Abstract
Background.
Little is known about viral hepatitis testing and infection prevalence among persons in private healthcare organizations (HCOs) in the United States.
Methods.
To determine the frequency of and characteristics associated with viral hepatitis testing and infection prevalence among adults with access to care, we conducted an observational cohort study among 1.25 million adults from 4 US HCOs and included persons with ≥1 clinical encounter during 2006–2008 and ≥12 months of continuous follow-up before 2009. We compared the number of infections identified with the number expected based on adjusted data from the National Health and Nutrition Examination Survey (NHANES).
Results.
Of 866 886 persons without a previous hepatitis B virus (HBV) diagnosis, 18.8% were tested for HBV infection, of whom 1.4% tested positive; among 865 659 without a previous hepatitis C virus (HCV) diagnosis, 12.7% were tested, of whom 5.5% tested positive. Less than half of those with ≥2 abnormal alanine aminotransferase (ALT) levels were subsequently tested for HBV or HCV. When tested, Asians (adjusted odds ratio [aOR] 6.33 relative to whites) were most likely HBV infected, whereas those aged 50–59 years were most likely HCV infected (aOR 6.04, relative to age <30 years). Based on estimates from NHANES, nearly one-half of HCV and one-fifth of HBV infections in this population were not identified.
Conclusions.
Even in this population with access to care and lengthy follow-up, only a fraction of expected viral hepatitis infections were identified. Abnormal ALT levels often but not consistently triggered testing. These findings have implications for the identification and care of 4–5 million US residents with HBV and HCV infection.
In the United States, chronic viral hepatitis infections are 3 to 5 times more prevalent than human immunodeficiency virus (HIV) infection [1]. Current estimates are that 800 000 to 1.4 million persons are living with chronic hepatitis B virus (HBV) infection and 2.7–3.9 million are living with chronic hepatitis C virus (HCV) infection; this is approximately 1%–% of the US population. Almost half of the liver transplants and approximately 18 000 recorded deaths annually in the United States result from HBV- and HCV-associated liver disease [2, 3]. Although a few limited studies suggest that 65% of persons with hepatitis B and 75% of those with hepatitis C are unaware of their infection, this parameter has not been examined in a geographically diverse managed care population with access to specialty care [4, 5].
Identification of persons chronically infected with HBV and HCV is necessary to prevent transmission to close contacts and to reduce the risk of progression to chronic liver disease and hepatocellular carcinoma through medical treatment [1]. Access to testing, prevention services, and treatment may be adversely affected by factors related to the patient, to the clinician, and to the healthcare system [6–10]. The most important barriers to receipt of existing services are inadequacy of health insurance coverage and lack of money to pay for services [1]. However, even among persons with health insurance, high deductibles, benefit limits, and the fragmentation of services are major obstacles to the effective delivery of care and services. In the public sector, the Department of Veterans Affairs has made considerable progress in the integration of viral hepatitis-related care [11]. Private sector integrated health systems, such as health maintenance (HMOs) or healthcare organizations (HCOs), have similar advantages of control of providers and care settings, coverage that reduces financial barriers, and information systems to track and share data [1].
Nonetheless, even among persons enrolled in large, private sector HCOs, relatively little is known about the sociodemographic and clinical characteristics associated with viral hepatitis testing and infection prevalence. For example, limited data suggest that only a fraction of viral hepatitis infections among patients within large HMOs—where access to care is relatively favorable—are identified [6]. To examine more fully these practices and to identify characteristics of persons in need of improved care and service, we determined characteristics associated with having a viral hepatitis test performed and with having a positive test result for HBV or HCV infection among persons enrolled in 4 US HCOs.
METHODS
Selection of Study Population
To ensure assessment of persons with recent interaction and sustained follow-up with the HCO (and, therefore, sufficient “risk” for being tested), we included in the analysis adults aged ≥18 years from the 4 participating HCOs (Geisinger Health System, Danville, Pennsylvania; Henry Ford Health System, Detroit, Michigan; Kaiser Permanente-Northwest, Portland, Oregon; Kaiser Permanente, Honolulu, Hawaii) of the HMO Research Network with: (1) at least 12 months of continuous enrollment at any time prior to 1 January 2009, and (2) at least 1 admission or outpatient provider, laboratory, or emergency department encounter from 1 January 2006 through 31 December 2008. In order to remove from the assessment of testing practices persons with prevalent, or previously documented, HBV or HCV infection, we excluded those with either a hepatitis B or hepatitis C International Classification of Diseases, Ninth Revision, diagnosis code within 6 months of their first encounter at the HCO.
Data Collection and Classification
Patient data collected from electronic medical records including age (age as of last encounter before 1 January 2009), gender, race/ethnicity, annual income (derived from census tract data based on zip code), and serum alanine aminotransferase (ALT) level (elevated values were relative to the upper limit of normal value specific to each laboratory that performed the test) were analyzed to determine the frequency and factors associated with testing for and infection with HBV and HCV. Data were collected from the date of the patient’s earliest health plan enrollment through the last health plan encounter, ending 31 December 2008. Electronic data were available retrospectively to 1 January 1997 from the Detroit and Portland sites, 1 January 1998 from the Honolulu site, and 1 January 2001 from the Danville site.
Persons classified as “tested” were those who, as of 31 December 2008, had at least 1 test performed during HCO enrollment for HBV surface antigen (HBsAg), immunoglobulin G antibody to HCV (anti-HCV), or a qualitative or quantitative test for HBV DNA or HCV RNA. Those classified “infected” with HBV or HCV had at least 1 positive result during HCO enrollment for any of the aforementioned tests.
The ethical conduct of the study underwent review by the institutional review boards of each local site and the US Centers for Disease Control and Prevention (CDC).
Estimate of the Number of Identified vs Number of Expected HBV and HCV Infections
To estimate the number of HBV and HCV infections “expected” in the 4 HCOs, we applied race-specific (for HBV) and age-specific (for HCV) national prevalence estimates according to the demographic composition of the HCO cohort. The prevalence estimates applied were obtained from the National Health and Nutrition Examination Survey (NHANES) and closely matched the time period from which our patients were selected (2006–2008) [12, 13].
Statistical Analysis
Univariate analysis was performed initially to study characteristics associated with having a test performed and with having a positive test result for HBV and HCV infection, followed by multivariate modeling using a logistic regression model. The final model retained variables with a P value < .05. To adjust for differences among the sites in the variable availability of retrospective electronic data, all analyses were adjusted by HCO site and member length of coverage. To study the impact of ALT elevations on having a test performed and for testing positive for infection, a similar multivariate model was developed but limited to persons with an initial elevated ALT result recorded before HBV or HCV infection testing. All analyses were performed using SAS, version 9.2 (SAS Institute Inc, Cary, NC).
RESULTS
Study Population
From 1 January 2006 through 31 December 2008, there were 1 248 558 adults enrolled in the 4 HCOs. Of these, 867 589 (69.5%) had at least 12 months of continuous follow-up any time before 2009 and at least 1 clinical encounter during 2006–2008. Among these 867 589 adults, the median length of cumulative enrollment was 87 months (interquartile range [IQR], 36–132). Most were under age 60 years (75%), female (54%), white (51%), and had an annual income based on census tract of between $30 000 and $75 000 (71%). Of these persons, 866 886 and 865 659 had no previous hepatitis B- or hepatitis C-related diagnosis code within 6 months of their first HCO encounter, respectively.
Testing for and Prevalence of HBV and HCV Infection
Among persons without a previous hepatitis B diagnosis code, 18.8% were tested at least once for HBV infection during enrollment; 1.4% of all persons tested had at least 1 positive result. Persons tested for HBV infection had a median cumulative enrollment of 102 months (IQR, 51–132) vs 83 months (IQR, 36–132) for persons not tested for HBV infection.
Of those without a previous hepatitis C diagnosis code, 12.7% were tested at least once for HCV infection during HCO enrollment; 5.5% of persons tested had at least 1 positive result. Persons tested for HCV infection had a median cumulative enrollment of 111 months (IQR, 54–139) vs 84 months (IQR, 36–132) for persons not tested for HCV infection.
Characteristics Associated With Being Tested and Testing Positive for HBV Infection
The sociodemographic characteristics associated with having a test performed and for testing positive for HBV infection, adjusted for length of HCO enrollment, are shown in Table 1. Among all characteristics, the proportion of persons tested for HBV infection ranged from 9.5% (those aged ≥80 years) to 29.7% (those of black or other race). Characteristics independently associated with being tested for HBV infection included female gender (adjusted odds ratio [aOR] 1.72, relative to males), age group 30–39 years (aOR 1.53, relative to age <30 years), black race (aOR 1.24, relative to whites), and Asian race (aOR 1.12, relative to whites).
Table 1.
Multivariate Analysis of Factors Associated With Testing and Testing Positive for Hepatitis B Virus Infection Among 866 886 Adults With No Previous Hepatitis B Diagnosis
| Characteristic | Total (N) | Tested N (%) | Adjusted OR for Being Tested (95% CI) | Tested Positive N (% Tested) | Adjusted OR for Testing Positive (95% CI) |
|---|---|---|---|---|---|
| Age Group (years) | |||||
| <30 | 161 894 | 34 314 (21.2%) | Ref | 344 (1.0%) | Ref |
| 30–39 | 143 699 | 40 453 (28.2%) | 1.53 (1.50–1.56) | 476 (1.2%) | 1.16 (1.01–1.33) |
| 40–49 | 163 074 | 31 497 (19.3%) | 0.84 (0.82–0.85) | 548 (1.7%) | 1.57 (1.37–1.80) |
| 50–59 | 173 821 | 27 642 (15.9%) | 0.61 (0.60–0.62) | 533 (1.9%) | 1.75 (1.52–2.02) |
| 60–69 | 116 030 | 16 673 (14.4%) | 0.51 (0.50–0.52) | 284 (1.7%) | 1.54 (1.31–1.82) |
| 70–79 | 63 904 | 7801 (12.2%) | 0.41 (0.40–0.42) | 83 (1.2%) | 0.94 (0.74–1.21) |
| >80 | 44 464 | 4206 (9.5%) | 0.29 (0.28–0.30) | 58 (1.4%) | 1.11 (0.83–1.48) |
| Missing/Unknown | 0 | — | — | — | — |
| Gender | |||||
| Male | 395 684 | 56 347 (14.2%) | Ref | 1146 (2.0%) | Ref |
| Female | 471 177 | 106 236 (22.6%) | 1.72 (1.70–1.74) | 1180 (1.1%) | 0.65 (0.62–0.69) |
| Unknown | 25 | 3 (12.0%) | 2.08 (0.61–7.07) | 0 | — |
| Race | |||||
| White | 439 545 | 78 742 (17.9%) | Ref | 483 (0.6%) | Ref |
| Black | 60 828 | 18 048 (29.7%) | 1.24 (1.21–1.26) | 207 (1.2%) | 1.82 (1.52–2.19) |
| Asian | 60 097 | 16 044 (26.7%) | 1.12 (1.10–1.15) | 671 (4.2%) | 6.33 (5.53–7.24) |
| American Indian/Alaska Native | 3936 | 890 (22.6%) | 1.01 (0.93–1.09) | 9 (1.0%) | 1.56 (0.79–2.99) |
| Native Hawaiian/Pacific Islander | 28 492 | 7294 (25.6%) | 0.89 (0.86–0.92) | 180 (2.5%) | 3.64 (2.99–4.42) |
| Othera | 17 206 | 5105 (29.7%) | 1.28 (1.23–1.33) | 54 (1.1%) | 2.38 (1.73–3.28) |
| Unknowna | 256 782 | 36 463 (14.2%) | 0.56 (0.55–0.57) | 722 (2.0%) | 2.90 (2.48–3.38) |
| Ethnicity | |||||
| Not Hispanic | 493 135 | 96 936 (19.7%) | Ref | 1,207 (1.3%) | Ref |
| Hispanic | 18 501 | 5660 (30.6%) | 1.09 (1.07–1.11) | 49 (0.9%) | 1.22 (1.13–1.33) |
| Unknown | 355 250 | 59 990 (16.9%) | 1.41 (1.35–1.46) | 1070 (1.8%) | 1.20 (0.98–1.46) |
| Annual Incomeb | |||||
| <$30 K | 59 450 | 12 781 (21.5%) | Ref | 190 (1.5%) | Ref |
| $30–49 K | 316 496 | 61 859 (19.5%) | 0.97 (0.95–0.99) | 888 (1.4%) | 0.82 (0.75–0.90) |
| $50–74 K | 298 588 | 58 453 (19.6%) | 0.94 (0.92–0.96) | 894 (1.5%) | 0.66 (0.60–0.73) |
| ≥$75 K | 74 946 | 15 859 (21.2%) | 0.96 (0.93–0.98) | 285 (1.8%) | 0.56 (0.49–0.64) |
| Unknown | 117 406 | 13 634 (11.6%) | 0.78 (0.74–0.82) | 69 (0.5%) | 0.82 (0.65–1.03) |
| ALT Levelc | |||||
| 1 Elevated ALT | 43 321 | 6444 (14.9%) | Ref | 45 (0.7%) | Ref |
| >2 Elevated ALTs | 67 370 | 28 410 (42.2%) | 4.59 (4.44–4.74) | 489 (1.7%) | 2.16 (1.58–2.96) |
Abbreviations: ALT, alanine aminotransferase; CI, confidence interval; OR, odds ratio; Ref, referent.
“Other” race refers to persons who self-identify as being of a race other than white/Caucasian, black/African American, Asian, American Indian/Alaska Native, Native Hawaiian/Pacific Islander, or who claim race as “other.” “Unknown” race refers to persons who self-identify as being of mixed or multiple racial backgrounds or who decline to self-identify with any race or to persons with race data missing.
Based on census tract geocode.
Results from a separate multivariate model developed to study the impact of ALT elevations on having a test performed and for testing positive for infection. This separate model was limited to persons with an initial elevated ALT result recorded before hepatitis B virus (HBV) infection testing. Persons tested for HBV infection had a median cumulative enrollment of 102 months (interquartile range [IQR], 51–132) vs 83 months (IQR, 36–132) for persons not tested for HBV infection.
The proportion of persons tested who had a positive test result for HBV infection ranged from 0.6% (white race) to 4.2% (Asian race). Characteristics independently associated with testing positive included Asian race (aOR 6.33, relative to whites), Native Hawaiian/Pacific Islander race (3.64, relative to whites), unknown race (aOR 2.90, relative to whites), and other race (aOR 2.38, relative to whites).
Characteristics Associated With Being Tested and Testing Positive for HCV Infection
Sociodemographic characteristics associated with having a test and with testing positive for HCV infection adjusted for length of HCO enrollment are shown in Table 2. The proportion of persons tested for HCV infection ranged from 8.5% (those aged ≥80 years) to 22.4% (persons of black race). The characteristics independently associated with being tested for HCV infection were age group 30–39 years (aOR 1.36, relative to age <30 years), black race (aOR 1.31, relative to whites), age group 40–49 years (aOR 1.28, relative to age <30 years), age group 50–59 years (aOR 1.26, relative to age <30 years), and American Indian/Native Alaskan race (aOR 1.25, relative to whites).
Table 2.
Multivariate Analysis of Factors Associated With Testing and Testing Positive for Hepatitis C Virus Infection Among 865 659 Adults With No Previous Hepatitis C Diagnosis
| Characteristic | Total (N) | Tested N (%) | Adjusted OR for Being Tested (95% CI) | Tested Positive N (%) | Adjusted OR for Testing Positive (95% CI) |
|---|---|---|---|---|---|
| Age Group (years) | |||||
| <30 | 161 877 | 17 132 (10.6%) | Ref | 355 (2.1%) | Ref |
| 30–39 | 143 665 | 18 966 (13.2%) | 1.36 (1.33–1.39) | 407 (2.2%) | 0.98 (0.85–1.14) |
| 40–49 | 162 765 | 22 469 (13.8%) | 1.28 (1.25–1.31) | 1328 (5.9%) | 2.88 (2.56–3.25) |
| 50–59 | 173 127 | 25 067 (14.5%) | 1.26 (1.23–1.28) | 2766(11.0%) | 6.04 (5.38–6.77) |
| 60–69 | 115 877 | 15 077 (13.0%) | 1.06 (1.03–1.08) | 798 (5.3%) | 2.86 (2.51–3.26) |
| 70–79 | 63 878 | 7075 (11.1%) | 0.84 (0.81–0.86) | 246 (3.5%) | 1.98 (1.67–2.35) |
| >80 | 44 470 | 3789 (8.5%) | 0.61 (0.59–0.63) | 108 (2.9%) | 1.69 (1.36–2.12) |
| Missing/Unknown | 0 | – | – | – | – |
| Gender | |||||
| Male | 394 953 | 50 225 (12.7%) | Ref | 3474 (6.9%) | Ref |
| Female | 470 681 | 59 347 (12.6%) | 0.96 (0.95–0.97) | 2534 (4.3%) | 0.65 (0.62–0.69) |
| Unknown | 25 | 3 (12.0%) | 1.41 (0.42–4.73) | 0 (0%) | – |
| Race | |||||
| White | 438 745 | 55 787 (12.7%) | Ref | 2876 (5.2%) | Ref |
| Black | 60 693 | 13 587 (22.4%) | 1.31 (1.28–1.35) | 909 (6.7%) | 1.70 (1.54–1.87) |
| Asian | 60 177 | 9025 (15.0%) | 1.10 (1.07–1.13) | 391 (4.3%) | 0.88 (0.78–0.99) |
| American Indian/Alaska Native | 3913 | 596 (15.2%) | 1.25 (1.14–1.36) | 52 (8.7%) | 1.51 (1.12–2.02) |
| Native Hawaiian/Pacific Islander | 28 494 | 3999 (14.0%) | 0.97 (0.93–1.00) | 175 (4.4%) | 1.00 (0.84–1.19) |
| Othera | 17 180 | 2517 (14.7%) | 1.15 (1.10–1.21) | 129 (5.1%) | 0.86 (0.70–1.08) |
| Unknowna | 256 457 | 24 064 (9.4%) | 0.79 (0.77–0.81) | 1476 (6.1%) | 0.95 (0.87–1.04) |
| Ethnicity | |||||
| Not Hispanic | 492 387 | 70 459 (14.3%) | Ref | 3532 (5.0%) | Ref |
| Hispanic | 18 475 | 2684 (14.5%) | 0.95 (0.93–0.97) | 147 (5.5%) | 1.22 (1.13–1.33) |
| Unknown | 354 797 | 36 432 (10.3%) | 1.11 (1.06–1.17) | 2329 (6.4%) | 1.20 (0.98–1.46) |
| Annual Incomeb | |||||
| <$30 K | 59 317 | 9053 (15.3%) | Ref | 689 (7.6%) | Ref |
| $30–49 K | 315 981 | 40 633 (12.9%) | 0.93 (0.90–0.95) | 2613 (6.4%) | 0.82 (0.75–0.90) |
| $50–74 K | 298 259 | 37 306 (12.5%) | 0.86 (0.84–0.89) | 1908 (5.1%) | 0.66 (0.60–0.73) |
| ≥$75 K | 74 914 | 10 503 (14.0%) | 0.84 (0.82–0.87) | 430 (4.1%) | 0.56 (0.49–0.64) |
| Unknown | 117 188 | 12 080 (10.3%) | 0.71 (0.67–0.75) | 368 (3.1%) | 0.82 (0.65–1.03) |
| ALT Levelc | |||||
| 1 ALT Elevation | 42 955 | 6397 (14.9%) | Ref | 190 (3.0%) | Ref |
| ≥2 ALT Elevations | 65 778 | 28 871 (43.9%) | 4.97 (4.81–5.14) | 2359 (8.2%) | 2.96 (2.54–3.45) |
Abbreviations: ALT, alanine aminotransferase; CI, confidence interval; OR, odds ratio; Ref, referent.
“Other” race refers to persons who self-identify as being of a race other than white/Caucasian, black/African American, Asian, American Indian/Alaska Native, Native Hawaiian/Pacific Islander, or who claim race as “other.” “Unknown” race refers to persons who self-identify as being of mixed or multiple racial backgrounds or who decline to self-identify with any race or to persons with race data missing.
Based on census tract geocode.
Results from a separate multivariate model developed to study the impact of ALT elevations on having a test performed and for testing positive for infection. This separate model was limited to persons with an initial elevated ALT result recorded before hepatitis C virus (HCV) infection testing. Persons tested for HCV infection had a median cumulative enrollment of 111 months (interquartile range [IQR], 54–139) vs 84 months (IQR, 36–132) for persons not tested for HCV infection.
The proportion of persons testing positive for HCV infection ranged from 2.1% (age group <30 years) to 11.0% (age group 50–59 years). The sociodemographic characteristics independently associated with testing positive were age related (aOR 6.04 for age group 50–59 years, aOR 2.88 for age group 40–49 years, aOR 2.86 for age group 60–69 years, relative to age <30 years).
Testing Based on Elevated ALT Levels
To study the impact of ALT elevation on having a test performed and for testing positive for infection, we used a separate multivariate model limited to persons with an initial elevated ALT result recorded before HBV (n = 110 691) or HCV (n = 108 693) infection testing (Tables 1 and 2). Among adults with ≥2 elevated ALT levels, 42.2% were later tested for HBV (aOR 4.59, relative to 1 ALT elevation) and 43.9% were later tested for HCV infection (aOR 4.97, relative to 1 ALT elevation). Of those tested after ≥2 elevated ALT levels, 1.7% were positive for HBV (aOR 2.16, relative to persons with 1 ALT elevation) and 8.2% were positive for HCV infection (aOR 2.96, relative to persons with 1 ALT elevation).
Estimate of Identified vs Expected HBV and HCV Infections
As NHANES prevalence estimates are adjusted either by race or age, but not both, we applied race-specific HBsAg prevalence from NHANES to estimate the number of expected HBV infections in our cohort (Figure 1) and applied age-specific anti-HCV prevalence from NHANES to estimate the number of expected (past and present) HCV infections (Figure 2). As we assumed for our estimate that all adults with previous hepatitis B or C diagnosis codes were truly prevalent chronic infections, we estimated conservatively that at least 21.1% of HBV and 43.1% of HCV infections in the study population remained unidentified.
Figure 1.
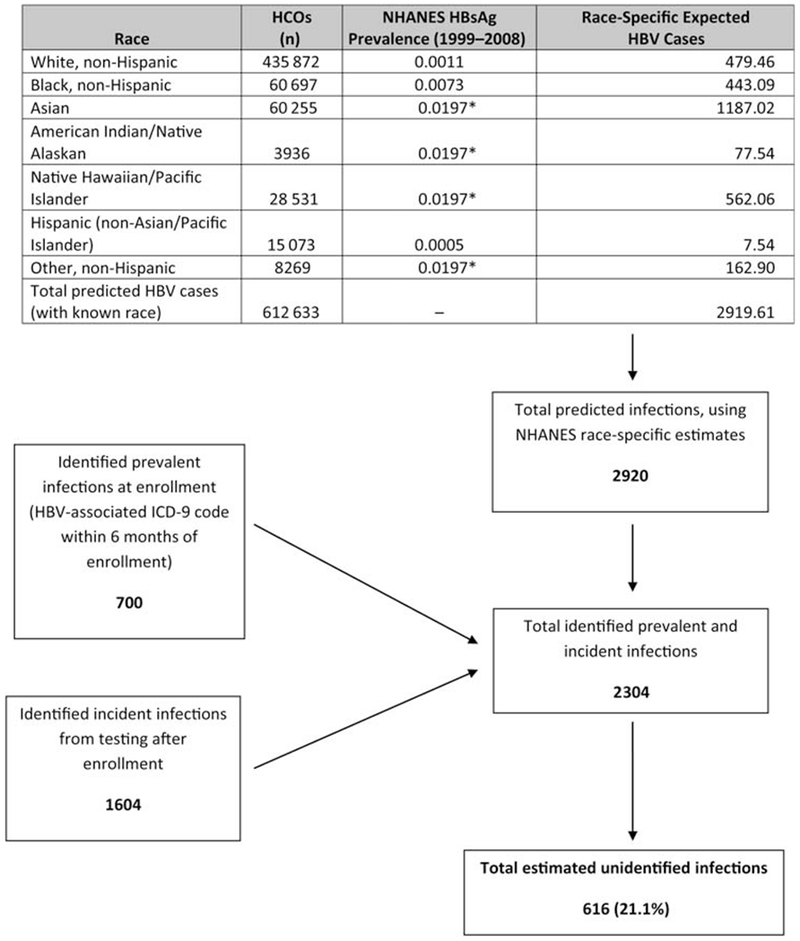
Estimated proportion of unidentified hepatitis B virus (HBV) infections among persons enrolled during 2006–2008 in 4 US healthcare organizations. Race-specific HBV surface antigen (HBsAg) prevalence from the National Health and Nutrition Examination Survey (NHANES) was used to estimate the number of expected HBV infections. Limiting analysis to the 71% of persons with known race, the total number identified with HBV infection was the sum of the number found through testing (1604 incident infections) and the number initially excluded from the testing frequency analysis due to a hepatitis B diagnosis code (700 prevalent infections), or approximately 2304 infections. Using NHANES race-specific estimates (where HBsAg prevalence for NHANES “other” race* corresponds to Asians, Native Hawaiian/Pacific Islanders, and American Indian/Alaska Natives in our cohort), approximately 2920 persons would be expected to test positive for HBsAg. Therefore, 616 (21.1%) of 2920 expected HBV infections in our cohort were unidentified. Abbreviations: HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCOs, healthcare organizations; ICD-9, International Classification of Diseases, Ninth Revision; NHANES, National Health and Nutrition Examination Survey.
Figure 2.
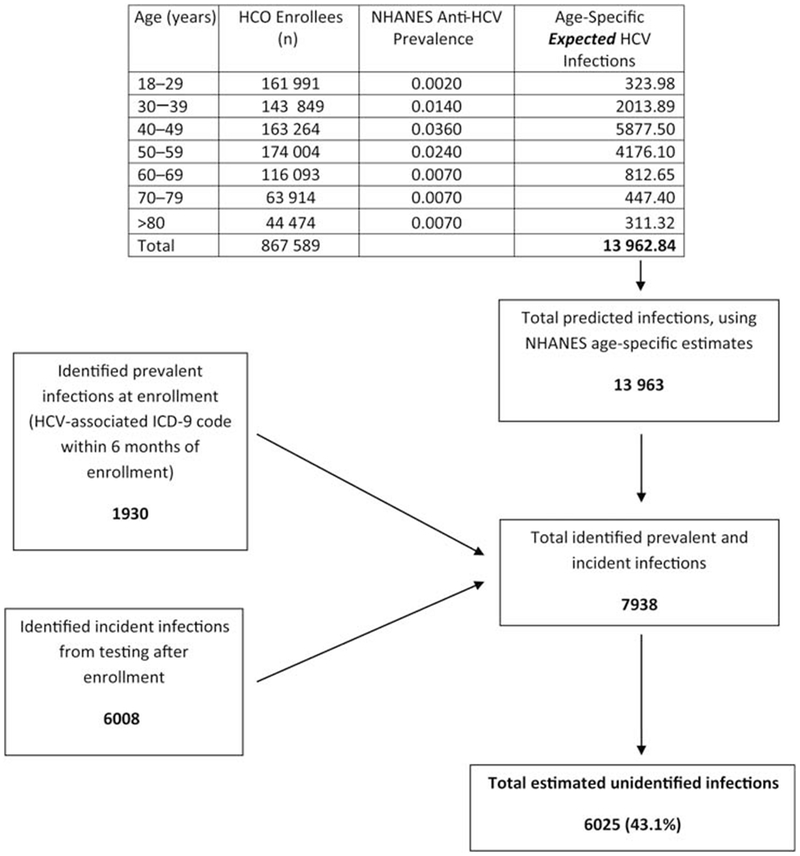
Estimated proportion of unidentified hepatitis C virus (HCV) infections among 867 589 adults enrolled during 2006–2008 in 4 US healthcare organizations. Age-specific anti-HCV prevalence from the National Health and Nutrition Examination Survey (NHANES) was used to estimate the number of expected HCV infections. The total number of persons identified with HCV infection was the sum of the number identified through testing (6008) and the number initially excluded from the testing frequency analysis due to a hepatitis C diagnosis code (1930), or approximately 7938 infections. Using age-specific NHANES estimates of HCV infection prevalence, the total number of persons predicted to test positive for anti-HCV in the cohort was approximately 13 963. Therefore, approximately 6025 (43.1%) of 13 963 HCV infections were unidentified. Abbreviations: Anti-HCV, IgG antibody to hepatitis C virus; HCO, healthcare organizations; HCV, hepatitis C virus; ICD-9, International Classification of Diseases, Ninth Revision; NHANES, National Health and Nutrition Examination Survey.
DISCUSSION
We found that among adults in 4 US HCOs with a median length of follow-up >7 years, approximately 1 in 5 were tested for HBV infection and 1 of 8 for HCV infection. Of these, a small minority tested positive for infection—1.4% for HBV and 5.5% for HCV. Based on NHANES-specific prevalence estimates applied to the composition of our cohort (also a civilian, noninstitutionalized population), a substantial proportion of HBV (one-fifth) and HCV (one-half) infections had not been identified. Because we had neither risk factor nor country of birth data, we could not identify the persons who “should” have been tested according to guidelines. However, our study suggests that irrespective of risk factor and country of birth information, there was a sizable gap between the number of HBV and HCV infections found and the number expected to be present in this large population of persons with access to care who were enrolled in 4 typical US HCOs.
Some persons in our cohort may have been vaccinated for hepatitis B or had tested negative for HBV or HCV infection in the past, in another healthcare setting prior to our period of study, and arguably might not “need” to be tested again. After testing negative for previous or chronic infection, persons at risk for HBV infection should be vaccinated. Because we only captured laboratory reports created within the participating HCOs, we could not identify persons who provided a verbal report of previous testing or vaccination to their HCO provider. Nonetheless, such persons may have been tested in the past because of risk factors and therefore might benefit from repeat testing because of ongoing risk (as with HIV testing, in some instances) or simply to have a documented test result in the current medical record.
The CDC and the American Association for the Study of Liver Diseases (AASLD) guidelines recommend testing persons with unexplained abnormal liver-enzyme tests for HBV and HCV infection [1]. The proportion of persons tested increased with the number of abnormal ALT levels accrued, that is, persons with ≥2 elevated ALT levels were more frequently tested than those with 1 elevated ALT level (and the odds of being tested and of testing positive were 4–5 and 2–3 times higher, respectively, for those with only 1 elevation vs ≥2 elevations). Nonetheless, among those with unknown infection status and ≥2 elevated ALT levels, fewer than 45% were tested for HBV or HCV infection. Many clinicians may attribute abnormal ALT levels to other causes such as alcohol use or medications and not immediately consider viral hepatitis. Asians and Native Hawaiian/Pacific Islanders were most likely to test positive for HBV infection, whereas persons aged 40–69 years were most likely to test positive for HCV infection.
Although the incidence of acute hepatitis B in the United States has declined substantially in recent years, the high prevalence of chronic hepatitis B among Asian Americans and Native Hawaiian/Pacific Islanders appears to have remained relatively constant, most having been born or having had a mother born in HBV-endemic regions [14, 15]. CDC guidelines recommend HBV infection screening for pregnant women, persons with high-risk behavior, and those born in Asia, Africa, and other geographic areas where HBsAg prevalence exceeds 2% [16]. In our cohort, we found that a comparatively high proportion of females (23%) and persons aged 30–39 years (28%) were tested, consistent with recommendations for prenatal hepatitis B testing. Persons of Asian or Native Hawaiian/Pacific Islander descent were tested more frequently for HBV infection than whites (26% combined vs 18%) but less frequently than blacks (30%) or those of “other” race (30%; persons of “other” race self-identified with a race other than white/Caucasian, black/African American, Asian, American Indian/Native Alaskan, Native Hawaiian/Pacific Islander). Given the strong association between Asian/Native Hawaiian/Pacific Islander race and testing positive for HBV infection, heightened attention to persons of such descent, particularly among those born outside the United States, is essential, irrespective of healthcare setting, in order to improve the identification of persons with HBV infection in the general population.
In 1998, the CDC recommended a process of screening persons for HCV risk factors followed by laboratory testing for those potentially exposed to HCV (risk-based approach) [17]. These recommendations were endorsed by a number of organizations, including AASLD, the Infectious Diseases Society of America, and the American College of Physicians [18, 19]. Improved strategies are needed, however, to determine the optimal approaches for reaching persons who might not identify themselves as being at risk for HCV infection. Persons at risk through limited or occasional drug use, particularly in the remote past, might not be aware of their risk or might not typically receive services in settings where viral hepatitis screening is performed, such as HIV counseling and testing sites and treatment programs for drugs and sexually transmitted diseases. Strategies incorporating the consideration of increased ALT levels have also been suggested in order to enhance identification of persons with HCV infection [20, 21], but such an approach also has limitations. Approximately 30% of HCV-infected persons will have persistently normal ALT levels despite the progression of hepatic fibrosis [22]. Furthermore, there is a lack of standardization of normal limits in laboratory ALT testing, particularly with regard to age, race, gender, and body mass index [23, 24]. A more recent suggestion is that age-based, birth cohort screening (of baby boomers) would provide a more cost-effective approach than risk-based screening because it would identify HCV infections earlier (ie, before the onset of chronic liver disease) and thereby reduce expenses related to advanced liver disease [25]. Although our analysis did not include a cost effectiveness component, we did find that the factor most strongly associated with testing positive for HCV infection was being in the age group of 40–69 years.
Our study had the following limitations. Persons tested for HBV or HCV infection had approximately 2 more years of cumulative enrollment than did persons not tested. Consequently, there may have been greater opportunity for those persons to be tested simply on the basis of greater “exposure” to care and follow-up. However, even among persons not tested, the median length of enrollment was approximately 7 years, which is a reasonably sufficient period of exposure for assessment and testing. Furthermore, because of the possibility of confounding, we adjusted for length of enrollment in the multivariate analysis to determine demographic and clinical factors associated with having a test performed and for testing positive. From a case definition standpoint, we used a single HBsAg or HBV DNA and a single anti-HCV or HCV RNA result to define testing for and infection with HBV or HCV, respectively, thereby including both past (resolved) and current infections. We could have misclassified some acute or resolved infections and overestimated the number of chronic infections in the cohort identified through testing. However, if we had, we would have overestimated the number of identified infections and underestimated the number of unidentified HCV and HBV infections, resulting in a greater fraction of unidentified infections than we actually predicted. African Americans, who have a higher prevalence of HCV infection than the rest of the US population, and Hispanics were underrepresented in our cohort, whereas persons of Asian and Native Hawaiian/Pacific Island descent, who have generally higher prevalence of HBV infection than the rest of the US population, were over-represented in our cohort. We do not purport that the estimates of testing practices and infection prevalence in our cohort are necessarily reflective of all private and public healthcare systems in the US. However, our 4 study sites, which had more than 1.2 million adults enrolled during the entire follow-up period, are largely typical of other HCOs and are located in geographically disparate regions in the US. Finally, use of NHANES data to estimate the expected number of infections in our cohort is limited because we could not adjust our expected number of cases for both age and sex, and because the categories of race in NHANES and our cohort were not the same.
In summary, we found that even in these HCOs, which provided comprehensive care, many who had 2 or more elevated ALT levels were not tested for HBV and HCV infection. Asian race and being middle-aged were independently associated with testing positive for HBV and HCV infection, respectively. Thus, although implementation of the healthcare reform legislation of 2010 may improve access to care, more aggressive policies in hepatitis testing to identify all infected persons are warranted.
Acknowledgments
Financial support. CHeCS was funded by the CDC Foundation, which received grants from Abbott Laboratories; Genentech, A Member of the Roche Group; Janssen Pharmaceutical Companies of Johnson & Johnson; and Vertex Pharmaceuticals.
Appendix
The CHeCS investigators include the following investigators and sites: Scott D. Holmberg, Eyasu H. Teshale, Philip R. Spradling, and Anne C. Moorman, Division of Viral Hepatitis, National Centers for HIV, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention, Atlanta, Georgia; Stuart C. Gordon, David R. Nerenz, Mei Lu, Lois Lamerato, Loralee B. Rupp, Nonna Akkerman, Nancy Oja-Tebbe, Chad Cogan, and Dana Larkin, Henry Ford Health System, Detroit, Michigan; Joseph A. Boscarino, Zahra S. Daar, Joe B. Leader, and Robert E. Smith, Geisinger Health System, Danville, Pennsylvania; Cynthia C. Nakasato, Vinutha Vijayadeva, Kelly E. Sylva, John V. Parker, and Mark M. Schmidt, Kaiser Permanente-Hawaii, Honolulu, Hawaii; Emily M. Henkle, Tracy L. Dodge, and Erin M. Keast, Kaiser Permanente-Northwest, Portland, Oregon.
Footnotes
See Appendix.
Ethical considerations. The investigation followed the guidelines of the US Department of Health and Human Services regarding protection of human subjects. The study protocol was approved and renewed annually by each participating institution’s institutional review board.
Potential conflicts of interest. S. C. G. receives grant/research support from Abbott Pharmaceuticals, Anadys Pharmaceuticals, Bristol-Myers Squibb, Conatus, Eiger Biopharmaceuticals, Inc, Exalenz BioScience, Gilead Pharmaceuticals, GlaxoSmithKline, GlobeImmune, Intercept Pharmaceuticals, Merck, Roche Pharmaceuticals, Tibotec, Vertex Pharmaceuticals, and Zymogenetics; serves as a consultant for Achillion, Bristol-Myers Squibb, CVS Caremark, Gilead Pharmaceuticals, Merck, Salix Pharmaceuticals, Johnson and Johnson, and Vertex; serves on the Data Monitoring Board for Tibotec; and serves as a speaker for Bayer, Gilead, Roche, Merck, and Vertex. M. L. receives grant support to institution from Henry Ford Health System. J. A. B. has consulted for Pfizer and Janssen, and receives grant support from NIH. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.IOM (Institute of Medicine). Hepatitis and liver cancer: a national strategy for prevention and control of hepatitis B and C. Washington, DC: The National Academies Press, 2010. [PubMed] [Google Scholar]
- 2.Kim WR, Terrault NA, Pedersen RA, et al. Trends in waitlist registration for liver transplantation for viral hepatitis in the US. Gastroenterology 2009; 137:1680–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC. Viral hepatitis: statistics and surveillance, 2009. Available at: http://www.cdc.gov/hepatitis/Statistics.htm. Accessed 19 May 2011.
- 4.Hagan H, Campbell J, Thiede H, et al. Self-reported hepatitis C virus antibody status and risk behavior in young injectors. Public Health Reports 2006; 121:710–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin SY, Chang ET, So SK. Why we should routinely screen Asian American adults for hepatitis B: a cross-sectional study of Asians in California. Hepatology 2007; 46:1034–40. [DOI] [PubMed] [Google Scholar]
- 6.Kim WR. The dawn of a new era: transforming our domestic response to hepatitis B and C. Activity 6: reports from today’s health care environment on the implementation of screening, diagnosis, and treatment recommendations. J Fam Pract (Supp) 2010; 59:S43–9. [PubMed] [Google Scholar]
- 7.Butt AA, Justice AC, Skanderson M, Rigsby MO, Good CB, Kwoh CK. Rate and predictors of treatment prescription for hepatitis C. Gut 2007; 56:385–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rousseau CM, Ioannou GN, Todd-Stenberg JA, et al. Racial differences in the evaluation and treatment of hepatitis C among veterans: a retrospective cohort study. Am J Pub Health 2008; 98:846–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trooskin SB, Navarro VJ, Winn RJ, et al. Hepatitis C risk assessment, testing, and referral for treatment in urban primary care: role of race and ethnicity. World J Gastroenetrol 2007; 13:1074–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanwal F, Schnitzler MS, Bacon BR, Hoang T, Buchanan PM, Asch SM. Quality of care in patients with chronic hepatitis C virus infection. Ann Intern Med 2010; 153:231–9. [DOI] [PubMed] [Google Scholar]
- 11.Groom H, Dieperink E, Nelson DB, et al. . Outcomes of a hepatitis C screening program at a large urban VA medical center. J Clin Gastroenterol 2008; 42:97–106. [DOI] [PubMed] [Google Scholar]
- 12.Ioannou GN. Hepatitis B virus in the United States: infection, exposure, and immunity rates in a nationally representative survey. Ann Intern Med 2011; 154:319–28. [DOI] [PubMed] [Google Scholar]
- 13.NCHS data brief number 27, March 2010, viral hepatitis. Available at: http://www.cdc.gov/nchs/data/databriefs. Accessed 5 June 2011.
- 14.Fleming DT, Zambrowski A, Fong F, et al. Surveillance programs for chronic viral hepatitis in three health departments. Public Health Rep 2006; 121:23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shepard CW, Simard EP, Finelli L, Fiore AE, Bell BP. Hepatitis B virus infection: epidemiology and vaccination. Epidemiol Rev 2006; 28:112–25. [DOI] [PubMed] [Google Scholar]
- 16.CDC. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR 2008; 57(RR-8):1–20. [PubMed] [Google Scholar]
- 17.CDC. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR 1998; 47(RR-19). [PubMed] [Google Scholar]
- 18.Alter MJ, Seeff LB, Bacon BR, Thomas DL, Rigsby MO, Di Bisceglie AM. Testing for hepatitis C virus infection should be routine for persons at increased risk for infection. Ann Intern Med 2004; 141:715–7. [DOI] [PubMed] [Google Scholar]
- 19.Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology 2009; 49:1335–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999–2002. Ann Intern Med 2006; 144:705–14. [DOI] [PubMed] [Google Scholar]
- 21.Arnold DT, Bentham LM, Jacob RP, Lilford RJ, Girling AJ. Should patients with abnormal liver function tests in primary care be tested for chronic viral hepatitis: cost minimization analysis based on a comprehensively tested cohort. BMC Family Practice 2011; 12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zapata R Clinical approach to the patient with chronic hepatitis C infection and normal aminotransferases. Ann Hepatol 2010; 9 (Suppl):72–9. [PubMed] [Google Scholar]
- 23.Brillanti S, Foli M, Gaiani S, Masci C, Miglioli M, Barbara L. Persistent hepatitis C viraemia without liver disease. Lancet 1993; 341: 464–5. [DOI] [PubMed] [Google Scholar]
- 24.Dufour DR, Lott JA, Nolte FS, Gretch DR, Koff RS, Seeff LB. Diagnosis and monitoring of hepatic injury. Performance characteristics of laboratory tests. Clin Chem 2000; 46:2027–49. [PubMed] [Google Scholar]
- 25.Deuffic-Burban S, Yazdanpanah Y. It is time to change the paradigm for hepatitis C virus testing. Clin Infect Dis 2012; 54:1272–4. [DOI] [PubMed] [Google Scholar]


