Abstract
OBJECTIVE
The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study demonstrated the beneficial effects of intensive therapy on atherosclerosis and clinical cardiovascular disease (CVD) outcomes. The current analyses evaluated the relationship between longitudinal changes in insulin dose and CVD risk factors and outcomes.
RESEARCH DESIGN AND METHODS
A total of 1,441 participants were randomly assigned to intensive or conventional diabetes therapy during the DCCT. After an average of 6.5 years of follow-up, 96% of the surviving cohort enrolled in the EDIC observational study, which included annual visits with detailed medical history, physical examination, and laboratory testing. CVD events were adjudicated by a review committee. Generalized linear mixed models and Cox proportional hazards regression models were used to assess the association between insulin dose and cardiometabolic risk factors and CVD risk, respectively, over a total of 30 years.
RESULTS
Higher insulin doses were significantly associated with a less favorable cardiometabolic risk profile (higher BMI, pulse rate, and triglycerides and lower HDL cholesterol) with the exception of lower diastolic blood pressure and lower LDL cholesterol. In a minimally adjusted model, a 0.1 unit/kg body wt/day increase in insulin dose was associated with a 6% increased risk of any CVD (95% CI 3, 9). However, the association with insulin dose was no longer significant after adjustment for other CVD risk factors.
CONCLUSIONS
During DCCT/EDIC, higher insulin doses were associated with adverse trends in several cardiometabolic risk factors, even after multivariable adjustment, but not with incident CVD outcomes.
Introduction
Type 1 diabetes is associated with an increased risk of cardiovascular disease (CVD) morbidity and mortality (1). Some studies have suggested that hyperinsulinemia may be associated with an increased CVD risk in type 2 diabetes (2,3); however, data from landmark trials such as the UK Prospective Diabetes Study (UKPDS) and the Kumamoto studies (4,5) indicate that the therapeutic use of exogenous insulin for glycemic control in patients with type 2 diabetes is not associated with an increased risk of CVD events. More recently, the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study demonstrated that higher insulin dose was not associated with an increased risk of CVD death after adjustment for baseline covariates (6).
The Diabetes Control and Complications Trial (DCCT) and its follow-up, the Epidemiology of Diabetes Interventions and Complications (EDIC) study, demonstrated that compared with conventional treatment, intensive insulin therapy was associated with significantly decreased long-term risk of atherosclerosis and major CVD events in type 1 diabetes (7–10). Mean insulin dose was a significant predictor of CVD events after minimal adjustment for age and updated mean HbA1c (hazard ratio [HR] for insulin dose per units/kg/day = 3.72, P = 0.001) (11).
Insulin doses vary over time in individuals with type 1 diabetes, most likely due to aging, weight gain, changes in body composition, and insulin resistance, among other factors (12,13). However, the association of longitudinal changes in insulin dose with markers of cardiometabolic risk have not been well studied in type 1 diabetes. The DCCT/EDIC study provides the opportunity to explore the long-term interrelationships of insulin dose and CVD risk factors and outcomes in a carefully studied cohort of participants with type 1 diabetes.
Research Design and Methods
The DCCT/EDIC study has previously been described (14–16). Briefly, 1,441 subjects with type 1 diabetes and aged 13–39 years at baseline were enrolled in the DCCT between 1983 and 1989. Approximately one-half of the cohort (N = 711) was randomly assigned to intensive therapy with the goal of maintaining blood glucose and HbA1c levels as close to the nondiabetic range as safely possible. The remainder (N = 730) were assigned to conventional therapy with the goal of clinical well-being and freedom from symptoms related to hyper- and hypoglycemia. Two cohorts were recruited: the primary prevention cohort (N = 726) with 1–5 years of diabetes duration, no retinopathy, and a urine albumin excretion rate <40 mg/24 h, and the secondary intervention cohort (N = 715) with 1–15 years of diabetes duration, mild-to-moderate nonproliferative diabetic retinopathy, and an albumin excretion rate ≤200 mg/24 h. Subjects with a history of CVD or with hypertension (blood pressure >140/90 mmHg or medication) or hyperlipidemia (fasting serum cholesterol ≥3 SD above age- and sex-specific means) were not eligible to participate.
At the end of the DCCT in 1993, after an average of 6.5 years (range 3–9) of follow-up, 1,422 subjects completed a closeout visit (99% of the original cohort). Subjects who were originally assigned to receive conventional therapy were taught and encouraged to adopt intensive therapy, and both original treatment groups returned to their own health care providers for routine care. In 1994, 96% of the surviving DCCT cohort enrolled in the EDIC observational study, and 1,251 of those participants (94% of the surviving cohort) have been followed for an additional 20 years. The present analyses focus on the data obtained during the combined mean 27 years (up to 30 years maximum) of DCCT and EDIC follow-up.
DCCT/EDIC Evaluations
Annual visits included a detailed medical history with demographic and behavioral risk factors, medical outcomes, and a physical examination with measurements of height, weight, waist circumference, sitting blood pressure, and pulse rate (14,16). Pulse pressure was defined as the difference between the systolic and diastolic blood pressure readings. Insulin doses were self-reported and expressed as the average total daily dose in units per kilogram of body weight. Blood samples were obtained, and fasting lipids (triglycerides and total and HDL cholesterol) and HbA1c were assayed centrally (17). Fasting lipids were measured annually during DCCT and every 2 years during EDIC. HbA1c was measured quarterly during DCCT and annually during EDIC. LDL cholesterol was calculated using the Friedewald equation. Concurrent medication usage was collected during EDIC but not during the DCCT.
For each cardiometabolic risk factor, the associations with insulin dose and CVD are presented for the current and time-weighted mean values. The current values represent the measurements at each annual visit. The time-weighted arithmetic mean values were computed using the quarterly DCCT and annual EDIC values weighted by 3 and 12 months, respectively, and represent the running average at each annual visit.
Cardiovascular Outcomes
The primary CVD outcome (any CVD) is defined as the time to the first occurrence of cardiovascular death, nonfatal myocardial infarction (MI), nonfatal stroke, subclinical MI detected on an annual electrocardiogram, angina confirmed by ischemic changes with exercise tolerance testing or by clinically significant obstruction on coronary angiography, revascularization (with angioplasty or coronary artery bypass), or congestive heart failure (paroxysmal nocturnal dyspnea, orthopnea, or marked limitation of physical activity caused by heart disease). The secondary CVD outcome, major adverse cardiovascular events (MACE) is defined as the time to cardiovascular death, nonfatal MI, or nonfatal stroke. Cardiovascular events reported during study visits prompted the clinic staff to obtain medical records, including electrocardiograms, diagnostic procedures, and cardiac enzymes. Cardiovascular events were centrally adjudicated by the Mortality and Morbidity Review Committee, which was masked to DCCT treatment assignment, HbA1c, and glucose levels.
Statistical Analysis
At DCCT baseline, quantitative and categorical characteristics were compared between treatment groups using the Wilcoxon rank sum and χ2 tests, respectively. For characteristics repeated over time (e.g., weight), the average mean over 30 years of DCCT/EDIC annual follow-up was computed using generalized linear mixed models for repeated measures.
Generalized linear mixed models were also used to assess the association between current and time-weighted mean insulin dose and the mean of each quantitative risk factor over repeated time points. Current (or annual) values of each risk factor were correlated with current insulin dose. Time-weighted mean values of each risk factor were correlated with time-weighted mean insulin dose. The DCCT/EDIC study year was included as a class effect, and the models assumed a compound symmetry covariance structure. Each model was minimally adjusted for DCCT baseline age, primary prevention versus secondary intervention cohort, and sex (when appropriate). The fully adjusted models also included treatment group, smoking status, drinking status, physical activity, duration of diabetes, BMI, systolic and diastolic blood pressure, pulse pressure, pulse rate, triglycerides, HDL and LDL cholesterol, and HbA1c levels. Additional models further adjusted for medication use. Covariates measured repeatedly over time entered the models as time-dependent covariates. The signed t statistic was used as a measure of the magnitude and direction of the association between an outcome and a covariate.
Cox proportional hazards regression models were used to estimate the effect of current and time-weighted mean insulin dose on the risk of CVD, with adjustment separately for each cardiometabolic risk factor as a time-dependent covariate. The models used time to the first occurrence of any CVD or MACE. Models using current insulin dose were adjusted for the current value of each risk factor. Models using time-weighted mean insulin dose were adjusted for the time-weighted mean value of each risk factor up until the event or censoring. All models were adjusted for DCCT baseline age, primary prevention versus secondary intervention cohort, and treatment group. The HRs and unsigned covariate Z values are presented. Since Z ≥ 3.28 is equivalent to a P value <0.001, the Z value is a better representation of the significance of the covariate effect in the model than is the P value. All analyses were performed using SAS software (version 9.3; SAS Institute, Cary, NC). Two-sided P ≤ 0.05 was considered statistically significant.
Results
Participant Characteristics
The characteristics of the DCCT/EDIC participants at baseline and after 30 years of total follow-up are presented in Table 1. There were no major differences between the intensive and conventional treatment groups at DCCT baseline, except for a slightly higher systolic blood pressure and higher weight in males in the conventional group. Over the 30-year study period, females in the conventional treatment group had a lower overall mean weight than females in the intensive group (mean difference of 2.7 kg, P = 0.0037). In addition, subjects in the conventional treatment group had a higher overall mean pulse rate (73.4 ± 7.0 vs. 72.3 ± 7.0 beats per minute [bpm], P = 0.0023) over all visits combined, higher triglycerides (75.1 ± 27.4 vs. 73.9 ± 27.0 mg/dL, P = 0.0002), lower insulin dose requirements (0.65 ± 0.18 vs. 0.67 ± 0.17 units/kg/day, P = 0.0069), and higher HbA1c values (8.4 ± 1.0% vs. 7.9 ± 1.0%, P < 0.0001). The significant difference between groups in HbA1c over the duration of the study is largely accounted for by the lower HbA1c maintained by design in the intensive treatment group during the DCCT.
Table 1.
Clinical characteristics of the DCCT/EDIC cohort at DCCT baseline (1983–1989) and by the 30th year of DCCT/EDIC follow-up, by treatment group
| DCCT baseline (1983–1989) |
Average over DCCT/EDIC |
|||||
|---|---|---|---|---|---|---|
| Intensive |
Conventional | P* | Intensive | Conventional | P* | |
| N | 711 | 730 | 711 | 730 | ||
| Demographic | ||||||
| Cohort (% primary prevention)† | 49 | 52 | 0.2818 | |||
| Sex (% female)† | 49 | 46 | 0.3169 | |||
| Age (years)‡ | 27.1 ± 7.1 | 26.5 ± 7.1 | 0.1383 | 41.8 ± 7.7 | 40.8 ± 7.8 | 0.0122 |
| Weight (kg)§ | ||||||
| Male | 73.8 ± 10.8 | 75.8 ± 11.7 | 0.0091 | 86.6 ± 18.3 | 86.1 ± 17.8 | 0.6160 |
| Female | 62.7 ± 8.6 | 62.1 ± 9.5 | 0.2966 | 73.7 ± 17.3 | 71.0 ± 17.8 | 0.0037 |
| BMI (kg/m2) | ||||||
| Male | 23.4 ± 2.6 | 23.9 ± 2.9 | 0.0045 | 27.3 ± 4.9 | 26.9 ± 4.8 | 0.1118 |
| Female | 23.3 ± 2.8 | 22.9 ± 2.9 | 0.0610 | 27.2 ± 5.8 | 26.0 ± 6.0 | 0.0001 |
| Traditional | ||||||
| Systolic blood pressure (mmHg) | 113.4 ± 11.5 | 114.7 ± 11.7 | 0.0116 | 118.8 ± 8.4 | 118.9 ± 8.5 | 0.8323 |
| Diastolic blood pressure (mmHg) | 72.3 ± 8.8 | 72.8 ± 8.9 | 0.2574 | 73.9 ± 5.2 | 73.8 ± 5.2 | 0.8186 |
| Pulse pressure (mmHg) | 41.1 ± 9.5 | 41.9 ± 9.8 | 0.0639 | 44.9 ± 6.2 | 45.0 ± 6.2 | 0.6451 |
| Pulse rate (bpm) | 76.1 ± 11.1 | 76.2 ± 11.2 | 0.7269 | 72.3 ± 7.0 | 73.4 ± 7.0 | 0.0023 |
| Total cholesterol (mg/dL) | 177.1 ± 32.8 | 175.7 ± 33.6 | 0.5289 | 181.7 ± 24.5 | 181.6 ± 24.5 | 0.9708 |
| Triglycerides (mg/dL) | 80.8 ± 43.3 | 81.8 ± 51.3 | 0.8151 | 73.9 ± 27.0 | 75.1 ± 27.4 | 0.0002 |
| HDL cholesterol (mg/dL) | 50.8 ± 12.3 | 50.3 ± 12.3 | 0.5048 | 55.8 ± 12.5 | 55.5 ± 12.5 | 0.7070 |
| LDL cholesterol (mg/dL) | 110.3 ± 28.7 | 109.1 ± 29.4 | 0.4967 | 108.8 ± 21.2 | 108.7 ± 21.2 | 0.9385 |
| Diabetes related | ||||||
| Duration of diabetes (years)‡ | 5.8 ± 4.2 | 5.5 ± 4.1 | 0.1441 | 20.5 ± 4.8 | 19.7 ± 5.1 | 0.0100 |
| Insulin dose (units/kg/day) | 0.67 ± 0.25 | 0.66 ± 0.25 | 0.3264 | 0.67 ± 0.17 | 0.65 ± 0.18 | 0.0069 |
| HbA1c (%) | 9.1 ± 1.6 | 9.1 ± 1.6 | 0.5542 | 7.9 ± 1.0 | 8.4 ± 1.0 | <0.0001 |
| HbA1c (mmol/mol) | 75.8 ± 17.4 | 75.5 ± 17.9 | 62.5 ± 10.9 | 67.9 ± 10.9 | ||
Data are means ± SD unless otherwise indicated.
*At DCCT baseline, treatment group comparisons were made using the Wilcoxon rank sum test or the χ2 test. For characteristics repeated over time (e.g., weight), the average mean over 30 years of DCCT/EDIC annual follow-up was computed using generalized linear mixed models for repeated measures. SDs were estimated from the SEs using the following equation: SD = SE * square root(N). The SDs are smaller during the DCCT/EDIC follow-up period owing to the larger amount of information in the longitudinal models.
†Cohort and sex are fixed baseline characteristics.
‡Age and duration were not evaluated using longitudinal models, since each is a function of time itself. Instead, the average age and duration were computed for each subject over that subject’s length of follow-up. The average means ± SD for all subjects are presented. Treatment group comparisons were made using the Wilcoxon rank sum test.
§Data for males based on 366 intensive and 395 conventional group participants; data for females based on 345 intensive and 335 conventional group participants.
Insulin Dose Across Study Subgroups
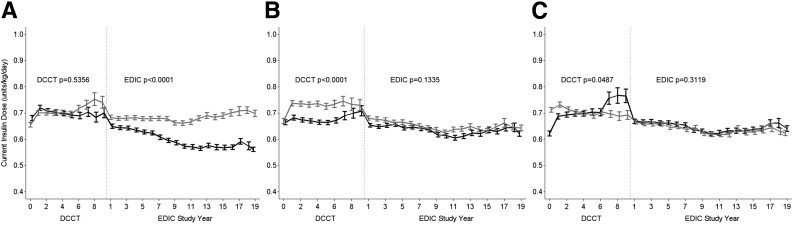
Figure 1 presents the means ± SE of insulin dose over time by sex, treatment group, and primary prevention versus secondary intervention cohort. Owing to staggered entry during the enrollment period into the DCCT and the fixed duration of the DCCT, the number of subjects evaluated declined during DCCT years 6–9. There were no differences in insulin dose, adjusted for weight, between males and females during DCCT (P = 0.5356); however, insulin dose was significantly higher in males over the subsequent 20 years of EDIC follow-up (mean for past 10 years 0.69 ± 0.23 vs. 0.57 ± 0.22 units/kg/day, P < 0.0001) (Fig. 1A). Insulin dose was higher in the intensive treatment group during the DCCT (0.71 ± 0.21 vs. 0.65 ± 0.21 units/kg/day, P < 0.0001) but similar between groups and declined slightly during EDIC (P = 0.1335) (Fig. 1B). There were no significant differences in insulin dose between the primary versus secondary cohorts during DCCT/EDIC follow-up (Fig. 1C).
Figure 1.
Current insulin dose (units/kg/day) during the DCCT/EDIC study by sex (black line for females) (A), DCCT intensive vs. conventional treatment group (black line for conventional) (B), and DCCT primary prevention vs. secondary intervention cohort (black line for primary prevention) (C). Data are means ± SE at each DCCT/EDIC follow-up year.
Relationship Between Insulin Dose and HbA1c
Supplementary Fig. 1 shows the relationship between the current (or annual) values of insulin dose and concurrent HbA1c values measured at the same annual visits. Insulin dose increased contemporaneously with increasing HbA1c in the intensive treatment group but remained stable with increasing HbA1c in the conventional group during DCCT (Supplementary Fig. 1A). Furthermore, insulin dose was significantly higher in the intensive treatment group than in the conventional group at all HbA1c levels >7% during the DCCT (P < 0.0001), consistent with the study design. During EDIC (Supplementary Fig. 1B and C), similar slightly increasing trends were observed in the intensive and conventional treatment groups.
Association of Insulin Dose With Cardiometabolic Risk Factors
The risk factor models for the association of insulin dose and cardiometabolic risk factors, including the insulin dose regression coefficient (slope), its SE, t statistic, and P value, are presented in Table 2. Insulin dose was associated with all of the cardiometabolic risk factors over the duration of the study in minimally and fully adjusted models. In both minimally and fully adjusted models, higher current (or annual) insulin dose was associated with increasing systolic blood pressure, pulse pressure, pulse rate, triglycerides, and HbA1c and decreasing diastolic blood pressure and HDL and LDL cholesterol. The strongest longitudinal associations for current insulin dose were with BMI among males (t = 22.59), pulse rate (t = 17.02), triglycerides (t = 21.18), and HDL cholesterol (t = −19.74). Similar, yet stronger, associations were observed for the time-weighted mean values of insulin dose and each of the risk factors (Table 2).
Table 2.
Longitudinal association of insulin dose (units/kg/day) with cardiometabolic risk factors during the DCCT/EDIC study
| Cardiometabolic risk factors (current or time weighted) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BMI (kg/m2) |
Systolic BP (mmHg) | Diastolic BP (mmHg) | Pulse pressure (mmHg) | Pulse rate (bpm) | Triglycerides (mg/dL)* | HDL cholesterol (mg/dL) | LDL cholesterol (mg/dL) | HbA1c (%) | ||
| Males | Females | |||||||||
| Minimally adjusted models† | ||||||||||
| Current insulin dose (units/kg/day) | ||||||||||
| β ± SE | 1.68 ± 0.07 | 1.45 ± 0.10 | 0.97 ± 0.32 | −0.72 ± 0.22 | 1.80 ± 0.27 | 4.18 ± 0.25 | 30.76 ± 1.66 | −6.06 ± 0.31 | −3.04 ± 0.81 | 0.11 ± 0.03 |
| t statistic | 22.59 | 14.34 | 3.04 | −3.31 | 6.71 | 17.02 | 21.18 | −19.74 | −3.75 | 3.59 |
| P | <0.0001 | <0.0001 | 0.0024 | 0.0009 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0002 | 0.0003 |
| Time-weighted mean insulin dose (units/kg/day) | ||||||||||
| β ± SE | 1.59 ± 0.08 | 1.72 ± 0.12 | 2.12 ± 0.24 | 0.47 ± 0.16 | 1.69 ± 0.19 | 6.15 ± 0.22 | 47.95 ± 1.92 | −8.23 ± 0.29 | −5.43 ± 0.75 | 0.44 ± 0.04 |
| t statistic | 18.93 | 14.64 | 9.01 | 2.87 | 8.66 | 28.36 | 30.25 | −28.80 | −7.29 | 12.20 |
| P | <0.0001 | <0.0001 | <0.0001 | 0.0041 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Fully adjusted models‡ | ||||||||||
| Current insulin dose (units/kg/day) | ||||||||||
| β ± SE | 1.47 ± 0.10 | 0.84 ± 0.12 | 1.45 ± 0.33 | −1.59 ± 0.23 | 1.84 ± 0.34 | 4.15 ± 0.31 | 18.45 ± 1.40 | −2.84 ± 0.30 | −7.11 ± 0.80 | 0.14 ± 0.04 |
| t statistic | 15.48 | 7.05 | 4.37 | −6.86 | 5.42 | 13.33 | 14.30 | −9.48 | −8.92 | 3.50 |
| P | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0005 |
| Time-weighted mean insulin dose (units/kg/day) | ||||||||||
| β ± SE | 1.23 ± 0.10 | 0.14 ± 0.14 | 1.47 ± 0.25 | −1.22 ± 0.18 | 1.66 ± 0.25 | 5.53 ± 0.29 | 28.80 ± 1.58 | −4.68 ± 0.28 | −12.66 ± 0.74 | 0.39 ± 0.05 |
| t statistic | 12.03 | 1.01 | 5.92 | −6.94 | 6.60 | 18.82 | 20.66 | −16.54 | −17.21 | 8.52 |
| P | <0.0001 | 0.3134 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Data are β estimates ± SE, t statistics, and P values from separate generalized linear mixed models assessing the longitudinal association of current and time-weighted mean insulin dose with each cardiometabolic risk factor. Current values of each risk factor were correlated with current insulin dose. Time-weighted mean values of each risk factor were correlated with time-weighted mean insulin dose. Insulin dose (current and time weighted) was entered into each model as a time-dependent covariate. β estimates are equal to the slope of the association. The signed t statistic corresponds with the magnitude and directionality of the association. BP, blood pressure.
*Triglyceride values were log transformed, and the percent change in triglycerides per 1-unit change in predictor is shown as [100(eβ − 1)] ± [100(eβ * SE)].
†Adjusted for DCCT baseline age, primary prevention vs. secondary intervention cohort, and sex (when appropriate).
‡Adjusted for DCCT baseline age, primary prevention vs. secondary intervention cohort, sex (when appropriate), treatment group, smoking status, drinking status, physical activity, duration of diabetes, and all other cardiometabolic risk factors listed above.
In multivariable models adjusting for all other risk factors, current and time-weighted mean insulin dose persisted as a significant predictor of the longitudinal changes in all of the cardiometabolic risk factors, with the exception of time-weighted insulin dose with time-weighted BMI in females. The magnitude and direction for each comparison in Table 2 remained the same after further adjustment for corresponding medications (e.g., antihypertensive medication for blood pressures, β-blockers for pulse rate, and lipid-lowering medications for triglycerides and HDL and LDL cholesterol).
Association of Insulin Dose With Incident Cardiovascular Events
There were 366 adjudicated CVD events in 184 participants and 226 MACE events in 88 participants during DCCT/EDIC. Table 3 presents the effects of insulin dose on the risk of CVD events and MACE with adjustment for the effects of potentially confounding variables. In minimally adjusted models (DCCT baseline age, primary prevention vs. secondary intervention, and treatment group),a 0.1 unit/kg/day increase in current insulin dose was associated with a 6% increased risk of any CVD (HR 1.06 [95% CI 1.03, 1.09], Z = 3.71) and a 5% increased risk of MACE (HR 1.05 [95% CI 1.00, 1.11], Z = 2.05). After adjustment for risk factors identified as significant predictors of any CVD and MACE in prior analyses, the HRs were attenuated and no longer significant (HR 1.03 [95% CI 0.99, 1.08], Z = 1.39, and HR 0.99 [95% CI 0.92, 1.08], Z = −0.13, respectively).
Table 3.
Effect of insulin dose (units/kg/day) on the risk of cardiovascular disease
| Any CVD |
MACE |
|||||||
|---|---|---|---|---|---|---|---|---|
| Effect of current insulin dose (per 0.1 unit/kg/day) |
Effect of time-weighted mean insulin dose (per 0.1 unit/kg/day) |
Effect of current insulin dose (per 0.1 unit/kg/day) |
Effect of time-weighted mean insulin dose (per 0.1 unit/kg/day) |
|||||
| HR (95% CI) | Z | HR (95% CI) | Z | HR (95% CI) | Z | HR (95% CI) | Z | |
| Cardiometabolic risk factors (current or time weighted) | ||||||||
| None | 1.06 (1.03, 1.09) | 3.71 | 1.17 (1.08, 1.27) | 3.80 | 1.05 (1.00, 1.11) | 2.05 | 1.15 (1.03, 1.30) | 2.37 |
| Systolic BP (mmHg) | 1.06 (1.03, 1.09) | 3.63 | 1.15 (1.06, 1.24) | 3.23 | 1.05 (1.00, 1.11) | 2.02 | 1.13 (1.00, 1.28) | 2.03 |
| Pulse rate (bpm) | 1.06 (1.03, 1.09) | 3.63 | 1.14 (1.04, 1.23) | 2.94 | 1.05 (1.00, 1.11) | 1.87 | 1.09 (0.97, 1.24) | 1.41 |
| Triglycerides (log) | 1.04 (1.00, 1.08) | 1.91 | 1.08 (0.99, 1.17) | 1.62 | 1.02 (0.95, 1.10) | 0.56 | 1.03 (0.90, 1.17) | 0.41 |
| HDL cholesterol (mg/dL) | 1.05 (1.01, 1.08) | 2.53 | 1.14 (1.05, 1.24) | 3.02 | 1.03 (0.96, 1.10) | 0.69 | 1.10 (0.97, 1.24) | 1.42 |
| LDL cholesterol (mg/dL) | 1.06 (1.03, 1.09) | 3.71 | 1.15 (1.06, 1.25) | 3.25 | 1.05 (1.00, 1.11) | 2.04 | 1.13 (1.00, 1.28) | 1.97 |
| HbA1c (%) | 1.06 (1.02, 1.09) | 3.32 | 1.12 (1.03, 1.22) | 2.71 | 1.05 (0.99, 1.11) | 1.69 | 1.09 (0.97, 1.24) | 1.41 |
| Fully adjusted model | 1.03 (0.99, 1.08) | 1.39 | 1.05 (0.95, 1.15) | 0.95 | 0.99 (0.92, 1.08) | −0.13 | 0.97 (0.84, 1.11) | −0.50 |
| Fully adjusted model without triglycerides | 1.04 (1.00, 1.09) | 2.14 | 1.07 (0.98, 1.17) | 1.47 | 1.01 (0.94, 1.10) | 0.33 | 0.99 (0.87, 1.14) | −0.09 |
Data are HRs (95% CI) and unsigned covariate Z values from separate Cox proportional hazards regression models assessing the effect of current and time-weighted mean insulin dose on the risk of CVD, with adjustment separately for each cardiometabolic risk factor as a time-dependent covariate. Models using current insulin dose were adjusted for the current value of each risk factor. Models using time-weighted mean insulin dose were adjusted for the time-weighted mean value of each risk factor. Insulin dose (current and time weighted) was entered into each model as a time-dependent covariate. All models were adjusted for DCCT baseline age, primary prevention vs. secondary intervention cohort, and treatment group. Any CVD: time to the first occurrence of cardiovascular death, nonfatal MI, nonfatal stroke, subclinical MI, angina confirmed by exercise tolerance testing or by significant obstruction on coronary angiography, revascularization, or congestive heart failure. MACE: time to cardiovascular death, nonfatal MI, or nonfatal stroke. BP, blood pressure.
Separate adjustments were made for each risk factor to identify which ones were responsible for attenuating the significant relationship between insulin dose and any CVD risk. Individual adjustments for systolic blood pressure, pulse rate, HDL and LDL cholesterol, and HbA1c did not significantly alter the minimally adjusted association (HR 1.06). There was partial mediation in the HR for insulin dose after adjustment for triglycerides (33% reduction of any CVD, HR 1.06–1.04). Similar findings were observed for time-weighted insulin dose; however, the HRs were higher in magnitude (any CVD, HR 1.17 [95% CI 1.08, 1.27], and MACE, HR 1.15 [95% CI 1.03, 1.29], respectively). Similarly, triglycerides explained a part of the significant effect between time-weighted insulin dose and any CVD (53% reduction of any CVD, HR 1.17–1.08).
For MACE, the association of current insulin dose and MACE was no longer significant after individual adjustment for each of the concurrent risk factors, with the exception of systolic blood pressure and LDL cholesterol. Similar observations were seen for the time-weighted mean values.
Conclusions
During the 30 years of the DCCT/EDIC study, higher insulin dose was strongly associated with worsening trends in several traditional CVD risk factors and CVD events. Among the risk factors, BMI (in males), pulse rate, triglycerides, and HDL cholesterol exhibited the strongest associations with insulin dose. However, after adjustment for known risk factors, we found no significant association between insulin dose and incident CVD events.
As DCCT/EDIC participants were almost completely deficient in endogenous insulin production, as documented by C-peptide studies (18), total daily insulin dose at a low targeted HbA1c in this population is likely to reflect insulin sensitivity. Thus, a greater insulin dose would be expected in individuals with insulin resistance compared with relatively insulin-sensitive individuals.
Several findings related to insulin dose emerged from our analyses. As expected, there was a linear relationship between insulin dose and HbA1c during DCCT in the intensive but not conventional therapy group, consistent with the treat-to-target approach among the former but not the latter (Supplementary Fig. 1A). The increased insulin dosing per unit HbA1c during the DCCT was also likely due to the increased weight gain observed in the intensive treatment group, which, in turn, may have increased insulin resistance. Compared with the DCCT period, insulin dose was similar and decreased in both treatment groups during EDIC follow-up, perhaps reflecting the cessation of study-directed active diabetes management in both treatment groups. It may also reflect the waning insulin resistance associated with pubertal status in our adolescents as they transitioned to adulthood (19). The higher overall insulin dose, adjusted for body mass, in males compared with females during decades of observation in the DCCT/EDIC is consistent with the known sex disparities in insulin sensitivity (20).
Our finding of a longitudinal association of higher insulin dose with lower HDL cholesterol levels and higher BMI, systolic blood pressure, and triglyceride levels is likely explained by underlying insulin resistance and concomitant insulin-related weight gain (13,21–24). However, the mechanism(s) underlying the inverse association between insulin dose and LDL cholesterol levels are probably more complex. As previously reported, during the DCCT, participants assigned to intensive therapy showed a 34% reduction in LDL cholesterol levels, lower apolipoprotein B, lower cholesterol content of dense LDL and VLDL, and higher content of buoyant LDL compared with subjects in the conventional treatment group (25,26). These findings are consistent with data from metabolic studies in humans showing decreased levels of VLDL triglycerides and LDL cholesterol and increased HDL2 cholesterol levels, along with upregulation of adipose tissue lipoprotein lipase, following insulin therapy (27–29).
Additionally, differences in macronutrient intake or other dietary practices (not captured in the present report) could have influenced the lipid profiles among our DCCT/EDIC participants. When weight gain was considered in the DCCT, the participants in the intensive treatment arm who gained the most weight displayed an adverse cardiometabolic profile characterized by higher blood pressure, triglycerides, total cholesterol, LDL cholesterol, and apolipoprotein B levels (12,21). Furthermore, a recent report from the DCCT/EDIC study showed that excessive weight gain attenuated the cardiovascular benefits of intensive therapy, with the subjects in the highest quartile of weight gain losing the cardioprotective effects of intensive therapy (30). Thus, the net effects of insulin therapy on lipid profiles, other CVD risk factors, and CVD events appear to be significantly influenced by the degree of weight gain. Whether the effects of insulin dose are mediated by insulin resistance cannot be ascertained from the current data.
The observed increase in pulse rate, which occurred in the setting of increased use of β-blockers over time, is consistent with prior demonstrations that sustained hyperinsulinemia, in the absence of hypoglycemia, increases sympathetic activity, with a resulting increase in pulse rate (31). Given that higher resting pulse rate in adults has been associated with decreased longevity, this correlation may have particular clinical relevance and merits consideration when assessing CVD outcomes in individuals with type 1 diabetes (22,32–35).
Based on the available evidence, efforts to intensify insulin therapy should continue in order to reduce the risk for microvascular and cardiovascular complications in type 1 diabetes. Regardless of the potential pernicious effects of higher insulin doses, intensive insulin therapy, with higher doses, was associated with ∼50% reduction in the incidence of CVD events in the DCCT/EDIC (10). However, given the deleterious effects of weight gain with insulin therapy (30), lifestyle counseling for prevention of weight gain is recommended. Given the adverse impact of weight gain, adjunctive lifestyle regimens that minimize weight gain would be advisable for patients receiving insulin therapy.
Supplementary Material
Article Information
Funding. The DCCT/EDIC has been supported by cooperative agreement grants (1982–1993, 2012–2017, and 2017–2022) and contracts (1982–2012) with the Division of Diabetes, Endocrinology, and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Diseases (current grant numbers U01-DK-094176 and U01-DK-094157) and through support by the National Eye Institute, the National Institute of Neurological Disorders and Stroke, the General Clinical Research Centers Program (1993–2007), and the Clinical Translational Science Center Program (2006–present), Bethesda, MD. Industry contributors have provided free or discounted supplies or equipment to support participants’ adherence to the study: Abbott Diabetes Care (Alameda, CA), Animas (West Chester, PA), Bayer Diabetes Care (North America Headquarters, Tarrytown, NY), Becton Dickinson (Franklin Lakes, NJ), Eli Lilly (Indianapolis, IN), Extend Nutrition (St. Louis, MO), Insulet Corporation (Bedford, MA), LifeScan (Milpitas, CA), Medtronic Diabetes (Minneapolis, MN), Nipro Home Diagnostics (Fort Lauderdale, FL), Nova Diabetes Care (Billerica, MA), Omron (Shelton, CT), Perrigo Diabetes Care (Allegan, MI), Roche Diabetes Care (Indianapolis, IN), and Sanofi (Bridgewater, NJ).
Industry contributors have had no role in the DCCT/EDIC study.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. B.H.B. wrote the manuscript and conducted the statistical analyses. S.D.-J., I.B., W.I.S., M.L., O.K., and J.M.L. wrote sections of the manuscript and reviewed and edited the manuscript. B.H.B. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. nos. NCT00360815 and NCT00360893, clinicaltrials.gov
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc18-1574/-/DC1.
A complete list of participants in the DCCT/EDIC Research Group is presented in the Supplementary Material published online for the article in N Engl J Med 2017;376:1507–1516.
References
- 1.Harjutsalo V, Forsblom C, Groop PH. Time trends in mortality in patients with type 1 diabetes: nationwide population based cohort study. BMJ 2011;343:d5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Després JP, Lamarche B, Mauriège P, et al. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J Med 1996;334:952–957 [DOI] [PubMed] [Google Scholar]
- 3.Hillson RM, Hockaday TD, Mann JI, Newton DJ. Hyperinsulinaemia is associated with development of electrocardiographic abnormalities in diabetics. Diabetes Res 1984;1:143–149 [PubMed] [Google Scholar]
- 4.Shichiri M, Kishikawa H, Ohkubo Y, Wake N. Long-term results of the Kumamoto Study on optimal diabetes control in type 2 diabetic patients. Diabetes Care 2000;23(Suppl. 2):B21–B29 [PubMed] [Google Scholar]
- 5.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 6.Siraj ES, Rubin DJ, Riddle MC, et al.; ACCORD Investigators . Insulin dose and cardiovascular mortality in the ACCORD trial. Diabetes Care 2015;38:2000–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group Intensive diabetes treatment and cardiovascular outcomes in type 1 diabetes: the DCCT/EDIC study 30-year follow-up. Diabetes Care 2016;39:686–69326861924 [Google Scholar]
- 8.Cleary PA, Orchard TJ, Genuth S, et al.; DCCT/EDIC Research Group . The effect of intensive glycemic treatment on coronary artery calcification in type 1 diabetic participants of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. Diabetes 2006;55:3556–3565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polak JF, Backlund JY, Cleary PA, et al.; DCCT/EDIC Research Group . Progression of carotid artery intima-media thickness during 12 years in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. Diabetes 2011;60:607–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nathan DM, Cleary PA, Backlund JY, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group . Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group Risk factors for cardiovascular disease in type 1 diabetes. Diabetes 2016;65:1370–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Purnell JQ, Hokanson JE, Marcovina SM, Steffes MW, Cleary PA, Brunzell JD. Effect of excessive weight gain with intensive therapy of type 1 diabetes on lipid levels and blood pressure: results from the DCCT. Diabetes Control and Complications Trial. JAMA 1998;280:140–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orchard TJ, Olson JC, Erbey JR, et al. Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10-year follow-up data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care 2003;26:1374–1379 [DOI] [PubMed] [Google Scholar]
- 14.The DCCT Research Group The Diabetes Control and Complications Trial (DCCT). Design and methodologic considerations for the feasibility phase. Diabetes 1986;35:530–545 [PubMed] [Google Scholar]
- 15.Nathan DM, Genuth S, Lachin J, et al.; Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 16.The DCCT/EDIC Research Group Epidemiology of Diabetes Interventions and Complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care 1999;22:99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The DCCT Research Group Lipid and lipoprotein levels in patients with IDDM: Diabetes Control and Complication Trial experience. Diabetes Care 1992;15:886–894 [DOI] [PubMed] [Google Scholar]
- 18.The DCCT Research Group Effects of age, duration and treatment of insulin-dependent diabetes mellitus on residual beta-cell function: observations during eligibility testing for the Diabetes Control and Complications Trial (DCCT). J Clin Endocrinol Metab 1987;65:30–36 [DOI] [PubMed] [Google Scholar]
- 19.Kelsey MM, Zeitler PS. Insulin resistance of puberty. Curr Diab Rep 2016;16:64. [DOI] [PubMed] [Google Scholar]
- 20.Magkos F, Wang X, Mittendorfer B. Metabolic actions of insulin in men and women. Nutrition 2010;26:686–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Purnell JQ, Zinman B, Brunzell JD; DCCT/EDIC Research Group . The effect of excess weight gain with intensive diabetes mellitus treatment on cardiovascular disease risk factors and atherosclerosis in type 1 diabetes mellitus: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study (DCCT/EDIC) study. Circulation 2013;127:180–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox K, Borer JS, Camm AJ, et al.; Heart Rate Working Group . Resting heart rate in cardiovascular disease. J Am Coll Cardiol 2007;50:823–830 [DOI] [PubMed] [Google Scholar]
- 23.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 24.Writing Group for the DCCT/EDIC Research Group Coprogression of cardiovascular risk factors in type 1 diabetes during 30 years of follow-up in the DCCT/EDIC study. Diabetes Care 2016;39:1621–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The DCCT Research Group Effect of intensive diabetes management on macrovascular events and risk factors in the Diabetes Control and Complications Trial. Am J Cardiol 1995;75:894–903 [DOI] [PubMed] [Google Scholar]
- 26.Purnell JQ, Marcovina SM, Hokanson JE, et al. Levels of lipoprotein(a), apolipoprotein B, and lipoprotein cholesterol distribution in IDDM. Results from follow-up in the Diabetes Control and Complications Trial. Diabetes 1995;44:1218–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taskinen MR, Kuusi T, Helve E, Nikkilä EA, Yki-Järvinen H. Insulin therapy induces antiatherogenic changes of serum lipoproteins in noninsulin-dependent diabetes. Arteriosclerosis 1988;8:168–177 [DOI] [PubMed] [Google Scholar]
- 28.Rabkin SW, Boyko E, Streja DA. Changes in high density lipoprotein cholesterol after initiation of insulin therapy in non-insulin dependent diabetes mellitus: relationship to changes in body weight. Am J Med Sci 1983;285:14–20 [DOI] [PubMed] [Google Scholar]
- 29.Agardh CD, Nilsson-Ehle P, Scherstén B. Improvement of the plasma lipoprotein pattern after institution of insulin treatment in diabetes mellitus. Diabetes Care 1982;5:322–325 [DOI] [PubMed] [Google Scholar]
- 30.Purnell JQ, Braffett BH, Zinman B, et al.; DCCT/EDIC Research Group . Impact of excessive weight gain on cardiovascular outcomes in type 1 diabetes: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. Diabetes Care 2017;40:1756–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rowe JW, Young JB, Minaker KL, Stevens AL, Pallotta J, Landsberg L. Effect of insulin and glucose infusions on sympathetic nervous system activity in normal man. Diabetes 1981;30:219–225 [DOI] [PubMed] [Google Scholar]
- 32.Kannel WB, Kannel C, Paffenbarger RS Jr, Cupples LA. Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J 1987;113:1489–1494 [DOI] [PubMed] [Google Scholar]
- 33.Kristal-Boneh E, Silber H, Harari G, Froom P. The association of resting heart rate with cardiovascular, cancer and all-cause mortality. Eight year follow-up of 3527 male Israeli employees (the CORDIS Study). Eur Heart J 2000;21:116–124 [DOI] [PubMed] [Google Scholar]
- 34.Dyer AR, Persky V, Stamler J, et al. Heart rate as a prognostic factor for coronary heart disease and mortality: findings in three Chicago epidemiologic studies. Am J Epidemiol 1980;112:736–749 [DOI] [PubMed] [Google Scholar]
- 35.Palatini P, Julius S. Elevated heart rate: a major risk factor for cardiovascular disease. Clin Exp Hypertens 2004;26:637–644 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.