Abstract
Type 1 diabetes mellitus is a difficult disease to treat due to the relative paucity of therapeutic options other than injectable insulin. The latter, however, can induce hypoglycemia, which has been linked to enhanced cardiovascular risk. Sodium glucose cotransporter 2 (SGLT2) inhibitors are a new class of oral anti-hyperglycemic medications that do not increase the hypoglycemia risk and are US Food and Drug Administration (FDA) approved in type 2 diabetes mellitus. SGLT2 inhibitors may also be of benefit in type 1 diabetic patients, in addition to insulin, although they have not yet been approved for this indication. By blocking SGLT2 in the early proximal tubules of the kidney, these drugs decrease renal glucose retention, which is enhanced in hyperglycemia, thereby improving blood glucose control, in type 1 and type 2 diabetic patents. Their low hypoglycemia risk is due to the compensating reabsorption capacity of another glucose transporter, SGLT1, in the downstream late proximal tubule and the body’s metabolic counter-regulation, which remains intact during SGLT2 inhibition. When insulin dosage is lowered too much, SGLT2 inhibitors can enhance ketogenesis to the extent that the risk of diabetic ketoacidosis increases, particularly in type 1 diabetic patients. SGLT2 inhibitors improve the renal and cardiovascular outcome in type 2 diabetic patients. The mechanisms likely include a reduction in glomerular hyperfiltration, blood pressure, volume overload, and body weight, as well as lowering blood glucose without increasing the hypoglycemia risk. The same mechanistic effects are induced in type 1 diabetic patients. More studies are needed with SGLT2 inhibitors in type 1 diabetic patients, including renal and cardiovascular clinical outcome trials, to fully evaluate their therapeutic potential in this specific population.
1. SGLT2 Inhibitors are Anti-hyperglycemic Drugs with Low Hypoglycemic Risk Approved in Type 2 Diabetic Patients
Sodium glucose cotransporter 2 (SGLT2) inhibitors are a new class of prescription drugs that are FDA approved for use in type 2 diabetic patients to lower blood glucose levels along with diet and exercise. They are currently not approved for use in type 1 diabetic patients. Their primary mechanism of action is the inhibition of SGLT2 in the early proximal tubule of the kidneys [1, 2]. The main function of this transporter is the reabsorption of glucose that has been filtered by the glomeruli of the kidneys. In normoglycemic conditions, SGLT2 accounts for about 97% of renal glucose reabsorption with the sodium glucose cotransporter 1 (SGLT1) located in the downstream late proximal tubule accounting for the rest, such that urine is nearly free of glucose in healthy individuals [2]. By inhibiting SGLT2, glucosuria is increased and serum glucose levels decline, particularly in hyperglycemic patients. The effect is enhanced in the setting of hyperglycemia since the latter increases the filtered load of glucose to the proximal tubule, which enhances glucose reabsorption via SGLT2 and as a consequence the glucosuric effect of SGLT2 inhibition. This glucosuric effect may further increase due to a diabetes-associated increase in renal SGLT2 expression, although this remains a matter of debate due to positive and negative data [2–5]. Thus, diabetes increases renal glucose reabsorption via SGLT2, which contributes to maintain hyperglycemia, and SGLT2 inhibition opposes these effects.
The first SGLT2 inhibitor was discovered over 100 years ago and called phlorizin, a flavonoid contained in the bark and fruit of fruit trees. This compound is a nonspecific SGLT inhibitor that inhibits both SGLT2 and SGLT1, the former with a tenfold higher affinity. Of note and in contrast to SGLT2, SGLT1 is expressed in various body tissues in addition to the renal tubules. This includes the small intestines where SGLT1 is expressed in the luminal membrane and responsible for glucose reabsorption [6]. As a result, phlorizin inhibition of SGLT1 can lead to extrarenal side effects such as diarrhea. To avoid SGLT1-dependent side effects, phlorizin derivates have been developed that are more specific inhibitors of SGLT2. Four of these compounds are currently FDA approved for use in type 2 diabetic patients in the USA: dapagliflozin (Forxiga™/Farxiga™), canagliflozin (Invokana™), ertugliflozin (Steglatro) and empagliflozin (Jardiance™). These inhibitors have an expected HbA1c-lowering effect of 0.7–0.8% from a baseline of around 8.0% [1, 2, 7].
Under normal conditions, SGLT1 is mostly inactive in the late proximal tubule due to the upstream reabsorption of filtered glucose via SGLT2, which allows very little glucose to pass by, and only a small fraction of the glucose transport capacity of SGLT1 is active. SGLT2 inhibition, however, increases the glucose load to the late proximal tubule. This in turn unmasks the reabsorptive capacity of SGLT1. As a consequence and due to SGLT1, renal glucose reabsorption remains at around 40–50% of filtered glucose when SGLT2 is inhibited in euglycemic conditions and with preserved glomerular filtration rate (GFR) [2]. Since the glucosuric effect of SGLT2 inhibitors is tied to the filtered load of glucose, they become ineffective once the filtered load falls below the transport capacity of SGLT1 (~ 80 g/day). As a result, in patients with severely reduced GFR, SGLT2 inhibitors are not FDA approved for use in type 2 diabetic patients. Dapagliflozin is approved for use for patients with a GFR>60 mL/min/1.73 m2 while empagliflozin and canagliflozin for GFR>45 mL/ min/1.73 m2. Additionally, SGLT2 inhibitors trigger counter-regulatory mechanisms that prevent a further reduction in blood glucose levels, including reductions in insulin release and increases in glucagon levels, which enhances hepatic gluconeogenesis. Thus, the glucose reabsorption capacity of SGLT1 in the downstream late proximal tubule and the metabolic counter-regulation induced by SGLT2 inhibitors explain their low hypoglycemia risk. Lowering blood glucose without increasing the hypoglycemia risk is a potential mechanism contributing to the protective effects of these compounds on the renal and cardiovascular system [2, 8] discussed further below.
2. SGLT2 Inhibitors have Protective Renal and Cardiovascular Effects in Type 2 Diabetic Patients
In addition to their glucose-lowering effect, SGLT2 inhibitors have beneficial cardiovascular and renal effects in type 2 diabetic patients. This was shown in the EMPAREG OUTCOME trial, which assessed the effects of empagliflozin on cardiovascular and renal outcomes in 7020 high cardiovascular risk type 2 diabetic patients with an estimated GFR (eGFR) of ≥ 30 mL/min−1 (1.73 m2)−1 [9]. Empagliflozin, along with standard of care therapy, which included angiotensin converting enzyme (ACE) inhibitor or angiotensin II receptor blocker (ARB), reduced the rate of nephropathy, which was defined as “progression to macro-albuminuria, doubling of serum creatinine, initiation of renal replacement therapy or death from renal disease”, with a relative risk reduction of 39%. It also reduced the rate of doubling of creatinine and progression to end stage renal disease by 44 and 55%, respectively [9]. In addition to renal benefits, the SGLT2 inhibitor also reduced the rate of death from cardiovascular disease, hospitalization for heart failure, and death from any cause by 38, 35 and 32%, respectively [10].
The CANVAS program, involving 10142 type 2 diabetic patients, was the second trial which showed significant cardiovascular and renal benefits of an SGLT2 inhibitor— in this case canagliflozin, compared to placebo. In terms of renal benefit, “the composite outcome of sustained reduction in eGFR, the need for renal replacement therapy, or death from renal causes” was seen less in the canagliflozin group compared to the placebo group with a hazard ratio of 0.6—similar to the EMPA-REG-OUTCOME trial. As in the EMPA-REG OUTCOME trial, the rate of heart failure was significantly lower in the canagliflozin arm versus the placebo group. There were no significant differences in the canagliflozin and placebo groups in terms of cardiovascular death [11]. The primary outcome in both trials was a composite of death from cardiovascular causes, nonfatal myocardial infarction or nonfatal stroke. The relative risk reduction of this outcome between the treatment and placebo arms was significant at 14% in both trials.
The difference between these two trials in terms of cardiovascular death may stem from the fact that the EMPA-REG OUTCOME trial included a higher prevalence of cardiovascular disease at baseline compared to the CANVAS program (99 vs 65%). The main difference in terms of adverse effects between the two trials was the increased risk of amputations seen with canagliflozin. The patients taking canagliflozin had nearly 2 times higher risk of needing an amputation, mainly toe and metatarsal, compared to placebo [11]. This was not seen in previous trials involving canagliflozin, and further studies are needed to better understand this issue.
3. Use of SGLT2 Inhibitors in Type 1 Diabetic Patients: Effects on Blood Glucose Control
In type 1 diabetic patients, the progressive loss of pancreatic islet β cells through an autoimmune mechanism results in insulin deficiency and ultimately hyperglycemia. Because of this deficiency, insulin is a required part of the treatment regimen for type 1 diabetic patients. However, 75% of adults with type 1 diabetes fail to achieve the glycemic control goal (HbA1c<7.0%) recommended by the American Diabetes Association [12]. Furthermore, insulin has the disadvantage of potentially causing hypoglycemia, which acutely can lead to death and in the long term enhance cardiovascular risk in these patients [13]. The only non-insulin drug currently FDA approved for use in type 1 diabetic patients is pramlintide, which inhibits glucagon secretion thereby lowering glucose levels. However, pramlintide can also cause hypoglycemia [14]. Type 1 diabetic patients also have a high cardiovascular risk and are in need of efficient therapies [15]. In poorly controlled type 1 diabetic patients who are compliant with their insulin therapy and suffer from frequent episodes of hypoglycemia, SGLT2 inhibitors may provide an attractive addition to the typical insulin-only regimens prescribed for these patients. Moreover, these patients should have a low risk of ketoacidosis but a high awareness of this problem (see below). Three prospective, well powered, doubleblind, placebo-controlled trials have been completed and published that have assessed the efficacy SGLT2 inhibitors in type 1 diabetic patients. The baseline characteristics of patients in these trials are summarized in Table 1. The first trial, performed by Henry and colleagues, consisted of 351 patients with type 1 diabetes who were randomized into three groups receiving daily doses of 100 or 300 mg of canagliflozin or placebo. The primary endpoint was the proportion of patients who achieved HbA1c reduction from baseline of more than 0.4% and no weight gain. In this 18-week trial, significantly more patients in the 100 and 300 mg canagliflozin groups achieved this goal compared to placebo (36.9 and 41.4 vs 14.5%, respectively; p<0.001). Moreover, both doses of canagliflozin significantly reduced HbA1c, body weight and the total required insulin dose compared to placebo (see Table 2) [16].
Table 1.
Baseline characteristics of three prospective, double-blind, placebo-controlled SGLT2 inhibitor trials in type 1 diabetic patients
| First author, year | Patient number | Treatment (mg/day) | Study duration (weeks) | Total insulin (units/ kg/day) | Age (y) | Men (%) | Baseline HbA1c (%) |
|---|---|---|---|---|---|---|---|
| Henry (2015) [16] | 351 | Canagliflozin 300, canagliflozin 100, placebo | 18 | 0.69 | 42 | 56 | 7.9 |
| Dandona (2017) [17] | 833 | Dapagliflozin 10, dapagliflozin 5, placebo | 24 | 0.74 | 42 | 48 | 8.5 |
| Garg (2017) [18] | 1402 | Sotagliflozin 400, placebo | 24 | 0.70 | 42 | 50 | 8.2 |
Table 2.
Efficacy of treatment in three prospective, double-blind, placebo-controlled SGLT2 inhibitor trials in type 1 diabetic patients
| First author, year | Treatment (mg/day) | Reduction in HbA1c% compared to baseline | Reduction in total insulin dose compared to placebo (units/day) | Weight loss compared to placebo (%) | BP reduction compared to placebo (mmHg) in patients with baseline systolic BP > 130 |
|---|---|---|---|---|---|
| Henry (2015) [16] | Canagliflozin 300, canagliflozin 100, placebo | 0.24 vs 0.27 vs 0 | 7.6 and 4.1 | 5.3 and 3.4 | N/A |
| Dandona (2017) [17] | Dapagliflozin 10, dapagliflozin 5, placebo | 0.47 vs 0.45 vs 0.03 | 7.63 and 7.18 | 3.72 and 2.96 | N/A |
| Garg (2017) [18] | Sotagliflozin 400, placebo | 0.79 vs 0.33 | 5.3 | 4.0 | 3.5 |
All three studies show a significant reduction in HbA1c, total insulin required, and body weight for the SGLT2 inhibitors compared to placebo in type 1 diabetic patients
The second study was the DEPICT1 trial where 833 patients were randomized into three different treatment arms: doses of 5 and 10 mg/day of dapagliflozin were compared with placebo. Primary outcome was the change in HbA1c. After 24 weeks of treatment, both doses of dapagliflozin significantly and to a similar extent reduced HbA1c levels compared to placebo: a 0.42% and a 0.45% reduction in the 5 and 10 mg dapagliflozin arms, respectively. Total daily insulin dose and body weight were likewise significantly reduced in the treatment arms compared to placebo [17].
The third study was the inTandem3 trial. It was the biggest trial assessing the efficacy and safety of an SGLT2 inhibitor in type 1 diabetic patients to date, and was published in September 2017 [18]. This was a Phase 3 trial comprising 1402 patients assigned to insulin therapy plus placebo versus insulin therapy plus sotagliflozin (400 mg per day), a combined SGLT2 and SGLT1 inhibitor, for 24 weeks. The primary endpoint was HbA1c of less than 7% with no episodes of severe hypoglycemia or diabetic ketoacidosis (DKA) after randomization. In the sotagliflozin group, 28.6% of patients met the primary end point versus 15.2% of patients in the placebo group. Moreover, the patients in the sotagliflozin group achieved a significantly lower HbA1c, systolic blood pressure and body weight and required significantly less daily insulin compared to the placebo group [18]. The efficacy results of these three main trials are summarized in Table 2.
As noted above, sotagliflozin is not a highly selective SGLT2 inhibitor, but can inhibit both SGLT2 and SGLT1 at therapeutic doses. Therefore, comparisons and similarities between the inTandem3 trial and the previous two trials mentioned should be made with caution. Sotagliflozin has a 20-times higher affinity for SGLT2 compared to SGLT1 [19]. In comparison, the selectivity for SGLT2 over SGLT1 for dapagliflozin, canagliflozin, empagliflozin is approximately 1167:1, 263:1 and 2667:1, respectively [20]. When sotagliflozin is applied orally, SGLT1 inhibition in the small intestine decreases glucose absorption, which causes glucose lowering especially postprandially. This is an added benefit of sotagliflozin compared to the more selective SGLT2 inhibitors. The inhibition of SGLT1 in the small intestine can also induce a sustained postprandial increase in glucagon-like peptide 1 (GLP1), which enhances glucose-dependent insulin secretion in type 2 diabetes [6]. This effect becomes irrelevant in the absence of endogenous insulin secretion. As stated previously, intestinal SGLT1 inhibition can cause diarrhea. The inTandem trial showed that twice as many patients reporting diarrhea were in the sotagliflozin group compared to the placebo group [18]; however, overall this risk seemed lower than expected indicating a potential therapeutic window. After reabsorption into the systemic circulation, sotagliflozin will inhibit SGLT2 in the kidney. It is unclear whether the tubular sotagliflozin concentrations following its oral application are high enough to inhibit SGLT1 in the kidneys. This would be helpful to know to better understand the role of the kidney in potential differences in side effects between very selective and less selective SGLT2 inhibitors (see below).
4. Use of SGLT2 Inhibitors in Type 1 Diabetic Patients: Safety Aspects
The above three trials also assessed the safety of these SGLT2 inhibitors in type 1 diabetic patients. One of the main concerns for any glucose-lowering drug is hypoglycemia. As shown in Table 3, none of the SGLT2 inhibitors in these three trials caused a significant increase in the hypoglycemia risk compared with the placebo group. As outlined above, this was expected for the specific SGLT2 inhibitors. Notably, no increase in the hypoglycemia risk was reported with sotagliflozin despite being an inhibitor of both SGLT2 and SGLT1. It may be that the inhibition of SGLT1 in the kidneys by sotagliflozin is minimal. Moreover, a stronger activation of metabolic counter-regulatory mechanisms, such as hepatic gluconeogenesis, may occur and help to counteract hypoglycemia.
Table 3.
Safety of treatment in three prospective, double-blind, placebo-controlled SGLT2 inhibitor trials in type 1 diabetic patients
| First author, year | Treatment (mg/day) | Documented hypoglycemia | Serious DKA (n) | Genital infections (n) | UTI (n) | Volume depletion (n) |
|---|---|---|---|---|---|---|
| Henry (2015) [16] | Canagliflozin 300, canagliflozin 100, placebo | No difference between Rx vs placebo | 7 vs 5 vs 0 | 11 vs 2 vs 3 | 6 vs 5 vs 2 | 1 vs 4 vs 0 |
| Dandona (2017) [17] | Dapagliflozin 10, dapagliflozin 5, placebo | No difference between Rx vs placebo | 4 vs 5 vs 3 | 34 vs 33 vs 7 | 19 vs 11 vs 13 | 0 vs 1 vs 2 |
| Garg (2017) [18] | Sotagliflozin 400, placebo | No difference between Rx vs placebo | 21 vs 4 | 45 vs 15 | 25 vs 27 | 13 vs 2 |
Diabetic ketoacidosis (DKA) is one of the main concerns related to using SGLT2 inhibitors, particularly in type 1 diabetic patients. DKA can occur in both type 1 and type 2 diabetic patients in the absence and presence of SGLT2 inhibitors. However, type 1 diabetic patients are more prone to DKA than type 2 diabetic patients, and therefore the use of SGLT2 inhibitors in the former population is of particular concern. It is not completely understood why SGLT2 inhibitors raise ketone levels in the serum and induce DKA. One proposed hypothesis is that due to their glucosuric and blood–glucose lowering effect, they decrease endogenous insulin release and increase glucagon levels [21]. While the drugs cannot reduce endogenous insulin levels in patients with type 1 diabetes, their blood–glucose lowering effect may cause the physician to lower the insulin dose. These changes in insulin and glucagon enhance lipolysis and release more free-fatty acids from adipose tissue, which are then used for ketogenesis by the liver. The ketone bodies are released into the systemic circulation to provide an alternative energy substrate when blood glucose is low. At high levels of plasma ketone bodies, SGLT2 inhibitors may also facilitate the renal retention of ketone bodies by lowering GFR (see below) and thereby reducing the filtered amount of ketone bodies below the renal tubular reabsorption capacity [22]. Hence, increased ketonemia and DKA can occur in response to SGLT2 inhibition in the absence of hyperglycemia [21, 22]. The low basal endogenous insulin levels typical of type 1 diabetes is thought to enhance the risk of DKA compared with type 2 diabetic patients. Several potential ketoacidosis triggers have been identified in patients with type 1 diabetes including major illness, reduced food and fluid intake, concomitant mild infection, increased physical activity, and/or reduced food intake and acute insulin dose reduction or omission, whereas in some cases, no contributing factors were identified [22, 23].
Of the adverse events reported to the FDA related to the use of SGLT2 inhibitors from March 2013 to May 2015, 73 cases of ketoacidosis were reported. All cases were treated in the emergency room or were admitted to hospital. Fortyfour of these reported at least one laboratory criteria supporting DKA such as high anion gap metabolic acidosis, ketonemia or low serum bicarbonate. Of the 73 cases, 44 were type 2 diabetic patients and 15 were type 1 diabetic patients; in 53 of the 73 patients another event occurred, which could have contributed to the DKA, e.g. dehydration, infection or changed insulin dosing. As a result, the FDA revised the label on SGLT2 inhibitors in May 2015 to include that SGLT2 inhibitors can potentially cause DKA and patients should stop taking the drug if DKA is diagnosed [24–26]. Notably, in the EMPA-REG OUTCOME trial (see above) no difference was seen in the rate of DKA between placebo and the treatment arms. There was a numerical increase in DKA with canagliflozin in the CANVAS study but it was not reported as statistically significant [11]. Overall, this is consistent with the lower DKA risk in type 2 diabetic patients with SGLT2 inhibitors, but may also relate to a well-controlled study group [10].
The three trials also provided insights regarding DKA and other adverse effects of SGLT2 inhibitors in patients with type 1 diabetes. In the canagliflozin trial, the SGLT2 inhibitor was associated with a higher rate of urinary tract infections, volume depletion and DKA [16]. Adjudication was not performed by an independent committee to assess the adverse events. In the DEPICT1 trial with dapagliflozin, the only adverse event that was reported in a significantly higher proportion of patients in the SGLT2 inhibitor arms was genital infection. In this study an independent DKA adjudication committee (not aware of the study group assignment) assessed all reported ketonerelated adverse events and classified them as “definitive DKA”, “possible DKA” or “unlikely to be DKA”. The number of patients with definitive DKA was similar across all treatment groups. The number of possible DKA events was higher in the treatment groups. However, of the 18 patients who had possible DKA, only 4 sought treatment for it at a medical facility. Of those 4, two did not meet the classical laboratory criteria for DKA and the other two did not have laboratory values available [17]. In the inTandem3 trial, there was a significantly higher rate of volume depletion, genital infection, urinary tract infection and serious/non-serious DKA in the sotagliflozin versus placebo group. An increased risk for volume depletion was not seen in the other two trials but has been reported with selective SGLT2 inhibitors in patients with type 2 diabetes and is not unexpected based on the diuretic and natriuretic potential of these compounds, especially when blood glucose levels are high. In addition, sotagliflozin may further facilitate volume depletion by inhibiting SGLT1 in the small intestine and potentially in the kidney. Volume depletion is a risk factor for DKA. Adjudication was done in this trial to confirm reported DKA events by a committee blinded to the treatment arm [18]. “Serious DKA” was defined in all 3 trials by any DKA case that required hospitalization [16–18]. In summary, two of the three trials showed an increased risk in serious DKA with SGLT2 inhibitors in type 1 diabetic patients compared to placebo.
5. How Can SGLT2 Inhibitors Protect the Kidney and Heart in Type 2 Diabetes and Should These Effects be Preserved in Patients with Type 1 Diabetes?
Glomerular hyperfiltration in the early phase of type 1 and 2 diabetes has been proposed to be a risk factor for the later development of albuminuria and diabetic nephropathy [27, 28]. An increase in single nephron GFR causes an increase in tubular transport work and oxygen need, since basically all filtered NaCl needs to be reabsorbed in the tubular system. Hyperfiltration can be found in as many as 75% of type 1 diabetic patients [27]. In type 2 diabetic patients, this proportion seems more variable likely due to the difference in glycemic control, duration of diabetes, age and GFR measurement method used for obese patients [27]. Moderate hyperglycemia induces a ‘primary’ increase in proximal tubular reabsorption by providing substrate for SGLTs or by causing the tubule to undergo hypertrophy [29]. This primary increase in proximal reabsorption reduces NaCl and fluid delivery to the downstream macula densa, causing GFR to increase via normal physiologic actions of tubulo-glomerular feedback. The tubuloglomerular feedback mechanism works on the single nephron level and adjusts the tone of the afferent arteriole, and thereby GFR, to stabilize the NaCl and fluid load to the macula densa. This contributes to the autoregulation of GFR and facilitates the fine regulation of NaCl and fluid balance in the distal nephron, downstream of the macula densa, by neurohumoral control. A primary increase in proximal reabsorption also reduces distal tubular flow rate, which can increase GFR by lowering tubular back pressure, i.e. the hydrostatic pressure in the Bowman’s space. Inhibiting SGLT2 attenuates the diabetes-induced process of tubular hyper-reabsorption and decreases glomerular hyperfiltration [2].
Preclinical studies have demonstrated the reduction in diabetic hyperfiltration by selective and non-selective SGLT2 inhibitors. In 1999, this was shown in micropuncture studies in rats using local application of phlorizin into Bowman’s space [30] and later by acute or chronic systemic application of selective SGLT2 inhibitors [31]. Hyperfiltration has been suppressed on the whole-kidney level in mouse models of diabetes by pharmacologic or genetic inhibition of SGLT2 [32]. In each case, diabetic hyperfiltration suppression was independent of effects on blood glucose [30, 32, 33], but was associated with an increase in NaCl concentration at the macula densa [30, 33] and in hydrostatic pressure in Bowman’s space [30]. Additionally, as observed in a genetic rodent model of type 1 diabetes, SGLT2 inhibitors can reduce renal growth, albuminuria and inflammation mainly through their glucose-lowering effect [34]. Similar results were reported in rodent models of type 2 diabetes [2].
Cherney and colleagues assessed in type 1 diabetic patients the relevance in the clinical setting. GFR was measured using inulin clearance in both type 1 diabetic patients with hyperfiltration and without hyperfiltration. This was done before and after administering empagliflozin (a selective SGLT2 inhibitor) 25 mg orally, daily for 8 weeks. This was a small study focusing on GFR and not powered to perform a detailed safety analysis. Few adverse events related to renal function were reported with no cases of acute renal failure or tubular necrosis. Two cases of diabetic ketoacidosis occurred within the first 3 days of drug exposure leading to discontinuation, one in the context of insulin pump failure and the other in the setting of acute gastroenteritis [35]. Forty patients completed the study. In the group with hyperfiltration, GFR was significantly decreased by 33 mL/min/1.73 m2, whereas empagliflozin had no significant effect in the group without hyperfiltration [35]. This is consistent with the assumption that SGLT2 inhibitors lower hyperfiltration by attenuating an increased tone of SGLT2-mediated tubular hyperreabsorption.
Thus, SGLT2 inhibitors can induce a rapid, functional and reversible reduction in GFR in type 1 and type 2 diabetic patients [2, 35]. They are currently approved for use in patients with a GFR>60 mL/min/1.73 m2 for dapagliflozin and a GFR>45 mL/min/1.73 m2 for empagliflozin and canagliflozin. However, even in patients with chronic kidney disease (CKD), the functional reduction in GFR occurs and can be beneficial. In these patients, the remaining intact nephrons are undergoing hyperfiltration. By reducing the hyperfiltration in these nephrons, SGLT2 inhibitors are expected to reduce the oxygen utilization for tubular transport and preserve their integrity, and thereby GFR in the long run [2]. None of the three discussed prospective and well-powered clinical trials on type 1 diabetic patients assessed whether eGFR was acutely reduced by SGLT2 inhibition (i.e. within first 1–2 weeks) and the follow-up time was too short to determine longterm GFR outcome [16–18].
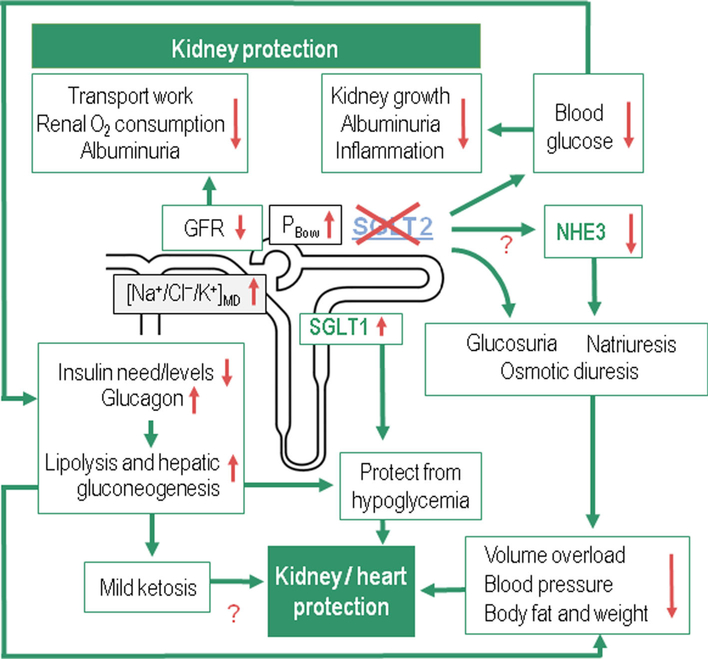
What other mechanism may contribute to the cardiovascular benefits of SGLT2 inhibitors in type 1 and type 2 diabetic patients beyond lowering blood glucose levels with little risk of hypoglycemia and preserving kidney function? Considering the prominent benefits related to heart failure, one explanation could be the reduction in blood pressure seen with these inhibitors in addition to body fat and weight loss. As mentioned above, sotagliflozin lowered blood pressure in type 1 diabetic patients [18], and all three trials in type 1 diabetic patients showed significant weight loss compared to placebo [16–18]. The mechanism of this blood pressure-lowering effect includes the glucose based osmotic diuresis (100–470 mL/day), natriuresis and weight loss [36, 37]. Sustained weight loss seems to be primarily due to increased lipolysis leading to a decrease in fat body content. In patients taking SGLT2 inhibitors, there is a shift from carbohydrate utilization to lipids primarily due to the reduction in glucose. This in turn leads to eventual lipolysis and weight loss. This has been demonstrated in rodents and clinical studies involving type 2 diabetic patients [38, 39]. Na+/H+-exchanger 3 (NHE3) is an early proximal tubule transporter that is co-expressed with SGLT2. Inhibition of SGLT2 may also inhibit NHE3 as recently proposed [2, 40]. This interaction between SGLT2 and NHE3 could be relevant to the blood pressurelowering effect of SGLT2. On the flip side, this interaction may modestly impair renal acid excretion. Another potential mechanism relates to the fact that SGLT2 inhibition shifts more glucose transport to SGLT1 in the late proximal tubule. This in turn may reduce the oxygen tension in the outer medulla leading to enhanced erythropoietin release and red blood cell production, which, together with the diuretic effect, increases hematocrit and could facilitate oxygen delivery to the kidney and the heart [41]. Cherney also reported a small but statistically significant increase in hematocrit in type 1 diabetic patients treated with the SGLT2 inhibitor empagliflozin [35]. As mentioned above, SGLT2 inhibitors can cause an increase in ketogenesis, which can have detrimental effects in the form of DKA. However, mild ketosis could potentially provide additional energy substrates in the form of ketone bodies for the heart and kidney, which could be organ protective [42]. Clearly, more studies are needed to better understand these issues and potential mechanisms, which all are expected to apply to both type 1 and type 2 diabetic patients. The pleiotropic effects of SGLT2 inhibitors are summarized in Fig. 1.
Fig. 1.
Proposed mechanisms of SGLT2 inhibition-induced kidney and heart protection in both type 1 and type 2 diabetic patients. The cartoon in the middle shows a nephron. PBow refers to the pressure in Bowman’s capsule. [Na+/Cl−/K+]MD refers to the concentration of sodium, chloride and potassium at the macula densa. Green arrows indicate consequences of SGLT2 inhibition and red arrows demonstrate changes in the associated variables. “?” indicate hypotheses which need further confirmation. This figure was modified with permission from [2]
6. Future Directions and Summary
More data are needed with regard to the use of SGLT2 inhibitors in type 1 diabetic patients, and various trials are ongoing. The first study is assessing the efficacy and safety of the selective SGLT2 inhibitor ipragliflozin in insulintreated type 1 diabetic patients [43]. The primary outcome measure is HbA1c. In the first part of the study ipragliflozin will be administered for 24 weeks under double-blind conditions to one group and the other will receive placebo. In the second part of the study, ipragliflozin will be administered under open-label conditions for 28 weeks to assess long-term safety and efficacy [44]. The same group of investigators will also administer ipragliflozin to type 1 diabetic patients in different doses for 2 weeks to assess pharmacokinetics, pharmacodynamics and safety at various doses, and placebo [43, 44]. Another trial that has been completed but the results have not yet been published, assessed the efficacy and safety of dapagliflozin in type 1 diabetic patients with a specific emphasis on ketogenesis, including the measurement of ketone bodies in urine and blood. There are three arms in this cross-over study, such that each patient will receive dapagliflozin, the glucagonlike peptide-1 agonist liraglutide, and placebo, each for 1 week [45]. The EASE trial involving the use of empagliflozin as an add-on to insulin in patients with type 1 diabetes has also been completed in both 26 and 52-week time intervals. Efficacy, safety and tolerability of low- and high-dose empagliflozin and placebo were assessed, and results are not yet published. These longer-term studies will provide important insights into the long-term safety and efficacy of these drugs in type 1 diabetes [46, 47].
There is a need for longer-term studies in type 1 diabetic patients. The duration of the 3 published trials discussed above were B 24 weeks in duration. The mentioned ipragliflozin trial and the EASE trial in type 1 diabetic patients will assess the long-term safety and efficacy of these drugs over a total of 52 weeks, which will provide important insights.
To what extent DKA is directly caused by an individual SGLT2 inhibitor? As observed in the 3 trials discussed above, dapagliflozin was not associated with an increased risk of DKA compared to placebo as opposed to sotagliflozin and canagliflozin. The latter two compounds are less selective for SGLT2. Another active ongoing clinical trial assesses the risk ofDKA in type 1 diabetic patients taking dapagliflozin versus liraglutide versus placebo and is expected to shed further light on this serious adverse event [45].
A key aspect for the future use and potential approval of SGLT2 inhibitors in type 1 diabetic patients will be the assessment of the risk-benefit relationship. Do the improvements in HbA1c and the potential beneficial effects on the kidney and heart outweigh the risks of diabetic ketoacidosis, which seems to be the most serious adverse event identified in type 1 diabetic patients. Previous studies have shown that SGLT2 inhibitors improve death from cardiovascular causes, hospitalization for heart failure and death from any cause in type 2 diabetic patients. Effects of SGLT2 inhibitors on cardiovascular outcomes have not yet been assessed in type 1 diabetic patients and these will require dedicated long-term trials. The mechanisms that we currently assume to contribute to the protective effect of SGLT2 inhibitors are expected to also occur in type 1 diabetic patients (Fig. 1).
In summary, high-powered prospective, double-blind and placebo-controlled clinical trials have shown that SGLT2 inhibitors are effective glucose-lowering drugs in addition to insulin in type 1 diabetic patients. SGLT2 inhibitors also have the potential to provide renal and cardioprotective benefits to type 1 diabetic patients through reduction of blood glucose levels with low hypoglycemia risk, reduction in glomerular hyperfiltration, decrease in blood pressure and volume overload, and weight loss. However, SGLT2 inhibitors enhance ketogenesis and can lead to DKA, especially in susceptible type 1 diabetic patients and in the presence of precipitating factors such as volume depletion. Further long-term trials and studies are needed to better understand how to prevent DKA episodes in these patients, whether dual inhibition of SGLT2 and SGLT1 has any added value, to elucidate if the renal and cardiovascular benefits of SGLT2 inhibitors shown in type 2 diabetic patients also occur in type 1 diabetic patients, and to determine whether these effects outweigh the risk and danger of DKA.
Key Points.
SGLT2 inhibitors are new anti-hyperglycemic therapies that are approved in type 2 diabetes mellitus, have a low hypoglycemia risk, and may also be of benefit for type 1 diabetic patients, as an add-on to insulin.
The mechanistic effects that have been linked to the renal and cardiovascular benefits of SGLT2 inhibitors in type 2 diabetic patients, have also been documented in type 1 diabetic patients.
More studies are underway and are needed to fully evaluate the therapeutic potential of SGLT2 inhibitors in type 1 diabetic patients, including the risk and prevention of diabetic ketoacidosis, and effects on relevant renal and cardiovascular outcomes.
Acknowledgements
The authors were supported by NIH Grants R01DK112042, R01DK106102, the UAB/UCSD O’Brien Center of Acute Kidney Injury NIH-P30DK079337, the NIH training Grant T32DK104717-02, and the Department of Veterans Affairs.
Footnotes
Compliance with Ethical Standards
Conflict of interest Over the past 36 months, V.V. has served as a consultant and received honoraria from Bayer, Boehringer Ingelheim, Intarcia Therapeutics, Astra-Zeneca, Janssen Pharmaceutical, Eli Lilly and Merck, and received grant support for investigator-initiated research from Astra-Zeneca, Bayer, Boehringer Ingelheim, Fresenius, and Janssen. H.F. has no conflicting interests to disclose.
References
- 1.Heerspink HJL, Perkins BA, Fitchett DH, et al. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus. Circulation. 2016;134:752–72. [DOI] [PubMed] [Google Scholar]
- 2.Vallon V, Thomson SC. Targeting renal glucose reabsorption to treat hyperglycaemia: the pleiotropic effects of SGLT2 inhibition. Diabetologia. 2017;60:215–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norton L, Shannon CE, Fourcaudot M, et al. Sodium-glucose cotransporter (SGLT) and glucose transporter (GLUT) expression in the kidney of type 2 diabetic subjects. Diabetes Obes Metab. 2017;19:1322–6. [DOI] [PubMed] [Google Scholar]
- 4.Solini A, Rossi C, Mazzanti CM, et al. Sodium-glucose cotransporter (SGLT)2 and SGLT1 renal expression in patients with type 2 diabetes. Diabetes Obes Metab. 2017;19:1289–94. [DOI] [PubMed] [Google Scholar]
- 5.Wang XX, Levi J, Luo Y, et al. SGLT2 Protein Expression Is Increased in Human Diabetic Nephropathy: SGLT2 PROTEIN INHIBITION DECREASES RENAL LIPID ACCUMULATION, INFLAMMATION, AND THE DEVELOPMENT OF NEPHROPATHY IN DIABETIC MICE. J Biol Chem. 2017;292:5335–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song P, Onishi A, Koepsell H, Vallon V. Sodium glucose cotransporter SGLT1 as a therapeutic target in diabetes mellitus. Exp Opin Ther Targets. 2016;20:1109–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheen AJ. Pharmacodynamics, efficacy and safety of sodiumglucose co-transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs. 2015;75:33–59. [DOI] [PubMed] [Google Scholar]
- 8.Vallon V, Thomson SC. Diabetes mellitus: cardiovascular and renal benefits of SGLT2 inhibition: insights from CANVAS. Nat Rev Nephrol. 2017;13:517–8. [DOI] [PubMed] [Google Scholar]
- 9.Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–34. [DOI] [PubMed] [Google Scholar]
- 10.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28. [DOI] [PubMed] [Google Scholar]
- 11.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–57. [DOI] [PubMed] [Google Scholar]
- 12.American Diabetes Association AD. 6. Glycemic targets: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41:S55–64. [DOI] [PubMed] [Google Scholar]
- 13.Khunti K, Davies M, Majeed A, et al. Hypoglycemia and risk of cardiovascular disease and all-cause mortality in insulin-treated people with type 1 and type 2 diabetes: a cohort study. Diabetes Care. 2015;38:316–22. [DOI] [PubMed] [Google Scholar]
- 14.American Diabetes Association AD. 8. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41:S73–85. [DOI] [PubMed] [Google Scholar]
- 15.De Ferranti SD, De Boer IH, Fonseca V, et al. Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Diabetes Care. 2014;37:2843–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henry RR, Thakkar P, Tong C, et al. Efficacy and safety of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as addon to insulin in patients with type 1 diabetes. Diabetes Care. 2015;38:2258–65. [DOI] [PubMed] [Google Scholar]
- 17.Dandona P, Mathieu C, Phillip M, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (DEPICT-1): 24 week results from a multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5:864–76. [DOI] [PubMed] [Google Scholar]
- 18.Garg SK, Henry RR, Banks P, et al. Effects of sotagliflozin added to insulin in patients with type 1 diabetes. N Engl J Med. 2017. 10.1056/nejmoa1708337. [DOI] [PubMed] [Google Scholar]
- 19.Sands AT, Zambrowicz BP, Rosenstock J, et al. Sotagliflozin, a dual SGLT1 and SGLT2 inhibitor, as adjunct therapy to insulin in type 1 diabetes. Diabetes Care. 2015;38:1181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grempler R, Thomas L, Eckhardt M, et al. Empagliflozin, a novel selective sodium glucose cotransporter-2 (SGLT-2) inhibitor: characterisation and comparison with other SGLT-2 inhibitors. Diabetes Obes Metab. 2012;14:83–90. [DOI] [PubMed] [Google Scholar]
- 21.Ogawa W, Sakaguchi K. Euglycemic diabetic ketoacidosis induced by SGLT2 inhibitors: possible mechanism and contributing factors. J Diabetes Investig. 2016;7:135–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiu H, Novikov A, Vallon V. Ketosis and diabetic ketoacidosis in response to SGLT2 inhibitors: basic mechanisms and therapeutic perspectives. Diabetes Metab Res Rev. 2017;33:e2886. [DOI] [PubMed] [Google Scholar]
- 23.Peters AL, Buschur EO, Buse JB, et al. Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium– glucose cotransporter 2 inhibition. Diabetes Care. 2015;38:1687–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.US Food and Drug Administration. FDA Drug Safety Communication: FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections. FDA Drug Saf Commun. 2015;2–4. [Google Scholar]
- 25.Fadini GP, Bonora BM, Avogaro A. SGLT2 inhibitors and diabetic ketoacidosis: data from the FDA Adverse Event Reporting System. Diabetologia. 2017;60:1385–9. [DOI] [PubMed] [Google Scholar]
- 26.Blau JE, Tella SH, Taylor SI, Rother KI. Ketoacidosis associated with SGLT2 inhibitor treatment: analysis of FAERS data. Diabetes Metab Res Rev. 2017;33:e2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jerums G, Premaratne E, Panagiotopoulos S, MacIsaac RJ. The clinical significance of hyperfiltration in diabetes. Diabetologia. 2010;53:2093–104. [DOI] [PubMed] [Google Scholar]
- 28.Magee GM, Bilous RW, Cardwell CR, et al. Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia. 2009;52:691–7. [DOI] [PubMed] [Google Scholar]
- 29.Vallon V, Blantz RC, Thomson S. Glomerular hyperfiltration and the salt paradox in early type 1 diabetes mellitus: a tubulocentric view. J Am Soc Nephrol. 2003;14:530–7. [DOI] [PubMed] [Google Scholar]
- 30.Vallon V, Richter K, Blantz RC, et al. Glomerular hyperfiltration in experimental diabetes mellitus: potential role of tubular reabsorption. J Am Soc Nephrol. 1999;10:2569–76. [DOI] [PubMed] [Google Scholar]
- 31.Thomson SC, Rieg T, Miracle C, et al. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. AJP Regul Integr Comp Physiol. 2012;302:R75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vallon V, Rose M, Gerasimova M, et al. Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. AJP Ren Physiol. 2013;304:F156–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vallon V, Thomson SC. Renal function in diabetic disease models: the tubular system in the pathophysiology of the diabetic kidney. Annu Rev Physiol. 2012;74:351–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vallon V, Gerasimova M, Rose MA, et al. SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. AJP Ren Physiol. 2014;306:F194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cherney DZI, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129:587–97. [DOI] [PubMed] [Google Scholar]
- 36.Scheen AJ, Delanaye P. Effects of reducing blood pressure on renal outcomes in patients with type 2 diabetes: focus on SGLT2 inhibitors and EMPA-REG OUTCOME. Diabetes Metab. 2017;43:99–109. [DOI] [PubMed] [Google Scholar]
- 37.Scheen AJ. Effects of reducing blood pressure on cardiovascular outcomes and mortality in patients with type 2 diabetes: focus on SGLT2 inhibitors and EMPA-REG OUTCOME. Diabetes Res Clin Pract. 2016;121:204–14. [DOI] [PubMed] [Google Scholar]
- 38.Bolinder J, Ljunggren O, Kullberg J, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012;97:1020–31. [DOI] [PubMed] [Google Scholar]
- 39.Yokono M, Takasu T, Hayashizaki Y, et al. SGLT2 selective inhibitor ipragliflozin reduces body fat mass by increasing fatty acid oxidation in high-fat diet-induced obese rats. Eur J Pharmacol. 2014;727:66–74. [DOI] [PubMed] [Google Scholar]
- 40.Pessoa TD, Campos LCG, Carraro-Lacroix L, et al. Functional role of glucose metabolism, osmotic stress, and sodium-glucose cotransporter isoform-mediated transport on Na+/H+ exchanger isoform 3 activity in the renal proximal tubule. J Am Soc Nephrol. 2014;25:2028–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Layton AT, Vallon V. SGLT2 inhibition in a kidney with reduced nephron number: modeling and analysis of solute transport and metabolism. Am J Physiol Renal Physiol. 2018. 10.1152/ajprenal.00551.2017 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferrannini E, Mark M, Mayoux E. CV protection in the EMPAREG OUTCOME trial: a thrifty substrate hypothesis. Diabetes Care. 2016;39:1108–14. [DOI] [PubMed] [Google Scholar]
- 43.Pharmacodynamics, pharmacokinetics, and safety of ASP1941 inpatients with type 1 diabetes mellitus—Full Text View—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02529449?cond=SGLT2+type+1+diabetes&rank=2. Accessed 28 Jul 2017.
- 44.A study of ASP1941 in combination with insulin in patients with type 1 diabetes mellitus—Full Text View—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02897219?cond=SGLT2+type+1+diabetes&rank=1. Accessed 28 Jul 2017.
- 45.Effects of single doses of liraglutide and dapagliflozin on hyperglycemia and ketogenesis in type 1 diabetes—Full Text View—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02777073?cond=SGLT2+type+1+diabetes&rank=3. Accessed 28 Jul 2017.
- 46.Empagliflozin as adjunctive to insulin therapy over 26 weeks in patients with T1DM (EASE-3)—Full Text View—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02580591?term=empagliflozin&cond=type+1+diabetes&rank=6. Accessed 20 Mar 2018.
- 47.Empagliflozin as Adjunctive to InSulin thErapy over 52 weeks in patients with type 1 diabetes mellitus (EASE-2)—Full Text View—linicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02414958?term=empagliflozin&cond=type+1+diabetes&rank=3. Accessed 20 Mar 2018.