Abstract
BACKGROUND
Fatty liver (FL) is now a worldwide disease. For decades, researchers have been kept trying to elucidate the mechanism of FL at the molecular level, but rarely involve the study of morphology and medical physics. Traditionally, it was believed that hemodynamic changes occur only when fibrosis occurs, but it has been proved that these changes already show in steatosis stage, which may help to reveal the pathogenesis and its progress. Because the pseudolobules are not formed during the steatosis stage, this phenomenon may be caused by the compression of the liver microcirculation and changes in the hemodynamics.
AIM
To understand the pathogenesis of hepatic steatosis and to study the hemodynamic changes associated with hepatic steatosis.
METHODS
Eight-week-old male C57BL/6 mice were divided into three groups randomly (control group, 2-wk group, and 4-wk group), with 16 mice per group. A hepatic steatosis model was established by subcutaneous injection of carbon tetrachloride in mice. After establishing the model, liver tissue from mice was stained with hematoxylin and eosin (HE), and oil red O stains. Blood was collected from the angular vein, and hemorheological parameters were estimated. A two-photon fluorescence microscope was used to examine the flow properties of red blood cells in the hepatic sinusoids.
RESULTS
Oil red O staining indicated lipid accumulation in the liver after CCl4 treatment. HE staining indicated narrowing of the hepatic sinusoidal vessels. No significant difference was observed between the 2-wk and 4-wk groups of mice on morphological examination. Hemorheological tests included whole blood viscosity (mPas, γ = 10 s-1/γ = 100 s-1) (8.83 ± 2.22/4.69 ± 1.16, 7.73 ± 2.46/4.22 ± 1.32, and 8.06 ± 2.88/4.22 ± 1.50), red blood cell volume (%) (51.00 ± 4.00, 42.00 ± 5.00, and 40.00 ± 3.00), the content of plasma fibrinase (g/L) (3.80 ± 0.50, 2.90 ± 0.80, and 2.30 ± 0.70), erythrocyte deformation index (%) (44.49 ± 5.81, 48.00 ± 15.29, and 44.36 ± 15.01), erythrocyte electrophoresis rate (mm/s per V/m) (0.55 ± 0.11, 0.50 ± 0.11, and 0.60 ± 0.20), revealing pathological changes in plasma components and red blood cells of hepatic steatosis. Assessment of blood flow velocity in the hepatic sinusoids with a laser Doppler flowmeter (mL/min per 100 g) (94.43 ± 14.64, 80.00 ± 12.12, and 67.26 ± 5.92) and two-photon laser scanning microscope (μm/s) (325.68 ± 112.66, 213.53 ± 65.33, and 173.26 ± 44.02) revealed that as the modeling time increased, the blood flow velocity in the hepatic sinusoids decreased gradually, and the diameter of the hepatic sinusoids became smaller (μm) (10.28 ± 1.40, 6.84 ± 0.93, and 5.82 ± 0.79).
CONCLUSION
The inner diameter of the hepatic sinusoids decreases along with the decrease in the blood flow velocity within the sinusoids and the changes in the systemic hemorheology.
Keywords: Hepatic steatosis, Hemodynamics, Hepatic sinusoids, Two-photon fluorescence microscopy, Carbon tetrachloride
Core tip: We evaluated the situation of blood flow in the hepatic sinusoids in hepatic steatosis mice, and found that both the velocity of the blood flow in the sinusoid and the diameter of the sinusoid decreased when the mice got fatty liver. Furthermore, we established the 3D imaging of the hepatic sinusoids to observe the sinusoids directly.
INTRODUCTION
Fatty liver (FL) is a reversible condition in which large vacuoles of triglyceride fat accumulate in the liver cells by the process of steatosis, including hepatic steatosis and steatohepatitis. It is a disease related to heredity, environment, and metabolic stress and is a chronic disease that impairs multiple systems[1]. Recent studies have reported that the pathogenesis of fatty liver is always accompanied by hepatic hemodynamic changes that induce abnormal fluid flow in the Disse space. The receptors or water channels on the surface of hepatocytes receive the signals of flow and then affect lipid metabolism in the liver cells, increasing hepatic lipid absorption and synthesis, thus resulting in the condition of hepatic steatosis[2]. However, there has been little research on the relationship between hemodynamic changes in hepatic sinusoids and the development of hepatic steatosis.
Carbon tetrachloride-induced hepatic steatosis is a classical experimental mouse model often used to study the molecular mechanisms of liver injury and the effects of drugs[3]. The CCl4-induced hepatic steatosis model can accurately demonstrate the changes in the morphology and the function of hepatocytes, and it is highly stable and cost-effective. The mechanism underlying CCl4-induced damage to the hepatocytes has been largely researched. The current consensus in the academic community is that the hepatotoxicity of CCl4 is multifaceted, including production of CCl4-derived reactive oxygen species (ROS), lipid peroxidation, covalent bonding of macromolecules, imbalance in calcium homeostasis, nucleic acid hypomethylation, inflammatory cytokines production and so on[4].
The two-photon laser scanning microscope (TPLSM) was developed by Webb and his colleagues in 1990 works by using a pulsed laser that emits light in the infrared spectrum[5]. With the progress of two-photon fluorescence microscopy technique[6], many studies were conducted to assess the characteristics of blood flow in the microcirculation of other organs using two-photon laser scanning microscopy[7]. However, there has been no particular study examining the characteristics of blood flow in the hepatic sinusoids.
The hemodynamic characteristics of the liver are closely related to the anatomical structure of the liver. The liver has dual blood supply systems, which are composed of the hepatic artery and portal vein. The blood flowing in the microcirculation of the liver is different from that in other organs. In general, one-third of the liver blood is supplied by the hepatic artery and approximately two-thirds is supplied by the portal vein. Although the proportion of blood supplied by the hepatic artery is relatively small, it supplies most of the oxygenated blood needed by the liver. As the blood in the portal vein is derived from intestinal venous blood, it is rich in nutrients, which are used by the body after being metabolized in the liver. Most previous studies have focused on the hemodynamic changes in the great vessels of the liver, such as the changes in the blood pressure of the hepatic portal vein and the changes in the blood flow of the hepatic portal vein, but they have rarely focused on the hemodynamic changes in hepatic sinusoidal microcirculation[8,9]. Therefore, we intended to use the two-photon fluorescence microscopy to study the morphological structure of the hepatic sinusoids and to assess the characteristics of blood flow in the sinusoids using the CCl4 hepatic steatosis mouse model. This study aimed to explore the changes in blood flow in the hepatic sinusoids under the condition of hepatic steatosis, thus providing support for the prevention and treatment of hepatic steatosis.
MATERIALS AND METHODS
Animal experiments and drug treatment
All experiments were approved by the Local Ethics Committee for Animal Research Studies at the Peking University Health Science Center. Eight-week-old male C57BL/6 mice, weighting 20-25 g, kept on standard laboratory chow and with free access to drinking water, were used in this study. They were housed in a restricted access room with controlled temperature (23 °C) and a light/dark (12 h/12 h) cycle. Forty-eight male mice were divided into three groups: control group, 2-wk treatment group, and 4-wk treatment group. In the control group, the mice received injections of olive oil every three days up to 4 wk. The 2-wk group was treated with olive oil injections during the first two weeks and with CCl4 injections (40% CCl4 in olive oil was injected subcutaneously on the back; dose calculated as 30 μL/g body weight) during last two weeks. The 4-wk group was treated with injections of 40% CCl4 in olive oil for 4 wk. After treatment with CCl4 or olive oil, eight mice of each group were anaesthetized for examining the velocity of blood flow in the superficial vessels of the liver by using a laser Doppler detector. Subsequently, these mice were sacrificed; their livers were collected and fixed in 10% neutral buffered formalin (NBF) for histological examination; the blood was collected in an anticoagulant tube for hemorheological investigation. Another eight mice were prepared for hemodynamic assessment by using a TPLSM to examine hepatic sinusoidal blood flow.
Estimation of blood flow velocity in superficial hepatic vessels
The mice were anaesthetized with 1% sodium pentobarbital (50 μg/g body weight, intraperitoneally [i.p.]). The abdomen was opened, and the liver was exposed; the left lobe was selected for estimation of blood flow velocity using a laser Doppler flowmeter (Advanced Laser Flowmeter ALF2, Japan). Ten positions of the liver were explored, and the average value was calculated after estimating for 3 times per position. Finally, all ten positions were explored and the results were analyzed.
Histology assay
For the best fixation effect, the liver sample tissue of about 10 mm × 5 mm × 5 mm was used. We used 10% NBF as the fixative. Hematoxylin and eosin (HE) and oil red O staining were performed using standard procedures. The oil red O positive areas were quantified as described previously.
Hemorheological investigation
The mice were anesthetized with 1% pentobarbital sodium (50 μg/g body weight, i.p.); the blood from the angular vein was collected with a 0.5-mm capillary tube into the anticoagulant tube. For the red blood cell-specific volume [hematocrit (HCT)], we used the capillary tube. Hemorheological parameters, such as the erythrocyte deformation index (STEELLEX LGB-190, Beijing), fibrinogen level, erythrocyte electrophoresis rate (LIANG-100 Red cell electrophoresis apparatus, Beijing), and whole blood viscosity (R80B Viscometer), were assessed.
Blood flow velocity in the hepatic sinusoids
After anesthetizing the mice, 200 μL of fluorescein isothiocyanate -dextran (wt 70000, Sigma, America) was injected intravenously (25 mg in 1 mL saline, intravenous injection via the tail vein)[10]. After 10 min when the fluorescent dye entered into the blood, it could not be taken up by blood cells due to the presence of blood cell membranes, therefore, under the laser excitation of the TPLSM, the dye in the blood fluoresced, while no such effect was seen in the cell due to no uptake of the dye. The inner side of the sinusoids often allows the pass-through of a single red blood cell when the red blood cells flow with the plasma. Therefore, the speed of red blood cells was used to represent the blood flow velocity within the sinusoids. The left lobe of the liver was surgically exposed with a small opening under the sternum. Keeping the lobe wet with saline, scanning of the lobe was performed under the TPLSM (inversion, Leica TCS SP8 MP) for estimating the velocity of blood in the sinusoids situated at 100-200 μm from the central vein[11]. In each mouse, ten blood vessels in different parts of the same lobe of the liver were selected for the estimation; meanwhile, the diameter of the sinusoids was measured.
Statistical analysis
All values are presented on graphs as the mean ± SEM. The comparison between multiple groups was performed by one-way ANOVA followed by Dunnett's multiple comparison test. P-values < 0.05 were considered statistically significant.
RESULTS
Weight
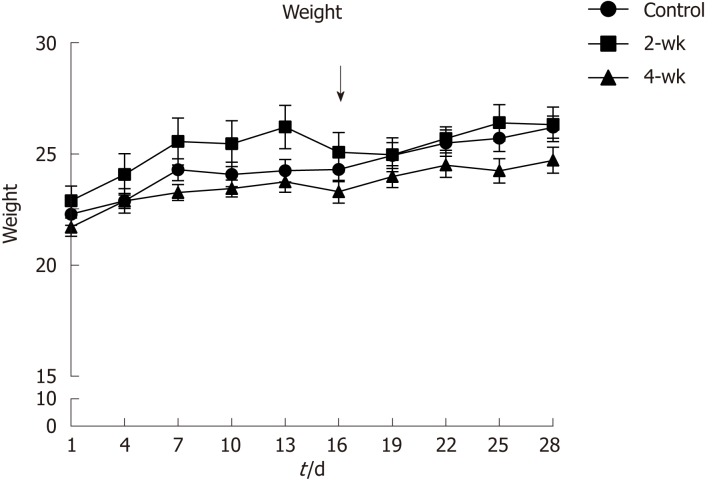
Before injection of CCl4 into the mice, their weights were recorded and monitored to maintain their healthy status (Figure 1). It was reported that the weights of the mice in the 2-wk group decreased during the third week, especially after injection of CCl4, which might have resulted from the toxic effect of CCl4. Thus, the mice experienced a weight loss in response to the stress induced by the injected drug.
Figure 1.
Change curves of mouse weights. The 2-wk group was treated with CCl4 injection after the second week of injecting olive oil. The results show that the weights of the mice decreased after CCl4 treatment.
Histological staining
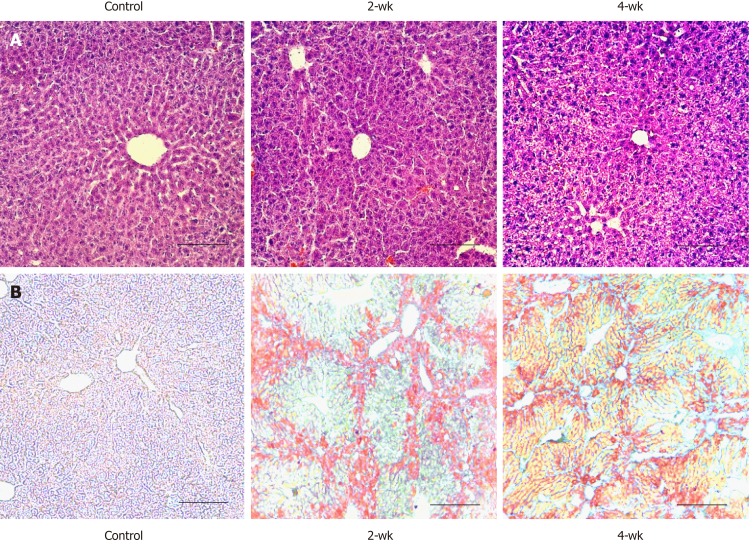
After four weeks of treatment with CCl4 or olive oil, the livers of the two CCl4-treated groups were larger, harder, and had more rough surfaces than those of the control group. HE staining of paraffin sections of the liver tissue (Figure 2A) in the control group revealed that the liver morphology was normal, the cytoplasmic staining was homogeneous, and the cell nuclei were distinct. The internal diameter of the hepatic sinusoid was larger near the central vein of the hepatic lobule. The liver morphology was also normal in the 2-wk group, and no obvious lipid molecules were observed. In the 4-wk group, there were vacuolar structures of different sizes in the liver cells. The internal diameters of sinusoids were difficult to be accurately estimated by HE staining. Oil red O staining (Figure 2B) of the frozen section of the liver tissue in the control group showed no positive region, indicating that no significant lipid accumulation in the liver. After the injections of CCl4 for two weeks, the mouse liver showed obvious orange-red lipid molecules after oil red O staining, and there were more positive areas around the central vein. Meanwhile, after CCl4 injection for four weeks, a large number of lipid vacuoles were seen in the internal hepatic regions, as well as in regions around the central vein, indicating that as the duration of CCl4 treatment increased, hepatic steatosis became more severe.
Figure 2.
Lipid deposition occurring in the liver after CCl4 treatment. A: Hematoxylin and eosin staining of paraffin section of liver tissue. The scale bar refers to 100 μm. B: Oil red O staining of frozen section of liver tissue. There was no lipid deposition in the control group; while in the 2-wk and 4-wk groups, a large amount of lipid deposition occurred after CCl4 injection. The scale bar refers to 100 μm.
Hemorheology characteristics
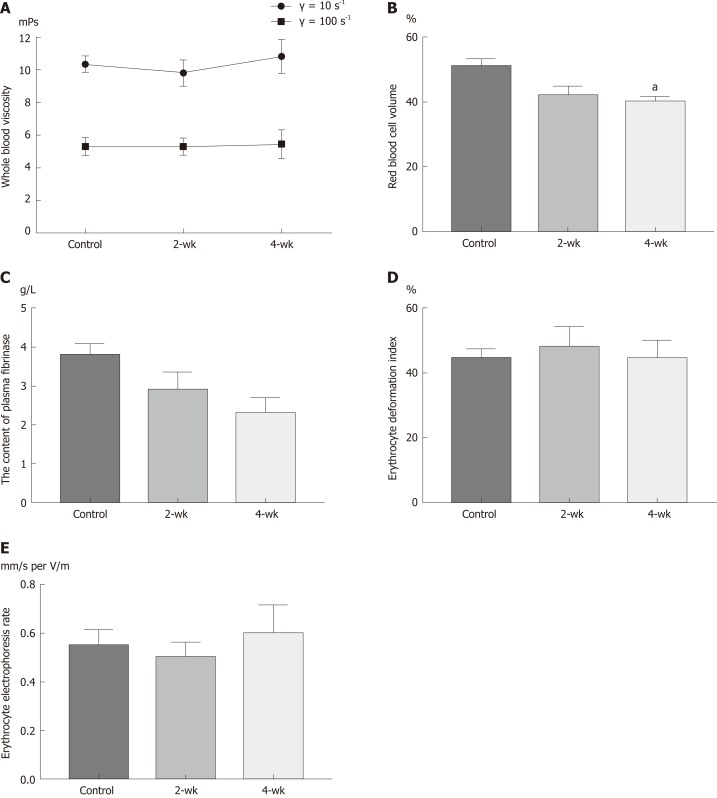
The changes in the viscosity of the blood are shown in Figure 3A, under the shear rates of γ = 10 s-1 and γ = 100 s-1. With the extension of time after CCl4 injection, the blood viscosity increased with a different shear rate; however, there was no statistical difference (P > 0.05). HCT decreased with the extension of time after CCl4 injection (Figure 3B). The HCT value was significantly decreased in the 4-wk group compared to that in the control group (P = 0.029). With the extension of time after CCl4 injection, the amount of fibrinogen in the blood decreased (Figure 3C), and the blood fibrinogen level in the 4-wk group was lower than that in the control group (P = 0.061). The results (Figure 3D) of estimating the erythrocyte deformation coefficient after CCl4 injection showed a downward trend, according to which, the erythrocyte deformation index of the 4-wk group was not statistically significant as compared to that of the control group (P = 0.121). The results (Figure 3E) of this study showed that with the extension of time after CCl4 injection, the erythrocyte electrophoresis rate in the mice showed an upward trend but without much statistical significance (P = 0.882).
Figure 3.
Changes of hemorheological parameters after CCl4 treatment. A: There was an upward trend in total blood viscosity, but there was no statistical difference. B: The hematocrit value decreased after CCl4 treatment. C: The fibrinogen levels decreased after CCl4 treatment. D: Erythrocyte deformation index decreased after CCl4 treatment. E: Erythrocyte electrophoresis rate showed an upward trend after CCl4 treatment. aP < 0.05 vs control.
Blood flow velocity and diameters of the sinusoids
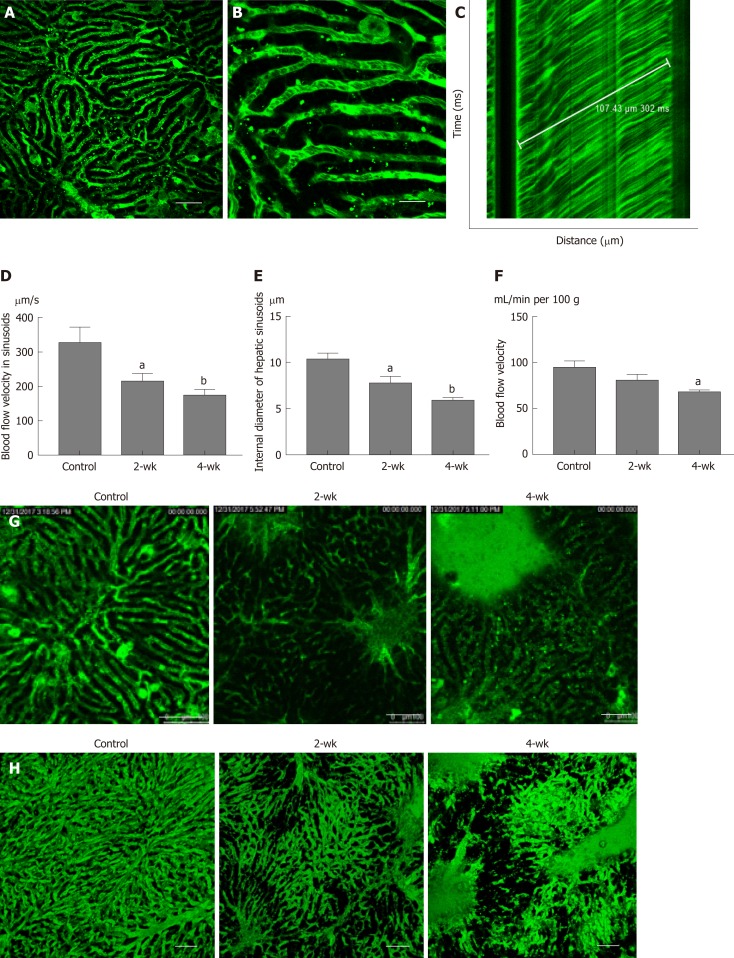
Under the microscope, the bright regions represented the location of the sinusoids (Figure 4A), whereas the dark spots in the bright regions represented the red blood cells (Figure 4B). A TPLSM could selectively scan the sinusoids located along a straight line and thus one-dimensional image changes along a straight line could be visualized. As the red blood cells appeared as dark spots in the field of vision, the black line in Figure 4C indicates the trajectory of red blood cells. In the graph, the time is plotted on the vertical axis to show the time-related changes in the image of the sinusoids scanned along the straight line by the TPLSM. If the figure shows that one erythrocyte moves 107.43 μm within 302 ms, the blood velocity in the sinusoid can be calculated as 354.4 μm/s. In this manner, the blood flow velocity in the left hepatic lobe of the control group, the 2-wk group, and the 4-wk group was measured (Figure 4D). It can be elucidated from the figure that, the blood flow velocity in hepatic sinusoids in 2-wk group (P < 0.05) and 4-wk group (P < 0.01) were less than that in the control group. This suggested that the rate of exchange of a single red blood cell in the sinusoids decreased significantly after hepatic steatosis in mice. At the same time, it was concluded that after measuring the hepatic sinusoidal diameters, the intravascular diameters of the hepatic sinusoids of the 2-wk (P < 0.05) and 4-wk (P < 0.01) groups were significantly lower than those of the control group (Figure 4E). Using a laser Doppler flow meter to measure the blood flow velocity of the superficial blood vessels of the mouse liver (Figure 4F), the blood flow velocity in the liver of mice decreased as a whole after CCl4 injection. There was a statistical difference in the blood flow velocity between the control group and the 4-wk group (P < 0.05); however, no significant difference was noted between the control group and the 2-wk group. The morphology of the hepatic sinusoids was directly observed under a two-photon fluorescence microscope. It was found that the mice in the control group had regular and uniform hepatic sinusoids, whereas the sinusoidal falsifications in the 2-wk and 4-wk groups were obvious, and the mean hepatic sinusoidal diameters in the respective groups were reduced. Fluorescent dye was exudated from the sinusoids, which resulted in decreased brightness, suggesting a change in vascular permeability (Figure 4G). Finally, using the high penetration of the TPLSM, XYZ scanning was performed to construct a 3D image of the hepatic sinusoids (Figure 4H).
Figure 4.
Changes of blood flow in the sinusoid after CCl4 treatment. A: After the injection of fluorescent dye, the mouse liver tissue structure was observed under a two-photon fluorescence microscope. The green luminescent area represents the liver sinusoid. Scale bar refers to 100 μm. B: On enlarging the image of the sinusoid, the darker dots appeared in the sinusoids, which represent red blood cells. Scale bar refers to 30 μm. C: The distance-time image was obtained by scanning with the two-photon laser scanning microscope, and the blood flow velocity of the liver was calculated based on the image. D-F: The blood flow velocity in the hepatic sinusoid, the internal sinusoidal diameter, and the velocity of blood flow in the superficial blood vessels of the liver were estimated in all three groups. After treatment with CCl4, the blood flow velocity both in the sinusoid and superficial blood vessels decreased significantly. The internal sinusoidal diameter also decreased. G: In the control group, hepatic sinusoid morphology was uniform, while in the 2-wk and the 4-wk groups, the shapes of the sinusoids were significantly zigzag and the internal diameters were significantly less than the average diameter. Scale bar refers to 100 μm. H: The 3D image of the hepatic sinusoids. aP < 0.05 vs control, bP < 0.01 vs control.
DISCUSSION
In this study, a two-photon fluorescence microscope was used to detect changes in blood flow in hepatic sinusoids in the CCl4-induced hepatic steatosis mouse model. The experimental results showed that in the presence of hepatic steatosis, the blood flow velocity in the hepatic sinusoids decreased along with the decrease in the internal diameter of the hepatic sinusoids. At the same time, blood flow velocity in the relatively large superficial hepatic vessels also decreased. Movement of red blood cells in the hepatic sinusoids was visually observed for the first time, to our knowledge, using a two-photon fluorescence microscope, and a 3D fluorescence image of the hepatic sinusoids was constructed.
At present, the common methods for modeling of hepatic steatosis in animals include induction with a high-fat diet, with a methionine- and choline-deficient diet, and with carbon tetrachloride[12]. Under normal circumstances of using carbon tetrachloride model, hepatic steatosis can be observed within two to four weeks[13]. Therefore, carbon tetrachloride has become a classical method of fast and stable modeling[14]. The mechanism of carbon tetrachloride-induced hepatic steatosis model can be explained as follows: carbon tetrachloride produces peroxidative stress in hepatocytes, which leads to changes in the lipid metabolism within hepatocytes and results in hepatic steatosis[15]. In our study of hepatic hemodynamics, it was needed to consider the changes in liver morphology only and explore the impact of these changes on liver and systemic blood flow. There was no need to consider the metabolic processes of the liver itself, hence CCl4-induced hepatic steatosis model was used.
Blood viscosity is the most intuitive and direct indicator of blood rheology[16]. Changes in blood viscosity can cause hemodynamic changes. It was found from the experimental results that the blood viscosity in mice did not change with the occurrence of fatty changes in the liver; however, an upward trend was observed among the changes in the blood viscosity. This indicated that during the early stage of hepatic steatosis, there was no significant difference in the blood viscosity. HCT is the most important factor affecting blood viscosity, and blood viscosity increases with increasing HCT[17]. In this experiment, after the injection of CCl4 into mice, HCT decreased to the level which was not enough to cause a significant decrease in blood viscosity. Plasma fibrinogen is an important factor affecting plasma viscosity, and plasma viscosity is an important component of overall blood viscosity[18]. The results of this experiment revealed that plasma fibrinogen levels decreased after hepatic steatosis, which may led to the decrease in plasma viscosity, as well as to the decrease in blood viscosity. Red blood cell deformability is also an important indicator of blood rheology, which explains the morphological structure of red blood cells[19]. The results of this study showed that after hepatic steatosis, the deformability of red blood cells decreased, while blood viscosity increased. The red blood cell electrophoresis rate is an index that affects the aggregation of red blood cells. Besides, the rate of red blood cell aggregation is closely related to blood viscosity. The experimental results showed that there was no significant change in the surface charge of red blood cells after hepatic steatosis, which might be explained by the fact that during the early stage of hepatic steatosis, the physiological structure and function of red blood cell membrane might not have changed.
Being the largest digestive organ and the center of all metabolic processes in the human body, the hemodynamic characteristics of the liver have always been the focus of research. Many clinical studies have found that early hepatic steatosis occurs in the center of hepatic lobules, and with the aggravation of the disease, the steatosis spreads to the whole hepatic lobule[20]. At the same time, experimental studies have observed that the enzymes of lipid de novo generation (such as FAS, SREBP1c, and SCD) in hepatocytes are overactive in the early fatty liver and lipid accumulating increased[21] . Lipids in hepatocytes are metabolized into various metabolic products, resulting in lipid toxicity, which in turn affects the transcription of hepatocyte genes, intracellular signaling pathways, production of reactive oxygen, and endoplasmic reticulum stress[22], and then induces liver fibrosis and even liver cirrhosis. The lipids in the cells gather to form large fat drops, and the hepatocytes expand, resulting in ballooning degeneration[23], which then squeeze the surrounding blood sinusoid, making the inner diameter of the blood sinus narrow and causing increased vascular resistance in the liver.
Most of previous studies of hepatic hemodynamics have focused on the portal vein and other large hepatic vessels, and much attention has been paid to the impact of the portal vein hypertension on the whole body under various pathological conditions. However, little research has been conducted on the characteristics of blood flow in the hepatic sinusoids. This study, through direct observation of the hepatic sinusoid, found that in the fatty liver state of mice, the hepatocytes swell in a larger volume and squeeze the hepatic sinusoid, making the diameter of the hepatic sinus smaller and making it more difficult for the blood cells to pass through the sinusoid. Meanwhile, the sinusoid is obviously tortuose, which makes the blood flow through the sinus more difficult. In addition, the permeability of the hepatic sinusoid also changes, making the components in the sinus more likely to leak out, thus affecting the blood flow of the liver. Under the influence of the above factors, hepatic hemodynamics changes. According to the Hagen-Poiseuille law, the blood pressure is related to both vascular resistance and the blood flow. In the fatty liver state, vascular resistance is increased, which is likely to cause non-cirrhotic portal hypertension[24], and then lead to serious consequences such as varicose veins, which is harmful to health.
Under normal circumstances, microcirculations play an important role in afterload, and decide the end diastolic blood flow status in organs. However, clinical imaging examinations cannot detect these microvessels[25,26]. Two-photon fluorescence microscopy provides us with another way to observe microcirculation in addition to electron microscopy. This observation method can link microscopic morphology with hemodynamics and complement laboratory imaging to fill gaps in clinical imaging. However, this method has limitations. For example, the test requires laparotomy in vivo and needs to inject fluorescent dyes via the vein, which directly limit its use on human specimens. Two-photon fluorescence microscopy can be used to display stereoscopic blood vessel morphology through 3D reconstruction. In the future, it can be combined with magnetic resonance imaging, ultrasound, and other fine imaging techniques to completely reconstruct the liver circulation through 3D printing, which will be a major breakthrough in the development of liver hemodynamics.
ARTICLE HIGHLIGHTS
Research background
Despite the high incidence of fatty liver, there was no specific diagnosis and treatment. And the study of morphological and medical physics changes in fatty liver have been ignored for many years. It has been reported that hemodynamic changes occur in steatosis stage, which might be caused by the compression of the liver microcirculation and changes in the hemorheology characteristics.
Research motivation
Re-examining steatosis from a new perspective - hemodynamics - may enhance our understanding of fatty liver and provide a new idea of treatment.
Research objectives
We mainly focused on the microcirculation of the liver in steatosis stage, which cannot be detected by clinical imaging technique. By the two-photon fluorescence microscopy imaging technique, we observed the structure and hemodynamic characteristics of the liver sinusoids, which linked microscopic morphology with hemodynamics and complemented laboratory imaging to fill gaps in clinical imaging.
Research methods
A hepatic steatosis model was established by subcutaneous injection of carbon tetrachloride in mice. After establishing the model, liver tissue from mice was stained with hematoxylin and eosin (HE) and oil red O stains. Blood was collected from the angular vein, and hemorheological parameters were estimated. A two-photon fluorescence microscope was used to examine the flow properties of red blood cells in the hepatic sinusoids. All result values are presented on graphs as the mean ± SEM. The comparison between multiple groups was performed by one-way ANOVA followed by Dunnett's multiple comparison Test. P-values < 0.05 were considered statistically significant.
Research results
Oil red O staining indicated lipid accumulation in the liver after CCl4 treatment. HE staining indicated narrowing of the hepatic sinusoidal vessels. No significant difference was observed between the 2-wk and 4-wk groups of mice on morphological examination. Hemorheological tests revealed pathological changes in plasma components and red blood cells of hepatic steatosis. Assessment of blood flow velocity in the hepatic sinusoids revealed that as the modeling time increased, the blood flow velocity in the hepatic sinusoids decreased gradually; meanwhile, the diameter of the hepatic sinusoids became smaller.
These results revealed that hemodynamic changes occurring during steatosis stage (at least in the early stage) were more likely caused by sinusoidal deformation, but the mechanism of these phenomena remains to be solved.
Research conclusions
We used two-photon fluorescence microscopy imaging technique to study hemodynamic changes in fatty liver. And we observed that hemorheological change occurred in hepatic steatosis stage, and manifested as changes in blood flow velocity. We found that this change may be mainly caused by sinusoidal deformation, although it is related to hemorheology characteristics, but there was no statistical difference.
Research perspectives
Two-photon fluorescence microscopy imaging technique provides us with another way to observe microcirculation in addition to electron microscopy, and can be used to display stereoscopic blood vessel morphology through 3D reconstruction. In the future, it can be combined with magnetic resonance imaging and other fine imaging techniques to completely reconstruct the liver circulation through 3D printing, which will be a major breakthrough in the development of liver hemodynamics.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional animal care and use committee statement: All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of the Peking University (IACUC protocol number: SCXK 2016-0010).
Conflict-of-interest statement: There is no conflict of interest in this study.
Data sharing statement: No additional data are available.
ARRIVE guidelines statement: The authors have read the ARRIVE guidelines, and the manuscript was prepared and revised according to the ARRIVE guidelines.
Peer-review started: December 21, 2018
First decision: January 23, 2019
Article in press: February 22, 2019
P- Reviewer: Saner F, Sijens PE S- Editor: Ma RY L- Editor: Wang TQ E- Editor: Huang Y
Contributor Information
Jing Fan, Department of Anatomy, Histology and Embryology, School of Basic Medical Sciences, Peking University Health Science Center, Beijing 100191, China.
Chong-Jiu Chen, Department of Anatomy, Histology and Embryology, School of Basic Medical Sciences, Peking University Health Science Center, Beijing 100191, China.
Yu-Chen Wang, Department of Anatomy, Histology and Embryology, School of Basic Medical Sciences, Peking University Health Science Center, Beijing 100191, China.
Wei Quan, Department of Anatomy, Histology and Embryology, School of Basic Medical Sciences, Peking University Health Science Center, Beijing 100191, China.
Jian-Wei Wang, Department of Anatomy, Histology and Embryology, School of Basic Medical Sciences, Peking University Health Science Center, Beijing 100191, China.
Wei-Guang Zhang, Department of Anatomy, Histology and Embryology, School of Basic Medical Sciences, Peking University Health Science Center, Beijing 100191, China. zhangwg@bjmu.edu.cn.
References
- 1.Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62:S47–S64. doi: 10.1016/j.jhep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 2.Poonkhum R, Showpittapornchai U, Pradidarcheep W. Collagen arrangement in space of Disse correlates with fluid flow in normal and cirrhotic rat livers. Microsc Res Tech. 2015;78:187–193. doi: 10.1002/jemt.22460. [DOI] [PubMed] [Google Scholar]
- 3.Donthamsetty S, Bhave VS, Mitra MS, Latendresse JR, Mehendale HM. Nonalcoholic fatty liver sensitizes rats to carbon tetrachloride hepatotoxicity. Hepatology. 2007;45:391–403. doi: 10.1002/hep.21530. [DOI] [PubMed] [Google Scholar]
- 4.Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003;33:105–136. doi: 10.1080/713611034. [DOI] [PubMed] [Google Scholar]
- 5.Huisken J, Swoger J, Del Bene F, Wittbrodt J, Stelzer EH. Optical sectioning deep inside live embryos by selective plane illumination microscopy. Science. 2004;305:1007–1009. doi: 10.1126/science.1100035. [DOI] [PubMed] [Google Scholar]
- 6.Schenke-Layland K, Riemann I, Damour O, Stock UA, König K. Two-photon microscopes and in vivo multiphoton tomographs--powerful diagnostic tools for tissue engineering and drug delivery. Adv Drug Deliv Rev. 2006;58:878–896. doi: 10.1016/j.addr.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Katona G, Szalay G, Maák P, Kaszás A, Veress M, Hillier D, Chiovini B, Vizi ES, Roska B, Rózsa B. Fast two-photon in vivo imaging with three-dimensional random-access scanning in large tissue volumes. Nat Methods. 2012;9:201–208. doi: 10.1038/nmeth.1851. [DOI] [PubMed] [Google Scholar]
- 8.Solhjoo E, Mansour-Ghanaei F, Moulaei-Langorudi R, Joukar F. Comparison of portal vein doppler indices and hepatic vein doppler waveform in patients with nonalcoholic fatty liver disease with healthy control. Hepat Mon. 2011;11:740–744. doi: 10.5812/kowsar.1735143X.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohammadinia AR, Bakhtavar K, Ebrahimi-Daryani N, Habibollahi P, Keramati MR, Fereshtehnejad SM, Abdollahzade S. Correlation of hepatic vein Doppler waveform and hepatic artery resistance index with the severity of nonalcoholic fatty liver disease. J Clin Ultrasound. 2010;38:346–352. doi: 10.1002/jcu.20696. [DOI] [PubMed] [Google Scholar]
- 10.Kang JJ, Toma I, Sipos A, McCulloch F, Peti-Peterdi J. Quantitative imaging of basic functions in renal (patho)physiology. Am J Physiol Renal Physiol. 2006;291:F495–F502. doi: 10.1152/ajprenal.00521.2005. [DOI] [PubMed] [Google Scholar]
- 11.Zipfel WR, Williams RM, Webb WW. Nonlinear magic: multiphoton microscopy in the biosciences. Nat Biotechnol. 2003;21:1369–1377. doi: 10.1038/nbt899. [DOI] [PubMed] [Google Scholar]
- 12.Etienne-Mesmin L, Vijay-Kumar M, Gewirtz AT, Chassaing B. Hepatocyte Toll-Like Receptor 5 Promotes Bacterial Clearance and Protects Mice Against High-Fat Diet-Induced Liver Disease. Cell Mol Gastroenterol Hepatol. 2016;2:584–604. doi: 10.1016/j.jcmgh.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seifert WF, Bosma A, Brouwer A, Hendriks HF, Roholl PJ, van Leeuwen RE, van Thiel-de Ruiter GC, Seifert-Bock I, Knook DL. Vitamin A deficiency potentiates carbon tetrachloride-induced liver fibrosis in rats. Hepatology. 1994;19:193–201. [PubMed] [Google Scholar]
- 14.Cunnane SC. Hepatic triacylglycerol accumulation induced by ethanol and carbon tetrachloride: interactions with essential fatty acids and prostaglandins. Alcohol Clin Exp Res. 1987;11:25–31. doi: 10.1111/j.1530-0277.1987.tb01255.x. [DOI] [PubMed] [Google Scholar]
- 15.Drasdo D, Hoehme S, Hengstler JG. How predictive quantitative modelling of tissue organisation can inform liver disease pathogenesis. J Hepatol. 2014;61:951–956. doi: 10.1016/j.jhep.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Késmárky G, Kenyeres P, Rábai M, Tóth K. Plasma viscosity: a forgotten variable. Clin Hemorheol Microcirc. 2008;39:243–246. [PubMed] [Google Scholar]
- 17.Yokoyama N, Sakota D, Nagaoka E, Takatani S. Alterations in red blood cell volume and hemoglobin concentration, viscoelastic properties, and mechanical fragility caused by continuous flow pumping in calves. Artif Organs. 2011;35:791–799. doi: 10.1111/j.1525-1594.2011.01317.x. [DOI] [PubMed] [Google Scholar]
- 18.Weisel JW, Litvinov RI. Fibrin Formation, Structure and Properties. Subcell Biochem. 2017;82:405–456. doi: 10.1007/978-3-319-49674-0_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohandas N, Chasis JA. Red blood cell deformability, membrane material properties and shape: regulation by transmembrane, skeletal and cytosolic proteins and lipids. Semin Hematol. 1993;30:171–192. [PubMed] [Google Scholar]
- 20.Chalasani N, Wilson L, Kleiner DE, Cummings OW, Brunt EM, Unalp A NASH Clinical Research Network. Relationship of steatosis grade and zonal location to histological features of steatohepatitis in adult patients with non-alcoholic fatty liver disease. J Hepatol. 2008;48:829–834. doi: 10.1016/j.jhep.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hijmans BS, Grefhorst A, Oosterveer MH, Groen AK. Zonation of glucose and fatty acid metabolism in the liver: mechanism and metabolic consequences. Biochimie. 2014;96:121–129. doi: 10.1016/j.biochi.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Duwaerts CC, Maher JJ. Mechanisms of Liver Injury in Non-Alcoholic Steatohepatitis. Curr Hepatol Rep. 2014;13:119–129. doi: 10.1007/s11901-014-0224-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rangwala F, Guy CD, Lu J, Suzuki A, Burchette JL, Abdelmalek MF, Chen W, Diehl AM. Increased production of sonic hedgehog by ballooned hepatocytes. J Pathol. 2011;224:401–410. doi: 10.1002/path.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarin SK, Kapoor D. Non-cirrhotic portal fibrosis: current concepts and management. J Gastroenterol Hepatol. 2002;17:526–534. doi: 10.1046/j.1440-1746.2002.02764.x. [DOI] [PubMed] [Google Scholar]
- 25.Topal NB. Orcan S, Sığırlı D, Orcan G, Eritmen Ü. Effects of fat accumulation in the liver on hemodynamic variables assessed by Doppler ultrasonography. J Clin Ultrasound. 2015;43:26–33. doi: 10.1002/jcu.22157. [DOI] [PubMed] [Google Scholar]
- 26.Elias J, Jr, Altun E, Zacks S, Armao DM, Woosley JT, Semelka RC. MRI findings in nonalcoholic steatohepatitis: correlation with histopathology and clinical staging. Magn Reson Imaging. 2009;27:976–987. doi: 10.1016/j.mri.2009.02.002. [DOI] [PubMed] [Google Scholar]