Abstract
Aims
Although there is evidence linking sugar-sweetened beverage (SSB) intake with the development of cardio-metabolic diseases, the underlying mechanisms remain unclear. The current study therefore evaluated the effects of SSB consumption by establishing a unique in-house in vivo experimental model.
Main methods
Male Wistar rats were divided into two groups: a) one consuming a popular local SSB (SSB- Jive), and b) a control group (Control-water) for a period of three and six months (n = 6 per group), respectively. Rats were gavaged on a daily basis with an experimental dosage amounting to half a glass per day (in human terms) (SSB vs. water). Cardiac function was assessed at baseline (echocardiography) and following ex vivo ischemia-reperfusion of the isolated perfused working rat heart. Oral glucose tolerance tests and mitochondrial respiratory analyses were also performed. In addition, the role of non-oxidative glucose pathways (NOGPs), i.e. the polyol pathway, hexosamine biosynthetic pathway (HBP) and PKC were assessed.
Key findings
These data show that SSB intake: a) resulted in increased weight gain, but did not elicit major effects in terms of insulin resistance and cardiac function after three and six months, respectively; b) triggered myocardial NOGP activation after three months with a reversion after six months; and c) resulted in some impairment in mitochondrial respiratory capacity in response to fatty acid substrate supply after six months.
Significance
SSB intake did not result in cardiac dysfunction or insulin resistance. However, early changes at the molecular level may increase risk in the longer term.
Keywords: Nutrition, Physiology, Cardiology, Molecular biology, Biochemistry
1. Introduction
Obesity is a global pandemic with developing nations often hardest hit [1]. In parallel, there is increased manifestation of obesity-related complications such as Type 2 Diabetes (T2D) and cardiovascular diseases (CVD) [2, 3, 4]. Although this rise is multi-factorial in nature, well-known risk factors include sedentary lifestyles and poor dietary habits e.g. increased caloric intake from sugar-sweetened beverages (SSBs) [5].
Numerous risk factors and adverse effects arise from increased SSB consumption [6] as the body does not compensate for the excess calories consumed. Studies have linked high SSB intake to numerous risk factors for cardio-metabolic diseases onset, e.g. increased visceral fat deposition, elevated triglyceride and cholesterol metabolites, and greater body weight [6, 7, 8]. The consumption of various carbohydrates (e.g. sugars and high fructose corn syrup) contributes to high dietary glycemic load, inflammation and insulin resistance irrespective of obesity [9, 10]. Moreover, high SSB consumption leads to increased blood glucose and insulin concentrations [8, 11, 12] that can directly impede hepatic insulin signaling and thereby promote insulin resistance [13]. SSB intake may also not trigger the satiety response and this may be an important link between elevated liquid calories and increased health risk.
Although there is evidence linking SSB intake with the development of cardio-metabolic diseases, the underlying mechanisms whereby it can result in such complications remain poorly understood. In light of this, we evaluated the effects of SSB consumption by establishing a unique in-house in vivo experimental model. Socio-economic factors and the recent implementation of a sugar tax in South Africa favor the consumption of more cost-effective SSBs among the poor and working class. As a result, we here investigated a popular, locally consumed SSB (Jive). In addition, it does not contain any caffeine that may result in effects on cardiac function and metabolism. For the current study, we investigated whether actual SSB consumption (as opposed to other completed studies where fructose and/or glucose intake were investigated) for 3 and 6 months, respectively, triggers metabolic dysregulation and putative links to mitochondrial and cardiac function. Here we specifically focused on the role of non-oxidative glucose pathways (NOGPs), i.e. the polyol pathway, hexosamine biosynthetic pathway (HBP), and PKC in this process, as such pathways were previously implicated in the onset of cardio-metabolic complications [14, 15, 16]. This was done as the NOGPs are branch pathways of glycolysis and are up-regulated in response to elevated glucose flux, thereby triggering detrimental downstream pathways that can lead to cardiac contractile dysfunction [17].
2. Materials and methods
2.1. Animals and experimental protocol
Male Wistar rats weighing ∼250 grams were divided into two groups: a) one consuming a popular local SSB (SSB- Jive), and b) a control group (Control-water) for periods of three and six months (n = 6 per group), respectively. The local SSB used was Jive granadilla flavor. This product contains 4 g sucrose per 100 ml, i.e. 176.7 KJ per 250 ml serving. Rats were gavaged on a daily basis with an experimental dosage depending on group allocations and body weight classification (refer Table 1). Dosage volumes were calculated using the surface area-to-volume ratio [18] and corrected for weight. Here dosages simulated the equivalent of 125 mL (54 calories) of SSB consumed per day for a 60 kg person. Rat chow was provided ad libitum and the animals regularly evaluated to assess their overall well-being. Food intake was monitored on a weekly basis by initially weighing normal rat chow and placing the pellets into rat cages, whereafter pellets were removed and re-weighed after seven days. The difference between the two measurements equated to food consumed per cage. The rats were weighed on a weekly basis to record the percentage weight gain to allow for an assessment of the development of obesity. Upon the completion of the experimental procedures, rats were euthanized and organs harvested, weighed and snap-frozen and stored at -80 °C until further analysis. All animals were treated in agreement with the Guide for the Care and Use of Laboratory Animals of the National Academy of Science (NIH publication No. 85–23, revised 1996). This study was executed with the approval of the Animal Ethics Committee of Stellenbosch University (Stellenbosch, South Africa), (Ethics # SU-ACUM13-00012).
Table 1.
SSB volumes gavaged according to weight classification.
| Treatment | 250–300 g | 300–350 g | 350–400 g | 400–450 g | >450 g |
|---|---|---|---|---|---|
| Control | 3.2 ml | 3.8 ml | 4.5 ml | 5.1 ml | 5.8 ml |
| Jive® | 2.4 ml | 2.9 ml | 3.4 ml | 3.8 ml | 4.4 ml |
2.2. Blood sampling
The animals were fasted overnight (at least 12 hours) and thereafter sedated using 3% isoflurane (Piramal, Bethlehem PA) before 1 mL of blood was drawn from the right jugular vein. The blood was then centrifuged to collect serum samples that were stored at −80 °C until it was sent to the National Health Laboratory Service (Tygerberg Hospital, Western Cape, South Africa) to evaluate for the measurement of: uric acid, alanine aminotransferase (ALT), hemoglobin A1c (HbA1c), triglycerides and total cholesterol levels (mmol/L) using standard procedures. Different methods were employed to determine HbA1c: National Glycohemoglobin Standardization Program (NGSP), the International Federation for Clinical Chemistry (IFCC) and estimated average glucose (eAG). The NGSP system is the most widely used and results are expressed as a percentage of glycation. The IFCC method relies on high performance liquid chromatography to separate glycated and non-glycated peptides. Thereafter mass spectrometry or capillary electrophoresis is employed to quantify each respective group and the results are expressed as mmol/mol. The eAG is calculated with a regression equation and measured in mmol/L (most contested method) [19]. Insulin levels were measured by using a commercially available ELISA kit (Mercodia AB, Sweden). The homeostatic model assessment for insulin resistance (HOMA-IR) was determined as before in order to quantify insulin resistance [20].
2.3. Oral glucose tolerance tests (OGTTs)
Baseline fasting glucose levels were initially evaluated whereafter glucose powder was dissolved in distilled water (0.86 g/kg body weight) and rats gavaged and monitored for 120 minutes. Readings were taken at the following time points (in minutes): 5, 10, 15, 30, 45, 60 and 120.
2.4. Mitochondrial respiration studies
Measurement of mitochondrial respiration was performed with a polarographic oxygen sensor in 2 mL glass chambers of an Oxygraph 2K (Oroboros Instruments, Innsbruck, Austria) as described before [21]. All substrates and chemicals were purchased from Sigma-Aldrich (St. Louis MO). Myocardial fibers (∼2 mg) were permeabilized using saponin and placed into the two oxygraph chambers, followed by determination of endogenous ROUTINE (R) respiration when oxygen flux stabilized. Pyruvate (5 mM), glutamate (10 mM), and malate (2 mM) were added to induce LEAK (L) respiration (for glucose oxidation), and octanoyl carnitine (0.2 mM) and malate (2 mM) for fatty acid (FA) oxidation.
Oxidative phosphorylation (OXPHOS [P]) was measured by adding 2.5 mM ADP, followed by the addition of 2.5 μM oligomycin to induce LEAK respiration by inhibiting ATP synthase. Next, titration with the uncoupling agent CCCP (steps of 0.5 M) increased respiration up to the maximal level and is referred to as electron transfer system (ETS) capacity.
Rotenone (complex I inhibitor) was then added to a final concentration of 0.5 M, while the addition of 2.5 M Antimycin A induced residual oxygen consumption (this inhibits complex III). Finally, titration of 0.5 mM TMPD and 2 mM ascorbate was performed to assess complex IV-linked respiration as a proxy for mitochondrial content. Oxygen flux at all respiratory states was normalized to the complex IV flux to correct for variations in cell content in the oxygraph chambers. Excess E-R capacity was calculated to determine the difference between the ETS capacity and R respiration.
2.5. Heart function
One week before the final experiments, the rats were slightly anesthetized with 1.5–2% isoflurane and positioned in the supine position on a warming pad. Closed chest echocardiography was performed with a VEVO 2100 ultrasound system (Fujifilm, Visualsonics, Ontario, Canada) and a 13–25 MHz linear array transducer. Left ventricular (LV) chamber size, ejection fraction and mass were obtained from 2-dimensional and M-mode measurements at the mid-papillary level. To assess LV diastolic function, both mitral E and A wave peak velocities were obtained from pulse Doppler in the apical 4-chamber view; the E/A ratio was then calculated. All measurements were made off-line on the mean of at least three consecutive cardiac cycles with the software resident on the ultrasound system.
For perfusion studies, rats were anesthetized using pentobarbitone sodium (Kyrone Laboratories, Johannesburg, South Africa) (160 mg/kg body weight). The heart was rapidly excised, arrested in ice-cold (4 °C) Krebs-Henseleit bicarbonate buffer and mounted through the aorta onto the aortic cannula. The left atrium was also cannulated via the pulmonary vein. Hearts were first retrogradely perfused (Langendorff mode), in a non-recirculating manner at constant hydrostatic pressure (100 cmH2O) for 10 minutes, followed by the working heart mode (preload 15 cmH2O, afterload 100 cmH2O) for 20 minutes. The hearts were not electrically paced, while the myocardial temperature was thermostatically controlled and monitored at regular intervals (constant at 37 °C during reperfusion and 36.5 °C during ischemia). Hearts were then subjected to 35 minutes regional ischemia induced by ligation of the proximal part of the left anterior descending coronary artery, followed by 2 hours of reperfusion.
Myocardial infarct size was determined as previously described [22]. At the end of 2 hours reperfusion, the silk suture around the coronary artery was securely tied and 1 mL Evans blue suspension (0.5%) was slowly injected using the aorta cannula. The heart was then frozen overnight before being cut into 2 mm thick slices that were subsequently stained with 1% w/v triphenyl tetrazolium chloride in phosphate buffer (pH 7.4 at 37 °C) for 15 minutes. Slices were fixed in 10% v/v formaldehyde solution at room temperature. The damage in each slice was visible with the blue area indicating viable tissue and the white or unstained area indicating the infarcted area (which was surrounded by a red area). The latter two areas constituted the area at risk (AR). All areas were drawn and scanned. For each slice the AR and the area of infarcted tissue were determined using computerized planimetry (UTHCSA Image Tool Program, University of Texas Health Science Center at San Antonio TX). The infarct size was expressed as a percentage of the area at risk (I/AR%).
2.6. Myocardial lipid and glucose metabolism
Tissue triglyceride levels were evaluated using the Picoprobe Fluorometric Triglyceride Quantification Assay Kit (Abcam, Cambridge MA). Myocardial glycogen and glycogen synthase 1 (cardiac enriched isoform) levels were determined using commercially available kits (Abcam, Global Biotech Company, San Francisco CA).
2.7. Myocardial NOGP analysis
Commercial kits were used to evaluate markers of the NOGP activation as done before in our laboratory [14, 15, 16]. Here PKC activity was determined using a commercially available kit (Abcam, Cambridge MA), while the polyol pathway was assessed using a D-sorbitol colorimetric detection kit (Biovision, Mountain View CA). We employed Western blotting to determine myocardial HBP activation as done before by us [14, 15, 16]. O-linked β–N-acetylglucosamine (O-GlcNAc) expression was determined by employing SDS-PAGE electrophoresis using the CTD110.6 antibody (Santa Cruz Biotechnology, Santa Cruz CA) together with an appropriate secondary antibody (anti-mouse IgG, HRP-linked, Cell Signaling Technology, Danvers MA). Final O-GlcNAc amounts were determined using Image Lab™ Software version 4.0 (BIO-RAD, Berkley CA). Protein was normalized using total protein content.
2.8. Oxidative stress analyses
2.8.1. NADPH oxidase activity assay
We employed an in-house NADPH oxidase activity assay as done before [15, 16]. Baseline luminescence measurement was performed on triplicate samples, followed by automatic addition of 100 μL of assay buffer (the luminometer was programmed to inject this volume into each well) and the light emission measured over a period of 20 minutes. The assay buffer consisted of: NaCl (120 mM), HEPES (250 mM, pH 7.4), CaCl2 (2H2O) (1.75 mM), KCl (1.2 mM), EDTA (0.5 mM), MgSO4 (7H2O) (1.2 mM), NADH (100 μL), glucose (11 mM) and lucigenin (5 μM). The data were expressed as relative light units (RLU)/μg protein.
2.8.2. Conjugated dienes (CDs) analysis
Heart samples were homogenized in PBS buffer. 100 μL of the homogenate was added to 300 μL chloroform/methanol mixture (2:1), vortexed briefly and centrifuged at 3, 000 × g for 1 minute. The bottom chloroform layer of each sample was pipetted into fresh microtubes and left open overnight at 4 °C to dry out. Thereafter, 700 μL cyclohexane was added to each of the microtubes, vortexed and 200 μL of sample was added to a UV 96-well plate that was read in a plate reader (Multiskan spektrum, Thermo Electron Corporation) at 240 nm. Cyclohexane was used as a blank and all samples were assayed in triplicate.
2.8.3. Glutathione redox analysis
Heart samples (∼200 μg) were homogenized on ice in 2 mL phosphate buffer (50 mM NaHPO4, 1 mM EDTA, pH 7.5). For GSSG analysis an additional 10 μL 1-methyl-2-vinyl-pyridinium trifluoromethane sulfonate (30 mM in 0.1 M hydrochloric acid) was added to every 1 mL phosphate buffer. Thus total (GSH) and oxidized (GSSG) glutathione samples were prepared separately. Samples were centrifuged at 15, 000 × g for 5 minutes and the supernatants were diluted (10–40×) and used for analysis. A standard curve was prepared using GSH (3 μM) and GSSG (1.5 μM) stock solutions and phosphate buffer. All other reagents were also prepared in Phosphate buffer. Standards and samples (50 μL of each in triplicate) were added to the 96-well plate. Next, 50 μL 0.3 mM 5,5′dithiobis-(2-nitrobenzoic acid) was added to each well followed by 50 μL glutathione reductase (0.02 U/μL). The plate was incubated for 5 minutes at room temperature after which 50 μL NADPH (1 mM) was added to each well. The absorbance was monitored at 410 nm for 5 minutes with 30 seconds intervals and levels calculated using pure GSH and GSSG as standards. Reduced glutathione (GSH) concentration was calculated as the difference between total glutathione and 2× GSSG.
2.9. Data analysis
Statistica 13.0 (StatSoft Inc., Dell Software, Tulsa OK) was used for statistical analyses. The weight gain and perfusion data were analyzed with a repeated measures ANOVA test. The rest of the normally distributed data (determined using Shapiro Wilk's test) was evaluated with Unpaired t-tests, while Mann-Whitney tests were used in the case of non-parametric data. These tests were performed to assess differences between Control and SSB groups at the two time points employed. Outliers were detected using Grubb's test and Levene's tests were employed to test for homogeneity of variances. A P-value <0.05 was considered significant. All the data are presented on Graphpad Prism 5.01 (Graphpad Software Inc., San Diego CA) as mean ± standard error of the mean (SEM) for parametric data and as median and interquartile range for non-parametric data.
3. Results
3.1. Body weight and organ changes
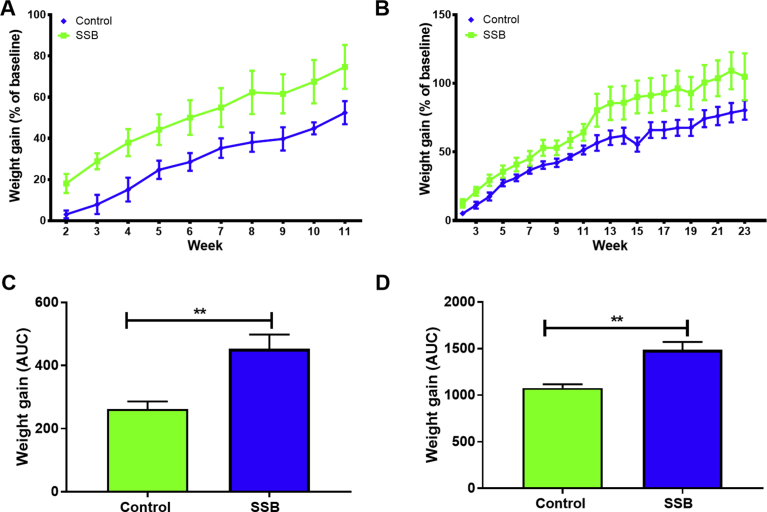
The SSB group gained more weight compared to the Control group, although this was not statistically significant when considering repeated measures analyses over time (Fig. 1A and B). However, area under the curve (AUC) analyses revealed significantly more weight gain in the SSB groups at three (p = 0.0035, Fig. 1C) and at six months, respectively (p = 0.0013, Fig. 1D). Moreover, we found no significant differences in food consumed by the two different groups here investigated (data not shown). No significant differences were observed for weights of various tissues (expressed as a percentage of the final body weight) (Table 2).
Fig. 1.
The impact of SSB consumption on weight gain. A) three months, and B) six months. The area under the curve was calculated at the C) 3 months and D) 6 months, time points. The animals were weighed on a weekly basis, and weight gained by each rat was then compared to its own baseline weight as a percentage Results are displayed as mean ± SEM. For all groups n = 6. **P < 0.01.
Table 2.
Organ weight after three and six months SSB treatment (expressed as % of final body weight).
| Organ | Month | Control | SSB |
|---|---|---|---|
| Heart (%) | Three | 0.249 ± 0.006 | 0.238 ± 0.006 |
| Six | 0.223 ± 0.006 | 0.230 ± 0.006 | |
| Liver (%) | Three | 2.911 ± 0.662 | 2.821 ± 0.543 |
| Six | 1.957 ± 1.175 | 1.985 ± 1.221 | |
| Muscle (%) | Three | 0.523 ± 0.139 | 0.521 ± 0.132 |
| Six | 0.462 ± 0.145 | 0.475 ± 0.176 | |
| Kidney (%) | Three | 0.280 ± 0.007 | 0.274 ± 0.007 |
| Six | 0.271 ± 0.007 | 0.266 ± 0.007 | |
| Visceral Fat (%) | Three | 1.630 ± 1.740 | 1.453 ± 1.775 |
| Six | 1.459 ± 1.740 | 1.594 ± 1.740 |
3.2. Blood metabolites and OGTTs
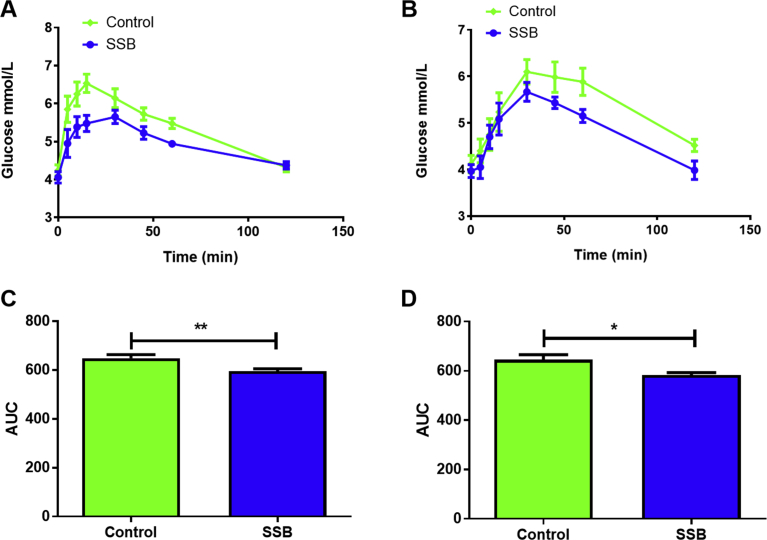
To gain further insights into the effects of SSB consumption, we also determined fasted serum levels for: uric acid, ALT, HbA1c, cholesterol, triglyceride and glucose (Table 3). Two of the HbA1c tests were increased in the SSB group after three months (p < 0.05 vs. Control) together with raised serum cholesterol levels (p < 0.01 vs. Control). By six months the HbA1c (IFCC) was still elevated in the SSB group (p < 0.05 vs. Control) while there were no significant differences for cholesterol levels (vs. Control). After six months the SSB group displayed higher uric acid levels vs. Controls (p < 0.05 vs. Control). The HOMA-IR data revealed no significant differences with SSB consumption at both experimental time points. The OGTT data showed a lower AUC in the SSB group vs. the Controls at both the three (p = 0.0027, Fig. 2C) and six months’ time points (p = 0.013, Fig. 2D).
Table 3.
Blood metabolites.
| Marker | Month | Control | SSB |
|---|---|---|---|
| Uric acid (mmol/L) | Three | 0.062 ± 0.005 | 0.062 ± 0.004 |
| Six | 0.075 ± 0.006 | 0.080 ± 0.006* | |
| ALT (U/L) | Three | 61.600 ± 3.027 | 53.500 ± 5.835 |
| Six | 54.170 ± 2.651 | 66.200 ± 7.612 | |
| HbA1c [NGSP] (%) | Three | 3.233 ± 0.099 | 3.500 ± 0.141 |
| Six | 3.133 ± 0.131 | 3.333 ± 0.0843 | |
| HbA1c [IFCC] (mmol/mol) | Three | 10.200 ± 0.583 | 15.830 ± 1.138 * |
| Six | 10.670 ± 0.907 | 13.000 ± 1.033 * | |
| HbA1c [eAG] (mmol/L) | Three | 2.400 ± 0.110 | 3.183 ± 0.166 * |
| Six | 2.417 ± 0.206 | 2.700 ± 0.139 | |
| Fasting blood glucose (mmol/L) | Three | 4.717 ± 0.145 | 4.400 ± 0.183 |
| Six | 5.525 ± 0.257 | 5.325 ± 0.209 | |
| HOMA-IR | Three | 7.999 ± 0.404 | 7.849 ± 0.441 |
| Six | 8.310 ± 0.430 | 7.778 ± 0.311 | |
| Insulin (mmol/L) | Three | 0.303 ± 0.012 | 0.294 ± 0.004 |
| Six | 0.317 ± 0.017 | 0.307 ± 0.008 | |
| Cholesterol (mmol/L) | Three | 1.250 ± 0.060 | 1.550 ± 0.060 ** |
| Six | 1.550 ± 0.067 | 1.650 ± 0.099 | |
| Triglycerides (mmol/L) | Three | 1.276 ± 0.140 | 1.350 ± 0.076 |
| Six | 1.150 ± 0.138 | 1.200 ± 0.137 |
ALT: alanine aminotransferase; HbA1c: glycosylated hemoglobin; NGSP: National Glycohemoglobin Standardization Program; IFCC: International Federation of Clinical Chemistry; eAG: Estimated Average Glucose; HOMA-IR: homeostasis model assessment of insulin resistance; *P < 0.05 vs. Control; **P < 0.01 vs. Control.
Fig. 2.
The effects of SSB consumption - oral glucose tolerance tests. A) OGTT - three months, B) OGTT - six months, C) AUC - 3 months, and D) AUC – 6 months. OGTTs were performed biweekly to monitor the animal's glycemic states and to evaluate the effects of SSB on postprandial hyperglycemic excursions. Glucose levels were tested at 0, 5, 10, 15, 30, 45, 60 and 120 minutes (n = 12 for baseline [week 1] to three months; n = 6 for the six -month time point). Values are expressed as mean ± SEM. Significantly different compared to Control group: **P < 0.005 and ***P < 0.001.
3.3. Mitochondrial respiration
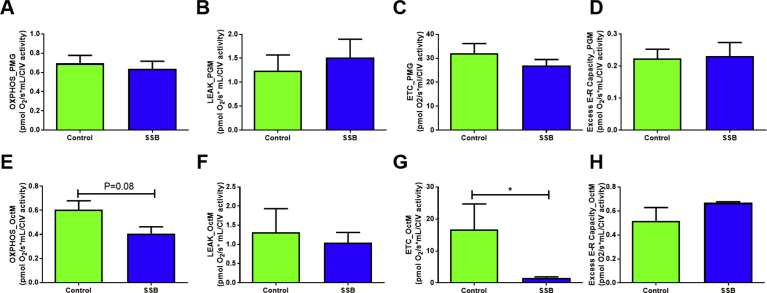
The results showed no significant differences between the groups for the various parameters tested for glucose oxidation at the six months’ time point (Fig. 3A–D). Here we employed pyruvate, glutamate and malate as oxidative substrates. Similar results were observed when examining the OXPHOS, LEAK and excess E-R ratios in response to FA oxidation (octanoyl-carnitine and malate substrates) (Fig. 3E, F and H). However, the ETS ratios were significantly decreased in the SSB group when supplied with the FA oxidation substrates (p = 0.03 vs. Controls, Fig. 3G). Due to technical issues no respiration data could be generated for the three month time point.
Fig. 3.
SSB intake and effects on mitochondrial respiratory function. A) – D) glycolytic substrates, and E) – H) FA substrates. A- LEAK, B- OXPHOS, C- ETS capacity and D- excess E-R. The substrates used for these experiments included pyruvate (P), glutamate (G) and malate (M). E- LEAK, F- OXPHOS, G- ETS capacity and H- excess E-R. The substrates used for these experiments included octanoyl carnitine (Oct) and malate (M). A total number of n = 6 was used for all groups. Results are displayed as mean ± SEM and significance is shown as *P < 0.01 compared to Control.
3.4. In vivo and ex vivo heart functional assessments
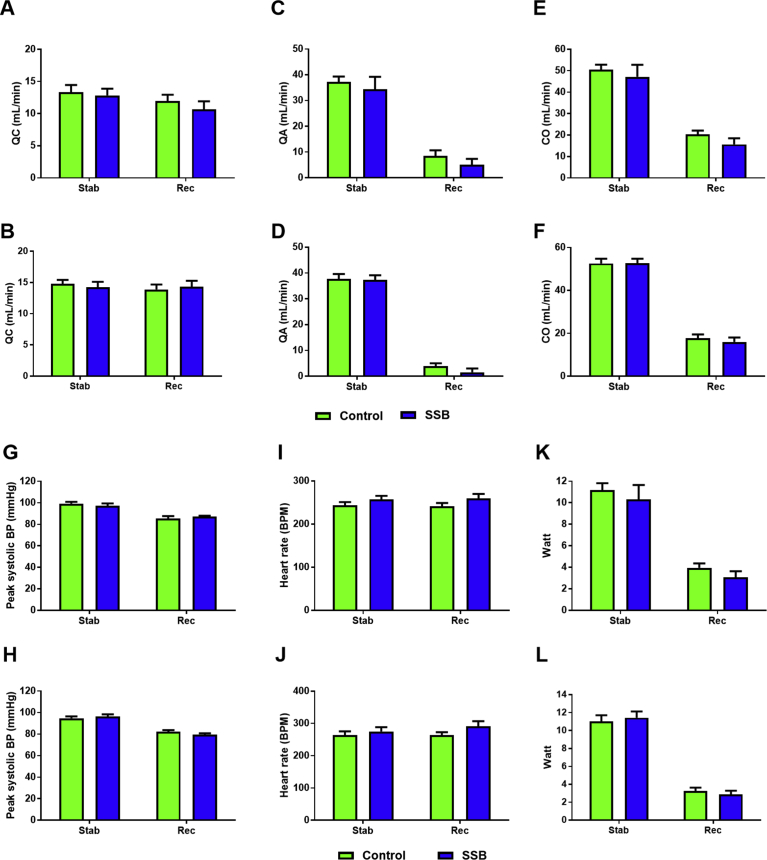
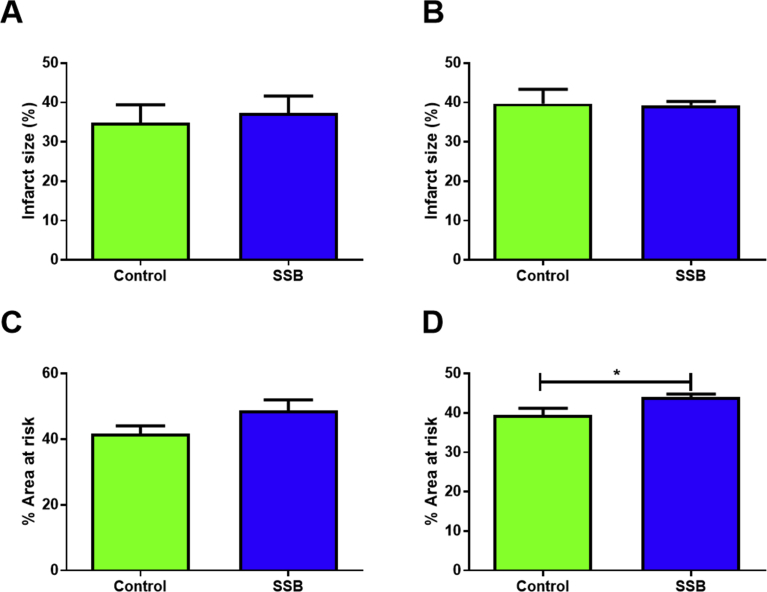
Echocardiographic analyses revealed no significant differences between the Control and SSB groups after three and six months, respectively (Table 4). In support, working heart perfusion data revealed no differences for any of the parameters examined between SSB-treated and Control groups during the stabilization phase and during recovery after simulated ischemia (Fig. 4). Infarct size determination revealed no differences between groups (Fig. 5A and B), although the AR was elevated in the SSB groups after six months (p = 0.02 vs. Controls, Fig. 5D).
Table 4.
Echocardiographic data.
| 3 months |
6 months |
|||||
|---|---|---|---|---|---|---|
| Control | SSB | p | Control | SSB | p | |
| Heart rate (beats/minute) | 378 ± 12 | 385 ± 7 | 0.675 | 401 ± 15 | 399 ± 13 | 0.777 |
| Left ventricle measures | ||||||
| Internal diameter diastole (mm) | 6.86 ± 0.11 | 6.82 ± 0.07 | 0.675 | 7.24 ± 0.19 | 7.03 ± 0.13 | 0.593 |
| Internal diameter systole (mm) | 3.68 ± 0.15 | 3.49 ± 0.18 | 0.965 | 3.93 ± 0.23 | 4.07 ± 0.20 | 0.600 |
| Ejection fraction (%) | 76 ± 2 | 79 ± 2 | 0.455 | 75 ± 2 | 71 ± 3 | 0.296 |
| mass (mg) | 780 ± 24 | 692 ± 41 | 0.065 | 854 ± 60 | 786 ± 55 | 0.679 |
| Mitral inflow | ||||||
| Peak E wave (cm/s) | 991 ± 64 | 968 ± 36 | 0.900 | 982 ± 67 | 904 ± 60 | 0.689 |
| Peak A wave (m/s) | 852 ± 68 | 867 ± 49 | >.999 | 914 ± 60 | 826 ± 49 | 0.446 |
| E/A ratio | 1.18 ± 0.06 | 1.12 ± 0.05 | 0.654 | 1.08 ± 0.01 | 0.94 ± 0.16 | 0.778 |
SSB: Sugar-sweetened beverage.
Fig. 4.
(part 1): The impact of SSB consumption on heart function (ex vivo Langendorff perfusions). Perfusion data, A, C, E), 3 months, B, D, F) 6 months. Cardiac output (QC) was calculated as the sum of aortic output (QA) and coronary flow (CO). The aortic systolic and diastolic pressure (mmHg) and HR [beats per minute (bpm)] were monitored and recorded on a computerized system through a side-arm of the aortic cannula, and total work performance was calculated as follows: pressure power + kinetic power = Watt. Results are displayed as mean ± SEM. A final n = 8 for all groups were used. (part 2): The impact of SSB consumption on heart function (ex vivo Langendorff perfusions). Perfusion data, G, I, K), 3 months, H, J, L) 6 months. The aortic systolic and diastolic pressure (mmHg) and HR [beats per minute (bpm)] were monitored and recorded on a computerized system through a side-arm of the aortic cannula connected to a Viggo-Spectramed pressure transducer coupled to the computer system. Total work performance was calculated as follows: pressure power + kinetic power = Watt. A final n = 8 for all groups were used.
Fig. 5.
The effects of SSB intake on infarct size. Infarct size and area at risk, A&C), 3 months, B&D) 6 months (n = 8). Infarct size is expressed as the percentage of the area at risk (I/AR%). Results are displayed as mean ± SEM and significance is shown as *P < 0.05 compared to Control.
3.5. Myocardial lipid and glucose metabolism
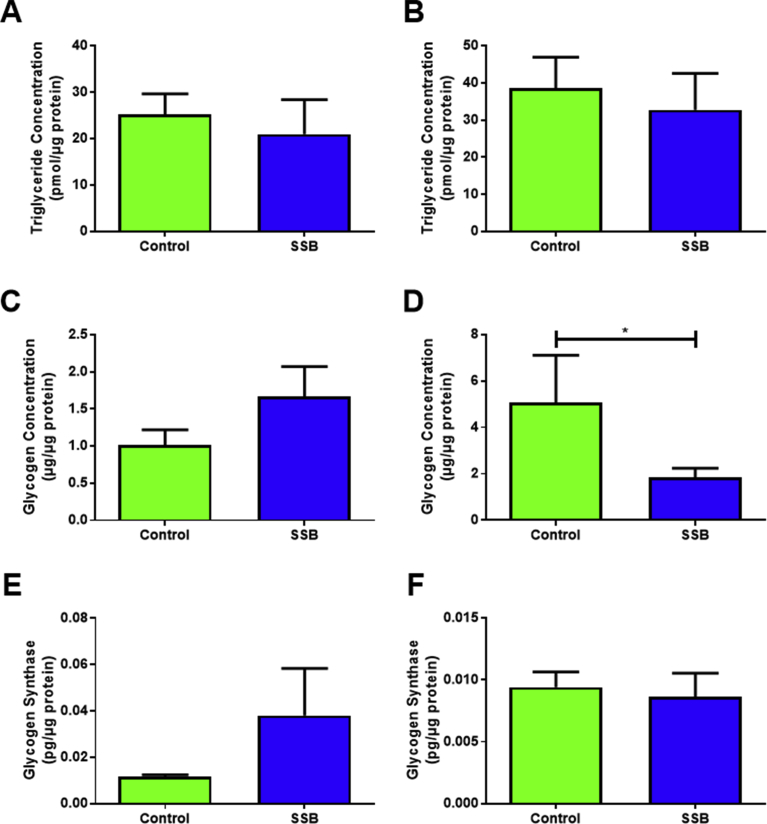
We next assessed several metabolic markers to gain an improved understanding of the mitochondrial respiration and heart functional data. Heart tissue triglyceride levels were unchanged between the groups at both time points (Fig. 6A and B). Myocardial glycogen concentrations were significantly reduced in the SSB group at six months (p = 0.02 vs. Control, Fig. 6D), however levels of glycogen synthase 1 were not significantly changed (Fig. 6E and F).
Fig. 6.
Markers of myocardial glucose and lipid metabolism following SSB intake. A, C and E is 3 Months and B, D and F represents 6 months. A and B show myocardial triglyceride stores with SSB consumption. C and D represent glycogen stores measured in the rat heart. E and F depict glycogen synthesis (n = 6 for all groups). Data are displayed as mean ± SEM and significance between the groups is displayed as *P < 0.05.
3.6. NOGP assessment
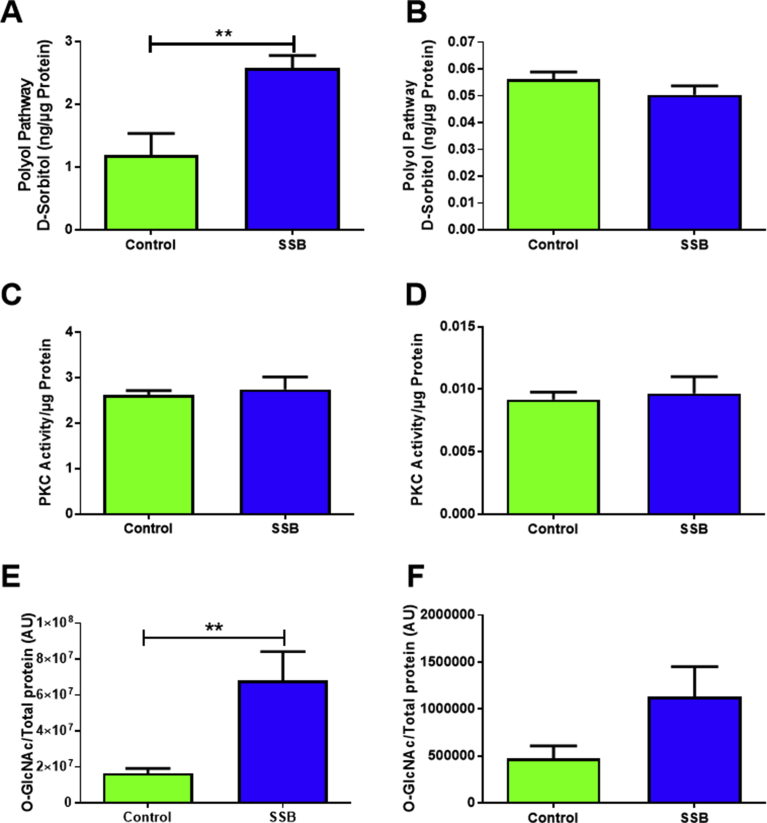
D-Sorbitol and O-GlcNAc levels were markedly elevated in the SSB treatment group (p = 0.002 and p = 0.009 vs. Controls, respectively) after three months (Fig. 7A and E), while PKC activity remained unchanged at both time points (Fig. 7C and D). Levels of D-Sorbitol in the SSB group were comparable to that of Controls after six months (Fig. 7B). Although O-GlcNAc levels remained ∼2.4-fold higher in the SSB animals at this time point, this change was not statistically significant (p = 0.09) (Fig. 7F).
Fig. 7.
NOGP regulation following SSB intake. NOGPs, A, C & E) 3 months, B, D & F) 6 months (n = 6). Values are expressed as mean ± SEM and significance is shown as **P < 0.01.
3.7. Oxidative stress and antioxidant defense markers
NADPH oxidase activity was unchanged at the three month time point (Table 5), but after six months it was lower in the SSB group compared to matched controls (p = 0.047 vs. Control, Table 5). CDs were decreased in the SSB group at three months (p = 0.014 vs. Control), while the opposite effect was observed after six months, with higher CDs in the SSB rats versus their matched controls (p = 0.056 vs. Control). The ratio of GSH:GSSG was not significantly altered after three months of SSB consumption (Table 5). However, it was higher in the SSB group after six months versus matched controls (p = 0.064 vs. Control).
Table 5.
Oxidative stress analyses.
| 3 months |
6 months |
|||||
|---|---|---|---|---|---|---|
| Control | SSB | p | Control | SSB | p | |
| NADPH oxidase activity (RLU/μg protein) | 0.165 ± 0.028 | 0.177 ± 0.031 | 0.389 | 0.176 (0.163–0.188) | 0.096 (0.089–0.172) | 0.047 |
| Conjugated dienes (μmol/g tissue) | 0.117 ± 0.014 | 0.072 ± 0.009 | 0.014 | 0.945 ± 0.192 | 1.279 ± 0.069 | 0.056 |
| Reduced glutathione (GSH) | 0.325 ± 0.067 | 0.292 ± 0.063 | 0.364 | 0.463 ± 0.008 | 0.477 ± 0.006 | 0.085 |
| Oxidized glutathione (GSSG) | 0.183 (0.134–0.199) | 0.182 (0.158–0.240) | 0.469 | 0.094 ± 0.004 | 0.088 ± 0.005 | 0.185 |
| GSH:GSSG ratio | 1.579 (1.152–4.393) | 1.749 (1.038–2.669) | 0.221 | 4.716 ± 0.198 | 5.44 ± 0.337 | 0.064 |
Data presented as mean ± SEM for parametric data or median (interquartile range) for non-parametric data. Differences were considered significant when p < 0.05.
4. Discussion
As the underlying mechanisms driving SSB-mediated development of cardio-metabolic diseases remain poorly understood, we further investigated this research question by employing a unique in-house in vivo experimental rat model. Here our data reveal that SSB intake: a) resulted in increased weight gain but did not elicit major effects in terms of insulin resistance and cardiac function after three and six months, respectively; b) triggered myocardial NOGP activation after three months that reversed after six months; and c) resulted in early changes on mitochondrial electron transport capacity in response to fatty acid substrate supply after six months.
4.1. SSB intake elicited increased weight gain but no major effects in terms of insulin resistance and cardiac function
Our data reveal that SSB consumption significantly changed body weight after three and six months, respectively, with minimal impact on organ weights. There are conflicting data in the literature in this regard (both animal-based and clinical studies), with some demonstrating increased weight gain following SSB consumption [6, 7, 23, 24, 25] while others found no significant changes [26, 27, 28, 29]. There are several reasons for such discrepancies, including the nature of the nutrient tested (e.g. fructose vs. sucrose vs. actual SSB), variations in daily amounts consumed, mode of nutrient intake (ad libitum availability vs. oral gavage), and the duration of the experiment. As the daily intake for the current model was roughly equivalent to half a glass of SSB consumed per day (in human terms) this is much lower than what would be typically used in animal studies of this nature. It is our opinion that this is a more realistic dosage to investigate in animal studies (vs. excessively high dosages) and that it together with the use of an actual SSB (versus fructose and/or glucose) resulted in relatively moderate alterations in terms of body weight.
SSB consumption did not significantly alter fasting blood glucose levels in our model. In agreement, others found that fructose (when consumed in moderate amounts) also did not adversely affect blood glucose levels [30]. This is in accordance with studies that employed sucrose as a sweetener [27, 28]. By contrast, some found that fructose consumption was linked to higher fasting blood glucose levels, although relatively higher dosages were employed in this instance [31]. Our data also show that SSB consumption increased HbA1c levels after three and six months, respectively, reflecting high glucose availability due to the continued SSB intake. In agreement, previous studies showed a positive correlation between SSB consumption and HbA1c levels, while others found that the latter is elevated when beverage servings increase to more than one per day [32]. The discordance between the fasting blood glucose and HbA1c data is likely due to glucose levels being tested after a prolonged (at least 12 hours) overnight fast, and hence differences between the groups may have been masked. The HbA1c results suggest that the SSB-consuming animals were likely in a state of (at least mild) hyperglycemia from as early as three months.
Our findings revealed variation in OGTTs. We are unclear what caused this and speculate that SSB consumption may trigger effects that impact either on pancreatic β–cell insulin secretion and/or insulin-mediated glucose uptake. However, further studies are required to obtain definitive answers in this regard. Popkin (2012) demonstrated that SSB consumption elicited adverse effects on insulin sensitivity and hence if such a response is continually stimulated it may eventually lead to the onset of IR [33]. However, this was not the case in our experimental model as there were no changes in the HOMA-IR index following SSB consumption. This is in agreement with others who found no significant changes in the HOMA-IR index in response to fructose intake [34]. Further studies are required to determine whether there are any alterations in terms of pancreatic β–cell function and insulin secretion in our model. Together these data show that relatively lower SSB intake does not trigger major effects on body weight and insulin sensitivity, and that the animals adapted quite well to such a chronic stimulus.
There were no significant differences for any of the functional parameters evaluated by echocardiography nor for the ex vivo ischemia-reperfusion experiments. The AR was the only parameter that was altered following ischemia-reperfusion (at both the three and six month time points) and we suggest that stressed SSB-treated rats display early signs of increased vulnerability to cardiac damage.
4.2. NOGP activation following SSB intake
High glucose availability can trigger downstream cardiac NOGP activation resulting in metabolic derangements, contributing to the onset of cardio-metabolic complications [14, 15, 16]. There was an upregulation of some the NOGPs (polyol and HBP) after three months and this is consistent with higher glucose availability as measured by elevated circulating HbA1c levels. Of note, we previously found that acute high glucose exposure (25 mmol/L) triggered marked upregulation of these pathways in cultured rat cardiomyoblasts and inhibitory studies also linked NOGP activation to IR [15]. Similar mechanisms were triggered in rat hearts subjected to high glucose (33 mmol/L vs. 11 mmol/L baseline controls) during ex vivo ischemia-reperfusion, with NOGP activation leading to downstream contractile dysfunction, greater infarct size and myocardial cell death [16]. However, as we found limited detrimental effects of SSB consumption on metabolic and cardiac function, it appears as if such pathway activation was not harmful in this particular context.
The degree of hyperglycemia (high experimental levels employed in the studies mentioned above) may be an important factor linking coordinate NOGP activity to detrimental functional effects - even acutely. Although our model shows increased availability of blood glucose over time (elevated HbA1c), the acute postprandial excursions still remain controlled in the SSB groups. We therefore propose that this may limit the translation of NOGP activation into detrimental downstream effects at the time points here employed. In fact, after six months there is some adaptation in response to continued SSB intake as displayed by lowered NOGP activation. However, the O-GlcNAc levels still remained elevated versus controls after six months (∼2.4-fold; p = 0.09). As fructose is a shared link between the polyol pathway and the HBP, increased D-Sorbitol levels (and by extension, higher endogenous fructose production) at least partially contributed to higher HBP activation after three months. However, normalization of D-Sorbitol levels after six months did not fully translate into fully lowered O-GlcNAc levels, suggesting that additional substrates may still feed into the HBP.
We propose that SSB-derived fructose could possibly contribute to the higher O-GlcNAc levels here observed. Rat cardiomyocytes express the fructose-specific transporter GLUT5 that allows for the in vitro uptake and utilization of fructose [35, 36]. Such fructose could provide F-6-P as an HBP substrate in addition to that provided by glycolysis and the polyol pathway. We also observed lower glycogen stores and that glucose oxidation was unaffected by SSB consumption after six months. As fructose downregulates cardiomyocyte glucose uptake via inhibition of insulin signaling [37, 38, 39], it is possible that its utilization may become more prominent (versus glucose) n hearts of SSB-treated rats at the six month time point.
These data therefore shows that the HBP is a relatively early and universal metabolic target that is triggered in response to SSB, but may be resistant to the adaptive response observed and hence remain elevated over a longer period. This is in accordance with a previous study where polyol and PKC markers were upregulated in hearts of pre-diabetic Otsuka Long Evans Diabetic Fatty rats, but reduced in overt diabetic counterparts [14]. However, the HBP remained elevated in both the pre-diabetic and diabetic groups. We concluded that NOGP activation (except the HBP) may occur in a “biphasic pattern” that are dependent on the severity of glycemia and IR, but that the HBP emerges as an important mediator that can exert detrimental effects under such conditions. For example, we have shown previously that increased O-GlcNAcylation (post-translational modification in response to HBP activity) of the apoptotic protein Bad leads to apoptosis in the hearts of high fat diet-fed IR rats [40]. Although the functional consequences appear minimal at both time points investigated, we postulate that SSB consumption in the long term (>six months) may translate into HBP-mediated cardio-metabolic complications, specifically if such consumption raises acute glycemic excursions and/or contributes to more pronounced glucose metabolic dysregulation.
4.3. Oxidative stress
Several studies indicated that increased oxidative stress plays a crucial role as the underlying mechanism leading to metabolic derangement/disease with high fructose intake [41, 42, 43, 44]. For example, rats placed on an eight-week high fructose diet displayed mitochondrial damage (lipid and protein components) and hepatic IR [45]. In parallel, they found decreased anti-oxidant defenses when examining superoxide dismutase levels.
For the current study, we surveyed induced cardiac oxidative damage following SSB consumption (NADPH oxidase, CDs). NADPH oxidase activity was decreased in the SSB groups after six months. The redox status of glutathione seemed to have improved after 6 months in the SSB group, although not significantly. When considering the level of oxidative lipid damage, decreased CD levels were shown at 3 months in the SSB group, but were increased after 6 months consumption of the sugar-sweetened beverage. Our findings are then in line with other studies also showing enhanced oxidative lipid damage in fructose-fed rats when examining long-term induced-oxidative stress (TBARS assay) [45, 46]. These studies employed relatively high amounts of pure fructose (30% of the total diet), while others found increased TBARS in rats receiving 10% fructose [47].
Uric acid can also stimulate ROS production through its action on NOX and can cause an increase in superoxide levels [48] and thus will further enhance oxidative stress, leading to downstream effects such as lipid peroxidation [49]. However, not all these pathways are activated in our model. Here uric acid levels were increased while NOX activity was downregulated after six months. These data are in agreement with other studies where fructose intake leads to increased uric acid formation [42, 50, 51].
We are unclear how and why decreased NOX activity occurs. It appears as if there are some early signs of oxidative stress after six months but that there is an adequate response to counter this. A possible explanation for these results and the overall relatively limited induced-oxidative stress in our model may be the compensatory rise in GSH:GSSG to increase overall, the redox balance. Here GSSG competes with NOX for substrate utilization, ultimately resulting in increased GSH anti-oxidative capacity.
4.4. Impact on mitochondrial respiration (FA β-oxidation)
Our data show that SSB consumption triggered a relatively early event, i.e. a degree of impairment in mitochondrial FA oxidation after six months, with the potential for longer-term consequences on heart function. As both in vivo and ex vivo heart functional analyses showed no significant changes, the alterations in mitochondrial FA metabolism do not seem to result in any functional effects yet. However, we propose that with sustained SSB consumption it is likely that cardiac energy production may begin to decline and eventually impact on the heart's function.
What are the underlying mechanisms in this instance? It is our opinion that this may occur at any of three steps (individually or in combination): a) decreased myocardial FA uptake, b) an impairment within the FA β-oxidation pathway, and c) dysregulation within the mitochondrial ETC. Our data show that myocardial triglyceride stores remained unaffected by SSB consumption thus ruling out increased or decreased breakdown. Alternatively, the SSBs may exert direct effects on the myocardial ETC system, e.g. ROS-induced damage can lead to peroxidation that has been linked with mitochondrial dysfunction in various tissues related to several pathophysiological conditions [52]. Increased HBP flux can also lead to enhanced O-GlcNAcylation of respiratory chain complex proteins and result in impaired mitochondrial respiratory function [53]. However, further studies are required to assess the impact of oxidative stress- and HBP-induced perturbations in our experimental model.
Our findings also show a significant decrease in myocardial glycogen following six months of SSB intake. What are the reasons and mechanisms responsible for this? As intracellular glycogen levels depend on the balance between its breakdown and synthesis, we tested whether glycogen synthase is implicated in this process. However, we found no significant changes. Thus the more likely explanation may be that increased SSB intake affects upstream regulators of glycogen breakdown with the aim to sustain energetic reserves due to lowered mitochondrial FA β-oxidation capacity. For example, studies demonstrated that fructose can increase flux through glycogen synthase to stimulate glycogen synthesis [54] and that this may be due to the activation of glucokinase [54, 55]. Glucokinase could therefore be affected in the SSB group and may account for the lowered glycogen stores observed.
4.5. Limitations
For the current study, we did not evaluate insulin levels during the OGTT experiments. Such information could have provided additional mechanistic insights together with various markers (e.g. PI3-kinase, Akt) of the insulin signaling pathway. Moreover, an exact breakdown of the sugars contained in the SSB here investigated could have provided additional insights regarding the findings generated.
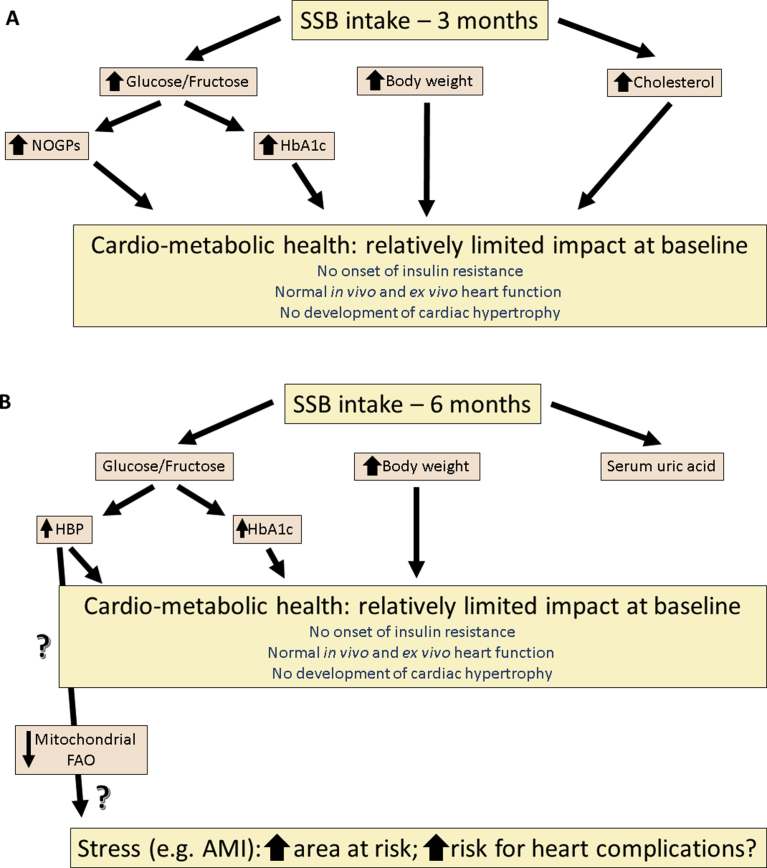
In conclusion, the current study found that SSB intake for a period of three and six months respectively, did not result in cardiac dysfunction or IR/T2DM (Fig. 8). However, despite such a lack of changes, early alterations at the molecular level may place such organisms at increased risk in the long term especially under stressful conditions (e.g. AMI).
Fig. 8.
Summary of findings of SSB-mediated effects. A) three months and B) six months. SSB- sugar-sweetened beverage, AMI- acute myocardial infarction, NOGP- Non-oxidative glucose pathways, HBP- Hexosamine biosynthetic pathway.
Declarations
Author contribution statement
Natasha Driescher: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Danzil E. Joseph: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Veronique R. Human, Edward Ojuka, Martin Cour, Nkanyiso Hadebe: Performed the experiments; Analyzed and interpreted the data.
Dirk Bester, Jeanine L. Marnewick, Sandrine Lecour, Amanda Lochner: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
M. Faadiel Essop: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This study was supported by grants from the South African National Research Foundation (IFR150119112480) and Stellenbosch University to M. F. Essop. Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors wish to thank Sonja Genade for her excellent assistance with the perfusion studies.
References
- 1.Yerlikaya A., Dagel T., King C., Kuwabara M., Lanaspa M.A., Andres-Hernando A., Covic A., Manitius J., Sag A.A., Kanbay M. Dietary and commercialized fructose: sweet or sour? Int. Urol. Nephrol. 2017;49:1611–1620. doi: 10.1007/s11255-017-1544-8. [DOI] [PubMed] [Google Scholar]
- 2.Bradshaw D., Norman R., Pieterse D., Levitt N.S. South African comparative risk assessment collaborating group, estimating the burden of disease attributable to diabetes in South Africa in 2000. South Afr. Med. J. 2007;97:700–706. http://www.ncbi.nlm.nih.gov/pubmed/17952227 [PubMed] [Google Scholar]
- 3.Guariguata L., Whiting D.R., Hambleton I., Beagley J., Linnenkamp U., Shaw J.E. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 2014;103:137–149. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Wild S., Roglic G., Green A., Sicree R. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 5.Lustig R.H., Mulligan K., Noworolski S.M., Tai V.W., Wen M.J., Erkin-Cakmak A., Gugliucci A., Schwarz J.-M. Isocaloric fructose restriction and metabolic improvement in children with obesity and metabolic syndrome. Obesity. 2016;24:453–460. doi: 10.1002/oby.21371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Ruyter J.C., Olthof M.R., Seidell J.C., Katan M.B. A trial of sugar-free or sugar-sweetened beverages and body weight in children. N. Engl. J. Med. 2012;367:1397–1406. doi: 10.1056/NEJMoa1203034. [DOI] [PubMed] [Google Scholar]
- 7.Ebbeling C.B., Feldman H.A., Chomitz V.R., Antonelli T.A., Gortmaker S.L., Osganian S.K., Ludwig D.S. A randomized trial of sugar-sweetened beverages and adolescent body weight. N. Engl. J. Med. 2012;367:1407–1416. doi: 10.1056/NEJMoa1203388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malik V.S., Popkin B.M., Bray G.A., Després J.-P., Willett W.C., Hu F.B. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care. 2010;33:2477–2483. doi: 10.2337/dc10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aragno M., Mastrocola R., Aragno M., Mastrocola R. Dietary sugars and endogenous formation of advanced glycation endproducts: emerging mechanisms of disease. Nutrients. 2017;9:385. doi: 10.3390/nu9040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ludwig D.S. The glycemic index. JAMA. 2002;287:2414–2423. doi: 10.1001/jama.287.18.2414. [DOI] [PubMed] [Google Scholar]
- 11.Anton S.D., Martin C.K., Han H., Coulon S., Cefalu W.T., Geiselman P., Williamson D.A. Effects of stevia, aspartame, and sucrose on food intake, satiety, and postprandial glucose and insulin levels. Appetite. 2010;55:37–43. doi: 10.1016/j.appet.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fagherazzi G., Vilier A., Saes Sartorelli D., Lajous M., Balkau B., Clavel-Chapelon F. Consumption of artificially and sugar-sweetened beverages and incident type 2 diabetes in the Etude Epidémiologique auprès des femmes de la Mutuelle Générale de l'Education Nationale–European Prospective Investigation into Cancer and Nutrition cohort. Am. J. Clin. Nutr. 2013;97:517–523. doi: 10.3945/ajcn.112.050997. [DOI] [PubMed] [Google Scholar]
- 13.Swarbrick M.M., Stanhope K.L., Elliott S.S., Graham J.L., Krauss R.M., Christiansen M.P., Griffen S.C., Keim N.L., Havel P.J. Consumption of fructose-sweetened beverages for 10 weeks increases postprandial triacylglycerol and apolipoprotein-B concentrations in overweight and obese women. Br. J. Nutr. 2008;100:947. doi: 10.1017/S0007114508968252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joseph D., Essop M.F. The effects of thiamine treatment on pre-diabetic versus overt diabetic rat hearts: role of non-oxidative glucose pathways. Int. J. Cardiol. 2014;176:1371–1373. doi: 10.1016/j.ijcard.2014.07.273. [DOI] [PubMed] [Google Scholar]
- 15.Joseph D., Kimar C., Symington B., Milne R., Essop M.F. The detrimental effects of acute hyperglycemia on myocardial glucose uptake. Life Sci. 2014;105:31–42. doi: 10.1016/j.lfs.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Mapanga R.F., Joseph D., Symington B., Garson K.-L., Kimar C., Kelly-Laubscher R., Essop M.F. Detrimental effects of acute hyperglycaemia on the rat heart. Acta Physiol. (Oxf). 2014;210:546–564. doi: 10.1111/apha.12184. [DOI] [PubMed] [Google Scholar]
- 17.Mapanga R.F., Essop M.F. Damaging effects of hyperglycemia on cardiovascular function: spotlight on glucose metabolic pathways. Am. J. Physiol. Heart Circ. Physiol. 2016;310:H153–H173. doi: 10.1152/ajpheart.00206.2015. [DOI] [PubMed] [Google Scholar]
- 18.Reagan-Shaw S., Nihal M., Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 19.Little R.R., Sacks D.B. HbA1c: how do we measure it and what does it mean? Curr. Opin. Endocrinol. Diabetes Obes. 2009;16:113–118. doi: 10.1097/MED.0b013e328327728d. [DOI] [PubMed] [Google Scholar]
- 20.Antunes L.C., Elkfury J.L., Jornada M.N., Foletto K.C., Bertoluci M.C., Antunes L.C., Elkfury J.L., Jornada M.N., Foletto K.C., Bertoluci M.C. Validation of HOMA-IR in a model of insulin-resistance induced by a high-fat diet in Wistar rats. Arch. Endocrinol. Metab. 2016;60:138–142. doi: 10.1590/2359-3997000000169. [DOI] [PubMed] [Google Scholar]
- 21.Ferko M., Kancirová I., Jašová M., Čarnická S., Muráriková M., Waczulíková I., Sumbalová Z., Kucharská J., Uličná O., Ravingerová T., Ziegelhöffer A. Remote ischemic preconditioning of the heart: protective responses in functional and biophysical properties of cardiac mitochondria. Physiol. Res. 2014;63:469–478. doi: 10.33549/physiolres.932933. www.biomed.cas.cz/physiolres [DOI] [PubMed] [Google Scholar]
- 22.Nduhirabandi F., Du Toit E.F., Blackhurst D., Marais D., Lochner A. Chronic melatonin consumption prevents obesity-related metabolic abnormalities and protects the heart against myocardial ischemia and reperfusion injury in a prediabetic model of diet-induced obesity. J. Pineal Res. 2011;50:171–182. doi: 10.1111/j.1600-079X.2010.00826.x. [DOI] [PubMed] [Google Scholar]
- 23.Mamikutty N., Thent Z.C., Sapri S.R., Sahruddin N.N., Mohd Yusof M.R., Haji Suhaimi F. The establishment of metabolic syndrome model by induction of fructose drinking water in male Wistar rats. BioMed Res. Int. 2014;2014:263897. doi: 10.1155/2014/263897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bocarsly M.E., Powell E.S., Avena N.M., Hoebel B.G. High-fructose corn syrup causes characteristics of obesity in rats: increased body weight, body fat and triglyceride levels. Pharmacol. Biochem. Behav. 2010;97:101–106. doi: 10.1016/j.pbb.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qi Q., Chu A.Y., Kang J.H., Jensen M.K., Curhan G.C., Pasquale L.R., Ridker P.M., Hunter D.J., Willett W.C., Rimm E.B., Chasman D.I., Hu F.B., Qi L. Sugar-sweetened beverages and genetic risk of obesity. N. Engl. J. Med. 2012;367:1387–1396. doi: 10.1056/NEJMoa1203039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Figlewicz D.P., Ioannou G., Bennett Jay J., Kittleson S., Savard C., Roth C.L. Effect of moderate intake of sweeteners on metabolic health in the rat. Physiol. Behav. 2009;98:618–624. doi: 10.1016/j.physbeh.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheludiakova A., Rooney K., Boakes R.A. Metabolic and behavioural effects of sucrose and fructose/glucose drinks in the rat. Eur. J. Nutr. 2012;51:445–454. doi: 10.1007/s00394-011-0228-x. [DOI] [PubMed] [Google Scholar]
- 28.Díaz-Aguila Y., Castelán F., Cuevas E., Zambrano E., Martínez-Gómez M., Muñoz A., Rodríguez-Antolín J., Nicolás-Toledo L. Consumption of sucrose from infancy increases the visceral fat accumulation, concentration of triglycerides, insulin and leptin, and generates abnormalities in the adrenal gland. Anat. Sci. Int. 2016;91:151–162. doi: 10.1007/s12565-015-0279-9. [DOI] [PubMed] [Google Scholar]
- 29.Lozano I., Van der Werf R., Bietiger W., Seyfritz E., Peronet C., Pinget M., Jeandidier N., Maillard E., Marchioni E., Sigrist S., Dal S. High-fructose and high-fat diet-induced disorders in rats: impact on diabetes risk, hepatic and vascular complications. Nutr. Metab. (Lond). 2016;13:15. doi: 10.1186/s12986-016-0074-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaby A.R. Adverse effects of dietary fructose. Altern. Med. Rev. 2005;10:294–306. http://archive.foundationalmedicinereview.com/publications/10/4/294.pdf [PubMed] [Google Scholar]
- 31.Rebollo A., Roglans N., Baena M., Sánchez R.M., Merlos M., Alegret M., Laguna J.C. Liquid fructose downregulates Sirt1 expression and activity and impairs the oxidation of fatty acids in rat and human liver cells. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2014;1841:514–524. doi: 10.1016/j.bbalip.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Sakurai M., Nakamura K., Miura K., Takamura T., Yoshita K., Nagasawa S.Y., Morikawa Y., Ishizaki M., Kido T., Naruse Y., Suwazono Y., Sasaki S., Nakagawa H. Sugar-sweetened beverage and diet soda consumption and the 7-year risk for type 2 diabetes mellitus in middle-aged Japanese men. Eur. J. Nutr. 2014;53:251–258. doi: 10.1007/s00394-013-0523-9. [DOI] [PubMed] [Google Scholar]
- 33.Popkin B.M. Sugary beverages represent a threat to global health. Trends Endocrinol. Metabol. 2012;23:591–593. doi: 10.1016/j.tem.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Abdelmalek M.F., Suzuki A., Guy C., Unalp-Arida A., Colvin R., Johnson R.J., Diehl A.M. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51:1961–1971. doi: 10.1002/hep.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mellor K.M., Bell J.R., Wendt I.R., Davidoff A.J., Ritchie R.H., Delbridge L.M.D. Fructose modulates cardiomyocyte excitation-contraction coupling and Ca2+ handling in vitro. PLoS One. 2011;6:e25204. doi: 10.1371/journal.pone.0025204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mirtschink P., Krishnan J., Grimm F., Sarre A., Hörl M., Kayikci M., Fankhauser N., Christinat Y., Cortijo C., Feehan O., Vukolic A., Sossalla S., Stehr S.N., Ule J., Zamboni N., Pedrazzini T., Krek W. HIF-driven SF3B1 induces KHK-C to enforce fructolysis and heart disease. Nature. 2015;522:444–449. doi: 10.1038/nature14508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mellor K.M., Bell J.R., Young M.J., Ritchie R.H., Delbridge L.M.D. Myocardial autophagy activation and suppressed survival signaling is associated with insulin resistance in fructose-fed mice. J. Mol. Cell. Cardiol. 2011;50:1035–1043. doi: 10.1016/j.yjmcc.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Cheng S.-M., Cheng Y.-J., Wu L.-Y., Kuo C.-H., Lee Y.-S., Wu M.-C., Huang C.-Y., Ting H., Lee S.-D. Activated apoptotic and anti-survival effects on rat hearts with fructose induced metabolic syndrome. Cell Biochem. Funct. 2014;32:133–141. doi: 10.1002/cbf.2982. [DOI] [PubMed] [Google Scholar]
- 39.Perret P., Slimani L., Briat A., Villemain D., Halimi S., Demongeot J., Fagret D., Ghezzi C. Assessment of insulin resistance in fructose-fed rats with 125I-6-deoxy-6-iodo-D-glucose, a new tracer of glucose transport. Eur. J. Nucl. Med. Mol. Imaging. 2007;34:734–744. doi: 10.1007/s00259-006-0267-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rajamani U., Joseph D., Roux S., Essop M.F. The hexosamine biosynthetic pathway can mediate myocardial apoptosis in a rat model of diet-induced insulin resistance. Acta Physiol. 2011;202:151–157. doi: 10.1111/j.1748-1716.2011.02275.x. [DOI] [PubMed] [Google Scholar]
- 41.Bagul P.K., Middela H., Matapally S., Padiya R., Bastia T., Madhusudana K., Reddy B.R., Chakravarty S., Banerjee S.K. Attenuation of insulin resistance, metabolic syndrome and hepatic oxidative stress by resveratrol in fructose-fed rats. Pharmacol. Res. 2012;66:260–268. doi: 10.1016/j.phrs.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 42.Lanaspa M.A., Sanchez-Lozada L.G., Choi Y.-J., Cicerchi C., Kanbay M., Roncal-Jimenez C.A., Ishimoto T., Li N., Marek G., Duranay M., Schreiner G., Rodriguez-Iturbe B., Nakagawa T., Kang D.-H., Sautin Y.Y., Johnson R.J. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress. J. Biol. Chem. 2012;287:40732–40744. doi: 10.1074/jbc.M112.399899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castro M.C., Massa M.L., Schinella G., Gagliardino J.J., Francini F. Lipoic acid prevents liver metabolic changes induced by administration of a fructose-rich diet. Biochim. Biophys. Acta Gen. Subj. 2013;1830:2226–2232. doi: 10.1016/j.bbagen.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 44.Castro M.C., Francini F., Gagliardino J.J., Massa M.L. Lipoic acid prevents fructose-induced changes in liver carbohydrate metabolism: role of oxidative stress. Biochim. Biophys. Acta Gen. Subj. 2014;1840:1145–1151. doi: 10.1016/j.bbagen.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 45.Crescenzo R., Bianco F., Falcone I., Coppola P., Liverini G., Iossa S. Increased hepatic de novo lipogenesis and mitochondrial efficiency in a model of obesity induced by diets rich in fructose. Eur. J. Nutr. 2013;52:537–545. doi: 10.1007/s00394-012-0356-y. [DOI] [PubMed] [Google Scholar]
- 46.Cioffi F., Senese R., Lasala P., Ziello A., Mazzoli A., Crescenzo R., Liverini G., Lanni A., Goglia F., Iossa S. Fructose-Rich diet affects mitochondrial DNA damage and repair in rats. Nutrients. 2017;9:323. doi: 10.3390/nu9040323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sangüesa G., Roglans N., Montañés J.C., Baena M., Velázquez A.M., Sánchez R.M., Alegret M., Laguna J.C. Chronic liquid fructose, but not glucose, supplementation selectively induces visceral adipose tissue leptin resistance and hypertrophy in female sprague-dawley rats. Mol. Nutr. Food Res. 2018;62:1800777. doi: 10.1002/mnfr.201800777. [DOI] [PubMed] [Google Scholar]
- 48.Glantzounis G.K., Tsimoyiannis E.C., Kappas A.M., Galaris D.A. Uric acid and oxidative stress. Curr. Pharmaceut. Des. 2005;11:4145–4151. doi: 10.2174/138161205774913255. http://www.ncbi.nlm.nih.gov/pubmed/16375736 [DOI] [PubMed] [Google Scholar]
- 49.Ejaz A.A., Mu W., Kang D.-H., Roncal C., Sautin Y.Y., Henderson G., Tabah-Fisch I., Keller B., Beaver T.M., Nakagawa T., Johnson R.J. Could uric acid have a role in acute renal failure? Clin. J. Am. Soc. Nephrol. 2007;2:16–21. doi: 10.2215/CJN.00350106. [DOI] [PubMed] [Google Scholar]
- 50.Johnson R.J., Perez-Pozo S.E., Sautin Y.Y., Manitius J., Sanchez-Lozada L.G., Feig D.I., Shafiu M., Segal M., Glassock R.J., Shimada M., Roncal C., Nakagawa T. Hypothesis: could excessive fructose intake and uric acid cause type 2 diabetes? Endocr. Rev. 2009;30:96–116. doi: 10.1210/er.2008-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chandramohan R., Pari L. Protective effect of umbelliferone on high-fructose diet-induced insulin resistance and oxidative stress in rats. Biomed. Aging Pathol. 2014;4:23–28. [Google Scholar]
- 52.Paradies G., Petrosillo G., Pistolese M., Ruggiero F.M. Reactive oxygen species affect mitochondrial electron transport complex I activity through oxidative cardiolipin damage. Gene. 2002;286:135–141. doi: 10.1016/s0378-1119(01)00814-9. [DOI] [PubMed] [Google Scholar]
- 53.Hu Y., Suarez J., Fricovsky E., Wang H., Scott B.T., Trauger S.A., Han W., Hu Y., Oyeleye M.O., Dillmann W.H. Increased enzymatic O-GlcNAcylation of mitochondrial proteins impairs mitochondrial function in cardiac myocytes exposed to high glucose. J. Biol. Chem. 2009;284:547–555. doi: 10.1074/jbc.M808518200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petersen K.F., Laurent D., Yu C., Cline G.W., Shulman G.I. Stimulating effects of low-dose fructose on insulin-stimulated hepatic glycogen synthesis in humans. Diabetes. 2001;50:1263–1268. doi: 10.2337/diabetes.50.6.1263. [DOI] [PubMed] [Google Scholar]
- 55.Shiota M., Postic C., Fujimoto Y., Jetton T.L., Dixon K., Pan D., Grimsby J., Grippo J.F., Magnuson M.A., Cherrington A.D. Glucokinase gene locus transgenic mice are resistant to the development of obesity-induced type 2 diabetes. Diabetes. 2001;50:622–629. doi: 10.2337/diabetes.50.3.622. [DOI] [PubMed] [Google Scholar]