Abstract
OBJECTIVE
Childhood and young adulthood may represent time periods in which cardiovascular risk factors (CVRFs) and their cumulative exposure lay the foundation for future risk of chronic diseases. We examined the longitudinal burden of CVRFs since childhood in men and women in whom diabetes did and did not develop at follow-up.
RESEARCH DESIGN AND METHODS
We included 1,530 participants (mean [SD] follow-up time 33.1 [8.2] years), who participated in the Bogalusa Heart Study and had been examined at least four times starting in childhood (mean age [SD] at first examination 9.4 [3.1] years). The area under the growth curve was used as a measure of cumulative exposure to CVRFs since childhood.
RESULTS
In childhood, boys and girls in whom diabetes did and did not develop at follow-up had similar CVRFs. Yet, over time, women during the transition from normoglycemia to diabetes experienced greater adverse changes in total cholesterol (TC), LDL cholesterol, and fasting plasma glucose (FPG) (noted as early as 23.5 years old and persisting across adulthood up to the age of the diagnosis of diabetes); a higher burden of exposure to BMI, TC, LDL cholesterol, and FPG from childhood to midlife; and a greater change in rates of BMI, TC, LDL cholesterol, and FPG since childhood than men during the same transition (interaction P values <0.05).
CONCLUSIONS
The greater exposure of women to and burden of CVRFs associated with diagnosis of diabetes may help to explain the stronger impact of diabetes as a major risk factor for cardiovascular events in women compared with men.
Introduction
The burden of cardiovascular disease (CVD) related to diabetes is a major public health challenge (1). There is a well-recognized CVD risk disparity by sex among individuals with diabetes (2–5). Women with diabetes are at markedly increased risk of CVD events compared with women without diabetes, whereas men with diabetes are at moderately increased risk compared with men without diabetes. For example, in the INTERHEART Study, an international case-control study including 15,152 case patients and 14,820 control subjects from 52 countries, women with diabetes were at a 4.3-fold risk for CVD events compared with women without diabetes, whereas men with diabetes had a little over twice the risk of CVD events, at 2.7-fold, versus their male counterparts without diabetes (6). More direct comparisons have also borne out this excess risk, with larger studies, including meta-analyses, reporting a 44% greater risk of incident coronary heart disease and a 27% greater risk of incident stroke among women with diabetes compared with men with diabetes (2,3,7).
Despite these numerous reports of sex differences in CVD risk among persons with diabetes, the reasons for the stronger effect of diabetes on CVD risk in women compared with men remain unclear. It has been hypothesized that the higher CVD risk among women with diabetes is a consequence either of diabetes inducing disproportionately greater increase in cardiovascular risk factors (CVRFs) in women (8–10) or of the poorer CVD risk profile in men without diabetes (11).
The majority of previous studies were conducted in midlife or later and were based on a single assessment or a few assessments of CVRFs. The developmental origins of health and disease hypothesis posits that exposures in early life are associated with the risk of chronic diseases in adulthood and later life. For the risk of CVD in later life, this hypothesis is supported by a large body of evidence (12–16). Yet, few studies have the ability to examine prospectively collected risk factors from childhood through young adulthood to midlife when the pathology associated with diabetes and CVD often culminates over decades. Examination of these factors by sex could have considerable implications with respect to the CVD pathogenesis in diabetes and could inform the design of more targeted and effective interventions for both sexes. In addition, because individual CVRFs vary throughout the life course, single measures of CVRFs may not reflect usual levels or cumulative burden, which could more accurately estimate the effects of these risk factors over several decades.
The Bogalusa Heart Study (BHS) is unique in having repeatedly measured prospectively collected CVRFs starting in childhood among men and women and extending over 40 years through midlife. In this study, we use data from the BHS to compare the childhood, young life, and midlife CVRFs; their cumulative burden; and rates of change since childhood in men and women with and without diabetes.
Research Design and Methods
The BHS is a series of long-term studies initiated in 1973 and designed to increase our understanding of the early natural history of CVD since childhood (17). Between 1973 and 2016, 9 cross-sectional surveys of children 4–18 years of age and 11 surveys of adults 19–58.1 years of age who had been previously examined as children were conducted in the semirural biracial (65% white and 35% black) community of Bogalusa, Louisiana. This panel design of repeated cross-sectional examinations conducted approximately every 3–4 years resulted in serial observations from childhood to adulthood and allowed an evaluation of the cumulative burden of CVRFs beginning in childhood. We used the area under the growth curve (AUC) to measure the cumulative burden of CVRFs from childhood to adulthood. Since the growth curves of some CVRFs are cubic models, a minimum of four measurements are needed. Therefore, we included 1,530 adults (1,020 whites and 510 blacks; 42.9% men; 42.6 years at follow-up, with a range of 20.1–56.9 years) who had been examined for the studied CVRFs, including BMI, systolic/diastolic blood pressure (BP), total cholesterol (TC), triglyceride (TG), LDL cholesterol (LDL-C), HDL cholesterol (HDL-C), and fasting plasma glucose (FPG) 4–16 times (8 times on average; at least 2 times in childhood and at least 2 times in adulthood). In all, 86% of subjects were examined for these CVRFs 6–12 times. The mean follow-up period was 33.1 years. Participants with a baseline diabetes diagnosis were excluded from this analysis.
Written informed consent was obtained from parents or guardians in childhood and from the participants themselves in adulthood. Study protocols were approved by the Institutional Review Board of the Tulane University Health Sciences Center (395283-3).
Measurements
Standardized protocols were followed by trained personnel across all surveys. Participants were instructed to fast for 12 h before the screening. For each participant, replicate measurements of height and weight were obtained, and the mean values were used for analysis. BMI was calculated as weight in kilograms divided by height in meters squared. BP was measured between 8:00 and 10:00 a.m. on the right arm in a relaxed sitting position by two trained technicians (triplicate each), using mercury manometers. Arm measurements, length and circumference, were made during the examination to ensure proper cuff size. The fourth Korotkoff phase was used for diastolic BP in children and adults to avoid bias resulting from using different phases for diastolic BP. The six readings were averaged.
Biochemical Laboratory Measurements
Between 1973 and 1986, TC and TG levels were determined with Technicon Auto Analyzer II (Technicon Instrument Corp., Tarrytown, NY) according to the laboratory manual of the Lipid Research Clinics Program (18). From 1987, these variables were measured using an Abbott VP instrument (Abbott Laboratories, Abbott Park, IL) by enzymatic procedures (19,20). Both chemical and enzymatic procedures met the performance requirements of the Lipids Standardization Program of the Centers for Disease Control and Prevention. Measurements on Centers for Disease Control and Prevention–assigned quality control samples showed no consistent bias over time within or between surveys. Serum lipoprotein cholesterol levels were analyzed by using a combination of heparin-calcium precipitation and agar-agarose gel electrophoresis procedures (21). Between 1978 and 1991, FPG was determined with a glucose oxidase method using a Beckman Glucose Analyzer (Beckman Instruments, Fullerton, CA). Since 1992, FPG has been measured enzymatically as part of a multichemistry profile. Between 2014 and 2016, HbA1c was measured with high-performance liquid chromatography using an NGSP-certified automated analyzer.
Statistical Analysis
All statistical analyses were performed with SAS version 9.2 (SAS Institute Inc., Cary, NC). Individuals were classified as having diabetes based on an FPG concentration ≥7.0 mmol/L (126 mg/dL) or the use of insulin or oral antidiabetic medications in accordance with 2018 American Diabetes Association criteria (22). In the sensitivity analysis, diabetes was also defined as an FPG concentration of ≥7 mmol/L or HbA1c ≥6.5% (48 mmol/mol) or the use of insulin or oral antidiabetic medications.
The first and last measurements of CVRFs were used as childhood and adulthood values, respectively. Because of positively skewed distribution, TGs were natural log transformed. The cumulative risk burden of CVRFs from childhood to adulthood was measured as the AUC, which was calculated using statistical models previously described (23). Growth curves of CVRFs measured repeatedly from childhood to adulthood were constructed for each race and sex group using a random-effects model in SAS Proc MIXED. Three different models were fitted that included 1) age only, 2) age and its quadratic term, and 3) age and its quadratic and cubic terms. Age and its higher-order terms were included one by one for model building. Age was centered to the mean age (23.5 years) to minimize the collinearity of age with its higher-order terms. The term age squared was divided by 10 and age cubed by 20 to improve the model fit (24). The mixed model incorporates fixed and random effects and allows the intercept, linear, and nonlinear parameters to vary from individual to individual. The random-effect coefficients represent the difference between fixed population parameters and the observed values for individuals. This model allows for repeated measurements and different numbers of unequally spaced observations across individuals. The mixed linear model computes maximum likelihood estimates of curve parameters for all of the study participants. Model selection was based on the Akaike information criterion. The most parsimonious growth curve model was selected. The higher-order terms of age would not be included in the equation if they were not at or below the significance level of 0.05, if they made lower-order terms not significant, or if they did not improve the goodness-of-fit of the model. A cubic curve was fitted for systolic/diastolic BP and TC. A quadratic curve was fitted for BMI, TG, and LDL-C. A linear model was fitted for HDL-C and FPG. The AUCs were calculated by adopting an integral calculus formula based on the fixed and random-effect parameters of the growth curve model during the follow-up period for each subject and divided by the number of follow-up years of each individual. The AUC measures have advantages over other longitudinal analysis models in that they measure cumulative burden. The linear change of each studied CVRF over age (hence, the rate of change) was calculated as the sum of the fixed and random coefficient of age for each individual.
Two-way ANCOVA with sex and diabetes status as the two main effects was performed to test for differences in the means of CVRFs and curve parameters. Bonferroni correction was applied to adjust P values for multiple comparisons. Statistical interactions for sex by follow-up diabetes status in their association with CVRFs were computed using generalized linear models to determine whether changes in means between those individuals in whom diabetes developed and those in whom it did not differed by sex. Significance was accepted at a two-tailed P value of <0.05.
Results
The representativeness of the study population participating in the study was examined by comparing the baseline differences between participants and nonparticipants (Supplementary Table 1). Participants were a little younger and more often female than nonparticipants, which is common among long-term observational studies (25). Similar race and studied CVRFs were observed.
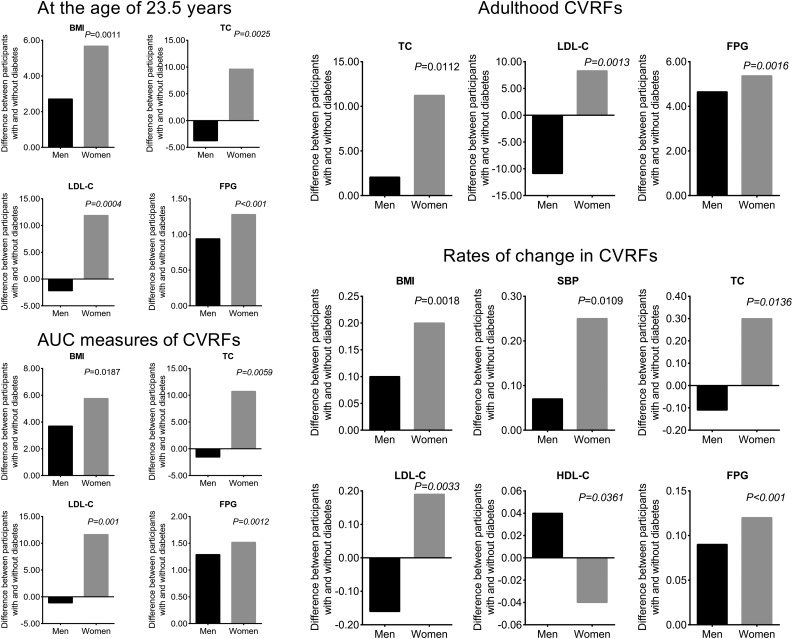
Major CVRFs measured from childhood to adulthood in the study cohort are presented in Table 1 by diabetes status, separately for men and women. At the earliest measurement, in childhood, no significant differences were seen for men and women in any of the studied CVRFs between those individuals in whom diabetes developed later in adulthood and those in whom it did not. Differences in all studied CVRFs as well as the prevalence of metabolic syndrome (MetS), a good summary of the burden of CVRFs, between those in whom diabetes developed and those in whom it did not were comparable between men and women (test for sex by diabetes status interaction was not significant). In middle-aged adults, both men and women with diabetes had significantly higher BMI, TG, FPG, and prevalence of MetS and lower HDL-C levels than their counterparts in whom diabetes did not develop. Diabetes status was associated with higher systolic/diastolic BP in women but not in men. Men who did not have diabetes had significantly higher systolic/diastolic BP, TG, and prevalence of MetS and lower HDL-C than women who did not have diabetes, but the magnitude of differences in these CVRFs and the prevalence of MetS between men and women in whom diabetes developed were less marked. Among women, those in whom diabetes developed in adulthood had more adverse changes in TC, LDL-C, and FPG over time. As expected, more women in whom diabetes developed experienced MetS. There was strong statistical evidence for sex difference in the association among TC, LDL-C, FPG, and the prevalence of MetS with eventual development of diabetes (P values <0.05 for effect modification of sex on the relationship between these CVRFs and the later diabetes status). The AUC values of all the studied CVRFs showed significant differences by diabetes status except for AUCs of TC and LDL-C among men. The difference in cumulative exposure burden represented as AUCs of TC and LDL-C among women with diabetes compared with women without diabetes was significantly greater than for their male counterparts (the P interaction terms for sex with diabetes status were 0.0066 for TC and 0.0016 for LDL-C, respectively). Men in whom diabetes did not develop in adulthood had significantly higher AUC values of systolic/diastolic BP and TG, and lower HDL-C than women in whom diabetes did not develop. However, there was no statistically significant evidence for sex differences by diabetes status in the associations of these CVRFs from childhood to adulthood. For BMI, women in whom diabetes went on to develop experienced much larger changes in cumulative exposure to adiposity over time than men in whom diabetes went on to develop. This was demonstrated by significantly greater differences in the AUCs of BMI between women in whom diabetes developed versus those in whom it did not develop compared with men in whom diabetes developed versus those in whom it did not develop (test for the effect of sex on the relationship between BMI and diabetes status interaction P = 0.0148). Similarly, there was a greater difference in AUCs for FPG among women in whom diabetes developed in adulthood and women in whom diabetes did not develop compared with comparable groups of men, with statistically significant sex interactions for FPG (P = 0.0011).
Table 1.
Mean levels of studied CVRFs measured since childhood in men and women according to diabetes status at follow-up
| Men |
P value* | Women |
P value* | Women vs. men | P interaction§ | ||||
|---|---|---|---|---|---|---|---|---|---|
| Without diabetes (N = 580) | With diabetes (N = 76) | Without diabetes (N = 796) | With diabetes (N = 78) | P (without diabetes) | P (with diabetes) | ||||
| Childhood (first examination) | |||||||||
| Age (years) | 9.68 ± 3.12 | 9.80 ± 2.99 | 0.22 | 9.33 ± 3.03 | 8.67 ± 3.04 | 0.4106 | 0.2238 | 0.1288 | 0.1322 |
| BMI (kg/m2) | 17.49 ± 3.28 | 18.15 ± 3.75 | 0.6378 | 17.19 ± 3.24 | 17.71 ± 4.10 | 0.811 | 0.5837 | 0.3147 | 0.8034 |
| SBP (mmHg) | 100.68 ± 10.53 | 102.44 ± 10.18 | 0.67 | 98.64 ± 10.00 | 100.43 ± 10.66 | 0.3563 | 0.17 | 0.503 | 0.8084 |
| DBP (mmHg) | 61.42 ± 7.79 | 61.80 ± 7.90 | 0.9593 | 60.70 ± 8.17 | 61.42 ± 8.2 | 0.8385 | 0.6224 | 0.8125 | 0.9871 |
| TC (mg/dL) | 162.94 ± 31.20 | 160.21 ± 30.5 | 0.89 | 165.79 ± 28.40 | 171.73 ± 32.83 | 0.5475 | 0.4827 | 0.0979 | 0.0868 |
| Ln TG (mg/dL) | 4.10 ± 0.41 | 4.16 ± 0.48 | 0.9843 | 4.15 ± 0.40 | 4.18 ± 0.42 | 0.996 | 0.1286 | 0.8417 | 0.7064 |
| LDL-C (mg/dL) | 88.50 ± 25.59 | 88.62 ± 26.30 | 0.943 | 92.40 ± 24.28 | 96.44 ± 29.19 | 0.0692 | 0.0271 | 0.3198 | 0.3599 |
| HDL-C (mg/dL) | 67.01 ± 21.13 | 63.00 ± 26.05 | 0.7589 | 65.21 ± 21.3 | 66.25 ± 22.05 | 0.7514 | 0.7505 | 0.1348 | 0.1664 |
| FPG (mmol/L) | 4.89 ± 0.50 | 5.03 ± 0.45 | 0.4152 | 4.77 ± 0.48 | 4.88 ± 0.52 | 0.3384 | 0.053 | 0.172 | 0.1192 |
| MetS (%)† | 23.45 | 32.89 | 0.3981 | 21.26 | 31.65 | 0.221 | 0.81 | 0.93 | 0.8953 |
| Adulthood (last examination) | |||||||||
| Age (years) | 42.16 ± 8.44 | 46.46 ± 5.97 | 0.0001 | 42.32 ± 8.46 | 44.97 ± 6.76 | 0.0404 | 0.7913 | 0.0685 | 0.2398 |
| BMI (kg/m2) | 29.67 ± 6.76 | 34.40 ± 7.17 | <0.001 | 29.78 ± 7.94 | 36.06 ± 8.45 | <0.001 | 0.4852 | 0.4097 | 0.1008 |
| SBP (mmHg) | 122.84 ± 14.81 | 127.08 ± 13.62 | 0.1499 | 116.32 ± 15.83 | 123.19 ± 18.3 | 0.0011 | <0.001 | 0.7076 | 0.3167 |
| DBP (mmHg) | 80.36 ± 10.66 | 82.93 ± 9.39 | 0.2783 | 76.18 ± 10.69 | 79.69 ± 9.87 | 0.0301 | <0.001 | 0.3398 | 0.6027 |
| TC (mg/dL) | 191.26 ± 39.44 | 184.95 ± 53.5 | 0.0871 | 193.21 ± 37.66 | 203.56 ± 48.62 | 0.1673 | 0.5645 | 0.0224 | 0.0139 |
| Ln TG (mg/dL) | 4.77 ± 0.56 | 5.09 ± 0.65 | <0.001 | 4.57 ± 0.51 | 4.95 ± 0.65 | <0.001 | <0.001 | 0.6069 | 0.5795 |
| LDL-C (mg/dL) | 121.72 ± 35.20 | 110.58 ± 41.76 | 0.0565 | 118.09 ± 33.75 | 125.74 ± 40.66 | 0.3891 | 0.3516 | 0.0437 | 0.0016 |
| HDL-C (mg/dL) | 46.25 ± 14.85 | 39.96 ± 11.48 | 0.0029 | 55.21 ± 15.27 | 47.01 ± 11.24 | <0.001 | <0.001 | 0.018 | 0.4486 |
| FPG (mmol/L) | 5.14 ± 0.74 | 9.79 ± 3.74 | <0.001 | 4.96 ± 0.68 | 10.34 ± 3.68 | <0.001 | 0.0693 | 0.0747 | 0.0015 |
| MetS (%)‡ | 28.97 | 71.05 | <0.001 | 20.88 | 77.22 | <0.001 | 0.003 | 0.72 | 0.047 |
| AUC measures∫ | |||||||||
| BMI (kg/m2) | 25.18 ± 4.69 | 28.93 ± 5.32 | <0.001 | 24.72 ± 5.33 | 30.62 ± 6.56 | <0.001 | 0.6609 | 0.2526 | 0.0148 |
| SBP (mmHg) | 116.01 ± 7.57 | 119.82 ± 7.82 | 0.0001 | 109.92 ± 7.16 | 115.36 ± 7.63 | <0.001 | <0.001 | 0.001 | 0.1935 |
| DBP (mmHg) | 73.30 ± 5.72 | 76.72 ± 5.58 | <0.001 | 71 ± 4.97 | 74.10 ± 5.18 | <0.001 | <0.001 | 0.0129 | 0.7187 |
| TC (mg/dL) | 177.29 ± 28.36 | 175.62 ± 30.06 | 0.7613 | 177.07 ± 23.64 | 187.5 ± 30.66 | 0.0046 | 0.9852 | 0.0293 | 0.0066 |
| Ln TG (mg/dL) | 4.48 ± 0.36 | 4.65 ± 0.40 | <0.001 | 4.37 ± 0.29 | 4.54 ± 0.36 | <0.001 | <0.001 | 0.1698 | 0.9725 |
| LDL-C (mg/dL) | 111.10 ± 24.96 | 109.68 ± 23.86 | 0.921 | 108.59 ± 20.99 | 119.56 ± 26.76 | 0.0003 | 0.2804 | 0.0461 | 0.0016 |
| HDL-C (mg/dL) | 51.37 ± 11.31 | 46.66 ± 10.45 | 0.0012 | 56.00 ± 9.79 | 52.34 ± 8.25 | 0.0166 | <0.001 | 0.004 | 0.5527 |
| FPG (mmol/L) | 4.84 ± 0.26 | 6.13 ± 1.06 | <0.001 | 4.63 ± 0.21 | 6.15 ± 1.19 | <0.001 | <0.001 | 0.8763 | 0.0011 |
Values are the mean ± SD, unless otherwise indicated. DBP, diastolic BP; SBP, systolic BP. Diabetes is defined as having an FPG concentration of ≥7 mmol/L or using insulin or oral antidiabetic medications in accordance with 2018 American Diabetes Association criteria.
*P value for difference between those with diabetes and those without diabetes.
§Interaction of sex by diabetes status.
†In the absence of a consensus pediatric MetS definition, we used the definition according to published reports. BMI was used as the measure of adiposity, because waist circumference was not measured at baseline. We generated age-, sex-, and race-specific percentiles of BMI, SBP, DBP, HDL-C, TG, and FPG from each participant. A participant was categorized as having MetS if the participant had any three of the following five components: BMI ≥75th percentile, SBP or DBP ≥75th percentile, HDL-C ≤25th percentile, TG ≥75th percentile, or FPG ≥75th percentile.
‡In adulthood, MetS was defined according to the consensus criteria in 2009 as the presence of three or more of the following criteria: elevated waist circumference (≥102 cm in men and ≥88 cm in women); TG ≥150 mg/dL; HDL-C <40 mg/dL in men and <50 mg/dL in women; BP ≥130/85 mmHg or receiving antihypertensive drug treatment in a patient with a history of hypertension; or FPG ≥5.6 mmol/L.
∫AUC is the cumulative risk burden of the relevant risk factor from childhood to adulthood. The AUCs were calculated as the integral of the growth curve parameter during the follow-up period in each subject. Since each individual had a different follow-up period, the AUC values were divided by the number of years of each individual’s follow-up time.
The sum of the fixed and random intercepts of each studied CVRF equals the respective CVRF level at the age of 23.5 years because age was centered to this mean value. As shown in Fig. 1, it is noteworthy that the differences in BMI, TC, LDL-C, and FPG in women in whom diabetes developed versus those in whom it did not develop become significantly greater than the difference between the comparable groups of men (test for sex × diabetes status interaction P values <0.05) as early as 23.5 years of age.
Figure 1.
Differences in mean values of studied CVRFs at the age of 23.5 years according to diabetes status at follow-up in men and women. Diabetes is defined as having an FPG concentration of ≥7 mmol/L or using insulin or oral antidiabetic medications in accordance with 2018 American Diabetes Association criteria. P values are interactions of sex by diabetes status. DBP, diastolic BP; SBP, systolic BP.
Rates of change in studied CVRFs from childhood to adulthood by sex and eventual diabetes status are summarized in Table 2. Among those in whom diabetes did not develop, men had significantly faster rates of change in all studied variables except for BMI and FPG than women. Among women, the rates of change in all studied CVRFs except for TC, LDL-C, and HDL-C were significantly faster for those in whom diabetes went on to develop compared with those in whom it did not. Although absolute levels were not higher, women in whom diabetes developed had greater relative differences in rates of change in systolic BP, TC, LDL-C, and HDL-C than men in whom diabetes developed when compared with their counterparts in whom diabetes did not develop (sex interaction P values <0.05). The difference in the rates of change in BMI and FPG were much more striking for women in whom diabetes went on to develop compared with men (P interaction term for sex differences by diabetes status was 0.0011 for rates of change in BMI and <0.001 for rates of change in FPG, respectively).
Table 2.
Mean levels of rates of change in studied CVRFs over a mean follow-up time of 33.1 years in men and women according to diabetes status at follow-up
| Men |
P value* | Women |
P value* | Women vs. men |
P interaction§ | ||||
|---|---|---|---|---|---|---|---|---|---|
| Without diabetes | With diabetes | Without diabetes | With diabetes | P (Without diabetes) | P (With diabetes) | ||||
| BMI | 0.42 ± 0.01 | 0.53 ± 0.02 | <0.001 | 0.41 ± 0.01 | 0.62 ± 0.03 | <0.001 | 0.9863 | 0.0174 | 0.0011 |
| SBP | 0.34 ± 0.02 | 0.43 ± 0.05 | 0.3850 | 0.13 ± 0.02 | 0.36 ± 0.06 | <0.001 | <0.001 | 0.416 | 0.0063 |
| DBP | 0.75 ± 0.01 | 0.85 ± 0.04 | 0.0199 | 0.44 ± 0.01 | 0.60 ± 0.03 | <0.001 | <0.001 | <0.001 | 0.179 |
| TC | 2.21 ± 0.05 | 2.07 ± 0.16 | 0.52 | 1.29 ± 0.03 | 1.52 ± 0.12 | 0.3339 | <0.001 | 0.0048 | 0.0323 |
| Ln TG | 0.03 ± 0.001 | 0.04 ± 0.001 | 0.0006 | 0.01 ± 0.001 | 0.02 ± 0.001 | <0.001 | <0.001 | <0.001 | 0.6154 |
| LDL-C | 1.62 ± 0.04 | 1.44 ± 0.10 | 0.2459 | 1.11 ± 0.02 | 1.27 ± 0.07 | 0.3573 | <0.001 | 0.7332 | 0.0055 |
| HDL-C | −0.53 ± 0.01 | −0.49 ± 0.04 | 0.7967 | −0.27 ± 0.01 | −0.32 ± 0.02 | 0.3016 | <0.001 | 0.0003 | 0.0235 |
| FPG | 0.01 ± 0.001 | 0.10 ± 0.01 | <0.001 | 0.01 ± 0.001 | 0.13 ± 0.01 | <0.001 | 0.5388 | <0.001 | <0.001 |
Values are the mean ± SE, unless otherwise indicated. DBP, diastolic BP; SBP, systolic BP. Diabetes is defined as having an FPG concentration of ≥7 mmol/L or using insulin or oral antidiabetic medications in accordance with 2018 American Diabetes Association criteria.
*P value for difference between those with diabetes and those without diabetes.
§Interaction of sex by diabetes status.
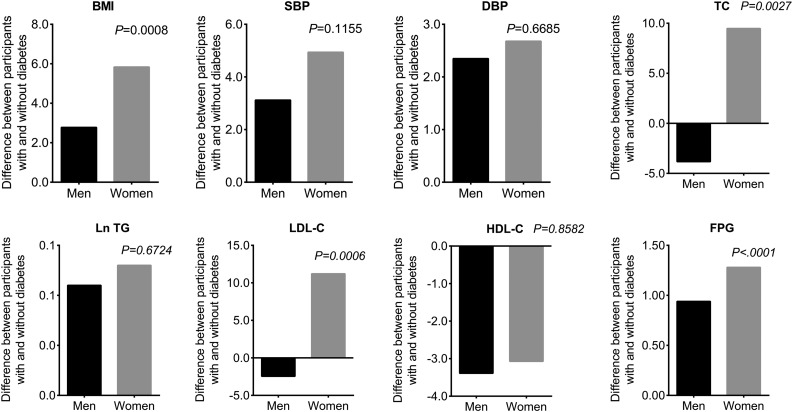
As shown in Fig. 2, sex differences for associations with adulthood TC; LDL-C; FPG; AUC levels of BMI, TC, LDL-C, and FPG; rates of change in BMI, systolic BP, TC, LDL-C, HDL-C, and FPG; and young adulthood BMI, TC, LDL-C, and FPG changed little with adjustment for race. The sex interaction for these parameters remained even after further adjustment for lipid-lowering, BP-lowering, and antidiabetic medication use (Supplementary Table 2). Further adjusting for parental diabetes history and adult smoking and alcohol use, qualitatively similar results were observed (Supplementary Fig. 1).
Figure 2.
Differences in race-adjusted mean levels of studied CVRFs measured since childhood in men and women according to diabetes status at follow-up. Diabetes is defined as having an FPG concentration of ≥7 mmol/L or using insulin or oral antidiabetic medications in accordance with 2018 American Diabetes Association criteria. P values are interactions of sex by diabetes status. SBP, systolic BP.
In each of the BHS race groups (black and white), the differences between those in whom diabetes developed and those in whom it did not develop were greater in women than in men for adulthood TC, LDL-C, and FPG; AUC levels of BMI, TC, LDL-C, and FPG; rates of change in BMI, systolic BP, TC, LDL-C, and FPG; and young adulthood BMI, TC, LDL-C, and FPG (data not shown).
Since participants who have received antihypertensive or lipid-lowering therapy represent a subgroup with the highest BP or lipid levels or with a high risk for the development of diabetes, we did not exclude these individuals. To avoid the effects of treatment on BP and lipid levels, two sensitivity analyses were performed. First, BP and lipid values were set as missing for participants who were taking antihypertensive or lipid-lowering medications at the time of the examinations, and the remaining values were used for analysis (Supplementary Fig. 2). Second, forced values of 140/90 mmHg were assigned to the measured systolic/diastolic BP for hypertensive patients under treatment, and values of 240 and 160 mg/dL were given for the measured TC and LDL-C levels for patients with dyslipidemia receiving lipid-lowering treatment (Supplementary Fig. 3). The results of the sensitivity analyses were qualitatively similar.
Results were remarkably similar when diabetes was defined as having FPG ≥7 mmol/L or HbA1c ≥6.5% (48 mmol/mol) or as the use of insulin or oral antidiabetic medications (Supplementary Tables 3–5).
Conclusions
In this longitudinal study spanning >40 years from childhood to adulthood, we identified significant sex differences in the major CVRFs among men and women in whom diabetes developed and did not develop over the follow-up period. Our findings that women in whom diabetes develops, compared with men, experience greater changes in the cumulative burden of CVRFs and faster rates of change over time, which likely contribute to their higher absolute risk of CVD events, have important public health implications.
CVRFs are identifiable in childhood and are predictive of adult CVD risk (12–16). However, data on sex differences in major CVRFs between individuals in whom diabetes did and did not develop spanning the periods of childhood and young adulthood have been lacking. Our study supports and extends previous work by examining sex differences in childhood, young adulthood, and midlife CVRFs between those in whom diabetes did and did not develop. Our findings are in keeping with the notion that women require more metabolic disturbances than men to transit from normoglycemia to a deranged glycemic state. As women transit from normoglycemia to diabetes, they put on more weight and experience deteriorations in their lipid and glycemic profiles (higher levels of TC, LDL-C, and FPG), and thus a higher prevalence of MetS, to a greater extent than do men. Importantly, despite the observed similar pattern extending back into young adulthood, detectable as early as the age of 23 years, the differences (despite no statistical significance) in girls in whom diabetes developed versus those in whom it did not develop were greater than in comparable groups of boys in systolic BP (1.79 vs. 1.76 mmHg), diastolic BP (0.72 vs. 0.38 mmHg), TC (5.94 vs. −2.13 mg/dL), LDL-C (4.04 vs. 0.12 mg/dL), and prevalence of MetS (10.4% vs. 9.4%). These findings characterize that sex differences in CVRFs and the prevalence of MetS between individuals in whom diabetes did and did not develop actually tracked back even into childhood.
This is, to the best of our knowledge, the first study to report differences between the cumulative burden of major CVRFs, which can more accurately capture persistence, longitudinal exposure, and rates of change in major CVRFs since childhood between individuals in whom diabetes did and did not develop. It is clear from emerging evidence that cumulative exposure to high levels of these CVRFs could impact subclinical outcomes such as carotid intima-media thickness and left ventricular hypertrophy (12–16), which are valid surrogate end points with which to assess CVD (26,27). A growing body of evidence has shown that rapid weight gain is predictive of related CVD risk (28–31). In the current study, women in whom diabetes developed had more marked differences in rates of change in BMI, systolic BP, TC, LDL-C, HDL-C, and FPG from childhood than men in whom diabetes developed when compared with their counterparts in whom diabetes did not develop. Differences between the long-term cumulative burden of exposure, represented as the AUC of BMI, TC, LDL-C, and FPG since childhood, between those in whom diabetes did and did not develop were more pronounced in women than in men. The greater differential cumulative burden of and rates of change in BMI, TC, LDL-C, and FPG associated with the development of diabetes in women compared with men is highly likely to contribute to the stronger impact of diabetes on CVD risk in women and begins as early as late adolescence and early young adulthood. Therefore, the observation that diabetes confers a greater risk for CVD in women compared with men is likely to result from a clustering of more exposure burden of CVRFs in women with diabetes (from which diabetes is an epiphenomenon), rather than the deleterious effect of diabetes itself.
In the current study, men in whom diabetes did not develop tended to have a higher burden of CVRFs and faster rates of change in systolic/diastolic BP, TC, TG, LDL-C, and HDL-C from childhood than their female counterparts. These faster rates of change in CVRFs in men without diabetes may result in a higher burden of CVRFs, which was noted in the present data. Available evidence indicates that a rapid increase in systolic/diastolic BP in childhood is a potent risk factor for adult hypertension and left ventricular hypertrophy (32,33). Further, a rapid increase in BMI independently predicted an increased risk of diabetes (34). Hence, the relatively faster rates of change in CVRFs since childhood, thus inducing a worse CVRF burden in men in whom diabetes did not develop compared with the burden in women in whom diabetes did not develop, may induce a higher absolute CVD risk in men in whom diabetes did not develop and lower the magnitude of the relative risk for CVD events among men in whom diabetes developed. These observations reinforce the idea that developing prevention and intervention strategies for these CVRFs earlier in childhood and increasing the intensity of prevention efforts among boys and girls might be effective in reducing the diabetes risk burden among mid-life adults, both men and women.
Determinants of the observed sex differences in rates of change in CVRFs remain largely unknown. Childhood growth and development undergo consecutive, programmed periods with hormones that may play a major role. The sexual maturation process during the adolescence period is characterized by a complex interplay among various gonadal and adrenal steroid hormones, growth hormones, and growth factors. It is well known that regional adipose tissue distribution as well as patterns of visceral fat deposition are different between men and women (35). Further studies are needed to explore how early-life growth and development programming or how fat distribution influences CVRFs and then alters adult glucose levels.
The strengths of our study include a well-characterized cohort with carefully prospectively collected follow-up data from childhood to adulthood. In addition, the repeated measures of CVRFs (at least two times in childhood and at least two times in adulthood) allow for reliable evaluation of the cumulative burden of exposure (represented by AUC) and rates of change in CVRFs. Furthermore, there was a vigorous quality assurance program and the application of the same stringent methodology used to ensure the quality of the data collection over the entire study period.
Although our study is unique in having life-course data on CVD risk profiles among men and women, its limitations also require careful consideration. First, a standard 75-g oral glucose tolerance test was not performed, which might suggest an underestimation of the prevalence of diabetes. Second, the sample is based in a semirural community of African and European ancestry; therefore, the results may not be generalizable to other groups not studied, for example, Hispanics in an urban environment. Finally, this is an observational study, not a randomized controlled trial, and although it represents our best evidence to date, the findings are subject to residual confounding, such as other lifestyle factors, and measurement error, as with all observational studies.
In summary, our study takes advantage of a unique data set to demonstrate that women during the transition from normoglycemia to diabetes experienced more severe deterioration in multiple CVRFs from young to mid-aged adulthood, a higher burden of exposure to multiple CVRFs from childhood to midlife, and greater change in rates of multiple CVRFs since childhood than men during the same transition. The combined effect of these multiple CVRFs in women with diabetes may explain the stronger impact of diabetes on cardiovascular events in women compared with men. Preventive strategies for cardiovascular risk and diabetes should include a focus on early life and young adulthood when these changes can initially be detected.
Supplementary Material
Article Information
Acknowledgments. The BHS is a joint effort of many investigators and staff members whose contribution is gratefully acknowledged. The authors thank the Bogalusa, LA, school system and, most importantly, the children and adults who have participated in this study over many years.
Funding. This work was supported by National Institutes of Health (NIH) grants R01AG041200, R21AG057983, R01HD069587, R01ES021724, and R01AG016592. M.K.-W. and L.B. were supported in part by NIH grants P20GM109036 and K12HD043451. T.D. was supported by National Natural Science Foundation of China grant 81700762. F.M.-J. was supported by NIH grants R01DK074970 and DK107444 and a VA Merit Review Award (BX003725).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. T.D. and L.B. conceived the study design, wrote the first draft of the manuscript, researched the data, contributed to the interpretation of results, commented on drafts of the manuscript, and approved the final version of the manuscript. C.F. and R.B. researched the data, contributed to the interpretation of results, and approved the final version of the manuscript. Y.G., M.K.-W., W.C., L.Q., E.H., F.M.-J., and V.F. contributed to the interpretation of results, commented on drafts of the manuscript, and approved the final version of the manuscript. L.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc18-2029/-/DC1.
References
- 1.American Diabetes Association 9. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018;41(Suppl. 1):S86–S104 [DOI] [PubMed] [Google Scholar]
- 2.Peters SA, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet 2014;383:1973–1980 [DOI] [PubMed] [Google Scholar]
- 3.Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ 2006;332:73–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Juutilainen A, Kortelainen S, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Gender difference in the impact of type 2 diabetes on coronary heart disease risk. Diabetes Care 2004;27:2898–2904 [DOI] [PubMed] [Google Scholar]
- 5.Peters SA, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia 2014;57:1542–1551 [DOI] [PubMed] [Google Scholar]
- 6.Yusuf S, Hawken S, Ounpuu S, et al.; INTERHEART Study Investigators . Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:937–952 [DOI] [PubMed] [Google Scholar]
- 7.Lyon A, Jackson EA, Kalyani RR, Vaidya D, Kim C. Sex-specific differential in risk of diabetes-related macrovascular outcomes. Curr Diab Rep 2015;15:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donahue RP, Rejman K, Rafalson LB, Dmochowski J, Stranges S, Trevisan M. Sex differences in endothelial function markers before conversion to pre-diabetes: does the clock start ticking earlier among women? The Western New York Study. Diabetes Care 2007;30:354–359 [DOI] [PubMed] [Google Scholar]
- 9.Wannamethee SG, Papacosta O, Lawlor DA, et al. Do women exhibit greater differences in established and novel risk factors between diabetes and non-diabetes than men? The British Regional Heart Study and British Women’s Heart Health Study. Diabetologia 2012;55:80–87 [DOI] [PubMed] [Google Scholar]
- 10.Williams K, Tchernof A, Hunt KJ, Wagenknecht LE, Haffner SM, Sniderman AD. Diabetes, abdominal adiposity, and atherogenic dyslipoproteinemia in women compared with men. Diabetes 2008;57:3289–3296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barrett-Connor EL, Cohn BA, Wingard DL, Edelstein SL. Why is diabetes mellitus a stronger risk factor for fatal ischemic heart disease in women than in men? The Rancho Bernardo Study. JAMA 1991;265:627–631 [PubMed] [Google Scholar]
- 12.Li S, Chen W, Srinivasan SR, et al. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: the Bogalusa Heart Study. JAMA 2003;290:2271–2276 [DOI] [PubMed] [Google Scholar]
- 13.Li X, Li S, Ulusoy E, Chen W, Srinivasan SR, Berenson GS. Childhood adiposity as a predictor of cardiac mass in adulthood: the Bogalusa Heart Study. Circulation 2004;110:3488–3492 [DOI] [PubMed] [Google Scholar]
- 14.Lai CC, Sun D, Cen R, et al. Impact of long-term burden of excessive adiposity and elevated blood pressure from childhood on adulthood left ventricular remodeling patterns: the Bogalusa Heart Study. J Am Coll Cardiol 2014;64:1580–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juonala M, Magnussen CG, Berenson GS, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med 2011;365:1876–1885 [DOI] [PubMed] [Google Scholar]
- 16.Juhola J, Magnussen CG, Berenson GS, et al. Combined effects of child and adult elevated blood pressure on subclinical atherosclerosis: the International Childhood Cardiovascular Cohort Consortium. Circulation 2013;128:217–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berenson GS, McMahan CA, Voors AW, et al. Cardiovascular risk factors in children. In The Early Natural History of Atherosclerosis and Essential Hypertension. Andrews C, Hester HE, Eds. New York, Oxford University Press, 1980, p. 47–123 [Google Scholar]
- 18.Lipid Research Clinics Program Manual of Laboratory Operations: Lipid and Lipoprotein Analysis. Bethesda, MD, National Institutes of Health, 1974 [Google Scholar]
- 19.Bucolo G, David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem 1973;19:476–482 [PubMed] [Google Scholar]
- 20.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem 1974;20:470–475 [PubMed] [Google Scholar]
- 21.Srinivasan SR, Berenson GS. Serum lipoproteins in children and methods for study. In CRC Handbook of Electrophoresis. Vol. 3: Lipoprotein Methodology and Human Studies. Lewis LA, Ed. Boca Raton, FL, CRC Press, 1983, p. 185–204 [Google Scholar]
- 22.American Diabetes Association 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018;41(Suppl. 1):S13–S27 [DOI] [PubMed] [Google Scholar]
- 23.Cook NR, Rosner BA, Chen W, Srinivasan SR, Berenson GS. Using the area under the curve to reduce measurement error in predicting young adult blood pressure from childhood measures. Stat Med 2004;23:3421–3435 [DOI] [PubMed] [Google Scholar]
- 24.Neter J, Wasserman W, Kutner MH. Applied linear statistical models. In Regression, Analysis of Variance and Experimental Designs. Homewood, IL, R.D. Irwin, 1990, p. 225–236 [Google Scholar]
- 25.Rovio SP, Pahkala K, Nevalainen J, et al. Cardiovascular risk factors from childhood and midlife cognitive performance: the Young Finns Study. J Am Coll Cardiol 2017;69:2279–2289 [DOI] [PubMed] [Google Scholar]
- 26.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 1990;322:1561–1566 [DOI] [PubMed] [Google Scholar]
- 27.Drazner MH, Rame JE, Marino EK, et al. Increased left ventricular mass is a risk factor for the development of a depressed left ventricular ejection fraction within five years: the Cardiovascular Health Study. J Am Coll Cardiol 2004;43:2207–2215 [DOI] [PubMed] [Google Scholar]
- 28.Li S, Chen W, Sun D, et al. Variability and rapid increase in body mass index during childhood are associated with adult obesity. Int J Epidemiol 2015;44:1943–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dyer AR, Stamler J, Greenland P. Associations of weight change and weight variability with cardiovascular and all-cause mortality in the Chicago Western Electric Company Study. Am J Epidemiol 2000;152:324–333 [DOI] [PubMed] [Google Scholar]
- 30.Lissner L, Odell PM, D’Agostino RB, et al. Variability of body weight and health outcomes in the Framingham population. N Engl J Med 1991;324:1839–1844 [DOI] [PubMed] [Google Scholar]
- 31.Rzehak P, Meisinger C, Woelke G, Brasche S, Strube G, Heinrich J. Weight change, weight cycling and mortality in the ERFORT Male Cohort Study. Eur J Epidemiol 2007;22:665–673 [DOI] [PubMed] [Google Scholar]
- 32.Shen W, Zhang T, Li S, et al. Race and sex differences of long-term blood pressure profiles from childhood and adult hypertension: the Bogalusa Heart Study. Hypertension 2017;70:66–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang T, Li S, Bazzano L, He J, Whelton P, Chen W. Trajectories of childhood blood pressure and adult left ventricular hypertrophy: the Bogalusa Heart Study. Hypertension 2018;72:93–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang T, Xu J, Li S, et al. Trajectories of childhood BMI and adult diabetes: the Bogalusa Heart Study. Diabetologia 2019;62:70–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kahn HS, Cheng YJ. Longitudinal changes in BMI and in an index estimating excess lipids among white and black adults in the United States. Int J Obes 2008;32:136–143 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.