Summary
Background
Statin therapy has been shown to reduce major vascular events and vascular mortality in a wide range of individuals, but there is uncertainty about its efficacy and safety among older people. We undertook a meta-analysis of data from all large statin trials to compare the effects of statin therapy at different ages.
Methods
In this meta-analysis, randomised trials of statin therapy were eligible if they aimed to recruit at least 1000 participants with a scheduled treatment duration of at least 2 years. We analysed individual participant data from 22 trials (n=134 537) and detailed summary data from one trial (n=12 705) of statin therapy versus control, plus individual participant data from five trials of more intensive versus less intensive statin therapy (n=39 612). We subdivided participants into six age groups (55 years or younger, 56–60 years, 61–65 years, 66–70 years, 71–75 years, and older than 75 years). We estimated effects on major vascular events (ie, major coronary events, strokes, and coronary revascularisations), cause-specific mortality, and cancer incidence as the rate ratio (RR) per 1·0 mmol/L reduction in LDL cholesterol. We compared proportional risk reductions in different age subgroups by use of standard χ2 tests for heterogeneity when there were two groups, or trend when there were more than two groups.
Findings
14 483 (8%) of 186 854 participants in the 28 trials were older than 75 years at randomisation, and the median follow-up duration was 4·9 years. Overall, statin therapy or a more intensive statin regimen produced a 21% (RR 0·79, 95% CI 0·77–0·81) proportional reduction in major vascular events per 1·0 mmol/L reduction in LDL cholesterol. We observed a significant reduction in major vascular events in all age groups. Although proportional reductions in major vascular events diminished slightly with age, this trend was not statistically significant (ptrend=0·06). Overall, statin or more intensive therapy yielded a 24% (RR 0·76, 95% CI 0·73–0·79) proportional reduction in major coronary events per 1·0 mmol/L reduction in LDL cholesterol, and with increasing age, we observed a trend towards smaller proportional risk reductions in major coronary events (ptrend=0·009). We observed a 25% (RR 0·75, 95% CI 0·73–0·78) proportional reduction in the risk of coronary revascularisation procedures with statin therapy or a more intensive statin regimen per 1·0 mmol/L lower LDL cholesterol, which did not differ significantly across age groups (ptrend=0·6). Similarly, the proportional reductions in stroke of any type (RR 0·84, 95% CI 0·80–0·89) did not differ significantly across age groups (ptrend=0·7). After exclusion of four trials which enrolled only patients with heart failure or undergoing renal dialysis (among whom statin therapy has not been shown to be effective), the trend to smaller proportional risk reductions with increasing age persisted for major coronary events (ptrend=0·01), and remained non-significant for major vascular events (ptrend=0·3). The proportional reduction in major vascular events was similar, irrespective of age, among patients with pre-existing vascular disease (ptrend=0·2), but appeared smaller among older than among younger individuals not known to have vascular disease (ptrend=0·05). We found a 12% (RR 0·88, 95% CI 0·85–0·91) proportional reduction in vascular mortality per 1·0 mmol/L reduction in LDL cholesterol, with a trend towards smaller proportional reductions with older age (ptrend=0·004), but this trend did not persist after exclusion of the heart failure or dialysis trials (ptrend=0·2). Statin therapy had no effect at any age on non-vascular mortality, cancer death, or cancer incidence.
Interpretation
Statin therapy produces significant reductions in major vascular events irrespective of age, but there is less direct evidence of benefit among patients older than 75 years who do not already have evidence of occlusive vascular disease. This limitation is now being addressed by further trials.
Funding
Australian National Health and Medical Research Council, National Institute for Health Research Oxford Biomedical Research Centre, UK Medical Research Council, and British Heart Foundation.
Research in context.
Evidence before this study
Before this meta-analysis, the evidence available from randomised trials on the effects of statin therapy in older people had been summarised only in meta-analyses of aggregated data from published reports. We searched MEDLINE, Embase, and PubMed for English-language publications published between Jan 1, 1996, and Dec 31, 2017, using the search terms “statins OR HMG CoA Reductase Inhibitors” and “Elderly OR Aged”, and found 14 meta-analyses with conflicting assessments of efficacy among older people (generally defined as >65 years). Because of a lack of access to the individual participant data, none of these previous meta-analyses were able to examine the effects of statins within particular older age groups (eg, >75 years) in primary and secondary prevention. We previously reported meta-analyses of the effects of statins by age, but these analyses were restricted in scope and some large randomised trials that included older individuals have been reported since they were published.
Added value of this study
We analysed individual participant data from 27 randomised controlled trials (n=174 149) and detailed summary data from one trial (n=12 705). During 4·9 years of follow-up, major vascular events were significantly reduced by statin therapy among all age groups by about a fifth per 1·0 mmol/L LDL cholesterol reduction, including among the 14 483 participants who were older than 75 years at randomisation. Older age groups were disproportionately represented in heart failure and dialysis trials (which did not show an overall benefit with statin therapy), and exclusion of those trials weakened an apparent trend with increasing age towards smaller relative risk reductions in vascular event and mortality outcomes. We found significant reductions in major coronary events in all age groups (including those older than 75 years at randomisation), but the trend towards smaller relative reductions with increasing age persisted, even after excluding heart failure and dialysis trials. However, as the absolute risk of these events was higher in older people, the absolute benefits were similar to, if not greater than, those at younger ages. We observed significant efficacy regardless of age among participants with previous vascular disease, whereas there was a weak trend towards smaller relative risk reductions with increasing age in the primary prevention setting (although there were too few such older participants for reliable assessment in that group alone). Despite previous concerns, we found no adverse effects on cancer or non-vascular mortality in any age group.
Implications of all the available evidence
Statins reduced vascular events irrespective of age, including in people older than 75 years. In the primary prevention setting, among people older than 75 years, there is less evidence of the effects of statin therapy. Ongoing trials are investigating this group directly.
Introduction
Meta-analyses of individual participant data from 27 randomised trials in the Cholesterol Treatment Trialists' (CTT) Collaboration database indicate that each 1·0 mmol/L reduction in LDL cholesterol with statin therapy reduces the risk of major vascular events by about a fifth, with similar proportional risk reductions among men and women,1 and across different levels of absolute risk.2 However, even among patients with established cardiovascular disease, rates of use of statin therapy have been shown to decline with increasing age, and are substantially lower in people older than 75 years, reflecting differences in both prescribing and compliance.3, 4 This decline is even more prominent among older patients with no evidence of occlusive vascular disease.5 One explanation for this observation might relate to uncertainty about applying the evidence for statin efficacy and safety from randomised trials to an older population, given that a relatively small number of people aged over 75 years were enrolled in such trials, and many older people have non-cardiovascular comorbidities.6, 7, 8, 9, 10 The CTT collaboration has age-specific data on vascular events, cause-specific mortality, and cancer from 28 randomised controlled trials of statin therapy among a total of nearly 187 000 participants, of whom about 14 500 were older than 75 years at baseline. We aimed to do a meta-analysis of data from all large statin trials to compare the effects of statin therapy at different ages and explore the effects of statin therapy among older individuals.
Methods
Study design and outcomes
The methods of the CTT Collaboration have been described previously,11 and were agreed before the reporting of any individual trial results. Randomised trials were eligible for inclusion if the main effect of at least one of the trial interventions was to lower LDL cholesterol, the trial was unconfounded with respect to this intervention (ie, no other differences in modification of risk factors between the relevant treatment groups were intended), and the trial aimed to recruit 1000 or more participants with a scheduled treatment duration of at least 2 years.11
As for all CTT analyses,1, 2, 11, 12, 13, 14, 15, 16 the risk of bias was low because of prespecified study methods, the ability to adjust for heterogeneity by weighting rate ratios (RRs) according to trial-level absolute differences in LDL cholesterol, and the low probability of publication bias due to a prospective design with prespecified study eligibility. Prespecified outcomes included major coronary events (defined as non-fatal myocardial infarction or coronary death), coronary revascularisation (angioplasty or bypass grafting), stroke (subdivided by type), site-specific cancers, and cause-specific mortality. Subsequently, we defined major vascular events as the composite of major coronary events, coronary revascularisation, and stroke.
Statistical analysis
Prespecified subgroup analyses included comparisons of the effects of statin therapy among people aged 65 years and younger and people older than 65 years at randomisation (along with several other subgroupings, including by history of vascular disease11). However, for the present more detailed analysis, we compared effects between six age groups (55 years or younger, 56–60 years, 61–65 years, 66–70 years, 71–75 years, and older than 75 years) and, where statistical power was limited because of low numbers of specific event types, between two retrospectively defined groups (≤75 years and >75 years). Because of disproportionate representation of older people (particularly >75 years) in four trials done exclusively among people with heart failure17, 18 or receiving renal dialysis,19, 20 for whom statin therapy shows little or no benefit, we also examined the effects after exclusion of these trials.
Analyses included all randomised patients, irrespective of whether they received their allocated treatment (intention to treat). Estimates of the effects on disease rates within each trial were derived from the log-rank (o – e) statistic and its variance (v) for first events. Meta-analyses were weighted by the absolute LDL cholesterol difference in that trial at 1 year (d mmol/L), and are reported as effects per 1·0 mmol/L reduction in LDL cholesterol.1, 2, 12, 13, 14, 15, 16 In a meta-analysis of several trials, the log of the RR per 1·0 mmol/L was then calculated as S/V with variance 1/V (and hence, for example, with 95% CI of S/V ± 1·96 ÷√V), where S is the sum over all trials of d(o – e) and V is the sum over all trials of d2v. We compared proportional risk reductions in different age subgroups by use of standard χ2 tests for heterogeneity when there were two groups, or trend when there were more than two groups.
To allow for multiple subdivisions of the data into subgroups, we present only summary RRs with 95% CIs; all other RRs are presented with 99% CIs. All analyses were done with SAS (version 9.3) and R (version 3.2.5).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The writing committee had full access to all the data in the study and takes final responsibility for its content and the decision to submit for publication.
Results
Individual participant data were provided by investigators or sponsors for 27 trials of statin therapy,2 and detailed summary data provided for one further trial,21 permitting meta-analyses of all 28 trials. Data were unavailable for four eligible trials (9264 participants), all of which exclusively enrolled people with known vascular disease.22, 23, 24, 25
In 23 trials, a statin-based regimen was compared with placebo or usual care (147 242 participants; median follow-up 4·8 years). In the other five trials, an intensive statin regimen was assessed against a standard statin regimen (39 612 participants; median follow-up 5·1 years).1 Across all 28 trials, the median follow-up duration was 4·9 years (range 2·0 to 7·0). 39 242 (21%) of 186 854 participants were aged 55 years or younger, 31 434 (17%) were 56–60 years, 37 764 (20%) were 61–65 years, 36 567 (20%) were 66–70 years, 27 314 (15%) were aged 71–75 years, and 14 483 (8%) were older than 75 years (appendix; age was unrecorded for 50 participants, none of whom had a major vascular event, died, or developed cancer during the trial).
We found significant differences in each of the measured baseline characteristics across the six age groups (p<0·001 for all comparisons; appendix). Older participants were more likely to be female and to have heart failure or hypertension and were less likely to be current smokers. 2893 (20%) of patients older than 75 years compared with 6692 (4%) of those aged 75 years or younger were patients in heart failure trials, and 482 (3%) of patients older than 75 years compared with 3546 (2%) of those aged 75 years or younger were patients in renal dialysis trials (table). We observed a trend towards lower baseline LDL cholesterol concentrations with increasing age in the statin therapy versus control trials and—to a lesser extent—in the more intensive versus standard statin trials (appendix). Mean LDL cholesterol differences between treatment arms at one year (overall difference 1·08 mmol/L) were slightly smaller among older participants (appendix).
Table.
Baseline characteristics
| ≤75 years (n=172 321) | >75 years (n=14 483) | ||
|---|---|---|---|
| Age (years) | 61·6 (8·0) | 78·8 (2·8) | |
| Sex | |||
| Male | 125 783 (73%) | 8476 (59%) | |
| Female | 46 538 (27%) | 6007 (41%) | |
| History of vascular disease | 96 088 (56%) | 8034 (55%) | |
| History of myocardial infarction | 59 654 (35%) | 4210 (29%) | |
| History of other symptomatic coronary heart disease | 61 767 (36%) | 4448 (31%) | |
| History of heart failure (New York Heart Association class II–IV)* | 6692 (4%) | 2893 (20%) | |
| On dialysis† | 3546 (2%) | 482 (3%) | |
| History of diabetes | 31 919 (19%) | 2499 (17%) | |
| Current smoker | 35 701 (21%) | 1485 (10%) | |
| Treated hypertension | 82 213 (48%) | 8696 (60%) | |
| Systolic blood pressure (mm Hg) | 138·1 (19·5) | 143·4 (21·4) | |
| Diastolic blood pressure (mm Hg) | 81·2 (10·6) | 78·9 (10·8) | |
| Body–mass index (kg/m2) | 27·1 (24·6–30·1) | 26·3 (23·8–29·1) | |
| Total cholesterol (mmol/L) | 5·4 (1·0) | 5·1 (1·0) | |
| LDL cholesterol (mmol/L) | 3·3 (0·9) | 3·2 (0·8) | |
| HDL cholesterol (mmol/L) | 1·2 (0·3) | 1·3 (0·3) | |
| Triglycerides (mmol/L) | 1·6 (1·2–2·2) | 1·4 (1·0–1·9) | |
| Creatinine (μmol/L)‡ | 94 (80–106) | 99 (88–117) | |
Data are mean SD, n (%), or median (IQR). HOPE-3 participant data for body-mass index (mean 27·2 kg/m2 [SD 4·8] among participants ≤75 years and 26·1 kg/m2 [4·8] among participants >75 years), triglycerides (1·7 mmol/L [1·1] among participants ≤75 years and 1·6 mmol/L [0·9] among participants >75 years), and creatinine (79 μmol/L [19] among participants ≤75 years and 82 μmol/L [20] among participants >75 years) not included. 50 participants with missing age (49 from MEGA and one from A to Z) were excluded.
All participants from GISSI-HF and CORONA trials only.
All participants from 4D and AURORA trials.
Excludes participants on dialysis at randomisation (ie, participants from 4D and AURORA).
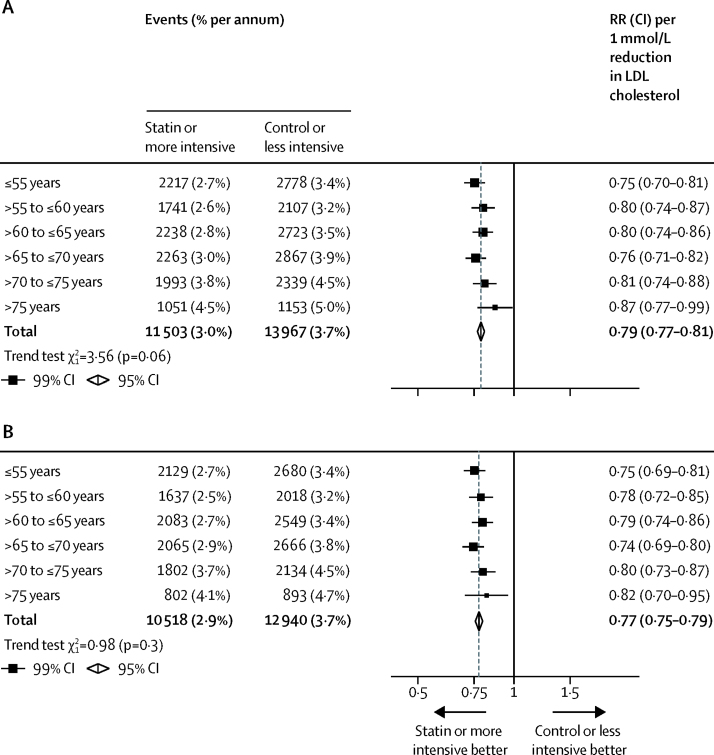
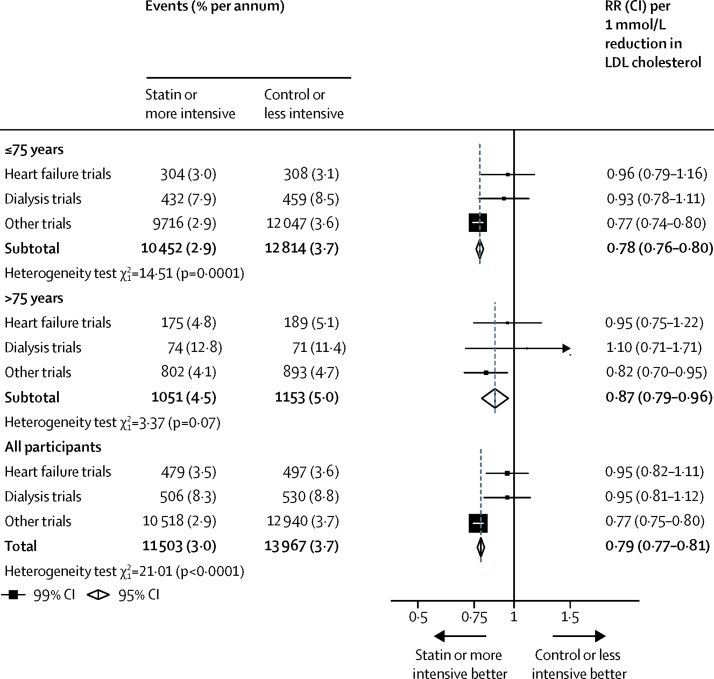
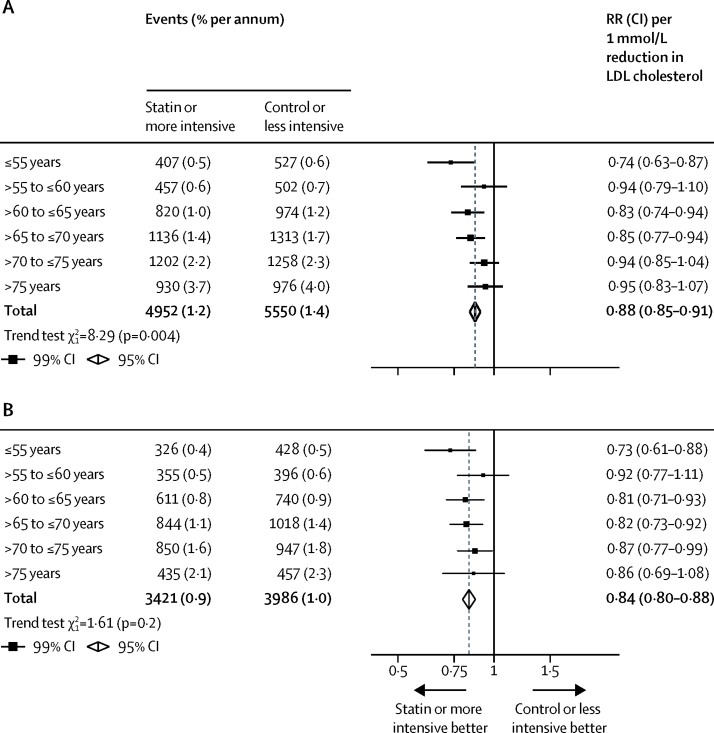
Among all 28 trials, statin therapy or a more intensive statin regimen compared with control therapy or a less intensive statin regimen produced a 21% (RR 0·79, 95% CI 0·77–0·81) proportional reduction in the risk of a first major vascular event per 1·0 mmol/L reduction in LDL cholesterol. We found independently significant risk reductions in each of the age groups considered, including among patients older than 75 years at the start of treatment (figure 1). Although proportional reductions in major vascular events diminished slightly with increasing age, this trend was not statistically significant (ptrend=0·06; figure 1). Previous studies have found that statin therapy does not reduce the rate of major vascular events among patients with moderate or severe heart failure or who are undergoing dialysis for renal failure16, 17, 18, 19, 20 (pheterogeneity<0·0001; figure 2). A disproportionate number of patients with heart failure or dialysis were in the older age groups of our overall meta-analysis population, which could confound comparisons between the effects of statin therapy by age. In exploratory analyses, after exclusion of 13 613 (7%) patients from four trials restricted to patients with heart failure or undergoing dialysis,17, 18, 19, 20 the non-significant trend towards smaller proportional reductions in major vascular events with increasing age was diminished (ptrend=0·3; figure 1). These patterns were unchanged after adjustment for sex, diabetes, hypertension, smoking, previous cardiovascular disease status, body–mass index (BMI), and renal function (adjusted ptrend=0·06 in 27 trials with individual participant data; p=0·4 after exclusion of four trials that exclusively included participants with heart failure or on dialysis).
Figure 1.
Effects on major vascular events per mmol/L reduction in LDL cholesterol by age at randomisation
All studies (A) and excluding four trials that exclusively included patients with heart failure or on dialysis (B). Data from participants with missing baseline data included in the totals. RR=rate ratio.
Figure 2.
Effects on major vascular events per mmol/L reduction in LDL cholesterol, subdivided by age at randomisation and particular trial populations
Data from participants with missing baseline data included in the totals. RR=rate ratio.
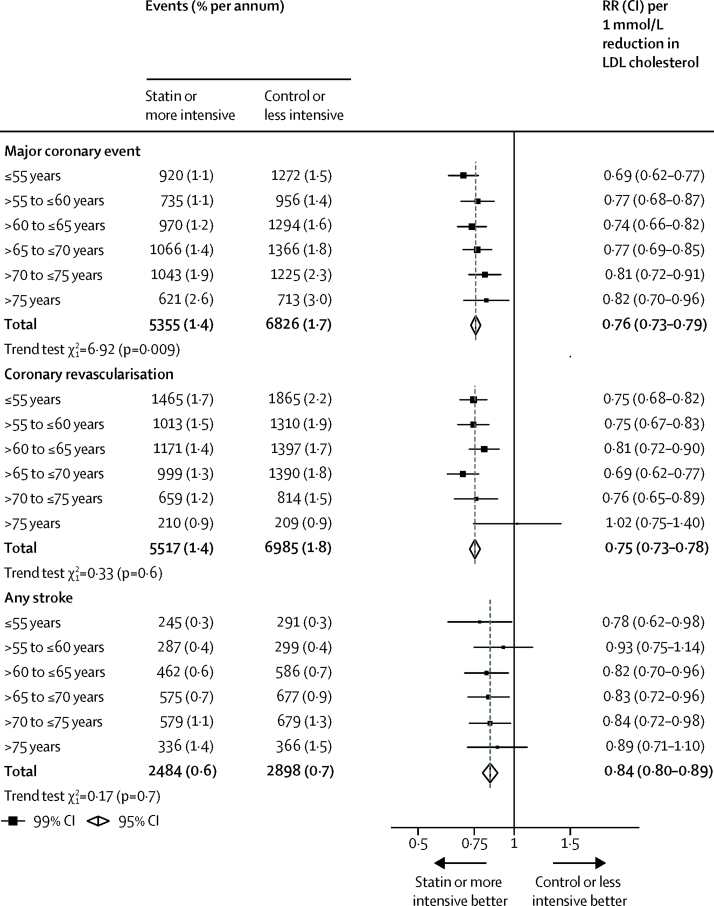
Overall, statin or more intensive therapy yielded a 24% (RR 0·76, 95% CI 0·73–0·79) proportional reduction in major coronary events per 1·0 mmol/L reduction in LDL cholesterol. We found a statistically significant trend towards smaller proportional reductions in major coronary events with increasing age (ptrend=0·009; figure 3), which remained significant after exclusion of the heart failure and dialysis trials (ptrend=0·01; appendix). These patterns were unchanged after adjustment for sex, diabetes, hypertension, smoking, previous cardiovascular disease status, BMI, and renal function (adjusted ptrend=0·008 in 27 trials with individual participant data; p=0·012 after exclusion of four trials that exclusively included participants with heart failure or on dialysis). Nevertheless, there was still a significant reduction in major coronary events among all patients older than 75 years (figure 3). When we compared patients aged 75 years or younger with patients older than 75 years, we found no significant differences between the proportional effects on major coronary events, or on non-fatal myocardial infarctions and coronary deaths considered separately (with or without exclusion of the heart failure or dialysis trials; appendix).
Figure 3.
Effects on components of major vascular events per mmol/L reduction in LDL cholesterol in all studies, by age at randomisation
Data from participants with missing baseline data included in the totals. RR=rate ratio.
Overall, we observed a 25% (RR 0·75, 95% CI 0·73–0·78) proportional reduction in the risk of coronary revascularisation procedures with statin therapy or a more intensive statin regimen per 1·0 mmol/L lower LDL cholesterol, which did not differ significantly across age categories (ptrend=0·6), although we found no apparent effect on the relatively small number of procedures done among patients older than 75 years (figure 3; appendix). Similarly, the proportional reductions in stroke of any type (RR 0·84, 95% CI 0·80–0·89) did not differ significantly by age group (ptrend=0·7; figure 3).
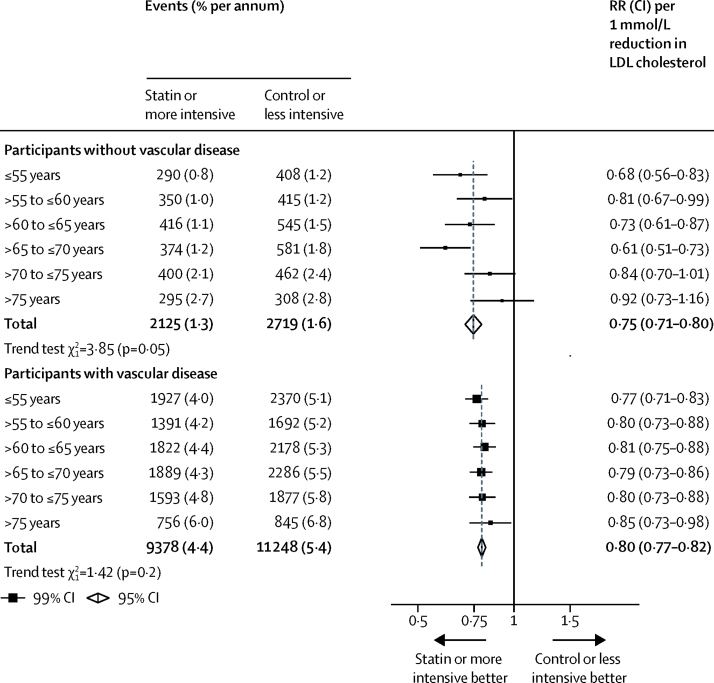
Among participants with known vascular disease at study entry, proportional reductions in major vascular events were similar across age groups, regardless of whether heart failure and dialysis trials were included or excluded (ptrend=0·2 when these trials were included, ptrend=0·9 when these trials were excluded; figure 4; appendix). However, among participants with no history of vascular disease, we observed a significant trend towards smaller proportional risk reductions with increasing age (ptrend=0·05; figure 4), which persisted after exclusion of the heart failure and dialysis trials (ptrend=0·03; appendix).
Figure 4.
Effects on major vascular events per mmol/L reduction in LDL cholesterol, subdivided by age at randomisation and by previous vascular disease
Data from participants with missing baseline data included in the totals. RR=rate ratio.
Overall, we found a 12% (RR 0·88, 95% CI 0·85–0·91) proportional reduction in vascular mortality per 1·0 mmol/L reduction in LDL cholesterol, with a trend towards smaller proportional reductions with older age (ptrend=0·004; figure 5). However, 1014 (53%) of 1906 vascular deaths among people older than 75 years occurred in the four heart failure or dialysis trials. After exclusion of these trials, we observed no apparent trend towards smaller proportional reductions in vascular mortality with older age (ptrend=0·2, figure 5). We found no effect of statin therapy on all non-vascular causes of death (RR 0·96, 95% CI 0·92–1·01) irrespective of age group (ptrend=0·7; appendix). We also found no effects on deaths due to cancer or on the larger numbers of incident cancer cases, overall or by age (appendix). Consequently, when the beneficial effect on vascular mortality and the absence of effect on non-vascular mortality were combined, we observed a significant reduction in all-cause mortality (RR 0·91, 95% CI 0·88–0.93; appendix). We also found a trend towards smaller proportional reductions in all-cause mortality with increasing age (ptrend=0·04; appendix), but this did not persist after exclusion of the heart failure and dialysis trials (ptrend=0·1, appendix).
Figure 5.
Effects on vascular death per mmol/L reduction in LDL cholesterol, subdivided by age at randomisation
All studies (A) and excluding four trials that exclusively included patients with heart failure or on dialysis (B). Data from participants with missing baseline data included in the totals. RR=rate ratio.
Discussion
Individual randomised trials of statin therapy have previously reported significant cardiovascular risk reductions among participants older than 65 years but younger than 70 years at the time of randomisation (who were therefore aged >70–75 years at the end of a median of 5 years of scheduled treatment).26, 27, 28, 29, 30 Meta-analyses among older people have consistently reported evidence for beneficial effects in secondary prevention, but the evidence has been less clear for primary prevention.31, 32, 33, 34 Availability of individual participant data in the CTT Collaboration database has permitted more detailed assessment of the effects of statin therapy at different ages.
In our meta-analysis of data from 28 trials among 186 854 people (with 14 483 [8%] older than 75 years at randomisation), we found slightly smaller proportional risk reductions in major vascular events (ptrend=0·06) and vascular deaths (ptrend=0·004) with increasing age. The exclusion criteria for 24 trials incorporated at least one of the following: a history of heart failure, poor ejection fraction, poor prognosis (other than from atherosclerotic disease), or the requirement for renal dialysis. However, two trials exclusively enrolled patients with moderate to severe (New York Heart Association Class II–IV) heart failure17, 18 and two exclusively enrolled patients with end-stage renal disease requiring dialysis.19, 20 Statin therapy has not been found to reduce the risk of major vascular events or vascular deaths in either of these patient populations,16, 17, 18, 19, 20 and consequently is not recommended for such patients in the absence of other indications.10, 35, 36 Therefore, we did exploratory analyses that excluded these four trials17, 18, 19, 20 to assess their contribution to the observed trends towards smaller relative reductions with increasing age. Among people without heart or renal failure, we found little evidence of any diminution of benefit with increasing age on major vascular events (ptrend=0·3) or on vascular death (ptrend=0·2).
Proportional reductions in major vascular events were similar irrespective of age among patients with a history of vascular disease (ie, secondary prevention), but we observed a trend towards smaller proportional risk reductions in those with no known vascular disease (ie, primary prevention), with no independently significant reductions in either of the two age groups including patients older than 70 years. Among patients older than 70 years, only around a fifth of the major vascular events occurred in those with no history of vascular disease, and this relative paucity of evidence has led to further primary prevention trials among older individuals. For example, the STAREE trial37 aims to assess the effects of atorvastatin 40 mg daily in 18 000 primary prevention patients aged 70 years or older at the time of recruitment.
We observed a trend towards smaller proportional reductions in major coronary events (ptrend=0·009) with increasing age, although a significant reduction remained among patients older than 75 years at randomisation. The reasons underlying this trend are unclear. Age-related factors—such as altered pharmacokinetics and pharmacodynamics and an increased risk of drug interactions in the setting of polypharmacy38—would be expected to influence absolute LDL cholesterol reductions from therapy, but these did not differ materially by age. This trend might reflect a reduced capacity for statins to impact on advanced atherosclerotic plaques, greater diagnostic uncertainty at older ages (eg, difficulty separating myocardial infarctions due to unstable atherosclerosis from supply–demand imbalances that occur with other illnesses), and poorer long-term adherence to the assigned study treatment among older people (since our weighted analyses are based on 1 year cholesterol differences). We observed no trends towards smaller proportional reductions in coronary revascularisation procedures or strokes with increasing age, but too few such events occurred among patients older than 75 for us to assess the effects on these outcomes directly. Statin therapy definitely decreases the overall risk of ischaemic stroke and any stroke, but might increase the risk of haemorrhagic stroke.14 Individual participant data were not available from one eligible trial22 in which a large number of strokes (especially haemorrhagic) occurred, and we cannot reliably comment on the relevance of age to the effects of statin therapy on stroke subtypes.
There is a paucity of information on the effects of statins on mortality in people at low risk of vascular disease, and very large trials (eg, the ongoing STAREE trial37) would be needed to provide direct evidence of a mortality reduction among older people in primary prevention. However, our overall analyses in a combined primary and secondary prevention population indicate that the proportional reductions in vascular mortality are similar irrespective of age. We have previously shown that statin therapy does not increase the incidence of cancer15 (as had been suggested8) or of non-vascular causes of death.14 Consequently, given the similar proportional reductions in vascular mortality in both primary and secondary prevention settings, and the complete absence of effect of statin therapy on non-vascular mortality, reductions in total mortality would be expected in both clinical settings.
Statins have been estimated to increase the risk of myopathy (defined as muscle pain or weakness combined with large increases in blood concentrations of creatine kinase) typically by one case per 10 000 patients treated with statins per year,9 but this risk can be increased by drug interactions and major comorbidities that are more common in older people.39 A meta-analysis40 of published data for participants older than 65 years at randomisation in statin trials reported no increased risk of less severe muscle-related adverse events. The CTT Collaboration is undertaking a prespecified analysis of reported adverse events in statin trials from original trial records,41 including examining whether age directly influences the small increase in risk of diabetes,42, 43 and whether statins adversely influence cognition (noting that no excess risks have been identified in any large randomised trials29, 30, 44, 45).
The selection criteria used among the 28 trials contributing to this meta-analysis, and differences in when they were conducted, mean that the absolute risk of major vascular events and mortality in our overall study population is not likely to be representative of any contemporary population. Therefore, we have not produced estimates of the absolute effects of statin therapy directly from the numbers of events observed in these trials. By contrast, the proportional effects observed in this meta-analysis are likely to be widely generalisable. Consequently, as the proportional reductions in major vascular events appeared to diminish only slightly (if at all) with increasing age, while untreated absolute risks of major vascular events in the general population increase exponentially with age, the absolute benefits of a given absolute reduction in LDL cholesterol with statin therapy would be expected to be substantially greater among older individuals. For example, in the primary prevention setting, two individuals aged 63 years and 78 years with otherwise identical risk factors might have projected major vascular event rates of 2·5% versus 4·0% per year, respectively. Reducing those risks by a fifth with a 1·0 mmol/L LDL cholesterol reduction would prevent first major vascular events from occurring each year in 50 individuals aged 63 years and 80 individuals aged 78 years per 10 000 people treated.
In conclusion, statin therapy produces significant reductions in major vascular events, irrespective of age. There is less definitive direct evidence of benefit in the primary prevention setting among patients older than 75 years, but evidence supports the use of statin therapy in older people considered to have a sufficiently high risk of occlusive vascular events.
Correspondence to: CTT Secretariat, National Health and Medical Research Council (NHMRC) Clinical Trials Centre, Camperdown, NSW 2050, Australia ctt@ctc.usyd.edu.au
or
Clinical Trial Service Unit and Epidemiological Studies Unit (CTSU), Nuffield Department of Population Health, Oxford OX3 7LF, UK ctt@ndph.ox.ac.uk
Acknowledgments
Acknowledgments
Although individual trials that contributed data to these analyses were funded by research grants from the pharmaceutical industry, charities, and government organisations, the Cholesterol Treatment Trialists' Collaboration has not been funded by industry. This collaboration was jointly coordinated by the National Health and Medical Research Council Clinical Trials Centre (CTC) in Australia, and by the Medical Research Council Population Health Research Unit and Clinical Trial Service Unit & Epidemiological Studies Unit (CTSU) in the UK. The current meta-analysis work at the CTC was supported by a programme grant from the Australian National Health and Medical Research Council and a grant from the National Heart Foundation, Australia; and at the CTSU was supported by the UK Medical Research Council, British Heart Foundation, and Cancer Research UK. BM acknowledges support from the National Institute for Health Research Oxford Biomedical Research Centre.
Contributors
AK, CB, JS, and RC established the collaboration. AK, JF, BM, CB, RC, and JS devised the study concept. RC, CB, CR, JE, LB, AK, JS, and EB collected the data. AK, JF, BM, JE, CB, RC, and JS specified how data should be analysed and interpreted the analyses. BM, RO'C, JF, and LB did the statistical analysis and created the figures. AK and JF produced the first draft of the manuscript. All authors contributed to manuscript revisions. All collaborators had an opportunity to contribute to the interpretation of the results and to drafting of the report. AK, JF, BM, and CB had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of data analyses.
Cholesterol Treatment Trialists' (CTT) Collaboration
Writing Committee: Jordan Fulcher*, Borislava Mihaylova*, Rachel O'Connell, Jonathan Emberson, Lisa Blackwell, Christina Reith, Michael Koren, Andrew Tonkin, Paul Ridker, Elizabeth Barnes, Ian Ford, Chris Packard, Eva Lonn, Christoph Wanner, Wolfgang Koenig, Antonio Gotto, John Kjekshus, Salim Yusuf, Rory Collins, John Simes, Colin Baigent, Anthony Keech, on behalf of the Cholesterol Treatment Trialists' Collaboration (*contributed equally).
CTT Collaborators contributing trial data to analyses in this paper: A to Z trial (phase Z): J de Lemos, E Braunwald, M Blazing, S Murphy; AFCAPS/TexCAPS (AirForce/Texas Coronary Atherosclerosis Prevention Study): J R Downs, A Gotto, M Clearfield; ALERT (Assessment of Lescol in Renal Transplantation): H Holdaas; ALLHAT (Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial): D Gordon, B Davis; ALLIANCE (Aggressive Lipid-Lowering Initiation Abates New Cardiac Events): M Koren; ASCOT (Anglo-Scandinavian Cardiac Outcomes Trial): B Dahlöf, N Poulter, P Sever; ASPEN (Atorvastatin Study for the Prevention of Coronary Heart Disease Endpoints in Non-Insulin Dependent Diabetes Mellitus): R H Knopp (deceased); AURORA (A study to evaluate the Use of Rosuvastatin in subjects On Regular haemodialysis: an Assessment of survival and cardiovascular events): B Fellström, H Holdaas, A Jardine, R Schmieder, F Zannad; CARDS (Collaborative Atorvastatin Diabetes Study): H M Colhoun, D J Betteridge, P N Durrington, G A Hitman, J Fuller, A Neil; CARE (Cholesterol And Recurrent Events Study): F Sacks, L Moyé, M Pfeffer, C M Hawkins, E Braunwald; CORONA (Controlled Rosuvastatin Multinational Trial in Heart Failure): J Kjekshus, H Wedel, J Wikstrand; 4D (Die Deutsche Diabetes Dialyse Studie): C Wanner, V Krane; GISSI (Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico)—Heart Failure: L Tavazzi, A Maggioni; GISSI-Prevenzione: R Marchioli, G Tognoni, M G Franzosi, A Maggioni; HOPE-3: S Yusuf, E Lonn; HPS (Heart Protection Study): R Collins, J Armitage, L Bowman, M J Landray, A Keech, S Parish, R Peto, P Sleight; IDEAL (Incremental Decrease in Endpoints through Aggressive Lipid-lowering): T R Pedersen; JUPITER (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin): P M Ridker; LIPID (Long-term Intervention with Pravastatin in Ischaemic Disease): J Simes, A Keech, S MacMahon, I Marschner, A Tonkin, J Shaw; LIPS (Lescol Intervention Prevention Study): P W Serruys; MEGA (Management of Elevated cholesterol in the primary prevention Group of Adult Japanese): H Nakamura; Post-CABG (Post-Coronary Artery Bypass Graft Study): G Knatterud (deceased); PPP (Pravastatin Pooling Project): C Furberg, R Byington; PROSPER (Prospective Study of Pravastatin in the Elderly at Risk): N Sattar, I Ford, J W Jukema, S Kean, S Trompet, P Macfarlane; PROVE-IT (Pravastatin or Atorvastatin Evaluation and Infection Therapy): E Braunwald, C Cannon, S Murphy; SEARCH (Study of Effectiveness of Additional Reductions in Cholesterol and Homocysteine): R Collins, J Armitage, L Bowman, S Parish, R Peto, P Sleight; 4S (Scandinavian Simvastatin Survival Study): J Kjekshus, T R Pedersen, L Wilhelmsen; TNT (Treating to New Targets): J LaRosa; WOSCOPS (West of Scotland Coronary Prevention Study): I Ford, C Packard, P Macfarlane, N Sattar, S Kean, M Robertson, R Young. Other members: M Flather, S Goto, J Kastelein, C Newman, C Shear, J Tobert, J Varigos, H White. Further details of membership of the CTT Collaboration can be found at: https://www.cttcollaboration.org.
CTT secretariat: Jane Armitage, Colin Baigent, Elizabeth Barnes, Lisa Blackwell, Rory Collins, Kelly Davies, Jonathan Emberson, Jordan Fulcher, Heather Halls, Charlie Harper, Will Herrington, Lisa Holland, Anthony Keech, Adrienne Kirby, Borislava Mihaylova, Rachel O'Connell, David Preiss, Christina Reith, John Simes, Enti Spata, Kate Wilson.
Declaration of interests
RO'C, EB, IF, CW, and JS have nothing to disclose. JF reports personal fees from Amgen, Bayer, Pfizer, Boehringer Ingelheim, Sanofi, and AstraZeneca, outside the submitted work; and non-financial support from Amgen, Bayer, and Pfizer, outside the submitted work. BM reports grants from the Medical Research Council, British Heart Foundation, and the National Institute for Health Research Oxford Biomedical Research Centre during the conduct of the study, and grants from Merck outside the submitted work. CR report grants from the Medical Research Council and British Heart Foundation during the conduct of the study; and grants from Merck, outside the submitted work. JE reports grants from the Medical Research Council and the British Heart Foundation during the conduct of the study, and a grant from Boehringer Ingelheim outside the submitted work. LB reports grants from the Medical Research Council and the British Heart Foundation during the conduct of the study. MK is an employee of a company that has received study grants and consulting fees from manufacturers of PCSK9 inhibitors and treatments for lipid disorders, outside the submitted work. AT reports personal fees from Amgen and Sanofi, outside the submitted work. PR reports a research grant from AstraZeneca during the conduct of the study; and research grants from Novartis, Pfizer, and Kowa, outside the submitted work. CP reports a grant from Merck, outside the submitted work; and personal fees from Merck, Pfizer, Sanofi, Amgen, and Daiichi-Sankyo, outside the submitted work. EL reports grants from AstraZeneca, Bayer, Boehringer Ingelheim, Amgen, and Merck, outside the submitted work; and personal fees from Bayer, Amgen, Novartis, and Sanofi, outside the submitted work. WK reports grants and non-financial support from Roche, Beckmann, Singulex, and Abbott, outside the submitted work; and personal fees from AstraZeneca, Novartis, Pfizer, The Medicines Company, GlaxoSmithKline, Dalcor, Sanofi, Berlin-Chemie, Kowa, and Amgen, outside the submitted work. AG reports personal fees from Aegerion Pharmaceuticals, Arisaph Pharmaceuticals, DuPont, Esperion Therapeutics, Kowa, Merck, Roche, Vatera Capital, ISIS Pharmaceuticals, Weill Cornell Medicine, and Amgen, outside the submitted work. SY reports a grant from AstraZeneca, outside the submitted work. RC reports support from the Nuffield Department of Population Health, during the conduct of the study; grants from the British Heart Foundation, Cancer Research UK, Medical Research Council, Merck, National Institute for Health Research, and the Wellcome Trust, outside the submitted work; personal fees from the British Heart Foundation and UK Biobank, outside the submitted work; other support from Pfizer to the Nuffield Department of Population Health (prize for independent research); and a patent for a statin-related myopathy genetic test licensed to University of Oxford from Boston Heart Diagnostics (RC has waived any personal reward). CB reports grants from the Medical Research Council and British Heart Foundation, during the conduct of the study; and grants from Pfizer, Merck, Novartis, and Boehringer Ingelheim, outside the submitted work. AK reports grants from Abbott and Mylan, outside the submitted work; and personal fees from Abbott, Amgen, AstraZeneca, Mylan, and Pfizer, outside the submitted work. LB reports grants from UK Medical Research Council and the British Heart Foundation during the conduct of the study.
Contributor Information
Cholesterol Treatment Trialists' Collaboration:
Jane Armitage, Colin Baigent, Elizabeth Barnes, D John Betteridge, Lisa Blackwell, Michael Blazing, Louise Bowman, Eugene Braunwald, Robert Byington, Christopher Cannon, Michael Clearfield, Helen Colhoun, Rory Collins, Björn Dahlöf, Kelly Davies, Barry Davis, James de Lemos, John R Downs, Paul Durrington, Jonathan Emberson, Bengt Fellström, Marcus Flather, Ian Ford, Maria Grazia Franzosi, Jordan Fulcher, John Fuller, Curt Furberg, David Gordon, Shinya Goto, Antonio Gotto, Heather Halls, Charlie Harper, C Morton Hawkins, Will Herrington, Graham Hitman, Hallvard Holdaas, Lisa Holland, Alan Jardine, J Wouter Jukema, John Kastelein, Sharon Kean, Anthony Keech, Adrienne Kirby, John Kjekshus, Genell Knatterud (deceased), Robert Knopp (deceased), Wolfgang Koenig, Michael Koren, Vera Krane, Martin J Landray, John LaRosa, Eva Lonn, Peter MacFarlane, Stephen MacMahon, Aldo Maggioni, Roberto Marchioli, Ian Marschner, Borislava Mihaylova, Lemuel Moyé, Sabina Murphy, Haruo Nakamura, Andrew Neil, Connie Newman, Rachel O'Connell, Chris Packard, Sarah Parish, Terje Pedersen, Richard Peto, Marc Pfeffer, Neil Poulter, David Preiss, Christina Reith, Paul Ridker, Michele Robertson, Frank Sacks, Naveed Sattar, Roland Schmieder, Patrick Serruys, Peter Sever, John Shaw, Charles Shear, John Simes, Peter Sleight, Enti Spata, Luigi Tavazzi, Jonathan Tobert, Gianni Tognoni, Andrew Tonkin, Stella Trompet, John Varigos, Christoph Wanner, Hans Wedel, Harvey White, John Wikstrand, Lars Wilhelmsen, Kate Wilson, Robin Young, Salim Yusuf, and Faiez Zannad
Supplementary Material
References
- 1.Cholesterol Treatment Trialists (CTT) Collaboration Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174 000 participants in 27 randomised trials. Lancet. 2015;385:1397–1405. doi: 10.1016/S0140-6736(14)61368-4. [DOI] [PubMed] [Google Scholar]
- 2.Cholesterol Treatment Trialists' (CTT) Collaborators The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koopman C, Vaartjes I, Heintjes EM. Persisting gender differences and attenuating age differences in cardiovascular drug use for prevention and treatment of coronary heart disease, 1998–2010. Eur Heart J. 2013;34:3198–3205. doi: 10.1093/eurheartj/eht368. [DOI] [PubMed] [Google Scholar]
- 4.Salami JA, Warraich H, Valero-Elizondo J. National trends in statin use and expenditures in the US adult population from 2002 to 2013: insights from the Medical Expenditure Panel Survey. JAMA Cardiol. 2017;2:56–65. doi: 10.1001/jamacardio.2016.4700. [DOI] [PubMed] [Google Scholar]
- 5.O'Keeffe AG, Petersen I, Nazareth I. Initiation rates of statin therapy for the primary prevention of cardiovascular disease: an assessment of differences between countries of the UK and between regions within England. BMJ Open. 2015;5:e007207. doi: 10.1136/bmjopen-2014-007207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foody JM, Rathore SS, Galusha D. Hydroxymethylglutaryl-CoA reductase inhibitors in older persons with acute myocardial infarction: evidence for an age-statin interaction. J Am Geriatr Soc. 2006;54:421–430. doi: 10.1111/j.1532-5415.2005.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alonzo CB. Myths and facts concerning the use of statins in very old patients. Cardiovasc Hematol Disord Drug Targets. 2011;11:17–23. doi: 10.2174/187152911795945141. [DOI] [PubMed] [Google Scholar]
- 8.Mangin D, Sweeney K, Heath I. Preventive health care in elderly people needs rethinking. BMJ. 2007;335:285–287. doi: 10.1136/bmj.39241.630741.BE1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins R, Reith C, Emberson J. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532–2561. doi: 10.1016/S0140-6736(16)31357-5. [DOI] [PubMed] [Google Scholar]
- 10.Stone NJ, Robinson J, Lichtenstein AH. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 11.Cholesterol Treatment Trialists' (CTT) Collaboration Protocol for a prospective collaborative overview of all current and planned randomized trials of cholesterol treatment regimens. Am J Cardiol. 1995;75:1130–1134. doi: 10.1016/s0002-9149(99)80744-9. [DOI] [PubMed] [Google Scholar]
- 12.Cholesterol Treatment Triallists' (CTT) Collaborators Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90 056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 13.Cholesterol Treatment Triallists' (CTT) Collaborators Efficacy of cholesterol-lowering therapy in 18 686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117–125. doi: 10.1016/S0140-6736(08)60104-X. [DOI] [PubMed] [Google Scholar]
- 14.Cholesterol Treatment Trialists' (CTT) Collaborators Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170 000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cholesterol Treatment Trialists' (CTT) Collaboration Lack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175 000 people in 27 randomised trials of statin therapy. PLoS One. 2012;7:e29849. doi: 10.1371/journal.pone.0029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cholesterol Treatment Trialists (CTT) Collaboration Impact of renal function on the effects of LDL cholesterol lowering with statin-based regimens: a meta-analysis of individual participant data from 28 randomised trials. Lancet Diabetes Endocrinol. 2016;4:829–839. doi: 10.1016/S2213-8587(16)30156-5. [DOI] [PubMed] [Google Scholar]
- 17.Tavazzi L, Maggioni AP, Marchioli R. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1231–1239. doi: 10.1016/S0140-6736(08)61240-4. [DOI] [PubMed] [Google Scholar]
- 18.Kjekshus J, Apetrei E, Barrios V. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248–2261. doi: 10.1056/NEJMoa0706201. [DOI] [PubMed] [Google Scholar]
- 19.Fellström BC, Jardine AG, Schmieder RE. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395–1407. doi: 10.1056/NEJMoa0810177. [DOI] [PubMed] [Google Scholar]
- 20.Wanner C, Krane V, März W. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 21.Yusuf S, Bosch J, Dagenais G. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med. 2016;374:2021–2031. doi: 10.1056/NEJMoa1600176. [DOI] [PubMed] [Google Scholar]
- 22.Amarenco P, Bogousslavsky J, Callahan A., 3rd High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–559. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 23.Athyros VG, Papageorgiou AA, Mercouris BR. Treatment with atorvastatin to the National Cholesterol Educational Program goal versus ‘usual’ care in secondary coronary heart disease prevention. The Greek atorvastatin and coronary-heart-disease evaluation (GREACE) study. Curr Med Res Opin. 2002;18:220–228. doi: 10.1185/030079902125000787. [DOI] [PubMed] [Google Scholar]
- 24.Zhao SP, Yu BL, Peng DQ, Huo Y. The effect of moderate-dose versus double-dose statins on patients with acute coronary syndrome in China: results of the CHILLAS trial. Atherosclerosis. 2014;233:707–712. doi: 10.1016/j.atherosclerosis.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Hosomi N, Nagai Y, Kohriyama T. The Japan statin treatment against recurrent stroke (J-STARS): a multicenter, randomized, open-label, parallel-group study. EBioMedicine. 2015;2:1071–1078. doi: 10.1016/j.ebiom.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miettinen TA, Pyörälä K, Olsson AG. Cholesterol-lowering therapy in women and elderly patients with myocardial infarction or angina pectoris: findings from the Scandinavian Simvastatin Survival Study (4S) Circulation. 1997;96:4211–4218. doi: 10.1161/01.cir.96.12.4211. [DOI] [PubMed] [Google Scholar]
- 27.Hunt D, Young P, Simes J. Benefits of pravastatin on cardiovascular events and mortality in older patients with coronary heart disease are equal to or exceed those seen in younger patients: Results from the LIPID trial. Ann Intern Med. 2001;134:931–940. doi: 10.7326/0003-4819-134-10-200105150-00007. [DOI] [PubMed] [Google Scholar]
- 28.Lewis SJ, Moye LA, Sacks FM. Effect of pravastatin on cardiovascular events in older patients with myocardial infarction and cholesterol levels in the average range. Results of the Cholesterol and Recurrent Events (CARE) trial. Ann Intern Med. 1998;129:681–689. doi: 10.7326/0003-4819-129-9-199811010-00002. [DOI] [PubMed] [Google Scholar]
- 29.Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 30.Shepherd J, Blauw GJ, Murphy MB. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–1630. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 31.Savarese G, Gotto AM, Jr, Paolillo S. Benefits of statins in elderly subjects without established cardiovascular disease: a meta-analysis. J Am Coll Cardiol. 2013;62:2090–2099. doi: 10.1016/j.jacc.2013.07.069. [DOI] [PubMed] [Google Scholar]
- 32.Teng M, Lin L, Zhao YJ. Statins for primary prevention of cardiovascular disease in elderly patients: systematic review and meta-analysis. Drugs Aging. 2015;32:649–661. doi: 10.1007/s40266-015-0290-9. [DOI] [PubMed] [Google Scholar]
- 33.Lowe RN, Vande Griend JP, Saseen JJ. Statins for the primary prevention of cardiovascular disease in the elderly. Consult Pharm. 2015;30:20–30. doi: 10.4140/TCP.n.2015.20. [DOI] [PubMed] [Google Scholar]
- 34.Afilalo J, Duque G, Steele R, Jukema JW, de Craen AJ, Eisenberg MJ. Statins for secondary prevention in elderly patients: a hierarchical bayesian meta-analysis. J Am Coll Cardiol. 2008;51:37–45. doi: 10.1016/j.jacc.2007.06.063. [DOI] [PubMed] [Google Scholar]
- 35.Catapano AL, Graham I, De Backer G. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37:2999–3058. doi: 10.1093/eurheartj/ehw272. [DOI] [PubMed] [Google Scholar]
- 36.Kidney Disease: Improving Global Outcomes (KDIGO) Lipid Work Group KDIGO Clinical Practice Guideline for Lipid Management in Chronic Kidney Disease. Kidney Int Suppl. 2013;3:259–305. [Google Scholar]
- 37.ClinicalTrials.gov. A clinical trial of Statin therapy for reducing events in the elderly (STAREE) https://clinicaltrials.gov/ct2/show/NCT02099123 (accessed Dec 1, 2017).
- 38.Wilmot KA, Khan A, Krishnan S, Eapen DJ, Sperling L. Statins in the elderly: a patient-focused approach. Clin Cardiol. 2015;38:56–61. doi: 10.1002/clc.22338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Armitage J. The safety of statins in clinical practice. Lancet. 2007;370:1781–1790. doi: 10.1016/S0140-6736(07)60716-8. [DOI] [PubMed] [Google Scholar]
- 40.Iwere RB, Hewitt J. Myopathy in older people receiving statin therapy: a systematic review and meta-analysis. Br J Clin Pharmacol. 2015;80:363–371. doi: 10.1111/bcp.12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cholesterol Treatment Trialists' (CTT) Collaboration Protocol for analyses of adverse event data from randomized controlled trials of statin therapy. Am Heart J. 2016;176:63–69. doi: 10.1016/j.ahj.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sattar N, Preiss D, Murray HM. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–742. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 43.Preiss D, Seshasai SR, Welsh P. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA. 2011;305:2556–2564. doi: 10.1001/jama.2011.860. [DOI] [PubMed] [Google Scholar]
- 44.Feldman HH, Doody RS, Kivipelto M. Randomized controlled trial of atorvastatin in mild to moderate Alzheimer disease: LEADe. Neurology. 2015;74:956–964. doi: 10.1212/WNL.0b013e3181d6476a. [DOI] [PubMed] [Google Scholar]
- 45.McGuinness B, Craig D, Bullock R, Passmore P. Statins for the prevention of dementia. Cochrane Database Syst Rev. 2016;1 doi: 10.1002/14651858.CD003160.pub3. CD003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.