Abstract
Electroencephalographic (EEG) research has suggested relatively reduced brain activity in the left frontal and right posterior region trait-markers of depression. However, inconsistent results have been reported. Based on previous studies reporting the heart rate variability (HRV) as an index of emotional regulation, this study makes a novel investigation of the role of heart rate variability (HRV) as a moderator in the relationship between frontal and parietal alpha asymmetry and depression. Resting EEG (eyes open) was recorded in 38 patients with MDD and 34 healthy subjects. Frontal and parietal alpha asymmetries were calculated at total (8–12 Hz), high (10–12 Hz), and low (8–10 Hz) alpha frequency bands. Three vagally mediated HRV (vmHRV) components (LF, HF, and the LF/HF ratio) were calculated in the frequency domain. Relatively greater right parietal alpha activity significantly predicted the severity of depression only when HF was low (or the LF/HF ratio was high) at low alpha frequency band. The interaction effect of parietal alpha asymmetry and vmHRV remained significant after including anxiety score as a covariate. No moderation effect of vmHRV was found for frontal sites and other frequency bands, as well as healthy subjects. These findings suggest that vmHRV moderates the association between parietal alpha asymmetry at low frequency band and depression for MDD patients. We suggest that the interaction between parietal alpha asymmetry and vmHRV may be a biomarker of MDD.
Keywords: Clinical psychology, Neuroscience, Psychiatry
1. Introduction
Electroencephalography (EEG) studies have suggested abnormal alpha asymmetry, or the relative difference in EEG alpha power between homologous right and left electrodes [1], as a trait marker of vulnerability to depression. Specifically, reduced relative activity (inferred from relatively high alpha power [2]) in the left frontal [3, 4] and right posterior [5, 6] regions of the brain has been reported to characterize depression. According to Davidson's approach-withdrawal model [7], relatively greater left frontal activity is associated with approach-related positive emotion, whereas relatively greater right frontal activity is related to withdrawal-related negative emotion. Previous studies have found that relatively reduced left frontal activity distinguishes depressed and euthymic individuals with a history of depression from never-depressed individuals [3, 8]. Frontal alpha asymmetry was also associated with greater depression severity [9, 10], and was reported to be temporally stable and state-independent [11, 12]. In contrast, reduced right parietal activity is thought to reflect low emotion-related arousal [13, 14]. Relatively reduced right parietal activity has been found in previously depressed patients [5] as well as in children with both parents with major depressive disorder (MDD) [15].
In summary, alpha asymmetry has been discussed in the context of emotion, motivation, and arousal in affective disorders. However, several other studies have produced contradictory findings. For instance, no significant difference was found in frontal asymmetry between non-depressed and depressed individuals [16]. The inconsistency of findings is more apparent in parietal asymmetry, where a reverse pattern (i.e. greater right parietal activity) was reported in several studies [17, 18]. One of the possible explanations for the inconsistent results is the role of emotional regulation, or the ability to appropriately modulate emotion in response to environmental changes [19], in the relationship between alpha asymmetry and depression. Recent theories of affective disorders have emphasized the importance of emotional regulation in the onset and development of depression [19, 20]. As frontal and parietal asymmetries are suggested to be indicators of emotion responding and emotion-related arousal, respectively, one's ability to regulate emotion and arousal may have an effect on the relationship between alpha asymmetry and depression.
From this perspective, we suggest heart rate variability (HRV) as a variable that may moderate the association between alpha asymmetry and depression. HRV indicates a function of the autonomic nervous system, in which high HRV reflects healthy cardiac activity and low HRV reflects low parasympathetic activity and relatively excessive sympathetic activity [21, 22, 23]. In particular, previous research indicated that vagally mediated HRV (vmHRV) may reflect emotional and arousal regulation, higher vmHRV indicating better emotional regulation [24, 25]. Previous studies have reported a significant inverse correlation between self-reports on difficulties in emotional regulation and vmHRV [26, 27, 28]. Moreover, HRV has been suggested as an index of “flexible dynamic regulation of autonomic activity [29],” reflecting an integration of neural feedback mechanisms of central nervous system and autonomic nervous system. vmHRV is also associated with brain regions associated with emotional regulation, such as the amygdala and the ventromedial prefrontal cortex, and reduced vmHRV is reported in subjects with psychopathologies including depression [29, 30, 31, 32]. Adopting the view that vmHRV is an index of emotional regulation and interplays with the brain, the current study aimed to investigate the role of vmHRV on the relationship between alpha asymmetry and depression.
Furthermore, recent studies have suggested the need to consider anxiety comorbidity and alpha frequency range when examining alpha asymmetry. Depression is often associated with anxiety, which tends to show a different pattern of alpha asymmetry, or greater right parietal activity and greater left frontal activity [33, 34], compared to that of depression alone. In addition, narrow alpha sub-bands (low, 8–10 Hz; high, 10–12 Hz) may differ in functional characteristics and thus should be interpreted differently in clinical studies [35, 36, 37]. The low alpha band has been reported to be non-task specific and more sensitive to attention and cognitive performance, whereas the higher alpha band is more related to semantic or task-specific effects [38, 39]. However, the role of different alpha frequency ranges for alpha asymmetry in depression is unclear. Hence, the consideration of the severity of anxiety and the use of alpha sub-bands may account for the inconsistent results of the previous studies and may help further clarify the relationship between alpha asymmetry and depression. Furthermore, while previous studies on frontal and parietal asymmetry most commonly analyzed and reported the F3-F4 and P3-P4 component, this may be due to publication bias [40]. Other methodological differences such as length of measurement and sex-specific differences [41, 42] may have attributed to the previous inconsistent results as well.
In sum, the present study aimed to explore the interaction of alpha asymmetry with vmHRV in MDD patients and healthy controls. We analyzed alpha sub-bands to examine if they show different patterns in their relation to depression.
2. Methods
2.1. Participants
A total of 72 subjects between ages of 20 and 65 participated in this study. Patients with MDD were recruited from Ilsan Paik hospital and community mental health centers in South Korea. They were diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition [43], by board-certified psychiatrists, and had no history of psychotic symptoms or bipolar disorder. The healthy participants were volunteers from the local community, and had no current or previous diagnosis of mental health. Yet, it should be noted that volunteer bias may threaten the external validity and the participants may have been affected by factors specific to the local community. Only right-handed participants were included in the study, and those with epilepsy, impaired vision, brain injury, or other severe medical conditions were excluded. In addition, to control for the possible effect of heart disease on vmHRV, subjects with a history of heart disease were excluded from the study. The final sample included 38 patients diagnosed with MDD and 34 healthy subjects. MDD patients consisted of 28 women and 10 men with a mean age of 42.24 years (SD = 13.76) and mean education of 12.11 years (SD = 2.99). Healthy subjects consisted of 24 women and 10 men with a mean age of 37.37 (SD = 13.28) and mean education of 14.49 years (SD = 2.92).
Most of the MDD patients were being treated with antidepressants: es-citalopram (N = 19), venlafaxine (N = 6), bupropion (N = 3), sertraline (N = 3), paroxetine (N = 2), and fluoxetine (N = 2). Patients were also treated with one of the three types of benzodiazepine: .5–1 mg of lorazepam (N = 12), .25–1.5 mg of alprazolam (N = 11), and .125–.5 mg of etizolam (N = 10). All procedures were approved by the Institutional Review Board of Inje University Ilsan Paik Hospital (2015-03-212). Informed consent was obtained prior to participation.
2.2. Psychological assessment
The severity of depression was assessed by the Korean version of the Beck Depression Inventory (BDI)-II, a commonly used self-reported rating inventory that includes 21 items rated on a 4-point scale. The Korean version of BDI-II showed high Cronbach's alpha (α = .85) and test-retest reliability (r = .75) [44]. The internal consistency for the participants was excellent (α = .95). Anxiety was measured by the Korean version of the Beck Anxiety Inventory (BAI), a self-reported questionnaire comprising 21 items. BAI assessment showed internal consistency and test-retest reliability of .93 and .84, respectively [45]. The BAI Cronbach's alpha for the participants was .94.
2.3. Measures and analysis
2.3.1. EEG measures
Participants wore an electrode cap and were seated in a comfortable chair in a sound-attenuated room. EEG was recorded for 3 minutes at rest for both eyes open and eyes closed conditions. EEG signals were recorded using a NeuroScan SynAmps 2 amplifier (Compumedics, El Paso, TX, USA) from 62 Ag/AgCl surface electrodes mounted on a Quik-Cap (Compumedics, El Paso, TX, USA) according to the extended International 10–20 system. The electrode was referenced at Cz and the ground electrode was placed on the forehead. A vertical electrooculogram (EOG) was recorded using bipolar electrodes, one located above and one below the right eye. A horizontal EOG was recorded at the outer canthus of each eye. The impedance of the electrodes was maintained at less than 5 kΩ. EEG data were recorded with a 0.1–100-Hz bandpass filter at a sampling rate of 1,000 Hz, with 60 Hz noise removed using a notch filter.
The recorded EEG data were preprocessed using Scan 4.3 software (Compumedics, El Paso, TX, USA). Gross artifacts, such as movement artifacts, were rejected by visual inspection by a trained person with no knowledge of the origin of the data. In this study, eyes-open EEG data were used. No significant difference between eyes open and closed conditions have been reported in previous studies in frontal alpha asymmetry [4, 16, 42, 46]. For instance, a study for investigating EEG frontal alpha asymmetry in patients with mental illness and 1908 healthy controls revealed that frontal alpha asymmetry had equal distribution across individuals during both eyes-open and eyes-closed conditions [46]. Furthermore, Choi et al. (2011) [47] suggested that the effect of asymmetry neurofeedback training should be measured in the eyes open condition as they found a change in alpha asymmetric brain activity pattern only in the eyes-open condition. Thus, although the eyes-closed condition may be more broadly used, the eyes-open condition is equally appropriate for measuring alpha asymmetry.
The data were segmented into ∼ 2 s (2,048 points) epochs, and the epochs with signals exceeding ±100 μV on any of the 62 electrode sites were excluded from further analysis. A total of 30 epochs (∼60 s) were prepared for each participant. A Fast Fourier transformation was performed on the 62 electrodes to form the low (8–10 Hz), high (10–12 Hz), and total (8–12 Hz) frequency alpha bands [48]. We considered three paired sites for the frontal region (FP1-FP2, F3-F4, F7-F8) [40,49] and four for the parietal region (P1-P2, P3-P4, P5-P6, P7-P8) [6].
Alpha symmetry was defined as the lateral index by comparing the corresponding frequency band percentages of the left and right hemispheres [50, 51, 52]. This method includes measuring the difference between the two hemispheres divided by their sum, and multiplying the result by 100 (A = (Pleft – Pright)/(Pleft + Pright) × 100, where Pleft and Pright are the absolute powers of the corresponding frequency band in the appropriate brain electrode. A positive value indicates greater alpha or reduced brain activity in the left hemisphere, whereas a negative value indicates greater alpha or reduced brain activity in the right hemisphere.
2.3.2. HRV measures
A 5-min single-channel (3-lead) electrocardiogram (ECG) signal was measured. Participants were instructed to avoid smoking and caffeine intake 6 h prior to the measurement. The ECG electrode sensors were attached to the left and right wrists and left ankle. Prior to recordings, each subject was given approximately 5 minutes of rest in order to control for the environmental effects on heartbeat. Then, recordings were performed in seated position at complete rest using the SA-3000P HRV analyzer (Medicore Co., Ltd, Seoul, Korea). The analyzer detected signals at 500 Hz, and the ECG signal was amplified and digitized. A band-pass filter with default passband of 5–200 Hz was applied to the recorded ECG data for artifact rejection. The following vmHRV parameters were calculated by frequency domain spectral analysis: low-frequency power (LF; .04–0.15 Hz), high-frequency power (HF; .15–0.4 Hz), and LF/HF ratio. These parameters were chosen on the basis of previous studies indicating that low high-frequency and high LF/HF are associated with depression [53, 54]. HF is considered as an index of parasympathetic modulation [55], whereas LF is often proposed to reflect both sympathetic and parasympathetic activity [56].
2.4. Statistical analyses
Normality was first tested for each variable. Distributions with a skewness over 2.0 and a kurtosis over 7.0 are considered moderately non-normal [57]. All variables except LF/HF were within the range of a normal distribution. Therefore, LF/HF ratio was log transformed for further analysis. Covariate variables were determined using Pearson's correlation analysis. As a result, education, which had a significant effect on the dependent variable (r = .405, p = .012) was entered as a covariate in further analyses. Sex was also included as a covariate for its differential impact on alpha asymmetry [58, 59]; female tend to show stronger and consistent alpha asymmetry patterns than males.
For both MDD patients and healthy controls, the zero-order correlation of main variables revealed that several asymmetry indices at frontal and parietal areas were correlated. To reduce multicollinearity due to correlation between independent variables, mean centering was performed for all variables included in regression analysis. There was no other significant correlation among the main variables.
Three-step hierarchical regression analysis was performed for all subjects, and the patients and healthy subjects separately to examine the interaction effects of the alpha asymmetry and vmHRV indices on depression. Education and sex were entered as the covariates in the first block, alpha asymmetry and vmHRV index in the second block, and the interaction between alpha asymmetry index and vmHRV in the third block. The BDI total score was entered as the dependent variable. In addition, to investigate the effect of anxiety comorbidity, as described in previous studies [34, 60], we executed additional regression analysis including the total BAI score as the covariate.
In addition, we tested a model with depression as the independent variable and alpha asymmetry as the dependent variable to examine the possibility of depression affecting alpha asymmetry, instead of alpha asymmetry affecting depression. We also tested a model with depression as the independent variable and vmHRV as the dependent variable to examine if depression affects vmHRV. These analyses were done in order to ensure that our model best described the relationship among alpha asymmetry, vmHRV, and depression.
3. Results
3.1. Descriptive statistics
3.1.1. Demographic variables
Among a total of 72 participants, there were more women than men (52:20), and age of the participants ranged from 20 to 65. Healthy participants had significantly longer education years than MDD patients (p = .001). There were no significant group differences in sex and age. Mean BDI-II and BAI scores for MDD patients and healthy controls are presented in Table 1. MDD patients had significantly higher BDI (p < .001) and BAI (p < .001) scores.
Table 1.
The descriptive statistics of mean vmHRV and alpha asymmetry index for low, high, and total frequency bands at frontal and parietal lobes.
| MDD patients | Healthy subjects | |||||
|---|---|---|---|---|---|---|
| BDI-II | 19.76 ± 14.84 | 7.91 ± 5.26 | ||||
| BAI |
38.58 ± 13.51∗∗∗ |
18.40 ± 10.84∗∗∗ |
||||
|
Heart rate variability | ||||||
| HR | 76.57 ± 12.74 | 77.31 ± 10.31 | ||||
| HF | 115.09 ± 88.13∗ | 348.69 ± 386.77∗ | ||||
| Ratio | 2.06 ± 2.11 | 1.58 ± 1.07 | ||||
| RMSSD |
19.16 ± 7.57∗∗ |
29.68 ± 18.10∗∗ |
||||
| Alpha asymmetry at frontal lobe | ||||||
|
Low |
High |
Total |
Low |
High |
Total |
|
| FP1-2 | −.13 ± 7.77∗ | .06 ± 7.01∗ | −.01 ± 6.99∗ | −5.20 ± 8.69∗ | −4.66 ± 7.47∗ | −4.92 ± 7.44∗ |
| F3-4 | 2.24 ± 5.67∗ | 1.43 ± 4.74 | 1.83 ± 4.43∗ | −1.98 ± 10.02∗ | −0.85 ± 8.21 | −1.29 ± 8.19∗ |
| F7-8 |
2.65 ± 9.15 |
2.74 ± 7.48 |
2.73 ± 7.74 |
2.87 ± 12.50 |
1.23 ± 14.95 |
2.48 ± 12.72 |
|
Alpha asymmetry at parietal lobe | ||||||
|
Low |
High |
Total |
Low |
High |
Total |
|
| P1-2 | −.83 ± 12.07 | .88 ± 11.92 | .25 ± 11.77 | 3.52 ± 13.86 | −0.02 ± 18.77 | 0.90 ± 14.96 |
| P3-4 | .05 ± 10.72 | 1.74 ± 10.32 | 1.08 ± 10.19 | 1.78 ± 16.50 | −2.46 ± 21.94 | −1.03 ± 17.41 |
| P5-6 | −.16 ± 8.35 | 1.12 ± 8.73 | .54 ± 8.39 | −0.43 ± 17.94 | −3.04 ± 23.86 | −2.89 ± 19.66 |
| P7-8 | −1.35 ± 10.72 | −.82 ± 7.51∗ | −.30 ± 8.08∗ | −6.08 ± 21.35 | −10.26 ± 25.14∗ | −9.47 ± 23.46∗ |
Note. BDI = Beck's depression inventory; BAI = Beck's anxiety inventory; HR = Heart rate; HF = high frequency; RMSSD = Root Mean Square of the Successive Differences.
*p < .05, **p < .01, ***p < .001.
3.1.2. vmHRV measures
In general, descriptive statistics for vmHRV indexes were slightly lower than the published norm values (see [61]). The mean heart rate for all participants was 76.927, (SD = 11.565, range: 48–122), mean HF was 222, (SD = 294.397, range = 3.21–1674.43), mean LF/HF was 2.083(SD = 1.05, range .04–20.20), and mean RMSSD was 24.203 (SD = 11.565, range = 4.89–98.19). Consistent with previous studies (e.g. [62]), there was a strong correlation between RMSSD and HF (r = .84, p < .001). MDD patients had significantly lower HF (p = .003), and significantly lower RMSSD (p = .002) than healthy subjects.
3.1.3. EEG measures
MDD patients had significantly greater FP1-FP2 alpha asymmetry index score at total (F = 8.34, p = .005), low (F = 6.82, p = .011) and high (F = 7.61, p = .007) frequency bands, greater F3-F4 alpha asymmetry index at total (F = 4.16, p = .045) and low (F = 4.98, p = .029) frequency bands, and significantly lower P7-P8 alpha asymmetry index at total (F = 5.13, p = .027) and high (F = 6.72, p = .012) frequency band than healthy subjects. Mean HF and LF/HF ratio as well as the mean alpha asymmetry index at the frontal lobe and parietal lobe for each group are presented in Table 1.
3.2. Interaction between alpha asymmetry and vmHRV
For the frontal region, hierarchical regression analyses revealed no significant interaction effects of frontal alpha asymmetry index and vmHRV on the BDI score for total, MDD patients and healthy subjects. Specifically, no interactions between any of the frontal alpha asymmetry sites and vmHRV significantly predicted the BDI score.
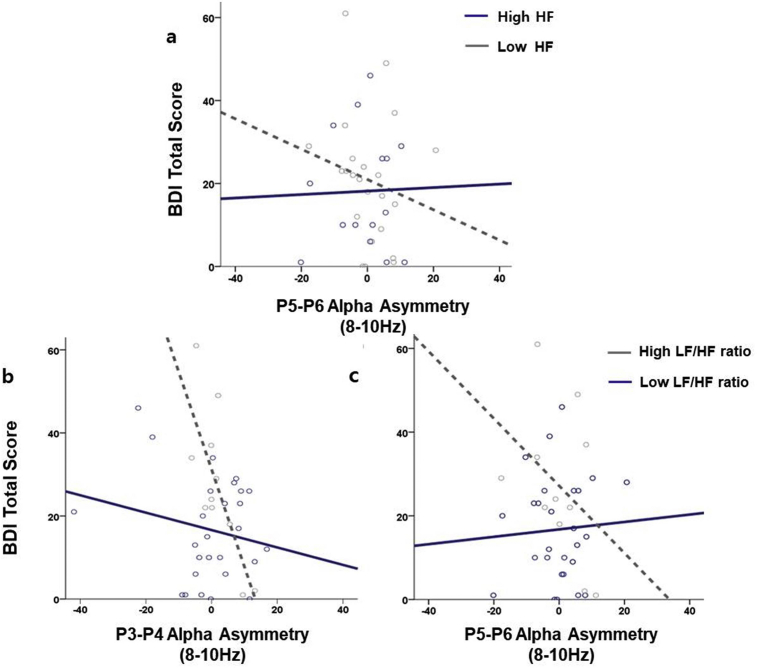
For the parietal region, the regression model (F (16) = 2.291, p = .038) of hierarchical regression analyses revealed significant interaction effect between low-band alpha asymmetry index at P5-P6 and HF (β = .485; p = .018), and marginally significant interactions between low-band alpha asymmetry index at P3-P4 and LF/HF (β = -.419; p = .074) and P5-P6 and LF/HF (β = .412; p = .072) on the BDI score for MDD patients (Table 2, Fig. 1). Additional regression analysis with the BAI score as the covariate showed that the interaction between parietal alpha asymmetry index at P3-P4 and LF/HF ratio remained statistically significant (β = -.548; p = .016). No significant results were found at the high and total alpha bands, or for total participants and healthy controls. Furthermore, the results were insignificant when alpha asymmetry index and vmHRV were entered as dependent variables and depression as the independent variable, demonstrating that the current model best describes the relationship among alpha asymmetry, vmHRV, and depression.
Table 2.
Summary of hierarchical regression analyses for parietal alpha asymmetry index at low frequency band and vmHRV variables predicting the BDI-II total score.
| Predicting variables | F-value | R2 | β | t-value | df | |
|---|---|---|---|---|---|---|
| Model 1 | 3.989** | .186 | 2 | |||
| Sex education | −.148 .381 |
−.960 2.467 |
||||
| Model 2 | 1.800 | .332 | 8 | |||
| P1-P2 | .285 | 1.547 | ||||
| P3-P4 | −.299 | −1.428 | ||||
| P5-P6 | .087 | .461 | ||||
| P7-P8 | −.159 | −.808 | ||||
| HF | −.071 | −.382 | ||||
| Ratio | .220 | 1.108 | ||||
| Model 3 | 2.291** | .636 | 16 | |||
| P1-P2 HF | .295 | .968 | ||||
| P3-P4 HF | .015 | .058 | ||||
| P5-P6 HF | .485** | 2.564 | ||||
| P7-P8 HF | −.035 | −.150 | ||||
| P1-P2 Ratio | −.076 | −.384 | ||||
| P3-P4 Ratio | −.419* | −1.878 | ||||
| P5-P6 Ratio | .412* | 1.892 | ||||
| P7-P8 Ratio | .065 | .301 |
Note. HF, high-frequency heart rate; Ratio, the LF/HF heart rate variability ratio.
*p < 1.0, **p < 0.5.
Fig. 1.
The effects of interactions between a) HF and P5-P6 asymmetry index, b) LF/HF ratio and P3-P4 asymmetry index, and c) LF/HF ratio and P5-P6 asymmetry index on the BDI-II total score at the low alpha band (8–10 Hz). The gray and blue lines indicate the line of best fit (i.e. line that best represents the data), while the gray and blue dots indicate individual data values.
4. Discussion
The primary aim of this study was to explore the effect of the interaction between alpha asymmetry and vmHRV on depression severity in both MDD patients and health subjects. Overall, our results indicate that the lower the HF (or the higher the LF/HF ratio), the greater the influence of parietal alpha asymmetry index of low frequency band on depression in MDD patients; a more negative asymmetry index refers to relatively greater right parietal alpha power or reduced right brain activity. The interaction effect of parietal alpha asymmetry and vmHRV remained statistically significant after including the BAI total score as a covariate. No significant results were found in healthy participants. Furthermore, no moderation effect of vmHRV was found for frontal sites and other frequency bands in both patients and healthy subjects. Nevertheless, there were significant group differences in frontal (FP1-FP2, F3-F4) and parietal (P7-P8) asymmetries between patient and healthy participants, corroborating the results of the previous studies suggesting that reduced left frontal alpha activity and reduced right parietal alpha activity distinguish depressed from non-depressed individuals [3, 8, 12, 60].
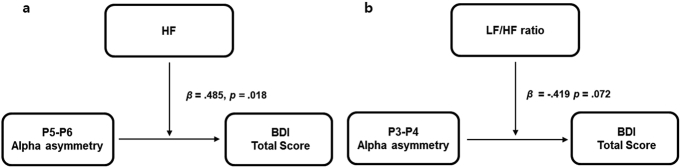
Our results suggest a role for vmHRV as a moderator in the relationship between parietal alpha asymmetry and depressive symptoms (Fig. 2). Specifically, higher HF or lower LF/HF ratios significantly weakened the negative relationship between parietal alpha asymmetry index of the low frequency band (P5-P6 and P3-P4, respectively) and the BDI score. These findings suggest that high vmHRV may be protective against the influence of parietal alpha asymmetry on depression. In other words, individuals who have both relatively reduced right parietal activity and high vmHRV may be less susceptible to depression. Conversely, the combination of relatively reduced right parietal activity and decreased vmHRV could confer vulnerability to depression.
Fig. 2.
a) HF and b) LF/HF ratio moderate the relationship between parietal alpha asymmetry at the low alpha band (8–10 Hz) and the BDI-II total score.
Previous studies have reported that vmHRV may reflect the ability to generate regulated emotional and arousal responses through the autonomic nervous system [29, 63, 64], and can be an indicator of individual differences in emotion regulatory capacity [25, 65]. HF, which reflects parasympathetic tone, is involved in emotion and arousal regulation by keeping the heart rate stable [66] and inhibiting autonomic arousal in emotional expression [25]. The LF/HF ratio, which reflects sympatho-vagal balance [67], may moderate depressive vulnerability via autonomic regulation. An increased LF/HF ratio (sympathetic dominance) is usually related to autonomic dysregulation, such as increased overnight urinary cortisol, proinflammatory cytokines, and acute-phase proteins [68, 69, 70]. The possible role of vmHRV as a moderator in the relationship between relatively reduced right parietal activity and depression may therefore reflect the role of autonomic regulation. Yet, it should be noted that there has been critical discussion on the validity of LF/HF ratio as an index of sympatho-vagal balance, and thus should be interpreted with caution (for more information, see [71]).
Based on the postulation that parietal alpha asymmetry reflects emotion-related arousal [13, 14], increased vmHRV may mitigate depressive vulnerability related to parietal alpha activity by enhancing regulatory capability. Indeed, several clinical studies have shown regulatory effects of HRV-based interventions in a variety of clinical symptoms [72]. This increased vmHRV could reflect greater prefrontal neuronal inhibitory activity [29], suggesting that the greater the ability to inhibit response levels of emotional arousal, the lower the effect of abnormal parietal alpha asymmetry on depression. This view of vmHRV may underlie the insignificant interaction between vmHRV and frontal alpha asymmetry reported in this study. In particular, the ventral striatum, which has been identified as a neural component associated with frontal asymmetry during reward processing [73], is also suggested as a neural correlate of HF [74]. Thus, frontal asymmetry and vmHRV may be closely associated with each other, playing an integrated role in depression, rather than vmHRV moderating the relationship between frontal asymmetry and depression.
Yet, the insignificant results could be due to other factors such as the experimental condition of the current study, which assessed alpha asymmetry only at rest. According to the capability model, individual difference in frontal alpha asymmetry is best revealed when the situational demands are taken into account [75]. Stewart et al. (2011) have demonstrated that the relationship between frontal alpha asymmetry and depression status was stronger during directed facial action task compared to resting state [76]. Thus, the insignificant results in the present study may be attributed to using only resting state condition, which may reflect characteristics of depression less pronouncedly than during emotionally evoking tasks. However, this may be applicable to parietal alpha asymmetry as well, although there are not enough studies to confirm the difference between resting and task-elicited parietal asymmetry. Meanwhile, several studies have noted that age may influence HRV, for instance, a negative correlation between age and HRV was found [77, 78]. The impact of age may be applicable for this study considering the range of the age of participants.
Furthermore, the moderating effect of vmHRV remained significant when the BAI score was included as a covariate. It is noteworthy that the moderation effect still existed even after controlling for anxiety symptoms, given that many prior studies have reported the possibility of depression and anxiety comorbidity affecting the results between parietal alpha asymmetry and anxiety. Previous studies have shown that depressed patients with anxiety had greater right than left activity in posterior region, whereas depressed patients without anxiety comorbidity had greater left than right activity in posterior region [60, 79]. In addition, anxious arousal has been linked to right parietal hyperactivation, an opposite pattern to that seen in depression [33, 80]. However, our results remaining significant after controlling for the level of anxiety indicates that the interaction results can be attributed to depression, rather than anxiety.
Yet, different patterns of results were found depending on alpha frequency bands and patient status. Specifically, the moderation effect of vmHRV on depression was observed only for alpha asymmetry at low frequency band in patients with MDD, but not other frequency bands (high and total) or healthy participants. Such different results can be due to alpha asymmetry at low—as opposed to high or total—frequency band being a more suitable indicator of clinical symptoms. For instance, the abnormal alpha asymmetry pattern in ADHD patients was found to be the most predominant at low frequency band [81]. Although the role of alpha frequency sub-bands in depression is still ambivalent, individuals with high risk for depression also showed reduced left frontal alpha activity at low alpha frequency range compared to those with low risk [18]. Also, as suggested by previous EEG studies, high and low alpha sub-bands may have different functions in which the low frequency band is less task-specific than the high frequency band [82, 83]. Finally, the insignificant results found in healthy subjects show that the interaction effect of vmHRV and parietal alpha asymmetry on depression may only pertain to individuals with clinical level of depression.
The present study has several limitations. First, most of the participants were under medication; antidepressants and benzodiazepines have been reported to decrease and increase vmHRV, respectively [84, 85, 86]. Second, the number of participants was relatively small to adequately address the moderation effect. Third, other factors that may affect vmHRV, such as cardiovascular training and body mass, were not controlled in the current study. Cardiovascular training [77] and body mass [87] could enhance and reduce HRV, respectively. In addition, although this study used the BAI to statistically control for level of anxiety, tools to measure more specific domains of anxiety such as anxious apprehension and anxious arousal were not included. Yet, previous alpha asymmetry studies have reported different frontal asymmetry patterns between the two domains of anxiety [33, 80]. Future studies should therefore employ symptom measures of anxious apprehension and arousal in order to more effectively account for the potential effect of anxiety on the relationship between alpha asymmetry and depression. Lastly, only single short-term HRV was assessed in this study and therefore may be affected by situational factors [88]. Future studies using repeated measurements of HRV would be necessary to confirm the results. Despite these limitations, it is believed that this study represents an important first step in the investigation of the relationship between alpha asymmetry and vmHRV.
5. Conclusion
The results of the present study suggest that vmHRV and parietal alpha asymmetry interaction may be a plausible candidate of a biomarker of MDD. Increased vmHRV seems to decrease the depression-predicting effect of reduced right parietal activity. vmHRV may play a role as a potential moderator of parietal alpha asymmetry in patients with MDD. Larger studies, controlled for medication and comorbidity, are needed to confirm the findings.
Declarations
Author contribution statement
Seung Yeon Baik: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Cholong Kim: Analyzed and interpreted the data; Wrote the paper.
Sungkean Kim, Dong-Wook Yook: Contributed reagents, materials, analysis tools or data.
Hyang Sook Kim, Hyein Chang: Analyzed and interpreted the data.
Seung-Hwan Lee: Conceived and designed the experiments.
Funding statement
This work was supported by the 2017 creative research program of Inje University, and a grant from the Korea Science and Engineering Foundation (KOSEF), funded by the Korean government (NRF-2018R1A2A2A05018505).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Reznik S.J., Allen J.J. Frontal asymmetry as a mediator and moderator of emotion: an updated review. Psychophysiology. 2018;55(1) doi: 10.1111/psyp.12965. [DOI] [PubMed] [Google Scholar]
- 2.Nelson B.D. Frontal brain asymmetry in depression with comorbid anxiety: a neuropsychological investigation. J. Abnorm. Psychol. 2012;121(3):579. doi: 10.1037/a0027587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen J.J. Regional electroencephalographic asymmetries in bipolar seasonal affective disorder before and after exposure to bright light. Biol. Psychiatry. 1993;33(8):642–646. doi: 10.1016/0006-3223(93)90104-l. [DOI] [PubMed] [Google Scholar]
- 4.Henriques J.B., Davidson R.J. Left frontal hypoactivation in depression. J. Abnorm. Psychol. 1991;100(4):535. doi: 10.1037//0021-843x.100.4.535. [DOI] [PubMed] [Google Scholar]
- 5.Henriques J.B., Davidson R.J. Regional brain electrical asymmetries discriminate between previously depressed and healthy control subjects. J. Abnorm. Psychol. 1990;99 doi: 10.1037//0021-843x.99.1.22. [DOI] [PubMed] [Google Scholar]
- 6.Stewart J.L. The oft-neglected role of parietal EEG asymmetry and risk for major depressive disorder. Psychophysiology. 2011;48(1):82–95. doi: 10.1111/j.1469-8986.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davidson R.J. Anterior electrophysiological asymmetries, emotion, and depression: conceptual and methodological conundrums. Psychophysiology. 1998;35(5):607–614. doi: 10.1017/s0048577298000134. [DOI] [PubMed] [Google Scholar]
- 8.Gotlib I.H. EEG alpha asymmetry, depression, and cognitive functioning. Cognit. Emot. 1998;12(3):449–478. [Google Scholar]
- 9.Diego M.A., Field T., Hernandez-Reif M. CES-D depression scores are correlated with frontal EEG alpha asymmetry. Depress. Anxiety. 2001;13(1):32–37. [PubMed] [Google Scholar]
- 10.Saletu B., Anderer P., Saletu-Zyhlarz G. EEG topography and tomography (LORETA) in diagnosis and pharmacotherapy of depression. Clin. EEG Neurosci. 2010;41(4):203–210. doi: 10.1177/155005941004100407. [DOI] [PubMed] [Google Scholar]
- 11.Allen J.J. The stability of resting frontal electroencephalographic asymmetry in depression. Psychophysiology. 2004;41(2):269–280. doi: 10.1111/j.1469-8986.2003.00149.x. [DOI] [PubMed] [Google Scholar]
- 12.Blackhart G.C., Minnix J.A., Kline J.P. Can EEG asymmetry patterns predict future development of anxiety and depression?: a preliminary study. Biol. Psychol. 2006;72(1):46–50. doi: 10.1016/j.biopsycho.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 13.Moratti S. Hypofunction of right temporoparietal cortex during emotional arousal in depression. Arch. Gen. Psychiatr. 2008;65(5):532–541. doi: 10.1001/archpsyc.65.5.532. [DOI] [PubMed] [Google Scholar]
- 14.Heller W. Neuropsychological mechanisms of individual differences in emotion, personality, and arousal. Neuropsychology. 1993;7(4):476. [Google Scholar]
- 15.Bruder G.E. Electroencephalographic measures of regional hemispheric activity in offspring at risk for depressive disorders. Biol. Psychiatry. 2005;57(4):328–335. doi: 10.1016/j.biopsych.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Reid S.A., Duke L.M., Allen J.J. Resting frontal electroencephalographic asymmetry in depression: inconsistencies suggest the need to identify mediating factors. Psychophysiology. 1998;35(4):389–404. [PubMed] [Google Scholar]
- 17.Mathersul D. Investigating models of affect: relationships among EEG alpha asymmetry, depression, and anxiety. Emotion. 2008;8(4):560. doi: 10.1037/a0012811. [DOI] [PubMed] [Google Scholar]
- 18.Tomarken A.J. Resting frontal brain activity: linkages to maternal depression and socio-economic status among adolescents. Biol. Psychol. 2004;67(1):77–102. doi: 10.1016/j.biopsycho.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Aldao A., Nolen-Hoeksema S., Schweizer S. Emotion-regulation strategies across psychopathology: a meta-analytic review. Clin. Psychol. Rev. 2010;30(2):217–237. doi: 10.1016/j.cpr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Rive M.M. Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neurosci. Biobehav. Rev. 2013;37(10):2529–2553. doi: 10.1016/j.neubiorev.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 21.Bilchick K.C., Berger R.D. Heart rate variability. J. Cardiovasc. Electrophysiol. 2006;17(6):691–694. doi: 10.1111/j.1540-8167.2006.00501.x. [DOI] [PubMed] [Google Scholar]
- 22.Kemp A.H. Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol. Psychiatry. 2010;67(11):1067–1074. doi: 10.1016/j.biopsych.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Kleiger R.E. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am. J. Cardiol. 1987;59(4):256–262. doi: 10.1016/0002-9149(87)90795-8. [DOI] [PubMed] [Google Scholar]
- 24.Fox N.A. 1989. Heart-rate Variability and Behavioral Reactivity: Individual Differences in Autonomic Patterning and Their Relation to Infant and Child Temperament. [Google Scholar]
- 25.Appelhans B.M., Luecken L.J. Heart rate variability as an index of regulated emotional responding. Rev. Gen. Psychol. 2006;10(3):229. [Google Scholar]
- 26.Berna G., Ott L., Nandrino J.-L. Effects of emotion regulation difficulties on the tonic and phasic cardiac autonomic response. PLoS One. 2014;9(7):e102971. doi: 10.1371/journal.pone.0102971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beauchaine T.P. Future directions in emotion dysregulation and youth psychopathology. J. Clin. Child Adolesc. Psychol. 2015;44(5):875–896. doi: 10.1080/15374416.2015.1038827. [DOI] [PubMed] [Google Scholar]
- 28.Williams D.P. Resting heart rate variability predicts self-reported difficulties in emotion regulation: a focus on different facets of emotion regulation. Front. Psychol. 2015;6:261. doi: 10.3389/fpsyg.2015.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thayer J.F., Lane R.D. A model of neurovisceral integration in emotion regulation and dysregulation. J. Affect. Disord. 2000;61(3):201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- 30.Thayer J.F. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev. 2012;36(2):747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 31.Rechlin T. Are affective disorders associated with alterations of heart rate variability? J. Affect. Disord. 1994;32(4):271–275. doi: 10.1016/0165-0327(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 32.Carney R.M. Change in heart rate and heart rate variability during treatment for depression in patients with coronary heart disease. Psychosom. Med. 2000;62(5):639–647. doi: 10.1097/00006842-200009000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Heller W. Patterns of regional brain activity differentiate types of anxiety. J. Abnorm. Psychol. 1997;106(3):376. doi: 10.1037//0021-843x.106.3.376. [DOI] [PubMed] [Google Scholar]
- 34.Nitschke W.H.J.B. The puzzle of regional brain activity in and anxiety: the importance of subtypes and comorbidity. Cognit. Emot. 1998;12(3):421–447. [Google Scholar]
- 35.Klimesch W. Induced alpha band power changes in the human EEG and attention. Neurosci. Lett. 1998;244(2):73–76. doi: 10.1016/s0304-3940(98)00122-0. [DOI] [PubMed] [Google Scholar]
- 36.Klimesch W., Pfurtscheller G., Schimke H. Pre-and post-stimulus processes in category judgement tasks as measured by event-related desynchronization (ERD) J. Psychophysiol. 1992 [Google Scholar]
- 37.Velasques B. Changes in slow and fast alpha bands in subjects submitted to different amounts of functional electrostimulation. Neurosci. Lett. 2008;441(2):149–152. doi: 10.1016/j.neulet.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 38.Pfurtscheller G., Neuper C., Krausz G. Functional dissociation of lower and upper frequency mu rhythms in relation to voluntary limb movement. Clin. Neurophysiol. 2000;111(10):1873–1879. doi: 10.1016/s1388-2457(00)00428-4. [DOI] [PubMed] [Google Scholar]
- 39.Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res. Rev. 1999;29(2):169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- 40.Gold C., Fachner J., Erkkilä J. Validity and reliability of electroencephalographic frontal alpha asymmetry and frontal midline theta as biomarkers for depression. Scand. J. Psychol. 2013;54(2):118–126. doi: 10.1111/sjop.12022. [DOI] [PubMed] [Google Scholar]
- 41.Thibodeau R., Jorgensen R.S., Kim S. Depression, anxiety, and resting frontal EEG asymmetry: a meta-analytic review. J. Abnorm. Psychol. 2006;115(4):715. doi: 10.1037/0021-843X.115.4.715. [DOI] [PubMed] [Google Scholar]
- 42.Stewart J.L. Resting frontal EEG asymmetry as an endophenotype for depression risk: sex-specific patterns of frontal brain asymmetry. J. Abnorm. Psychol. 2010;119(3):502. doi: 10.1037/a0019196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Association A.P. American Psychiatric Association; 2013. DSM 5. [Google Scholar]
- 44.Lee Y., Song J. A study of the reliability and the validity of the BDI, SDS, and MMPI-D scales. Kor. J. Clin. Psychol. 1991;10(1):98–113. [Google Scholar]
- 45.Kwon S.-M. University of Queensland; 1992. Differential Roles of Dysfunctional Attitudes and Automatic Thoughts in Depression: an Integrated Cognitive Model of Depression. [Google Scholar]
- 46.Gordon E., Palmer D.M., Cooper N. EEG alpha asymmetry in schizophrenia, depression, PTSD, panic disorder, ADHD and conduct disorder. Clin. EEG Neurosci. 2010;41(4):178–183. doi: 10.1177/155005941004100404. [DOI] [PubMed] [Google Scholar]
- 47.Choi S.W. Is alpha wave neurofeedback effective with randomized clinical trials in depression? A pilot study. Neuropsychobiology. 2011;63(1):43–51. doi: 10.1159/000322290. [DOI] [PubMed] [Google Scholar]
- 48.Dekker M.K. Feasibility of eyes open alpha power training for mental enhancement in elite gymnasts. J. Sports Sci. 2014;32(16):1550–1560. doi: 10.1080/02640414.2014.906044. [DOI] [PubMed] [Google Scholar]
- 49.Carvalho A. EEG frontal asymmetry in the depressed and remitted elderly: is it related to the trait or to the state of depression? J. Affect. Disord. 2011;129(1):143–148. doi: 10.1016/j.jad.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 50.Jin S.-H. Hemispheric laterality and dimensional complexity in schizophrenia under sound and light stimulation. Int. J. Psychophysiol. 2003;49(1):1–15. doi: 10.1016/s0167-8760(03)00078-3. [DOI] [PubMed] [Google Scholar]
- 51.Cherbuin N. Mild cognitive disorders are associated with different patterns of brain asymmetry than normal aging: the PATH through Life Study. Front. Psychiatry. 2010;1 doi: 10.3389/fpsyt.2010.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anokhin A.P., Heath A.C., Myers E. Genetic and environmental influences on frontal EEG asymmetry: a twin study. Biol. Psychol. 2006;71(3):289–295. doi: 10.1016/j.biopsycho.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Udupa K. Alteration of cardiac autonomic functions in patients with major depression: a study using heart rate variability measures. J. Affect. Disord. 2007;100(1):137–141. doi: 10.1016/j.jad.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 54.van der Kooy K.G. Differences in heart rate variability between depressed and non-depressed elderly. Int. J. Geriatr. Psychiatry. 2006;21(2):147–150. doi: 10.1002/gps.1439. [DOI] [PubMed] [Google Scholar]
- 55.Hedman A. The high frequency component of heart rate variability reflects cardiac parasympathetic modulation rather than parasympathetic ‘tone’. Acta Physiol. 1995;155(3):267–273. doi: 10.1111/j.1748-1716.1995.tb09973.x. [DOI] [PubMed] [Google Scholar]
- 56.Thayer J.F., Lane R.D. The role of vagal function in the risk for cardiovascular disease and mortality. Biol. Psychol. 2007;74(2):224–242. doi: 10.1016/j.biopsycho.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 57.Curran P.J., West S.G., Finch J.F. The robustness of test statistics to nonnormality and specification error in confirmatory factor analysis. Psychol. Methods. 1996;1(1):16. [Google Scholar]
- 58.Davidson R.J. Sex differences in patterns of EEG asymmetry. Biol. Psychol. 1976;4(2):119–138. doi: 10.1016/0301-0511(76)90012-0. [DOI] [PubMed] [Google Scholar]
- 59.Earle J.B., Pikus A.A. The effect of sex and task difficulty on EEG alpha activity in association with arithmetic. Biol. Psychol. 1982;15(1-2):1–14. doi: 10.1016/0301-0511(82)90027-8. [DOI] [PubMed] [Google Scholar]
- 60.Kentgen L.M. Electroencephalographic asymmetries in adolescents with major depression: influence of comorbidity with anxiety disorders. J. Abnorm. Psychol. 2000;109(4):797. doi: 10.1037//0021-843x.109.4.797. [DOI] [PubMed] [Google Scholar]
- 61.Nunan D., Sandercock G.R., Brodie D.A. A quantitative systematic review of normal values for short-term heart rate variability in healthy adults. Pacing Clin. Electrophysiol. 2010;33(11):1407–1417. doi: 10.1111/j.1540-8159.2010.02841.x. [DOI] [PubMed] [Google Scholar]
- 62.Massin M.M., Derkenne B., von Bernuth G. Correlations between indices of heart rate variability in healthy children and children with congenital heart disease. Cardiology. 1999;91(2):109–113. doi: 10.1159/000006889. [DOI] [PubMed] [Google Scholar]
- 63.Thayer J.F., Siegle G.J. Neurovisceral integration in cardiac and emotional regulation. IEEE Eng. Med. Biol. Mag. 2002;21(4):24–29. doi: 10.1109/memb.2002.1032635. [DOI] [PubMed] [Google Scholar]
- 64.Sack M., Hopper J.W., Lamprecht F. Low respiratory sinus arrhythmia and prolonged psychophysiological arousal in posttraumatic stress disorder: heart rate dynamics and individual differences in arousal regulation. Biol. Psychiatry. 2004;55(3):284–290. doi: 10.1016/s0006-3223(03)00677-2. [DOI] [PubMed] [Google Scholar]
- 65.Geisler F.C. The impact of heart rate variability on subjective well-being is mediated by emotion regulation. Pers. Indiv. Differ. 2010;49(7):723–728. [Google Scholar]
- 66.Hughes J.W., Stoney C.M. Depressed mood is related to high-frequency heart rate variability during stressors. Psychosom. Med. 2000;62(6):796–803. doi: 10.1097/00006842-200011000-00009. [DOI] [PubMed] [Google Scholar]
- 67.Berntson G.G. Cardiac autonomic balance versus cardiac regulatory capacity. Psychophysiology. 2008;45(4):643–652. doi: 10.1111/j.1469-8986.2008.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thayer J.F., Sternberg E. Beyond heart rate variability. Ann. N. Y. Acad. Sci. 2006;1088(1):361–372. doi: 10.1196/annals.1366.014. [DOI] [PubMed] [Google Scholar]
- 69.Sgoifo A. Cardiac autonomic reactivity and salivary cortisol in men and women exposed to social stressors: relationship with individual ethological profile. Neurosci. Biobehav. Rev. 2003;27(1):179–188. doi: 10.1016/s0149-7634(03)00019-8. [DOI] [PubMed] [Google Scholar]
- 70.Mujica-Parodi L., Renelique R., Taylor M. Higher body fat percentage is associated with increased cortisol reactivity and impaired cognitive resilience in response to acute emotional stress. Int. J. Obes. 2009;33(1):157–165. doi: 10.1038/ijo.2008.218. [DOI] [PubMed] [Google Scholar]
- 71.Billman G.E. The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front. Physiol. 2013;4:26. doi: 10.3389/fphys.2013.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ratanasiripong P., Ratanasiripong N., Kathalae D. Biofeedback intervention for stress and anxiety among nursing students: a randomized controlled trial. ISRN Nurs. 2012;2012 doi: 10.5402/2012/827972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nusslock R., Alloy L.B. Reward processing and mood-related symptoms: an RDoC and translational neuroscience perspective. J. Affect. Disord. 2017;216:3–16. doi: 10.1016/j.jad.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lane R.D. Neural correlates of heart rate variability during emotion. Neuroimage. 2009;44(1):213–222. doi: 10.1016/j.neuroimage.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 75.Coan J.A., Allen J.J., McKnight P.E. A capability model of individual differences in frontal EEG asymmetry. Biol. Psychol. 2006;72(2):198–207. doi: 10.1016/j.biopsycho.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stewart J.L. Frontal EEG asymmetry during emotional challenge differentiates individuals with and without lifetime major depressive disorder. J. Affect. Disord. 2011;129(1-3):167–174. doi: 10.1016/j.jad.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leicht A.S., Allen G.D., Hoey A.J. Influence of age and moderate-intensity exercise training on heart rate variability in young and mature adults. Can. J. Appl. Physiol. 2003;28(3):446–461. doi: 10.1139/h03-033. [DOI] [PubMed] [Google Scholar]
- 78.Reardon M., Malik M. Changes in heart rate variability with age. Pacing Clin. Electrophysiol. 1996;19(11):1863–1866. doi: 10.1111/j.1540-8159.1996.tb03241.x. [DOI] [PubMed] [Google Scholar]
- 79.Bruder G.E. Regional brain asymmetries in major depression with or without an anxiety disorder: a quantitative electroencephalographic study. Biol. Psychiatry. 1997;41(9):939–948. doi: 10.1016/S0006-3223(96)00260-0. [DOI] [PubMed] [Google Scholar]
- 80.Nitschke J.B. Contrasting patterns of brain activity in anxious apprehension and anxious arousal. Psychophysiology. 1999;36(5):628–637. [PubMed] [Google Scholar]
- 81.Hale T.S. Atypical alpha asymmetry in adults with ADHD. Neuropsychologia. 2009;47(10):2082–2088. doi: 10.1016/j.neuropsychologia.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Haegens S. Thalamocortical rhythms during a vibrotactile detection task. Proc. Natl. Acad. Sci. Unit. States Am. 2014;111(17):E1797–E1805. doi: 10.1073/pnas.1405516111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Klimesch W. Event-related desynchronization (ERD) and the Dm effect: does alpha desynchronization during encoding predict later recall performance? Int. J. Psychophysiol. 1996;24(1):47–60. doi: 10.1016/s0167-8760(96)00054-2. [DOI] [PubMed] [Google Scholar]
- 84.Howell S. Effects of propofol and thiopentone, and benzodiazepine premedication on heart rate variability measured by spectral analysis. Br. J. Anaesth. 1995;74(2):168–173. doi: 10.1093/bja/74.2.168. [DOI] [PubMed] [Google Scholar]
- 85.Agelink M.W. Short-term effects of intravenous benzodiazepines on autonomic neurocardiac regulation in humans: a comparison between midazolam, diazepam, and lorazepam. Crit. Care Med. 2002;30(5):997–1006. doi: 10.1097/00003246-200205000-00008. [DOI] [PubMed] [Google Scholar]
- 86.Glassman A.H. Heart rate variability in acute coronary syndrome patients with major depression: influence of sertraline and mood improvement. Arch. Gen. Psychiatr. 2007;64(9):1025–1031. doi: 10.1001/archpsyc.64.9.1025. [DOI] [PubMed] [Google Scholar]
- 87.Lutfi M.F., Sukkar M.Y. Relationship of height, weight and body mass index to heart rate variability. Sudan Med. J. 2011;47(1):14–19. [Google Scholar]
- 88.Sandercock G.R., Bromley P.D., Brodie D.A. The reliability of short-term measurements of heart rate variability. Int. J. Cardiol. 2005;103(3):238–247. doi: 10.1016/j.ijcard.2004.09.013. [DOI] [PubMed] [Google Scholar]