QTOF-MS2. Food Res. Int. 2015;70:101–109. doi: 10.1016/j.foodres.2015.01.012. [DOI] [Google Scholar]
- 60.Hasan A., Hussain A., Khan M.A. Flavonol glycosides from leaves of Bergenia himalaica. Asian J. Chem. 2005;17:822–828. [Google Scholar]
- 61.Polasek J., Queiroz E.F.K. On-line identification of phenolic compounds of Trifolium, species using HPLC-UV-MS and post-column UV-derivatisation. Phytochem. Anal. 2007;18:13–23. doi: 10.1002/pca.946. [DOI] [PubMed] [Google Scholar]
- 62.Sun F., Shen L.M., Ma Z.J. Screening for ligands of human aromatase from mulberry (Mori alba L.) leaf by using high-performance liquid chromatography/tandem mass spectrometry. Food Chem. 2011;126:1337–1343. doi: 10.1016/j.foodchem.2010.11.096. [DOI] [Google Scholar]
- 63.Faizi S., Siddiqui B.S., Saleem R., Siddiqui S., Aftab K., Gilani A.H. Isolation and Structure Elucidation of New Nitrile and Mustard Oil Glycosides from Moringa oleifera and Their Effect on Blood Pressure. J. Nat. Prod. 1994;57:1256–1261. doi: 10.1021/np50111a011. [DOI] [PubMed] [Google Scholar]
- 64.Wang S.P., Liu L., Wang L.L., Hu Y.H., Zhang W.D., Liu R.H. Structural characterization and identification of major constituents in Jitai tablets by high-performance liquid chromatography/diode-array detection coupled with electrospray ionization tandem mass spectrometry. Molecules. 2012;17:10470–10493. doi: 10.3390/molecules170910470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Y.F., Hu L.M., Liu Y.N. A rapid method for qualitative and quantitative analysis of major constituents in dengzhanxixin injection by LC-DAD-ESI-MSn. Chromatographia. 2010;71:845–853. doi: 10.1365/s10337-010-1540-y. [DOI] [Google Scholar]
- 66.Tumer T.B., Rojas-Silva P., Poulev A., Raskin L., Waterman C. Direct and Indirect Antioxidant Activity of Polyphenol- and Isothiocyanate-Enriched Fractions from Moringa oleifera. J. Agric. Food Chem. 2015;63:1505–1513. doi: 10.1021/jf505014n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.He C.M., Cheng Z.H., Chen D.F. Qualitative and quantitative analysis of flavonoids in Sophora tonkinensis by LC/MS and HPLC. Chin. J. Nat. Med. 2013;11:690–698. doi: 10.3724/SP.J.1009.2013.00690. [DOI] [PubMed] [Google Scholar]
- 68.Yan G.L., Zou D., Zhang A.H., Tan Y.L., Sun H., Wang X.J. UPLC-Q-TOF-MS/MS fingerprinting for rapid identification of chemical constituents of Ermiao Wan. Anal. Methods. 2015;7:846–862. doi: 10.1039/C4AY01215A. [DOI] [Google Scholar]
- 69.Mekonnen A. Chemical Investigation of the Leaves of Moringa Stenopetala. Bull. Chem. Soc. Ethiopi. 2000;14:197–201. doi: 10.4314/bcse.v14i1.72018. [DOI] [Google Scholar]
- 70.Strutt K.D., Keay S., Millett M. The Hinterland of Portus. Integrated Analysis of Geophysical Survey Data and Remotely Sensed Imagery in the Tiber Delta. Chem. Pharm. Bull. 2012;39:1551–1555. [Google Scholar]
- 71.Falowo A.B., Mukumbo F.E., Idamokoro E.M., Lorenzo J.M., Afolayan A.J., Muchenje V. Multi-functional application of Moringa oleifera Lam. in nutrition and animal food products: A review. Food Res. Int. 2018;106:317–334. doi: 10.1016/j.foodres.2017.12.079. [DOI] [PubMed] [Google Scholar]
- 72.Xu X.F., Cheng X.L., Lin Q.H., Li S.S., Jia Z., Han T., Lin R.C., Wang D., Wei F., Li X.R. Identification of mountain-cultivated ginseng and cultivated ginseng using UPLC/oa-TOF MSE with a multivariate statistical sample-profiling strategy. J. Ginseng Res. 2016;40:344–350. doi: 10.1016/j.jgr.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rai A., Hameed A., Noreen R. Antioxidant Potential and Biochemical Analysis of Moringa oleifera Leaves. Int. J. Agric. Biol. 2017;19:941–950. doi: 10.17957/IJAB/15.0366. [DOI] [Google Scholar]