Abstract
Background
Analysis of mixed microbial communities using metagenomic sequencing experiments requires multiple preprocessing and analytical steps to interpret the microbial and genetic composition of samples. Analytical steps include quality control, adapter trimming, host decontamination, metagenomic classification, read assembly, and alignment to reference genomes.
Results
We present a modular and user-extensible pipeline called Sunbeam that performs these steps in a consistent and reproducible fashion. It can be installed in a single step, does not require administrative access to the host computer system, and can work with most cluster computing frameworks. We also introduce Komplexity, a software tool to eliminate potentially problematic, low-complexity nucleotide sequences from metagenomic data. A unique component of the Sunbeam pipeline is an easy-to-use extension framework that enables users to add custom processing or analysis steps directly to the workflow. The pipeline and its extension framework are well documented, in routine use, and regularly updated.
Conclusions
Sunbeam provides a foundation to build more in-depth analyses and to enable comparisons in metagenomic sequencing experiments by removing problematic, low-complexity reads and standardizing post-processing and analytical steps. Sunbeam is written in Python using the Snakemake workflow management software and is freely available at github.com/sunbeam-labs/sunbeam under the GPLv3.
Electronic supplementary material
The online version of this article (10.1186/s40168-019-0658-x) contains supplementary material, which is available to authorized users.
Keywords: Sunbeam, Shotgun metagenomic sequencing, Software, Pipeline, Quality control
Background
Metagenomic shotgun sequencing involves isolating DNA from a mixed microbial community of interest, then sequencing deeply into DNAs drawn randomly from the mixture. This is in contrast to marker gene sequencing (e.g., the 16S rRNA gene of bacteria), where a specific target gene region is amplified and sequenced. Metagenomic sequencing has enabled critical insights in microbiology [1–9], especially in the study of virus and bacteriophage communities [10–15]. However, an ongoing challenge is analyzing and interpreting the resulting large datasets in a standard and reliable fashion [16–23].
A common practice to investigate microbial metagenomes is to use Illumina sequencing technology to obtain a large number of short (100–250 base pair) reads from fragmented DNA isolated from a sample of interest. After sequence acquisition, several post-processing steps must be carried out before the sequences can be used to gain insight into the underlying biology [21, 24].
Researchers have many tools at their disposal for accomplishing each post-processing step and will frequently encounter multiple parameters in each tool that can change the resulting output and downstream analysis. Varying parameters, tools, or reference database versions between analyses makes it challenging to compare the results of different metagenomic sequencing experiments [25]. Conversely, employing a consistent workflow across studies ensures that experiments are comparable and that the downstream analysis is reproducible, as emphasized in [21]. Documentation of software, databases, and parameters used is an essential element of this practice; otherwise, the benefits of consistent and reproducible workflows are lost.
A metagenomic post-processing workflow should have the following qualities to maximize its utility and flexibility: it should be deployable on shared computing systems and in the cloud, it should allow simple configuration of software parameters and reference databases, it should provide error handling and the ability to resume after interruptions, it should be modular so that unnecessary steps can be skipped or ignored, and it should allow new procedures to be added by the user. The ability to deploy the workflow on both institutional and cloud platforms enables workflows to be repeated in different labs with different computing setups and provides flexibility for researchers to choose between computing resources at the institution and in the cloud. Similarly, the ability to record running parameters through the use of configuration files allows for the use of experiment-specific software parameters and serves as documentation for future reference.
Several features contribute to efficient data analysis. It is beneficial if errors or interruptions in the workflow do not require restarting from the beginning: as sequencing experiments produce large amounts of data, having to repeat steps in data processing can be time-consuming and expensive. In addition, not all steps in a workflow will be necessary for all experiments, and some experiments may require custom processing. To handle experiments appropriately, the workflow should provide an easy way to skip unnecessary steps but run them later if necessary. To make the framework widely useful, users must be able to straightforwardly add new steps into the workflow as needed and share them with others. Several pipelines have been developed that achieve many of these goals [26–29], but did not meet our needs for greater flexibility in processing metagenomic datasets and long-term reproducibility of analyses.
Here, we introduce Sunbeam, an easily deployable and configurable pipeline that produces a consistent set of post-processed files from metagenomic sequencing experiments. Sunbeam is self-contained and installable on GNU/Linux systems without administrator privileges. It features error handling, task resumption, and parallel computing capabilities thanks to its implementation in the Snakemake workflow language [30]. Nearly all steps are configurable, with reasonable pre-specified defaults, allowing rapid deployment without extensive parameter tuning. Sunbeam can run either using local data directly or using external data from the National Center for Biotechnology Information (NCBI)’s Sequence Read Archive (SRA) [31]. Sunbeam is extensible using a simple mechanism that allows new procedures to be added by the user.
In addition, Sunbeam features custom software that allows it to process data from challenging sample types, including samples with high proportions of low-quality or host-derived sequences. These include custom-tuned host-derived read removal steps for any number of host or contaminant genomes, and Komplexity, a novel sequence complexity analysis program that rapidly and accurately removes problematic low-complexity reads before downstream analysis. Microsatellite DNA sequences make up a significant proportion of the human genome and are highly variable between individuals [32–34], compounding the difficulty of removing them by alignment against a single reference genome. We developed Komplexity because existing tools for analyzing nucleotide sequence complexity [35–37] did not meet our needs in terms of speed, removal of spurious hits, and natively processing fastq files. We have used Sunbeam in published and ongoing studies of host-associated, low-microbial biomass body sites [19, 38, 39], the virome [40], and longitudinal sampling of the microbiome [41, 42].
Sunbeam is implemented in Python, Bash, and Rust. It is licensed under the GPLv3. It is freely available at https://github.com/sunbeam-labs/sunbeam and documentation is available at http://sunbeam.readthedocs.io.
Implementation
Installation
Sunbeam is installable on GNU/Linux distributions that meet the initial hardware and software requirements listed in the “Availability and requirements” section. Installation is performed by downloading the software from its repository and running “install.sh.” Sunbeam does not require administrator privileges to install or run. We verified that Sunbeam installed and ran a basic analysis workflow on Debian 9; CentOS 6 and 7; Ubuntu 14.04, 16.04, 18.04, and 18.10; Red Hat Enterprise 6 and 7; and SUSE Enterprise 12.
Sunbeam utilizes the Conda package management system [43] to install additional software dependencies beyond those listed in the “Availability and requirements” section. Conda provides automatic dependency resolution, facilitates software installation for non-administrative users, and uses a standardized system for packaging additional software and managing third-party software channels. Conda also provides an isolated environment for additional software dependencies, to avoid conflicts with existing software outside the pipeline. Conda is installed by the Sunbeam installation script if needed. The isolated software environment used by Sunbeam is also created by the installation script.
Sunbeam architecture
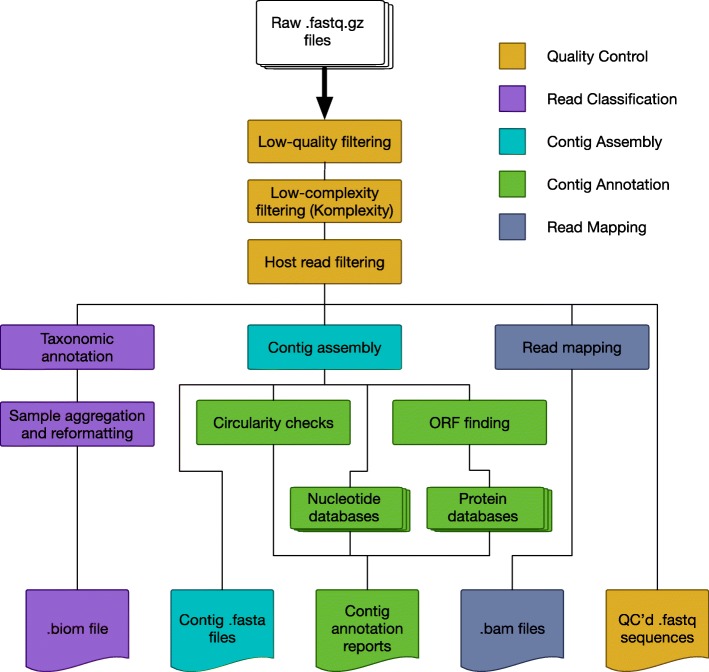
Sunbeam is comprised of a set of discrete steps that take specific files as inputs and produce other files as outputs (Fig. 1). Each step is implemented as a rule in the Snakemake workflow framework [30]. A rule specifies the input files needed, the output files produced, and the command or code needed to generate the output files. Implementation of the workflow in Snakemake offered several advantages, including workflow assembly, parallelism, and ability to resume following an interruption. Snakemake determines which steps require outputs from other steps in order to form a directed acyclic graph (DAG) of dependencies when the workflow is started, so the order of execution can change to suit available resources. This dependency DAG prevents ambiguities and cyclic dependencies between steps in the workflow. The DAG structure also allows Snakemake to identify steps that can operate in parallel (e.g., on other processors or compute nodes) if requested by the user. Snakemake manages the scheduling and is compatible with job submission systems on shared clusters.
Fig. 1.
Inputs, processes, and outputs for standard steps in the Sunbeam metagenomics pipeline
The input to the Sunbeam pipeline consists of raw, demultiplexed Illumina sequencing reads, either local files or samples available through the SRA. By default, Sunbeam performs the following preliminary operations on reads in the following order:
Quality control: Optionally, reads are downloaded from the SRA using grabseqs [44] and sra-tools [31]. Adapter sequences are removed, and bases are quality filtered using the Cutadapt [45] and Trimmomatic [46] software. Read pairs surviving quality filtering are kept. Read quality is assessed using FastQC [47] and summarized in separate reports.
Low-complexity masking: Sequence complexity in each read is assessed using Komplexity, a novel complexity scoring algorithm described below. Reads that fall below a user-customizable sequence complexity threshold are removed. Logs of the number of reads removed are written for later inspection.
Host read decontamination: Reads are mapped against a user-specified set of host or contaminant sequences using bwa [48]. Reads that map to any of these sequences within certain identity and length thresholds are removed. The numbers of reads removed are logged for later inspection.
After the initial quality-control process, multiple optional downstream steps can be performed in parallel. In the classify step, the decontaminated and quality-controlled reads are classified taxonomically using Kraken [49] and summarized in both tab-separated and BIOM [50] format. In the assembly step, reads from each sample are assembled into contigs using MEGAHIT [51]. Contigs above a pre-specified length are annotated for circularity. Open reading frames (ORFs) are predicted using Prodigal [52]. The contigs (and associated ORFs) are then searched against any number of user-specified nucleotide or protein BLAST [53] databases, using both the entire contig and the putative ORFs. The results are summarized into reports for each sample. Finally, in the mapping step, quality-controlled reads are mapped using bwa [48] to any number of user-specified reference genomes or gene sets, and the resulting BAM files are sorted and indexed using SAMtools [54].
Sunbeam is structured in such a way that the output files are grouped conceptually in different folders, providing a logical output folder structure. Standard outputs from Sunbeam include fastq files from each step of the quality-control process, taxonomic assignments for each read, contigs assembled from each sample, gene predictions, and alignment files of all quality-controlled reads to any number of reference sequences. Most rules produce logs of their operation for later inspection and summary. Users can request specific outputs separately or as a group, and the pipeline will run only the steps required to produce the desired files. This allows the user to skip or re-run any part of the pipeline in a modular fashion.
Error handling
Most of the actual operations in Sunbeam are executed by third-party bioinformatics software as described above. The error handling and operational stability of these programs vary widely, and random non-reproducible errors can arise during normal operation. In other cases, execution in a cluster environment can result in resources being temporarily and stochastically unavailable. These circumstances make it essential that Sunbeam can handle these errors in a way that minimizes lost computational effort. This is accomplished in two ways: first, the dependency DAG created by Snakemake allows Sunbeam’s execution to continue with other steps executing in parallel if a step fails. If that step failed due to stochastic reasons, or is interrupted, it can then be re-tried without having to re-execute successful upstream steps. Second, Sunbeam wraps execution of many of these programs with safety checks and error-handling code. Where possible, we have identified common failure points and taken measures to allow downstream steps to continue correctly and uninterrupted. For instance, some software will occasionally fail to move the final output file to its expected location. We check for this case and perform the final operation as necessary. In other cases, some programs may break if provided with an empty input file. In rules that use these programs, we have added checks and workarounds to mitigate this problem. Finally, in cases where throwing an error is unavoidable, we halt the execution of the rule and provide any error messages generated during the failure. These cases include instances where a step requires more memory than allocated, a variety of error that can be difficult to diagnose. To recover, the user can allocate more memory in the configuration file or on the command line and re-execute.
Sunbeam also inherits all of Snakemake’s error-handling abilities, including the ability to deal with NFS filesystem latency (via the “--latency-wait” option), and automatically re-running failed rules (via the “--restart-times” option). These options are frequently used to overcome issues that arise as part of execution in a cluster environment: in particular, on NFS-based clusters, an upstream rule may complete successfully but the output file(s) are not visible to all nodes, preventing downstream rule execution and an error concerning missing files. The “--latency-wait” option forces Snakemake to wait extra time for the filesystem to catch up. Other workflow systems, such as the Common Workflow Language, allow the workflow to be specified separately from the file paths, and for this reason may offer more safety than the Snakemake framework [55]. In normal use, however, we found that the precautions taken by the Snakemake framework were sufficient to run on shared NFS filesystems at our institutions under typical load.
In addition to runtime error mitigation, Sunbeam performs a series of “pre-flight checks” before performing any operations on the data to preempt errors related to misconfiguration or improper installation. These checks consist of (1) verifying that the environment is correctly configured, (2) verifying that the configuration file version is compatible with the Sunbeam version, and (3) verifying that all file paths specified in the configuration file exist and are accessible.
Versioning and development
We have incorporated an upgrade and semantic versioning system into Sunbeam. Specifically, the set of output files and configuration file options are treated as fixed between major versions of the pipeline to maintain compatibility. Any changes that would change the format or structure of the output folder or would break compatibility with previous configuration files only occur during a major version increase (i.e., from version 1.0.0 to version 2.0.0). Minor changes, optimizations, or bug fixes that do not alter the output structure or configuration file may increase the minor or patch version number (i.e., from v1.0.0 to v1.1.0). Sunbeam stable releases are issued on an as-needed basis when a feature or bug fix is tested and benchmarked sufficiently.
To prevent unexpected errors, the software checks the version of the configuration file before running to ensure compatibility and will stop if it is from a previous major version. To facilitate upgrading between versions of Sunbeam, the same installation script can also install new versions of the pipeline in place. We provide a utility to upgrade configuration files between major version changes.
To ensure the stability of the output files and expected behavior of the pipeline, we built an integration testing procedure into Sunbeam’s development workflow. This integration test checks that Sunbeam is installable, produces the expected set of output files, and correctly handles various configurations and inputs. The test is run through a continuous integration system that is triggered upon any commit to the Sunbeam software repository, and only changes that pass the integration tests are merged into the “stable” branch used by end users.
Extensions
The Sunbeam pipeline can be extended by users to implement new features or to share reproducible reports. Extensions take the form of supplementary rules written in the Snakemake format and define the additional steps to be executed. Optionally, two other files may be provided: one listing additional software requirements and another giving additional configuration options. Extensions can optionally run in a separate software environment, which enables the use of software dependencies that conflict with Sunbeam’s. Any package available through Conda can be specified as an additional software dependency for the extension. To integrate these extensions, the user copies the files into Sunbeam’s extensions directory, where they are automatically integrated into the workflow during runtime. The extension platform is tested as part of our continuous integration test suite.
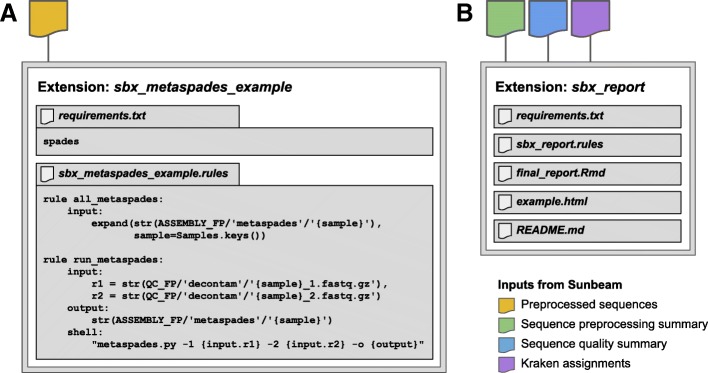
User extensions can be as simple or complex as desired and have minimal boilerplate. For example, an extension to run the MetaSPAdes assembly program [56] is shown in Fig. 2a. The file sbx_metaspades_example.rules specifies the procedure necessary to generate assembly results from a pair of decontaminated, quality-controlled FASTQ input files. The pattern for the input files is taken directly from the Sunbeam documentation. The path of the output directory is created by specifying the output directory and the sample name; it was created by modifying the pattern for the standard Sunbeam assembly output, given in the documentation. The shell command at the bottom of the rule is taken directly from the MetaSPAdes documentation at https://biosphere.france-bioinformatique.fr/wikia2/index.php/MetaSPAdes. The extension requires one additional file to run: a file named requirements.txt, containing one line, “spades”, which is the name of the Conda package providing the MetaSPAdes software. In all, the extension comprises a minimal specification of how to install the program, run the program, and name the output files.
Fig. 2.
Schematics of example extension inputs and contents. a Files for extension sbx_metaspades_example, which uses MetaSPAdes to assemble reads from quality-controlled fastq.gz files. sbx_metaspades_example.rules lists procedure necessary to generate assembly results from a pair of decontaminated, quality-controlled FASTQ input files. requirements.txt lists the software requirements for the package to be installed through Conda. b Files contained within the sbx_report extension: requirements.txt lists the software requirements for the package to be installed through Conda; sbx_report.rules contains the code for the rule as above, final_report.Rmd is a R markdown script that generates and visualizes the report, example.html is an example report, and README.md provides instructions for installing and running the extension. Sunbeam inputs required for each extension are shown as colored shapes above the extensions
As a second example, we show the file structure of the sbx_report extension, which generates a report from preprocessing, sequence quality, and taxonomic assignment summary files generated in the default workflow (Fig. 2b). This extension includes additional files for the report template, an example output report, and a README file with instructions for the user.
Because extensions are integrated directly into the main Sunbeam environment, they have access to the same environmental variables and resources as the primary pipeline and gain the same error-handling benefits. The manner in which the extensions are integrated into the dependency graph means that a valid extension can only extend, not alter, the primary workflow. Invalid extensions that violate the acyclic dependency requirements will prevent Sunbeam from running. This helps minimize conflicts between extensions as long as unique naming conventions are followed.
To make it easy for users to create their own extensions, we provide documentation, an extension template on our GitHub page (https://github.com/sunbeam-labs/sbx_template), and a number of useful prebuilt extensions available at http://sunbeam-labs.org. We created extensions that allow users to run alternate metagenomic read classifiers like Kaiju [57] or MetaPhlAn2 [58], visualize read mappings to reference genomes with IGV [59], co-assemble contigs from user-specified groups of samples, and even format Sunbeam outputs for use with downstream analysis pipelines like Anvi’o [60]. Users can publish Sunbeam extensions at our website, http://sunbeam-labs.org.
Komplexity
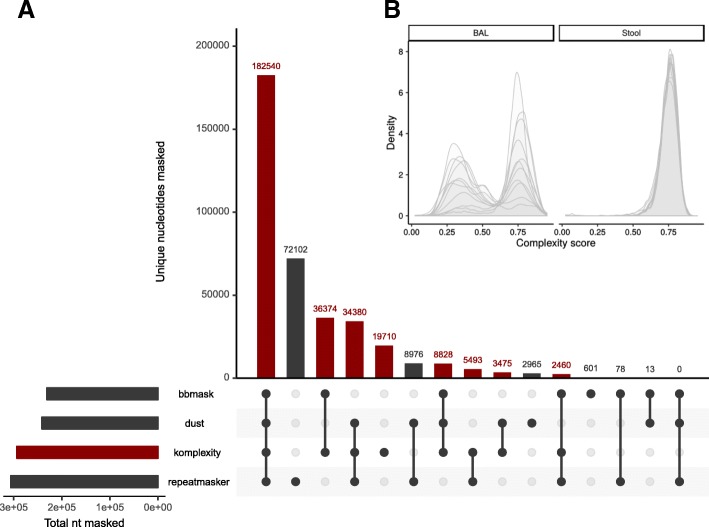
We regularly encounter low-complexity sequences comprised of short nucleotide repeats that pose problems for downstream taxonomic assignment and assembly [12, 39], for example, by generating spurious alignments to unrelated repeated sequences in database genomes. These are especially common in low-microbial biomass samples associated with vertebrate hosts. To avoid these potential artifacts, we created a novel, fast read filter called Komplexity. Komplexity is an independent program, implemented in the Rust programming language for rapid, standalone performance, designed to mask or remove problematic low-complexity nucleotide sequences. It scores sequence complexity by calculating the number of unique k-mers divided by the sequence length. Example complexity score distributions for reads from ten stool virome samples (high microbial biomass; [15]) and ten bronchoalveolar lavage (BAL) virome samples (low-biomass, high-host; [12]) are shown in Fig. 4b—low-complexity reads are often especially problematic in low-microbial biomass samples like BAL. Komplexity can either return this complexity score for the entire sequence or mask regions that fall below a score threshold. The k-mer length, window length, and complexity score cutoff are modifiable by the user, though default values are provided (k-mer length = 4, window length = 32, threshold = 0.55). Komplexity accepts FASTA and FASTQ files as input and outputs either complexity scores or masked sequences in the input format. As integrated in the Sunbeam workflow, Komplexity assesses the total read complexity and removes reads that fall below the default threshold. Although low-complexity reads are filtered by default, users can turn off this filtering or modify the threshold in the Sunbeam configuration file. Komplexity is also available as a separate open-source program at https://github.com/eclarke/komplexity.
Fig. 4.
a Comparison between Komplexity and similar software (BBMask, DUST, and RepeatMasker). The small bar plot in the lower left shows the total nucleotides masked by each tool. The central bar plot shows the number of unique nucleotides masked by every tool combination; each combination is shown by the connected dots below. Bars displaying nucleotides masked by tool combinations that include Komplexity are colored red. b Example complexity score distributions calculated by Komplexity for reads from ten stool virome samples (high microbial biomass; [15]) and ten bronchoalveolar lavage (BAL) virome samples (low-biomass, high-host; [12]) using the default parameters
Results and discussion
Sunbeam implements a core set of commonly required tasks supplemented by user-built extensions. Even so, the capabilities of Sunbeam compare favorably with features offered in existing pipelines such as SURPI (Sequence-based Ultra-Rapid Pathogen Identification) [26], EDGE (Empowering the Development of Genomics Expertise) [27], ATLAS (Automatic Tool for Local Assembly Structures) [28], and KneadData [29]. A detailed feature comparison is shown in Table 1. Where Sunbeam’s primary advancements lie are in its extension framework and novel software to address the issues of low-complexity or host-derived sequence filtering. The Sunbeam extensions framework facilitates the addition of new features to Sunbeam without adding overhead to the core pipeline—once developed, a Sunbeam extension can be discovered and used by anyone through our extensions website (www.sunbeam-labs.org). Sunbeam extensions that generate figures can also be used to promote reproducible science (examples below). As Sunbeam can work directly from SRA data, regenerating figures and analyses from a study is as painless as installing the extension, initializing using the BioProject or SRA project identifier, then running the pipeline as usual.
Table 1.
Feature comparison for metagenomic pipelines
| Sunbeam | SURPI | KneadData | EDGE | ATLAS | |
|---|---|---|---|---|---|
| Architecture/usage | |||||
| Dependency management | Conda | Bash | Pip (partial) | Conda | |
| Modularity | Snakemake | Perl modules | Snakemake | ||
| Results reporting | Tables, coverage maps, figures | Tables, coverage maps | Tables, coverage maps | ||
| Extension framework | Sunbeam extensions | ||||
| Clinical certification | CLIA | ||||
| Data source | Local, SRA | Local | Local | Local | Local |
| Quality control | |||||
| Adapter trimming | Trimmomatic, Cutadapt | Cutadapt | Trimmomatic | FaQCs | BBDuk2 |
| Error correction | Tadpole | ||||
| Read quality | Fastqc | Cutadapt | Fastqc | FaQCs | BBDuk2 |
| Host filtering | Any | Human | Any | Any | Any |
| Low complexity | Komplexity | DUST | TRF | Mono- or dinucleotide repeats | BBDuk2 |
| Read subsampling/rarefaction | VSEARCH (extension) | ||||
| Sequence analysis | |||||
| Reference alignment | BWA | Bowtie2, MUMmer + JBrowse | BBMap | ||
| Classification | Kraken, (MetaPhlAn2, Kaiju extensions) | SNAP | GOTTCHA, Kraken, MetaPhlAn | DIAMOND | |
| Assembly | MEGAHIT | Minimo | IDBA-UD, SPAdes | MEGAHIT, SPAdes | |
| ORFs (aa) | Prodigal, BLASTp | Prokka | |||
| Full contig (nt) | Circularity, BLASTn | RAPSearch | BWA | DIAMOND | |
| Functional annotation | eggNOG (extension) | ENZYME/eggNOG/ dbCAN | |||
| Phylogeny reconstruction | PhaMe, FastTree/RAxML | ||||
| Primer design | BW, Primer3 | ||||
Feature comparison for metagenomic pipelines. Tools used by each pipeline: trimmomatic [46], cutadapt [45], tadpole [78], fastqc [47], FaQCs [79], BBDuk2 [80], DUST [36], TRF [81], VSEARCH [82], bwa [48], bowtie2 [83], BBMap [84], KRAKEN [49], SNAP [85], MUMmer [86], JBrowse [87], GOTTCHA [88], MetaPhlAn [58], DIAMOND [89], FastTree [90], MEGAHIT [51], SPAdes [91], Minimo [92], Prodigal [52], BLASTp [53], Prokka [93], BLASTn [53], eggNOG [94], ENZYME [95], dbCAN [96], Primer3 [97], RAPSearch [98], RAxML [99], conda [43], PhaME [100], Snakemake [30], SAMtools [54]
To demonstrate the development of extensions and the use of Sunbeam on real-world data, we reproduced key results from three published studies and tested Sunbeam on an internal pilot study of shallow shotgun sequencing (Fig. 3). These studies span multiple research areas (high-biomass human gut microbiome, soil microbiome, virome, and shallow shotgun methodology) and emphasize Sunbeam’s versatility. Each of these analyses is packaged and distributed as a Sunbeam extension. The extension workflow begins with downloading data from the SRA and ends with generation of a final report. We verified that the example workflows produced identical results on our institutional systems and on cloud-based systems.
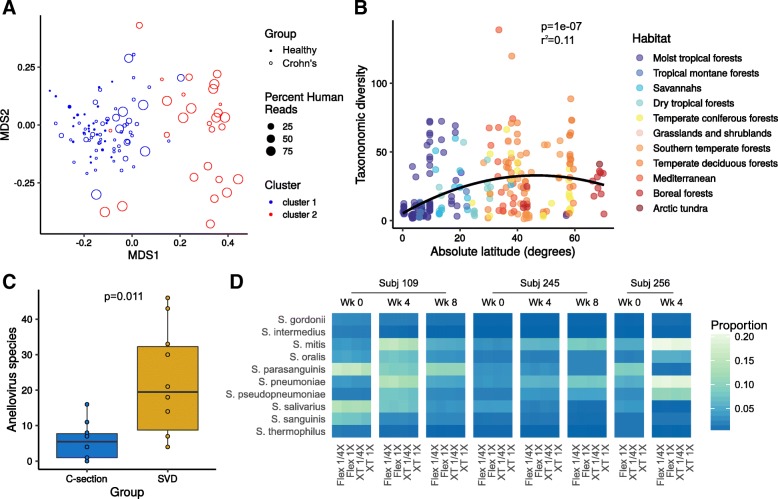
Fig. 3.
a Nonmetric multidimensional scaling plots generated using the vegan package in R [76], using MetaPhlAn2 classifications of data from Lewis et al. [61]. Each point is colored by the cluster in which it was annotated in the Lewis et al. metadata—cluster 2 (red) is the dysbiotic cluster, while cluster 1 (blue) is the healthy-like cluster. b Inverse Simpson diversity by absolute latitude calculated using the vegan package in R from the Kraken classification output of Sunbeam for Bahram et al. [63]. Points are colored by habitat. The polynomial regression line is shown in black. c Boxplots of unique Anelloviridae taxa in each sample from McCann et al. [64]. Each point corresponds to a single sample. d Heatmap from shallow shotgun analysis colored by proportional abundance. Each row corresponds to a bacterial taxon; each column represents a different reagent combination. Columns are grouped by time point, then by subject (top). All plots were generated using the ggplot2 R package [77]
In Fig. 3a, we reproduce a finding from Lewis et al. showing distinct gut microbial community clusters in individuals with Crohn’s disease, which track with human DNA abundance in stool [61]. As this finding depends on the use of read-based classification, we used Kraken (the Sunbeam built-in classifier) as well as extensions for Kaiju [57] and MetaPhlAn2 [58] to test the consistency of the conclusions across different tools—the original study used MetaPhlAn [62] to classify reads. All three classification methods recapitulated the previously reported clusters (MetaPhlAn2 results in Fig. 3a, full report in Additional file 1). This analysis can be re-run using the sbx_lewis2015 extension (https://github.com/louiejtaylor/sbx_lewis2015).
An example of a non-host-associated analysis is shown in Fig. 3b. Bahram et al. showed that soil bacterial diversity was highest in temperate latitudes, but lower at the equator and in artic regions [63]. We used Sunbeam to process and classify reads from this dataset and found similar results (Fig. 2b; P < 0.001, R2 = 0.11; Bahram et al. P < 0.001, R2 = 0.16). This analysis can be reproduced using the sbx_bahram2018 extension (https://github.com/louiejtaylor/sbx_bahram2018, Additional file 2).
In Fig. 3c, we reproduce results from the virome study published by McCann et al., which found differences according to mode of birth in gut viral communities of 1-year-olds [64]. One salient finding was that Anelloviridae, a family of ubiquitous human commensal viruses [65], were much more diverse in children born by spontaneous vaginal delivery (SVD) compared to those born via C-section. We used Sunbeam to classify the reads from this dataset and also found more unique anelloviruses in SVD compared to C-section (Fig. 3c, Additional file 3; P = 0.011; McCann et al. P = 0.014). The finding of McCann et al. was recovered using different approaches to identify viruses: McCann et al. used a translated nucleotide query against a database of anellovirus ORF1 proteins, while we used Kraken-based classification. This analysis can be reproduced using the sbx_mccann2018 extension (https://github.com/louiejtaylor/sbx_mccann2018).
Figure 3d shows results from a methods development pilot study conducted at the CHOP Microbiome Center. Here, we sequenced tongue swab specimens from three healthy children, collected at 4-week intervals. DNA from the tongue swabs was prepared for sequencing using two library preparation kits: the Nextera XT kit and the Nextera DNA Flex kit. For each kit and specimen, the DNA library was prepared using a full-scale (1×) and quarter-scale (1/4×) reagent volumes relative to standard Illumina protocols. We conducted shotgun metagenomic sequencing and recovered approximately 500,000 reads per sample, after host filtering. Thus, our pilot study is an example of “shallow shotgun sequencing,” which is emerging as a cost-effective alternative to 16S rRNA marker gene studies [66]. In our analysis of the pilot study, the type of kit had a small but measurable effect on the abundance of Streptococcus species, but the estimated effect size for the kit (R2 = 0.003 for Streptococcus mitis) was orders of magnitude less than that between specimens (R2 = 0.995). This analysis can be reproduced using the sbx_shallowshotgun_pilot extension (https://github.com/junglee0713/sbx_shallowshotgun_pilot; report HTML file in Additional file 4).
Sunbeam’s extension framework promotes reproducible analyses and greatly simplifies performing the same type of analysis on multiple datasets. Extension templates, as well as a number of pre-built extensions for metagenomic analysis and visualization software like Anvi’o [60], MetaPhlAn [58], and Pavian [67], are available on our GitHub page (https://github.com/sunbeam-labs). Sunbeam’s ability to use data directly from the SRA facilitates reproducibility: final figure generation can be integrated into a Sunbeam extension, greatly lowering the barrier to reproducing analyses and studies.
Comparing low-complexity filtering program filtering and performance
Low-complexity reads often cross-align between genomes, and commonly elude standard filters in use today. The gold standard of such filtering programs, RepeatMasker [35], uses multiple approaches to identify and mask repeat or low complexity DNA sequences, including querying a database of repetitive DNA elements (either Repbase [68] or Dfam [69]). Another program, used in the BLAST+ suite, DUST [36] employs an algorithm which scores and masks nucleotide sequence windows that exceed a particular complexity score threshold (with lower complexity sequences assigned higher scores) such that no subsequence within the masked region has a higher complexity score than the entire masked region. BBMask, developed by the Joint Genome Institute, masks sequences that fall below a threshold of k-mer Shannon diversity [37].
Many of these tools were not optimal for our use with shotgun metagenomic datasets. RepeatMasker uses databases of known repeat sequences to mask repetitive nucleotide sequences, but runs too slowly to be feasible for processing large datasets. An option to filter reads falling below a certain complexity threshold is not available in DUST, RepeatMasker, or BBMask (although filtering is available in the BBMask companion tool BBDuk). Finally, the memory footprint of BBMask scales with dataset size, requiring considerable resources to process large shotgun sequencing studies. Therefore, we designed Komplexity to mask or filter metagenomic reads as a rapid, scalable addition to the Sunbeam workflow that can also be installed and run separately. It accepts FASTA/Q files as input, can mask or remove reads below a specified threshold, and operates with a constant memory footprint. Our goal was to achieve quality comparable to RepeatMasker in a reasonable timeframe.
To compare the performance of all the low-complexity-filtering tools discussed above, we used pIRS [70] to simulate Illumina reads from the human conserved coding sequence dataset [71] as well as human microsatellite records from the NCBI nucleotide database [72] with the following parameters: average insert length of 170 nucleotides with a 5% standard deviation, read length of 100 nucleotides, and 5× coverage. To ensure compatibility with all programs, we converted the resulting files to FASTA format, then selected equal numbers of reads from both datasets for a total of approximately 1.1 million bases in the simulated dataset (available at https://zenodo.org/record/2541222) [73]. We processed the reads using Komplexity, RepeatMasker, DUST, and BBMask and used GNU Time version 1.8 [74] to measure peak memory usage and execution time for six replicates (Table 2). Komplexity and RepeatMasker mask a similar proportion of microsatellite nucleotides, while none of the four tools masks a large proportion of coding nucleotides. Komplexity runs faster and has a smaller memory footprint than other low-complexity filtering programs. The memory footprint of Komplexity and DUST are also relatively constant across datasets of different sizes (data not shown).
Table 2.
Memory usage, speed, and nucleotides masked for each program
| Tool | Microsatellite nucleotides masked (%) | Conserved coding sequence nucleotides masked (%) | Speed (kilobase/second) | Peak memory usage (megabytes) |
|---|---|---|---|---|
| Komplexity | 54.6 | 0.68 | 10,100 ± 560 | 3.50 ± 0.06 |
| RepeatMasker | 57 | 0.75 | 0.64 ± 0.02 | 624 ± 3.0 |
| BBMask | 43 | 0.029 | 1690 ± 440 | 385 ± 52 |
| DUST | 44.9 | 0.74 | 795 ± 10 | 17.0 ± 0.18 |
Columns show the percentage of nucleotides (microsatellite or conserved coding sequence) from reads masked by each tool, as well as the normalized time taken and peak memory usage of each tool while processing the dataset (1.1 megabases). The top-performing tool in each category is shown in italics
To understand the extent to which different tools might synergize to mask a larger proportion of overall nucleotides, we visualized nucleotides from the microsatellite dataset masked by each tool or combinations of multiple tools using UpSetR [75] (Fig. 4a). Komplexity masks 78% of the nucleotides masked by any tool, and 96% excluding nucleotides masked by only RepeatMasker. This suggests that there would only be a marginal benefit to running other tools in series with Komplexity. Komplexity in combination with Sunbeam’s standard host removal system resulted in the removal of over 99% of the total simulated microsatellite reads.
Conclusions
Here, we introduce Sunbeam, a Snakemake-based pipeline for analyzing shotgun metagenomic data with a focus on reproducible analysis, ease of deployment, and use. We also present Komplexity, a tool for rapidly filtering and masking low-complexity sequences from metagenomic sequence data, and show its superior performance in comparison with other tools for masking human microsatellite repeat sequences. Sunbeam’s scalability, customizability, and facilities for deployment simplify the processing of shotgun metagenomic sequence data, while its extension framework enables customized reproducible analyses. We have already used Sunbeam in multiple published [19, 38–42] and ongoing studies.
Availability and requirements
Project name: Sunbeam
Project home page: https://github.com/sunbeam-labs/sunbeam
Operating system: GNU/Linux; verified on the following distributions: Debian 9; CentOS 6 and 7; Ubuntu 14.04, 16.04, 18.04, and 18.10; Red Hat Enterprise 6 and 7.
Programming languages: Python, Rust, and Bash.
Other requirements: Software: Python (version 2.7, 3.4, 3.5, or 3.6), git (version ≥ 2), GNU Coreutils, wget, bzip2, and Bash (version ≥ 3) required for installation. At least 100 GB hard drive space and 16 GB memory are recommended to run the pipeline, dependent on databases and input file sizes used.
License: GPLv3
Restrictions to use by non-academics: No
Additional files
Lewis et al. 2015 report. Report with figures reproducing results from Lewis et al. 2015 [61]. (HTML 1031 kb)
Bahram et al. 2018 report. Report with figures reproducing results from Bahram et al. 2018 [63]. (HTML 954 kb)
McCann et al. 2018 report. Report with figures reproducing results from McCann et al. 2018 [64]. (HTML 812 kb)
Shallow shotgun pilot report. Report with figures relating to the shallow shotgun pilot study described above. (HTML 1252 kb)
Acknowledgements
Thanks to members of the Bushman lab, Penn-CHOP Microbiome Center, and Penn Bioinformatics Code Review communities for helpful suggestions, discussions, and beta testing.
Funding
This work was supported by the NIH grants U01HL112712 (Site-Specific Genomic Research in Alpha-1 Antitrypsin Deficiency and Sarcoidosis (GRADS) Study), R01HL113252, and R61HL137063, and received assistance from the Penn Center for AIDS Research (P30AI045008), T32 Training Grant (T32AI007324, LJT), and the PennCHOP Microbiome Program (Tobacco Formula grant under the Commonwealth Universal Research Enhancement (C.U.R.E) program with the grant number SAP # 4100068710).
Availability of data and materials
Sunbeam is available at https://github.com/sunbeam-labs/sunbeam. Komplexity is available at https://github.com/eclarke/komplexity. Pre-built extensions referenced can be found at https://github.com/sunbeam-labs/. The dataset of simulated microsatellite and conserved coding sequence reads is archived in Zenodo at https://zenodo.org/record/2541222. Shotgun data from the shallow shotgun pilot study is deposited in SRA under project identifier SRP178101. The analyses in Fig. 3 can be reproduced by the following Sunbeam extensions: https://github.com/louiejtaylor/sbx_lewis2015, https://github.com/louiejtaylor/sbx_bahram2018, https://github.com/louiejtaylor/sbx_mccann2018, https://github.com/junglee0713/sbx_shallowshotgun_pilot.
Abbreviations
- ATLAS
Automatic Tool for Local Assembly Structures
- BAM
Binary alignment map
- BLAST
Basic Local Alignment Search Tool
- DAG
Directed acyclic graph
- EDGE
Empowering the Development of Genomics Expertise
- GPL
(GNU) General Public License
- ORF(s)
Open reading frame(s)
- SDUST
Symmetric DUST
- SRA
Sequence Read Archive
- SURPI
Sequence-based Ultra-Rapid Pathogen Identification
- SVD
Spontaneous vaginal delivery
Authors’ contributions
ELC, CZ, FDB, and KB conceived and designed Sunbeam. ELC, LJT, CZ, AC, and KB developed Sunbeam. ELC and LJT conceived and developed Komplexity. LJT performed the low-complexity sequence masking analysis, reproduced results from previously published studies, and wrote the associated extensions. ELC, LJT, AC, and KB wrote the manuscript. BF conducted the shallow shotgun pilot study presented here; JJL conducted the analysis and wrote the shallow shotgun Sunbeam extension. All authors read, improved, and approved the final manuscript.
Ethics approval and consent to participate
The study protocol was approved by The Children’s Hospital of Philadelphia Institutional Review Board. All subjects gave written, informed consent prior to participation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Erik L. Clarke, Email: ecl@pennmedicine.upenn.edu
Louis J. Taylor, Email: louist@pennmedicine.upenn.edu
Chunyu Zhao, Email: chunyu.zhao@czbiohub.org.
Andrew Connell, Email: ancon@pennmedicine.upenn.edu.
Jung-Jin Lee, leej39@email.chop.edu.
Bryton Fett, fettb@email.chop.edu.
Frederic D. Bushman, Email: bushman@pennmedicine.upenn.edu
Kyle Bittinger, bittingerk@email.chop.edu.
References
- 1.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449:804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 3.Muegge BD, Kuczynski J, Knights D, Clemente JC, Gonzalez A, Fontana L, et al. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science. 2011;332:970–4. [DOI] [PMC free article] [PubMed]
- 4.Morgan XC, Tickle TL, Sokol H, Gevers D, Devaney KL, Ward DV, et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012;13:R79. doi: 10.1186/gb-2012-13-9-r79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee STM, Kahn SA, Delmont TO. Shaiber A, Esen özcan C, Hubert NA, et al. Tracking microbial colonization in fecal microbiota transplantation experiments via genome-resolved metagenomics. Microbiome. 2017;5:1–10. doi: 10.1186/s40168-017-0270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinsdale EA, Edwards RA, Hall D, Angly F, Breitbart M, Brulc JM, et al. Functional metagenomic profiling of nine biomes. Nature. 2008;452:629–632. doi: 10.1038/nature06810. [DOI] [PubMed] [Google Scholar]
- 7.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abubucker S, Segata N, Goll J, Schubert AM, Izard J, Cantarel BL, et al. Metabolic reconstruction for metagenomic data and its application to the human microbiome. PLoS Comput Biol. 2012;8:e1002358. [DOI] [PMC free article] [PubMed]
- 9.Fierera N, Leff JW, Adams BJ, Nielsend UN, Bates ST, Lauber CL, et al. Cross-biome metagenomic analyses of soil microbial communities and their functional attributes. Proc Natl Acad Sci. 2012;109:21390–21395. doi: 10.1073/pnas.1215210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breitbart M, Hewson I, Felts B, Mahaffy JM, Nulton J, Salamon P, et al. Metagenomic analyses of an uncultured viral community from human feces. J Bacteriol. 2003;185:6220–6223. doi: 10.1128/JB.185.20.6220-6223.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwards RA, Rohwer F. Viral metagenomics. Nat Rev Microbiol. 2005;3:801–805. doi: 10.1038/nrmicro1163. [DOI] [PubMed] [Google Scholar]
- 12.Abbas AA, Diamond JM, Chehoud C, Chang B, Kotzin JJ, Young JC, et al. The perioperative lung transplant virome: torque teno viruses are elevated in donor lungs and show divergent dynamics in primary graft dysfunction. Am J Transplant. 2017;17:1313–1324. doi: 10.1111/ajt.14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emerson JB, Thomas BC, Andrade K, Allen EE, Heidelberg KB, Banfielda JF. Dynamic viral populations in hypersaline systems as revealed by metagenomic assembly. Appl Environ Microbiol. 2012;78:6309–6320. doi: 10.1128/AEM.01212-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma Y, Madupu R, Karaoz U, Nossa CW, Yang L, Yooseph S, et al. Human papillomavirus community in healthy persons, defined by metagenomics analysis of human microbiome project shotgun sequencing data sets. J Virol. 2014;88:4786–4797. doi: 10.1128/JVI.00093-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minot S, Bryson A, Chehoud C, Wu GD, Lewis JD, Bushman FD. Rapid evolution of the human gut virome. Proc Natl Acad Sci. 2013;110:12450–12455. doi: 10.1073/pnas.1300833110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meisel JS, Hannigan GD, Tyldsley AS, SanMiguel AJ, Hodkinson BP, Zheng Q, et al. Skin microbiome surveys are strongly influenced by experimental design. J Invest Dermatol. 2016;136:947–956. doi: 10.1016/j.jid.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiss S, Amir A, Hyde ER, Metcalf JL, Song SJ, Knight R. Tracking down the sources of experimental contamination in microbiome studies. Genome Biol. 2014;15:1–3. doi: 10.1186/s13059-014-0564-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim D, Hofstaedter CE, Zhao C, Mattei L, Tanes C, Clarke E, et al. Optimizing methods and dodging pitfalls in microbiome research. Microbiome. 2017;5:1–14. doi: 10.1186/s40168-017-0267-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lauder AP, Roche AM, Sherrill-Mix S, Bailey A, Laughlin AL, Bittinger K, et al. Comparison of placenta samples with contamination controls does not provide evidence for a distinct placenta microbiota. Microbiome. 2016;4:1–11. doi: 10.1186/s40168-016-0172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nayfach S, Pollard KS. Toward accurate and quantitative comparative metagenomics. Cell. 2016;166:1103–1116. doi: 10.1016/j.cell.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knight R, Vrbanac A, Taylor BC, Aksenov A, Callewaert C, Debelius J, et al. Best practices for analysing microbiomes. Nat Rev Microbiol. 2018;16:410–422. doi: 10.1038/s41579-018-0029-9. [DOI] [PubMed] [Google Scholar]
- 22.Delmont TO, Eren AM. Identifying contamination with advanced visualization and analysis practices: metagenomic approaches for eukaryotic genome assemblies. PeerJ. 2016;4:e1839. doi: 10.7717/peerj.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kjartansdóttir KR, Friis-Nielsen J, Asplund M, Mollerup S, Mourier T, Jensen RH, et al. Traces of ATCV-1 associated with laboratory component contamination. Proc Natl Acad Sci. 2015;112:E925–E926. doi: 10.1073/pnas.1423756112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quince C, Walker AW, Simpson JT, Loman NJ, Segata N. Shotgun metagenomics, from sampling to analysis. Nat Biotechnol. 2017;35:833–844. doi: 10.1038/nbt.3935. [DOI] [PubMed] [Google Scholar]
- 25.Nasko DJ, Koren S, Phillippy AM, Treangen TJ. RefSeq database growth influences the accuracy of k-mer-based lowest common ancestor species identification. Genome Biol. 2018;19:1–10. doi: 10.1186/s13059-018-1554-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naccache SN, Federman S, Veeraraghavan N, Zaharia M, Lee D, Samayoa E, et al. A cloud-compatible bioinformatics pipeline for ultrarapid pathogen identification from next-generation sequencing of clinical samples. Genome Res. 2014;24:1180–1192. doi: 10.1101/gr.171934.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li PE, Lo CC, Anderson JJ, Davenport KW, Bishop-Lilly KA, Xu Y, et al. Enabling the democratization of the genomics revolution with a fully integrated web-based bioinformatics platform. Nucleic Acids Res. 2017;45:67–80. doi: 10.1093/nar/gkw1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White RAI, Brown J, Colby S, Overall CC, Lee J-Y, Zucker J, et al. ATLAS (Automatic Tool for Local Assembly Structures) - a comprehensive infrastructure for assembly, annotation, and genomic binning of metagenomic and metatranscriptomic data. Peer J Prepr. 2017;5:e2843v1 Available from: https://peerj.com/preprints/2843.pdf.
- 29.KneadData. 2017 [cited 2018 Feb 1]. Available from: https://bitbucket.org/biobakery/kneaddata
- 30.Koster J, Rahmann S. Snakemake—a scalable bioinformatics workflow engine. Bioinformatics. 2012;28:2520–2522. doi: 10.1093/bioinformatics/bts480. [DOI] [PubMed] [Google Scholar]
- 31.Leinonen R, Sugawara H, Shumway M. The sequence read archive. Nucleic Acids Res. 2011;39:2010–2012. doi: 10.1093/nar/gkq1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ellegren H. Microsatellites: simple sequences with complex evolution. Nat Rev Genet. 2004;5:435–445. doi: 10.1038/nrg1348. [DOI] [PubMed] [Google Scholar]
- 33.Payseur BA, Nachman MW. Microsatellite variation and recombination rate in the human genome. Genetics. 2000;156:1285–1298. doi: 10.1093/genetics/156.3.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Subramanian S, Mishra RK, Singh L. Genome-wide analysis of microsatellite repeats in humans: their abundance and density in specific genomic regions. Genome Biol. 2003;4:R13 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC151303/. [DOI] [PMC free article] [PubMed]
- 35.Smit A, Hubley R, Green P. RepeatMasker Open-4.0. 2013; Available from: http://www.repeatmasker.org
- 36.Morgulis A, Gertz EM, Schäffer AA, Agarwala R. A fast and symmetric DUST implementation to mask low-complexity DNA sequences. J Comput Biol. 2006;13:1028–1040. doi: 10.1089/cmb.2006.13.1028. [DOI] [PubMed] [Google Scholar]
- 37.JGI. BBMask. 2018; Available from: https://jgi.doe.gov/data-and-tools/bbtools/bb-tools-user-guide/bbmask-guide/
- 38.Leiby JS, Mccormick K, Sherrill-Mix S, Clarke EL, Kessler LR, Taylor LJ, et al. Lack of detection of a human placenta microbiome in samples from preterm and term deliveries. Microbiome. 2018;6:196. [DOI] [PMC free article] [PubMed]
- 39.Clarke EL, Lauder AP, Hofstaedter CE, Hwang Y, Fitzgerald AS, Imai I, et al. Microbial lineages in sarcoidosis: A metagenomic analysis tailored for low-microbial content samples. Am J Respir Crit Care Med. 2018;197:225–34. [DOI] [PMC free article] [PubMed]
- 40.Abbas AA, Young JC, Clarke EL, Diamond JM, Imai I, Haas AR, et al. Bidirectional transfer of Anelloviridae lineages between graft and host during lung transplantation. Am J Transplant. 2018; Available from: http://doi.wiley.com/10.1111/ajt.15116. [DOI] [PMC free article] [PubMed]
- 41.Clarke EL, Connell AJ, Six E, Kadry NA, Abbas AA, Hwang Y, et al. T cell dynamics and response of the microbiota after gene therapy to treat X-linked severe combined immunodeficiency. Genome Med. 2018;10:70. doi: 10.1186/s13073-018-0580-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor JM, Lefkowitz E, Clarke EL, Baker K, Lauder A, Kim D, et al. Evaluation of a therapy for Idiopathic Chronic Enterocolitis in rhesus macaques (Macaca mulatta) and linked microbial community correlates. PeerJ. 2018;6:e4612. [DOI] [PMC free article] [PubMed]
- 43.Anaconda INC. Conda. 2018. Available from: https://anaconda.org
- 44.Taylor LJ, Abbas AA. grabseqs: a utility for easy downloading of reads from next-gen sequencing repositories. 2019. Available from: https://github.com/louiejtaylor/grabseqs
- 45.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2015;17:1–3.
- 46.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.BabrahamBioinformatics. FastQC. 2018. Available from: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/
- 48.Li H, Li H, Durbin R, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–60. [DOI] [PMC free article] [PubMed]
- 49.Wood DE, Salzberg SL. Kraken: ultrafast metagenomic sequence classification using exact alignments. Genome Biol. 2014;15:R46. [DOI] [PMC free article] [PubMed]
- 50.McDonald D, Clemente JC, Kuczynski J, Rideout JR, Stombaugh J, Wendel D, et al. The Biological Observation Matrix (BIOM) format or: how I learned to stop worrying and love the ome-ome. Gigascience. 2012;464:1–6. doi: 10.1186/2047-217X-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li D, Luo R, Liu CM, Leung CM, Ting HF, Sadakane K, et al. MEGAHIT v1.0: a fast and scalable metagenome assembler driven by advanced methodologies and community practices. Methods. 2016;102:3–11. doi: 10.1016/j.ymeth.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 52.Hyatt D, Chen G-L, LoCascio PF, Land ML, Larimer FW, Hauser LJ. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics. 2010;11:119. [DOI] [PMC free article] [PubMed]
- 53.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 54.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chapman B, Chilton J, Heuer M, Kartashov A, Leehr D, Ménager H, et al. Common workflow language, v1.0. Specification, common workflow language working group. Amstutz P, Crusoe MR, Tijanić N, editors. 2016.
- 56.Nurk S, Meleshko D, Korobeynikov APP. metaSPAdes: a new versatile metagenomic assembler. Genome Res. 2017;1:30–47. doi: 10.1101/gr.213959.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Menzel P, Ng KL, Krogh A. Fast and sensitive taxonomic classification for metagenomics with Kaiju. Nat Commun. 2016;7:1–9. doi: 10.1038/ncomms11257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Truong DT, Franzosa EA, Tickle TL, Scholz M, Weingart G, Pasolli E, et al. MetaPhlAn2 for enhanced metagenomic taxonomic profiling. Nat Methods. 2015;12:902–903. doi: 10.1038/nmeth.3589. [DOI] [PubMed] [Google Scholar]
- 59.Robinson JT, Thorvaldsdóttir H, Winckler W, Guttman M, Lander ES, Getz G, et al. Integrative genomics viewer. Nat Biotechnol. 2011;29:24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eren AM, Esen ÖC, Quince C, Vineis JH, Morrison HG, Sogin ML, et al. Anvi’o: an advanced analysis and visualization platform for ‘omics data. Peer J. 2015;3:e1319. doi: 10.7717/peerj.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lewis JD, Chen EZ, Baldassano RN, Otley AR, Griffiths AM, Lee D, et al. Inflammation, antibiotics, and diet as environmental stressors of the gut microbiome in pediatric Crohn’s disease. Cell Host Microbe. 2015;18:489–500. doi: 10.1016/j.chom.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Segata N, Waldron L, Ballarini A, Narasimhan V, Jousson O, Huttenhower C. Metagenomic microbial community profiling using unique clade-specific marker genes. Nat Methods. 2012;9:811. doi: 10.1038/nmeth.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bahram M, Hildebrand F, Forslund SK, Anderson JL, Soudzilovskaia NA, Bodegom PM, et al. Structure and function of the global topsoil microbiome. Nature. 2018;560:233–237. doi: 10.1038/s41586-018-0386-6. [DOI] [PubMed] [Google Scholar]
- 64.McCann A, Ryan FJ, Stockdale SR, Dalmasso M, Blake T, Ryan CA, et al. Viromes of one year old infants reveal the impact of birth mode on microbiome diversity. Peer J. 2018;6:e4694. doi: 10.7717/peerj.4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Spandole S, Cimponeriu D, Berca LM, Mihăescu G, Miha G. Human anelloviruses: an update of molecular, epidemiological and clinical aspects. Arch Virol. 2015;160:893–908. doi: 10.1007/s00705-015-2363-9. [DOI] [PubMed] [Google Scholar]
- 66.Hillmann B, Al-ghalith GA, Shields-Cutler RR, Zhu Q, Gohl DM, Beckman KB, et al. Evaluating the information content of shallow shotgun metagenomics Benjamin. mSystems. 2018;3:1–12. doi: 10.1128/mSystems.00069-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Breitwieser FP, Salzberg SL. Pavian: interactive analysis of metagenomics data for microbiomics and pathogen identification. bioRxiv. 2016:084715 Available from: https://www.biorxiv.org/content/early/2016/10/31/084715. [DOI] [PMC free article] [PubMed]
- 68.Jurka J, Kapitonov VV, Pavlicek A, Klonowski P, Kohany O, Walichiewicz J. Repbase Update, a database of eukaryotic repetitive elements. Cytogenet Genome Res. 2005;110:462–467. doi: 10.1159/000084979. [DOI] [PubMed] [Google Scholar]
- 69.Hubley R, Finn RD, Clements J, Eddy SR, Jones TA, Bao W, et al. The Dfam database of repetitive DNA families. Nucleic Acids Res. 2016;44:D81–D89. doi: 10.1093/nar/gkv1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hu X, Yuan J, Shi Y, Lu J, Liu B, Li Z, et al. pIRS: profile-based Illumina pair-end reads simulator. Bioinformatics. 2012;28:1533–5. [DOI] [PubMed]
- 71.Pruitt KD, Harrow J, Harte RA, Wallin C, Diekhans M, Maglott DR, et al. The consensus coding sequence (CCDS) project: identifying a common protein-coding gene set for the human and mouse genomes. Genome Res. 2009;19:1506. doi: 10.1101/gr.080531.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Coordinators NR Database resources of the National Center for biotechnology information. Nucleic Acids Res. 2015;44:7–19. doi: 10.1093/nar/gku1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Clarke EL, Taylor LJ, Zhao C, Connell A, Lee J-J, Fett B, et al. Example data for “Sunbeam: an extensible pipeline for analyzing metagenomic sequencing experiments” [Version 2]. Zenodo. 2019; [DOI] [PMC free article] [PubMed]
- 74.GNU Time. Available from: https://www.gnu.org/software/time/
- 75.Conway JR, Lex A, Gehlenborg N. UpSetR: an R package for the visualization of intersecting sets and their properties. Bioinformatics. 2017;33:2938–2940. doi: 10.1093/bioinformatics/btx364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, et al. vegan: community ecology package. 2018.
- 77.Wickham H. ggplot2: elegant graphics for data analysis. New York: Springer-Verlag; 2016. [Google Scholar]
- 78.JGI. Tadpole. 2018. Available from: https://jgi.doe.gov/data-and-tools/bbtools/bb-tools-user-guide/tadpole-guide/
- 79.Lo CC, Chain PSG. Rapid evaluation and quality control of next generation sequencing data with FaQCs. BMC Bioinformatics. 2014;15:1–8. doi: 10.1186/s12859-014-0366-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.JGI. BBDuk. 2018. Available from: https://jgi.doe.gov/data-and-tools/bbtools/bb-tools-user-guide/bbduk-guide/
- 81.Benson G. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Research. 1999;27:573–80. [DOI] [PMC free article] [PubMed]
- 82.Rognes T, Flouri T, Nichols B, Quince C, Mahé F. VSEARCH: a versatile open source tool for metagenomics. Peer J. 2016;4:e2584. doi: 10.7717/peerj.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.JGI. BBMap. 2018. Available from: https://jgi.doe.gov/data-and-tools/bbtools/bb-tools-user-guide/bbmap-guide/
- 85.Zaharia M, Bolosky WJ, Curtis K, Fox A, Patterson D, Shenker S, et al. Faster and more accurate sequence alignment with SNAP. arXiv. 2011;1111:e5572v1.
- 86.Marçais G, Delcher AL, Phillippy AM, Coston R, Salzberg SL, Zimin A. MUMmer4: a fast and versatile genome alignment system. PLoS Comput Biol. 2018;14:1–14. doi: 10.1371/journal.pcbi.1005944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Skinner ME, Uzilov AV, Stein LD, Mungall CJ, Holmes IH. JBrowse: a next-generation genome browser. Genome Res. 2009;19:1630–1638. doi: 10.1101/gr.094607.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Freitas TAK, Li P-E, Scholz MB, Chain PSG. Accurate read-based metagenome characterization using a hierarchical suite of unique signatures. Nucleic Acids Res. 2015;43:e69. [DOI] [PMC free article] [PubMed]
- 89.Buchfink B, Xie C, Huson DH. Fast and sensitive protein alignment using DIAMOND. Nat Methods. 2014;12:59–60. doi: 10.1038/nmeth.3176. [DOI] [PubMed] [Google Scholar]
- 90.Price MN, Dehal PS, Arkin AP. FastTree 2 - approximately maximum-likelihood trees for large alignments. PLoS One. 2010;5:e9490. [DOI] [PMC free article] [PubMed]
- 91.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Treangen TJ, Sommer DD, Angly FE, Koren S, Pop M. Next generation sequence assembly with AMOS. Curr Protoc Bioinforma. 2011;33:11.8.1–11.8.18. [DOI] [PMC free article] [PubMed]
- 93.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 94.Jensen LJ, Julien P, Kuhn M, von Mering C, Muller J, Doerks T, et al. eggNOG: automated construction and annotation of orthologous groups of genes. Nucleic Acids Res. 2008;36:250–254. doi: 10.1093/nar/gkm796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bairoch A. The ENZYME database in 2000. Nucleic Acids Res. 2000;28:304–305. doi: 10.1093/nar/28.1.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yin Y, Mao X, Yang J, Chen X, Mao F, Xu Y. DbCAN: a web resource for automated carbohydrate-active enzyme annotation. Nucleic Acids Res. 2012;40:445–451. doi: 10.1093/nar/gks479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, et al. Primer3-new capabilities and interfaces. Nucleic Acids Res. 2012;40:1–12. doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ye Y, Choi JH, Tang H. RAPSearch: a fast protein similarity search tool for short reads. BMC Bioinformatics. 2011;12:159. [DOI] [PMC free article] [PubMed]
- 99.Stamatakis A, Ludwig T, Meier H. RAxML-II: a program for sequential, parallel and distributed inference of large phylogenetic trees. Concurr Comput Pract Exp. 2005;17:1705–1723. doi: 10.1002/cpe.954. [DOI] [Google Scholar]
- 100.Ahmed SA, Lo C-C, Li P-E, Davenport KW, Chain PSG. From raw reads to trees: whole genome SNP phylogenetics across the tree of life. bioRxiv. 2015:032250 Available from: http://biorxiv.org/content/early/2015/11/19/032250.abstract.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Lewis et al. 2015 report. Report with figures reproducing results from Lewis et al. 2015 [61]. (HTML 1031 kb)
Bahram et al. 2018 report. Report with figures reproducing results from Bahram et al. 2018 [63]. (HTML 954 kb)
McCann et al. 2018 report. Report with figures reproducing results from McCann et al. 2018 [64]. (HTML 812 kb)
Shallow shotgun pilot report. Report with figures relating to the shallow shotgun pilot study described above. (HTML 1252 kb)
Data Availability Statement
Sunbeam is available at https://github.com/sunbeam-labs/sunbeam. Komplexity is available at https://github.com/eclarke/komplexity. Pre-built extensions referenced can be found at https://github.com/sunbeam-labs/. The dataset of simulated microsatellite and conserved coding sequence reads is archived in Zenodo at https://zenodo.org/record/2541222. Shotgun data from the shallow shotgun pilot study is deposited in SRA under project identifier SRP178101. The analyses in Fig. 3 can be reproduced by the following Sunbeam extensions: https://github.com/louiejtaylor/sbx_lewis2015, https://github.com/louiejtaylor/sbx_bahram2018, https://github.com/louiejtaylor/sbx_mccann2018, https://github.com/junglee0713/sbx_shallowshotgun_pilot.