Abstract
The hallmark of Polycythemia vera (PV) is the presence of JAK2V617F mutation and increased RBC mass. Chronic myelomonocytic leukemia (CMML) is defined as persistent blood absolute monocyte count (AMC) >/= 1 × 109/L for at least 3 months with myeloid cell dysplasia. Few cases of evolved CMML from PV have been described. We present a case of PV that progressed to CMML. We demonstrated the CMML clone was most likely derived from PV- JAK2V617F clone. This clone carried a complex genetic mutations of ASXL1, RUNX1, SRSF2 and TET2, NRAS, KRAS, plus CMML cells were of the classical phenotype CD14+ CD16−by flow cytometry.
Keywords: Chronic myelomonocytic leukemia, Polycythemia vera, Monocytosis
1. Introduction
Polycythemia vera (PV) diagnostic criteria was updated in the 2016 World Health Organization (WHO) [1] revision and the criteria for transformation to post PV-Myelofibrosis (MF) that was developed by the International Working Group for Myeloproliferative neoplasms Research and Treatment (IWG-MRT) [2]. The post-PV fibrotic transformation and evolution to AML/MDS represent a major cause of death in PV patients [3]. A rare phenomenon is clonal evolution of PV to chronic myeloid leukemia [4]. Even rarer are cases of PV transformation to CMML. The clinical presentation of overt CMML from PV has been reported previously in two cases [5]. Peripheral monocytosis is not pathognomonic of CMML and it can be observed in other hematologic conditions such as PV [6]. Here, we believe, are the first to present a case of JAK2V617F positive PV that possibly transformed into CMML proven by clonal analysis and the characteristic gene mutation pattern seen in CMML along with flow cytometric studies demonstrating the classic type CD14+ CD16−. monocytes.
2. Materials and methods
Detection of JAK2V617F mutation: Peripheral blood was obtained from the patient and the CD14+ cells were isolated using CD14+ isolation kits (Miltenyi Biotec, Germany). The cells were then analyzed at Genoptix Company (Carlsbad, CA) for the JAK2V617F mutation analysis. JAK2 mutation analysis includes isolation of genomic DNA, gene amplification by quantitative PCR and probe analysis to determine the presence of theV617F (1849G > T) mutation. As assessed by mutant DNA dilution experiments, this assay can detect this mutation when present at levels as low as 1%.
3. Case report
The patient is an 80-year-old African American man who was first evaluated by the hematology department because of elevated hemoglobin (Hb) in 2015. Past medical history was significant for early stage prostate cancer treated with radiation therapy 9 years prior to the presentation and prosthetic bovine aortic valve replacement for severe aortic stenosis. At presentation, Hb was 16.9 g/dL, hematocrit (Hct) 53%, white blood cells (WBC) 14 × 10⁹/L and monocytes 1 × 10⁹/L. Platelet count was normal 153 × 10⁹/L.
Physical examination was remarkable only for plethora. Spleen size was normal at 10.5 cm by abdominal ultrasound. Erythropoietin serum level was low 1.53 U/ml (normal range 2.6–18.5 U/ml). Peripheral blood smear review showed hypochromic RBC, increased neutrophils and monocytes, no immature cells or dysplasia, and no tear drop cells. Peripheral blood molecular analysis was positive for JAK2V617F mutation with allele burden of 20.93%. Extended mutation analysis also included: CALR, CSF3R, SETBP1, PDGFRᵃ, and PDGFRᵝ, BCR-ABL, were all negative.
Due to his age and cardiovascular risk factors he was considered to be high risk patient and was started on cytoreductive therapy with hydroxyurea (HU) (1 g a day) with serial monitoring of blood count.
He was on hydroxyurea for 2 weeks but experienced severe thrombocytopenia with platelet count as low as 15 × 10⁹/L. He was then treated with therapeutic phlebotomy alone with a goal Hct < 45%. WBC fluctuated between 15 and 60 × 10⁹/L. One year after diagnosis, peripheral blood JAK2V617F allele burden was increased to 56.33%. Due to progressive increase in WBC to 50 × 109/L, patient underwent bone marrow aspirate and biopsy in January 2017 that showed hypercellular bone marrow with left shifted granulocytes hyperplasia, minimal reticulin fibrosis and markedly reduced storage iron attributed to repeated phlebotomy. Bone marrow JAK2V617F allele burden was similar to peripheral blood, 57.96%. Cytogenetic analysis was normal 46, XY [20] as well as the FISH studies.
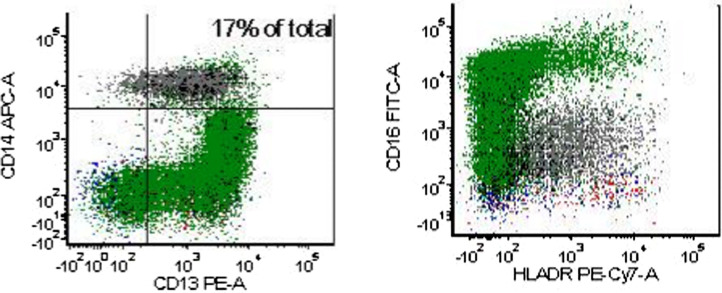
By October 2017, there was marked increase in WBC 124 × 109/L, with sustained peripheral blood monocytosis >1 × 10⁹/L and platelets of 773 × 109/L. Hb and HCT was 13.1 g/dL and 45.9% respectively with MCV 74.6 fL. Clinically there was evidence of splenomegaly confirmed by CT abdomen and pelvis. Peripheral blood smear showed increase in all 3 cell lines: markedly increased RBC, left shift neutrophilia, monocytosis (17%) with dysplastic features and normal and large platelets (Fig. 1). Cytogenetic analysis was normal 46, XY [20] again. However, FISH molecular studies showed acquired somatic mutations of the ASXL1, RUNX1, SRSF2 and TET2, NRAS, KRAS. FISH for BCR/ABL, PDGFR-alpha, PDGFR-beta and FGFR1 was negative. Peripheral blood flow cytometry was positive for CD14+ and CD16− monocytes (classical subtype) which by calculation represented 95% of the CD14⁺ cells (Fig. 2), supporting the diagnosis of CMML [11]. We further isolated the CD 14+ cells and assayed for JAK2V617F mutation and confirmed that the CMML cells carried JAK2 V617F (Fig. 3) suggesting the same clone as identified in the original PV clone, supporting our hypothesis that CMML evolved from PV with JAK2V617F mutation clone . Patient was started on azacytidine. He responded to the treatment well, as evidenced by decreasing WBC count on serial testing and general status improvment til the day of the report.
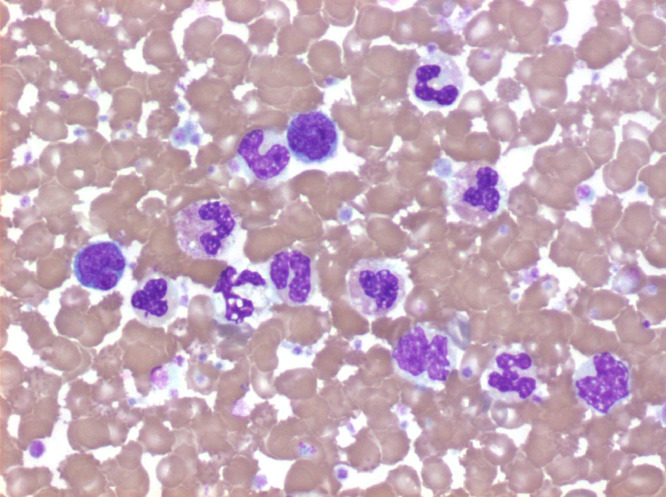
Fig. 1.
Peripheral blood smear showed marked monocytosis and dysplastic featured of myeloid cells, characteristic of CMML.
Fig. 2.
Images of monocytes in flow cytometric analysis: they were CD14+ CD 16−, the classical type, characteristic of CMML. (Grey population represents monocytes, Green events represents granulocytes). Calculation of CD14+ CD 16− cells represents 95% of total CD14+ cells.
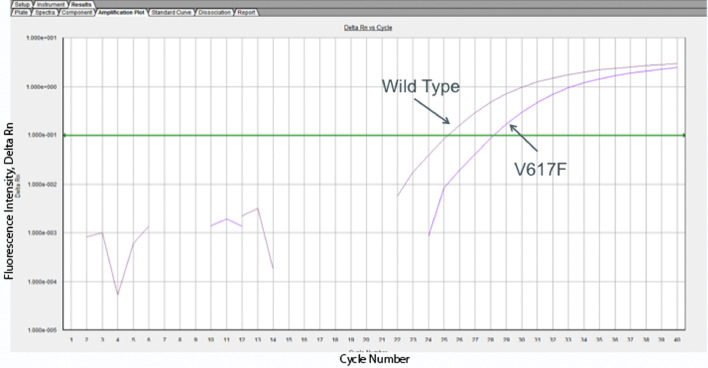
Fig. 3.
Detection of JAK2V617F mutation. CD14+ cells were isolated using CD14+ isolation kits (Miltenyi Biotec, Germany), then cells were analyzed at Genoptix Company (Carlsbad, CA) for the JAK2V617F mutation analysis. The real time PCR amplification of wildtype or JAK2V617F mutation is shown as cycle number (X-axis) vs. fluorescence intensity (Y-axis). The darker purple curve furthest of the upper right is the WT probe and the lower right curve is the mutant probe. The Ct for the wild type probe was 25.2032 and the Ct for the V617F probe was 28.1533. The% mutated in this patient was 33.58%.
4. Discussion
The key event in the pathogenesis of myeloproliferative neoplasms (MPNs) is a somatic mutation within hematopoietic stem and progenitor cells, JAK2V617F being the most prevalent mutation in PV [7]. Approximately one third of patients with MPN will acquire additional somatic mutations that influence subsequent clonal expansion and drive the disease evolution. [8] Historically, persistent peripheral monocytsis is the hallmark of CMML with at least 1 × 10⁹/L monocytes. [9] However, peripheral monocytosis can be seen in other hematologic malignancies, including MDS and MPN, as well as benign reactive conditions. Immunophenotypically, monocytes are divided in 3 groups: the dominant classical monocytes (CD14⁺-CD16⁻), a small percentage of intermediate monocytes (CD14⁺-CD16⁺) and nonclassical monocytes (CD14⁻-CD16⁺). [10] Talati et al. [11] demonstrated that CMML can be accurately diagnosed by the presence of classical monocytes by flow cytometry regardless of mutation status or cytogenetic abnormalities, more specifically to distinguish CMML vs MDS subgroup with classical monocytes. MDS with classical monocytosis is associated with favorable prognosis and SF3B1 mutation is present with greater frequency.
The clinical implications of monocytosis in PV are not well defined. Analysis of 267 PV patients by Barraco et al. [6] revealed that elderly PV-patients (>60 years) have a higher prevalence of monocytosis (21%), as well higher number of WBC associated with TET2/SRSF2 mutations (57%/29%). Unlike MDS with classical monocytosis, the presence of monocytosis in PV is associated with more aggressive behavior. However, further analysis of monocytes subsets was not included in the study. It still remains a matter of debate if there is a concomitant small CMML clone in early phase of PV which potentially becomes dominant as disease progresses to CMML. .
Over time the clinical course of PV might be complicated by either progression to AML/MDS with an incidence that ranges between 5–15% after 10 years [12] or myelofibrosis with an estimated 15-year risk of 6%. [13] Transformation of PV to CMML appears to be less common, most cases present as de novo disease and a small subset evolved from preexisting MDS. Reported cytogenetic abnormalities associated with AML/MDS transformation in PV patients are complex karyotype, trisomy 1q or acquired new myelodysplasia-related clones. [14] The role of JAK2V617F is of particular interest at the time of transformation. JAK2V617F mutation is reported in 7.8% of de novo CMML. [15] In a preliminary report in abstract form by Dr Xu et al., MPN—CMML, JAK2V617F mutation was seen in 57% of cases (4 of 7 cases). Of note, 2 cases were associated with disappearance of JAK2V617F clone at the time of CMML transformation, however the details were not pubished. [16] The other case report by Holcombe [5] was published in 1991, so analysis of JAK2 mutation or clonal analysis was not available. In our case, we isolated the CD14⁺ cells, when he was at the CMML stage, and showed that CD14⁺ cells carried the JAK2V617F mutation (Fig. 3). Since CMML clone (CD14⁺ CD16⁻) represents 95% of the CD14+ cells (Fig. 2), this suggests that CMML clone that carried JAK2V617F was mostly likely derived from the same JAK2V617F positive original PV clone and carried additional gene mutation that included ASXL1, RUNX1, SRSF2 and TET2, NRAS, KRAS. The possibility that a small CMML clone did not originate from preexisting JAK2+ PV clone can't be totally ruled out as isolated CD14⁺ cells did not express 100% JAK2V617F mutations. The other reason that favors our theory is that CMML usually doesn't carry the JAK2V617F mutations [15], therefore the positive JAK2V617F in the CMML clone will likely derived from the JAK2V617F mutation of original PV clone.
Our patient had a history of prostate carcinoma treated with radiation 9 years prior and this raises an possibility of therapy-related CMML. In general, therapy related CMML usually is associated with abnormal cytogenetic such as del 5q/−5, del 7q/−7, 8 +, 11q23 deletion [17], but our patient had normal cytogenetic studies at the time of CMML transformation. Therefore, it is suggestive that progression to CMML is unlikely to be therapy-related in the setting of normal cytogenetic analysis.
5. Conclusion
Clonal evolution of JAK2V617F PV to CMML is a rare phenomenon. Therefore, we aimed to describe a CMML case that likely shared a common clonal origin of early polycythemia phenotype, as demonstrated by the CMML cells carrying the JAK2V617F positive mutation and acquired molecular alterations late during disease progression, such as ASXL1, RUNX1, SRSF2 and TET2, NRAS, KRAS. Lastly, we demonstrated by flow cytometry analysis that the CMML clone was of the classical CD14+ CD16− monocyte subtype.
References
- 1.Arber D.A., Orazi A., Hasserjan R. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 2.Barosi G., Mesa R.A., Thiele J. International Working Group for Myeloproliferative neoplasms Research and Treatment (IWG-MRT). Proposed Criteria for the Diagnosis of Post-Polycythemia Vera and Post-Essential Thrombocythemia Myelofibrosis: A Consensus Statement From the International Working Group for Myeloproliferative neoplasms Research and Treatment; Leukemia; 2008. pp. 437–438. [DOI] [PubMed] [Google Scholar]
- 3.Tefferi A., Guglielmelli P., Larson D.R. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood. 2014;124(16):2507–2513. doi: 10.1182/blood-2014-05-579136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tam C.S., Nussenzveiq R.M., Popat U. The natural history and treatment outcome of blast phase BCR-ABL- myeloprolifeerative neoplasms. Blood. 2008;112(5):1628–1637. doi: 10.1182/blood-2008-02-138230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holcombe R.F., Treseler P.A., Rosenthal D.S. Chronic myelomonocytic leukemia transformation in polycythemia vera. Leukemia. 1991;5(7):606–610. [PubMed] [Google Scholar]
- 6.Barraco D., Cerquozzi S., Gangat N. Monocytosis in polycythemia vera: clinical and molecular correlates. Am. J. Hematol. 2017;92(7):640–645. doi: 10.1002/ajh.24740. [DOI] [PubMed] [Google Scholar]
- 7.James C., Ugo V., Le Couedic J.P. A unique clonal JAK2 mutation leading to constitutive signaling causes polycythemia vera. Nature. 2005;434(7037):1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 8.Nangalia J., Massie C.E., Baxter E.J. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013;369(25):2391–2405. doi: 10.1056/NEJMoa1312542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arber D.A., Orazi A., Hasserjian R. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 10.Wong K.L., Tai J.J., Wong W.C. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood. 2011;118(5):e16–e31. doi: 10.1182/blood-2010-12-326355. [DOI] [PubMed] [Google Scholar]
- 11.Talati C., Zhang L., Shaheen G. Monocytes subset analysis accurately distinguishes CMML from MDS and is associated with a favorable MDS prognosis. Blood. 2016;128:2009. doi: 10.1182/blood-2016-12-753210. [DOI] [PubMed] [Google Scholar]
- 12.Finazzi G., Caruso V., Marchioli R. Acute leukemia in polycythemia vera. An analysis of 1638 patients enrolled in a prospective observational study. Blood. 2005;105:2664–2670. doi: 10.1182/blood-2004-09-3426. [DOI] [PubMed] [Google Scholar]
- 13.Passamonti F., Rumi E., Caramella M. A dynamic prognostic model to predict survival in post-polycythemia vera myelofibrosis. Blood. 2008;111:3383–3387. doi: 10.1182/blood-2007-11-121434. [DOI] [PubMed] [Google Scholar]
- 14.Lopez J.E.H., Carballo-Zarate A.A., Medeiros L.J. Cytogenetic abnormalities may predict transformation to acute myeloid leukemia in polycythemia vera patients. Blood. 2016;128:4258. [Google Scholar]
- 15.Levine R.L., Loriaux M., Huntly B.J. The JAK2V617F activating mutation occurs in chronic myelomonocytic leukemia and acute myeloid leukemia, but not in acute lymphoblastic leukemia or chronic lymphocytic leukemia. Blood. 2005;106:3377–3379. doi: 10.1182/blood-2005-05-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Y., Yung A., Kwok B. Chronic myelomonocytic leukemia with preexisting myelodysplastic syndrome or myeloproliferative neoplasm: two stages of one disease with poor prognosis. Blood. 2013;122:1342. [Google Scholar]
- 17.Takahashi K., Pemmaraju N., Strati P. Clinical characteristics and outcomes of therapy-related chronic myelomonocytic leukemia. Blood. 2013;122:2807–2811. doi: 10.1182/blood-2013-03-491399. [DOI] [PMC free article] [PubMed] [Google Scholar]