Highlights
-
•
The GIRAFE trial will evaluate an adaptive radiotherapy method.
-
•
48 patients will be included.
-
•
They will undergo 4 intermediate re-planning CTs ( iCTs) and 35 daily MVCT.
-
•
MVCT adaptive segmentation will be compared with manual recontouring.
-
•
Deformable contour deformation on iCTs will be compared with manual recontouring.
Abbreviations: ART, adaptive radiotherapy; CT, computed tomography; CTV, clinical target volume; DIR, deformable image registration; DSC, Dice similarity coefficient; GTV, gross tumor volume; H&N, head and neck; iCT, intermediate computed tomography; ICRU, international commission on radiation units and measurements; IMRT, intensity-modulated radiotherapy; IGRT, image-guided radiotherapy; IUCT, Institut Universitaire du cancer de Toulouse; MVCT, megavoltage computed tomography; OAR, organ at risk; PET, positron emission tomography; PTV, planning target volume
Abstract
During exclusive curative radiotherapy for head and neck tumors, the patient’s organs at risk (OAR) and target volumes frequently change size and shape, leading to a risk of higher toxicity and lower control than expected on planned dosimetry. Adaptive radiotherapy is often necessary but 1) tools are needed to define the optimal time for replanning, and 2) the subsequent workflow is time-consuming.
We designed a prospective study to evaluate 1) the validity of automatically deformed contours on the daily MVCT, in order to safely use the “dose-of the day” tool to check daily if replanning is necessary; 2) the automatically deformed contours on the replanning CT and the time gained in the replanning workflow.
Forty-eight patients with T3-T4 and/or involved node >2 cm head and neck squamous cell carcinomas, planned for curative radiotherapy without surgery, will be enrolled. They will undergo treatment with helical IMRT including daily repositioning MVCTs. The contours proposed will be compared weekly on intermediate planning CTs (iCTs) on weeks 3, 4, 5 and 6. On these iCTs both manual recontouring and automated deformable registration of the initial contours will be compared with the contours automatically defined on the MVCT. The primary objective is to evaluate the Dice similarity coefficient (DSC) of the volumes of each parotid gland. The secondary objectives will evaluate, for target volumes and all OARs: the DSC, the mean distance to agreement, and the average surface-to-surface distance. Time between the automatic and the manual recontouring workflows will be compared.
1. Introduction
The prognosis of locally advanced head and neck (H&N) cancer patients has improved over the last few decades with better remission rates and local tumor control, thanks to the improvement of diagnostic imaging, surgical and radiotherapy techniques. There is a trend toward a wider use of exclusive radiotherapy with or without concomitant chemotherapy, in a curative intent for locally advanced oropharyngeal, hypo-pharyngeal or laryngeal tumors [1], [2]. Moreover, the increasing number of HPV induced oropharyngeal carcinomas and their excellent prognostic with exclusive radio-chemotherapy increased the proportion of these non-surgical treatments.
The management of these locally advanced tumors must now take into account the patient’s quality of life in the long term. The development and use of IMRT has resulted in better quality of life and decreased late toxicity by protecting healthy tissue and organs at risks [3], [4], [5], particularly salivary glands.
Image guidance facilitates precise patient positioning, thereby allowing a decrease of PTV margins. However, this highlights the need to address questions regarding anatomical changes during treatment [6]. Indeed, complex and progressive changes of the patient’s anatomy occur during the seven weeks of treatment in the curative setting. The patient’s external contour is modified in the case of weight loss and the morphology of organs at risk may also change. In particular, the parotid and submandibular salivary glands can shrink and migrate medially [7]. The parotids have been described to lose between 5 and 32% of their volume at mid-treatment and 13–41% by the end of treatment. [8] In a prospective study, Castelli et al. described an average increase of 3.7 Gy to the parotids with an average increase of 8.2% of the risk of xerostomia [9]. Similarly, gross tumor and pathological lymph nodes may also respond early during treatment – a decrease in GTV was estimated between 21 and 75% by the end of treatment [8], [10]. Significant dose delivery differences may arise from these anatomical changes because of the steep dose fall-off inherent in the IMRT technique, and the actual delivered dose may not correspond to the planned dose [11]. The consequences can be an increase in delivered doses to the organs at risk (OAR), and/or a decrease in the delivered dose to the tumor, resulting in an increased risk of toxicity and/or recurrence [12].
Adaptive radiotherapy (ART) can be defined as all the processes that contribute to the modification of a treatment plan based on the observation of individual variations during the whole treatment. ART is becoming a major area of investigation in modern radiotherapy [13], aiming to optimize the delivered dose distribution to the daily anatomy of the patient based on the anatomic variations during the treatment. In case of significant variations in planned dose to OARs or PTVs, replanning is performed. The right timing should be personalized as theses variations are non-linear. A recent literature review analyzed 8 in silico studies and 5 clinical studies: ART decreased dose to the parotid gland from 0.6 to 6 Gy and maximum dose to the spinal cord form 1.1 to 4 Gy. The conclusion is that largest early anatomical and dose variations are the best candidates for ART [14], [15]. A study using weekly CTs for 85 patients describes non-linear parotid gland shrinkage with a preponderance of anatomical changes during the first half of treatment [16].
Currently, at least five ongoing prospective clinical trials (Table 1) on adaptive radiotherapy are recruiting with different re-scanning schedules, all of them based on new planning CTs.
Table 1.
Current trials of head and neck ART on clinical trials.gouv (September 2018).
|
NCT02545322 Graz |
18 | Unresected | Deformable registration at week 3 & week 5 | PTV D98% |
|
NCT03215719 New York UM |
25 | Unresected HPV+ | De-escalation at week 4 if >40% nodal shrinkage | 2 years progression free survival |
|
NCT03096808 MSKCCC |
65 | Unresected SCCHNC | Unknown (data will be asked to coordinator) | loco regional event free survival |
|
NCT01874587 Rennes cancer center France ARTIX |
174 | Unresected Oropharyngeal | Every week re-planning | Increase in salivary flow 25% & Non inferiority in EFS |
ART strategies require many additional steps as compared with a standard treatment workflow: multiple simulation CTs, CT registration, re-contouring, new provisional dosimetry and finally decision-making before applying a new treatment plan. This time-consuming workflow limits routine implementation in many centers. Hence, development of automated adaptive tools is crucial for the assessment and deployment of this technique.
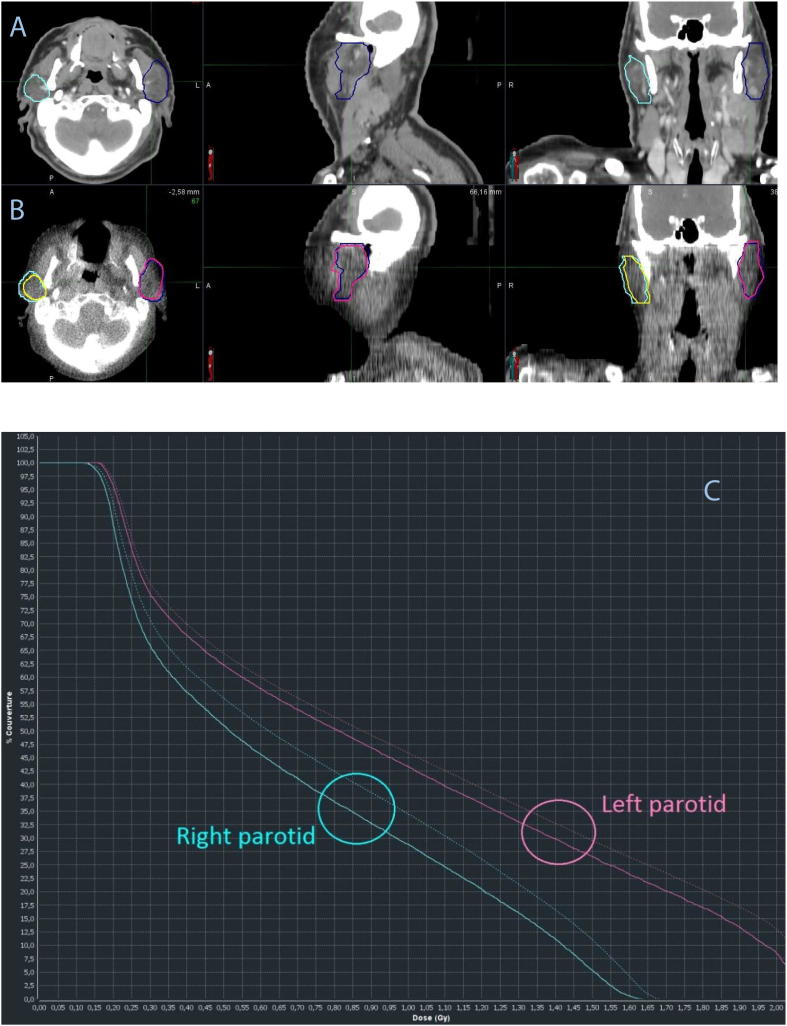
The most recent radiation therapy delivery systems provide built-in hardware and software capabilities for daily ART. Accuray© (Sunnyvale, CA) recently released such tools for use with TomoTherapy systems. With the adaptive radiotherapy software (PreciseART™), a dose-volume histogram comparison between daily administered dose and planned fraction dose called “dose-of-the-day” is then available. At one time-point of the treatment, it can also compare the total dose that was planned with the sum of “dose of the day”, and can project the dose for the whole treatment with the hypothesis of an unchanging anatomy since the last fraction delivered, called “projected total dose” (see Fig. 1). A recent publication by Branchini et al. described that “dose of the day” distributions calculated on deformed CTs proved to be statistically equivalent to the calculated dose on the same day KvCT, validating the methodology of deformable dosimetry [17]. This approach may allow detection of delivered dose variations to the tumor and organs at risk and offers a decision-making tool for re-planning if the dose constraints are not reached due to anatomical changes.
Fig. 1.
The process begins with an automated rigid alignment, possibly manually corrected if needed, between the reference planning CT(A) and the daily in-room MVCT (B). The planning contours (dark cyan for the right parotid and dark blue for the left parotid) are overlaid to the daily MVCT to verify setup accuracy and to evaluate if there are changes in current anatomy relative to baseline. A deformable image registration can be performed to propagate original planning contours onto current anatomy. Both rigid and deformed contours are available simultaneously and automatically. The deformed contours are used by default (B) in yellow for the right parotid and pink for the left parotid on the images. Then a dose-of-the day is proposed (C) showing the dose difference between OARs, the plain line is for the initial contours, the dotted line for the deformed contours on the MVCT. Here we see a dose difference for the right (blue) and left (pink) parotid due to their shrinkage. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
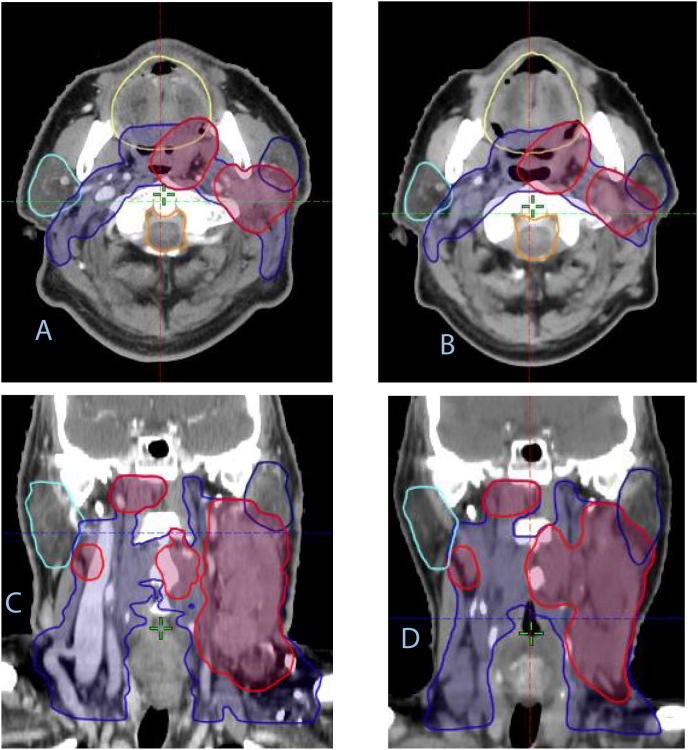
If re-planning is needed, based on a modified projected total dose of one or several OARs above the constraints, a replanning tool (PreciseRTX™) provides immediate deformable co-registration of both planning CTs and contours (see Fig. 2).
Fig. 2.
If re-planning is indicated, the gold standard is to rigidly register the re-planning CT with the initial CT and correct slice by slice the co-registered initial contours. Another possibility to be evaluated in this study is to transfer the re-planning CT into the PRECISE RTX console where a deformable registration is performed on initial CT (A, C) with propagation of the initial contours (A, C) to the registered new CT: the resultant contours on the re-planning CT are shown on B and D, in axial (AB) and coronal planes (CD).
A fast and trustworthy method of automatic contour deformation on MVCT will allow the implementation of adaptive radiotherapy into daily practice. However, three questions arise when considering ART on a daily use: Who might benefit from ART? When should we consider ART? How should we implement ART? These questions prompted us to design a prospective monocenter phase II study, the GIRAFE trial.
First, which patients can clinically benefit from this approach the most? Not all patients need an ART strategy. This question has already been addressed and we may assume that ART is particularly indicated for patients with large tumors and patients with small parotids, two categories of patients who present a high risk of CTV under-dosage with parotid over-dosage [13], [18]. We will then evaluate this method for patients with onsite voluminous tumors or nodes and take into account the analysis of the initial parotid volume.
Secondly, when and how many times should we consider re-planning? The frequency of re-planning is not well defined yet in the literature. In a recent review, Brouwer et al. [19] cited at least 38 studies evaluating volume variations during RT with different set-up. One to seven intermediate CT and re-plans were used. In general in our clinic we rarely observe anatomical changes within the first two weeks; and replanning on the last week of treatment for a few fractions is not justified. Hence, to answer this question, we decided to acquire four re-planning CTs (iCTs) before the 3rd, 4th, 5th and 6th week of treatment. Each iCT will be compared with the daily in-room MVCT systematically acquired as IGRT before daily Tomotherapy treatment [20].
The last question is “How should we proceed for ART in order to avoid a time-consuming, complex and unwieldy process?” Two key components for this are automatic deformation and re-planning automatization. Before considering an implementation of ART workflow in clinical setting, the tools described above must be validated for deformable image registration and volume adaptations on low-definition imaging such as MVCT [21] and for contour deformation on the new planning CT.
In order to use the “dose of the day” tool, we will perform a study to evaluate the automatic DIR and the subsequent contour deformation propagations on MVCT by comparing them with the gold standard method, which is manually delineated parotid glands from an iCT. The secondary goal will be to evaluate the newly delineated volumes of the other organs at risk with the automated DIR after each MVCT and each re-planning CT. The time needed for every step of this workflow will be recorded. The ultimate goal is to define a clinical workflow for ART for head and neck cancer, defining the scenarios of the “How?” and the “When?”
2. Methods
2.1. Study design
The GIRAFE study will be a monocenter open-label phase II prospective study, taking place in our hospital, Institut Claudius Regaud, at the Institut Universitaire du Cancer de Toulouse-Oncopole (IUCT-Oncopole) which is dedicated to cancer care.
2.2. Eligibility criteria
Patients with histologically proven locally advanced H&N cancer with nodal involvement (at least T3 or involved node >2 cm) in curative intent with an Eastern Cooperative Oncology Group performance status 0 or 1 will be enrolled. Exclusion criteria include metastatic disease, adjuvant treatment and recurrent disease, unfit patients due to comorbidities, pregnant women, and inability to follow procedures during treatment or follow-up.
2.3. Treatment preparation procedures
Pre-therapeutic imaging assessment will include FDG-PET and MRI for patients with oropharyngeal and nasopharyngeal cancer (T2-weighted and gadolinium injected T1-weighted sequences).
The initial Planning CTs (CT0) will be performed with and without intravenous iodine contrast agent, using 2.5 mm slice thickness from the vertex to the diaphragm, with a five-point immobilization mask.
Target volumes will be delineated based on international recommendations [22], [23] after manual rigid registration with the PET planning CT and an MRI if available.
Two target volumes will be defined: CTV70 corresponding to the gross tumor volume and the positive lymph nodes with a 5 mm 3D margin corrected to the air and bones free from tumor invasion, CTV56 corresponding to the low risk microscopic spread volume. The planning target volumes (PTVs) will be created by expanding CTVs by a 3 mm 3D margin.
Delineated organs at risk (OAR) will include at least the right parotid, left parotid, right submandibular salivary gland, left submandibular salivary gland, brainstem, spinal canal, mandible, oral cavity, pharyngeal constrictors and larynx [24].
Target volumes and OARs manual delineation will be validated by the radiation oncologist in charge of the patient.
International recommendations for dose prescription and OAR dose constraints will be applied: 95% of the PTV must be covered by the 95% isodose line, 98% of the PTV must be covered by the 90% isodose line according to ICRU83.
Dose constraints for OARs will follow published values: Parotids Dmean <26 Gy for at least one parotid, submandibular salivary glands out of fields Dmean <42 Gy, brainstem Dmax <50 Gy, spinal canal Dmax <45 Gy, oral cavity Dmax ≤70 Gy and Dmean <45 Gy (except for larynx cancers where Dmean <30 Gy), larynx in extra-laryngeal location Dmean <25 Gy with D5% <45–50 Gy if possible, constrictor muscles Dmean <50 Gy [20]. Simultaneous integrated boost with helical intensity-modulated radiotherapy will be delivered in 35 fractions, 5 fractions a week for 7 weeks and not modified unless it appears necessary.
Each patient will undergo four weekly intermediate non-contrast CTs starting at the third week of treatment (iCTw3 to iCTw6) with the same protocol as CT0. This frequency is chosen because during the first two weeks changes in the tumor size or weight are exceptional and in the 7th week, re-planning is not feasible. The need for a new immobilization mask will be evaluated before performing each iCT. For each iCT, CTVs and OARs will be manually delineated. The additional dose received from these four CTs is evaluated at Computed Tomography Dose index in the volume (CTDIvol) of 15 mGy and dose-length-product (DLP) of 600 mGy cm and considered negligible compared to the 70 Gy delivered to the tumor.
The image-guided radiotherapy (IGRT) modality will consist of a daily MVCT – a daily 3 mm positioning tolerance will be accepted. MVCT will be performed in « normal » quality except for the days of intermediate CTs when the patient will be placed in treatment position with a MVCT in « fine » quality. In that case the maximal absorbed dose will be 3 cGy.
As in our usual clinical routine, the referring radiation oncologist will decide whether a re-plan is warranted based on clinical MVCT and iCT criteria. This will not impact the endpoint of the study, as all contours comparisons will be done on the same date between MVCT and iCT, after completion of the treatment.
Concomitant chemotherapy or targeted therapy may be proposed according to the recommendations of the tumor board. We will avoid acquiring the iCT during the first, second or third day of concomitant chemotherapy as hydration during chemotherapy was described as inducing geometric changes in parotid glands (mean variations of 7.2%, 10% and 7% respectively) [25].
Patients who need emergency surgery will be excluded from the study.
3. Experimental intervention
3.1. Contours
Anatomical structures will be delineated manually on CT0 and on the 4 intermediate KvCT, i.e. iCTw3, iCTw4, iCTw5 and iCTw6. All manual delineation on planning CT0 and iCTs will be performed after the end of the whole treatment by the same experienced junior radiation oncologist. All image registrations and contours on CT0 and iCTs will be validated by the senior radiation oncologist coordinator of the study with a double check by another head and neck experienced senior radiation oncologist.
The initial volumes contoured on CT0 are propagated to each daily MVCT using automated DIR within PreciseART. The accuracy of this automatic deformable volume definition on MVCTs will be assessed by comparison to the manual volumes delineated on the 4 iCTs.
An additional comparison will be made between manual re-contouring on iCT and deformable propagation of the contours from CT0 to iCTw3, iCTw4, iCTw5, iCTw6 using automated DIR on PreciseRTX.
All comparisons will use The Dice Similarity Coefficient (DSC) to compare the volumes. Ideally, when two volumes overlap perfectly, the DSC equals 1. A null DSC would correspond to two disjoint volumes. The DSC between a volume A and a volume B is defined as follows:
The DSC will be calculated between manual and automatic volumes the day of each iCT, and also for each treatment day, for all OARs and for gross tumor volumes if possible [26].
3.2. Statistical analysis
In a previous study, Goldberg-Zimring et al. suggested that the satisfactory volume matching should be 70% (DSC of 0.7) or more for brain target volumes for adaptive radiotherapy application and Tong et al. described an average DSC for parotids of median 0.835 when comparing manual segmentation with deep neural network segmentation method on volumetric CT scans [27], [28].
The primary endpoint of this study is to evaluate the proportion of patients presenting with a DSC ≥0.85 for both left and right parotid glands with MVCT deformed contours compared with the gold standard of manual contouring.
The following hypotheses are used for p0 = 80%, maximal unacceptable rate of patient with comparison success for whom the automatic recontouring on the MVCT after deformable registration will be considered as insufficiently feasible, and p1 = 95%, minimal acceptable rate of patients with comparison success for whom the automatic recontouring on the MVCT after deformable registration will be considered as sufficiently feasible.
Using a A’Hern design (alpha = 2.5%, 1-beta = 90%) and (p0 = 80%; p1 = 95%), 48 patients evaluable need to be included [29].
The secondary endpoints are:
-
–
The evaluation of DSCs for all other OARs
-
–
The evaluation of the distance between centers of gravity for all OARs and target volumes
-
–
The analysis of surface-to-surface distance of the OARs
-
–
The difference in time for automatic deformable contour segmentation vs manual segmentation.
4. Conclusion
A key challenge for adaptive therapy is the determination of the optimal time for replanning. A daily MVCT and “dose-of-the-day” potentially offers an efficient workflow for plan adaptation.
In our method, contouring differences could be caused by factors other than MVCT image quality and deformation accuracy, such as:
-
–
Variability in contouring between contourers or the same contour over time
-
–
Issues with the rigid registration between the MVCT and the iCT
The study attempts to control for these effects by having the senior reviewers check all contours and registrations to eliminate/minimize variation due to these factors.
If we validate the approach used, a quick daily check of the target volumes and organs at risk daily dose and cumulated doses should allow the physician to decide the proper timing for replanning. Moreover, if we validate the automatic deformable recontouring on the iCT, we could describe an efficient workflow in order to keep maximizing clinical control without jeopardizing quality of life.
Ethics
The protocol is under submission to the national French ethics committee for the protection of persons participating in clinical research. An informed consent will be obtained during the first medical consultation with a radiation oncologist.
Co-author specific contributions
ALa: corresponding author; primary investigator; conceived, coordinated, and directed all study activities, responsible for data collection, project integrity, manuscript content and editorial oversight and correspondence; direct oversight of trainee personnel (BG, VE, JD).
MM, MP, JPD: clinical trial activation.
TF, ALu: Clinical protocol development, manuscript drafting and editing, statistical analysis.
EG, PG, AM, MR, BG, VE: Clinical protocol development.
JD, ALa, MR: contours, validation and double-check of the contours,
ALa, GH, EG, FXA, VE, BG: adaptive workflow development.
ALa, GH, EG, PG, AM, MR, FXA, VE, BG, ML, MM: manuscript editing.
Funding
This research is supported by an Accuray Grant.
Conflicts of interest
A. Laprie performed workshops for Varian, and conferences with funding of travel expenses from Merck-Serono and Accuray.
Acknowledgment
We thank Accuray for English editing.
Footnotes
Trial Sponsor: Institut Claudius Regaud, Institut Universitaire du Cancer de Toulouse – Oncopole.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2019.02.006.
Contributor Information
Vincent Esteyrie, Email: Esteyrie.vincent@iuct-oncopole.fr.
Amélie Lusque, Email: lusque.amelie@iuct-oncopole.fr.
Pierre Graff, Email: graff.pierre@iuct-oncopole.fr.
Anouchka Modesto, Email: modesto.anouchka@iuct-oncopole.fr.
Michel Rives, Email: rives.michel@iuct-oncopole.fr.
Michel Lapeyre, Email: michel.lapeyre@cjp.fr.
Jacques Desrousseaux, Email: desrousseaux.jacques@iuct-oncopole.fr.
Eliane Graulières, Email: graulieres.eliane@iuct-oncopole.fr.
Gregory Hangard, Email: hangard.gregory@iuct-oncopole.fr.
François-Xavier Arnaud, Email: arnaud.francois-xavier@iuct-oncopole.fr.
Regis Ferrand, Email: ferrand.regis@iuct-oncopole.fr.
Jean-Pierre Delord, Email: delord.jean-pierre@iuct-oncopole.fr.
Muriel Poublanc, Email: poublanc.muriel@iuct-oncopole.fr.
Muriel Mounier, Email: mounier.muriel@iuct-oncopole.fr.
Thomas Filleron, Email: filleron.thomas@iuct-oncopole.fr.
Anne Laprie, Email: laprie.anne@iuct-oncopole.fr.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Chen A.Y., Zhu J., Fedewa S. Temporal trends in oropharyngeal cancer treatment and survival: 1998–2009. Laryngoscope. 2014;124:131–138. doi: 10.1002/lary.24296. [DOI] [PubMed] [Google Scholar]
- 2.Machtay M. Organ preservation therapy using induction plus concurrent chemoradiation for advanced resectable oropharyngeal carcinoma: a University of Pennsylvania Phase II Trial. J Clin Oncol. 2002;20:3964–3971. doi: 10.1200/JCO.2002.11.026. [DOI] [PubMed] [Google Scholar]
- 3.Nutting C.M. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12:127–136. doi: 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graff P. Impact of intensity-modulated radiotherapy on health-related quality of life for head and neck cancer patients: matched-pair comparison with conventional radiotherapy. Int J Radiat Oncol. 2007;67:1309–1317. doi: 10.1016/j.ijrobp.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 5.Marta G.N. Intensity-modulated radiation therapy for head and neck cancer: systematic review and meta-analysis. Radiother Oncol. 2014;110:9–15. doi: 10.1016/j.radonc.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Lim-Reinders S., Keller B.M., Al-Ward S., Sahgal A., Kim A. Online adaptive radiation therapy. Int J Radiat Oncol Biol Phys. 2017;99:994–1003. doi: 10.1016/j.ijrobp.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 7.Bhide S.A. Weekly volume and dosimetric changes during chemoradiotherapy with intensity-modulated radiation therapy for head and neck cancer: a prospective observational study. Int J Radiat Oncol. 2010;76:1360–1368. doi: 10.1016/j.ijrobp.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Castadot P., Geets X., Lee J.A., Grégoire V. Adaptive functional image-guided IMRT in pharyngo-laryngeal squamous cell carcinoma: is the gain in dose distribution worth the effort? Radiother Oncol. 2011;101:343–350. doi: 10.1016/j.radonc.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Castelli J. Impact of head and neck cancer adaptive radiotherapy to spare the parotid glands and decrease the risk of xerostomia. Radiat Oncol. 2015;10:1–10. doi: 10.1186/s13014-014-0318-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barker J.L. Quantification of volumetric and geometric changes occurring during fractionated radiotherapy for head-and-neck cancer using an integrated CT/linear accelerator system. Int. J. Radiat. Oncol. 2004;59:960–970. doi: 10.1016/j.ijrobp.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz D.L., Dong L. Adaptive radiation therapy for head and neck cancer-can an old goal evolve into a new standard? J Oncol. 2011;2011 doi: 10.1155/2011/690595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grégoire V., Jeraj R., Lee J.A., O’Sullivan B. Radiotherapy for head and neck tumours in 2012 and beyond: conformal, tailored, and adaptive? Lancet Oncol. 2012;13:e292–e300. doi: 10.1016/S1470-2045(12)70237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brouwer C.L., Steenbakkers R.J.H.M., Langendijk J.A., Sijtsema N.M. Identifying patients who may benefit from adaptive radiotherapy: Does the literature on anatomic and dosimetric changes in head and neck organs at risk during radiotherapy provide information to help? Radiother Oncol. 2015;115:285–294. doi: 10.1016/j.radonc.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 14.Castelli J. Adaptive radiotherapy for head and neck cancer. Acta Oncol (Madr) 2018;57:1284–1292. doi: 10.1080/0284186X.2018.1505053. [DOI] [PubMed] [Google Scholar]
- 15.Castelli J. Adaptive radiotherapy in head and neck cancer is required to avoid tumor underdose. Acta Oncol (Madr) 2018;57:1267–1270. doi: 10.1080/0284186X.2018.1468086. [DOI] [PubMed] [Google Scholar]
- 16.Sanguineti G., Ricchetti F., Wu B., McNutt T., Fiorino C. Parotid gland shrinkage during IMRT predicts the time to Xerostomia resolution. Radiat Oncol. 2015;10:19. doi: 10.1186/s13014-015-0331-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Branchini M. Validation of a method for “dose of the day” calculation in head-neck tomotherapy by using planning ct-to-MVCT deformable image registration. Phys Medica. 2017;39:73–79. doi: 10.1016/j.ejmp.2017.05.070. [DOI] [PubMed] [Google Scholar]
- 18.Mahmoud O. Prospective pilot study comparing the need for adaptive radiotherapy in unresected bulky disease and in postoperative patients with head and neck cancer. Technol Cancer Res Treat. 2017;16:1014–1021. doi: 10.1177/1533034617717624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brouwer C.L. Selection of head and neck cancer patients for adaptive radiotherapy to decrease xerostomia. Radiother Oncol. 2016;120:36–40. doi: 10.1016/j.radonc.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 20.Den R.B. Daily Image guidance with cone-beam computed tomography for head-and-neck cancer intensity-modulated radiotherapy: a prospective study. Int J Radiat Oncol. 2010;76:1353–1359. doi: 10.1016/j.ijrobp.2009.03.059. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L., Wang Z., Shi C., Long T., Xu X.G. The impact of robustness of deformable image registration on contour propagation and dose accumulation for head and neck adaptive radiotherapy. J Appl Clin Med Phys. 2018;19:185–194. doi: 10.1002/acm2.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grégoire V. Delineation of the primary tumour Clinical Target Volumes (CTV-P) in laryngeal, hypopharyngeal, oropharyngeal and oral cavity squamous cell carcinoma: AIRO, CACA, DAHANCA, EORTC, GEORCC, GORTEC, HKNPCSG, HNCIG, IAG-KHT, LPRHHT, NCIC CTG, NCRI, NRG Oncology, PHNS, SBRT, SOMERA, SRO, SSHNO, TROG consensus guidelines. Radiother Oncol. 2018;126:3–24. doi: 10.1016/j.radonc.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 23.Grégoire V. Delineation of the neck node levels for head and neck tumors: A 2013 update. DAHANCA, EORTC, HKNPCSG, NCIC CTG, NCRI, RTOG, TROG consensus guidelines. Radiother Oncol. 2014;110:172–181. doi: 10.1016/j.radonc.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Noël G., Antoni D., Barillot I., Chauvet B. Délinéation des organes à risque et contraintes dosimétriques. Cancer/Radiothérapie. 2016;20:S36–S60. doi: 10.1016/j.canrad.2016.07.032. [DOI] [PubMed] [Google Scholar]
- 25.Kager P.M. Geometric changes of parotid glands caused by hydration during chemoradiotherapy. Radiat Oncol. 2015 doi: 10.1186/s13014-015-0554-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.García-Mollá R. Validation of a deformable image registration produced by a commercial treatment planning system in head and neck. Phys Med. 2015;31:219–223. doi: 10.1016/j.ejmp.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 27.Goldberg-Zimring D. Statistical validation of brain tumor shape approximation via spherical harmonics for image-guided neurosurgery. Acad Radiol. 2005;12 doi: 10.1016/j.acra.2004.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tong N., Gou S., Yang S., Ruan D., Sheng K. Fully automatic multi-organ segmentation for head and neck cancer radiotherapy using shape representation model constrained fully convolutional neural networks. Med Phys. 2018 doi: 10.1002/mp.13147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.A’Hern R.P. Sample size tables for exact single-stage phase II designs. Stat Med. 2001;20:859–866. doi: 10.1002/sim.721. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.