Abstract
Objective
To identify all publications from the ‘Treatment for Adolescents With Depression Study (TADS)’ and assess the findings regarding occurrence of any adverse effects in the treatment groups both for the short-term and long-term study stages.
Design
Descriptive analysis of TADS publications with any information on adverse effects.
Results
We identified 48 publications describing various aspects of the TADS, in which 439 adolescent patients received treatment with fluoxetine, cognitive–behavioural therapy, cognitive–behavioural therapy plus fluoxetine or placebo. Eight publications were assessed as providing some data on adverse effects. Risk of suicidal behaviour was the only adverse effect that was addressed in all publications. Several psychiatric and physical adverse effects were reported during the first 12 weeks, but not mentioned in reports from later study stages. Common adverse effects of fluoxetine, such as weight changes or sexual problems, were not identified or mentioned in the publications.
Conclusions
The TADS publications do not present a comprehensive assessment of treatment risk with fluoxetine in adolescents, especially for more than 12 weeks of treatment. Risk of suicidality was the only adverse effect that was reported over time. Reporting of adverse effects was incomplete with regard to the long-term safety profile of fluoxetine.
Keywords: clinical pharmacology, adverse events, clinical trials
Strengths and limitations of this study.
This is the first systematic assessment of adverse effects reporting in publications from the Treatment for Adolescents With Depression Study (TADS).
The analysis encompasses all adverse events mentioned in publications from the TADS.
An extensive literature search was conducted and we believe that all relevant studies have been identified.
We cannot exclude the possibility that some publications may have been overlooked.
Introduction
The safety profile of selective serotonin reuptake inhibitors (SSRIs) in adolescents has been extensively debated. Several systematic reviews have analysed what is known about the risk of suicidal behaviour1–3 as well as other psychiatric and somatic adverse risks and the perceived benefit/risk balance. The reviews have highlighted considerable variations in assessment, definitions and reporting of adverse effects in the clinical trials.
The Norwegian Regional Medicines Information and Pharmacovigilance Centres and the Center for Psychopharmacology at Diakonhjemmet Hospital regularly receive queries from hospital doctors and general practitioners regarding the safety of fluoxetine (FLX) and other SSRIs in adolescent patients.
One of the major clinical studies of efficacy and safety of FLX in adolescents is the ‘Treatment for Adolescents With Depression Study (TADS)’, which is often referred to in textbooks and reviews.
In 1998, the US National Institute of Mental Health (NIMH) issued a request for proposals (RFP-NIH-NIMH 98-DS-0008) with the objective of launching a clinical trial to address the effectiveness of treatment for adolescents with major depression.4 The subsequent study, ‘TADS’ was coordinated by the Department of Psychiatry and Behavioral Sciences and the Duke Clinical Research Institute, both at Duke University Medical Center, collaborating with and funded by NIMH,5 and carried out in the period 2000–2003.6 The study included 439 youths who were randomised to one of four treatment groups; (1) FLX, (2) cognitive–behavioural therapy (CBT), (3) cognitive–behavioural therapy plus fluoxetine (COMB) or (4) placebo (PBO) for 12 weeks (stage I).6 Double-blind treatment was performed among patients treated with FLX and PBO only, while patients treated with CBT with or without FLX received open treatment. Stage II and III were maintenance phases for the active treatment groups, with the option of intensifying treatment for partial responders. Patients in the PBO group were offered open active treatment of FLX, CBT or both. Stage IV consisted of an additional year of open follow-up.5
The two primary outcome measures in the TADS were Children’s Depression Rating Scale-Revised (CDRS-R) total scores, and responder status on the Clinical Global Impressions-Improvement scale. According to protocol, all analyses would be performed by intention to treat (ITT), regardless of later events.
Adverse events during the acute and maintenance phases were defined as secondary outcomes.7 Patients were monitored for safety regarding affective disorders, need for mental health treatment, need for concomitant medications, occurrence of adverse events and serious adverse events and use of adjunctive services and attrition prevention. Most assessments were based on both patient and parent information.8
The TADS has been described as the largest and arguably the highest quality acute-phase randomised PBO controlled trial of an antidepressant drug for adolescent depression.9 We understand from the protocol and monitoring procedures that the TADS team intended to evaluate the tolerability of treatment, and that the study was expected to provide improved insight into the potential adverse effects of antidepressant treatment in this age group, due to its study size and duration. Several publications from the TADS have addressed risks of adverse effects. Despite this, concerns have been raised regarding under-reporting of suicidal risk,10 study size and an increased risk of psychiatric adverse effects.11
In the TADS, adverse events were defined as an unfavourable medical change that occurred after beginning or during the study that might or might not be related to or caused by the study drug or CBT treatment. This was further specified as any medical event that caused clinically significant interference with functioning (eg, headache that caused school absence or otherwise caused clinically significant activity restriction), any event that required medical attention, and any medical event associated with impairment in functioning and induced the patient to take a concomitant medication. Conditions that did not lead to clinically significant interference with functioning or did not require medical attention were not defined as adverse events.7 8 The protocol specified that new-onset psychiatric symptoms, such as emerging mania or panic attacks, would be recorded if they caused clinically significant interference with functioning.8 It follows that such conditions would not be recorded unless a certain severity threshold was reached.
Harm-related adverse events were defined as involving harm to self, which could include a non-suicidal event. Examples given are cutting, worsening of suicidal ideation, suicide attempt or harm to others. Suicide-related adverse events were defined as worsening suicidal ideation and/or suicide attempt. Adverse event forms were to be used throughout the study and it must be assumed that such data were collected, as well as clinical scoring data for possible psychiatric adverse events.
Our objective in the present study was to identify all publications from the TADS and assess the findings regarding occurrence of any adverse effects in the treatment groups both for the short-term and long-term study stages. The TADS was chosen because of the non-industrial funding and because it is considered as a high-quality study.9
Methods
Literature search
Publications from the TADS were identified through searches in PubMed, EMBASE, PsycINFO, Google Scholar, ClinicalTrials.gov, NIMH website nimh.nih.gov, the Duke Clinical Research Institute TADS website (http://tads.dcri.org), by hand searching of references in identified publications, and by searching other publications by the main authors (snowballing). Search terms in Google Scholar were either «TADS team» or «Treatment for adolescents with depression study». Search term in PsycINFO was «Treatment for adolescents with depression study». Search term in PubMed was the phrase Treatment for adolescents with depression study. The initial publications with data from the TADS study were identified and used to search for similar publications, limited to 2004 to 1 September 2017, Clinical Trial or Randomized Controlled Trial and age group Child 0–18. Search term in Embase was «Treatment for adolescents with depression study». The final main search in all databases was conducted on 5 September 2017. An additional literature search in PubMed for any recent TADS publications was conducted in February 2018 and updated in January 2019.
Inclusion and exclusion criteria
Identified TADS publications were assessed and classified according to publication topic and reported outcomes. Inclusion criteria: All publications that reported on results from the TADS and provided some information on adverse effects. Publications on efficacy or non-primary or non-secondary outcomes were excluded if they gave no information on adverse events.
Data assessment
Adverse effects were defined as psychiatric or somatic diagnoses or complaints arising during treatment, as described in the publications. In addition, we have included worsening of depression as an adverse effect if described in the publications. Publications describing any adverse events during treatment were analysed in detail regarding the types and frequency estimates of adverse events. Two researchers (TW and SN) evaluated each publication independently. All researchers (TW, SN and MK) discussed any ambiguity and the data extraction tables.
Patient and public involvement
Patients or the public were not involved in this literature review.
Results
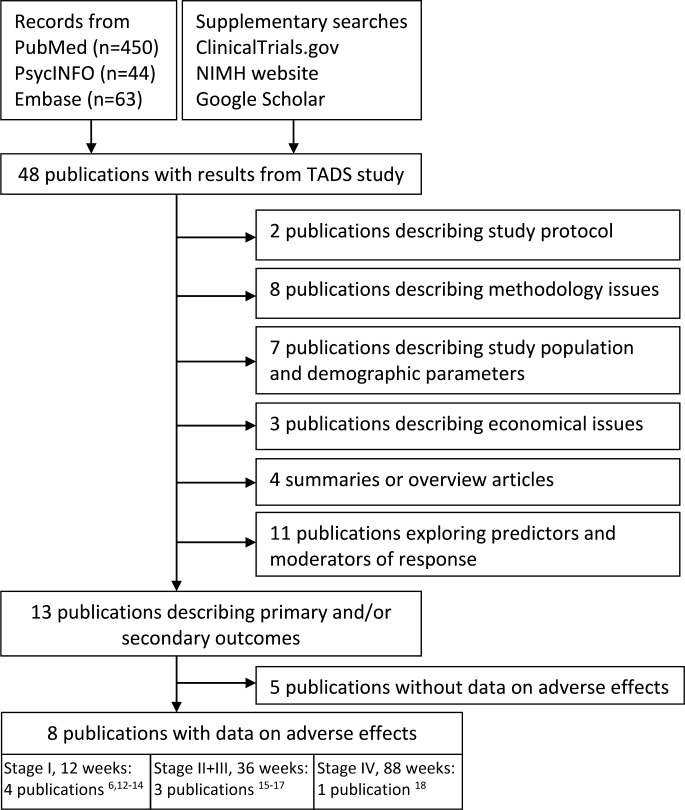
We identified 48 publications that reported on the study protocol and/or various outcomes in the TADS population. The selection process and publication characteristics are described in figure 1.
Figure 1.
Selection and characteristics for publications from the TADS. NIMH, National Institute of Mental Health; TADS, Treatment for Adolescents With Depression Study.
Eight publications were assessed as providing at least some data on adverse effects,6 12–18 of which four publications reported possible adverse effects for subgroups of patients only; patients who responded to treatment,13 patients originally assigned to PBO treatment,16 patients who had at least one suicidal event17 and patients using attrition prevention services,14 respectively. Reporting of adverse effects was most detailed in the two initial results publications from stage I (0–12 weeks),6 12 and included a wide range of adverse effects, including several psychiatric and gastrointestinal reactions. One stage I publication did not address adverse effects explicitly; however, symptoms that may be associated with adverse effects were described as residual symptoms of depression.13
The publications that reported on adverse effects in the later study stages II–IV listed few adverse effects except suicidal behaviour (table 1). The publication that purported to report on long-term effectiveness and safety outcomes only included reporting of suicide-related adverse events.15
Table 1.
Reporting of adverse events in publications from the TADS
| Reported event | Stage 1 (12 weeks) | Stage 2+3 (36 weeks) | Stage 4 (88 weeks) | |||||
| TADS team6 | Emslie et al12 | Kennard et al13* | May et al14† | TADS team15 | Kennard et al16‡ | Vitiello et al17§ | TADS team18 | |
| Harm-related adverse event | x | x | x | |||||
| Suicide-related adverse event | x | x | x¶ | x | x | x | x | x |
| Attempted suicide | x | x | x | x | x | x | ||
| Homicidality | x | x | ||||||
| Mania | x | x | x | x | ||||
| Hypomania | x | x | x | |||||
| Elevated mood | x | x | ||||||
| Trouble attention/concentration | x | x¶ | ||||||
| Racing thoughts | x | |||||||
| Excessive talking/talking very fast | x | |||||||
| Increase in activities | x | |||||||
| Impulsivity | x | |||||||
| Hypersensitivity** | x | x | ||||||
| Irritability | x | x | x¶ | x | ||||
| Anger | x | x | ||||||
| Worsening of depression | x | x | x | x | x | x | ||
| Psychomotor | x¶ | |||||||
| Guilt | x¶ | |||||||
| Mood | x¶ | |||||||
| Interest | x¶ | |||||||
| Crying | x | x | ||||||
| Agitation | x | x | ||||||
| Akathisia | x | x | ||||||
| Nervousness | x | x | ||||||
| Restlessness | x | x | ||||||
| Hyperactivity | x | x | ||||||
| Panic attacks | x | x | ||||||
| Anxiety | x | x | ||||||
| Excessive sweating | x | |||||||
| Difficulty breathing | x | |||||||
| Hearing problems | x | |||||||
| Somnolence/feeling drowsy | x | x | ||||||
| Insomnia/sleeplessness | x | x | x | |||||
| Sleep | x | x | x¶ | |||||
| Nightmare | x | x | ||||||
| Night sweats | x | |||||||
| Sedation | x | x | ||||||
| Fatigue | x | x¶ | ||||||
| Tremor | x | x | ||||||
| Behaviour/feeling abnormal | x | x | ||||||
| Social problems | x | x | ||||||
| Headache | x | x | ||||||
| Upper abdominal pain | x | x | ||||||
| Stomach pain | x | |||||||
| Diarrhoea | x | x | ||||||
| Influenza/sinusitis | x | |||||||
| Cold, sore throat, cough/wheeze | x | |||||||
| Allergies | x | |||||||
| Dry mouth | x | |||||||
| Nausea/vomiting | x | x | ||||||
| Fever | x | |||||||
| Muscle aches or cramps | x | |||||||
| Joint pain | x | |||||||
| Numbness or tingling arms or legs | x | |||||||
| Weight | x¶ | |||||||
| Chest pain | x | |||||||
| Racing/pounding heart, skip beats | x | |||||||
| Urination frequency or pain | x | |||||||
| Constipation, feeling bloated | x | |||||||
| Skin rash/hives | x | |||||||
*Reporting limited to responders subgroup, regardless of treatment arm.
†Reporting limited to subgroup of patients seeking attrition prevention.
‡Reporting limited to ITT placebo group.
§Reporting limited to patients with a suicidal event.
¶Reported as residual symptoms of depression.
**Understood as mood hypersensitivity.
ITT, intention to treat; TADS, Treatment for Adolescents With Depression Study.
Patient population and treatment modifications during the study
In the TADS, 439 patients were randomised to one of the four treatment groups. By the end of stage I (12 weeks), 351 patients remained for assessment, of them 270 patients in active treatment groups. The rest of the patients had either withdrawn their consent, or been classified as premature terminators due to need for additional treatment.6 15 It is not specified to what extent drop-outs or premature terminations were due to adverse events in the initial study population and if those adverse events were included in the reports. By week 36 (end of stage III), 178 patients remained in the group to which they had been randomised, specifically 68 for COMB, 55 for FLX and 55 for CBT.15 Patients who terminated their assigned treatment prematurely did in many cases continue their assessments and were included in the ITT analyses for their original group, although they received an active treatment other than that specified for the group they were assigned to.12 15 19 Between 34% and 46% of patients in the monotherapy groups did not remain in their assigned treatment arm by the end of stage II, and 43 of the 111 patients (38%) in the CBT group were receiving another SSRI or antidepressant by the end of stage III (36 weeks).19
Reporting of suicidality in TADS publications
Suicidality symptoms were monitored using an affective disorders screening procedure (ADS), Reynolds Adolescent Depression Scale, a revised CDRS-R, a Suicide Ideation Questionnaire-Junior (SIQ-Jr) as well as adverse event/serious adverse event forms. All the TADS publications classified as reporting adverse effects6 12–18 describe the risk of suicidal events, defined as discrete episodes of suicidal ideation, suicidal attempts or preparatory acts towards an imminent attempt. Injury to self was not included if there was no suicidal intent. Reporting of suicidal events and risk is described in the online supplementary file. Data on suicidality are presented as either counts of discrete episodes, mean scores, score changes or proportion of patients reaching threshold values on scoring tools.
bmjopen-2018-026089supp001.pdf (103.5KB, pdf)
By week 12, CDRS-R item 13 scores are reported as per cent of patients with score ≥2 for the total study population,6 per cent of patients with score worsening ≥1 point and per cent of patients with score increase from 1-2 to ≥5 for each treatment group.12 SIQ-Jr scores are reported as per cent of patients with scores ≥31 for the total study population6 and each treatment group,15 per cent of patients with score increase to ≥3112 and mean score for each treatment group.6 12
By week 36, CDRS-R scores are not described in any of the publications. For SIQ-Jr scores, results are described for patients who had completed the SIQ-Jr assessment at week 36 and for a smaller number of patients who both completed the assessment and were still in their assigned treatment group.15 Results are presented as the percentage of patients with score ≥31 for each treatment group. Patients with score increases and mean scores are not reported.
Suicidal events are presented for all three treatment groups, and reported for ITT and observed cases groups. The frequency of suicidal events was calculated using the group size according to the original randomisation, with no reference to the reduction in study group sizes.15
The publication by Vitiello et al17 analyses suicidal events in more detail. Patients with high or increased scores, but not classified as having an event, were not included in the analysis. Nine cases of suicidal behaviour were presented as occurring in the PBO group, even though the patients were using FLX at the time and the PBO period had ended. The paper reports on the number of cases, but does not include results from the suicidality scoring tools CDRS-R Item 13 and SIQ-Jr. The number of suicidal episodes was greater than it appears, as seven patients had more than one episode,17 and only the most severe episode was included in the analysis.
The long term phase IV publication18 present SIQ-Jr scores for a total of 66 patients who had at least one stage IV assessment. The paper refers to the baseline ITT groups of 327 patients (excluding PBO), but due to withdrawals any changes in scores may be biased, and reflect a selected study population rather than a treatment effect.
Reporting of psychiatric adverse effects/mania across TADS publications
The TADS group found higher rates for psychiatric adverse events in patients receiving FLX than in patients receiving CBT or PBO.6 12 The psychiatric adverse events included symptoms classified as mania spectrum, irritability/depression spectrum, agitation spectrum, anxiety or other. Of these, mania spectrum symptoms were described in greater detail in the 2006 safety publication.12 We have therefore assessed and summarised the reporting of mania spectrum symptoms across the TADS publications (table 2).
Table 2.
Reporting of mania spectrum symptoms in publications from the TADS
| Reporting parameter | Stage 1 (12 weeks) | Stage 2+3 (36 weeks) | Stage 4 (88 weeks) | |||||
| TADS team6 | Emslie et al12 | Kennard et al13 | May et al14 | TADS team15 | Kennard et al16 | Vitiello et al17 | TADS team18 | |
| ADS Mania subscale score | Baseline: All: 2.4±2.3 COMB: 2.6±2.4 FLX: 2.2±2.2 CBT: 2.5±2.4 PBO: 2.2±2.3 12 weeks: All: 0.9±1.4 COMB: 0.5±0.8 FLX: 1.1±1.0 CBT: 1.0±1.2 PBO: 1.1±0.1 |
Baseline: 2.5±2.2 | ||||||
| Prior to suicidal event: 1.6±2.2 | ||||||||
| Mean change before event: −0.6±2.3 | ||||||||
| ADS Mania subscale score increase (≥3 points) | All: 65/424 (15.3%) | |||||||
| COMB: 20% (n=21) | ||||||||
| FLX: 14.2% (n=15) | ||||||||
| CBT: 12.3% (n=13) | ||||||||
| PBO: 15.0% (n=16) | ||||||||
| Patients with attrition prevention due to mania/hypomania | 1.28% (1/78) | |||||||
| Mania | COMB: n=0 FLX: n=1 CBT: n=0 PBO: n=1 |
FLX: n=1 | ||||||
| Hypomania | COMB: n=1 FLX: n=2 CBT: n=0 PBO: n=1 |
COMB: n=1 FLX: n=2 PBO: n=1 |
||||||
| Elevated mood | COMB: n=0 FLX: n=1 CBT: n=0 PBO: n=0 |
FLX: n=1 | ||||||
ADS, affective disorders screening; CBT, cognitive behavioural therapy; COMB, cognitive behavioural therapy plus fluoxetine; FLX, fluoxetine; PBO, placebo; TADS, Treatment for Adolescents With Depression Study.
Mania spectrum symptoms (mania, hypomania and elevated mood) were monitored using an ADS procedure, as well as adverse event or serious adverse event forms. Due to the adverse event definition threshold, new cases of emerging mania were not recorded unless the symptoms caused clinically significant interference with functioning.7
Mania spectrum symptoms were mentioned in three of the four publications that reported on adverse effects in TADS during 0–12 weeks of treatment (stage I). The initial 2004 publication by the TADS group reported a total of seven patients with mania spectrum symptoms as an adverse effect; four in the FLX group, one in the COMB group, none in the CBT group and two in the PBO group.6 In the 2006 safety results publication,12 occurrences of mania spectrum symptoms were reported based on both spontaneous reports and assessment by physician using a formal symptom checklist (ADS mania items). According to this publication, six patients spontaneously reported a mania spectrum disorder; four in the FLX group, one in the COMB group and one in the PBO group. On the ADS mania scoring scale, however, 65 of 424 patients across all treatment groups reportedly had an increase of 3 points or more. The absolute score increase for each patient or treatment group is not provided. The analysis of patients with at least one suicidal event (n=44) describes mean ADS mania score prior to the suicidal event for 31 of the 44 patients during 36 weeks of treatment.17
We did not identify any publication describing mania spectrum symptoms in the entire study population that received treatment for more than 12 weeks (stages II–IV) (table 2).
The publications from stage II–IV failed to mention psychiatric adverse effects that were identified during stage I, such as restlessness, nervousness and sleep difficulties (table 1).
Other adverse effects
Adverse effects other than suicidality were summed up by the TADS team in 2004,6 reported in further detail in 200612 and mentioned in the two other publications from study stage I to a varying extent.13 14 19 According to the most extensive publication with regard to safety data at 12 weeks,12 sedation, insomnia, vomiting and upper abdominal pain occurred at least twice as often in patients receiving FLX with or without CBT than with PBO. We did not identify any publication describing non-psychiatric adverse effects in the study population that received treatment for more than 12 weeks (stages II–IV) (table 1).
Adverse effects of FLX, as acknowledged at present, are listed in table 3. The adverse effects are classified according to whether they were reported in any of the eight TADS publications or not. Several well-known adverse effects of FLX were not reported in the TADS publications, among them weight and appetite changes. Effects on sexual functioning are not mentioned in this group of young patients.
Table 3.
TADS reporting of presently acknowledged common adverse effects of fluoxetine30
| Mentioned in publications from the TADS * | Not mentioned in publications from the TADS |
| Insomnia | Decreased appetite, incl. anorexia |
| Sleep disorder | Weight decreased |
| Abnormal dreams, incl. nightmares | Tension |
| Anxiety | Libido decreased, incl. loss of libido |
| Somnolence, incl. hypersomnia, sedation | Gynaecological bleeding, incl. menstrual bleeding disorders |
| Nervousness | Erectile dysfunction |
| Restlessness | Ejaculation disorder |
| Headache | Dizziness |
| Disturbance in attention | Dysgeusia |
| Tremor | Lethargy |
| Palpitations | Vision blurred |
| Diarrhoea | ECG QT prolonged |
| Nausea | Flushing, incl. hot flushes |
| Vomiting | Yawning |
| Dry mouth | Dyspepsia |
| Rash | Chills |
| Urticaria (hives) | Feeling jittery |
| Pruritus | |
| Hyperhidrosis | |
| Arthralgia | |
| Frequent urination | |
| Fatigue |
*Not necessarily identified as an adverse effect of fluoxetine treatment.
TADS, Treatment for Adolescents With Depression Study.
Discussion
The TADS protocol included a threshold limit on what would be considered an adverse event, specifying that the event must cause clinically significant interference with functioning, require medical attention or cause a need to take medication.6 As an example, emerging mania was not recorded unless symptoms exceeded this threshold.7 It must be assumed that this reduced the number of reported adverse effects, which may not have been severe enough to reduce daily functioning or cause a need for additional treatment. We have not been able to find a published version of the questionnaires that were used and consequently do not have information as to which adverse effects were specifically asked for. The protocol does not define how the scoring parameters for adverse events should be analysed. The number of suicidal events is described, but other parameters, such as absolute or worsening scores on risk assessment scales, are not consistently reported. An example is the SIQ-Jr scores, where week 12 publications report mean scores and number of patients with score increase to ≥31,6 12 while the follow-up publication by week 36 reported per cent of patients with SIQ-Jr score ≥31.15 Scoring of mania symptoms is described as inconsistent and varying between clinicians.12 It is conceivable that some patients may have had worsening scores without passing the threshold score for suicidality or mania, respectively. Conversion into dichotomous scales, as was done for SIQ-Jr scores ≥31 and ADS Mania subscale score change increase ≥3 points, does not give insight into the magnitude in case of increased scores.
All analyses were planned as ITT, regardless of later events.7 Nine cases of suicidal behaviour were presented as occurring in the PBO group17 although the patients were using FLX at the time and the PBO period had ended. As pointed out by Högberg et al,10 the risk of suicidal behaviour will not appear to be increased for FLX compared with PBO if patients using FLX are assessed in the PBO group. ITT analyses of adverse events may be biased towards finding no differences between groups.20 This is especially relevant in studies with large drop-out rates and in study groups where patients received add-on treatment that differed from the assigned medication, as was the case in the TADS.19 Other authors have questioned whether the TADS may have under-reported adverse effects due to small numbers and patients leaving the study early.11 Use of ITT analyses will have led to underestimation of the frequency of psychiatric and other adverse events, a fact which has been little discussed.
Risk of suicidal behaviour was the only adverse effect that was addressed during all four treatment stages. Several psychiatric and physical adverse effects were reported during the first 12 weeks, but not mentioned in publications from the further treatment stages. Examples are sedation, insomnia, vomiting and upper abdominal pain, which occurred in more than 2% of patients in the first 12 weeks.12 The 2% occurrence is described as infrequent (≤5%), but should more correctly be classified as common.21 The risk of psychiatric adverse events such as mania, irritability, agitation and anxiety is given as 11% in the FLX group and 5.6% in the COMB group.12 In the review by Jane Garland et al, the occurrence of emotional/behavioural adverse effects is given as 10%–25%,3 but the numbers may not be comparable due to different inclusion criteria. Other adverse effects of SSRI treatment, such as appetite changes, weight changes and sexual problems, are not mentioned in any publication. Growth issues were not addressed. Changes in weight or appetite may have occurred without reaching the severity threshold. Sexual adverse effects may not have been relevant to many patients at the time due to their age, or may not have been forthcoming in interviews, especially as many patients were interviewed in the company of caregivers.12 Risk of sexual adverse effects was discussed in the adverse event monitoring protocol22 and procedures in case of pregnancies were established,23 so it is reasonable to assume a that certain proportion of patients were sexually active. Prolonged treatment into adulthood may well increase the relevance of such concerns.
To our knowledge, this is the first systematic assessment of adverse effects reporting in publications from the TADS. We conducted an extensive literature search and believe that all relevant studies have been identified, however, we cannot exclude the possibility that some publications may have been overlooked. Our findings regarding adverse effect reporting and potential for bias are based on analysis of only one study and do not give information on adverse effects reporting or bias in other studies of SSRIs in adolescents. However, discrepancies and weaknesses in the reporting of adverse events in such studies have also been noted by other authors.24 25 We have not had access to primary data.
A previous assessment of the adverse effects reporting in TADS focused on the occurrence of suicidal events and increased risk of suicidal behaviour10 and this is reflected in the most recent Cochrane review.1 Like Högberg et al,10 we have noted the misleading PBO group classification of patients with a suicidal event who were using FLX at the time. Our analysis encompasses all adverse events mentioned in publications from the TADS. Gaps and discrepancies in coding, transcription and reporting of harms in clinical trials have been reported, and the number of adverse events may differ between study reports and published papers.24 26 Several barriers to accurate harms reporting24 are relevant to the TADS, notably the severity threshold, conversions from continuous to dichotomous outcomes, individual judgements of association between event and medication, handling of adverse events in patients who discontinued treatment and the extensive use of concomitant medications. In future studies, the potential for bias may be substantially reduced by avoiding severity thresholds and defining a consistent method of describing adverse effects such as suicidal risk and mania score worsening. Occurrence or worsening of mania and other psychiatric adverse effects for individual patients should be reported in more detail. We would also suggest that if risk is presented as percentages, it should be calculated based on the number of patients who were receiving treatment at the time the adverse event occurred. This will be of particular importance in studies with large drop-out rates and treatment changes. The full spectrum of adverse effects should be reported for all study stages. A plan for data sharing should be in place to facilitate reanalysis and evaluation by other researchers, as practised by the BMJ.27
Due to its long duration (36 weeks) and follow-up (1 year), the TADS could have provided valuable information on the long-term occurrence of adverse effects both in frequency and severity. The adverse effects profile of FLX in the TADS has only been reported in detail for stage 1, where approximately 200 patients received FLX for 12 weeks. The raw data from the trial have been requested28 and planned for release into the public domain,29 but we have not been able to ascertain that these have been made publicly available. The incomplete reporting of adverse effects in a major study like TADS may lead to bias and erroneous conclusions regarding the safety profile of FLX when given to minors. The risk of suicidal behaviour has been the subject of many discussions and regulatory actions, but there has been considerably less focus on other clinically important adverse effects. This may have clinically important implications, since the benefit/risk estimations regarding FLX use in adolescents will be biased. If adverse effects are not acknowledged as such, there is a risk that symptoms may be misinterpreted and treated as more serious illnesses.
Supplementary Material
Footnotes
Contributors: SN suggested the research question. All authors discussed and defined the project. TW and SN researched the literature and made the initial assessments. All authors discussed the publications included in the study, including interpretation and presentation of results. TW drafted and finalised the manuscript as lead author. SN and MK commented on the draft and revised the manuscript at all stages. All authors agreed to the final version of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
Patient consent for publication: Not required.
References
- 1. Hetrick SE, McKenzie JE, Cox GR, et al. Newer generation antidepressants for depressive disorders in children and adolescents. Cochrane Database Syst Rev 2012;11:Cd004851 10.1002/14651858.CD004851.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Locher C, Koechlin H, Zion SR, et al. Efficacy and safety of selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and placebo for common psychiatric disorders among children and adolescents: a systematic review and meta-analysis. JAMA Psychiatry 2017;74:1011–20. 10.1001/jamapsychiatry.2017.2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jane Garland E, Kutcher S, Virani A, et al. Update on the use of SSRIs and SNRIs with children and adolescents in clinical practice. J Can Acad Child Adolesc Psychiatry 2016;25:4–10. [PMC free article] [PubMed] [Google Scholar]
- 4. National Institute of Mental Health. NIH Guide: Effectiveness of treatment for adolescents with major depression. 1998. https://grants.nih.gov/grants/guide/notice-files/not98-059.html (accessed 20 Mar 2017).
- 5. Treatment for Adolescents With Depression Study Team. Treatment for Adolescents With Depression Study (TADS): rationale, design, and methods. J Am Acad Child Adolesc Psychiatry 2003;42:531–42. 10.1097/01.CHI.0000046839.90931.0D [DOI] [PubMed] [Google Scholar]
- 6. March J, Silva S, Petrycki S, et al. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents With Depression Study (TADS) randomized controlled trial. JAMA 2004;292:807–20. 10.1001/jama.292.7.807 [DOI] [PubMed] [Google Scholar]
- 7. The TADS Team Duke University Medical Center. Protocol RFP-NIH-NIMH 98-DS-0008 Treatment for Adolescents with Depression Study (TADS): Duke Clinical Research Institute. 2000. tads.dcri.org (accessed 20 Mar 2017).
- 8. The TADS Team Duke University Medical Center. Adverse event monitoring and adjunctive services and attrition prevention (ASAP) in the NIMH Treatment of Adolescent Depression Study (TADS) vs 4.1: Duke Clinical Research Institute. 2005. tads.dcri.org (accessed 20 Mar 2017).
- 9. Walkup JT. Antidepressant efficacy for depression in children and adolescents: industry- and NIMH-funded studies. Am J Psychiatry 2017;174:430–7. 10.1176/appi.ajp.2017.16091059 [DOI] [PubMed] [Google Scholar]
- 10. Högberg G, Antonuccio DO, Healy D. Suicidal risk from TADS study was higher than it first appeared. Int J Risk Saf Med 2015;27:85–91. 10.3233/JRS-150645 [DOI] [PubMed] [Google Scholar]
- 11. Jureidini J, Tonkin A, Mansfield PR. TADS study raises concerns. BMJ 2004;329:1343–4. 10.1136/bmj.329.7478.1343-d [DOI] [Google Scholar]
- 12. Emslie G, Kratochvil C, Vitiello B, et al. Treatment for Adolescents with Depression Study (TADS): safety results. J Am Acad Child Adolesc Psychiatry 2006;45:1440–55. 10.1097/01.chi.0000240840.63737.1d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kennard B, Silva S, Vitiello B, et al. Remission and residual symptoms after short-term treatment in the Treatment of Adolescents with Depression Study (TADS). J Am Acad Child Adolesc Psychiatry 2006;45:1404–11. 10.1097/01.chi.0000242228.75516.21 [DOI] [PubMed] [Google Scholar]
- 14. May DE, Kratochvil CJ, Puumala SE, et al. A manual-based intervention to address clinical crises and retain patients in the Treatment of Adolescents With Depression Study (TADS). J Am Acad Child Adolesc Psychiatry 2007;46:573–81. 10.1097/chi.0b013e3180323342 [DOI] [PubMed] [Google Scholar]
- 15. March JS, Silva S, Petrycki S, et al. The Treatment for Adolescents With Depression Study (TADS): long-term effectiveness and safety outcomes. Arch Gen Psychiatry 2007;64:1132–43. 10.1001/archpsyc.64.10.1132 [DOI] [PubMed] [Google Scholar]
- 16. Kennard BD, Silva SG, Mayes TL, et al. Assessment of safety and long-term outcomes of initial treatment with placebo in TADS. Am J Psychiatry 2009;166:337–44. 10.1176/appi.ajp.2008.08040487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vitiello B, Silva SG, Rohde P, et al. Suicidal events in the Treatment for Adolescents With Depression Study (TADS). J Clin Psychiatry 2009;70:741–7. 10.4088/JCP.08m04607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. March J, Silva S, Curry J, et al. The Treatment for Adolescents With Depression Study (TADS): outcomes over 1 year of naturalistic follow-up. Am J Psychiatry 2009;166:1141–9. 10.1176/appi.ajp.2009.08111620 [DOI] [PubMed] [Google Scholar]
- 19. Kennard BD, Silva SG, Tonev S, et al. Remission and recovery in the Treatment for Adolescents with Depression Study (TADS): acute and long-term outcomes. J Am Acad Child Adolesc Psychiatry 2009;48:186–95. 10.1097/CHI.0b013e31819176f9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. CIOMS Working Group VI. Management of safety information from clinical trials. Geneva: Council for International Organizations of Medical Sciences (CIOMS). 2005. https://cioms.ch/wp-content/uploads/2017/01/Mgment_Safety_Info.pdf (accessed 14 Jan 2019).
- 21. Uppsala Monitoring Centre. Glossary of pharmacovigilance terms. 2018. https://www.who-umc.org/global-pharmacovigilance/global-pharmacovigilance/glossary/ (accessed 14 Jan 2019).
- 22. The TADS Team Duke University Medical Center. Treatment for adolescents with depression study (TADS) Pharmacotherapy Treatment Manual Final Version (5.1). 2000. http://tads.dcri.org/tads-manuals (accessed 10 Jan 2019).
- 23. The TADS Team Duke University Medical Center. Procedures for managing pregnancy risk in the NIMH treatment of adolescent depression study (TADS). 2001. http://tads.dcri.org/tads-manuals (accessed 11 Jan 2019).
- 24. Le Noury J, Nardo JM, Healy D, et al. Restoring Study 329: efficacy and harms of paroxetine and imipramine in treatment of major depression in adolescence. BMJ 2015;351:h4320 10.1136/bmj.h4320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de Vries TW, van Roon EN. Low quality of reporting adverse drug reactions in paediatric randomised controlled trials. Arch Dis Child 2010;95:1023–6. 10.1136/adc.2009.175562 [DOI] [PubMed] [Google Scholar]
- 26. Maund E, Tendal B, Hróbjartsson A, et al. Benefits and harms in clinical trials of duloxetine for treatment of major depressive disorder: comparison of clinical study reports, trial registries, and publications. BMJ 2014;348:g3510 10.1136/bmj.g3510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Loder E, Groves T. The BMJ requires data sharing on request for all trials. BMJ 2015;350:h2373 10.1136/bmj.h2373 [DOI] [PubMed] [Google Scholar]
- 28. Mann H. Public release of raw data from the TADS trial. BMJ 2005;330:730. [Google Scholar]
- 29. March JS. TADS Group. Authors of TADS study reply to letter raising concerns. BMJ 2005;330:730.3–1. 10.1136/bmj.330.7493.730-b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Prozac. Summary of Product Characteristics: Eli Lilly and Company Limited. 2018. https://www.medicines.org.uk/emc/product/3768/smpc#PRODUCTINFO (accessed 31 May 2018).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-026089supp001.pdf (103.5KB, pdf)