Abstract
Ultrasound-assisted extraction (UAE) is widely used; however, the efficiency of extraction depends on the raw materials. Therefore, optimization of UAE must be investigated for each type of plant material. By-products from soursop fruit have not been studied as a source of bioactive compounds. In this work, the optimization of UAE conditions (extraction time (5, 10, and 15 min), pulse cycle (0.4, 0.7, and 1 s), and sonication amplitude (40%, 70%, and 100%)) for the extraction of phenolic compounds (soluble, hydrolyzable, condensed tannins, and total polyphenols) from soursop by-products (seed, peel, and columella) and pulp was evaluated using response surface methodology. The optimal conditions for UAE to obtain the highest total polyphenol content from by-products and pulp was dependent on the raw material. Peel resulted in the highest content of total polyphenols (187.32 mg/g dry matter [DM]) followed by columella (164.14 mg/g DM), seed (36.15 mg/g DM), and pulp (33.24 mg/g DM). The yield of polyphenolic content from peel and columella obtained with UAE was higher (32–37%) than conventional extraction for 2 h under stirring (14–16%). The contents of gallic acid (0.36–15.86 µg/g DM), coumaric acid (0.07–1.37 µg/g DM), and chlorogenic acid (9.18–32.67 µg/g DM) in the different parts of the fruit were higher in the extracts obtained by UAE compared with a conventional extraction method (0.08–0.61, 0.05–0.08, 3.15–13.08 µg/g DM, respectively), although it was dependent on the raw materials. Soursop by-products can be functionally important if they are used to extract bioactive compounds by UAE; a technology with high potential for commercial extraction on a large scale.
Keywords: soursop fruit, phenolic compounds, pulp and by-products, ultrasound-assisted extraction
1. Introduction
Soursop (Annona muricata L.) belongs to the family Annonaceae, and it is found in different tropical and subtropical areas worldwide. Its components (stems, leaves, and roots) and some parts of the fruit (pulp and seeds) have been used medicinally against human diseases such as cancer, diabetes, cardiovascular and anti-inflammatory problems, and parasitic infections [1,2].
The consumption of soursop fruits has increased around the world due to its importance for health [3]. The soursop fruit presents an irregular form (typically heart-shaped) that is dark green when unripe and pale green when ripe. The fruits measure 20 to 25 cm in length and 10 to 12 cm in diameter, and weigh measure from 0.25 to 5 kg. The surface of the fruit is covered with small conical protuberances [3,4]. Soursop fruit consist of 67% edible components, and it is principally consumed as fresh fruit, although in an increasing percentage is processed as juice, nectar, and puree; however, 33% of the whole fruit is discarded as waste (20% peel, 8.5% seeds, and 4% columella) [3]. As a result, there is a high level of by-products, especially peels and seeds; amounts of 660 kg of by-products/day at processing plants could result in local pollution [5]. Soursop leaves, pulp, and seeds have been recognized as sources of valuable bioactive compounds such as polyphenols, alkaloids, and acetogenins [2,6,7]. Soursop by-products such as columella and peel have not been studied. In some studies, it has been reported that many fruit by-products may contain higher levels of bioactive compounds than the edible parts [8,9]. Therefore, soursop by-products could be considered as a potential source to extract natural bioactive compounds with antioxidant activity or pharmaceutical applications.
Phenolic compounds are strong antioxidants, although their protective action goes beyond the modulation of oxidative stress; thus, they have been used as nutritional supplements, additives in functional foods, pharmaceutical products to prevent chronic diseases such as cardiovascular diseases, type 2 diabetes, and some types of cancers [9]. The polyphenolic extract has antioxidant activity by different mechanisms and was shown to inhibit the pro-inflammatory enzymes (cyclooxygenase, lipoxygenase, and phospholipase A2) in metabolic syndrome, oxidative stress, and inflammatory processes [10]. Furthermore, it has been reported that polyphenols act primarily through modulation of the PI3K/AKT pathway, demonstrating that the polyphenols are inflammatory mediators in an in vitro colon cancer model [11].
Traditionally, phenolic compounds from plant materials have been extracted by conventional extraction methods (extraction with stirring, maceration, cold pressing, squeezing, and hydro-distillation). However, most of these techniques are based on the extracting power of different solvents along with the application of heat and long extraction times. These approaches can cause loss of polyphenol content due to oxidation, ionization, and hydrolysis [12,13,14]. Rasheed et al. [14] evaluated different extraction methods (decoction, infusion, and maceration) and their effect on the bioactive compound profile of Hibiscus sabdariffa (roselle) extracts. Cold maceration proved to be better for preserving anthocyanins, whereas the infusion method was more suitable for recovering organic acids. In that context, the impact of the extraction method plays an important role on the extractable compounds and their related biological activities [14].
As an alternative technology, ultrasound (US) is being used increasingly. Ultrasound may produce a cavitation effect, the action of which can cause physical and mechanical changes in raw materials facilitating extraction of compounds [15,16]. US has been applied for polyphenolic extraction from muicle leaves [16], starfruit leaves [13], as well as pomegranate [17] and orange [18] peel, among many other plants with nutritional or pharmaceutical effects [9]. Anaya-Esparza et al. [16] obtained a higher phenolic content (54 mg/g) using ultrasound-assisted extraction (UAE, 2 min extraction time and 0.7 s pulse cycle at 55% of sonication amplitude) than with stirring (46.4 mg/g, 2 h using a magnetic stirrer at room temperature) and thermal decoction (47.7 mg/g, boiled at 60 °C for 5 min) in muicle leaves. UAE can improve the extraction rate, reduce the extraction time, and increase the stability of compounds compared with conventional extraction methods such as stirring and thermal decoction [16]. Although there is a lot research on UAE in vegetal material, the optimization of US extraction on soursop pulp or by-products has not been investigated; the extraction efficiency depends on the UAE conditions and raw materials. Therefore, the optimization of UAE must be investigated for each type of plant material [19].
The Box-Behnken design (BBD) tool is used for optimization of extraction procedures of biological compounds and is an important response surface methodology (RSM) [20]. BBD is a rotatable second-order design based on a three-level incomplete factorial design. This design is used extensively as an economic method for extracting a large amount of information with a small number of experiments [21]. On the other hand, RSM allows the interaction of the independent variables with the response variables to be monitored using a collection of statistical and mathematical methods [20,21]. The aim of this study was to evaluate the effect of UAE conditions on the extraction of phenolic compounds from soursop seed, peel, columella, and pulp and to use RSM to optimize the UAE parameters (extraction time, pulse cycle, and sonication amplitude) for extracting polyphenolic compounds from pulp and by-products of soursop fruits using BBD. In addition, the optimal UAE conditions are compared with a conventional extraction method for extraction of polyphenolic compounds and their yield.
2. Results and Discussion
2.1. Ultrasound-Assisted Extraction of Soluble Polyphenols from Soursop Samples
The experimental data for soluble polyphenols (SP) obtained by UAE from soursop peel, columella, seeds, and pulp are shown in Table 1. Statistical differences (p < 0.01) were observed between treatments (experimental conditions: XET = extraction time (5, 10, and 15 min), XPC = pulse cycle [0.4, 0.7, and 1 s], and XSA = sonication amplitude [40%, 70%, and 100%]) and raw materials (seeds, peel, columella, and pulp). The extraction of SP from soursop pulp and by-products was dependent on the experimental conditions. The highest SP contents from peel were 157.40 and 156.29 mg/g DM in samples treated for 5 and 15 min (XPC, 0.7 s; XSA, 100%), respectively. Under the same experimental conditions (XET = 5 min, XPC = 0.7 s, XSA = 100%), the highest SP content in seeds was obtained (28.38 mg/g DM), and comparable values were observed in powder seeds after 10 min of exposure at 100% sonication amplitude (XSA) and 0.7 s pulse cycle (XPC). In contrast to peel and seeds, the highest SP values for columella (143.35 mg/g DM) and pulp (23.62 mg/g DM) were observed when the lowest values for the experimental conditions were applied (XET = 10 min, XPC = 0.4 s, XSA = 40% and XET = 5 min, XPC = 0.7 s, XSA = 40%, respectively). León-Fernández et al. [22] reported an SP content of 35.6 mg/g (DM) in soursop pulp extract after applying extraction with an ultrasonic bath (3 h, 42 kHz at 25 °C) and using methanol as solvent. In this work, a 11-fold reduction in extraction time was achieved compared with the report of León-Fernández et al. [22]. Several studies have shown that UAE could be used as an efficient method for the extraction of polyphenolic compounds from plant material [13,16].
Table 1.
Effect of process variables of ultrasound-assisted extraction on soluble polyphenols from peel, seeds, columella, and pulp from soursop fruit.
| Run | UAE Conditions | Final Temperature (°C) | Soluble Polyphenols (mg/g Dry Matter) | |||||
|---|---|---|---|---|---|---|---|---|
| XET | XSA | XPC | Peel | Seed | Columella | Pulp | ||
| 1 | 5 | 40 | 0.7 | 23.38 ± 1.84 | 74.26 ± 0.84 i,B | 24.27 ± 0.18 c,d,C | 115.01 ± 4.76 b,A | 23.62 ± 0.16 a,C |
| 2 | 15 | 40 | 0.7 | 22.25 ± 2.72 | 126.45 ± 8.42 e,f,A | 16.19 ± 0.42 h,C | 84.06 ± 1.30 f,g,B | 20.00 ± 0.69 e,f,C |
| 3 | 5 | 100 | 0.7 | 24.88 ± 1.89 | 157.40 ± 6.03 a,A | 28.38 ± 0.98 a,C | 106.59 ± 3.93 c,B | 19.50 ± 0.66 f,g,C |
| 4 | 15 | 100 | 0.7 | 24.50 ± 1.08 | 156.29 ± 4.14 a,b,A | 21.99 ± 0.54 f,C | 91.67 ± 2.53 d,e,B | 19.26 ± 0.47 g,C |
| 5 | 5 | 70 | 0.4 | 22.25 ± 2.10 | 116.07 ± 7.90 f,g,A | 25.07 ± 0.58 b,c,B | 106.44 ± 5.55 c,A | 19.54 ± 0.19 f,g,B |
| 6 | 15 | 70 | 0.4 | 23.38 ± 2.32 | 141.68 ± 5.25 b,c,d,A | 15.85 ± 0.22 h,C | 105.82 ± 5.05 c,B | 19.24 ± 0.11 g,C |
| 7 | 5 | 70 | 1 | 24.75 ± 1.55 | 154.51 ± 9.22 a,b,c,A | 24.91 ± 0.64 b,c,C | 91.64 ± 0.21 d,e,B | 20.62 ± 0.29 d,C |
| 8 | 15 | 70 | 1 | 24.63 ± 2.43 | 156.76 ± 5.71 a,A | 23.81 ± 0.58 d,e,C | 104.48 ± 2.22 c,B | 19.35 ± 0.34 g,C |
| 9 | 10 | 40 | 0.4 | 22.88 ± 1.93 | 135.92 ± 2.35 d,e,A | 20.85 ± 0.61 g,B | 143.35 ± 1.52 a,A | 21.45 ± 0.62 c,B |
| 10 | 10 | 100 | 0.4 | 24.50 ± 1.08 | 141.50 ± 6.60 c,d,A | 20.29 ± 0.52 g,C | 116.47 ± 0.36 b,B | 20.42 ± 0.77 d,e,C |
| 11 | 10 | 40 | 1 | 24.88 ± 1.31 | 129.23 ± 3.30 d,e,f,A | 23.11 ± 0.52 e,C | 91.08 ± 0.17 d,e,B | 21.33 ± 0.27 c,C |
| 12 | 10 | 100 | 1 | 25.13 ± 1.65 | 138.20 ± 8.03 d,e,A | 21.26 ± 0.76 f,g,C | 81.46 ± 1.86 f,g,B | 20.40 ± 0.18 d,e,C |
| 13 | 10 | 70 | 0.7 | 23.88 ± 0.75 | 110.39 ± 7.12 g,h,A | 25.39 ± 0.50 b,C | 93.66 ± 1.13 d,B | 22.19 ± 0.58 b,C |
| 14 | 10 | 70 | 0.7 | 24.63 ± 1.03 | 99.18 ± 0.87 h,A | 24.45 ± 0.55 b,c,d,C | 86.74 ± 0.59 e,f,B | 22.63 ± 0.38 b,C |
| 15 | 10 | 70 | 0.7 | 24.63 ± 1.25 | 103.26 ± 7.24 g,h,A | 24.21 ± 0.87 c,d,C | 78.53 ± 0.51 g,B | 19.83 ± 0.49 e,f,g,C |
UAE, ultrasound-assisted extraction; XET, exposition time (min); XSA, sonication amplitude (%); XPC, pulse cycle. Data are expressed as means ± standard deviation (n = 3). Different lowercase letters indicate significant statistical differences between treatments (α = 0.05); different capital letters indicate significant statistical differences between raw materials (α = 0.05).
Other authors have argued that the mechanical effects generated by UAE modify the morphology of the cell walls, and a more porous surface is obtained, thus facilitating the extraction [23]. Thus, the components in the free state migrate from the material to the solution. In addition, the ultrasonic treatment accelerates the molecular motion of the solution and hence helps to quickly and effectively combine the components with the solution [24].
On the other hand, peel and columella showed higher (p < 0.01) SP content than pulp and seeds after UAE. These results may be attributable to the composition and complexity of each matrix and because the peel protects the fruit from external factors, and the columella is a conduit of nutrients and protects the seeds [25]. Similar trends were previously reported by Albuquerque et al. [26] who reported higher phenolic content in peel (1.7–1.9 g/kg) than in pulp (0.30–1.20 g/kg) and seeds (0.30–0.40 g/kg) from Annona cherimoya Mill.
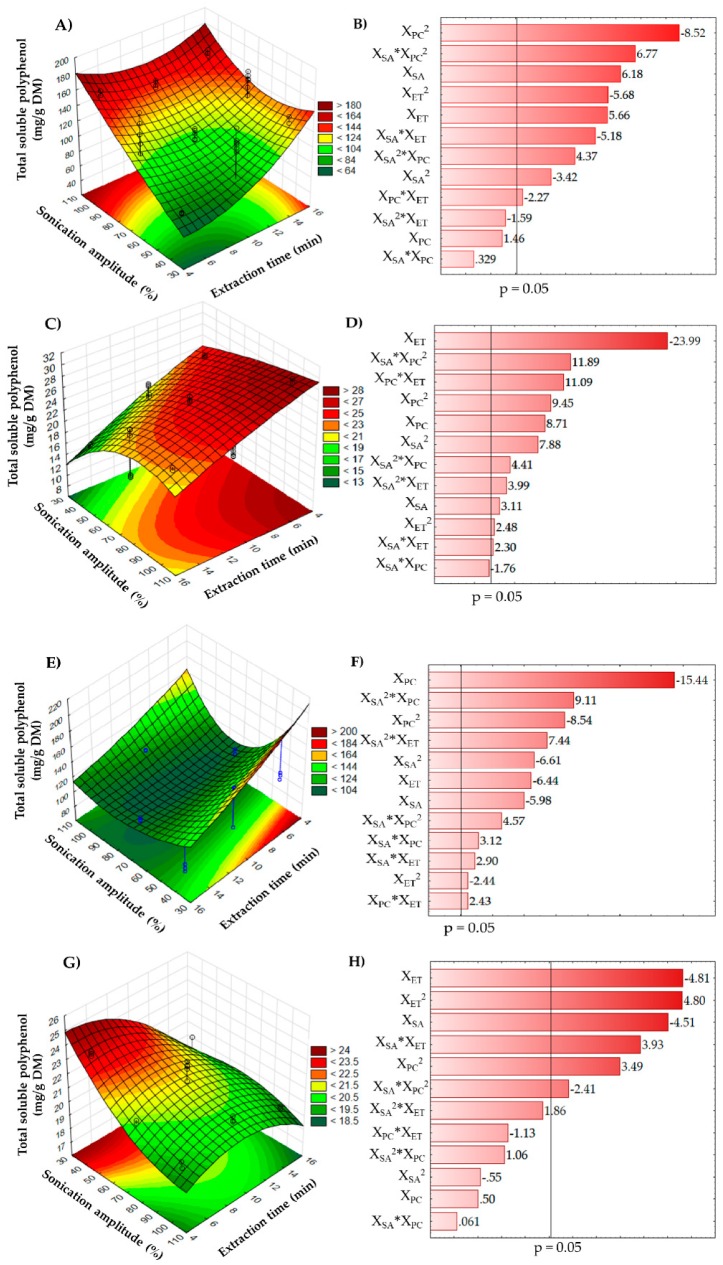
The significant interaction effects (p < 0.01) of the UAE parameters on the SP content in peel (Figure 1A), seeds (Figure 1C), columella (Figure 1E), and pulp (Figure 1G) are shown in three-dimensional response surface plots, where the shapes contour plots (elliptical form) indicate the interactions between the corresponding variables [27]. Results indicated that SP extraction by UAE was observed in the whole experimental domain and in all vegetal material studied, independent of the extraction time, pulse cycle, and sonication amplitude used. However, the highest SP content from peel (Figure 1A) and seeds (Figure 1C) can be obtained at 15 and 5 min, respectively, with medium pulse cycles (0.7 s) and sonication amplitude (100%). Conversely, the highest SP values in columella (Figure 1E) and pulp (Figure 1G) can obtained at lower experimental conditions (XET = 7.5 min, XPC = 0.4 s and XET = 5 min, XPC = 0.7 s, respectively), in particular for the sonication amplitude (XSA, 40%). In addition, the Pareto charts (Figure 1B,D,F,H) show the effect of independent variables on the extraction of SP at a confidence level of 95%. The effect (negative or positive) of the variables on SP extraction is dependent on each matrix, and the effect of the independent variables (and their interactions) on each vegetal material could be ranked as follows: for pulp, XET > XSA > XPC; peel, XPC > XSA > XET; columella, XPC > XSA > XET; and seeds, XET > XPC > XSA. Xu; and Pan [28] reported that extraction efficiency for all-trans-lycopene from red grapefruit is a function of the independent variables (extraction time, temperature, solvent/material ratio, ultrasonic intensity, and duty cycle). As in this study, the UAE conditions for SP compounds from soursop samples were dependent on the plant matrix [25].
Figure 1.
Response surface plots and Pareto charts for soursop peel (A,B), seeds (C,D), columella (E,F), and pulp (G,H) showing the effect of ultrasound-assisted extraction on the soluble polyphenol content. DM, dry matter; XET, exposition time (min); XSA, sonication amplitude (%); XPC, pulse cycle.
RSM for the extraction of SP from soursop pulp, peel, columella, and pulp by UAE as a function of extraction time, pulse cycle, and sonication amplitude was developed using a multiple regression technique. A second-order polynomial equation (Table 2) for each material was obtained, which described the effect of the test variables as well as the combined effect of all test variables on the response [21]. The analysis of variance of the SP content showed that the experimental data from pulp, columella, peel, and seeds have good correlation (R2 = 0.77, R2 = 0.94, R2 = 0.91, and R2 = 0.97, respectively), and thus, adequate adjustment of the experimental data to the model was observed (lack of fit, p > 0.05), except for pulp, which showed weak adjustment (R2 = 0.77) (Table 2). The lack of fit demonstrated the fitness of the model, giving an indication of approximation to the real system. Comparable results were reported by Bashi et al. [29] during optimization (extraction temperature, pH, liquid/solid ratio, and extraction time) of UAE of phenolic compounds from Achillea biebersteinii using RSM (R2 = 0.85).
Table 2.
The predicted mathematical model for the extraction of soluble polyphenols (mg/g) from Annona muricata peel, seeds, columella, and pulp after ultrasound-assisted extraction.
| Sample | Polynomial Equation | R2 |
|---|---|---|
| Peel | 594.18 − 8.08XSA + 0.04XSA2 − 1459.15XPC + 891.37XPC2 + 4.51XET + 0.61XET2 + 21.09XSA*XPC − 9.12XSA*XPC2 − 0.06XSA2*XPC − 0.27XSA*XET + 0.00XSA2*XET − 3.89XPC*XET | 0.91 |
| Seeds | 74.23 − 0.98XSA + 0.004XPC2 − 109.02XPC + 59.83XPC2 − 2.40XET − 0.01XET2 + 2.15XSA*XPC − 1.14XSA*XPC2 − 0.004XSA2*XPC + 0.035XSA*XET − 0.001XSA2*XET + 1.35XPC*XET | 0.97 |
| Columella | 778 − 14.68XSA + 0.08XSA2 − 906.85XPC + 367.59XPC2 − 21.45XET + 0.14XET2 + 14.32XSA*XPC − 3.30XSA*XPC2 − 0.06XSA2*XPC + 0.47XSA*XET − 0.003XSA2*XET + 2.24XPC*XET | 0.94 |
| Pulp | 24.40 − 0.27XSA + 0.002XSA2 + 35.55XPC − 27.78XPC2 − 0.11XET − 0.04XET2 − 0.21XSA*XPC + 0.27XSA*XPC2 − 0.001XSA2*XPC + 0.02XSA*XET − 0.001XSA2*XET − 0.16XPC*XET | 0.77 |
XSA, sonication amplitude (%); XPC, pulse cycle (s); XET, extraction time (min); R2, regression coefficient.
Some coefficients (Table 3) were not significant (p > 0.05) to the model: for peel, XET and XSA2*XET, in pulp, XSA, XET, XSA*XPC, XSA2*XPC, and XSA2*XET. On the other hand, all coefficients for seed and columella were significant (p < 0.05) to the model.
Table 3.
Regression coefficients of predicted quadratic polynomial models with the ultrasound-assisted extraction conditions on the soluble phenolic content from Annona muricata peel, seeds, columella, and pulp.
| Source | Regression Coefficients | |||
|---|---|---|---|---|
| Total Soluble Phenolic Content β Coefficient | ||||
| Peel | Seed | Columella | Pulp | |
| Mean/intercept | 594.18 | 74.23 | 778.00 | 20.48 |
| XSA | −8.08 + | −0.98 + | −14.68 + | −0.29 ++ |
| XSA2 | 0.04 + | −0.004 + | 0.08 + | 0.002 + |
| XPC | −1459.15 + | −109.02 + | −906.85 + | 35.55 + |
| XPC2 | 891.37 + | 59.83 + | 367.59 + | −27.78 + |
| XET | 4.51 ++ | −2.40 + | −21.45 + | −0.11 ++ |
| XET2 | 0.61 + | −0.01 + | 0.14 + | −0.04 + |
| XSA*XPC | 21.09 + | 2.15 + | 14.35 + | −0.21 ++ |
| XSA*XPC2 | −9.12 + | −1.14 + | −3.30 + | 0.27 + |
| XSA2*XPC | −0.06 + | −0.004 + | −0.06 + | −0.001 ++ |
| XSA*XET | −0.27 + | 0.03 + | 0.47 + | 0.023 + |
| XSA2*XET | 0.00 ++ | −0.0002 + | −0.003 + | −0.0001 ++ |
| XPC*XET | −3.89 + | 1.35 + | 2.24 + | −0.05 ++ |
| R-square | 0.91 | 0.97 | 0.94 | 0.77 |
| R-adjust | 0.87 | 0.96 | 0.92 | 0.69 |
XET, extraction time; XPC, pulse cycle; XSA, sonication amplitude. + Significant (p < 0.05); ++ non-significant (p > 0.05).
Pan et al. [16] mentioned that when UAE is applied in plant materials (e.g., pomegranate peel) the intensity level and pulse duration has a prominent effect on the polyphenolic extraction yield; and a long extraction time had a negative effect. However, the authors mentioned that efficacy of treatment depended on the matrix composition and the cellulose-hemicellulose-lignin ratio. In addition, a slight increase in temperature from 1 to 2.88 °C (Table 1) for all UAE runs was observed in a normal reaction when UAE was applied as a consequence of interaction of cavitation and extraction time. This temperature increase can be also attributable to heat transference from cavitation bubbles, which may cause a gradual temperature increase in the medium and facilitate extraction of the compounds. Similar trends were reported previously during UAE (extraction time, 2 min; pulse cycle, 0.7 s; sonication amplitude, 55%) of polyphenolic compounds from muicle leaf extracts [16]. However, there was no drastic increase in temperature because the temperature was controlled during the experiments.
2.2. Evaluation of Model Reliability and Comparison of Ultrasound-Assisted Extraction with Conventional Extraction
The optimum UAE conditions for SP extraction from A. muricata peel, seeds, columella, and pulp are shown in Table 4. To verify the reliability of the model, treatments were performed using the optimal conditions for each sample as recommended by Aydar et al. [19]. The experimental values of the SP content (Table 5) for all samples were in agreement with the predicted values under the optimal conditions (see Table 4). Therefore, an efficient extraction of polyphenols from soursop samples using UAE was possible, as has been reported by other studies where RSM was used to optimize the extraction of polyphenols by UAE from pomegranate [17], orange [18] peel, muicle [16], and starfruit leaves [13].
Table 4.
Optimal conditions of ultrasonic-assisted extraction on soluble polyphenols from Annona muricata peel, seed, columella, and pulp.
| Parameter | Peel | Seeds | Columella | Pulp |
|---|---|---|---|---|
| Extraction time (min) | 15 | 5 | 7.5 | 5 |
| Pulse cycle (s) | 0.4 | 0.7 | 0.4 | 0.7 |
| Sonication amplitude (%) | 99.95 | 100 | 40 | 40 |
| Optimal response (mg/g DM) | 161.76 | 28.38 | 153.65 | 23.62 |
| −95% Confidence limit | 148.21 | 27.63 | 147.64 | 22.74 |
| +95% Confidence limit | 175.30 | 29.13 | 159.65 | 24.50 |
| Confidence interval (±) | 27.09 | 1.5 | 11.98 | 1.76 |
−95% confidence limit, lower limit; +95% confidence limit, upper limit; confidence interval, difference between upper and lower limits; DM, dry matter.
Table 5.
Soluble and hydrolyzable polyphenols, condensed tannins, total polyphenols, and yield from Annona muricata peel, seed, columella, and pulp using the optimal conditions for ultrasound-assisted extraction and conventional extraction (extraction for 2 h under stirring, see Section 3.6.1).
| Parameter | Ultrasound-Assisted Extraction | Conventional Extraction | ||||||
|---|---|---|---|---|---|---|---|---|
| Peel | Seeds | Columella | Pulp | Peel | Seeds | Columella | Pulp | |
| Soluble polyphenols (mg/g dry matter) | 171.26 ± 8.83 a | 24.92 ± 0.33 d | 152.97 ± 1.01 b | 24.70 ± 1.52 d | 133.96 ± 0.03 c | 23.58 ± 2.14 d | 124.91 ± 8.81 c | 24.76 ± 1.72 d |
| Hydrolyzable polyphenols (mg/g dry matter) | 8.40 ± 0.14 c | 11.05 ± 0.38 a | 6.14 ± 0.96 d | 5.68 ± 0.21 e | 6.51 ± 0.23 d | 9.01 ± 0.41 b | 7.31 ± 0.14 d | 5.47 ± 0.28 e |
| Condensed tannins (mg/g dry matter) | 7.66 ± 0.74 a | 0.18 ± 0.02 d | 5.13 ± 0.20 b | 2.86 ± 0.22 c | 8.21 ± 0.49 a | 0.72 ± 0.07 d | 4.90 ± 0.69 b | 0.91 ± 0.02 d |
| Total polyphenols (mg/g dry matter) | 187.32 ± 3.23 a | 36.15 ± 0.24 d | 164.14 ± 0.70 b | 33.24 ± 0.65 e | 148.68 ± 0.25 c | 33.31 ± 0.87 e | 137.12 ± 3.21 c | 31.14 ± 3.01 e |
| Polyphenolic yield (%) | 37.51 ± 2.87 a | 7.13 ± 0.14 c | 32.34 ± 0.31 a | 6.59 ± 0.25 c | 16.68 ± 0.15 b | 6.67 ± 0.48 c | 14.87 ± 1.43 b | 5.42 ± 1.01 c |
All values are means ± standard deviation of three determinations. Different letters in each column indicate significant statistical differences between treatments (α = 0.01). Experimental conditions for ultrasound-assisted extraction: (1) peel: XET = 15 min, XPC = 0.4 s, XSA = 40%; (2) seeds: XET = 5 min, XPC = 0.7 s, XSA = 100%; (3) columella: XET = 7.5 min, XPC = 0.4 s, XSA = 40%; (4) pulp: XET = 5 min, XPC = 0.7 s, XSA = 40%.
The SP content from peel and columella, hydrolyzable polyphenol (HP) content from peel and seeds, and condensed tannin (CT) content from pulp were significantly higher (p < 0.01) when UAE was used compared with conventional extraction (Table 5). However, there was no significant difference (p > 0.01) in the SP content from seeds and pulp, HP content from columella and pulp, or CT content from peel, seeds, or columella for both extraction methods; however, the extraction time was significantly reduced to 45–55 min. Also, the total polyphenol content was higher using UAE in all samples except pulp, and the yield of phenolic content from peel and columella was higher under UAE (32.34–37.51%) than for organic aqueous extraction (14.87–16.68%). Cvjetko Bubalo et al. [30] reported an increase (more than 30%) in the extraction of SP in grape peel when the samples were treated by UAE (15 min, 60 W, 20 °C) compared with conventional extraction. Velásquez-García et al. [31] added a pretreatment with ultrasound in the extraction of bioactive compounds from mesocarp of chontaduro (Bactris gasipaes) using supercritical CO2, which did not increase the extraction yield, but the operation time was shorter than for conventional extraction. Sousa et al. [32] found that UAE was inefficient for extracting ellagitannins. These authors mentioned that this can be justified because the UAE process produces a large amount of energy, which may lead to cleavage or oxidation of molecules, mainly those with a higher molecular mass.
The effect of ultrasound is attributed to interaction with the plant material, altering its physical and chemical properties, and to a cavitation effect, which facilitates the release of extractable compounds and enhances mass transport by disrupting the plant cell walls [15]. Our results are in agreement with Jovanović et al. [33], who compared UAE and maceration extraction methods for extraction of polyphenols from Thymus serpyllum L. herb. They reported that the polyphenolic yield obtained by UAE was higher (22.81 mg/L) than the maceration extraction method (18.12 mg/L), and indicated that UAE could be used for extraction of polyphenols in the future due to the high yield of polyphenolic and flavonoid content, short extraction time, and low degradation of the extracted compounds compared with other extraction methods.
2.3. Soluble Polyphenols Profile
A total of nine phenolic compounds (gallic, coumaric, cinnamic, caffeic, chlorogenic, protocateic, 4-hydroxybenzoic, syringic, and neochlorogenic acids) were identified (Table 6). The type of polyphenol and their concentration were dependent on the raw material and the extraction method, but it was clear that the UAE led to the highest concentration of some polyphenolic compounds such as gallic, coumaric, and chlorogenic acids compared with conventional extraction. Extraction under conventional techniques is usually characterized by poor quality of extracts because thermodegradation of bioactive compounds is induced [34]; UAE is an attractive and green alternative. However, phenolic profile from seeds was not different (p < 0.01) using both methods, probably because the SP content from seeds was very low.
Table 6.
Profile of soluble polyphenols from Annona muricata peel, seed, columella, and pulp using the optimal conditions of ultrasound-assisted extraction and conventional extraction (extraction for 2 h under stirring, see Section 3.6.1).
| No. | Compound | Retention Time (min) | Ultrasound-Assisted Extraction (μg/g Dry Matter) | Conventional Extraction (μg/g Dry Matter) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Peel | Seed | Columella | Pulp | Peel | Seed | Columella | Pulp | |||
| 1 | Gallic acid | 20.66 | 14.50 ± 0.44 a | 0.36 ± 0.01 c | 12.16 ± 0.37 b | 15.86 ± 1.68 a | 1.10 ± 0.08 c | 0.46 ± 0.03 c | 0.61 ± 0.12 c | 0.08 ± 0.01 c |
| 2 | Coumaric acid | 46.61 | 1.37 ± 0.09 a | 0.07 ± 0.00 c,d | 0.08 ± 0.01 bc | 0.07 ± 0.01 c,d | 0.15 ± 0.04 b | 0.07 ± 0.02 c,d | 0.08 ± 0.01 b,c | 0.05 ± 0.01 c,d |
| 3 | Cinnamic acid | 52.91 | 45.51 ± 1.88 a | 40.48 ± 1.21 a,b | 30.36 ± 1.98 b | 42.04 ± 8.72 a,b | 37.16 ± 1.33 a,b | 42.51 ± 0.59 a | 14.51 ± 3.88 c | 7.67 ± 1.14 c |
| 4 | Caffeic acid | 37.17 | 43.68 ± 1.78 a | 32.62 ± 1.22 b | nd | nd | 30.20 ± 8.38 b | 34.44 ± 0.46 b | 11.83 ± 3.19 c | nd |
| 5 | Chlorogenic acid | 34.23 | 32.67 ± 0.53 a | 12.33 ± 0.46 b,c | 9.18 ± 0.59 c | 12.80 ± 2.72 b | 12.25 ± 2.87 b,c | 13.08 ± 0.18 b | nd | 3.15 ± 0.25 d |
| 6 | Protocateic acid | 23.02 | 150.46 ± 6.62 a | 133.47 ± 5.02 a | nd | nd | 123.12 ± 4.28 a | 140.97 ± 1.62 a | nd | 25.37 ± 3.77 b |
| 7 | 4-Hydroxybenzoic acid | 31.16 | 145.98 ± 6.47 a | nd | 94.45 ± 6.15 a,b | 131.63 ± 2.87 a,b | 117.37 ± 32.65 b | nd | 45.96 ± 12.43 c | 24.22 ± 3.60 c,d |
| 8 | Syringic acid | 41.62 | 883.71 ± 3.94 a | 780.77 ± 9.31 b | nd | nd | nd | 824.05 ± 1.89 b | nd | 148.83 ± 2.14 c |
| 9 | Neochlorogenic acid | 24.05 | 78.86 ± 3.48 a | 69.70 ± 2.62 a | 51.90 ± 3.38 b | 72.32 ± 1.31 a | nd | 73.56 ± 0.97 a | nd | 13.30 ± 1.98 c |
All values are means ± standard deviation of three determinations. Different letters in each column indicate significant statistical differences between treatments and between samples (α = 0.01). nd, not detected.
3. Materials and Methods
The experimental work was carried out in two stages. In the first stage, the optimal experimental conditions were determined to extract the highest SP content from soursop pulp and by-products under UAE by using RSM. The second stage consisted of evaluation of the accuracy of the model by measuring the SP, HP, and CT contents, total polyphenols, and the profile of the polyphenolic compounds obtained under optimal UAE conditions. The yield obtained with UAE versus conventional extraction was compared.
3.1. Plant Material and Chemicals
Soursop fruits (Annona muricata L.) were collected in orchards located in Compostela, Nayarit, Mexico, at the consumption maturity stage (15–19 °Brix). The pulp and by-products (peel, seeds, and columella) were separated manually. The samples were freeze-dried (Labconco 77522020, Kansas City, MO, USA) at −50 °C under pressure of 0.12 mbar. The dried samples were then ground in a food processor (Nutribullet NB-101B, Los Angeles, CA, USA) and sieved using a stainless steel mesh (no. 35; Fisher Scientific, Hampton, NH, USA) to a particle size of 500 µm. Phenolic standards, Folin-Ciocalteu phenol reagent, methanol, water, and acetic acid of HPLC grade were purchased from Sigma-Aldrich (St Louis, MO, USA).
3.2. Experimental Design
A BBD was used to determine the optimal UAE conditions for extracting polyphenols from soursop fruit pulp and by-products with three levels for each factor. The goal was to evaluate the individual and interactive effects of extraction time (XET, min), pulse cycle (XPC, s), and sonication amplitude (XSA, %) at 40%, 70%, and 100% on SP content. A randomized experimental order was used to reduce the effect of unexplained variability on the observed response. Table 1 shows the experimental design used in the study; the three independent variables were studied at three levels: XET (5, 10, and 15 min), XPC (0.4, 0.7, and 1.0), XSA (40%, 70%, and 100%).
3.3. Ultrasound-Assisted Extraction Procedure
The extraction of polyphenolic compounds from soursop pulp and by-products was performed using a UP400S ultrasonic system (400 W, 24 kHz frequency) (Hielscher Ultrasonics, Teltow, Germany). Extraction solutions were according to Pérez-Jiménez et al. [35]. The ultrasonic probe (H7 Tip 7, Hielscher, Teltow, Germany) with 100% maximum amplitude corresponded to 175 μm and acoustic power density of 300 W/cm2. The ultrasonic probe was submerged 2 cm into the extraction solution. The procedure started by weighing the lyophilized samples (0.5 g), placing them in an extraction tube with 20 mL of a water-methanol solution (50:50 v/v) acidified to pH 2 with HCl (0.8% v/v 16 mM HCl was added), and then the samples were subjected to UAE (according to the experimental design; see Table 1). A recirculating cold water bath (Thermo Scientific 2870, Waltham, MA, USA) was used to maintain the extraction temperature in a range of 25 ± 2 °C. The samples were centrifuged (Hermle Z32HK, Wehingen, Germany) at 8000× g for 10 min at 4 °C, the supernatants were recovered, and the residues were resuspended in 20 mL of acetone–water solution (70:30 v/v). The samples were processed again under the same controlled UAE conditions and centrifuged. The supernatants were combined and analyzed to measure SP.
3.4. Soluble Polyphenolic Compounds
The SP content was determined using Folin–Ciocalteu reagent following the Montreau procedure [36] with slight modifications. The supernatants (250 µL) were mixed with 1250 µL of Folin-Ciocalteu reagent and incubated in tubes for 5 min. Sodium carbonate solution (1000 µL, 75 g/L) was then added and the tubes were incubated in a water bath for 15 min at 50 °C. Then 270 µL were transferred into the wells of a 96-well microplate reader (Synergy HT Multi-Detection Microplate Reader, BioTek Instruments, Winooski, VT, USA), and the absorbance was measured at 765 nm. SP content was calculated with a standard curve (R2 = 0.998) for gallic acid, and the results were expressed as milligrams of gallic acid equivalents per gram of sample of dry matter (mg/g DM). Each standard and sample was analyzed in triplicate.
3.5. Response Surface Methodology Analysis
Once the data was obtained (SP), RSM was applied to obtain the optimal processing conditions for the four samples. A second-order polynomial equation derived from RSM was used to calculate the predicted response (Equation (1)).
| (1) |
where Y is the predicted response (SP), Xi are the uncoded or coded values for the factors (XET, XPC, and XSA), β0 is a constant, βi are the main effect coefficients for each variable, and βij are the interaction effect coefficients. Model adequacy was evaluated by analysis of variance (ANOVA) to determine the effects of significant interactions in the model (p < 0.01) and by quantification of the coefficient of determination (R-squared and R-adjust).
3.6. Model Reliability and Comparison of Ultrasound-Assisted Extraction with Conventional Extraction
The optimal UAE conditions by RSM analysis for extracting polyphenolic compounds from soursop pulp and by-products were used to verify the accuracy of the model. The content of SP, HP, and CT were measured, and the total polyphenols and the yield were calculated. In addition, the profile of the polyphenolic compounds was determined by HPLC. The results for phenolic compound using the optimal UAE conditions were compared with the results with conventional extraction.
3.6.1. Conventional Extraction of Polyphenols
Organic aqueous extraction was performed with 0.5 g of lyophilized samples in 20 mL of methanol-water solution (50:50, v/v) acidified with HCl (0.8% v/v 16 mM HCl). The mixture was stirred at room temperature (25 ± 2 °C) at a moderate speed (10× g) for 1 h in a shaker (Heidolph Rex 2, Heidoplh Instruments, Schuwabach, Germany). Then the extracts were centrifuged at 8000× g for 10 min at 4 °C. The supernatants were recovered and the residues were resuspended in 20 mL of an acetone-water solution (70:30, v/v); the samples were stirred for 1 h at room temperature and centrifuged under the same conditions. The method of extraction was according Pérez-Jiménez et al. [35]. The supernatants were combined and used to measure SP; the residues were used to measure HP and CT.
3.6.2. Soluble Polyphenols, Hydrolyzable Polyphenols, and Condensed Tannins
Polyphenols were extracted under optimal UAE conditions or conventional extraction. Supernatants (soluble polyphenolic extracts) and residues were obtained. SPs were measured in the supernatants following the methodology described earlier. The residues were obtained in two lots: one lot was used to evaluate HP and the other to measure CT.
HPs were determined with the method described by Hartzfeld et al. [37]. Hydrolysis of the samples (0.5 g) with 20 mL methanol/concentrated H2SO4 (90:10 v/v) at 85 °C for 20 h was performed. The samples were then centrifuged (10 min, 25 °C, 5000× g). The supernatants were saved and the residues were resuspended in distilled water twice, and the mixture was centrifuged at each dilution. The supernatants (extracts) were combined. The extracts (500 µL) were mixed with 500 µL of Folin-Ciocalteu reagent and incubated for 5 min at room temperature. Then, 1000 µL of sodium carbonate solution (75 g/L) was added to the mixture and incubated in a water bath for 15 min at 50 °C. Subsequently, absorbance was measured at 765 nm. HPs were calculated using a standard curve for gallic acid. The results were reported as mg/g DM. CTs were determined in the residues according to Reed et al. [38]. Ten milliliters of butanol/HCl/ FeCl3 (97.5:2.5 v/v) solution was added to the residues (0.5 g) and incubated at 100 °C for 3 h. The samples were centrifuged (10 min, 4 °C, 6000× g) and CTs were determined in the supernatants. Absorbance was measured at 555 nm using a spectrophotometer (Jenway 6705, Dunmow, UK). CTs were calculated from a standard curve of proanthocyanidins from Mediterranean carob pods (Ceratonia siliqua L.). The CT content was expressed as mg/g DM.
3.6.3. Polyphenol Profile by HPLC
The partial identification of phenolic acids was performed in the SP extracts under optimal UAE conditions and with conventional extraction. SP extracts were concentrated in a rotary evaporator and resuspended in 1 mL of eluent A. The samples (20 μL) were injected into an HPLC system (Agilent Technologies 1260 Infinity, Waldbronn, Germany) equipped with a photodiode array detector and a C18 reverse-phase column (5 μm particle size, 4.6 mm in diameter, 250 mm long; Thermo Scientific, Sunnyvale, CA, USA). The mobile phases were acidified water with 2% acetic acid (eluent A) and acidified water (0.5% acetic acid)–methanol (10:90, eluent B). Standards and samples were analyzed using a gradient program: 0% B; 0–35 min, 35% B; 35–55 min, 75% B; 55–60 min, 100% B; 60–70 min; 0% B, at a flow rate of 0.4 mL/min. The peak areas of the samples were detected at 280 and 320 nm, and a calibration curve (0.5–300 μg/mL) of gallic, chlorogenic, caffeic, cinnamic, caffeic, chlorogenic, protocateic, syringic, neochlorogenic, 4-hydroxybenzoic, and p-coumaric acids was used to identify and quantify the polyphenols [7]. Total phenolic compounds were determined as the sum of SP, HP, and CT.
3.6.4. Yield of Polyphenolic Compounds
Yield was defined as the percentage of the total extracted polyphenols (SP, HP, and CT) from the total weight of the sample (g). The extraction yield was calculated (Equation (2)) as suggested by Aydar et al. [19].
| (2) |
3.7. Statistical Analysis
Data were expressed as means ± standard deviation (n = 6). In the first stage, data were analyzed with RSM. In the second stage, the experiments were conducted in a one-factorial experimental design. The analyses were performed using ANOVA (p < 0.01) with STATISTICA v. 10 (Statsoft, Tulsa, OK, USA) software. Fisher’s least significant difference method was used to examine the differences between means (α = 0.01).
4. Conclusions
The optimal UAE conditions are dependent on the type of raw material. The results in this study confirmed that short extraction times using UAE were sufficient to achieve swelling of the cells, leading to increased permeability of vegetal cell walls, and more phenolic compounds could be extracted from soursop in decreasing order of peel followed by columella, seeds, and pulp. The efficiency of UAE was higher than the conventional extraction method. The polyphenolic profile of the extracts showed the presence of nine polyphenolic compounds including gallic, coumaric, cinnamic, caffeic, chlorogenic, protocateic, 4-hydroxybenzoic, syringic, and neochlorogenic acids. The content of phenolic acids was higher for UAE than for conventional extraction. The present study highlights that by-products (peel and columella) from soursop fruits could be a potential source of polyphenolic compounds and that UAE is an efficient technology to extract these bioactive compounds.
Acknowledgments
The authors gratefully acknowledge the scholarship from CONACYT-Mexico to G.A.H. This work is part of the activities of the RED TEMATICA CONACYT 12.3, to Reduce and Valorize Food Losses and Waste: Towards Sustainable Food Systems” (294768).
Author Contributions
Conceptualization, G.A.-H. and E.M.G.; Methodology, G.A.-H., M.d.L.G.-M., S.G.S.-A., E.M.G.; Software, J.A.S.-B. and L.M.A.-E.; Validation, G.A.-H., L.M.A.-E., and E.M.G.; Formal Analysis, G.H.-A., S.G.S.-A., M.d.L.G.-M., and E.M.G.; Funding Acquisition, E.M.G.; Project Administration, E.M.G.; Writing—Original Draft Preparation, G.A.-H., L.M.A.-E., and E.M.G.; Writing—review and editing, M.d.l.Á.V.-V., J.M.-C., and E.M.G.
Funding
This research was funded by Tecnológico Nacional de México grant number 2019-P.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Sample Availability: Standards of phenolic acids are commercially available.
References
- 1.Zorofchian-Moghadamtousi S., Fadaeinasab M., Nikzad S., Mohan G., Mohd-Ali H., Kadir H.A. Annona muricata (Annonaceae): A review of its traditional uses, isolated acetogenins and biological activities. Int. J. Mol. Sci. 2015;16:15625–15658. doi: 10.3390/ijms160715625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coria-Téllez A.V., Montalvo-Gónzalez E., Yahia E.M., Obledo-Vázquez E.N. Annona muricata: A comprehensive review on its traditional medicinal uses, phytochemicals, pharmacological activities, mechanisms of action and toxicity. Arabian J. Chem. 2018;11:662–691. doi: 10.1016/j.arabjc.2016.01.004. [DOI] [Google Scholar]
- 3.Badrie N., Schauss A.G. Soursop (Annona muricata L.): Composition, nutritional value, medicinal uses, and toxicology. In: Ross-Watson R., Preedy R.V., editors. Bioactive Foods in Promoting Health. 1st ed. Academic Press; Sand Diego, CA, USA: 2010. pp. 621–643. [Google Scholar]
- 4.Pinto A.C.Q., Cordeiro M.C.R., Andrade S.R.M., Ferreira F.R., Filgueiras H.A.C., Alves R.E., Kinpara D.I. Annona Species. International Centre for Underutilised Crops, University of Southampton; Southampton, UK: 2005. pp. 3–18. [Google Scholar]
- 5.SIAP Servicio de Información Agroalimentaria y Pesquera. [(accessed on 18 December 2018)];2018 Available online: https://www.gob.mx/siap/acciones-y-programas/produccion-agricola-33119.
- 6.Moreno-Hernández C., Sáyago-Ayerdi S., García-Galindo H., Mata-Montes De Oca M., Montalvo-González E. Effect of the application of 1-methylcyclopropene and wax emulsions on proximate analysis and some antioxidants of soursop (Annona muricata L.) Sci. World J. 2014;1:1–7. doi: 10.1155/2014/896853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiménez V.M., Gruschwitz M., Schweiggert R.M., Carle R., Esquivel P. Identification of phenolic compounds in soursop (Annona muricata) pulp by high-performance liquid chromatography with diode array and electrospray ionization mass spectrometric detection. Food Res. Int. 2014;65:1–18. doi: 10.1016/j.foodres.2014.05.051. [DOI] [Google Scholar]
- 8.Ajila C.M., Bhat S.G., Rao U.J.S.P. Valuable components of raw and ripe peels from two Indian mango varieties. Food Chem. 2007;102:1006–1011. doi: 10.1016/j.foodchem.2006.06.036. [DOI] [Google Scholar]
- 9.Bamba B., Shi J., Tranchant C., Xue S., Forney C., Lim L.T. Influence of extraction conditions on ultrasound-assisted recovery of bioactive phenolics from blueberry pomace and their antioxidant activity. Molecules. 2018;23:1685. doi: 10.3390/molecules23071685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costamagna M.S., Zampini I.C., Alberto M.R., Cuello S., Torres S., Pérez J., Quispe C., Schemeda-Hirschmann G., Isla M.I. Polyphenols rich fraction from Geoffroea decorticans fruits flour affects key enzymes involved in metabolic syndrome, oxidative stress and inflammatory process. Food Chem. 2016;190:392–402. doi: 10.1016/j.foodchem.2015.05.068. [DOI] [PubMed] [Google Scholar]
- 11.Bucio-Noble D., Kautto L., Krisp C., Ball M.S., Molloy M.P. Polyphenol extracts from dried sugarcane inhibit inflammatory mediators in an in vitro colon cancer model. J. Proteomics. 2018;177:1–10. doi: 10.1016/j.jprot.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Azmir J., Zaidul I.S.M., Rahman M.M., Sharif K.M., Mohamed A., Sahena F., Jahurul M.H.A., Ghafoor K., Norulaini N.A.N., Omar A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013;117:426–436. doi: 10.1016/j.jfoodeng.2013.01.014. [DOI] [Google Scholar]
- 13.Zamora-Gasga V.M., Serafín-García M.S., Sánchez-Burgos J.A., Estrada-Velazquez R.M., Sáyago-Ayerdi S.G. Optimization of ultrasonic-assisted extraction of antioxidant compounds from starfruit (Averrhoa carambola L) leaves. J. Food Process. Preserv. 2016;41:1–10. doi: 10.1111/jfpp.13093. [DOI] [Google Scholar]
- 14.Rasheed D.M., Porzel A., Frolov A., El Seedi H.R., Wessjohann L.A., Farag M.A. Comparative analysis of Hibiscus sabdariffa (roselle) hot and cold extracts in respect to their potential for α-glucosidase inhibition. Food Chem. 2018;250:236–244. doi: 10.1016/j.foodchem.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 15.Chemat F., Rombaut N., Sicaire A.G., Meullemiestre A., Fabiano-Tixier A.S., Abert-Vian M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2016;34:540–560. doi: 10.1016/j.ultsonch.2016.06.035. [DOI] [PubMed] [Google Scholar]
- 16.Anaya-Esparza L.M., Ramos-Aguirre D., Zamora-Gasga V.M., Yahia E., Montalvo-González E. Optimization of ultrasonic-assisted extraction of phenolic compounds from Justicia spicigera leaves. Food Sci. Biotechnol. 2018;27:1093–1102. doi: 10.1007/s10068-018-0350-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan Z., Qu W., Ma H., Atungulu G.G., McHugh T.H. Continuous and pulsed ultrasound-assisted extractions of antioxidants from pomegranate peel. Ultrason. Sonochem. 2011;18:1249–1257. doi: 10.1016/j.ultsonch.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Khan M.K., Abert-Vian M., Fabiano-Tixier A.S., Dangles O., Chemat F. Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chem. 2010;119:851–858. doi: 10.1016/j.foodchem.2009.08.046. [DOI] [Google Scholar]
- 19.Aydar A.Y., Bagdathiglu N., Koseoglu O. Effect on olive oil extraction and optimization of ultrasound-assisted extraction of extra virgin olive oil by response surface methodology (RSM) Grasas Aceites. 2017;67:l89. doi: 10.3989/gya.1057162. [DOI] [Google Scholar]
- 20.Box G.E.P., Wilson K.B. On the experimental attainment of optimum conditions. J. R. Stat. Soc. 1951;13:1–45. doi: 10.1111/j.2517-6161.1951.tb00067.x. [DOI] [Google Scholar]
- 21.Aslan N., Cebeci Y. Application of Box-Behnken design and response surface methodology for modeling of some Turkish coals. Fuel. 2007;86:90–97. doi: 10.1016/j.fuel.2006.06.010. [DOI] [Google Scholar]
- 22.León-Fernández A.L., Sáyago-Ayerdi S.G., Velázquez-Estrada R.M., Zepeda-Vallejo L.G., Yahia E., Montalvo-González E. In vitro antioxidant capacity of crude extracts and acetogenin fraction of soursop fruit pulp. Pharm. Anal. Acta. 2017;8:1–7. doi: 10.4172/2153-2435.1000550. [DOI] [Google Scholar]
- 23.Rodríguez-Riera Z., Robaina-Mesa M., Jáuregui-Haza U., Blanco-González A., Rodríguez-Chanfrau J.E. Empleo de la radiación ultrasónica para la extracción de compuestos bioactivos provenientes de fuentes naturales. Estado actual y perspectivas. Revista CENIC Ciencias Químicas. 2014;45:139–147. [Google Scholar]
- 24.Heydari-Majd M., Rajaei A., Bashi D.S., Mortazavi S.A., Bolourian S. Optimization of ultrasonic-assisted extraction of phenolic compounds from bovine pennyroyal (Phlomidoschema parviflorum) leaves using response surface methodology. Ind. Crops Prod. 2014;57:195–202. doi: 10.1016/j.indcrop.2014.03.031. [DOI] [Google Scholar]
- 25.Mihailović N.R., Mihailović V.B., Kreft S., Ćirić A.R., Joksović L.G., Đurđević P.T. Analysis of phenolics in the peel and pulp of wild apples (Malus sylvestris (L.) Mill.) J. Food Compos. Anal. 2018;67:1–9. doi: 10.1016/j.jfca.2017.11.007. [DOI] [Google Scholar]
- 26.Albuquerque T.G., Santos F., Sanches-Silva A., Oliveira M.B., Bento A.C., Costa H.S. Nutritional and phytochemical composition of Annona cherimola Mill. fruits and by-products: Potential health benefits. Food Chem. 2016;193:187–195. doi: 10.1016/j.foodchem.2014.06.044. [DOI] [PubMed] [Google Scholar]
- 27.Yan Y.L., Yu C.H., Chen J., Li X.X., Wang W., Li S.Q. Ultrasonic-assisted extraction optimized by response surface methodology, chemical composition and antioxidant activity of polysaccharides from Tremella mesenterica. Carbohydr. Polym. 2011;83:217–224. doi: 10.1016/j.carbpol.2010.07.045. [DOI] [Google Scholar]
- 28.Xu Y., Pan S. Effects of various factors of ultrasonic treatment on the extraction yield of all-trans-lycopene from red grapefruit (Citrus paradise Macf.) Ultrason. Sonochem. 2013;20:1026–1032. doi: 10.1016/j.ultsonch.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Bashi D.S., Mortazavi S.A., Rezaei K., Rajaei A., Karimkhani M.M. Optimization of ultrasound-assisted extraction of phenolic compounds from yarrow (Achillea biebersteinii) by response surface methodology. Food Sci. Biotechnol. 2012;21:1005–1011. doi: 10.1007/s10068-012-0131-0. [DOI] [Google Scholar]
- 30.Cvjetko-Bubalo M., Curko N., Tomaševic M., Ganic K.K., Redovnikovic I.R. Green extraction of grape skin phenolics by using deep eutectic solvents. Food Chem. 2016;200:159–166. doi: 10.1016/j.foodchem.2016.01.040. [DOI] [PubMed] [Google Scholar]
- 31.Velásquez-García J., Gutierrez D.J., Andrade-Mahecha M.M., Martínez-Correa H.A. Pretratamiento con ultrasonido en extracción de compuestos bioactivos de mesocarpio de chontaduro (Bactris gasipaes) usando CO2 supercrítico. Agron. Colom. 2016;34:1370–1373. doi: 10.15446/agron.colomb.v34n1supl.58458. [DOI] [Google Scholar]
- 32.Sousa A.D., Maia A.I.V., Rodrigues T.H.S., Canuto K.M., Ribeiro P.R.V., Pereira R.C.A., Vieira R.F., Brito E.S. Ultrasound-assisted and pressurized liquid extraction of phenolic compounds from Phyllanthus amarus and its composition evaluation by UPLC-QTOF. Ind. Crops Prod. 2016;79:91–103. doi: 10.1016/j.indcrop.2015.10.045. [DOI] [Google Scholar]
- 33.Jovanović A.A., Đorđević V.B., Zdunić G.M., Pljevljakušić D.S., Šavikin K.P., Gođevac D.M., Bugarski B.M. Optimization of the extraction process of polyphenols from Thymus serpyllum L. herb using maceration, heat- and ultrasound-assisted techniques. Sep. Purif. Technol. 2017;179:369–380. doi: 10.1016/j.seppur.2017.01.055. [DOI] [Google Scholar]
- 34.Poodi Y., Bimakr M., Ganjloo A., Zarringhalami S. Intensification of bioactive compounds extraction from Feijoa (Feijoa sellowiana Berg.) leaves using ultrasonic waves. Food Bioprod. Process. 2017;108:37–50. doi: 10.1016/j.fbp.2017.12.004. [DOI] [Google Scholar]
- 35.Pérez-Jiménez J., Arranz S., Tabernero M., Díaz-Rubio M.E., Serrano J. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: Extraction, measurement and expression of results. Food Res. Int. 2008;41:274–285. doi: 10.1016/j.foodres.2007.12.004. [DOI] [Google Scholar]
- 36.Montreau F. Sur le dosage des composés phénoliques totaux dans les vins par la methode Folin-Ciocalteau. Connaiss Vigne Vin. 1972;24:397–404. doi: 10.20870/oeno-one.1972.6.4.2071. [DOI] [Google Scholar]
- 37.Hartzfeld P.W., Forkner R., Hunter M.D., Hagerman A.E. Determination of hydrolyzable tannins (gallotannins and ellagitannins) after reaction with potassium iodate. J. Agric. Food Chem. 2002;50:1785–1790. doi: 10.1021/jf0111155. [DOI] [PubMed] [Google Scholar]
- 38.Reed J.D., McDowell R.T.E., Van Soest P.J., Horvath P.R.J. Condensed tannins: A factor limiting the use of cassava forage. J. Sci. Food Agric. 1982;33:213–220. doi: 10.1002/jsfa.2740330302. [DOI] [Google Scholar]