Abstract
We investigate the self-assembly dynamics of an imine-based pentafoil knot and related pentameric circular helicates, each derived from a common bis(formylpyridine)bipyridyl building block, iron(II) chloride, and either monoamines or a diamine. The mixing of circular helicates derived from different amines led to the complete exchange of the N-alkyl residues on the periphery of the metallo-supramolecular scaffolds over 4 days in DMSO at 60 °C. Under similar conditions, deuterium-labeled and nonlabeled building blocks showed full dialdehyde building block exchange over 13 days for open circular helicates but was much slower for the analogous closed-loop pentafoil knot (>60 days). Although both knots and open circular helicates self-assemble under thermodynamic control given sufficiently long reaction times, this is significantly longer than the time taken to afford the maximum product yield (2 days). Highly effective error correction occurs during the synthesis of imine-based pentafoil molecular knots and pentameric circular helicates despite, in practice, the systems not operating under full thermodynamic control.
Introduction
Self-assembled metallo-supramolecular architectures are often the most stable structures in a distribution of many possible products.1−5 The dynamic bonding in such systems provides a means of “error correction”, generally interpreted as the thermodynamically preferred species being selected from a landscape of possible alternatives that equilibrate during the course of the reaction.2−7 Some of the most celebrated examples are Lehn’s circular helicates,2 typically derived from tris-bipyridine ligand strands and iron(II) or nickel(II) salts, used to exemplify such self-assembly processes in numerous textbooks and university courses. However, while it is recognized3 that “self-assembly under-thermodynamic-control” is likely an oversimplified description for a number of complex supramolecular assembly processes,3−5 the dynamics of such systems have rarely been investigated experimentally. Reports of the self-assembly of metallo-supramolecular structures under nonequilibrium conditions remain rare.8−10
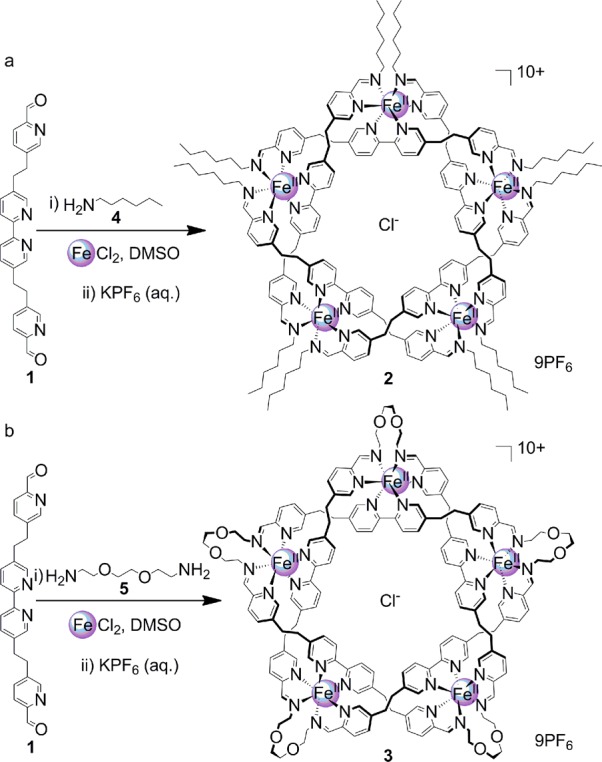
Here we investigate the process by which aldehyde 1 forms imine-based pentameric circular helicates11 (such as 2) and a molecular pentafoil knot (3)12 (Scheme 1). The assembly of these structures occurs spontaneously from 21 individual components in the case of circular helicate 2 (16 for knot 3) when dialdehyde 1, amine 4 (or diamine 5), and FeCl2 are combined in an appropriate stoichiometry in dimethyl sulfoxide (DMSO).11,12 Initially formed linear oligomers and polymers (which give rise to the initial very broad 1H NMR spectra of these reactions11,12) rearrange to form essentially a single product over 48 h at 60 °C,13 a process accompanied by the appearance and growth over time of a single set of sharp 1H NMR signals, reflecting the high conversion of the oligomeric and polymeric intermediates to the low-molecular-weight, high-symmetry, circular helicate/knot.11,12
Scheme 1. Self-Assembly of (a) Open Pentameric Circular Helicate 2 and (b) Pentafoil Knot 3.
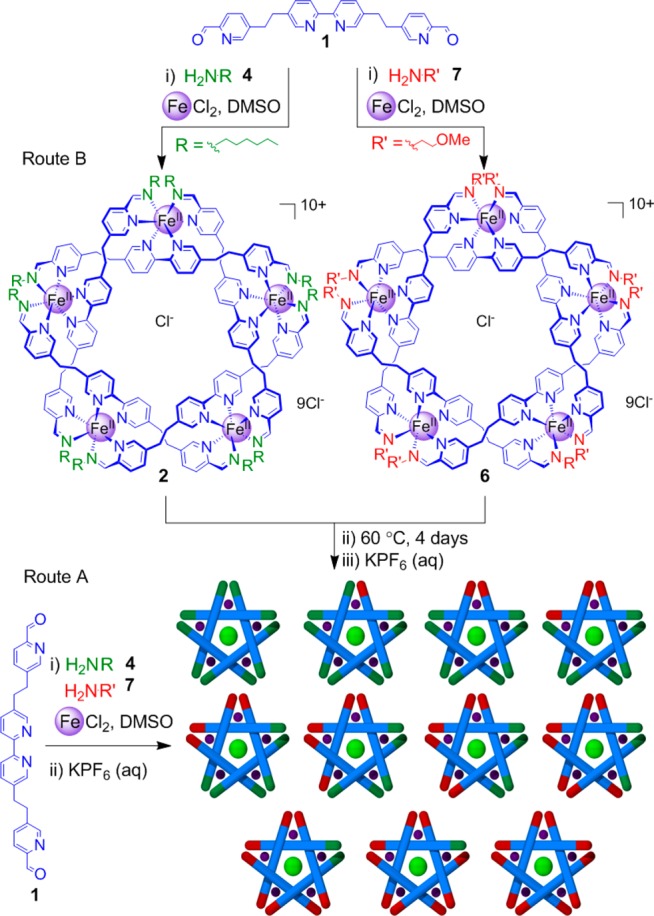
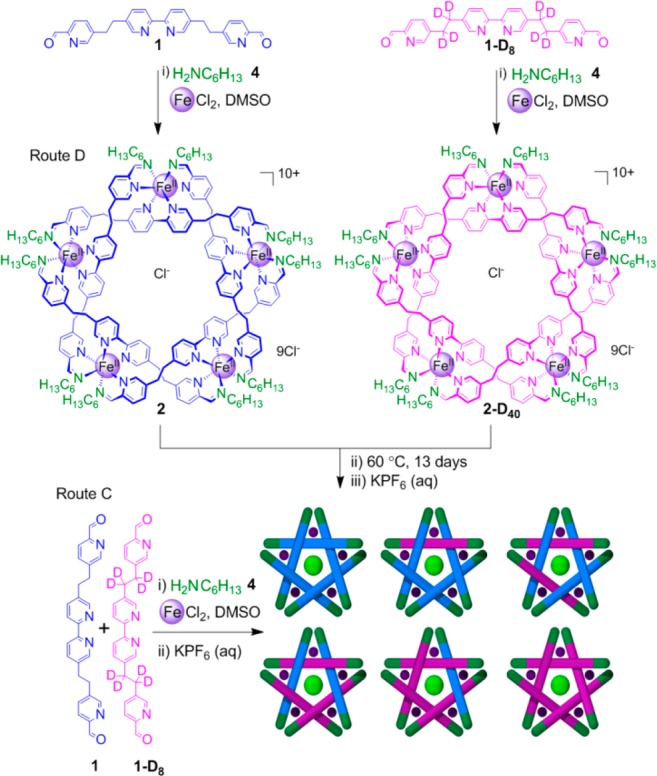
To investigate the reversibility and dynamic nature of these remarkable self-assembling systems, we proposed a two-pronged approach. First, exchange14 of the imine N-alkyl moieties was probed by reacting dialdehyde 1 and FeCl2 with different, but chemically similar, amines (Scheme 2). Second, exchange of the central aldehyde residues15 was investigated through the use of deuterium-labeled (1-D8) and unlabeled (1) derivatives (Scheme 3).
Scheme 2. Exchange of Amine Residues on a Pentameric Circular Helicate.
Scheme 3. Exchange of Labeled and Unlabeled Dialdehyde Residues That Form the Core of a Pentameric Circular Helicate.
Results and Discussion
The addition of excess primary amine to pentameric cyclic helicate 2 led to the partial decomposition of the complex,11 limiting the information that could be gathered about the building block exchange processes. However, by mixing two preformed pentameric helicates derived from different, but chemically similar, amines (e.g., circular helicates 2 and 6, Scheme 2) the exchange process could be studied in the absence of significant amounts of free amine.
To follow the exchange processes, two reactions were monitored (Routes A and B, Scheme 2). A control reaction (Route A) used a 1:1 ratio of hexylamine 4 and methoxyethylamine 7 for the reaction with dialdehyde 1 and FeCl2 to ensure that there was no thermodynamic bias between the two resulting structures (see the SI for experimental details). After anion exchange with aqueous potassium hexafluorophosphate and the take up of the product in acetonitrile, the sample was analyzed by 1H NMR and electrospray ionization mass spectrometry (ESI-MS). The 1H NMR spectrum showed broad peaks indicative of the formation of a large number of similar species (Figures S1 and S3a), and ESI-MS (Figure 1a) confirmed the expected statistical distribution of 11 (not including regional isomers) pentameric circular helicates bearing n hexylamine residues and (10 – n) 2-methoxyethyl amine residues (for n = 0–10). The results show that there is no statistical preference for incorporating hexyl or methoxyethyl chain amines into the circular helicates under the reaction conditions.
Figure 1.
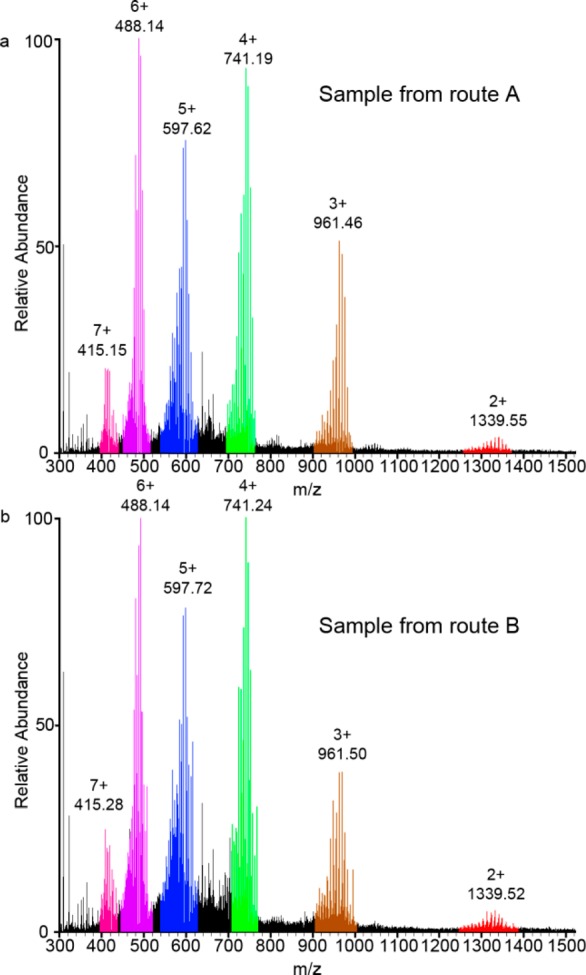
Electrospray ionization mass spectrometry (ESI–MS) analysis following the anion exchange of (a) a control sample of circular pentameric helicates from route A, where amines 4 and 7 were mixed prior to addition to the reaction mixture and (b) circular pentahelicates from route B, where amines 4 and 7 were reacted separately with aldehyde 1 to generate helicates 2 and 6 which were subsequently mixed and heated for 4 days at 60 °C. Peaks corresponding to helicates bearing n hexylamine residues and (10 – n) 2-methoxyethyl amine residues (for n = 0–10) with varying numbers of PF6– counterions.
The second reaction (Route B, Scheme 2) monitors the exchange of amine residues between two preformed circular helicates, 2 and 6. After 24 h of heating reaction mixtures to form 2 and 6 separately, the reactions were combined and heated for another 4 days. Additional signals in the 1H NMR spectra appeared over time (Figure S1), indicative of the formation of mixed-amine circular pentameric helicates. The exchange of amine groups, which may proceed by either direct attack by free amine or by hydrolysis, was confirmed by ESI-MS (Figures S2 and 1). After 4 days, 1H NMR and ESI-MS showed no further changes in the amine-group distribution. The products were precipitated by the addition of aqueous KPF6, collected, washed, and taken up in CD3CN. A comparison of the products from this route (B) with those of the control reaction (A) indicated that full scrambling of the amine residues had occurred: 1H NMR (Figure S3) and ESI MS (Figure 1b) spectra for the two samples are indistinguishable, confirming that the exchange of amines via imines is dynamic under the experimental conditions, resulting in a statistical distribution of amines around and between the circular helicates. Samples of isolated (pure) helicates 2 and 6 were not found to undergo significant component exchange under similar reaction conditions, indicating that the presence of some reaction constituents (e.g., free amine, anions, and/or metal centers) is required for component exchange.
Having established the dynamic nature of the imine groups on the periphery of the structure, the exchange of the dialdehyde residues that form the central core of the helicate was examined. Unlike imine exchange, the exchange of a single dialdehyde building block requires major structural reorganization involving a significant number of other building blocks (amine groups, dialdehydes and metal ions). This contrasts with most complex metallosupramolecular assemblies in which the exchange of individual components can occur stepwise without requiring the disassembly of a large proportion of the structure.16 Such systems can remain largely intact throughout the ligand exchange process, resulting in a high degree of kinetic stability.
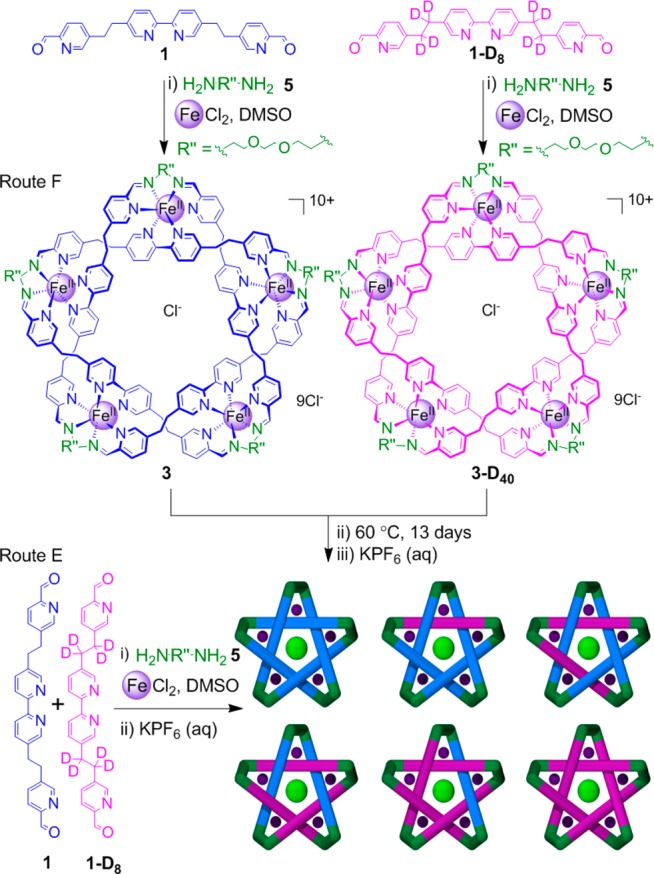
A deuterated analogue of aldehyde 1 (1-D8) was prepared through a modification of the synthesis route to 1 (Scheme S2).15 Using deuterium-labeled and nonlabeled dialdehydes, it was possible to probe the dynamics of forming both the open pentameric circular helicates and the closed-loop pentafoil knot (Schemes 3 and 4).
Scheme 4. Exchange of Labeled and Unlabeled Dialdehyde Residues That Form the Core of a Pentafoil Knot.
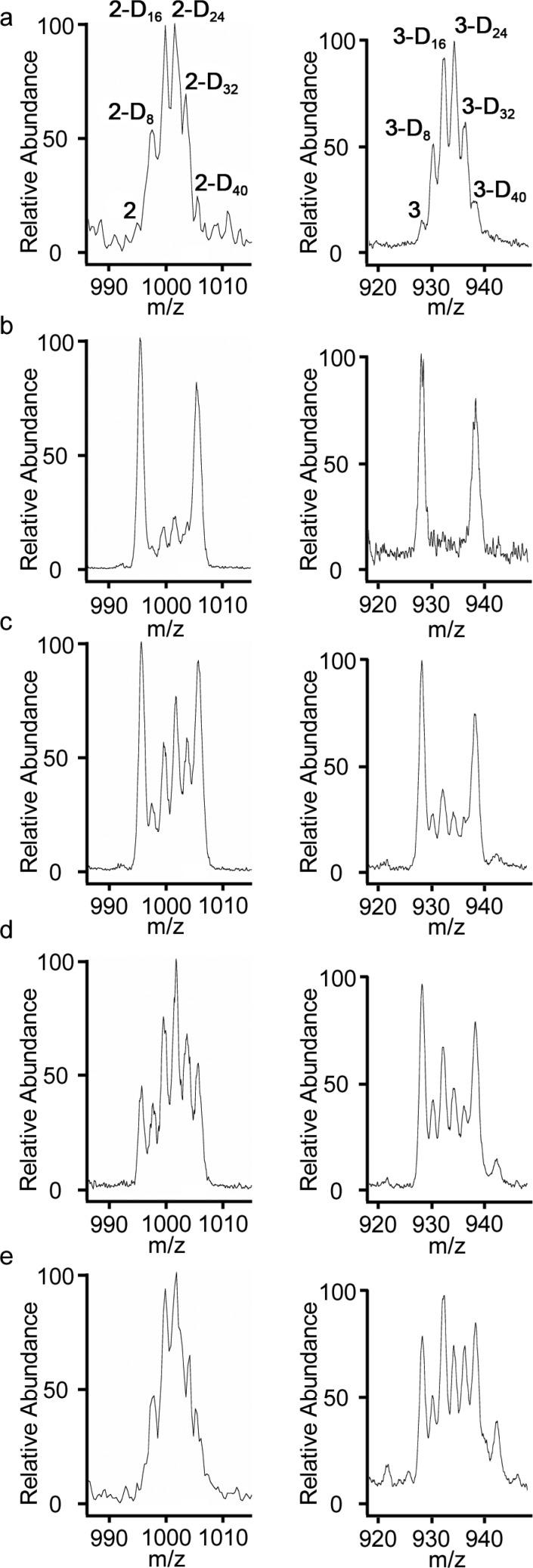
The assembly of pentameric circular helicate 2 was investigated by a time-dependent mixing experiment (Scheme 3). A control reaction (Route C) of 0.5 equiv of aldehyde 1, 0.5 equiv of aldehyde 1-D8, 2.2 equiv of hexylamine 4, and 1.1 equiv of FeCl2 in DMSO-d6 was monitored over 14 days at 60 °C, with the analysis of the product confirming the statistical incorporation of 1 and 1-D8 into the isotopomers of 2. ESI-MS showed a 1:5:10:10:5:1 mixture of 2/2-D8/2-D16/2-D24/2-D32/2-D40 (Figure 2a, left) after 48 h. This distribution remained unchanged under the reaction conditions for another 12 days at 60 °C.
Figure 2.
Electrospray ionization mass spectrometry (ESI-MS) analysis following anion exchange of (left, a) a control sample of pentameric circular helicate isotopomers from route C, where aldehydes 1 and 1-D8 were mixed prior to the addition of amine 4. (b–e) Pentameric circular helicate isotopomers from route D, where aldehydes 1 and 1-D8 were reacted separately with amine 4 to generate helicates 2 and 2-D40, which were subsequently mixed and held at 60 °C. After (b) 1 day, (c) 3 days, (d) 6 days, and (e) 13 days. (Right, a) Control sample of pentafoil knot isotopomers from route E, where aldehydes 1 and 1-D8 were mixed prior to the addition of diamine 5. (b–e) Pentafoil knot isotopomers from route F, where aldehydes 1 and 1-D8 were reacted separately with diamine 5 to generate pentafoil knots 3 and 3-D40, which were subsequently mixed and held at 60 °C. After (b) 1 day, (c) 3 days, (d) 6 days, and (e) 13 days.
To monitor the exchange of dialdehyde components between labeled and nonlabeled circular helicates, dialdehyde 1 was reacted with FeCl2 and hexylamine 4 under the standard conditions for 48 h. Dialdehyde 1-D8 was reacted under similar conditions in a separate reaction. The two reaction mixtures were combined (Route D, Scheme 3) and monitored over the course of 13 days at 60 °C by ESI–MS. After 24 h, predominately two species, homoligand strand circular helicates 2 and 2-D40, were present. The number of mixed-ligand-strand circular helicates increased steadily over the next 12 days (Figure 2b–e, left) until an essentially fully scrambled statistical distribution was reached (Figure 2e, left). 1H NMR analysis closely matched the spectrum of the control sample and showed no significant degradation of the circular helicates (Figure S5).
A similar set of experiments was carried out to probe the dialdehyde residue exchange from the core of pentafoil knots (Scheme 4). A control reaction (Route E) of 0.5 equiv of aldehyde 1 and 0.5 equiv of aldehyde 1-D8 with 1.1 equiv of diamine 5 and FeCl2 in DMSO was held at 60 °C and monitored by 1H NMR and ESI-MS. After 48 h, ESI-MS showed the expected 1:5:10:10:5:1 statistical distribution of mixed-ligand-strand pentafoil knots (3/3-D8/3-D16/3-D24/3-D32/3-D40) (Figure 2a, right). The isotopomer distribution remained constant over longer reaction periods with no evidence of further changes in composition.
To monitor the exchange of dialdehyde components between labeled and nonlabeled pentafoil knots, dialdehyde 1 was reacted with FeCl2 and diamine 5 under the standard conditions for 48 h. Dialdehyde 1-D8 was reacted under similar conditions in a separate reaction. The two reaction mixtures were combined (Route F, Scheme 4), maintained at 60 °C, and monitored by ESI-MS. After 24 h, little exchange of the labled and unlabeled dialdehyde building blocks between the 3 and 3-D40 pentafoil knots was observed (Figure 2b, right). After 3 and 6 days, a small amount of exchange had occurred (Figure 2c,d, right), but after 13 days, the amount of dialdehyde exchange between the closed-loop pentafoil knots (Figure 2e, right) is comparable only to the amount exchanged between the open pentameric circular helicates after 6 days (Figure 2d, left). Even after 60 days under the reaction conditions at 60 °C, a fully scrambled statistical distribution was not reached. 1H NMR indicated that by this time significant decomposition of the knots had occurred.
Implications for the Mechanism of an Imine-Based Circular Helicate and Pentafoil Knot Self-Assembly
The experimental observations regarding building block exchange shed light on the process of supramolecular assembly of imine-based circular helicates and pentafoil knots. Although both amine and dialdehyde components undergo intercomplex exchange under the conditions used for their synthesis from the parent building blocks, the time scale required for complete scrambling (13 days in the case of open pentameric circular helicates and >60 days for the pentafoil knot) is far longer than the reaction time that gives the maximum yield of the products (2 days), indicating that neither self-assembly reaction is under thermodynamic control under the most effective conditions for synthesis. Rather, the slow kinetics of component exchange (particularly of the core dialdehyde-derived units) in the circular helicate and knot act as kinetic traps as the initially formed linear oligomeric and polymeric intermediates undergo more rapid rearrangements and component exchange.
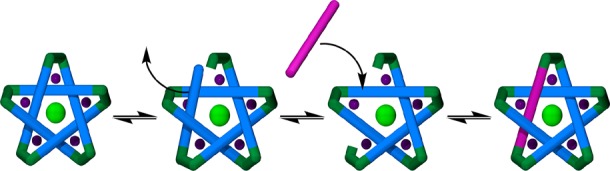
The difference in the exchange rates between the components on the periphery of the circular helicate (the amines) and those that form the core (the dialdehydes) can be rationalized in terms of the number of bonds and stabilizing interactions that have to be broken during the exchange of each type of component. The hydrolysis of an imine bond (or direct displacement by a free amine) and the dissociation of the amine are the only requirements for the exchange of amine components (Scheme 5a). An intermediate aldehyde group is still able to coordinate to the iron(II) center (Scheme 5a, central structure), so amine exchange can occur without significantly destabilizing the supramolecular complex as a whole. However, the exchange of one of the core dialdehyde-derived components requires the breaking of two Fe–N(imine) and four Fe–N(pyridine) coordination bonds in addition to the hydrolysis (or direct amine exchange) of two imine covalent bonds (Scheme 5b).17 This is obviously a far more energetically demanding process and probably destabilizes the intermediate complex to the extent that further component exchange processes occur more rapidly on that intermediate than on the more kinetically stable circular helicate. In DMSO over the course of 13 days at 60 °C, both peripheral and core component exchange processes occur with sufficient frequency to generate a statistical distribution from isotopically labeled components in the products, and the assembly process is under complete thermodynamic control.
Scheme 5. (a) Amine and (b and c) Dialdehyde Component Exchange Mechanisms for (a and b) Imine-Based Pentameric Cyclic Helicates and (c) Closed-Loop Pentafoil Knots.
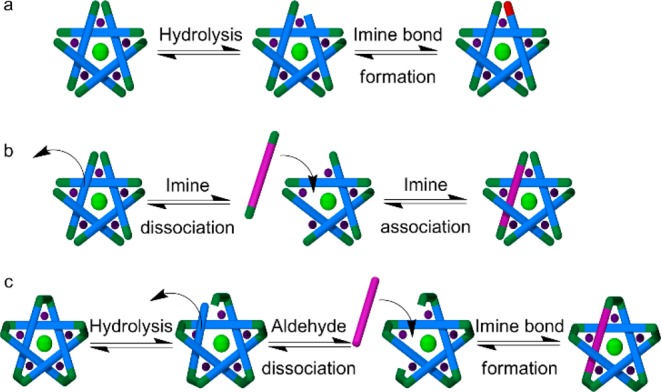
Component exchange processes with an imine-based pentafoil knot require even more disruption to the structure as a whole (Scheme 5c). The pentafoil knot is so kinetically stable that even though its self-assembly from the original building blocks, involving the rearrangement of initially formed linear oligomers and polymers, is complete after 48 h at 60 °C in DMSO, under the same conditions the components of the knot core have not been exchanged between knot molecules sufficiently to become statistically distributed after 60 days.
Conclusions
The high-yielding synthesis of imine-based pentameric circular helicates and pentafoil knots from amine and dialdehyde building blocks is a remarkable example of metallosupramolecular assembly. The products form as a result of numerous well-defined effects and interactions: octahedral metal-ion helicate formation entwines the ligand strands, short linkers between the chelating groups favor cyclic double helicates over linear triple helicates, chloride anions template the size (pentamer) of circular helicate, and reversible imine bond formation enables error correction of initially formed linear oligomeric and polymeric species.11,12 Monitoring the exchange of chemically similar, but distinguishable, amines allows the dynamics of the N-alkyl groups that form the periphery of the self-assembled structures to be probed. Similarly, isotopic labeling enables the exchange of dialdehyde-derived components at the core of the circular helicates and knot to be monitored. The results show that these self-assembly reactions are not under thermodynamic control on the time scale and conditions generally used to synthesize these (supra)molecular structures. This finding illustrates the potential pitfalls in assuming that complex self-assembly processes proceed in a particular way without corroborating experimental evidence. In doing so, it also highlights the potential for supramolecular systems assembled using what are individually reversible and dynamic coordination bonds to be governed by a key kinetically slow, or irreversible, step (or steps), thereby delivering a particular type of nonequilibrium self-assembly process often exploited in nature.
Acknowledgments
We thank the Engineering and Physical Sciences Research Council (EPSRC) (EP/P027067/1) and the European Research Council (ERC) (Advanced Grant No. 339019) for funding. D.A.L. is a Royal Society Research Professor.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.8b12800.
Experimental procedures and spectroscopic data for all compounds (PDF)
Author Present Address
§ School of Chemistry, UNSW Sydney, NSW 2052 Australia.
The authors declare no competing financial interest.
Supplementary Material
References
- For overviews on self-assembly, see; a Lehn J.-M. Programmed chemical systems: Multiple subprograms and multiple processing/expression of molecular information. Chem. - Eur. J. 2000, 6, 2097–2102. . [DOI] [PubMed] [Google Scholar]; b Lehn J.-M. Perspectives in chemistry—Steps towards complex matter. Angew. Chem., Int. Ed. 2013, 52, 2836–2850. 10.1002/anie.201208397. [DOI] [PubMed] [Google Scholar]; c Lehn J.-M. Perspectives in chemistry—Aspects of adaptive chemistry and materials. Angew. Chem., Int. Ed. 2015, 54, 3276–3289. 10.1002/anie.201409399. [DOI] [PubMed] [Google Scholar]
- a Hasenknopf B.; Lehn J.-M.; Kneisel B. O.; Baum G.; Fenske D. Self-assembly of a circular double helicate. Angew. Chem., Int. Ed. Engl. 1996, 35, 1838–1840. 10.1002/anie.199618381. [DOI] [Google Scholar]; b Hasenknopf B.; Lehn J.-M.; Boumediene N.; Dupont-Gervais A.; Van Dorsselaer A.; Kneisel B.; Fenske D. Self-assembly of tetra- and hexanuclear circular helicates. J. Am. Chem. Soc. 1997, 119, 10956–10962. 10.1021/ja971204r. [DOI] [Google Scholar]; c Hasenknopf B.; Lehn J.-M.; Boumediene N.; Leize E.; Van Dorsselaer A. Kinetic and thermodynamic control in self-assembly: sequential formation of linear and circular helicates. Angew. Chem., Int. Ed. 1998, 37, 3265–3268. . [DOI] [PubMed] [Google Scholar]
- For discussions of the thermodynamics of metallosupramolecular self-assembly, see; a Zeckert K.; Hamacek J.; Rivera J.-P.; Floquet S.; Pinto A.; Borkovec M.; Piguet C. A simple thermodynamic model for rationalizing the formation of self-assembled multimetallic edifices: application to triple-stranded helicates. J. Am. Chem. Soc. 2004, 126, 11589–11601. 10.1021/ja0483443. [DOI] [PubMed] [Google Scholar]; b Piguet C.; Borkovec M.; Hamacek J.; Zeckert K. Strict self-assembly of polymetallic helicates: the concepts behind the semantics. Coord. Chem. Rev. 2005, 249, 705–726. 10.1016/j.ccr.2004.08.023. [DOI] [Google Scholar]; c Hamacek J.; Borkovec M.; Piguet C. A simple thermodynamic model for quantitatively addressing cooperativity in multicomponent self-assembly processes—Part 1: Theoretical concepts and application to monometallic coordination complexes and bimetallic helicates possessing identical binding sites. Chem. - Eur. J. 2005, 11, 5217–5226. 10.1002/chem.200500290. [DOI] [PubMed] [Google Scholar]; d Hamacek J.; Borkovec M.; Piguet C. A simple thermodynamic model for quantitatively addressing cooperativity in multicomponent self-assembly processes-Part 2: Extension to multimetallic helicates possessing different binding sites. Chem. - Eur. J. 2005, 11, 5227–5237. 10.1002/chem.200500289. [DOI] [PubMed] [Google Scholar]; e Hamacek J.; Borkovec M.; Piguet C. Simple thermodynamics for unravelling sophisticated self-assembly processes. Dalton Trans. 2006, 1473–1490. 10.1039/b518461d. [DOI] [PubMed] [Google Scholar]; f Riis-Johannessen T.; Dalla Favera N.; Todorova T. K.; Huber S. M.; Gagliardi L.; Piguet C. Understanding, controlling and programming cooperativity in self-assembled polynuclear complexes in solution. Chem. - Eur. J. 2009, 15, 12702–12718. 10.1002/chem.200900904. [DOI] [PubMed] [Google Scholar]; g Piguet C. Five thermodynamic describers for addressing serendipity in the self-assembly of polynuclear complexes in solution. Chem. Commun. 2010, 46, 6209–6231. 10.1039/c0cc00811g. [DOI] [PubMed] [Google Scholar]; h Aboshyan-Sorgho L.; Cantuel M.; Bernardinelli G.; Piguet C. Looking for the origin of the switch between coordination-captured helicates and catenates. Dalton Trans. 2012, 41, 7218–7226. 10.1039/c2dt30414g. [DOI] [PubMed] [Google Scholar]
- For selected reviews on metallosupramolecular self-assembly, see; a Holliday B. J.; Mirkin C. A. Strategies for the construction of supramolecular compounds through coordination chemistry. Angew. Chem., Int. Ed. 2001, 40, 2022–2043. . [DOI] [PubMed] [Google Scholar]; b Fujita M.; Tominaga M.; Hori A.; Therrien B. Coordination assemblies from a Pd(II)-cornered square complex. Acc. Chem. Res. 2005, 38, 369–378. 10.1021/ar040153h. [DOI] [PubMed] [Google Scholar]; c Beves J. E.; Blight B. A.; Campbell C. J.; Leigh D. A.; McBurney R. T. Strategies and tactics for the metal-directed synthesis of rotaxanes, knots, catenanes, and higher order links. Angew. Chem., Int. Ed. 2011, 50, 9260–9327. 10.1002/anie.201007963. [DOI] [PubMed] [Google Scholar]; d Forgan R. S.; Sauvage J.-P.; Stoddart J. F. Chemical topology: Complex molecular knots, links, and entanglements. Chem. Rev. 2011, 111, 5434–5464. 10.1021/cr200034u. [DOI] [PubMed] [Google Scholar]; e Chakrabarty R.; Mukherjee P. S.; Stang P. J. Supramolecular coordination: Self-assembly of finite two- and three-dimensional ensembles. Chem. Rev. 2011, 111, 6810–6918. 10.1021/cr200077m. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Smulders M. M. J.; Riddell I. A.; Browne C.; Nitschke J. R. Building on architectural principles for three-dimensional metallosupramolecular construction. Chem. Soc. Rev. 2013, 42, 1728–1754. 10.1039/C2CS35254K. [DOI] [PubMed] [Google Scholar]; g Hardy J. G. Metallosupramolecular grid complexes: Towards nanostructured materials with high-tech applications. Chem. Soc. Rev. 2013, 42, 7881–7899. 10.1039/c3cs60061k. [DOI] [PubMed] [Google Scholar]; h Han M.; Engelhard D. M.; Clever G. H. Self-assembled coordination cages based on banana-shaped ligands. Chem. Soc. Rev. 2014, 43, 1848–1860. 10.1039/C3CS60473J. [DOI] [PubMed] [Google Scholar]; i Cook T. R.; Stang P. J. Recent developments in the preparation and chemistry of metallacycles and metallacages via coordination. Chem. Rev. 2015, 115, 7001–7045. 10.1021/cr5005666. [DOI] [PubMed] [Google Scholar]; j Zarra S.; Wood D. M.; Roberts D. A.; Nitschke J. R. Molecular containers in complex chemical systems. Chem. Soc. Rev. 2015, 44, 419–432. 10.1039/C4CS00165F. [DOI] [PubMed] [Google Scholar]; k Gil-Ramírez G.; Leigh D. A.; Stephens A. J. Catenanes: Fifty years of molecular links. Angew. Chem., Int. Ed. 2015, 54, 6110–6150. 10.1002/anie.201411619. [DOI] [PMC free article] [PubMed] [Google Scholar]; l Lu Y.; Deng Y.-X.; Lin Y.-J.; Han Y.-F.; Weng L.-H.; Li Z.-H.; Jin G.-X. Molecular Borromean rings based on dihalogenated ligands. Chem. 2017, 3, 110–121. 10.1016/j.chempr.2017.06.006. [DOI] [Google Scholar]; m Fielden S. D. P.; Leigh D. A.; Woltering S. L. Molecular knots. Angew. Chem., Int. Ed. 2017, 56, 11166–11194. 10.1002/anie.201702531. [DOI] [PMC free article] [PubMed] [Google Scholar]; n Saha S.; Regeni I.; Clever G. H. Structure relationships between bis-monodentate ligands and coordination driven self-assemblies. Coord. Chem. Rev. 2018, 374, 1–14. 10.1016/j.ccr.2018.06.010. [DOI] [Google Scholar]
- For selected recent examples of metallosupramolecular self-assembly, see; a Leigh D. A.; Pritchard R. G.; Stephens A. J. A Star of David catenane. Nat. Chem. 2014, 6, 978–982. 10.1038/nchem.2056. [DOI] [PubMed] [Google Scholar]; b Wood C. S.; Ronson T. K.; Belenguer A. M.; Holstein J. J.; Nitschke J. R. Two-stage directed self-assembly of a cyclic [3]catenane. Nat. Chem. 2015, 7, 354–358. 10.1038/nchem.2205. [DOI] [PubMed] [Google Scholar]; c Samanta S. K.; Rana A.; Schmittel M. Conformational slippage determines rotational frequency in five-component nanorotors. Angew. Chem., Int. Ed. 2016, 55, 2267–2272. 10.1002/anie.201509108. [DOI] [PubMed] [Google Scholar]; d Marcos V.; Stephens A. J.; Jaramillo-Garcia J.; Nussbaumer A. L.; Woltering S. L.; Valero A.; Lemonnier J.-F.; Vitorica-Yrezabal I. J.; Leigh D. A. Allosteric initiation and regulation of catalysis with a molecular knot. Science 2016, 352, 1555–1559. 10.1126/science.aaf3673. [DOI] [PubMed] [Google Scholar]; e Danon J. J.; Krüger A.; Leigh D. A.; Lemonnier J.-F.; Stephens A. J.; Vitorica-Yrezabal I. J.; Woltering S. L. Braiding a molecular knot with eight crossings. Science 2017, 355, 159–162. 10.1126/science.aal1619. [DOI] [PubMed] [Google Scholar]; f Shyshov O.; Brachvogel R.-C.; Bachmann T.; Srikantharajah R.; Segets D.; Hampel F.; Puchta R.; von Delius M. Adaptive behavior of dynamic orthoester cryptands. Angew. Chem., Int. Ed. 2017, 56, 776–781. 10.1002/anie.201609855. [DOI] [PubMed] [Google Scholar]; g Hong C. M.; Kaphan D. M.; Bergman R. G.; Raymond K. N.; Toste F. D. Conformational selection as the mechanism of guest binding in a flexible supramolecular host. J. Am. Chem. Soc. 2017, 139, 8013–8021. 10.1021/jacs.7b03812. [DOI] [PubMed] [Google Scholar]; h Song B.; Zhang Z.; Wang K.; Hsu C.-H.; Bolarinwa O.; Wang J.; Li Y.; Yin G. Q.; Rivera E.; Yang H.-B.; Liu C.; Xu B.; Li X. Direct Self-Assembly of a 2D and 3D Star of David. Angew. Chem., Int. Ed. 2017, 56, 5258–5262. 10.1002/anie.201701417. [DOI] [PubMed] [Google Scholar]; i Bloch W. M.; Holstein J. J.; Hiller W.; Clever G. H. Morphological control of heteroleptic cis- and trans-Pd2L2L′2 cages. Angew. Chem., Int. Ed. 2017, 56, 8285–8289. 10.1002/anie.201702573. [DOI] [PMC free article] [PubMed] [Google Scholar]; j Datta S.; Saha M. L.; Stang P. J. Hierarchical assemblies of supramolecular coordination complexes. Acc. Chem. Res. 2018, 51, 2047–2063. 10.1021/acs.accounts.8b00233. [DOI] [PMC free article] [PubMed] [Google Scholar]; k Kim T. Y.; Vasdev R. A. S.; Preston D.; Crowley J. D. Strategies for reversible guest uptake and release from metallosupramolecular architectures. Chem. - Eur. J. 2018, 24, 14878–14890. 10.1002/chem.201802081. [DOI] [PubMed] [Google Scholar]; l Käseborn M.; Holstein J. J.; Clever G. H.; Lützen A. A Rotaxane-like cage-in-ring structural motif for a metallosupramolecular Pd6L12 aggregate. Angew. Chem., Int. Ed. 2018, 57, 12171–12175. 10.1002/anie.201806814. [DOI] [PubMed] [Google Scholar]; m Zhang L.; Stephens A. J.; Nussbaumer A. L.; Lemonnier J.-F.; Jurček P.; Vitorica-Yrezabal I. J.; Leigh D. A. Stereoselective synthesis of a composite knot with nine crossings. Nat. Chem. 2018, 10, 1083–1088. 10.1038/s41557-018-0124-6. [DOI] [PubMed] [Google Scholar]; n Danon J. J.; Leigh D. A.; Pisano S.; Valero A.; Vitorica-Yrezabal I. J. A six-crossing doubly interlocked [2]catenane with twisted rings, and a molecular granny knot. Angew. Chem., Int. Ed. 2018, 57, 13833–13837. 10.1002/anie.201807135. [DOI] [PMC free article] [PubMed] [Google Scholar]; o Zhang L.; August D. P.; Zhong J.; Whitehead G. F. S.; Vitorica-Yrezabal I. J.; Leigh D. A. Molecular trefoil knot from a trimeric circular helicate. J. Am. Chem. Soc. 2018, 140, 4982–4985. 10.1021/jacs.8b00738. [DOI] [PubMed] [Google Scholar]; p Bloch W. M.; Holstein J. J.; Dittrich B.; Hiller W.; Clever G. H. Hierarchical assembly of an interlocked M8L16 Container. Angew. Chem., Int. Ed. 2018, 57, 5534–5538. 10.1002/anie.201800490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For selected reviews on stimuli-responsive chemical systems, see; a McConnell A. J.; Wood C. S.; Neelakandan P. P.; Nitschke J. R. Stimuli-responsive metal–ligand assemblies. Chem. Rev. 2015, 115, 7729–7793. 10.1021/cr500632f. [DOI] [PubMed] [Google Scholar]; b Kassem S.; van Leeuwen T.; Lubbe A. S.; Wilson M. R.; Feringa B. L.; Leigh D. A. Artificial molecular motors. Chem. Soc. Rev. 2017, 46, 2592–2621. 10.1039/C7CS00245A. [DOI] [PubMed] [Google Scholar]; For selected reviews on dynamic covalent chemistry, see; c Lehn J.-M. Dynamic combinatorial chemistry and virtual combinatorial libraries. Chem. - Eur. J. 1999, 5, 2455–2463. . [DOI] [Google Scholar]; d Rowan S. J.; Cantrill S. J.; Cousins G. R. L.; Sanders J. K. M.; Stoddart J. F. Dynamic covalent chemistry. Angew. Chem., Int. Ed. 2002, 41, 898–952. . [DOI] [PubMed] [Google Scholar]; e Corbett P. T.; Leclaire J.; Vial L.; West K. R.; Wietor J.-L.; Sanders J. K. M.; Otto S. Dynamic combinatorial chemistry. Chem. Rev. 2006, 106, 3652–3711. 10.1021/cr020452p. [DOI] [PubMed] [Google Scholar]; f Hunt R. A. R.; Otto S. Dynamic combinatorial libraries: new opportunities in systems chemistry. Chem. Commun. 2011, 47, 847–858. 10.1039/C0CC03759A. [DOI] [PubMed] [Google Scholar]; g Belowich M. E.; Stoddart J. F. Dynamic imine chemistry. Chem. Soc. Rev. 2012, 41, 2003–2024. 10.1039/c2cs15305j. [DOI] [PubMed] [Google Scholar]; h Li J.; Nowak P.; Otto S. Dynamic combinatorial libraries: From exploring molecular recognition to systems chemistry. J. Am. Chem. Soc. 2013, 135, 9222–9239. 10.1021/ja402586c. [DOI] [PubMed] [Google Scholar]; i Mattia E.; Otto S. Supramolecular systems chemistry. Nat. Nanotechnol. 2015, 10, 111–119. 10.1038/nnano.2014.337. [DOI] [PubMed] [Google Scholar]
- For other dynamically responsive metal coordination systems, see; a Livoreil A.; Dietrich-Buchecker C. O.; Sauvage J.-P. Electrochemically triggered swinging of a [2]-catenate. J. Am. Chem. Soc. 1994, 116, 9399–9400. 10.1021/ja00099a095. [DOI] [PubMed] [Google Scholar]; b Campbell V. E.; de Hatten X.; Delsuc N.; Kauffmann B.; Huc I.; Nitschke J. R. Cascading transformations within a dynamic self-assembled system. Nat. Chem. 2010, 2, 684–687. 10.1038/nchem.693. [DOI] [PubMed] [Google Scholar]; c Zheng Y.-R.; Zhao Z.; Wang M.; Ghosh K.; Pollock J. B.; Cook T. R.; Stang P. J. A facile approach toward multicomponent supramolecular structures: Selective self-assembly via charge separation. J. Am. Chem. Soc. 2010, 132, 16873–16882. 10.1021/ja106251f. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Zheng Y.-R.; Lan W.-J.; Wang M.; Cook T. R.; Stang P. J. Designed post-self-assembly structural and functional modifications of a truncated tetrahedron. J. Am. Chem. Soc. 2011, 133, 17045–17055. 10.1021/ja207217t. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Vantomme G.; Jiang S.; Lehn J.-M. Adaptation in constitutional dynamic libraries and networks, switching between orthogonal metalloselection and photoselection processes. J. Am. Chem. Soc. 2014, 136, 9509–9518. 10.1021/ja504813r. [DOI] [PubMed] [Google Scholar]; f Boiocchi M.; Fabbrizzi L. Double-stranded dimetallic helicates: assembling–disassembling driven by the CuI/CuII redox change and the principle of homochiral recognition. Chem. Soc. Rev. 2014, 43, 1835–1847. 10.1039/C3CS60428D. [DOI] [PubMed] [Google Scholar]; g Beves J. E.; Blanco V.; Blight B. A.; Carrillo R.; D’Souza D. M.; Howgego D. C.; Leigh D. A.; Slawin A. M. Z.; Symes M. D. Toward metal complexes that can directionally walk along tracks: Controlled stepping of a molecular biped with a palladium(II) foot. J. Am. Chem. Soc. 2014, 136, 2094–2100. 10.1021/ja4123973. [DOI] [PubMed] [Google Scholar]; h Wang W.; Wang Y.-X.; Yang H.-B. Supramolecular transformations within discrete coordination-driven supramolecular architectures. Chem. Soc. Rev. 2016, 45, 2656–2693. 10.1039/C5CS00301F. [DOI] [PubMed] [Google Scholar]; i Holub J.; Vantomme G.; Lehn J.-M. Training a constitutional dynamic network for effector recognition: Storage, recall, and erasing of information. J. Am. Chem. Soc. 2016, 138, 11783–11791. 10.1021/jacs.6b05785. [DOI] [PubMed] [Google Scholar]; j Bloch W. M.; Abe Y.; Holstein J. J.; Wandtke C. M.; Dittrich B.; Clever G. H. Geometric complementarity in assembly and guest recognition of a bent heteroleptic cis-[Pd2LA2LB2] coordination cage. J. Am. Chem. Soc. 2016, 138, 13750–13755. 10.1021/jacs.6b08694. [DOI] [PubMed] [Google Scholar]; k Pramanik S.; Aprahamian I. Hydrazone switch-based negative feedback loop. J. Am. Chem. Soc. 2016, 138, 15142–15145. 10.1021/jacs.6b10542. [DOI] [PubMed] [Google Scholar]; l Preston D.; Barnsley J. E.; Gordon K. C.; Crowley J. D. Controlled formation of heteroleptic [Pd2(La)2(Lb)2]4+ cages. J. Am. Chem. Soc. 2016, 138, 10578–10585. 10.1021/jacs.6b05629. [DOI] [PubMed] [Google Scholar]; m Burke M. J.; Nichol G. S.; Lusby P. J. Orthogonal selection and fixing of coordination self-assembly pathways for robust metallo-organic ensemble construction. J. Am. Chem. Soc. 2016, 138, 9308–9315. 10.1021/jacs.6b05364. [DOI] [PubMed] [Google Scholar]; n Zhao D.; van Leeuwen T.; Cheng J.; Feringa B. L. Dynamic control of chirality and self-assembly of double-stranded helicates with light. Nat. Chem. 2017, 9, 250–256. 10.1038/nchem.2668. [DOI] [PubMed] [Google Scholar]; o Rizzuto F. J.; Nitschke J. R. Stereochemical plasticity modulates cooperative binding in a CoII12L6 cuboctahedron. Nat. Chem. 2017, 9, 903–908. 10.1038/nchem.2758. [DOI] [PubMed] [Google Scholar]; p Men G.; Lehn J.-M. Higher order constitutional dynamic networks: [2 × 3] and [3 × 3] networks displaying multiple, synergistic and competitive hierarchical adaptation. J. Am. Chem. Soc. 2017, 139, 2474–2483. 10.1021/jacs.6b13072. [DOI] [PubMed] [Google Scholar]; q Chakraborty S.; Hong W.; Endres K. J.; Xie T.-Z.; Wojtas L.; Moorefield C. N.; Wesdemiotis C.; Newkome G. R. Terpyridine-based, flexible tripods: From a highly symmetric nanosphere to temperature-dependent, irreversible, 3D isomeric macromolecular nanocages. J. Am. Chem. Soc. 2017, 139, 3012–3020. 10.1021/jacs.6b11784. [DOI] [PubMed] [Google Scholar]; r Mittal N.; Pramanik S.; Paul I.; De S.; Schmittel M. Networking nanoswitches for ON/OFF control of catalysis. J. Am. Chem. Soc. 2017, 139, 4270–4273. 10.1021/jacs.6b12951. [DOI] [PubMed] [Google Scholar]; s McConnell A. J.; Aitchison C. M.; Grommet A. B.; Nitschke J. R. Subcomponent exchange transforms an FeII4L4 cage from high- to low-spin, switching guest release in a two-cage system. J. Am. Chem. Soc. 2017, 139, 6294–6297. 10.1021/jacs.7b01478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For kinetic control in metallosupramolecular self-assembly, see; a Fujita M.; Nagao S.; Ogura K. Guest-induced organization of a three-dimensional palladium(II) cagelike complex. A prototype for ″induced-fit″ molecular recognition. J. Am. Chem. Soc. 1995, 117, 1649–1650. 10.1021/ja00110a026. [DOI] [PubMed] [Google Scholar]; b Ibukuro F.; Kusukawa T.; Fujita M. A thermally switchable molecular lock. Guest-templated synthesis of a kinetically stable nanosized cage. J. Am. Chem. Soc. 1998, 120, 8561–8562. 10.1021/ja980853f. [DOI] [Google Scholar]; c Hasenknopf B.; Lehn J.-M.; Boumediene N.; Leize E.; Van Dorsselaer A. Kinetic and thermodynamic control in self-assembly: sequential formation of linear and circular helicates. Angew. Chem., Int. Ed. 1998, 37, 3265–3268. . [DOI] [PubMed] [Google Scholar]; d Tashiro S.; Tominaga M.; Kusukawa T.; Kawano M.; Sakamoto S.; Yamaguchi K.; Fujita M. Pd(II)-directed dynamic assembly of a dodecapyridine ligand into end-capped and open tubes: The importance of kinetic control in self-assembly. Angew. Chem., Int. Ed. 2003, 42, 3267–3270. 10.1002/anie.200351397. [DOI] [PubMed] [Google Scholar]; e Hori A.; Yamashita K.-I.; Fujita M. Kinetic self-assembly: Selective cross-catenation of two sterically differentiated PdII-coordination rings. Angew. Chem., Int. Ed. 2004, 43, 5016–5019. 10.1002/anie.200460671. [DOI] [PubMed] [Google Scholar]; f Albrecht M.; Dehn S.; Fröhlich R. A nonanuclear Gallium(III) cluster: An intermediate in the formation of dinuclear triple-stranded helicates?. Angew. Chem., Int. Ed. 2006, 45, 2792–2794. 10.1002/anie.200600123. [DOI] [PubMed] [Google Scholar]; g Yamanaka M.; Yamada Y.; Sei Y.; Yamaguchi K.; Kobayashi K. Selective formation of a self-assembling homo or hetero cavitand cage via metal coordination based on thermodynamic or kinetic control. J. Am. Chem. Soc. 2006, 128, 1531–1539. 10.1021/ja0555365. [DOI] [PubMed] [Google Scholar]; h Pentecost C. D.; Chichak K. S.; Peters A. J.; Cave G. W. V.; Cantrill S. J.; Stoddart J. F. A molecular Solomon link. Angew. Chem., Int. Ed. 2007, 46, 218–222. 10.1002/anie.200603521. [DOI] [PubMed] [Google Scholar]; i Cangelosi V. M.; Carter T. G.; Zakharov L. N.; Johnson D. W. Observation of reaction intermediates and kinetic mistakes in a remarkably slow self-assembly reaction. Chem. Commun. 2009, 5606–5608. 10.1039/b914750k. [DOI] [PubMed] [Google Scholar]; j Crowley J. D.; Goldup S. M.; Lee A.-L.; Leigh D. A.; McBurney R. T. Active metal template synthesis of rotaxanes, catenanes and molecular shuttles. Chem. Soc. Rev. 2009, 38, 1530–1541. 10.1039/b804243h. [DOI] [PubMed] [Google Scholar]; k Goldup S. M.; Leigh D. A.; Long T.; McGonigal P. R.; Symes M. D.; Wu J. Active metal template synthesis of [2]catenanes. J. Am. Chem. Soc. 2009, 131, 15924–15929. 10.1021/ja9070317. [DOI] [PubMed] [Google Scholar]; l Beves J. E.; Leigh D. A. Linking rings without templates. Nat. Chem. 2010, 2, 708–710. 10.1038/nchem.745. [DOI] [PubMed] [Google Scholar]; m Hasell T.; Wu X.; Jones J. T. A.; Bacsa J.; Steiner A.; Mitra T.; Trewin A.; Adams D. J.; Cooper A. I. Triply interlocked covalent organic cages. Nat. Chem. 2010, 2, 750–755. 10.1038/nchem.739. [DOI] [PubMed] [Google Scholar]; n Goldup S. M.; Leigh D. A.; McGonigal P. R.; Ronaldson V. E.; Slawin A. M. Z. Two axles threaded using a single template site: Active metal template macrobicyclic [3]rotaxanes. J. Am. Chem. Soc. 2010, 132, 315–320. 10.1021/ja9080716. [DOI] [PubMed] [Google Scholar]; o Stefankiewicz A. R.; Harrowfield J.; Madalan A.; Rissanen K.; Sobolevc A. N.; Lehn J.-M. Structural and metallo selectivity in the assembly of [2 × 2] grid-type metallosupramolecular species: Mechanisms and kinetic control. Dalton Trans. 2011, 40, 12320–12332. 10.1039/c1dt11226k. [DOI] [PubMed] [Google Scholar]; p Cheng H. M.; Leigh D. A.; Maffei F.; McGonigal P. R.; Slawin A. M. Z.; Wu J. En route to a molecular sheaf: Active metal template synthesis of a [3]rotaxane with two axles threaded through one ring. J. Am. Chem. Soc. 2011, 133, 12298–12303. 10.1021/ja205167e. [DOI] [PubMed] [Google Scholar]; q Brusilowskij B.; Dzyuba E. V.; Troff A. W.; Schalley C. A. Effects of subtle differences in ligand constitution and conformation in metallo-supramolecular self-assembled polygons. Dalton Trans. 2011, 40, 12089–12096. 10.1039/c1dt10621j. [DOI] [PubMed] [Google Scholar]; r Chepelin O.; Ujma J.; Barran P. E.; Lusby P. J. Sequential, kinetically controlled synthesis of multicomponent stereoisomeric assemblies. Angew. Chem., Int. Ed. 2012, 51, 4194–4197. 10.1002/anie.201108994. [DOI] [PubMed] [Google Scholar]; s Hoekman S.; Kitching M. O.; Leigh D. A.; Papmeyer M.; Roke D. Goldberg active template synthesis of a [2]rotaxane ligand for asymmetric transition-metal catalysis. J. Am. Chem. Soc. 2015, 137, 7656–7659. 10.1021/jacs.5b04726. [DOI] [PubMed] [Google Scholar]; t Fujita D.; Yokoyama H.; Ueda Y.; Sato S.; Fujita M. Geometrically restricted intermediates in the self-assembly of an M12L24 cuboctahedral complex. Angew. Chem., Int. Ed. 2015, 54, 155–158. 10.1002/anie.201409216. [DOI] [PubMed] [Google Scholar]; u Danon J. J.; Leigh D. A.; McGonigal P. R.; Ward J. W.; Wu J. Triply threaded [4]rotaxanes. J. Am. Chem. Soc. 2016, 138, 12643–12674. 10.1021/jacs.6b07733. [DOI] [PubMed] [Google Scholar]; v Nishino T.; Yamada Y.; Akine S.; Sugimotod K.; Tanaka K. Kinetically “locked” metallomacrocycle. Dalton Trans. 2016, 45, 3831–3837. 10.1039/C5DT04635A. [DOI] [PubMed] [Google Scholar]; w Neal E. A.; Goldup S. M. Kinetic self-sorting approach to heterocircuit [3]rotaxanes. Angew. Chem., Int. Ed. 2016, 55, 12488–12493. 10.1002/anie.201606640. [DOI] [PMC free article] [PubMed] [Google Scholar]; x Lewis J. E. M.; Winn J.; Cera L.; Goldup S. M. Iterative synthesis of oligo[n]rotaxanes in excellent yield. J. Am. Chem. Soc. 2016, 138, 16329–16336. 10.1021/jacs.6b08958. [DOI] [PubMed] [Google Scholar]; y Huang C.-B.; Xu L.; Zhu J.-L.; Wang Y.-X.; Sun B.; Li X.; Yang H.-B. Real-Time Monitoring the Dynamics of Coordination-Driven Self-Assembly by Fluorescence-Resonance Energy Transfer. J. Am. Chem. Soc. 2017, 139, 9459–9462. 10.1021/jacs.7b04659. [DOI] [PubMed] [Google Scholar]; z Bogie P. M.; Holloway L. R.; Lyon Y.; Onishi N. C.; Beran G. J. O.; Julian R. R.; Hooley R. J. A Springloaded Metal-Ligand Mesocate Allows Access to Trapped Intermediates of Self-Assembly. Inorg. Chem. 2018, 57, 4155–4163. 10.1021/acs.inorgchem.8b00370. [DOI] [PubMed] [Google Scholar]
- For examples of kinetic controlled supramolecular assembly in biology, see; a Jennings P.; Wright P. Formation of a molten globule intermediate early in the kinetic folding pathway of apomyoglobin. Science 1993, 262, 892–896. 10.1126/science.8235610. [DOI] [PubMed] [Google Scholar]; b Pellarin R.; Schuetz P.; Guarnera E.; Caflisch A. Amyloid fibril polymorphism is under kinetic control. J. Am. Chem. Soc. 2010, 132, 14960–14970. 10.1021/ja106044u. [DOI] [PubMed] [Google Scholar]
- For key examples of the quantitative analysis of self-assembly, see; a Tsujimoto Y.; Kojima T.; Hiraoka S. Rate-determining step in the self-assembly process of supramolecular coordination capsules. Chem. Sci. 2014, 5, 4167–4172. 10.1039/C4SC01652A. [DOI] [Google Scholar]; b Kai S.; Martí-Centelles V.; Sakuma Y.; Mashiko T.; Kojima T.; Nagashima U.; Tachikawa M.; Lusby P. J.; Hiraoka S. Quantitative Analysis of Self-Assembly Process of a Pd2L4 Cage Consisting of Rigid Ditopic Ligands. Chem. - Eur. J. 2018, 24, 663–671. 10.1002/chem.201704285. [DOI] [PubMed] [Google Scholar]; c Tateishi T.; Zhu W.; Foianesi-Takeshige L. H.; Kojima T.; Ogata K.; Hiraoka S. Self-Assembly of a Pd4L8 Double-Walled Square Partly Takes Place Through the Formation of Kinetically Trapped Species. Eur. J. Inorg. Chem. 2018, 2018, 1192–1197. 10.1002/ejic.201800037. [DOI] [Google Scholar]; d Kai S.; Kojima T.; Thorp-Greenwood F. L.; Hardie M. J.; Hiraoka S. How does chiral self-sorting take place in the formation of homochiral Pd6L8 capsules consisting of cyclotriveratrylene-based chiral tritopic ligands?. Chem. Sci. 2018, 9, 4104–4108. 10.1039/C8SC01062E. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Nakagawa M.; Kai S.; Kojima T.; Hiraoka S. Energy-Landscape-Independent Kinetic Trap of an Incomplete Cage in the Self-Assembly of a Pd2L4 Cage. Chem. - Eur. J. 2018, 24, 8804–8808. 10.1002/chem.201801183. [DOI] [PubMed] [Google Scholar]; f Tateishi T.; Kai S.; Sasaki Y.; Kojima T.; Takahashi S.; Hiraoka S. Two dominant self-assembly pathways to a Pd3L6 double-walled triangle. Chem. Commun. 2018, 54, 7758–7761. 10.1039/C8CC02608D. [DOI] [PubMed] [Google Scholar]; g Kai S.; Tateishi T.; Kojima T.; Takahashi S.; Hiraoka S. Self-Assembly of a Pd4L8 Double-Walled Square Takes Place through Two Kinds of Metastable Species. Inorg. Chem. 2018, 57, 13083–13086. 10.1021/acs.inorgchem.8b02470. [DOI] [PubMed] [Google Scholar]
- Ayme J.-F.; Beves J. E.; Leigh D. A.; McBurney R. T.; Rissanen K.; Schultz D. Pentameric circular Iron(II) double helicates and a molecular pentafoil knot. J. Am. Chem. Soc. 2012, 134, 9488–9497. 10.1021/ja303355v. [DOI] [PubMed] [Google Scholar]
- Ayme J.-F.; Beves J. E.; Leigh D. A.; McBurney R. T.; Rissanen K.; Schultz D. A synthetic molecular pentafoil knot. Nat. Chem. 2012, 4, 15–20. 10.1038/nchem.1193. [DOI] [PubMed] [Google Scholar]
- Longer reaction times at 60 °C lead to lower yields of the circular helicate/knot, apparently as a result of side reactions involving the decomposition of the building blocks.
- a Rowan S. J.; Stoddart J. F. Thermodynamic synthesis of rotaxanes by imine exchange. Org. Lett. 1999, 1, 1913–1916. 10.1021/ol991047w. [DOI] [Google Scholar]; b Ro S.; Rowan S. J.; Pease A. R.; Cram D. J.; Stoddart J. F. Dynamic hemicarcerands and hemicarceplexes. Org. Lett. 2000, 2, 2411–2414. 10.1021/ol005962p. [DOI] [PubMed] [Google Scholar]; c Leigh D. A.; Lusby P. J.; Teat S. J.; Wilson A. J.; Wong J. K. Y. Benzylic imine catenates: Readily accessible octahedral analogues of the Sauvage catenates. Angew. Chem., Int. Ed. 2001, 40, 1538–1543. . [DOI] [PubMed] [Google Scholar]; d Chichak K. S.; Cantrill S. J.; Stoddart J. F. Dynamic nanoscale Borromean links. Chem. Commun. 2005, 3391–3393. 10.1039/b503717d. [DOI] [PubMed] [Google Scholar]
- The synthesis of labeled versions of hexylamine helicate 2-D40 and pentafoil knot 3-D40 gave products in yields comparative to that of their nonlabeled derivatives. (See the SI for details.)
- a Ziegler M.; Davis A. V.; Johnson D. W.; Raymond K. N. Supramolecular chirality: A reporter of structural memory. Angew. Chem., Int. Ed. 2003, 42, 665–668. 10.1002/anie.200390183. [DOI] [PubMed] [Google Scholar]; b Sato S.; Ishido Y.; Fujita M. Remarkable stabilization of M12L24 spherical frameworks through the cooperation of 48 Pd(II)–pyridine interactions. J. Am. Chem. Soc. 2009, 131, 6064–6065. 10.1021/ja900676f. [DOI] [PubMed] [Google Scholar]
- a Hogg L.; Leigh D. A.; Lusby P J.; Morelli A.; Parsons S.; Wong J. K. Y. A simple general ligand system for assembling octahedral metal-rotaxane complexes. Angew. Chem. 2004, 43, 1218–1221. 10.1002/ange.200353186. [DOI] [PubMed] [Google Scholar]; b Leung K. C.-F.; Wong W.-Y.; Arico F.; Haussmann P. C.; Stoddart J. F. The stability of imine-containing dynamic [2]rotaxanes to hydrolysis. Org. Biomol. Chem. 2010, 8, 83–89. 10.1039/B915864B. [DOI] [PubMed] [Google Scholar]; c Hasell T.; Schmidtmann M.; Stone C. A.; Smith M. W.; Cooper A. I. Reversible water uptake by a stable imine-based porous organic cage. Chem. Commun. 2012, 48, 4689–4691. 10.1039/c2cc31212c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.