Abstract
Background:
Studies of emergent neuroimaging in the management of patients presenting with a breakthrough seizure are lacking. We sought to determine how often emergent computed tomography (CT) scans are obtained in patients with known epilepsy presenting with a seizure and how often acute abnormalities are found.
Methods:
This multicenter retrospective cohort study was performed in the emergency department at 2 academic medical centers. The primary outcomes were percentage of visits where a CT scan was obtained, whether CT findings represented acute abnormalities, and whether these findings changed acute management.
Results:
Of the 396 visits included, CT scans were obtained in 39%, and 8% of these scans demonstrated acute abnormalities. Patients who were older, had status epilepticus, a brain tumor, head trauma, or an abnormal examination were all significantly more likely to undergo acute neuroimaging (P < .05). In the multivariable model, only history of brain tumor (odds ratio [OR] 5.88, 95% confidence interval [CI], 1.33-26.1) and head trauma as a result of seizure (OR 3.92, 95% CI, 1.01-15.2) reached statistical significance in predicting an acutely abnormal scan. The likelihood of an acute imaging abnormality in visits for patients without a history of brain tumor or head trauma as a result of the seizure was 2.7% (2 visits). Both of these patients had abnormal neurological examinations.
Conclusion:
Obtaining an emergent CT scan for patients with epilepsy presenting with a seizure may be avoidable in most cases, but might be indicated for patients with a history of brain tumor or head trauma as a result of seizure.
Keywords: seizure, computed tomography, CT, emergency, epilepsy
Introduction
There are an estimated 3.4 million people with epilepsy in the United States.1 The estimated annual direct medical cost of epilepsy in the United States is $9.6 billion,2 with emergency department (ED) visits making up a substantial portion of this cost at 1.15 billion dollars.3 Patients with epilepsy average 0.98 to 1.12 visits to the ED annually.4,5
Studies of emergent neuroimaging in the acute management of patients presenting with a breakthrough seizure are lacking. Recent guidelines recommend performing neuroimaging in the ED in patients with a first unprovoked seizure.6,7 American Academy of Neurology guidelines concluded that the evidence is inadequate to support or refute emergent imaging for patients with epilepsy presenting with a breakthrough seizure while the American College of Emergency Physicians guidelines do not address this issue at all.6, 7 Previous guidelines recommended considering emergent neuroimaging when the provider suspects a serious structural lesion—with features such as a new seizure type, new focal deficits, persistent altered mental status (with or without intoxication), fever, recent trauma, persistent headache, or history of cancer, anticoagulation, or HIV all considered to be suggestive of an underlying structural lesion.8
We sought to determine how often patients with known epilepsy presenting with a seizure undergo emergent neuroimaging and the yield of imaging in changing acute management. We hypothesized that a large percentage of patients with seizures would undergo computed tomography (CT), and that in the majority of cases, the imaging results would not lead to a change in acute management. Patients with medically refractory epilepsy may undergo a multitude of unnecessary scans over their lifetime, which represents a potentially substantial cost, inconvenience, and radiation burden.
Methods
Study Design and Setting
We performed a retrospective review of all ED visits for adults (age ≥18) who presented with a seizure between October 1, 2012, and September 30, 2013,at 2 academic hospitals, Stanford University (hospital A, a level 1 trauma center) and the University of California, San Francisco (hospital B).
Selection of Participants
Visits were identified using a query of ED encounters with an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis of seizure or status epilepticus anywhere in the chart (ICD-9-CM codes 345.X, 780.3X). These charts were reviewed by 2 authors (KAK and JPB) and only patients with a history of seizure noted in the ED encounter documentation and currently on or previously recommended to be on antiepileptics were included. Patients with a first-time seizure, second lifetime seizure never placed on antiepileptics, exclusively alcohol withdrawal seizures, and those patients with spells of unclear etiology who were never placed on an antiepileptic were all excluded. For those patients meeting our definition of epilepsy, all ED encounters during the study period were reviewed and those where the patient presented with an acute seizure were included. Visits where the seizure occurred greater than 24 hours prior to presentation, the patient presented with aura alone, left prior to being fully evaluated or went straight to magnetic resonance imaging (MRI) were excluded. To determine the consistency of visit selection, 50 charts were reviewed for inclusion by both authors (KAK and JPB).
Methods of Measurement
Charts for included encounters were reviewed for demographic information, seizure semiology, antiepileptic history, past medical history, current medications, physical examination findings, laboratory results, neuroimaging results, discharge antiepileptic medications and doses, and whether neurology or neurosurgery consult was obtained. All variables to be collected as well as their definitions were determined a priori and entered into a standardized electronic data collection form. There were no missing data as it relates to the primary outcome measures. If the ED encounter documentation did not explicitly indicate a relevant medical problem (eg, cancer, HIV) or medication, it was assumed that the patient did not have these. If trauma was not recorded in the history but was found on examination, this was coded as trauma secondary to seizure unless otherwise specified in the chart. If the history and examination did not reveal any historical or clinical evidence of trauma, it was assumed there was no head trauma as a result of seizure. There were no other missing data fields.
Outcome Measures
The primary outcomes were (1) was acute neurologic imaging with a CT obtained during the visit? (2) was the neuroimaging finding deemed to be acute? and (3) did the neuroimaging finding result in an acute change in clinical management? Radiology reports were reviewed to determine results of any imaging obtained. We defined acute neuroimaging abnormalities broadly to include the following: traumatic intracranial hemorrhage (subarachnoid, intraparenchymal, subdural, or epidural) or skull or facial fracture, nontraumatic intracranial hemorrhage (subarachnoid, intraparenchymal, subdural, or epidural), stroke, new or enlarging tumor, new or worsening hydrocephalus, and brain abscess. Acute changes in management were defined as emergent referral or consultation, nonantiepileptic medication change (eg, prescription of steroids to reduce edema or blood product transfusion for intracranial hemorrhage), surgery, urgent/emergent radiation, observation and follow-up scan, or admission for further workup. We also reviewed charts for evidence of potential harm from obtaining an unnecessary scan, including incidental or artifactual findings that led to additional monitoring or testing. Using the data from hospital B, we measured the interrater agreement for the determination of which scans demonstrated an acute finding as well as which findings led to an acute change in management. For the single case where there was disagreement, a third author (VCD) reviewed the chart and the outcomes were adjudicated by discussion.
Primary Data Analysis
We examined whether any clinical variables were predictive of detecting an acute finding on neuroimaging in the ED. We also calculated the rate at which neuroimaging was obtained for encounters with each of these clinical variables. We chose the encounter as the unit of analysis rather than the patient, because the clinical situation can vary for a single patient presenting on different occasions with breakthrough seizures (eg, they may have head trauma on one occasion but not the other). However, there is also correlation between encounters for the same patient. To account for correlation between encounters for the same patient, we used generalized estimating equations with the patient as the group variable. Since the dependent variables (whether or not imaging was performed, and whether or not the scan showed acute findings) were categorical, we used the binomial family with a logit link function and exchangeable correlation. The threshold for statistical significance was set using a 2-tailed α at .05. Corrections for multiple comparisons were not made, since with the exception of demographics, each of the variables examined were chosen intentionally and a priori, since they might be predicted on a clinical basis to be associated with an acute abnormality on neuroimaging in patients with recurrent seizures. We constructed a multivariable model using all variables from the univariate analysis where the level of significance was P < .10. All statistical analysis was performed using a licensed copy of STATA, version 13 (Citation: StataCorp. 2013. Stata Statistical Software: Release 13. College Station, Texas: StataCorp LP).
Standard Protocol Approvals, Registrations, and Patient Consents
The Stanford University and University of California, San Francisco, institutional review boards independently approved the study and a waiver of informed consent was granted.
Results
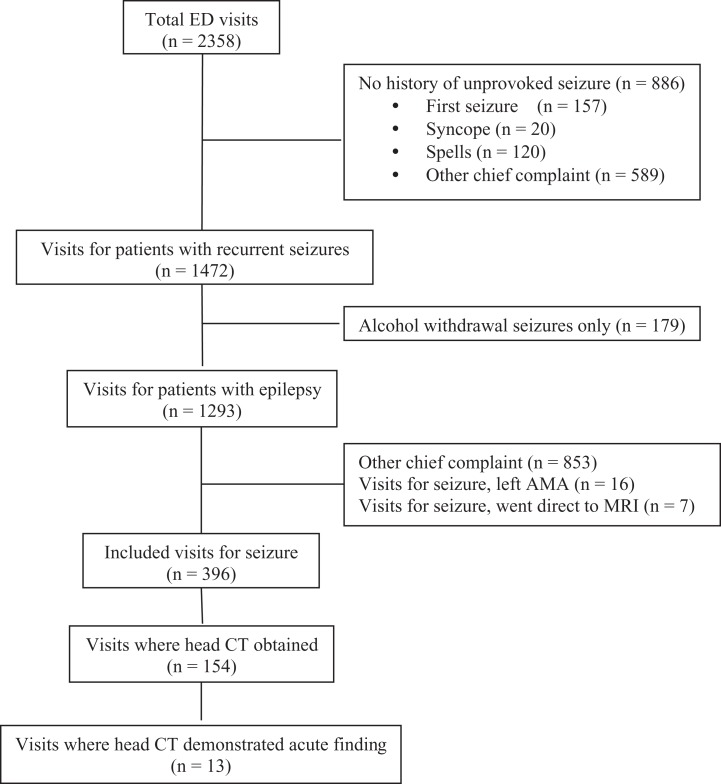
Our ICD-9-CM query revealed 436 unique patients for hospital A and 527 unique patients for hospital B with a total of 2358 ED visits between October 1, 2012, and September 30, 2013. Among those encounters, 396 met our inclusion criteria. Details of patient selection are outlined in Figure 1. There was 100% agreement on inclusion and exclusion in the 50 charts reviewed by 2 authors at hospital B. Hospital A was not included for interrater agreement for convenience (only one study author had access to the medical record at this site).
Figure 1.
Emergency department visit inclusion algorithm.
Characteristics of Study Patients
Of all the reviewed encounters, a total of 396 visits met the study inclusion criteria across 276 unique patients (196 visits from hospital A and 200 visits from hospital B). The mean age at the time of ED presentation was 43 (range 18-99); 157 visits were for females (40%) and 239 were for males (60%). A CT scan was obtained in 154 of the 396 (39%) encounters. Overall, 126 patients (46%) had at least one scan and 12 patients (4%) had more than one scan, 3 (2%) had 3 scans and 1 patient (0.8%) had 11 scans during the 1-year study period (Supplemental table 1).
Table 1 describes the demographic and clinical features of the visits with head CTs compared to those where none was performed. Patients who were scanned were older (P < .001), more likely to have a known history of brain tumor (P < .001), have a history of seizure-related or other recent trauma (P < .001 and P = .001, respectively), a change in seizure semiology (P = .05), multiple seizures or status epilepticus (P = .02), have focal findings on examination or encephalopathy without focal findings (P < .001 and P = .004, respectively), or have recent neuroimaging (P = .004). Patient sex, race, and primary language did not differ significantly between the 2 groups. The HIV status, antiplatelet, anticoagulation or immunosuppressant use, persistent headache, presence of fever or meningismus, and medication adherence also did not differ significantly between the 2 populations.
Table 1.
Comparison of Clinical Characteristics for Visits Where a CT was Obtained Compared to Those Where It was Not.
| No CT, n = 242 (%) | CT, n = 154 (%) | P Value | |
|---|---|---|---|
| Mean age (SD) | 40 (16) | 48 (17) | <.001 |
| Male sex | 145 (60) | 94 (61) | .87 |
| Race | .95 | ||
| White | 129 (53) | 82 (53) | |
| Black | 53 (22) | 37 (24) | |
| Hispanic | 32 (13) | 17 (11) | |
| Asian | 22 (9) | 13 (8) | |
| Other | 6 (2) | 5 (3) | |
| Seizure semiology | .66 | ||
| Generalized tonic clonic | 160 (66) | 108 (70) | |
| Focal aware | 31 (13) | 20 (13) | |
| Unknown | 51 (21) | 26 (17) | |
| Change in semiology | 8 (3) | 12 (8) | .06 |
| Seizure duration | .02 | ||
| Single, self-limited | 166 (69) | 92 (60) | |
| Multiple | 66 (27) | 44 (29) | |
| Status epilepticus | 10 (4) | 18 (12) | |
| Status epilepticus (by ICD-9-CM) | 2 (1) | 8 (5) | .02 |
| Cancer (current or prior) | |||
| Systemic malignancy | 5 (2) | 7 (5) | .17 |
| Brain tumor | 16 (7) | 29 (19) | <.001 |
| HIV+ | 10 (4) | 8 (5) | .48 |
| Medications | |||
| Antiplatelet | 20 (8) | 18 (12) | .24 |
| Anticoagulation | 8 (3) | 8 (5) | .34 |
| Immunosuppression | 2 (1) | 5 (3) | .10 |
| Persistent headache | 45 (19) | 29 (19) | .91 |
| Fever or meningismus | 3 (1) | 3 (2) | .55 |
| Head trauma | |||
| As a result of seizure | 22 (9) | 49 (32) | <.001 |
| Other recent head trauma | 2 (1) | 10 (6) | .007 |
| Physical examination | |||
| Normal or baseline | 195 (81) | 87 (56) | <.001 |
| Encephalopathic but nonfocal | 40 (17) | 44 (29) | .005 |
| Focal neurological findings | 7 (3) | 23 (15) | <.001 |
| Recent neuroimaging (<1 month) | 32 (13) | 38 (25) | .006 |
| Nonadherence by self-report or low AED levels | 122 (50) | 77 (50) | .80 |
Abbreviations: AED, antiepileptic drug; CT, computed tomography; SD, standard deviation.
Main Results
Results of the CT scans are outlined in Table 2. None of the scans had more than one abnormal finding reported. Interrater agreement for determining which scans had an acute finding and which led to an acute change in management was 98% for both outcomes, with a κ of.91. The majority of the scans were either normal (34%) or demonstrated nonacute findings (58%). Acute abnormalities were found in 13 cases (8.4%) and included traumatic intracranial hemorrhage or fracture (4.5%), expanding tumor or spread (1.9%), and nontraumatic intracranial hemorrhage (1.9%).
Table 2.
Findings on CT.
| Neuroimaging Finding | Number of Scans, n = 154 (%) |
|---|---|
| Normal | 52 (34) |
| Diffuse atrophy | 10 (6.5) |
| Focal atrophy (ie, old stroke or trauma) | 47 (31) |
| Other nonacute finding | 31 (20) |
| Traumatic ICH or skull fracture | 7 (4.5) |
| Nontraumatic ICH | 3 (1.9) |
| Expanding tumor or spread | 3 (1.9) |
Abbreviations: CT, computed tomography; ICH, intracranial hemorrhage.
Male patients and those with a history of brain tumor or head trauma as a result of seizure were the most likely to show an acute abnormality although only male sex reached statistical significance (OR 9.42, 95% CI, 1.19-74.6). These results are summarized in Table 3. A multivariable model was constructed using male sex, history of brain tumor, and head trauma as a result of seizure to determine whether these 3 variables were independent predictors of acute neuroimaging findings, of which only brain tumor or head trauma were (Table 4). History of brain tumor or head trauma as a result of seizure predicted 11 out of the 13 acutely abnormal scans, and in 75 of the 154 visits where scans were obtained, the patient had one or both of these characteristics. Conversely, acute abnormalities were found in only 2 of the 79 visits (2.5%) where scans were obtained and the patient did not have either of these 2 characteristics.
Table 3.
Predictors of Acute Neuroimaging Findings.
| No Acute Findings, n (%) | Acute Findings, n (%) | P Value | OR for Positive Scan (95% CI) | |
|---|---|---|---|---|
| n = 141 | n = 13 | |||
| Mean age (SD) | 48 (18) | 51 (13) | .57 | 1.01 (0.98-1.04) |
| Male sex | 82 (58) | 12 (92) | .03 | 9.42 (1.19-74.6) |
| Race | .23 | |||
| White | 76 (54) | 6 (46) | 1 | |
| Black | 35 (25) | 2 (15) | 0.84 (0.16-4.41) | |
| Hispanic | 13 (9) | 4 (31) | 3.85 (0.95-15.6) | |
| Asian | 12 (9) | 1 (8) | 1.03 (0.11-9.33) | |
| Other | 5 (4) | 0 (0) | ||
| Seizure semiology | .89 | |||
| Generalized tonic clonic | 97 (69) | 11 (85) | 1 | |
| Focal | 18 (13) | 2 (15) | 0.92 (0.19-4.49) | |
| Unknown | 26 (18) | 0 (0) | ||
| Change in semiology | 11 (8) | 1 (8) | .94 | 0.92 (0.11-7.69) |
| Seizure duration | .89 | |||
| Single self-limited | 85 (60) | 7 (54) | 1 | |
| Multiple | 40 (28) | 4 (31) | 1.19 (0.34-4.15) | |
| Status epilepticus | 16 (11) | 2 (15) | 1.44 (0.28-7.38) | |
| Status epilepticus (by ICD-9-CM) | 7 (5) | 1 (8) | .71 | 1.50 (0.17-13.1) |
| Cancer (current or prior) | ||||
| Systemic malignancy | 6 (4) | 1 (8) | .62 | 1.75 (0.19-15.7) |
| Brain tumor | 24 (17) | 5 (38) | .08 | 2.93 (0.88-9.74) |
| HIV+ | 7 (5) | 1 (8) | .67 | 1.61 (0.18-14.3) |
| Medications | ||||
| Antiplatelet | 16 (11) | 2 (15) | .69 | 1.38 (0.28-6.83) |
| Anticoagulation | 6 (4) | 2 (15) | .12 | 3.94 (0.71-22.0) |
| Immunosuppression | 5 (4) | 0 (0) | .49 | |
| Persistent headache | 26 (18) | 3 (23) | .72 | 1.28 (0.33-4.88) |
| Fever or meningismus | 3 (2) | 0 (0) | .60 | |
| Head trauma | ||||
| As a result of seizure | 41 (29) | 8 (62) | .05 | 2.99 (0.99-9.07) |
| Other recent head trauma | 10 (7) | 0 (0) | .32 | |
| Physical examination | .63 | |||
| Normal or baseline | 81 (57) | 6 (46) | 1 | |
| Encephalopathic but nonfocal | 40 (28) | 4 (31) | 1.48 (0.41-5.36) | |
| Focal neurological findings | 20 (14) | 3 (23) | 1.96 (0.46-8.43) |
Abbreviations: CI, confidence interval; OR, odds ratio; SD, standard deviation.
Table 4.
Multivariable Analysis of Predictors for Positive Head Imaging.
| Patient Characteristic | OR for Positive Scan (95% CI) | |
|---|---|---|
| Male sex | 7.84 | (0.95-65.0) |
| Brain tumor (current or prior) | 5.88 | (1.33-26.1) |
| Head trauma as a result of seizure | 3.92 | (1.01-15.2) |
Abbreviations: CI, confidence interval; OR, odds ratio.
In the additional 2 patients with acute CT abnormalities, the neurological examination was abnormal. One patient had metastatic lung cancer, was encephalopathic, and was found to have a subarachnoid density that was consistent with either hemorrhage or possible artifact, but no follow-up imaging was obtained as this was not concordant with the patient’s goals of care. The other patient presented in convulsive status epilepticus with a third nerve palsy and was found to have a large subdural hematoma.
Using our broad definition of a change in management, all of the scans with acute findings changed acute management. Eight led to an admission (5%), 4 led to a follow-up scan (3%), 5 led to urgent referral (3%), 2 led to a medication change (1%), and 1 led to a surgery (0.7%).
Among the 13 patients with acute neuroimaging abnormalities, 3 had nondisplaced fractures that prompted an otolaryngology consult and antibiotics but required no further inpatient workup. Two others were patients already being followed by neurooncology with surveillance outpatient imaging who had findings of worsening leptomeningeal disease and progressive radiation treatment effect respectively and required urgent but not emergent treatment decisions. One patient with active metastatic lung cancer had a CT with possible scant subarachnoid hemorrhage or artifact for which repeat imaging was recommended but not pursued due to a change in the patient’s goals of care. In total, we counted only 7 patients (4.5%) where the acute findings might have led to an acute neurological decompensation: 5 traumatic intracranial hemorrhages, 1 patient with glioblastoma on therapeutic enoxaparin with an intratumoral hemorrhage, and 1 patient with melanoma and brain metastases with intratumoral hemorrhage.
In our study population, 3 scans led to overutilization and potential harm. In 2, the preliminary CT read suggested an acute finding which upon further consideration the final read of the initial CT was ultimately deemed normal. The preliminary interpretation led to hospitalization for observation in both patients and a follow-up scan in one. In the third, a possible cortical vein thrombosis was read on the final report, so the patient was called back to the ED the next day for an MRI/magnetic resonance venography, which was normal.
Discussion
The CT scans were performed in 39% of encounters for breakthrough seizure. Only 8% of the scans obtained, accounting for 3% of all encounters, demonstrated an acute neuroimaging abnormality, all of which led to a change in management. The incidence of acute CT abnormalities found in our study is less than that seen in prior studies evaluating utility of neuroimaging in first-time seizure or mixed populations. In the evaluation of first-time seizure, 34% to 56% of patients had an abnormal CT scan,9-14 but only 9% to 17% of these resulted in a change in acute management.10,11,13 In the 2 previous studies with mixed populations that included patients with established epilepsy, the rate of acute imaging abnormalities was 21% to 25%.15,16 To our knowledge, there is only one other published study evaluating incidence of acute neuroimaging findings in patients with epilepsy presenting with seizure. The authors found that 3% of the 381 scans demonstrated a finding that led to an acute change in management with definition of acute change in management defined more narrowly than in our study.17 In one study of children with epilepsy and breakthrough seizure, 17% had a CT scan and none showed any acute abnormalities.18 A priori, we chose to define acute neuroimaging abnormalities conservatively to ensure all patients with an acute finding were captured. However, this likely led to an overestimate of the true utility of emergent CT in the evaluation of seizures in our population of patients with epilepsy.
This study has some important limitations. As a retrospective chart review, we were reliant on information documented in the chart. Information about the indication for obtaining a neuroimaging scan was not always apparent and therefore not coded. Although it may be common practice to wait for labs, including drug levels, prior to ordering neuroimaging, we were unable to capture this medical decision-making process retrospectively. Abstractors were not blinded to the study hypothesis which may have led to some bias in variable abstraction. To minimize this effect, variables were defined a priori. Additionally, interpreters of neuroimaging studies were not blinded to the clinical situation, which might have led to bias regarding whether the result was acute or acutely changed management. For the variables that predicted an abnormal scan, we should note that due to the small numbers, the study was likely underpowered to detect the significance of several variables—particularly HIV status, antiplatelet, anticoagulant or immunosuppressant use, or infectious symptoms in predicting an acutely abnormal scan. By contrast, because our study was performed at 2 tertiary centers with a neurooncology practice, our population was likely more enriched with brain tumors than a community hospital setting and this likely influenced both the frequency of CTs obtained and the likelihood of an abnormal CT. In addition, not all patients underwent scans, and we are unable to be certain that some acute findings were missed in patients who were not scanned. For patients who had trauma as a cause or consequence of their seizure, we could not determine whether clinicians were using a validated tool, such as the Canadian Heat CT rules, to determine need for neuroimaging. These scores were not reported explicitly in the chart and some of the necessary clinical information to calculate these scores was missing (duration of amnesia, mechanism). Accordingly, we were unable to validate these tools in our cohort.19 The more recently validated Nexus II criteria for neuroimaging in blunt trauma similarly could not be applied to our retrospective population as the necessary clinical information was variably documented. Abnormal level of alertness is a consistent variable in both prediction rules, but how this applies to patients with seizure who may have loss of awareness due to seizure itself and postictal impairments has not yet been studied.20
This study has important implications for the evaluation of patients with epilepsy presenting to the ED with a seizure. Epilepsy is a common disorder and many patients with epilepsy present to the ED repeatedly and undergo multiple scans over time. Unnecessary scans are costly to the health-care system and result in unnecessary radiation exposure for patients. Additionally, unnecessary scans can also lead to harm if incidental findings are discovered that require additional testing. In our study, this led to unnecessary observation stays in 2 patients and an additional emergency room visit for follow-up imaging in another.
Our data help identify which patients have a higher likelihood of an abnormal scan that would impact acute management and may set the stage for further prospective studies aimed at defining potential criteria for when to consider acute neuroimaging in patients with epilepsy presenting with a seizure. This may pave the way for decision support tools to help reduce overutilization of CT scans.21 In our cohort, neuroimaging was more frequently obtained in patients who were older, had a known brain tumor, a history of seizure-related trauma, a change in seizure semiology, multiple or prolonged seizures, had focal findings on exam or encephalopathy or had recent neuroimaging. However, the factors most associated with an acute imaging finding were history of a brain tumor, head trauma as a result of seizure, and male sex. Unlike the other identified risk factors, it is not clear why being male would increase the likelihood of an acute imaging finding. It is possible this is the result of overfitting the multivariable model given the small number of outcomes, and male sex may only be associated with acute imaging abnormalities because both trauma and glioblastoma are also more common in males. Only 2 of the 13 scans with acute abnormalities did not have either a brain tumor or head trauma as a result of the seizure; this represented 2 of 79 (2.5%) scans obtained in patients without these characteristics, and 2 of the 284 encounters (0.07%) in the cohort without these characteristics. Furthermore, both of these patients had an abnormal neurological examination. This suggests that in the absence of these historical features, acute neuroimaging in patients with epilepsy presenting with a seizure may change management in only 0.07 to 2.5% of cases, and with the addition of a normal neurological exam, acute neuroimaging may not be warranted. In our population, there were 79 scans (51% of those obtained) that did not meet any of these criteria and could be considered as potentially avoidable cost and radiation exposure.
Focal neurological findings were not a significant predictor of an acute abnormality. This contrasts with what has been seen in other studies.9-11,22 of first time seizure and in the study of imaging yield in patients with known epilepsy17 but is similar to what was found in a previous retrospective study of epilepsy patients with trauma and seizure.23 This may reflect a difference in study population as many of the patients in our study had brain tumors and may have had focal neurological findings at baseline.
Notably, the rate of obtaining neuroimaging in this patient population was high across both centers in our study and similar to that of Salinksly et al.17 The American Epilepsy Society recently released a set of Choosing Wisely recommendations including a recommendation to avoid brain imaging in patients with acute seizure and established epilepsy.24 We believe our study highlights a key opportunity for developing a more robust evidence-based prediction tool for determining need for acute neuroimaging in this population.
In conclusion, in ED visits for patients with epilepsy who undergo emergent neuroimaging, acute findings are found in 8% of these scans. Obtaining an emergent CT scan for the patient with epilepsy presenting with a seizure may be of high yield for patients with a history of brain tumor or head trauma as a result of seizure but it might be avoidable in others.
Supplemental Material
Supplementary_Table for Yield of Emergent CT in Patients With Epilepsy Presenting With a Seizure by Kathryn A. Kvam, Vanja C. Douglas, William D. Whetstone, S. Andrew Josephson, and John P. Betjemann in The Neurohospitalist
Acknowledgments
WDW reports grant money to University of California, San Francisco, to conduct research conceived and written by William D. Whetstone from the department of defense and Neilsen foundation for activities unrelated to this manuscript. Study data were gathered from the Stanford University electronic medical records using STRIDE (Stanford Translational Research Integrated Database Environment). STRIDE is a research and development project at Stanford University to create a standards-based informatics platform supporting clinical and translational research.25 The project described was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1 TR001085. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Study data were obtained from the UCSF electronic medical records using THREDs. Study data were collected and managed using REDCap electronic data capture tools hosted at Stanford University and University of California, San Francisco.26 REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing: (1) an intuitive interface for validated data entry; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; (and 4) procedures for importing data from external sources.
Authors’ Note: KAK, VCD, and JPB conceived the study, defined the study variables, and created the data collection instrument. WDW and SAJ provided advice on study design. KAK performed the inclusion analysis and KAK and JPB performed the chart abstraction. VCD performed the statistics with additional input from SAJ. KAK drafted the manuscript, and all authors contributed substantially to its revision. KAK takes responsibility for the paper as a whole. Statistical analysis was performed by Vanja C. Douglas, MD, Department of Neurology, University of California, San Francisco, CA, USA. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Declaration of Conflicting Interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: VCD receives compensation as Editor-in-Chief of The Neurohospitalist. SAJ receives personal compensation in his role as Editor-in-Chief of JAMA Neurology and as Associate Editor for Continuum Audio. JPB receives personal compensation for serving as web editor of JAMA Neurology. KAK reports no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Kathryn A. Kvam  https://orcid.org/0000-0002-2532-8315
https://orcid.org/0000-0002-2532-8315
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Zack MM, Kobau R. National and state estimates of the numbers of adults and children with active epilepsy – United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(31):821–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yoon D, Frick KD, Carr DA, et al. Economic impact of epilepsy in the United States. Epilepsia. 2009;50(10):2186–2191. [DOI] [PubMed] [Google Scholar]
- 3. Singh G, Mithal A, Lingala B. Emergency department visits for uncontrolled epilepsy in US: a national perspective. Neurology. 2016;86(suppl P1.061). Abstract. [Google Scholar]
- 4. Faught RE, Weiner JR, Guerin A, et al. Impact of nonadherence to antiepileptic drugs on health care utilization and costs: findings from the RANSOM study. Epilepsia. 2009;50(3):501–509. [DOI] [PubMed] [Google Scholar]
- 5. Davis KL, Candrilli SD, Edin HM. Prevalence and cost of nonadherence with antiepileptic drugs in an adult managed care population. Epilepsia. 2008;49(3):446–454. [DOI] [PubMed] [Google Scholar]
- 6. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with seizures. Ann Emerg Med. 2004;43(5):605–625. [DOI] [PubMed] [Google Scholar]
- 7. Harden CL, Huff JS, Schwartz TH, et al. Reassessment: neuroimaging in the emergency patient presenting with seizure (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommitttee of the American Academy of Neurology. Neurology. 2007;69(18):1772–1780. [DOI] [PubMed] [Google Scholar]
- 8. Practice parameter: neuroimaging in the emergency patient presenting with seizure – summary statement. Neurology. 1996;47(1):288–291. [PubMed] [Google Scholar]
- 9. Ramirez-Lassepas M, Cipolle RJ, Morillo LR, et al. Value of computed tomographic scan in the evaluation of adult patients after their first seizure. Ann Neurol. 1984;15(6):536–543. [DOI] [PubMed] [Google Scholar]
- 10. Sempere AP, Villaverde FJ, Martinez-Menendez B, et al. First seizure in adults: a prospective study from the emergency department. Acta Neurol Scand. 1992;86(2):134–138. [DOI] [PubMed] [Google Scholar]
- 11. Schoenenberger RA, Heim SM. Indication for computed tomography of the brain in patients with first uncomplicated generalized seizure. BMJ. 1994;309(6960):986–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Henneman PL, DeRoos F, Lewis RJ. Determining the need for admission in patients with new onset seizures. Ann Emerg Med. 1994;24(6):1108–1114. [DOI] [PubMed] [Google Scholar]
- 13. Mower WR, Biros MH, Talan DA, et al. Selective tomographic imaging of patients presenting with new-onset seizure disorders. Acad Emerg Med. 2002;9(1):43–47. [DOI] [PubMed] [Google Scholar]
- 14. Tardy B, Lafond P, Convers P, et al. Adult first generalized seizure: etiology, biological tests, EEG, CT scan, in an ED. Am J Emerg Med. 1995;(13):1–5. [DOI] [PubMed] [Google Scholar]
- 15. Eisner RF, Turnbull TL, Howes DS, et al. Efficacy of the “standard” seizure workup in the emergency department. Ann Emerg Med. 1986;15(1):33–39. [DOI] [PubMed] [Google Scholar]
- 16. Reinus WR, Wippold FJ, Erickson KK. Seizure patient selection for emergency computed tomography. Ann Emerg Med. 1993;22(8):1298–1303. [DOI] [PubMed] [Google Scholar]
- 17. Salinsky M, Wong VSS, Motika P, et al. Emergency department neuroimaging for epileptic seizures. Epilepsia. 2018;59(9):1676–1683. [DOI] [PubMed] [Google Scholar]
- 18. Allen L, Jones CT. Emergency department use of computed tomography in children with epilepsy and breakthrough seizure activity. J Child Neurol. 2007;22(9):1099–1101. [DOI] [PubMed] [Google Scholar]
- 19. Stiell IG, Clement CM, Rowe BH, et al. Comparison of the Canadian head CT rule and the New Orleans criteria in patients with minor head injury. JAMA. 2005;294(12):1511–1518. [DOI] [PubMed] [Google Scholar]
- 20. Mower WR, Gupta M, Rodriguez R, Hendey GW. Validation of the sensitivity of the National Emergency X-Radiography Utilization Study (NEXUS) head computed tomography (CT) decision instrument for selective imaging of blunt head injury patients: an observational study. PLoS Med. 2017;14(7):e1002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Griffey RT, Jeffe DB, Bailey T. Emergency physicians’ attitudes and preferences regarding computed tomography, radiation exposure and imaging decision support. Acad Emerg Med. 2014;21(7):768–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Feussner JR, Linfors EW, Blessing CL, et al. Computed tomography brain scanning in alcohol withdrawal seizures. Ann Intern Med. 1981;94(4 pt 1):519–522. [DOI] [PubMed] [Google Scholar]
- 23. Neidlinger NA, Pal JD, Victorino GP. Head computed tomography scans in trauma patients with seizure disorder. Arch Surg. 2005;140(9):858–864. [DOI] [PubMed] [Google Scholar]
- 24. American Epilepsy Society. Five Things Physicians and Patients Should Question. 2018. http://www.choosingwisely.org/societies/american-epilepsy-society/ Accessed October 16, 2018.
- 25. Lowe HJ, Ferris TA, Hernandez PM, et al. STRIDE – an integrated standards-based translational research informatics platform. AMIA Annu Symp Proc. 2009;2009:391–395. [PMC free article] [PubMed] [Google Scholar]
- 26. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary_Table for Yield of Emergent CT in Patients With Epilepsy Presenting With a Seizure by Kathryn A. Kvam, Vanja C. Douglas, William D. Whetstone, S. Andrew Josephson, and John P. Betjemann in The Neurohospitalist