Abstract
It is well known that patent ductus arteriosus (PDA) in adults, especially in the elderly, differs from that in pediatric patients. A 68-year-old woman with a PDA with focal calcification at the aortic orifice of the ampulla with a minimum diameter of 4.0 mm and length of 14.8 mm, was treated with a 10/8-mm Amplatzer duct occluder (ADO) (St. Jude Medical Corp, St. Paul, MN, USA). After device implantation, systolic blood pressure (BP) increased to approximately 220 mmHg from 130 mmHg. She experienced transient dyspnea from hypertensive heart failure, which improved through continuous infusion of anti-hypertensive agents. She suddenly felt pressure on her chest 12 h post-procedure and collapsed. Surgical thoracotomy revealed an ascending aortic dissection into the pericardial space. In retrospective review, the ADO may have been slightly deformed by fluoroscopy. The complication may have been triggered by the resilience caused by device deformation, damage to the aortic wall due to the aortic side of the device, uneven elasticity of the arterial wall, and uncontrolled excessively high blood pressure.
<Learning objective: Although transcatheter patent ductus arteriosus (PDA) closure is an established, safe, and effective procedure when treating PDA of the elderly, wall damage due to the device may occur because of atherosclerotic changes different from that of younger patients and blood pressure will rise after closure. Therefore, it is necessary to carefully select the type and size of the device and to strictly control blood pressure in patients with a history of hypertension.>
Keywords: Aortic dissection, Transcatheter closure, Patent ductus arteriosus, Amplatzer duct occluder
Introduction
Transcatheter closure of a patent ductus arteriosus (PDA) with the Amplatzer ductal occluder (ADO) (St. Jude Medical Corp, St. Paul, MN, USA) is a well-established procedure. In general, transcatheter PDA closure improves left-ventricular hemodynamics with a low rate of complications, such as embolization, hemolysis, and infective endocarditis [1], [2]. However, transcatheter PDA closure in the elderly remains unclear. We experienced a transcatheter PDA closure case complicated by Stanford type A aortic dissection and cardiac tamponade occurring 12 h after device deployment. In this case, informed consent was obtained from the patient for publication of this case report and any accompanying images and all details that might disclose the identity of the patient were anonymized.
Case report
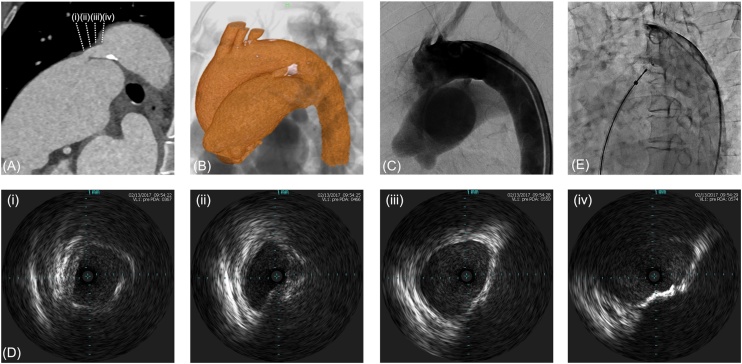
The patient, a 68-year-old female, was admitted to our hospital complaining of progressive shortness of breath, which was determined to be New York Heart Association (NYHA) symptom class II. She had a 10-year history of hypertension treated with an angiotensin-converting enzyme inhibitor (perindopril erbumine 4 mg), and untreated PDA diagnosed at the age of 37 years. She refused surgical closure for PDA until symptoms manifested. Echocardiography showed severe mitral regurgitation (MR) and PDA with continuous left-to-right shunt. Computed tomography angiography (CTA) demonstrated Krichenko type-A PDA with focal calcification at the aortic orifice of the ampulla with a minimum diameter of 4.0 mm and length of 14.8 mm (Fig. 1A, B). Right-heart catheterization showed pulmonary flow/systemic flow of 1.6 and mean pulmonary artery pressure of 31 mmHg. We decided to perform transcatheter PDA closure to relieve the volume-overload in the left-side heart and alleviate MR.
Fig. 1.
Computed tomography angiography (CTA) and aortography showing patent ductus arteriosus (PDA). (A) Long-axis cross-sectional image of PDA in CTA. (B) Calcification on the aortic side in CTA. (C) Preoperative control arteriography indicating PDA before device deployment. (D) Intravascular ultrasound images of PDA. (i) The diameter was 4.2 mm × 6.9 mm at the pulmonary end. (ii) The diameter was 4.0 mm at the narrowest point of the lesion. (iii) Calcified lesions occupied around half the circumference. (iv) The diameter was 6.5 mm × 11.8 mm at the aortic end. (E) Final angiography showing device position just before implantation.
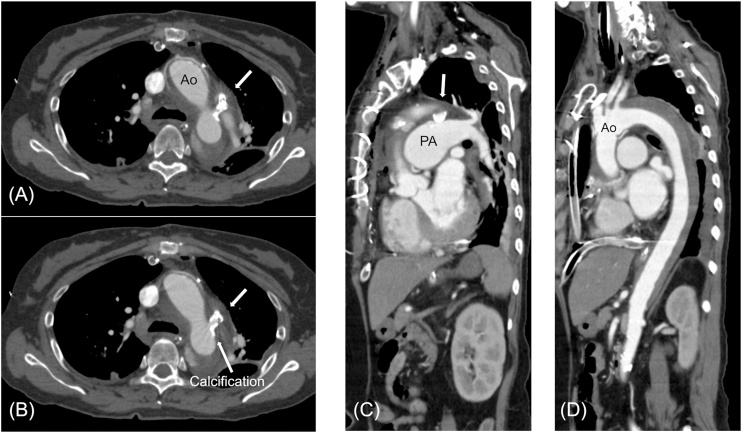
The closure procedure was performed under local anesthesia and fluoroscopic and intravascular ultrasound (IVUS) guidance. An initial biplane angiography was performed with a 5-Fr pigtail catheter positioned in the proximal descending aorta in the anteroposterior and 60° left anterior oblique projections (Fig. 1C). We crossed a 0.014″ guidewire from the descending aorta to the pulmonary artery, and IVUS was performed. IVUS revealed that the narrowest part of the PDA was 4.0 mm, which was found to be equivalent by CTA, and also revealed semicircular calcification and atherosclerotic findings on the aortic side (Fig. 1D). A 10/8-mm ADO was selected as it was expected to be approximately twice the size of the narrowest portion of the PDA. The procedure was then performed using the retrograde wire-assisted technique as described previously [3]. After the distal tip of the delivery sheath passed over the PDA without any resistance from the right femoral vein, the skirt of the device was expanded into the descending aorta. We attempted device implantation twice, but we removed it because of inappropriate positioning of the device owing to pulling tension. Finally, it was possible to place it at the ideal position during the third attempt; however, it was necessary to apply slightly more tension than usual in order to pull it toward the pulmonary artery side, since it was a relatively long PDA. The angiography image before device implantation revealed a good position for implantation, and residual shunts decreased just before indwelling (Fig. 1E). After device implantation, systolic blood pressure (BP) increased to approximately 220 mmHg from 130 mmHg and she experienced transient dyspnea from hypertensive heart failure. We immediately controlled the BP to reduce it to under 140 mmHg using continuous infusion of nitroglycerin with oxygen inhalation. The final angiography after device implantation was not performed in order to avoid progression of heart failure. We tried to control blood pressure to below 140 mmHg by continuous infusion of both nitroglycerin and nicardipine after transcatheter PDA closure. Her condition stabilized until 12 h after PDA closure; however, she suddenly felt chest pressure, lost consciousness, and collapsed. Echocardiography revealed overt cardiac tamponade. After drainage of pericardial blood, emergent hemostasis of cardiac tamponade was performed by surgical thoracotomy. Surgical findings confirmed the diagnosis of cardiac tamponade caused by perforation of the aorta due to ascending aortic dissection; however, no entry site was found in the ascending aorta. Ascending aortic replacement was performed and she recovered without neurological deficits. Contrast computed tomography after the surgical repair showed intramural hematoma due to dissection from the device (Fig. 2A–D).
Fig. 2.
Computed tomography angiography (CTA) showing aortic dissection. (A) Axial view of CTA indicating patent ductus arteriosus (PDA) device (thick arrow) after ascending aortic replacement. (B) The PDA device (thick arrow) is placed on the side of the pulmonary artery that was in the front of the calcification site (thin arrow) on the side of the aorta. (C), (D) Sagittal view of postoperative CTA demonstrating PDA and aortic dissection.
Discussion
Transcatheter closure has become a standard treatment in adult cases of PDA. Elimination of shunting flow after percutaneous closure is comparable to that following surgery. The ADO device appears to be safe and effective and is the most widely used device for PDA closure and the possibility of complications from transcatheter PDA closure, including embolization, hemolysis, infective endocarditis, and left pulmonary artery narrowing, are low [1]. Moreover, serious aortic dissection related to transcatheter closure of PDA is extremely rare and this is the first report, to the best of our knowledge, of a complication that caused acute aortic dissection after transcatheter PDA closure.
PDAs in the elderly have atherosclerotic characteristics, such as heavy calcification, severe tortuosity, and eccentricity of the ductal lumen, that differ from those in pediatric patients [4]. In this case, we examined the PDA size by computed tomography, fluoroscopy, and IVUS. IVUS precisely revealed that calcification was around 180° at the aortic side. The size of the occluder we selected was twice that of the narrowest portion of the PDA, which was acceptable based on a previous report [5]. Surgical findings and a contrast computed tomography scan after urgent surgery clearly showed an intramural hematoma and that the dissection cavity started from the periphery of the device; therefore, the device was suspected to be related to this event.
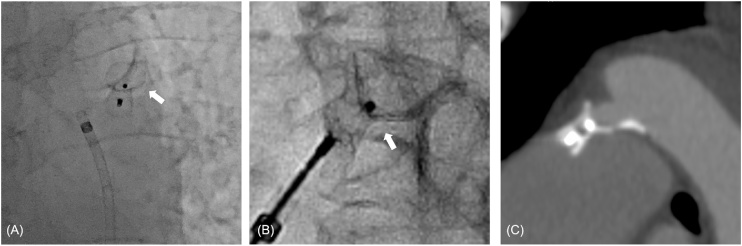
There are several possibilities for the cause of aortic dissection. First, damage to the aortic wall was possible due to the aortic side of the device and pulling the device too much toward the pulmonary artery side, owing to the long PDA, which may have contributed to aortic dissection. An angiogram after detachment revealed that the ADO was deployed with slight deformation of the device in the aortic skirts (Fig. 3A, B), and the implanted ADO was located closer to the pulmonary artery than the calcification on the aortic side (Fig. 3C). The damage caused by device deformation might have caused unevenness of the tension on the arterial wall, which might trigger dissection due to atherosclerotic changes in the elderly. Prevention of this complication may include selecting a larger size of ADO or other closure devices that are more suitable for this morphology. Previous reports showed that using the Amplatzer vascular plug (AVP) to treat long tubular or small PDAs is effective [6]. Second, it might have been the result of procedure-related complications, such as minor wall damage due to repositioning the device several times. The procedure was carried out smoothly, except when determining the indwelling position of the device. Due to the morphology of the semi-tubular PDA, it was difficult to determine the best indwelling position. Although transcatheter closure is the preferred approach even in the presence of calcification that is routinely seen in the older adult aorta, more attention should be paid to device delivery in consideration of the arteriosclerotic changes with calcification, which reduces the elasticity of the PDA. Third, a predisposition to dissection from aortic enlargement (a diameter of about 40 mm) may have led to aortic dissection and connective tissue abnormalities or hereditary diseases might have been present. There have been reports of an inheritable relationship between aortic dissection and PDA [7], [8]; however, there was no familial history of aortic dissection and PDA in this case. Since this patient had a long history of high BP, there was a greater possibility for dissociation to occur. It is necessary to strictly control BP, since it tends to rise after deployment of the device due to dramatic hemodynamic changes [9]. The aforementioned multifactorial contributions that overlapped with the placement of the device are considered to have led to the aortic dissection after transcatheter closure of PDA. This case highlights the importance of device selection for long PDAs, validation of the shape of the ADO device after deployment, and stabilization of BP during the procedure. Performing an analysis of the morphology and size of the PDA to select the appropriate type of closure device is significantly important.
Fig. 3.
Morphological evaluation of Amplatzer duct occluder after deployment.
(A, B) Slight deformation (white arrow) of the device on the aortic skirts in the anteroposterior projection. (C) Enlarged image of patent ductus arteriosus in computed tomography angiography.
Acknowledgments
The authors thank Yutaka Okita MD, PhD; Hiroshi Tanaka MD, PhD; Takeshi Inoue MD, PhD; Daisuke Terashita, MD, PhD; and Ryo Takeshige, MD for the excellent management of the patient’s operation and treatment.
Acknowledgments
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Pass R.H., Hijazi Z., Hsu D.T., Lewis V., Hellenbrand W.E. Multicenter USA Amplatzer patent ductus arteriosus occlusion device trial: initial and one-year results. J Am Coll Cardiol. 2004;44:513–519. doi: 10.1016/j.jacc.2004.03.074. [DOI] [PubMed] [Google Scholar]
- 2.Eerola A., Jokinen E., Boldt T., Pihkala J. The influence of percutaneous closure of patent ductus arteriosus on left ventricular size and function: a prospective study using two- and three-dimensional echocardiography and measurements of serum natriuretic peptides. J Am Coll Cardiol. 2006;47:1060–1066. doi: 10.1016/j.jacc.2005.09.067. [DOI] [PubMed] [Google Scholar]
- 3.Hsin H.T., Lin L.C., Hwang J.J., Ho S.G., Tseng C.D., Chiang F.T. Retrograde wire-assisted percutaneous transcatheter closure of persistent ductus arteriosus with Amplatzer duct occluder in the elderly: a new application. Catheter Cardiovasc Interv. 2004;61:264–267. doi: 10.1002/ccd.10762. [DOI] [PubMed] [Google Scholar]
- 4.Vita J.A., Bittl J.A., Selwyn A.P., Lock J.E. Transcatheter closure of a calcified patent ductus arteriosus in an elderly man. J Am Coll Cardiol. 1988;12:1382–1385. doi: 10.1016/0735-1097(88)92624-1. [DOI] [PubMed] [Google Scholar]
- 5.Gu X., Zhang Q., Sun H., Fei J., Zhang X., Kutryk M.J. Transcatheter closure of calcified patent ductus arteriosus in older adult patients: immediate and 12-month follow-up results. Congenit Heart Dis. 2017;12:289–293. doi: 10.1111/chd.12437. [DOI] [PubMed] [Google Scholar]
- 6.Cho E.H., Song J., Kang I.S., Huh J., Lee S.Y., Choi E.Y. Transcatheter closure of small ductus arteriosus with Amplatzer vascular plug. Korean J Pediatr. 2013;56:396–400. doi: 10.3345/kjp.2013.56.9.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khau Van Kien P., Wolf J.E., Mathieu F., Zhu L., Salve N., Lalande A. Familial thoracic aortic aneurysm/dissection with patent ductus arteriosus: genetic arguments for a particular pathophysiological entity. Eur J Hum Genet. 2004;12:173–180. doi: 10.1038/sj.ejhg.5201119. [DOI] [PubMed] [Google Scholar]
- 8.Glancy D.L., Wegmann M., Dhurandhar R.W. Aortic dissection and patent ductus arteriosus in three generations. Am J Cardiol. 2001;87:813–815. doi: 10.1016/s0002-9149(00)01515-0. [DOI] [PubMed] [Google Scholar]
- 9.Kimball T.R., Ralston M.A., Khoury P., Crump R.G., Cho F.S., Reuter J.H. Effect of ligation of patent ductus arteriosus on left ventricular performance and its determinants in premature neonates. J Am Coll Cardiol. 1996;27:193–197. doi: 10.1016/0735-1097(95)00452-1. [DOI] [PubMed] [Google Scholar]