Abstract
Aims
Troponin levels are commonly elevated among patients hospitalized for heart failure (HF), but the prevalence and prognostic significance of early post-discharge troponin elevation are unclear. This study sought to describe the frequency and prognostic value of pre-discharge and post-discharge troponin elevation, including persistent troponin elevation from the inpatient to outpatient settings.
Methods and results
The ASTRONAUT trial (NCT00894387; http://www.clinicaltrials.gov) enrolled hospitalized HF patients with ejection fraction of ≤40% and measured troponin I prior to discharge (i.e. baseline) and at 1-month post-discharge in a core laboratory (elevation defined as >0.04 ng/mL). This analysis included 1469 (91.0%) patients with pre-discharge troponin data. Overall, 41.5% and 29.9% of patients had elevated pre-discharge [median: 0.09 ng/mL; interquartile range (IQR): 0.06–0.19 ng/mL] and 1-month (median: 0.09 ng/mL; IQR: 0.06–0.15 ng/mL) troponin levels, respectively. Among patients with pre-discharge troponin elevation, 60.4% had persistent elevation at 1 month. After adjustment, pre-discharge troponin elevation was not associated with 12-month clinical outcomes. In contrast, 1-month troponin elevation was independently predictive of all-cause death [hazard ratio (HR) 1.59, 95% confidence interval (CI) 1.18–2.13] and cardiovascular mortality or HF hospitalization (HR 1.28, 95% CI 1.03–1.58) at 12 months. Associations between 1-month troponin elevation and outcomes were similar among patients with newly elevated (i.e. normal pre-discharge) and persistently elevated levels (interaction P ≥ 0.16). The prognostic value of 1-month troponin elevation for 12-month death was driven by a pronounced association among patients with coronary artery disease (interaction P = 0.009).
Conclusions
In this hospitalized HF population, troponin I elevation was common at both the index hospitalization and 1 month post-discharge. Elevated troponin I level at 1 month, but not pre-discharge, was independently predictive of increased clinical events at 12 months. Early post-discharge troponin I measurement may offer a practical means of risk stratification and troponin I level should be investigated as a therapeutic target.
Keywords: Heart failure, Clinical trials, Post-discharge, Troponin, Outcomes, Hospitalization
Introduction
Troponin is a central biomarker in cardiovascular medicine and plays a fundamental role in defining myocardial injury in patients with acute coronary syndrome (ACS). Data have also shown that troponin levels are elevated in a significant proportion of patients hospitalized for heart failure (HF), which suggests a degree of cardiac damage even in the absence of overt clinical myocardial ischaemia.1 Although the precise mechanism underlying troponin elevation among hospitalized HF patients is unclear and possibly multifactorial, it has been hypothesized that troponin elevation defines a state of ongoing end-organ injury that mediates further worsening HF.2‒4 However, despite strong biological plausibility, prior work examining the prognostic utility of various troponin assays in the hospitalized HF setting has yielded mixed results.5,6 For example, among patients in the biomarker subset of the ASCEND-HF (Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure) trial, a higher troponin I level was independently predictive of in-hospital death or worsening HF, but was not associated with 30-day or 180-day outcomes.5 In contrast, investigators in the RELAX-AHF (Relaxin in Acute Heart Failure) study found higher baseline and peak levels of high-sensitivity troponin T to be strongly associated with 180-day cardiovascular mortality (CVM).6
Despite available therapy, within 60–90 days of discharge, mortality and readmission rates for hospitalized HF patients approach 15% and 30%, respectively. Although a multitude of risk scores for patients hospitalized for HF have been described, models are generally complex and have modest discriminatory power, and uptake has been limited. Thus, there is an unmet need for a simple, practical means of risk stratification as patients transition to the post-discharge vulnerable phase following HF hospitalization. In this context, although troponin I testing is commonly performed during hospitalization, the prognostic significance of troponin abnormalities during early outpatient follow-up and persistent troponin elevation from the inpatient to outpatient setting is unclear. The ASTRONAUT (Aliskiren Trial on Acute Heart Failure Outcomes) trial database affords the opportunity to formally study these questions.7
Methods
Study design
The study design and primary results of the ASTRONAUT trial have been previously reported.7,8 Briefly, ASTRONAUT was a prospective, multicentre, global, placebo-controlled randomized trial investigating the effects of aliskiren, a direct renin inhibitor, on clinical outcomes among stable patients hospitalized for HF. All patients were aged ≥18 years and had a left ventricular ejection fraction (EF) of ≤40%, elevated admission natriuretic peptide level [B-type natriuretic peptide (BNP) ≥400 pg/mL or N terminal pro-B-type natriuretic peptide (NT-proBNP) ≥1600 pg/mL], and signs and symptoms of fluid overload that required hospitalization. The trial found aliskiren to have no influence on clinical outcomes compared with placebo. The trial did not specify exclusion criteria based on admission or baseline troponin levels. However, patients with myocardial infarction (MI) or coronary revascularization within 3 months of enrolment or with investigator-reported unstable coronary artery disease (CAD) likely to require revascularization were excluded.
ASTRONAUT was conducted in full accordance with the Declaration of Helsinki and with institutional review board and ethics committee approval at all sites. Informed consent was obtained from all patients. The present analysis included patients in both the aliskiren and placebo study arms. All patients for whom baseline troponin I data were available were included.
Troponin I measurement
The trial protocol included measurement of troponin I prior to discharge at the time of study randomization (i.e. baseline/Visit 2), which occurred at a median of 5 days after hospital admission and after patients had been haemodynamically stabilized. The protocol also included measurement of troponin I at 1 month post-randomization. All troponin samples in ASTRONAUT were measured at a central core laboratory blinded to clinical data (Quest Diagnostics, Valencia, CA, USA). Serum concentrations were measured using the TnI-Ultra assay (Siemens Healthcare Diagnostics, Tarrytown, NY, USA) with a 99th percentile upper reference limit of 0.04 ng/mL and an imprecision at this level of 0.004 ng/mL. In the trial database, the lowest observed value was 0.02 ng/mL and lesser values were imputed as 0.01 ng/mL.
Study endpoints and definitions
The prespecified co-primary endpoints for the present analysis were: (i) all-cause mortality within 12 months, and (ii) the composite of CVM or hospitalization for HF (HHF) within 12 months. All clinical endpoints were adjudicated by a blinded clinical event committee (Brigham and Women’s Hospital, Boston, MA, USA). The definition of HHF was presentation requiring overnight hospitalization with signs and symptoms of HF and treatment with i.v. medications (i.e. diuretics, vasodilators, inotropes), mechanical fluid removal, an intra-aortic balloon pump, or initiation or intensification (i.e. doubling) of the maintenance diuretic dose.
Statistical analysis
Patients were grouped by the presence or absence of pre-discharge troponin elevation (i.e. measured at study baseline). Likewise, patients were grouped by the presence or absence of troponin elevation at 1 month. All continuous variables were reported as the mean ± standard deviation (SD) or median and interquartile range (IQR). Baseline demographics, vital signs and laboratory values, medical and medication histories, and clinical events were compared between groups using chi-squared, analysis of variance and Kruskal–Wallis distribution-free tests as appropriate.
The primary predictor in the present study was troponin I elevation (i.e. >0.04 ng/mL) and analyses were conducted for pre-discharge and 1-month troponin elevation separately. For evaluation of 12-month all-cause mortality and 12-month CVM/HHF, Kaplan–Meier curves were constructed for each study group and compared using log-rank tests. For clinical endpoints, univariable and multivariable Cox proportional hazards models were used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for the primary predictor. For 1-month troponin models, clinical events prior to troponin measurement were either excluded (i.e. death) or ignored (i.e. rehospitalization). The proportional hazards assumption was confirmed by Kolmogorov-type supremum tests. Prespecified interaction analyses were performed within multivariable models to test for differential associations between troponin elevation and endpoints by history of CAD and history of diabetes. For 1-month troponin elevation, additional interaction testing with pre-discharge troponin status was performed.
Multivariable models for clinical outcomes were adjusted for 28 prespecified covariates: age; gender; race; geographic region; ischaemic HF aetiology; New York Heart Association (NYHA) functional class; EF; systolic blood pressure (SBP); heart rate; NT-proBNP; serum sodium; serum blood urea nitrogen (BUN); serum creatinine; QRS duration; body mass index (BMI); aliskiren randomization; medical history (prior HF hospitalization, hypertension, CAD, atrial fibrillation, diabetes, chronic obstructive pulmonary disease), and background therapy [angiotensin-converting enzyme inhibitor (ACEI)/angiotensin II receptor blocker (ARB), beta-blocker, mineralocorticoid receptor antagonist (MRA), digoxin, implantable cardioverter-defibrillator (ICD), cardiac resynchronizaton therapy (CRT)]. Covariate data reflected values collected at baseline, except for 1-month troponin models, which utilized covariate data collected at 1-month follow-up when possible (i.e. SBP, heart rate, NT-proBNP, serum sodium, BUN, serum creatinine and BMI) The multiple imputation procedure [fully conditional specification methods as implemented in the MI and MIANALYZE procedures in SAS (SAS Institute, Inc., Cary, NC, USA)] was used for missing covariate data (<5% for all variables). All statistical analyses were performed using SAS Version 9.3. A two-tailed P value of <0.05 was considered to indicate statistical significance.
Results
Patient characteristics
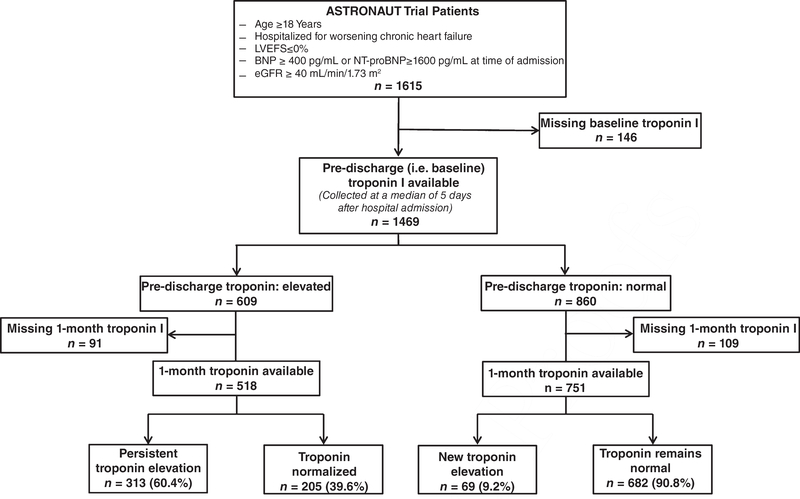
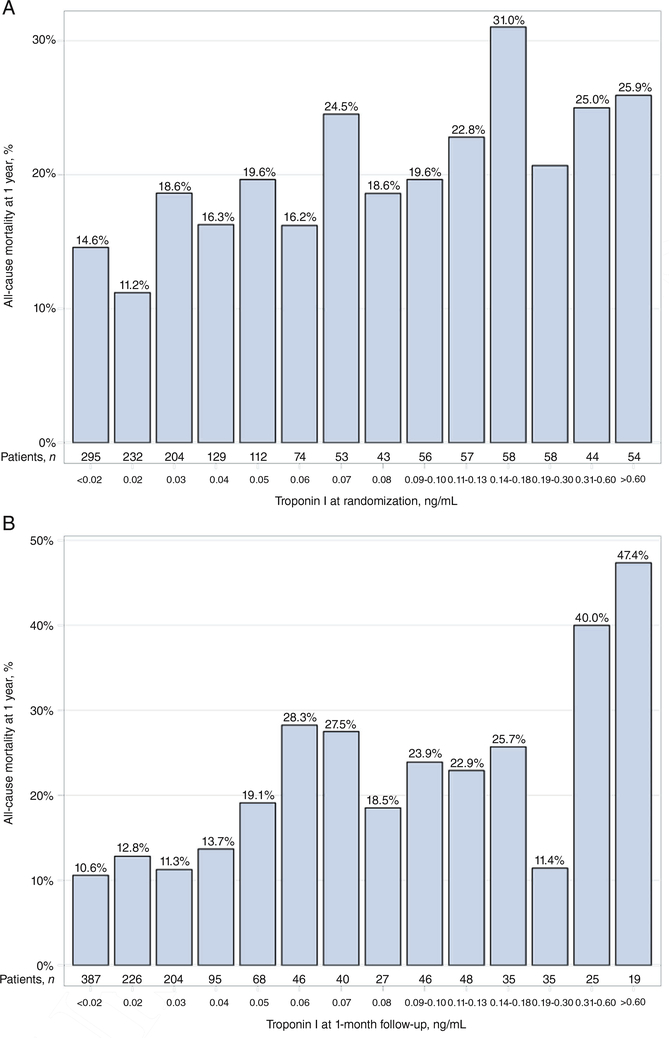
Of the 1615 patients in the ASTRONAUT efficacy cohort, data on troponin levels were available for 1469 (91.0%) patients at pre-discharge (i.e. baseline), 1301 (80.6%) at 1 month, and 1269 (78.6%) at both time-points (Figure 1). Figure 2 displays the distribution of patients by pre-discharge and 1-month troponin I levels and the corresponding rates of 12-month mortality. Table 1 displays baseline demographic, clinical and laboratory data for patients by pre-discharge troponin elevation status. Similar data by 1-month troponin elevation status and troponin trajectory from pre-discharge/baseline to 1 month are presented in supplementary material Tables S1 and S2, online, respectively. Among patients with elevated troponin I levels (pre-discharge, n = 860; 1 month, n = 389), median values at pre-discharge/baseline and 1 month were 0.09 ng/mL (IQR: 0.06–0.19 ng/mL) and 0.09 ng/mL (IQR: 0.06–0.15 ng/mL), respectively. Compared with those with normal values, patients with pre-discharge troponin elevation generally had higher SBP and NT-proBNP levels, and worse renal function. Notably, rates of CAD, coronary revascularization and prior MI did not differ by pre-discharge troponin status. In contrast, patients with 1-month troponin elevation were more likely to have established diabetes and CAD, but had rates of prior coronary revascularization and MI similar to those with normal troponin levels.
Figure 1.
Selection of the analytic cohort and breakdown of troponin testing at pre-discharge (baseline) through 1-month follow-up. BNP, B-type natriuretic peptide; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction
Figure 2.
All-cause mortality at 12 months by (A) pre-discharge and (B) 1-month post-discharge troponin I level
Table 1.
Baseline characteristics by pre-discharge troponin I elevation (>0.04ng/mL)
| Pre-discharge (baseline) troponin I value |
P value | ||
|---|---|---|---|
| Elevated | Normal | ||
| (n = 609) | (n = 860) | ||
| Median troponin I (ng/mL) | 0.09 (0.06–0.19) | 0.02 (0.01 –0.03) | <0.001 |
| Demographic data | |||
| Age, years, mean ± SD | 65.0 ± 12.0 | 64.0±12.3 | 0.119 |
| Male, n (%) | 482 (79.1%) | 652 (75.8%) | 0.134 |
| Race, n (%) | |||
| White | 41 0 (67.3%) | 608 (70.7%) | 0.167 |
| Black | 32 (5.3%) | 42 (4.9%) | 0.749 |
| Asian | 1 45 (23.8%) | 1 74 (20.2%) | 0.101 |
| Other | 22 (3.6%) | 36 (4.2%) | 0.578 |
| Region, n (%) | |||
| North America | 42 (6.9%) | 71 (8.3%) | 0.335 |
| Latin America | 52 (8.5%) | 96(11.2%) | 0.100 |
| Western Europe | 159 (26.1%) | 1 88 (21.9%) | 0.059 |
| Eastern Europe | 189 (31.0%) | 288 (33.5%) | 0.322 |
| Asia/Pacific | 1 67 (27.4%) | 21 7 (25.2%) | 0.347 |
| Time from admission to randomization, days, median (IQR) | 4 (2–7) | 5 (2–7) | 0.451 |
| Hospital length of stay, days, median (IQR) | 9 (5–14) | 8 (5–13) | 0.076 |
| Ejection fraction, %, mean ± SD | 27.6±7.8 | 27.9 ± 7.0 | 0.467 |
| NYHA class III/IV, n (%) | 384 (63.1%) | 552 (64.2%) | 0.657 |
| QRS duration on baseline ECG, ms, mean ± SD | 119±38 | 117±41 | 0.397 |
| Vital sign and laboratory data | |||
| Systolic blood pressure, mmHg, mean ± SD | 124.4 ± 14.0 | 122.5±12.5 | 0.010 |
| Heart rate, b.p.m., mean ± SD | 78.6 ± 16.6 | 77.1 ±15.2 | 0.077 |
| Weight, kg, mean ± SD | 76.3 ± 20.2 | 78.5±21.4 | 0.050 |
| BMI, kg/m2, mean ± SD | 26.8±5.9 | 27.3±6.3 | 0.121 |
| Haemoglobin, g/dL, mean ± SD | 13.7±2.0 | 13.7±2.0 | 0.890 |
| Serum sodium, mmol/L, mean ± SD | 138.9±3.7 | 138.7±3.6 | 0.445 |
| BUN, mmol/L, mean ± SD | 9.6±4.0 | 8.7±3.5 | <0.001 |
| Creatinine, mmol/L, mean ± SD | 103.5±27.2 | 98.2±27.0 | <0.001 |
| eGFR, mL/min/1.73 m2, mean ± SD | 64.6±19.0 | 68.4±20.7 | <0.001 |
| NT-proBNP at admission,a pg/mL, median (IQR) | 4876 (3000–10 828) | 3505 (2567–6679) | <0.001 |
| NT-proBNP at baseline,a pg/mL, median (IQR) | 3378 (1848–7025) | 2395 (1 341 –4409) | <0.001 |
| PRA, μIU/mL, pg/mL, median (IQR) | 3.3 (0.7–17.9) | 2.6 (0.4–14.9) | 0.050 |
| Past medical history, n (%) | |||
| Previous HF hospitalization | 41 9 (68.8%) | 569 (66.2%) | 0.288 |
| Coronary artery disease | 337 (55.3%) | 460 (53.5%) | 0.484 |
| Previous PCI | 114 (18.7%) | 1 65 (1 9.2%) | 0.822 |
| Previous CABG | 1 00 (1 6.4%) | 1 40 (1 6.3%) | 0.942 |
| Previous myocardial infarction | 266 (43.7%) | 353 (41.0%) | 0.314 |
| Previous stroke | 69 (11.3%) | 69 (8.0%) | 0.032 |
| Previous TIA | 17(2.8%) | 26 (3.0%) | 0.795 |
| Hypertension | 475 (78.0%) | 635 (73.8%) | 0.068 |
| Atrial fibrillation | 254 (41.7%) | 357 (41.5%) | 0.940 |
| Diabetes | 257 (42.2%) | 342 (39.8%) | 0.350 |
| COPD | 135 (22.2%) | 1 48 (1 7.2%) | 0.018 |
| Baseline therapies, n (%) | |||
| Diuretic | 584 (95.9%) | 825 (95.9%) | 0.973 |
| Beta-blocker | 489 (80.3%) | 732 (85.1%) | 0.015 |
| ACEI/ARB | 509 (83.6%) | 721 (83.8%) | 0.895 |
| MRA | 349 (57.3%) | 494 (57.4%) | 0.959 |
| MRA + ACEI/ARB | 286 (47.0%) | 41 2 (47.9%) | 0.721 |
| Digoxin | 265 (43.5%) | 323 (37.6%) | 0.022 |
| ICD | 94 (1 5.4%) | 137(15.9%) | 0.797 |
| CRT | 41 (6.7%) | 52 (6.0%) | 0.595 |
| Permanent pacemaker | 63 (1 0.3%) | 89 (1 0.3%) | 0.998 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; BNP, B-type natriuretic peptide; BUN, blood urea nitrogen; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; HF, heart failure; ICD, implantable cardioverter-defibrillator; IQR, interquartile range; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; PRA plasma renin activity; SD, standard deviation; TIA, transient ischaemic attack.
Data available for 676 patients at admission and all 1468 patients at pre-discharge (i.e. baseline).
Clinical outcomes
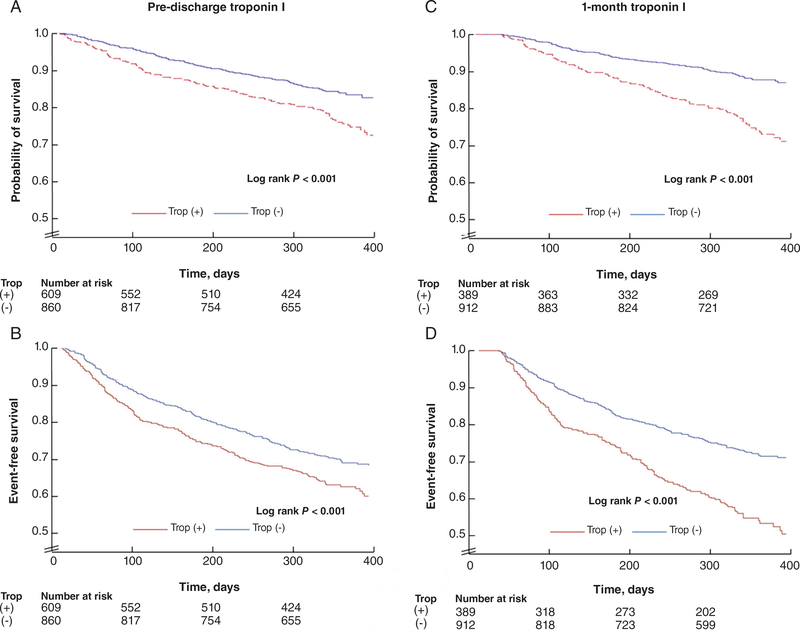
Event rates by pre-discharge and 1-month troponin status are displayed in Table 2 and supplementary material Table S3 (online), respectively. Kaplan–Meier time-to-first-event analysis demonstrated decreased event-free survival for both co-primary endpoints among patients with pre-discharge troponin elevation (log rank P ≤ 0.001) (Figure 3). Similar results were seen in the analysis of 1-month troponin status (log rank P < 0.001).
Table 2.
Event rates by pre-discharge troponin I elevation (>0.04ng/mL)
| Pre-discharge (baseline) troponin I value |
P value | ||
|---|---|---|---|
| Elevated | Normal | ||
| (n = 609) | (n = 860) | ||
| 12-month event rates, n (%) | |||
| All-cause mortality | 134 (22.0%) | 128 (14.9%) | <0.001 |
| CVM or HHF | 246 (40.4%) | 282 (32.8%) | 0.003 |
| CVM | 1 23 (20.2%) | 118 (13.7%) | 0.001 |
| Pump failure | 48 (7.9%) | 45 (5.2%) | 0.040 |
| Sudden cardiac death | 43 (7.1 %) | 39 (4.5%) | 0.038 |
| Fatal myocardial infarction | 10 (1.6%) | 4 (0.5%) | 0.028 |
| Presumed sudden death | 3 (0.5%) | 6 (0.7%) | 0.743 |
| Presumed CV death | 12 (2.0%) | 10 (1.2%) | 0.209 |
| Other CV death | 1 (0.2%) | 0 (0.0%) | 0.415 |
| Fatal stroke | 3 (0.5%) | 9 (1.0%) | 0.379 |
| CV procedural | 0 | 2 (0.2%) | 0.514 |
| Unknown | 3 (0.5%) | 3 (0.3%) | 0.697 |
| HHF | 1 72 (28.2%) | 223 (25.9%) | 0.325 |
| All-cause rehospitalization | 299 (49.1 %) | 391 (45.5%) | 0.169 |
| CV event | 261 (42.9%) | 295 (34.3%) | 0.001 |
| Myocardial infarction | 30 (4.9%) | 24 (2.8%) | 0.032 |
| Stroke | 12 (2.0%) | 29 (3.4%) | 0.108 |
| 6-month event rates, n (%) | |||
| All-cause mortality | 82 (1 3.5%) | 75 (8.7%) | 0.004 |
| CVM or HHF | 1 82 (29.9%) | 1 96 (22.8%) | 0.002 |
| CVM | 77 (12.6%) | 71 (8.3%) | 0.006 |
| HHF | 130 (21.3%) | 1 61 (1 8.7%) | 0.214 |
| All-cause rehospitalization | 239 (39.2%) | 312 (36.3%) | 0.247 |
| 30-day event rates, n (%) | |||
| All-cause mortality | 14 (2.3%) | 8 (0.9%) | 0.033 |
| HHF | 41 (6.7%) | 39 (4.5%) | 0.067 |
| All-cause rehospitalization | 93 (15.3%) | 99 (11.5%) | 0.035 |
CV cardiovascular; CVM, cardiovascular mortality; HHF, hospitalization for heart failure.
Figure 3.
Kaplan–Meier curves for (A) all-cause mortality and (B) cardiovascular mortality or hospitalization for heart failure at 12 months follow-up by pre-discharge troponin I status, and (C) all-cause mortality and (D) cardiovascular mortality or hospitalization for heart failure at 12 months follow-up by 1-month post-discharge troponin I status. Times to events were compared using log-rank tests
Unadjusted and adjusted outcome analyses are presented in Table 3. Both pre-discharge and 1-month troponin elevation were predictive of 12-month all-cause mortality and CVM/HHF in unadjusted analyses (all: P < 0.001). However, after accounting for potential confounders, associations between pre-discharge troponin status and either outcome were no longer significant (P ≥0.113). In contrast, 1-month troponin elevation maintained a robust and statistically significant association with heightened risk for 12-month all-cause mortality (HR 1.59, 95% CI 1.18–2.13; P =0.002) and CVM/HHF (HR 1.28, 95% CI 1.03–1.58; P =0.024). Interaction testing revealed a consistent prognostic value of 1-month troponin I elevation irrespective of pre-discharge troponin status. Associations between 1-month troponin elevation and 12-month all-cause mortality (P value for interaction: 0.764) and CVM/HHF (P value for interaction: 0.163) were similar among patients with newly elevated (i.e. normal pre-discharge) and persistently elevated levels.
Table 3.
Clinical outcomes by pre-discharge and 1-month post-discharge troponin I elevation (>0.04 ng/mL)a
| Endpoint (12months) | Unadjusted HR (95% CI) | Adjusted HR (95% CI)b |
|---|---|---|
| Pre-discharge troponin I elevation | ||
| All-cause mortality | 1.58 (1.24 – 2.01), P < 0.001 | 1.23 (0.95 – 1.59), P = 0.113 |
| CVM/HHF | 1.34 (1.13 – 1.59), P < 0.001 | 1.1 1 (0.93 – 1.33), P = 0.257 |
| 1-month troponin I elevationc | ||
| All-cause mortality | 2.28 (1.73 – 3.01), P < 0.001 | 1.59 (1.18 – 2.13), P = 0.002 |
| CVM/HHF | 1.86 (1.53 – 2.26), P < 0.001 | 1.28 (1.03 – 1.58), P = 0.024 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CI, confidence interval; CVM, cardiovascular mortality; HHF, hospitalization for heart failure; HR, hazard ratio; NT-proBNP, N terminal pro-B-type natriuretic peptide.
Data represent HRs and 95% CIs for risk for primary co-endpoints for patients with elevated troponin I levels compared with patients with non-elevated troponin I levels.
Adjusted forage, gender, race, geographic region, ischaemic heartfailure aetiology, NewYork HeartAssociation class III/IV, ejection fraction, systolic blood pressure, heart rate, NT-proBNP level, serum sodium, blood urea nitrogen, serum creatinine, QRS duration, aliskiren randomization, past medical history (prior HHF, coronary artery disease, atrial fibrillation, diabetes, chronic obstructive pulmonary disease), and medications (ACEI/ARB, beta-blocker, mineralocorticoid receptor antagonist, digoxin, implantable cardioverter-defibrillator, cardiac resynchronization therapy). For systolic blood pressure, heart rate, NT-proBNP, serum sodium, BUN, serum creatinine and body mass index, baseline data were used within the pre-discharge/baseline troponin model and 1-month data were used within the 1-month troponin model.
Outcome analyses reflect events occurring after 1 month of follow-up (i.e. landmark analysis).
Influences of CAD and diabetes
Results of interaction analysis by history of CAD and diabetesIn this large cohort of hospitalized HF patients with reduced EF, are displayed in Table 4. After multivariable adjustment, there was no significant interaction between history of CAD or diabetes and pre-discharge troponin status for either 12-month all-cause mortality or CVM/HHF. Similarly, the association between 1-month troponin elevation and outcomes was not significantly influenced by the presence or absence of diabetes. By comparison, there was a statistically significant interaction between CAD and 1-month troponin elevation for 12-month all-cause mortality (P value for interaction: 0.009) in which the predictive value was driven by a pronounced association among patients with established CAD (HR 2.12, 95% CI 1.47–3.07; P <0.001). The association between 1-month troponin elevation and 12-month mortality was non-significant among patients without documented CAD (HR 0.96, 95% CI 0.59–1.57; P =0.873). There was no significant interaction between CAD and 1-month troponin status for the 12-month composite endpoint (P value for interaction: 0.193).
Table 4.
Association between troponin elevation and study endpoints by coronary artery disease and diabetes status
| Pre-discharge (baseline) troponin I | |||
|---|---|---|---|
| Endpoint(12 months) | Subgroup | Adjusted HR (95% CI) | P for interaction |
| ACM | CAD | 1.36 (0.99–1.87) | 0.737 |
| Non-CAD | 1.05 (0.71 –1.58) | ||
| DM | 1.35 (0.92–1.97) | 0.427 | |
| Non-DM | 1.15 (0.82–1.61) | ||
| CVM/HHF | CAD | 0.98 (0.78–1.24) | 0.052 |
| Non-CAD | 1.29 (0.96–1.72) | ||
| DM | 1.07 (0.82–1.40) | 0.862 | |
| Non-DM | 1.11 (0.87–1.43) | ||
| 1-month troponin I | |||
| ACM | CAD | 2.12 (1.47–3.07) | 0.009 |
| Non-CAD | 0.96 (0.59–1.57) | ||
| DM | 1.79 (1.16–2.77) | 0.456 | |
| Non-DM | 1.43 (0.96–2.14) | ||
| CVM/HHF | CAD | 1.41 (1.09–1.83) | 0.1 93 |
| Non-CAD | 1.07 (0.76–1.51) | ||
| DM | 1.31 (0.97–1.78) | 0.787 | |
| Non-DM | 1.24 (0.93 –1.65) | ||
ACM, all-cause mortality; CAD, coronary artery disease; CI, confidence interval; CVM/HHF, cardiovascular mortality or hospitalization for heart failure; DM, diabetes mellitus; HR, hazard ratio.
Data represent interaction P values from multivariate analysis. Covariates included in the multivariate model: age, gender, race, geographic region, ischaemic heart failure aetiology, New York Heart Association class III/IV, ejection fraction, systolic blood pressure, heart rate, NT-proBNP level, serum sodium, blood urea nitrogen, serum creatinine, QRS duration, aliskiren randomization, past medical history (prior HHF, coronary artery disease, atrial fibrillation, diabetes, chronic obstructive pulmonary disease), and medications (ACEI/ARB, beta-blocker, mineralocorticoid receptor antagonist, digoxin, implantable cardioverter-defibrillator, cardiac resynchronization therapy).
Discussion
In this large cohort of hospitalized HF patients with reduced EF, troponin I level was elevated in >40% of in-hospital patients and remained persistently elevated in >60% of these patients during 1-month post-discharge follow-up. Rates of comorbid CAD and diabetes did not differ by pre-discharge troponin status, but were significantly higher among patients with 1-month troponin elevation. Elevated troponin level at 1-month follow-up, but not pre-discharge, was independently predictive of adverse post-discharge outcomes at 12 months. The prognostic value of 1-month troponin elevation was similar among patients with, respectively, newly elevated (i.e. normal pre-discharge level) and persistently elevated (i.e. elevated baseline level) levels. Like-wise, traditional markers of ischaemic heart disease, including existing CAD and diabetes, did not influence the relationship between troponin elevation and outcomes, with the exception of a significantly stronger association between 1-month troponin elevation and death among patients with a history of CAD.
Many prior studies have established the prognostic significance of troponin elevation during hospitalization for HF for short-term in-hospital outcomes.2,9,10 However, rates of in-hospital mortality in North American hospitalized HF registries are 2–4% and the vast majority of deaths occur after discharge from the index hospitalization.11,12 Thus, there is perhaps a more pressing need to define the prognostic link between troponin measurements and post-discharge outcomes in this population. Unfortunately, databases with the necessary longitudinal data capture are limited and, to the present authors’ knowledge, the only prior investigations of troponin levels in the hospitalized HF trial literature come from the PROTECT pilot, ASCEND-HF and RELAX-AHF studies.5,6,13,14 Of these analyses, only the RELAX-AHF investigators evaluated the predictive value of post-discharge troponin levels by measuring levels of high-sensitivity troponin T up to 14 days post-randomization.6 However, in that study, prognostic significance was assessed only for baseline and peak troponin levels and no consideration was given to troponin measurements at specific time-points, thus limiting the clinical application of the study findings.6 A recent subsequent study from the same authors supported the 14-day time-point as offering greater prognostic gains than models using earlier measurement time-points.6,14 In comparison, the present data from ASTRONAUT specifically compared the relative prognostic value of troponin I at baseline (i.e. pre-discharge) and extended 1-month follow-up and found only the 1-month measurement to be predictive of subsequent mortality and HHF. Coupled with the widespread availability of troponin I assays in comparison with high-sensitivity troponin T assays, these data from ASTRONAUT may more easily facilitate confirmatory study in routine clinical practice. Additional novel features of this analysis include the first investigation of a persistently elevated (i.e. elevated pre-discharge and at 1 month) vs. newly elevated troponin level as hospitalized HF patients transition from the inpatient to ambulatory settings and suggest there is no significant difference in clinical outcomes between these groups. Lastly, to the present group’s knowledge, these data are the first to assess the prognostic value of troponin testing in hospitalized HF with reduced EF patients with and without traditional risk factors for myocardial ischaemia and suggest that a strategy of post-discharge troponin I testing may be particularly informative among patients with comorbid CAD.
The mechanism underlying troponin elevation among HF patients is unclear and is potentially multifactorial, relating to microvascular dysfunction, CAD and/or elevated ventricular filling pressures.2‒4 Likewise, the mechanism underlying the potentially differing prognostic significance of baseline and 1-month troponin I measurements remains uncertain. Viewing prior data and the results of the present analysis in aggregate, it is notable that whereas studies of troponin measurement during hospitalization for HF have shown variable ability to predict long-term outcomes, analyses in chronic ambulatory HF populations have more consistently demonstrated strong independent prognostic value.2,5,15‒18 In explanation, it might be speculated that troponin elevation during hospitalization reflects the recent unstable period surrounding admission with attendant neurohormonal and haemodynamic perturbations, and thus reflects only a transient state of decompensation that tracks with other markers of worsening HF. Although some authors have argued that troponin elevation in this acute setting reflects critical end-organ injury that mediates subsequent HF progression, this hypothesis remains to be proven and short-term investigational therapies targeting end-organ salvage have failed to improve long-term outcomes.19‒22 Thus, although hospitalization in itself portends a poor subsequent long-term prognosis, among HF with reduced EF patients surviving to discharge, it remains unclear if troponin elevation near the time of acute decompensation offers incremental predictive value above traditional risk markers. In contrast, myonecrosis during the stable ambulatory phase may represent a chronic ongoing state of cardiac dysfunction despite clinical stability and evidence-based HF therapies, and therefore may conceivably be more likely to carry independent prognostic significance. The precise point in time following acute decompensation at which troponin levels may transition to the prognostic value seen in chronic HF populations is unclear, but the current results from ASTRONAUT suggest this may occur by 30 days.
Clinical research implications
The ‘vulnerable phase’ following HF hospitalization encompasses the first few months post-discharge, during which the risk for adverse events is highest.23 In an attempt to mitigate risk, early post-discharge clinic visits are recommended for all HF patients and represent a cornerstone of post-hospitalization care in many health systems.24,25 However, although these visits are strongly recommended, the precise structure and goals of these visits remain ill-defined and practice patterns may vary. This is likely to reflect a lack of sound evidence supporting specific interventions or diagnostic testing.
Serum troponin I represents a widely available, simple and inexpensive laboratory test that is frequently ordered while HF patients are in hospital, but traditionally is not ordered after discharge.9 The ASTRONAUT data highlight the fact that a substantial minority of HF patients have elevated troponin I levels at 1-month clinic follow-up and support this measure as an under-recognized, robust and independent predictor of risk for subsequent mortality and readmission, particularly among patients with comorbid CAD. Notably, this predictive value was not significantly influenced by the pre-discharge troponin measurement, which further suggests a relative prognostic importance of testing at 1-month follow-up over in-hospital testing. Thus, in the current setting of sparse evidence and primarily empiric approaches to contemporary post-hospitalization HF care, the present study supports early post-discharge troponin I testing as a simple means of risk stratification and a potential complement to a simultaneous measurement of natriuretic peptide level.26 These data support further prospective confirmatory study to determine the potential of post-discharge ambulatory troponin testing in guiding the allocation of health care resources, informing clinical management decisions and as a target for investigational therapies. This line of research may hold particular promise in patients with comorbid CAD.
In the current study, it is notable that nearly one in 10 patients in whom in-hospital troponin levels were normal showed elevated levels at 30 days. However, this proportion is lower than that seen in the PROTECT pilot study, in which 21% of patients with negative baseline troponin T developed elevated levels by day 7.13 Together, these analyses suggest that the window during which patients are vulnerable to the development of cardiac injury extends beyond the acute decompensated phase. In the context of the recent neutral findings from the TRUE-AHF (Trial of Ularitide Efficacy and Safety in Acute Heart Failure) trial and the topline results from the RELAX-AHF-2 study, the current results from ASTRONAUT suggest that a clinical trial strategy focused on targeting cardiac injury with short-term in-hospital therapy is unlikely to improve long-term outcomes.19,27 Such an approach may target only a fraction of the window surrounding the HF hospitalization during which patients are vulnerable to injury and neglects the significant burden of ongoing cardiac damage that may occur post-discharge. Rather, the prevalence and prognostic value of continued post-discharge troponin elevation seen here support a clinical trial design in which study therapies are initiated in-hospital and continued during the outpatient phase.23
Limitations
The limitations of the present study should be acknowledged. Firstly, ASTRONAUT patients were enrolled at a median of 5 days after admission and were required to be clinically stable. Thus, the present results for pre-discharge (baseline) troponin I levels should be viewed in this context and do not represent measurements during active decompensation. Likewise, it is possible that patients with elevated pre-discharge troponin I in ASTRONAUT had considerably different measurements at the time of initial hospital presentation. Secondly, despite rigorous multivariable modelling, this retrospective observational analysis is unable to definitively prove cause–effect relationships. Thirdly, the trial cohort was comprised completely of patients with reduced EF and these findings may not apply to patients with HF with preserved EF. Fourthly, it should be emphasized that patients diagnosed with active or recent ACS were excluded from ASTRONAUT and the current data should not be extrapolated to patients hospitalized with HF and concurrent MI. Moreover, although the presence of ACS represented a strict exclusion criterion, the possibility that some patients with troponin elevation had unrecognized ACS cannot be fully ruled out. Fifthly, the current analysis did not explore troponin level as a continuous variable and does not specifically address potential prognostic differences by varying degree of troponin elevation. However, the decision not to perform continuous variable analysis was prespecified because 50–60% of patients showed troponin levels within the three lowest values (i.e. 0.01, 0.02 and 0.03 ng/mL), and because of the existence of imputed data for values of <0.02 ng/mL (295 patients at baseline; 387 patients at 1 month) (Figure 2). Lastly, it is unclear if these results are generalizable to other troponin assays, including troponin T or high-sensitivity troponin assays. It is possible that some ASTRONAUT patients with normal troponin I would have demonstrated elevated troponin levels if a high-sensitivity assay had been used.
Conclusions
In this large clinical trial cohort of hospitalized HF patients with reduced EF, troponin I was elevated in >40% of hospitalized patients and remained persistently elevated in >60% of these patients early post-discharge. Elevated troponin I level at 1-month follow-up, but not pre-discharge troponin elevation, was independently predictive of increased clinical events at 12 months, particularly among patients with comorbid CAD. The prognostic value of troponin elevation at 1-month follow-up was similar across patients with previously normal and those with an elevated in-hospital troponin level. The measurement of troponin I during the early post-discharge vulnerable phase should be considered as a practical means of risk stratification and troponin level may be prospectively investigated as a therapeutic target.
Supplementary Material
AppendixS 1. Table S1. Patient characteristics by 1-month troponin I elevation (>0.04 ng/mL).
Table S2. Patient characteristics by troponin I trajectory from pre-discharge/baseline to 1-month post-discharge follow-up.
Table S3. Event rates by 1-month troponin I elevation (>0.04 ng/mL).
Acknowledgments
Funding
Financial and material support for the ASTRONAUT trial was provided by Novartis Pharma AG (Basel, Switzerland). H.P.S. conducted all final analyses for this report with funding from the Center for Cardiovascular Innovation, Northwestern University Feinberg School of Medicine, Chicago, IL, USA, and takes responsibility for the integrity of the data.
Footnotes
Conflict of interest: S.J.G. is supported by the National Heart, Lung and Blood Institute (NHLBI) T32 post-doctoral training grant (5T32HL069749–14). J.B. reports research support from the National Institutes of Health, European Union and the Patient-Centered Outcomes Research Institute, and has served a consultant for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Meyers Squib, Cardiocell, Janssen, Novartis, Relypsa, ZS Pharma, Medtronic, Merck and CVRx. G.C.F. reports significant consulting for Novartis, and modest consulting for Amgen, Bayer, Medtronic and Janssen, holds the Eliot Corday Chair of Cardiovascular Medicine at the University of California Los Angeles and is also supported by the Ahmanson Foundation (Los Angeles, CA, USA). M.V. is supported by the NHLBI T32 postdoctoral training grant (T32HL007604). S.D.S. has received grant funding, consultant fees and travel support from Novartis. A.P.M. has served on committees of clinical studies sponsored by Amgen, Bayer, Abbott Vascular, Cardiorentis, Johnson & Johnson and Novartis Pharma. M.B. has served as a consultant for AstraZeneca, Bayer, Boehringer-Ingelheim, Daiichi-Sankyo, AWD Dresden, Berlin-Chemie, MSD, Novartis, Pfizer, Sanofi-Aventis and Servier. F.Z. has received grant funding from Novartis, BG Medicine and Roche Diagnostics, served on a board for Boston Scientific and as a consultant for Novartis, Takeda, AstraZeneca, Boehringer-Ingelheim, GE Healthcare, Relypsa, Servier, Boston Scientific, Bayer, Johnson & Johnson and ResMed. M.G. has served as a consultant for Abbott Laboratories, Astellas, AstraZeneca, Bayer HealthCare, CorThera, Cytokinetics, DebioPharm, Errekappa Terapeutici, GlaxoSmithKline, Ikaria, Johnson & Johnson, Medtronic, Merck, Novartis Pharma, Otsuka Pharmaceuticals, Palatin Technologies, Pericor Therapeutics, Protein Design Laboratories, Sanofi-Aventis, Sigma Tau, Solvay Pharmaceuticals, Takeda Pharmaceutical and Trevena Therapeutics. All other authors have no conflicts to declare.
References
- 1.Januzzi JL Jr, Filippatos G, Nieminen M, Gheorghiade M. Troponin elevation in patients with heart failure: on behalf of the third Universal Definition of Myocardial Infarction Global Task Force: Heart Failure Section. Eur Heart J 2012;33:2265–2271. [DOI] [PubMed] [Google Scholar]
- 2.Kociol RD, Pang PS, Gheorghiade M, Fonarow GC, O’Connor CM, Felker GM. Troponin elevation in heart failure prevalence, mechanisms, and clinical implications. J Am Coll Cardiol 2010;56:1071–1078. [DOI] [PubMed] [Google Scholar]
- 3.DeVore AD, Greiner MA, Sharma PP, Qualls LG, Schulte PJ, Cooper LB, Mentz RJ, Pang PS, Fonarow GC, Curtis LH, Hernandez AF. Development and validation of a risk model for in-hospital worsening heart failure from the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J 2016;178:198–205. [DOI] [PubMed] [Google Scholar]
- 4.Shionimya H, Koyama S, Tanada Y, Takahashi N, Fujiwara H, Takatsu Y, Sato Y. Left ventricular end-diastolic pressure and ejection fraction correlate independently with high-sensitivity cardiac troponin-T concentrations in stable heart failure. J Cardiol 2015;65:526–530. [DOI] [PubMed] [Google Scholar]
- 5.Felker GM, Hasselblad V, Tang WH, Hernandez AF, Armstrong PW, Fonarow GC, Voors AA, Metra M, McMurray JJ, Butler J, Heizer GM, Dickstein K, Massie BM, Atar D, Troughton RW, Anker SD, Califf RM, Starling RC, O’Connor CM. Troponin I in acute decompensated heart failure: insights from the ASCEND-HF study. Eur J Heart Fail 2012;14:1257–1264. [DOI] [PubMed] [Google Scholar]
- 6.Felker GM, Mentz RJ, Teerlink JR, Voors AA, Pang PS, Ponikowski P, Greenberg BH, Filippatos G, Davison BA, Cotter G, Prescott MF, Hua TA, Lopez-Pintado S, Severin T, Metra M. Serial high sensitivity cardiac troponin T measurement in acute heart failure: insights from the RELAX-AHF study. Eur J Heart Fail 2015;17:1262–1270. [DOI] [PubMed] [Google Scholar]
- 7.Gheorghiade M, Böhm M, Greene SJ, Fonarow GC, Lewis EF, Zannad F, Solomon SD, Baschiera F, Botha J, Hua TA, Gimpelewicz CR, Jaumont X, Lesogor A, Maggioni AP; ASTRONAUT Investigators and Coordinators. Effect of aliskiren on postdischarge mortality and heart failure readmissions among patients hospitalized for heart failure: the ASTRONAUT randomized trial. JAMA 2013;309:1125–1135. [DOI] [PubMed] [Google Scholar]
- 8.Gheorghiade M, Albaghdadi M, Zannad F, Fonarow GC, Böhm M, Gimpelewicz C, Botha J, Moores S, Lewis EF, Rattunde H, Maggioni A; ASTRONAUT Investigators and Study Coordinators. Rationale and design of the multicentre, randomized, double-blind, placebo-controlled Aliskiren Trial on Acute Heart Failure Outcomes (ASTRONAUT). Eur J Heart Fail 2011;13:100–106. [DOI] [PubMed] [Google Scholar]
- 9.Peacock WFIV, De Marco T, Fonarow GC, Diercks D, Wynne J, Apple FS, Wu AH; ADHERE Investigators. Cardiac troponin and outcome in acute heart failure. N Engl J Med 2008;358:2117–2126. [DOI] [PubMed] [Google Scholar]
- 10.Waxman DA, Hecht S, Schappert J, Husk G. A model for troponin I as a quantitative predictor of in-hospital mortality. J Am Coll Cardiol 2006;48:1755–1762. [DOI] [PubMed] [Google Scholar]
- 11.Adams KF Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP; ADHERE Scientific Advisory Committee and Investigators. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J 2005;149:209–216. [DOI] [PubMed] [Google Scholar]
- 12.Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CS, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 2014;63:1123–1133. [DOI] [PubMed] [Google Scholar]
- 13.O’Connor CM, Fiuzat M, Lombardi C, Fujita K, Jia G, Davison BA, Cleland J, Bloomfield D, Dittrich HC, Delucca P, Givertz MM, Mansoor G, Ponikowski P, Teerlink JR, Voors AA, Massie BM, Cotter G, Metra M. Impact of serial troponin release on outcomes in patients with acute heart failure: analysis from the PROTECT pilot study. Circ Heart Fail 2011;4:724–732. [DOI] [PubMed] [Google Scholar]
- 14.Demissei BG, Cotter G, Prescott MF, Felker GM, Filippatos G, Greenberg BH, Pang PS, Ponikowski P, Severin TM, Wang Y, Qian M, Teerlink JR, Metra M, Davison BA, Voors AA. A multimarker multi-time point-based risk stratification strategy in acute heart failure: results from the RELAX-AHF trial. Eur J Heart Fail 2017;19:1001–1010. [DOI] [PubMed] [Google Scholar]
- 15.Latini R, Masson S, Anand IS, Missov E, Carlson M, Vago T, Angelici L, Barlera S, Parrinello G, Maggioni AP, Tognoni G, Cohn JN; Val-HeFT Investigators. Prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation 2007;116:1242–1249. [DOI] [PubMed] [Google Scholar]
- 16.Miller WL, Hartman KA, Burritt MF, Grill DE, Jaffe AS. Profiles of serial changes in cardiac troponin T concentrations and outcome in ambulatory patients with chronic heart failure. J Am Coll Cardiol 2009;54:1715–1721. [DOI] [PubMed] [Google Scholar]
- 17.Masson S, Latini R, Anand IS. An update on cardiac troponins as circulating biomarkers in heart failure. Curr Heart Fail Rep 2010;7:15–21. [DOI] [PubMed] [Google Scholar]
- 18.Masson S, Anand I, Favero C, Barlera S, Vago T, Bertocchi F, Maggioni AP, Tavazzi L, Tognoni G, Cohn JN, Latini R; Valsartan Heart Failure Trial (Val-HeFT) and Gruppo Italiano per lo Studio della Sopravvivenza nell’Insufficienza Cardiaca–Heart Failure (GISSI-HF) Investigators. Serial measurement of cardiac troponin T using a highly sensitive assay in patients with chronic heart failure: data from 2 large randomized clinical trials. Circulation 2012;125:280–288. [DOI] [PubMed] [Google Scholar]
- 19.Packer M, O’Connor C, McMurray JJV, Wittes J, Abraham WT, Anker SD, Dickstein K, Filippatos G, Holcomb R, Krum H, Maggioni AP, Mebazaa A, Peacock WF, Petrie MC, Ponikowski P, Ruschitzka F, van Veldhuisen DJ, Kowarski LS, Schactman M, Holzmeister J; TRUE-AHF Investigators. Effect of ularitide on cardiovascular mortality in acute heart failure. N Engl J Med 2017;376:1956–1964. [DOI] [PubMed] [Google Scholar]
- 20.O’Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJ, Nieminen MS, Reist CJ, Rouleau JL, Swedberg K, Adams KF Jr, Anker SD, Atar D, Battler A, Botero R, Bohidar NR, Butler J, Clausell N, Corbalan R, Costanzo MR, Dahlstrom U, Deckelbaum LI, Diaz R, Dunlap ME, Ezekowitz JA, Feldman D, Felker GM, Fonarow GC, Gennevois D, Gottlieb SS, Hill JA, Hollander JE, Howlett JG, Hudson MP, Kociol RD, Krum H, Laucevicius A, Levy WC, Mendez GF, Metra M, Mittal S, Oh BH, Pereira NL, Ponikowski P, Tang WH, Tanomsup S, Teerlink JR, Triposkiadis F, Troughton RW, Voors AA, Whellan DJ, Zannad F, Califf RM. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med 2011;365:32–43. [DOI] [PubMed] [Google Scholar]
- 21.Massie BM, O’Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, Weatherley BD, Cleland JG, Givertz MM, Voors A, DeLucca P, Mansoor GA, Salerno CM, Bloomfield DM, Dittrich HC; PROTECT Investigators and Committees. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. N Engl J Med 2010;363:1419–1428. [DOI] [PubMed] [Google Scholar]
- 22.Packer M, Holcomb R, Abraham WT, Anker S, Dickstein K, Filippatos G, Krum H, Maggioni AP, McMurray JJV, Mebazaa A, O’Connor C, Peacock F, Ponikowski P, Ruschitzka F, van Veldhuisen DJ, Holzmeister J; TRUE-AHF Investigators and Committees. Rationale for and design of the TRUE-AHF trial: the effects of ularitide on the short-term clinical course and long-term mortality of patients with acute heart failure. Eur J Heart Fail 2017;19:673–681. [DOI] [PubMed] [Google Scholar]
- 23.Greene SJ, Fonarow GC, Vaduganathan M, Khan SS, Butler J, Gheorghiade M. The vulnerable phase after hospitalization for heart failure. Nat Rev Cardiol 2015;12:220–229. [DOI] [PubMed] [Google Scholar]
- 24.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147–239. [DOI] [PubMed] [Google Scholar]
- 25.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; Authors/Task Force Members, Document Reviewers. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 26.Greene SJ, Maggioni AP, Fonarow GC, Solomon SD, Böhm M, Kandra A, Prescott MF, Reimund B, Hua TA, Lesogor A, Zannad F, Gheorghiade M; ASTRO-NAUT Investigators and Coordinators. Clinical profile and prognostic significance of natriuretic peptide trajectory following hospitalization for worsening chronic heart failure: findings from the ASTRONAUT trial. Eur J Heart Fail 2015;17:98–108. [DOI] [PubMed] [Google Scholar]
- 27.Teerlink JR, Voors AA, Ponikowski P, Pang PS, Greenberg BH, Filippatos G, Felker GM, Davison BA, Cotter G, Gimpelewicz C, Boer-Martins L, Wernsing M, Hua TA, Severin T, Metra M. Serelaxin in addition to standard therapy in acute heart failure: rationale and design of the RELAX-AHF-2 study. Eur J Heart Fail 2017;19:800–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
AppendixS 1. Table S1. Patient characteristics by 1-month troponin I elevation (>0.04 ng/mL).
Table S2. Patient characteristics by troponin I trajectory from pre-discharge/baseline to 1-month post-discharge follow-up.
Table S3. Event rates by 1-month troponin I elevation (>0.04 ng/mL).