Abstract
In the striatum, histamine H3 receptors (H3Rs) are co-expressed with adenosine A2A receptors (A2ARs) in the cortico-striatal glutamatergic afferents and the GABAergic medium-sized spiny neurons that originate from the indirect pathway of the basal ganglia. This location allows H3Rs and A2ARs to regulate the striatal GABAergic and glutamatergic transmission. However, whether these receptors can physically interact has not yet been assessed. To test this hypothesis, a heteromer-selective in vitro assay was used to detect functional complementation between a chimeric A2AR302-Gαqi4 and wild-type H3Rs in transfected HEK-293 cells. H3R activation with the agonist RAMH resulted in Ca2+ mobilization (pEC50 7.31 ± 0.23; maximal stimulation, Emax 449 ± 25% of basal) indicative of receptor heterodimerization. Functional H3R-A2AR heteromers were confirmed by co-immunoprecipitation and observations of differential cAMP signaling when both receptors were co-expressed in the same cell. In membranes from rat striatal synaptosomes, H3R activation decreased A2AR affinity for the agonist CGS-21680 (pKi values 8.10 ± 0.04 and 7.70 ± 0.04). Moreover, H3Rs and A2ARs co-immunoprecipitated in protein extracts from striatal synaptosomes. These results support the existence of a H3R-A2AR heteromer with possible physiological implications for the modulation of the intra-striatal transmission.
Keywords: Adenosine A2A receptor, histamine H3 receptor, striatum, GPCR heterodimers, basal ganglia
1. Introduction
The actions of histamine in the periphery on airway constriction, inflammation and gastric acid secretion are well known, whereas its function in the Central Nervous System (CNS) is not yet fully understood. In mammals, histamine actions are mediated by four (H1R-H4R) G protein-coupled receptors (GPCRs), of which the H3 receptor (H3R) displays the highest expression in the brain. In the CNS, histamine is released from the terminals and dendrites of neurons located in the hypothalamic tuberomammilary nucleus, from where they innervate most of the CNS, including nuclei belonging to the basal ganglia, a subcortical group of structures intimately related to the control of motor behavior (Panula and Nuutinen, 2013; Panula et al., 2015).
The striatum is the main input nucleus of the basal ganglia and integrates motor and sensory information. The primary striatal afferents are glutamatergic axons originating from neurons located in the cerebral cortex and thalamus, and dopaminergic axons of sustantia nigra pars compacta neurons (Bolam et al., 2000). In turn, the axons of the GABAergic medium-sized spiny neurons (MSNs), which account for more than 90% of the striatal neuronal population (Kemp and Powell, 1971), project to the globus pallidus and sustantia nigra pars reticulata (Bolam et al., 2000).
H3Rs are abundantly expressed in the striatum either as pre-synaptic auto- and hetero-receptors or as post-synaptic receptors, with the latter located on the bodies of the MSNs and GABAergic or cholinergic interneurons (Pillot et al., 2002; González-Sepúlveda et al., 2013; Bolam and Ellender, 2016). The understanding of the physiological role of striatal H3Rs is complicated by their ubiquitous expression and capability to engage in protein-protein interactions with other GPCRs (Nieto-Alamilla et al., 2016). Of particular interest in the striatum are dopamine and adenosine receptors, which are also highly expressed. Based on the selective expression of dopamine and adenosine receptor subtypes, MSNs can be segregated into two neuronal populations. Neurons that originate the basal ganglia direct pathway (dMSNs) are identified by the expression of D1-like receptors (D1Rs) whereas the indirect pathway population (iMSNs) is formed by neurons expressing adenosine A2A receptors (A2ARs) and D2-like receptors (D2Rs) (Schiffman et al., 1991; Ferre et al., 1997; Tapper et al., 2004). In the striatum, H3Rs have been shown to form heterodimeric protein-protein complexes with dopamine D2 and D1 receptors (Ellenbroek, 2013), and heterotrimers with σ1/D1 receptors and NMDA/D1 receptors (Moreno et al., 2014; Rodríguez-Ruíz et al., 2016). In turn, A2ARs can form dimers with D2, cannabinoid CB1 and adenosine A1 receptors (Ciruela et al., 2006; Carriba et al., 2007; Hillon et al., 2002) and oligomers with D2 and mGlu5 glutamate receptors (Cabello et al., 2009).
Both H3 and A2A receptors modulate intra-striatal synaptic transmission. The activation of Gαi/o-coupled H3Rs results in inhibition of GABA, dopamine, acetylcholine and glutamate release (Doreulee et al., 2001; Schlicker et al., 1993; Prast et al., 1999; Arias-Montaño et al., 2001), whereas the activation of Gαs-coupled A2ARs also inhibits GABA release but facilitates glutamatergic cortico-striatal transmission (Kirk and Richardson, 1994; Popoli et al., 1995).
Either individually or through their heteromeric interactions with other GPCRs, A2ARs and H3Rs have been proposed as targets for the treatment of basal ganglia-related disorders such as Parkinson’s disease and addiction (Ferré et al., 2004; Schwarzschild et al., 2006; Passani and Blandina, 2011; Ellenbroek, 2013). In this work, we hypothesized that striatal H3Rs and A2ARs can not only form heteromers with dopamine receptors, but also with each other, and that this interaction forms a unique pharmacological target that could be of potential therapeutic interest. Here we show that H3Rs co-immunoprecipitate with A2ARs in HEK293 cells, suggestive of oligomerization. Further, we studied the physical properties of the A2AR-H3R interaction by a functional complementation assay. We next showed that the A2AR-H3R heteromerization has unique functional pharmacology in terms of cAMP modulation in HEK-293 cells co-expressing the receptors. Using protein extracts from striatal synaptosomes, we confirmed the presence of A2AR-H3R heteromers in vivo by co-immunoprecipitation. Finally, we found that in the same preparation H3R activation resulted in decreased binding affinity of A2AR for the agonist CGS-21680.
A preliminary account of this work was presented in the abstract form to the European Histamine Research Society (Márquez-Gómez et al., 2016) and submitted to an electronic pre-print server (Márquez-Gómez et al., 2017).
2. Methods
2.1. Materials
The following drugs and reagents were purchased from Sigma Aldrich (St. Louis, MO, USA): (R)(−)-α-methylhistamine dihydrochloride, histamine dihydrochloride, adenosine deaminase (from bovine spleen), Percoll, quinpirole dihydrochloride. Dimaprit was from Axon MedChem (Reston, VA, USA). CGS-21680 was from Cayman Chemical (Ann Arbor, MI, USA). N-α-[methyl-3H]-histamine (78.3 Ci·mmol−1) and CGS-21680-[carboxyethyl-3H (N)]-(35.2 Ci·mmol−1) were from Perkin Elmer (Boston, MA, USA).
2.2. Molecular cloning
Truncated A2A or H3 receptors were generated by PCR using the primers indicated in Supplementary Table 1. After amplification and restriction with HindIII and BamHI enzymes, the truncated A2A and H3 receptors were ligated to a pcDNA3.1 plasmid that contained the chimeric Gαqs4 and Gαqi4 proteins. Both A2ARs and H3Rs (cDNA Resource Center, Bloomsberg, PA, USA) were labeled with a triple hemagglutinin tag (3xHA). To generate the 3xHA tagged receptors, A2AR and the H3R were amplified from nucleotide 377 and 458, respectively, towards their amino terminus. The 3xHA tag was amplified from a plasmid that codified for the tagged histamine H4R (3xHA-H4R in pcDNA3.1, cDNA Resource Center). The amplified DNA fragments (3xHA-A2AR377/H3R458) where then re-introduced into the pcDNA3.1-H3R or pcDNA3.1-A2AR backbone to obtain the tagged, full length receptors. The insertion and orientation of the amplified fragment was verified by automated sequencing performed at FESI-UNAM (Los Reyes Iztacala, Estado de México, México).
2.3. Cell culture and transfection
HEK-293T cells (American Type Culture Collection, Manassas, VA, USA) were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin (50 UI/ml) and streptomycin (0.1 mg/ml) under a humidified atmosphere (5% CO2 in air) at 37 °C.
For transfections, cells were seeded in 6-well plates (6×105 cells/well; up to passage 15) and incubated for 24 h at 37 °C in a 5% CO2/air mixture. The next day, 10 μl X-tremeGENE (Roche, Basilea, Switzerland) were mixed with 500 μl Optimem (Life Technologies, San Diego, CA, USA) and the mixture was incubated for 5 min at room temperature before the addition of cDNA in a 1:5 ratio with X-tremeGENE. For cAMP assays a mixture of DNA:Glosensor cAMP plasmid (1:2 ratio) was added to the Optimem/X-tremeGENE solution. When plasmids containing the A2AR or H3R were transfected alone, the amount of DNA was preserved by adding empty pcDNA3.1 vector. The transfection mixture was incubated for 20 min at room temperature and then added to the cells, which were incubated for 24 h at 37 °C under a humidified atmosphere (5% CO2/air).
For co-immunoprecipitation assays, HEK-293T cells, grown in 100-millimeter Petri dishes and at 80% confluence, were transfected using the Lipofectamine 2000 method. Briefly, a mixture of DNA/Lipofectamine 2000 (1:2 ratio) was incubated for 20 min at room temperature before being added to the cells. After incubation for 5 h at 37 °C under a humidified atmosphere (5% CO2/air), the medium was replaced by high-glucose DMEM supplemented with 10% FBS and 1% antibiotic/antimycotic. Incubation continued for a further 24 h under the same condition.
2.4. cAMP accumulation assay
cAMP assays were performed as described in detail elsewhere (Chiang et al., 2016). Briefly, HEK-293T cells transfected with 3xHA-A2AR, 3xHA-H3R or a mixture of both plasmids (1 μg each) together with Glosensor cAMP plasmid (Promega, Madison, WI, USA) were seeded in a white 384-well low-volume plate (25,000 cells/well in 7.5 μl medium) and incubated with Glo-equilibrium medium (7.5 μl, Promega). Drugs under test were added in a 5 μl volume (4x in HBSS solution) and endogenous cAMP luminescence was measured in real-time in a Flexstation 3 apparatus (Molecular Devices, Sunnyvale, CA, USA).
2.5. Calcium mobilization assay
HEK-293T cells transfected with 1 μg of DNA were seeded (2.5×104 cells/well) in a 384-well clear bottom black plate (25 μl-volume) and incubated for 24 h at 37 °C in a humidified 5% CO2/air atmosphere, covered with Areaseal film (Sigma, St. Louis, MO, USA). Cells were then loaded with 25 μl of the FLIPR Ca2+ dye (Molecular Devices, Sunnyvale, CA, USA) and incubated for 1 h at 37 °C. Agonists were added in a 20 μl volume and calcium mobilization was measured in real-time for 2 min in a Flexstation 3 apparatus (Molecular Devices, Sunnyvale, CA, USA) and analyzed as described in detail in van Rijn et al. (2013).
2.6. Synaptosome preparation
All procedures were approved and controlled by the Cinvestav Animal Care Committee and were in accord with the rules issued by the National Institutes of Health (NIH Publications No. 8023) and the Mexican Council for Animal Care. Wistar rats (males, 250–300 g, provided by Unidad de Producción y Experimentación para Animales de Laboratorio; Cinvestav, Mexico City) were decapitated, the brain was quickly removed from the skull and the forebrain was separated and deposited on a metal plate placed on ice. The striata from 3–5 animals were dissected using forceps and the whole tissue was placed in 5 ml 0.32 M sucrose solution containing 10 mM Hepes, 1 mg/ml bovine serum albumin and 1 mM EDTA (pH 7.4 with NaOH). The tissue was homogenized using 10 strokes of a hand-held homogenizer (400 rpm), the homogenate was centrifuged (1000xg, 10 min, 4 °C) and the supernatant was pelleted at 14,000xg (12 min, 4 °C). The pellet was re-suspended in 5 ml of a Percoll solution (45 %, v:v) in Krebs-Henseleit-Ringer buffer (in mM: NaCl 140, Hepes 10, D-glucose 5, KCl 4.7, EDTA 1, pH 7.3 with NaOH). After centrifugation (2 min, 14,000xg, 4 °C), the upper phase was collected and brought up to 20 ml with Krebs-Ringer-Hepes (KRH) solution (in mM: NaCl 113, NaHCO3 25, Hepes 20, D-glucose 15, KCl 4.7, CaCl2 1.8, MgCl2 1.2, KH2PO4 1.2, pH 7.4 with NaOH). The suspension was centrifuged (20,000xg, 20 min, 4 °C) and the pellet (synaptosomes) was re-suspended in KRH solution unless otherwise indicated.
2.7. Electron microscopy
Striatal synaptosomes were isolated by the Percoll method as above. Sample preparation and electron microscopy were performed as described in detail elsewhere (Morales-Figueroa et al., 2015).
2.8. Radioligand binding assays with striatal membranes
Striatal synaptosomes were re-suspended in lysis solution (Tris-HCl 10 mM, EGTA 1 mM, pH 7.4) and incubated for 20 min at 4 °C before centrifugation (20 min, 20,000xg, 4 °C). The pellet (synaptosomal membranes) was re-suspended in 1 ml KRH solution containing adenosine deaminase (2 U/ml). After incubation for 30 min at 37 °C, the suspension was brought to 20 ml with KRH solution and centrifuged (20 min, 20,000xg, 4 °C). The membranes were re-suspended in incubation solution (Tris-HCl 50 mM, MgCl2 5 mM, pH 7.4) and 130 μl aliquots (40 μg protein) were incubated with 10 μl of increasing concentrations of CGS-21680 or RAMH (20x) and 50 μl of [3H]-NMHA (8 nM) or [3H]-CGS-21680 (48 nM). After 1 h at 30 °C ([3H]-NMHA) or 2 h at 25°C ([3H]-CGS-21680), incubations were stopped by rapid filtration through Whatman GF/B filters pre-soaked (2 h) in 0.3 % polyethylenimine (PEI). Filters were washed 3 times with 1 ml ice-cold buffer solution (50 mM Tris-HCl, pH 7.4), soaked in 4 ml scintillation solution and the tritium content was determined by scintillation counting.
2.9. Co-immunoprecipitation assays
Striatal synaptosomes were obtained as described above and then re-suspended in 1 ml of lysis solution (Tris-HCl 50 mM, NaCl 150 mM, Triton X-100 1%, SDS 0.05%, protease inhibitor 1 μl/ml). HEK-293T cells were dislodged in RIPA solution. For both synaptosomal and cell samples, protein was extracted by sonication (3 cycles, 30 sec, 8 kHz). The sample was centrifuged (20 min, 6,000xg) and the protein extract (supernatant) was collected. Nonspecific binding was removed by incubation (1 h, 4 °C) with 10 μl of AG beads (Santa Cruz Biotechnology; Dallas, TX, USA) under rotatory rocking. The beads were pelleted (2 min, 6000xg) and the supernatant was used for the assay. Protein quantification was performed by the BCA method.
The protein extracts (500 μg protein from striatal synaptosomes and 200 μg from HEK-293T) were incubated with 2 μg of the primary antibodies (anti-A2AR Abcam, cat. ab3461, lot. GR238882–9; anti-H3R, Abcam, cat. ab84468, lot. GR27494–1, in a 1:100 dilution) together with AG beads (Santa Cruz Biotechnology sc-2003, 1:5 antibody to beads ratio) for 16 h at 4°C with constant rotational rocking. Antibodies against the CD-81 protein (Santa Cruz Biotechnology, cat. sc-70803, lot. B0609) or D2R (Santa Cruz Biotechnology, cat. sc-5303, lot. A03013) were used as negative controls for the striatal synaptosomes or HEK-293T cells, respectively. The bead-antibody complex was pelleted (2 min, 6000xg) and a 30 μl aliquot of the supernatant was used as a load control. In both protein extracts, 30 μl of the total protein was used as input. The complex was dissociated for 60 min at 48°C in loading buffer (10% β-mercaptoethanol and 50% Laemmli buffer in H2O) and separated by electrophoresis in a 10% SDS-polyacrylamide gel (20 min at 80 V and then 65 min at 120 V). Semi-dry transfer was performed at 15 V for 95 min. Membranes were blocked with 5% nonfat dry milk diluted in TBS-Twin 0.05% solution (overnight, 4°C). After extensive washing, 5 ml of TBS-Twin 0.05% solution containing 5% BSA and the blotting antibody (anti-A2AR, anti-HA, Cell Signaling, cat. C29F4, lot. 3724S or anti-H3R, in a 1:1000 dilution) were added to the membrane and incubated overnight at 4 °C. Membranes were incubated with the secondary antibody (Invitrogen, HRP-conjugated anti-rabbit IgG, cat. 65–6120, 1:5000, non-fat dry milk 5% in TBS-Twin 0.05%) at room temperature for 2 h. For the loading controls membranes were incubated for 1 h with antibodies directed to β-tubulin (Invitrogen, cat. 322600, lot. 1235662) or α-actin (Sigma, cat. A5228, lot. 128K4843) in a 1:5000 dilution in TBST 0.05%. After washing, membranes were incubated for 1 h with the secondary antibody (α-mouse, Santa Cruz, cat. sc-2005) diluted in TBST 0.05%. Blot images were obtained by chemiluminescence in an X-ray film (Kodak).
2.10. Statistical analysis
Data show the mean ± standard error of the mean (SEM). Statistical analysis was performed with student t-test with Prisma GraphPad software, version 5.0 (San Diego, CA, USA).
3. Results
3.1. Co-immunoprecipitation of A2ARs and H3Rs expressed in HEK-293 cells
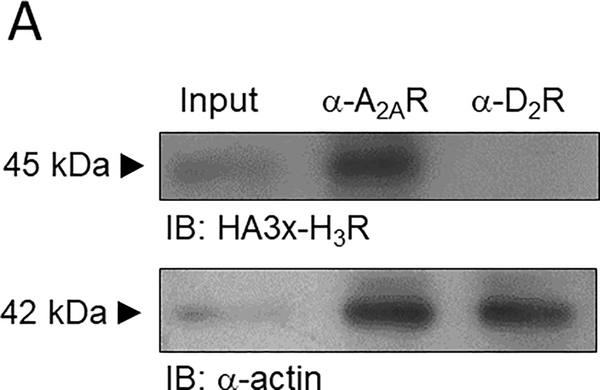
We first studied by co-immunoprecipitation whether H3Rs and A2ARs interact physically upon co-transfection in HEK-293T cells. 3xHA-H3Rs were detected after immunoprecipitation of wild type (WT) A2ARs (Figure 1A). HEK-293T cells lack expression of the D2 receptor, and an antibody targeting this receptor was therefore used as a negative control, yielding no signal. This result supports dimerization between H3Rs and A2ARs.
Figure 1.
Adenosine A2A and histamine H3 receptors co-immunoprecipitate in transfected HEK-293 cells. A. Co-immunoprecipitation of the hemagglutinin-tagged H3R (3xHA-H3R) with the A2AR in protein extracts from HEK-293 cells. The ~45 kDa band corresponds to the 3xHA-H3R, and was not observed with the negative control. Input represents 30% of the total protein. An antibody against the D2 receptor (α-D2R) was used as a negative control. The blot is representative of 3 independent experiments. The whole blot is shown in Supplementary Figure 5.
3.2. Functional complementation of A2ARs and H3Rs expressed in HEK-293 cells
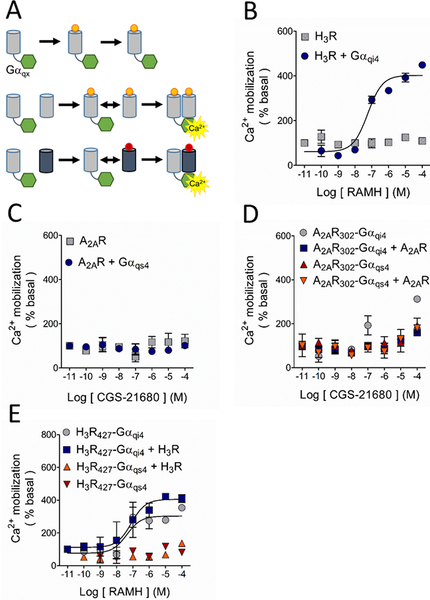
To further study the potential interaction between A2ARs and H3Rs in a recombinant cell system, we employed a functional complementation assay, previously used to study physical interactions between GPCRs (Han et al., 2009; van Rijn et al., 2013). This assay relies on the fusion of chimeric Gαqs4 or Gαqi4 proteins to the truncated C-terminal tail of a GPCR to generate a nonfunctional receptor, rescuable through homo- or hetero-dimerization with a WT, untruncated receptor. It was previously reported that fusion of a chimeric G protein to a GPCR truncated near helix 8 produces a receptor that is unable to signal by itself but can be rescued when transfected with a full-length receptor (van Rijn et al., 2013). Therefore, we first generated an A2AR truncated to 302 residues (A2AR302) and H3Rs of 427, 421 and 411 residues (H3R427, H3R421 and H3R411), and the truncated receptors were then fused to the chimeric G proteins (Figure 2A).
Figure 2.
Study of the possible A2AR-H3R dimerization by functional complementation assay. A. Basis of the assay. The G protein-coupled receptor (GPCR; grey cylinders) is truncated at its carboxyl terminus to generate a nonfunctional GPCR, which is then fused to the chimeric G protein (green hexagons). The chimeric G protein is formed by a Gαq protein in which 9 or 10 residues of the C-terminus were substituted by the corresponding sequence of a Gαs4 or Gαi4 protein (represented by X). Under these conditions, Ca2+ mobilization can only be elicited when the complex GPCR-Gαqx is in close proximity to a wild type (WT) GPCR, either the same (homodimerization) or a different receptor (heterodimerization). B. Activation of the H3R with its agonist RAMH resulted in Ca2+ mobilization only when it was co-expressed with the chimeric Gαqi4 but not when transfected alone. C. When activated with its agonist CGS-21680, the A2AR was uncapable to induce Ca2+ mobilization either alone or when co-transfected with the chimeric Gαqs4 protein. D. Functional complementation by homodimerization of the truncated A2AR302, either bound to Gαqs4 (A2AR302-Gαqs4) or Gαqi4 (A2AR302-Gαqi4), with the native A2AR was not observed. E. Activation of the truncated H3R427 bound to Gαqi4 (H3R427-Gαqi4) induced Ca2+ mobilization on its own and homodimerization with the WT-H3R did not modify the response. No signal was observed with activation of the H3R427-Gαqs4 construct when it was co-expressed with the WT-H3R. In all graphs data are means ± SEM from 3 replicates from a representative experiment. Where SEM bars are not visible, they are smaller than the symbol size. The quantitative analysis is shown in Tables 1 and Supplementary Table 2.
Activation of the H3R in transfected CHO-K1 cells and rat striatal neurons in primary culture induces Ca2+ mobilization (Cogé et al., 2001; Rivera-Ramirez et al., 2016), and in the striatum A2AR activation favors Ca2+ entry by modulating voltage-activated Ca2+ channels (Kirk and Richardson, 1995; Gubitz et al., 1996), which are endogenously expressed by HEK-293 cells (Berjukow et al., 1996; Thomas and Smart, 2005). In HEK-293 cells, the H3R selective agonist RAMH did not induce any discernible Ca2+ response but did so when the H3R was co-transfected with Gαqi4 proteins (Figure 2B). The A2AR did not induce Ca2+ signaling when activated by the selective agonist CGS-21680, but also no response was observed when co-transfected with Gαqs4 proteins, suggesting that A2ARs do not activate this chimeric protein (Figure 2C). It has been reported that not all GPCRs are amenable to signal through chimeric G-proteins (Conklin et al., 1996). A different Gαs-coupled receptor, the histamine H2 receptor, was capable to induce Ca2+ release when co-transfected with Gαqs4 proteins and stimulated with the selective agonist dimaprit (Supplementary Figure 1A), discarding Gαqs4 malfunction. Consistent with the finding that A2ARs did not signal efficiently via chimeric G-proteins, functional complementation by homodimerization of A2AR302-Gαqs4 or A2AR302-Gαqi4 with the full-length A2AR was not observed (Figure 2D).
The C-tail of the H3R was truncated such that the H3R-Gαqi4 fusion protein would display limited Ca2+ mobilization when expressed alone, but a pronounced Ca2+ signaling when co-expressed with WT-H3Rs. The H3R421-Gαqi4 and the H3R411-Gαqi4 constructs did not induce Ca2+ mobilization, and could not be rescued via homo-dimerization with WT-H3Rs (Supplementary Figure 1B). However, Ca2+ mobilization induced by H3R427-Gαqi4 upon activation and co-transfection with WT-H3Rs produced a similar increase in calcium release (Figure 2E). While not optimal, this behavior allowed for the study of the interaction between the H3R427-Gαqi4 and the A2AR as described below.
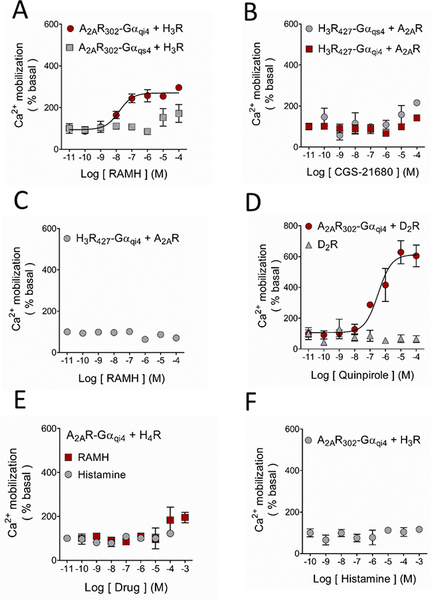
Co-transfection of the ‘inert’ A2AR302-Gαqi4 and H3Rs induced Ca2+ mobilization after activation of the latter receptors with RAMH, suggestive of close physical proximity between the H3R and both the A2AR and the fused Gαqi4-protein. H3R activation failed to induce functional complementation in cells co-transfected with A2AR302-Gαqs4 (Figure 3A). Given that H3Rs are Gαi/o-coupled, and the inability of the A2AR to signal through chimeric Gαqi4- or Gαqs4-proteins, it was not surprising that A2AR activation with the selective agonist CGS-21680 did not produce any response through H3R427-Gαqs4 or H3R427-Gαqi4 (Figure 3B). Interestingly, the co-expression of WT-A2ARs prevented RAMH-induced Ca2+ mobilization mediated by the H3R427-Gαqi4 (compare Figure 2E and Figure 3C). We briefly explored this pharmacological response further by analyzing Ca2+ mobilization in HEK-293T cells co-expressing the H3R427-Gαqi4 and the A2AR or the A2AR302-Gαqi4 and the H3R and activating both receptors, but did not observe any additive or antagonistic effect (Supplementary Figure 1C and 1D).
Figure 3.
Ligand modulation of the A2AR-H3R interaction in HEK-293 cells. Activation of the WT-H3R with its agonist RAMH led to functional complementation when co-expressed with the A2AR302-Gαqi4 but not with A2AR302-Gαqs4. B. Ca2+ mobilization was not observed when the WT-A2AR was co-transfected with the truncated H3R427-Gαqs4 or H3R427-Gαqi4, in accord with the incapability of the receptor to activate the chimeric proteins. C. As shown in Figure 1D, activation of the H3R427-Gαqi4 resulted in Ca2+ mobilization, and this response was prevented when it was co-expressed with the WT-A2AR, suggesting a preference of the H3R427-Gαqi4 to form heterodimers. D. The well-studied A2AR-D2R heterodimer was used as a positive control for these experiments. Activation of the D2R with increasing concentrations of the agonist quinpirole caused marked Ca2+ mobilization only when co-expressed with the chimeric A2AR302-Gαqi4. E. The histamine H4 receptor (H4R) as a negative control. No functional complementation was observed when the receptor was co-expressed with the A2AR302-Gαqi4, showing the specificity of the A2AR-H3R interaction. F. Functional complementation between the A2AR302-Gαqi4 and the WT-H3R was not observed when the latter receptor was activated with the endogenous agonist histamine. For all graphs data are means ± SEM from 3 replicates from representative experiments. Where SEM bars are not visible, they are smaller than the symbol size. The quantitative analysis is shown in Table 1 and Supplementary Table 2.
A2ARs have been shown to form heteromers with D2Rs (Ferré et al., 2003) and robust functional complementation was accordingly observed when A2AR-Gαqi4 was co-expressed with WT-D2Rs (Figure 3D), supporting that our results are due to heterodimerization and not to stochastic interactions.
3.3. The putative A2AR-H3R heteromer displays ligand bias
To test for the selective nature of the A2AR-H3R interaction in the functional complementation assay, we investigated if A2AR-Gαqi4 would also allow the structurally and physiologically similar histamine H4 receptor (H4R) to induce Ca2+ signaling. Co-activation with histamine of H3Rs and H4Rs elicited Ca2+ mobilization only when co-transfected with Gαqi4 (Supplementary Figure 2A and 2B, respectively). Neither histamine nor RAMH induced Ca2+ mobilization when the H4R was co-expressed with A2AR302-Gαqi4, supporting the specificity of the A2AR-H3R interaction (Figure 3E). Unexpectedly, whereas RAMH induced Ca2+ mobilization when the H3R was co-expressed with A2AR302-Gαqi4, histamine did not (compare Figure 3A with Figure 3F). This result suggests that the A2AR-H3R heteromer displays agonist bias.
3.4. A2AR-mediated signaling is increased by H3R co-activation
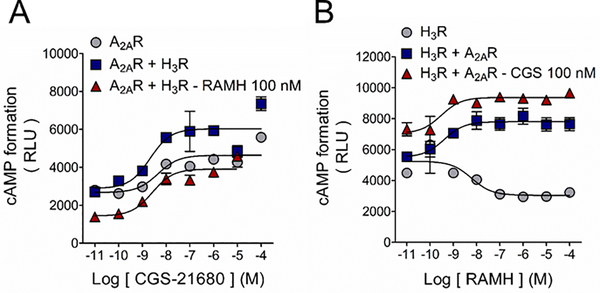
To study the pharmacology of the putative A2AR-H3R heteromer we next tested cAMP signaling using WT receptors in HEK-293T cells. In cells transfected with the A2AR, incubation with CGS-21680 resulted in a concentration-dependent increase in cAMP levels in accordance with the receptor’s coupling to the Gαs signaling pathway (Figure 3A). CGS-21680 induced a similar cAMP response in HEK293T-A2AR cells co-expressing H3Rs (Figure 4A). Co-activation of the receptors resulted in a decrease in baseline, in agreement with the Gαi/o coupling of the H3R, but led to an augmentation of A2AR-mediated cAMP formation as noticed by a 2.5-fold change of baseline (versus 1.5-fold in control). This result suggests that the A2AR signaling becomes more efficacious when heterodimerization with the H3R occurs (Figure 4A, Supplementary Figure 2C, and Table 2). The increase in the A2AR signaling was not observed in the absence of RAMH (1.5-fold of baseline) suggesting an agonist-dependency of the response (Figure 4A and Supplementary Figure 2C).
Figure 4.
H3R activation enhances A2AR-mediated cAMP signaling in HEK-293 cells. A. Activation of the Gαs-coupled A2AR with its agonist CGS-21680 induced cAMP formation and H3R co-activation enhanced A2AR efficacy. Co-expression of the H3R did not modified the A2AR functional response. Values for pEC50 and maximal effect (Emax) are given in Table 2. B. H3R activation with RAMH decreased forskolin-induced cAMP formation in accord with the inhibitory nature of the Gαi/o-coupled receptor. A2AR expression and receptors co-activation lead to a change in the H3R signaling. Data are means ± SEM from 5 replicates from a representative experiments. Where SEM bars are not visible, they are smaller than the symbol size. Values for pIC50 and maximal effect (Imax) are given in Table 2.
Table 2.
Pharmacological characteristics of the cAMP formation assay in transfected HEK-293 cells.
| Transfection | Agonist | pEC50 | Emax (%) | pIC50 | Imax (%) |
|---|---|---|---|---|---|
| A2AR | CGS | 8.0 ± 0.4 | 152 ± 7 | - | - |
| A2AR + H3R | CGS | 8.7 ± 0.5 | 153 ± 7 | - | - |
| A2AR + H3R | +RAMH | 8.0 ± 0.2 | 248 ± 9b | - | - |
| H3R | RAMH | - | - | 8.1 ± 0.3 | −26 ± 2 |
| H3R ± A2AR | RAMH | 10.6 ± 1 | 114 ± 3 | - | - |
| H3R ± A2AR | +CGS | 9.9 ± 0.6 | 120 ± 2 | - | - |
Data are means ± SEM from 3 experiments. Statistical comparisons were performed between two agonists for the same transfection, or between the transfection with the chimeric receptor and the transfection of the chimeric receptor plus the wild type receptor. ns, no significant difference
P < 0.05
P < 0.01, Student’s t test.
Both RAMH and CGS-21680 were assayed at 100 nM. RAMH, R-α-methylhistamine; CGS, CGS-21680.
In HEK293T-H3R cells, RAMH inhibited forskolin-stimulated cAMP formation congruent with the Gαi/o coupling of the H3R receptor. However, when the A2AR was co-transfected, RAMH-mediated H3R signaling shifted to facilitate cAMP formation instead. Activation of the A2AR caused an expected increase in the baseline but did not significantly modify the effect on cAMP formation. This result suggests that in the heterodimer, A2AR signaling prevails over the H3R signaling (Figure 4B and Supplementary Figure 2D; Table 2).
3.5. H3R activation decreases A2AR binding affinity in synaptosomal membranes
The identification of A2AR-H3R heteromers was performed in recombinant cell systems over-expressing the receptors. As previously mentioned A2ARs and H3Rs are co-expressed in iMSNs and cortico-striatal projections. We therefore used rat striatal synaptosomes to seek for pharmacological traces that supported the existence of native A2AR-H3R dimers.
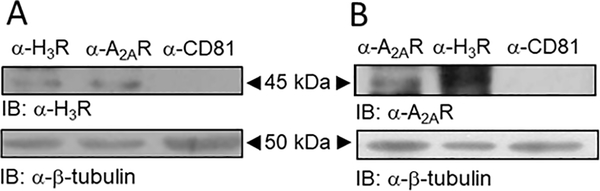
Electron microscopy confirmed in the purified synaptosomal preparation the vast presence and conserved structure of isolated nerve terminals, characterized by a delimited membrane and the presence of mitochondria and synaptic vesicles (Supplementary Figure 3A and B). In protein extracts from striatal synaptosomes, immunoprecipitation of the A2AR resulted in a band of ~45 kDa, corresponding to the expected migration of the H3R. No signal was detected when an irrelevant antibody (α-CD81) was tested. As a positive control the H3R was immunoprecipitated and detected as a band of ~45 kDa (Figure 5A). When the reverse approach was employed and the H3R was immunoprecipitated from the striatal protein extracts, a band of ~45 kDa corresponding to the expected migration of the A2AR was observed. This band was also observed when the A2AR was immunoprecipitated as a positive control but not detected in the negative control (Figure 5B). This result supports that the interaction A2AR-H3R is constitutively present in the striatal nerve terminals.
Figure 5.
Co-immunoprecipitation of the A2A and H3 receptors in striatal synaptosomes. A. The H3R co-immunoprecipitated with the A2AR in a protein extract of Percoll-purified striatal synaptosomes. The band of ~45 kDa corresponds to the expected migration of the H3R. No band was observed in the negative control (α-CD81). B. Co-immunoprecipitation of the A2AR with the H3R in a protein extract of striatal synaptosomes. The band of ~45 kDa corresponds to the expected migration of the A2AR. The figure depicts representative blots, repeated a further 4 times with similar results. Full blots are shown in Supplementary Figure 6.
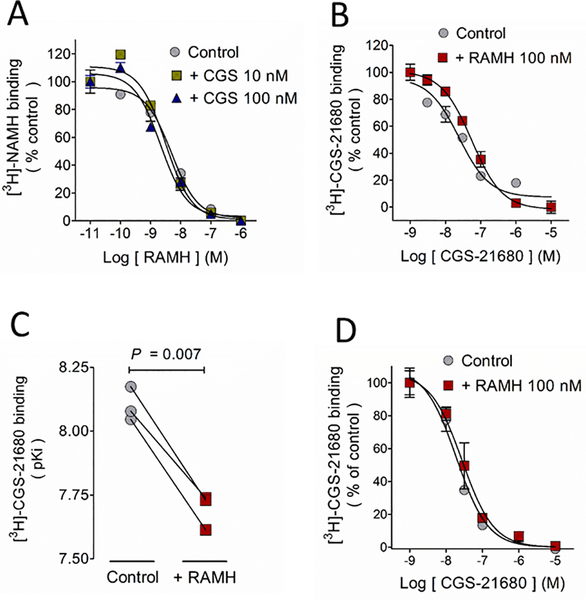
In binding studies with synaptosomal membranes the A2AR agonist CGS-21680 (10 and 100 nM) did not affect the affinity of the H3R for its agonist RAMH (Figure 6A), whereas RAMH (100 nM) decreased by two-fold the affinity of the A2AR for CGS-21680 (Figure 6B, 6C and Table 3), with no effect on maximal binding (Bmax). This pharmacology appears to be specific for pre-synaptic H3Rs and A2ARs, because we did not observe a similar decrease in A2AR affinity for CGS-21680 in membranes from the whole striatum, in which post-synaptic membranes constitute the major component (Figure 6D and Table 3).
Figure 6.
H3R activation decreases A2AR affinity for the agonist CGS-21680 in synaptosomal membranes but not in membranes from the whole striatum A. CGS-21680 (10 and 100 nM) did not modify the H3R affinity for its ligand RAMH in membranes isolated from striatal synaptosomes. B. In the same preparation, the H3R agonist RAMH (100 nM) decreased the A2AR affinity for [3H]-CGS-21680. Values are means ± SEM from 3 replicates from a representative experiment. C. Analysis of 3 independent experiments. The statistical analysis was performed with paired Student’s t test. D. H3R activation with RAMH (100 nM) failed to decreased the A2AR affinity for [3H]-CGS-21680 in membranes from the whole striatum. Values are means ± SEM from 3 replicates from a representative experiment. Where SEM bars are not visible, they are smaller than the symbol size.
Table 3.
Analysis of binding assays in rat striatal membranes
| pKi | Bmax (%) | |
|---|---|---|
| [3H]-NAMH | ||
| Synaptosomes | ||
| RAMH | 9.09 ± 0.22 | 100.0 ± 0.3 |
| RAMH + CGS 10 nM | 8.99 ± 0.02ns | 110.0 ± 4.0ns |
| RAMH + CGS 100 nM | 9.08 ± 0.06ns | 103.0 ± 4.1ns |
| [3H]CGS-21680 | ||
| Whole striatum | ||
| CGS | 8.06 ± 0.15 | 100.0 ± 14 |
| CGS + RAMH | 8.11 ± 0.04ns | 88.0 ± 6.0ns |
| Synaptosomes | ||
| CGS | 8.10 ± 0.04 | 100.0 ± 9.0 |
| CGS + RAMH | 7.70 ± 0.04a | 88.1 ± 10.0ns |
Data are means ± SEM from 3–5 experiments. For [3H]-NAMH binding assays the statistical analysis was performed with one-way Anova and Dunnett´s post hoc test. For [3H]-CGS-21680 binding, values in the presence of RAMH were compared with the corresponding control with Student’s t test.
P < 0.001, ns, not significant. CGS, CGS-21680. RAMH (R-α-methylhistamine) was tested at 100 nM in all the experiments.
4. Discussion
The H3R has been proposed as a potential novel drug target for the treatment of drug use disorders, depression, schizophrenia, and Parkinson’s disease. However, its wide brain expression and effects on other neurotransmitter systems may result in adverse effects when H3R selective drugs are administered systemically. The A2AR has also been proposed as an option for the treatment of Parkinson’s disease and possesses a strategic distribution in the striatum for targeting and modulating the cortico-dMSNs projections and the activity of iMSNs. The results presented herein may lead to an alternative to specifically target H3Rs located in either iMSNs or cortical afferents synapsing onto dMSNs projections.
4.1. A2AR-H3R interaction
The primary finding of this study was the identification, for the first time, of A2AR-H3R heteromers, not only in recombinant cell systems but also in rat striatal nerve terminals.
The basis of the functional complementation assays requires a receptor to be nonfunctional and this was obtained by truncation of the C-terminus. Herein we showed that truncation of the H3R from 445 to 411 or 421 residues was sufficient to prevent H3R-mediated G protein activation of the H3R-Gαqi4 fusion protein, yet we were unable to functionally rescue its signaling, indicating that the remaining C-tail may be too short to connect with an interacting GPCR. However, truncation to 427 amino acids allowed the H3R to remain functional.
Exposure of HEK-293T cells transfected with H3R427-Gαqi4 to the H3R agonist RAMH resulted in Ca2+ mobilization (Figure 1E). As mentioned in the Results section, this response was not expected. In an optimal scenario, the receptor truncation would have prevented or diminished the receptor capability to activate G proteins and the H3R427-Gαqi4 function was expected to be either complemented or increased by the co-expression and co-activation with the WT-H3R. However, these conditions allowed for the detection of the loss of H3R function with the solely presence of the A2AR, suggesting a preference of the H3R to form hetero-dimers over homo-dimers. It may be possible that truncating the H3R at the amino acids 421–427 will result in a receptor short enough to abolish the homo/monomeric signaling, but long enough to functionally complement with a full length GPCR.
In order for a GPCR to activate G proteins, the integrity of helix 8 appears to be required. This structural requirement has been demonstrated for D1Rs and opioid κ and μ receptors (van Rijn et al., 2013). The H3R third intracellular loop also appears to play a key role in the receptor-G protein coupling on the basis of the decreased signaling of the H3R365 isoform, which lacks 80 residues in the third intracellular loop (Riddy et al., 2016), and reduced signaling was induced by the A280V mutation in the same loop (Flores-Clemente et al., 2013). Further consideration to the H3R carboxyl tail should therefore be made when assessing the H3R-G protein coupling.
An important issue when describing a novel heterodimer is to show changes in the signaling profiles of the receptors involved in the dimer. In this regard, we found enhanced signaling efficacy of the agonist CGS-21680 when the H3R was co-transfected and co-activated in the HEK293T-A2AR cells. This effect can be explained by a H3R-mediated facilitation of the A2AR-G protein coupling, leading to an increase in the receptor efficacy to activate Gαs proteins and thus to produce cAMP. Given that in the absence of RAMH, CGS-21680 behaved the same in HEK293T-A2AR and HEK293T-A2AR/H3R cells, receptor expression does not appear to account for the observed effects.
Canonically H3Rs couple to Gαi/o proteins, which inhibit adenylyl cyclase activity and accordingly activation of the receptor with RAMH lead to a decrease in cAMP formation. Similar to the observed in the Ca2+ mobilization assays, expression of the A2AR shifted the H3R-mediated cAMP response from inhibition to an increase in the cAMP formation. In the Ca2+ mobilization assays we did not observe signaling of the H3R through Gαqs4 proteins bound to the truncated A2AR, and we are thus not considering a H3R change in signaling pathways as an explanation for this effect. Therefore, we hypothesized that in the A2AR-H3R heteromer the A2AR signaling prevails over that of the H3R, hampered possibly by a steric impediment. This is in line with the loss of Ca2+ signaling when the H3R-Gαqi4 was co-expressed with the A2AR.
An unexpected but interesting finding was the potential biased-signaling at the A2AR-H3R heteromer, as evidenced by the incapability of the endogenous ligand histamine to signal at the A2AR-H3R heteromer compared to the exogenous agonist RAMH. This discrepancy suggests that RAMH induces conformational changes in the H3R that allow the interaction to occur, whereas histamine-induced changes appear not sufficient for heteromerization, or at least for the heteromer to signal. Ligand bias could also be explained by agonist residence time, this is, the time that a particular drug remains in its binding pocket and that will directly determine the time that a given receptor maintains an active conformation. The active state induced by histamine may therefore have a shorter duration compared with that induced by RAMH, preventing the former from activating the chimeric G proteins bound to the A2AR302. Binding studies with striatal membranes and histamine show that the first hypothesis holds better because histamine induced an increase in the affinity of the A2AR for its agonist CGS-21680 (Supplementary Figure 4), opposite to the decrease in affinity change induced by RAMH in the same preparation. This result suggests that histamine and RAMH lock the H3R in different conformational states that affect its interaction with the A2AR.
4.2. Functional relevance of the A2AR-H3R heterodimer
Our results suggest a pre-synaptic location of the A2AR-H3R heterodimer in the striatum. A previous report indicates equal distribution of A2ARs in total and synaptosomal membranes from rat striatum (Rebola et al., 2005), with a preferential location on the post-synaptic density fraction over the pre-synaptic active zone fraction (49.2 ± 3.3 % and 26.9 ± 3.3 % of total immunoreactivity, respectively). H3Rs are expressed pre- and post-synaptically (Ellenbroek and Ghiabi, 2014), but they seem to be highly enriched in the terminals of striato-pallidal neurons (iMSNs) yielding a value of 1,327 ± 79 fmol/mg protein (Morales-Figueroa et al., 2014). This distribution supports our proposal for the heterodimer location and suggests a role for the A2AR-H3R heteromer in the pre-synaptic modulation of glutamatergic and GABAergic transmission.
In the striatum, A2ARs have a specific location in iMSNs (Schiffman et al., 1991) and cortico-dMSNs terminals, but not in the cortico-iMSNs projections (Quiróz et al., 2009) whereas the H3R is ubiquitously expressed throughout the striatum (Nieto-Alamilla et al., 2016). Both receptors can modulate the cortico-striatal glutamatergic and intra-striatal GABAergic transmission, but whereas the H3R consistently inhibits neurotransmitter release in either location, the A2AR facilitates cortico-striatal glutamate release but inhibits GABA release from the iMSNs collaterals.
Our data depicts thus a scenario where the A2AR and the H3R preserve their canonical signaling pathways (Gαs and Gαi/o, respectively), and upon dimerization the H3R facilitates the A2AR signaling. This receptor functionality is preserved in the cortico-dMSNs projections, with A2AR activation enhancing glutamate release (Popoli et al., 1995) and H3Rs exerting the opposite effect (Arias-Montaño et al., 2001). Activation of the A2AR-H3R heterodimer in these terminals may further facilitate glutamate release, resulting in hyper-stimulation of the dMSNs population.
Contrary to the A2AR and H3R antagonic modulation of glutamatergic transmission, both receptors inhibit GABA release from iMSNs collaterals. This may be due to the A2AR functional duality showed in this neuronal population, as demonstrated by its capability to activate Gαs and Gαq proteins (Gubitz et al., 1995; Kirk and Richardson, 1996). Therefore, we hypothesized that the activation of the A2AR-H3R heterodimer in this location will further inhibit GABA release from iMSN nerve terminals, facilitating the activation of the dMSNs. According to this hypothesis, the overall activation of the striatal A2AR-H3R heterodimer would increase dMSNs activity. It is therefore tempting to speculate that co-activation of the heterodimer may have relevance for the treatment of the autism and obsessive and compulsive disorder, in which alterations in cortico-striatal glutamatergic transmission and MSN excitability have been shown (Welch et al., 2008; Naaijen et al., 2017).
Furthermore, it has been reported that the A2AR antagonists KW-6002 and SCH-442416 can distinguish receptors located in the cortical afferents targeting dMSNs or iMSNs, respectively (Orrú et al., 2011). Although there are no agonists capable to differentiate between A2AR populations, this information may be a valuable tool for targeting and modulating the A2AR and H3R pharmacology in a location-specific manner, using bivalent ligands directed to the heterodimer.
5. Conclusion
This study presents for the first time evidence for an A2AR-H3R heterodimer based on functional complementation and co-immunoprecipitation assays in HEK-293 cells, where co-activation of the receptors leads to enhanced A2AR signaling and attenuation of H3R functionality. In rat striatal tissue, the interaction occurs in synapses where H3R activation modifies the binding affinity of the A2AR.
Supplementary Material
Table 1.
Pharmacological characteristics of the A2AR-H3R functional complementation assays in transfected HEK-293 cells
| Transfection | Agonist | Emax (%) | pEC50 |
|---|---|---|---|
| H3R + Gαqi4 | RAMH | 419 ± 25 | 7.73 ± 0.31 |
| Histamine | 504 ± 22b | 6.19 ± 0.17b | |
| H3R427-Gαqi4 | RAMH | 753 ± 41 | 7.14 ± 0.21 |
| H3R427-Gαqi4 + H3R | RAMH | 523 ± 41b | 7.38 ± 0.34 |
| A2AR302-Gαqi4 + H3R | RAMH | 449 ± 25 | 7.31 ± 0.23 |
| RAMH + CGS | 429 ± 14ns | 7.63 ± 0.15 | |
| H4R + Gαqi4 | Histamine | 725 ± 51 | 6.35 ± 0.25 |
| RAMH | 551 ± 27a | 6.15 ± 0.16 | |
| H2R + Gαqs4 | Dimaprit | 860 ± 37 | 6.15 ± 0.12 |
| A2AR302-Gαqi4 + D2R | Quinpirole | 612 ± 34 | 6.47 ± 0.22 |
Data are means ± SEM from 3 experiments. Statistical comparisons were performed between two agonists for the same transfection, or between the transfection with the chimeric receptor and the transfection of the chimeric receptor plus the wild type receptor. ns, no significant difference
P < 0.05
P < 0.01, Student’s t test.
Both RAMH and CGS-21680 were assayed at 100 nM. RAMH, R-α-methylhistamine; CGS, CGS-21680.
Acknowledgements
We thank Juan Escamilla-Sánchez and Raúl González-Pantoja for excellent technical assistance. We also thank Sirenia González for help in obtaining the synaptosomal MET images. R. M.-G. held a Conacyt graduate scholarship (244993).
Funding
This work was supported by Cinvestav, Conacyt (grant 220448 to J.-A. A.-M.), PAPIIT-UNAM (grant IN216215 to J.-M. A.), the National Institute on Alcohol Abuse and Alcoholism (grant AA20539 to R. M. v R.) and the Ralph W. and Grace M. Showalter Research Trust (to R. M. v R.). The funding sources were not involved at all in the study design, collection, analysis and interpretation of data, writing of the manuscript or the decision to submit this report.
Abbreviations
- A2AR
Adenosine A2A receptor
- cAMP
Cyclic adenosine monophosphate
- D1R
Dopamine D1 receptor
- D2R
Dopamine D2 receptor
- dMSNs
Direct pathway striatal medium-sized spiny neurons
- GABA
γ-Aminobutyric acid
- GPCRs
G-protein coupled receptors
- H2R
Histamine H2 receptor
- H3R
Histamine H3 receptor
- H4R
Histamine H4 receptor
- iMSNs
Indirect pathway striatal medium-sized spiny neurons
- MSNs
Striatal medium-sized spiny neurons
- RAMH
(R)-α-methylhistamine
- WT
Wild type
Footnotes
Conflicts of interest
The authors disclose no conflict of interest.
6. References
- 1.Arias-Montaño JA, Floran B, Garcia M, Aceves J, Young JM, 2001. Histamine H3 receptor-mediated inhibition of depolarization-induced, dopamine D1 receptor-dependent release of [3H]-gamma-aminobutyric acid from rat striatal slices. Br J Pharmacol 133:165–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berjukow S, Döring F, Froschmayr M, Grabner M, Glossmann H, Hering S, 1996. Endogenous calcium channels in human embryonic kidney (HEK293) cells. Br J Pharmacol 118:748–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolam JP, Hanley JJ, Booth PA, Bevan MD, 2000. Synaptic organization of the basal ganglia. J Anat 196:527–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolam PJ, Ellender TJ, 2016. Histamine and the striatum. Neuropharmacology 106:74–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cabello N, Gandía J, Bertarelli DCG, Watanabe M, Lluís C, Franco R, Ferré S, Luján R, Ciruela F, 2009. Metabotropic glutamate type 5, dopamine D2 and adenosine A2a receptors from higher-order oligomers in living cells. J Neurochem 109:1497–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carriba P, Ortiz O, Patkar K, Justinova Z, Stroik J, Themann A, Muller C, Woods AS, Hope BT, Ciruela F, Casadó V, Canela EI, Lluis C, Goldberg SR, Moratalla R, Franco R, Ferré S, 2007. Striatal adenosine A2A and cannabinoid CB1 receptors form functional heteromeric complexes that mediate the motor effects of cannabinoids. Neuropsycopharmacology 32:2249–2259 [DOI] [PubMed] [Google Scholar]
- 7.Chiang T, Sansuk K, van Rijn RM, 2016. β-arrestin 2 dependence of δ opioid receptor agonists is correlated with alcohol intake. Br J Pharmacol 173:332–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciruela F, Casadó V, Rodrigues RJ, Luján R, Burgueño J, Canals M, Borycz J, Rebola N, Goldberg SR, Mallol J, Cortés A, Canela EI, López-Giménez JF, Milligan G, Lluis C, Cunha RA, Ferré S, Franco R, 2006. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor heteromers. J Neurosci 26:2080–2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cogé F, Guénin SP, Audinot V, Renouard-Try A, Beauverger P, Macia C, Ouvry C, Nagel N, Rique H, Boutin JA, Galizzi JP, 2001. Genomic organization and characterization of splice variants of the human histamine H3 receptor. Biochem J 355:279–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conklin BR, Herzmark P, Ishida S, Voyno-Yasenetskaya TA, Sun Y, Farfel Z, Bourne HR, 1996. Carboxyl terminal mutations of Gq alpha and Gs alpha that alter the fidelity of receptor activation. Mol Pharmacol 50:885–890 [PubMed] [Google Scholar]
- 11.Doreulee N, Yanovsky Y, Flagmeyer I, Stevens DR, Haas HL, Brown RE, 2001. Histamine H3 receptors depress synaptic transmission in the corticostriatal pathway. Neuropharmacology 40:106–113 [DOI] [PubMed] [Google Scholar]
- 12.Ellenbroek BA, 2013. Histamine H3 receptors, the complex interaction with dopamine and its implications for addiction. Br J Pharmacol 170:46–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellenbroek BA, Ghiabi B, 2014. The other side of the histamine H3 receptor. Trends Neurosci 37:191–199 [DOI] [PubMed] [Google Scholar]
- 14.Ferré S, Ciruela F, Canals M, Marcellino D, Burqueno J, Casadó V, Hillion J, Torvinen M, Fanelli F, Benedetti PD, Goldberg SR, Bouvier M, Fuxe K, Agnati LF, Lluis C, Franco R, Woods A, 2004. Adenosine A2A-dopamine D2 receptor-receptor heteromers. Targets for neuro-psychiatric disorders Parkinsonism. Relat Disord 10:265–271 [DOI] [PubMed] [Google Scholar]
- 15.Ferré S, Fredholm BB, Moreli M, Popoli P, Fuxe K, 1997. Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci 20:482–487 [DOI] [PubMed] [Google Scholar]
- 16.Flores-Clemente C, Osorio-Espinoza A, Escamilla-Sánchez J, Leurs R, Arias JM, Arias-Montaño JA, 2013. A single-point mutation (Ala280Val) in the third intracellular loop alters the signaling properties of the human histamine H3 receptor stably expressed in CHO-K1 cells. Br J Pharmacol 170:127–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez-Sepulveda M, Rosell S, Hoffman HM, del Mar. Castillo-Ruíz Ma., Mignon V, Moreno-Delgado D, Vignes M, Diaz J, Sabria J, Ortiz J, 2013. Cellular distribution of the histamine H3 receptor in the basal ganglia: Functional modulation of dopamine and glutamate neurotransmission. Basal Ganglia 3:109–121 [Google Scholar]
- 18.Gubitz AK, Widdowson L, Kurokawa M, Kirkpatrick KA, Richardson PJ, 1996. Dual signaling by the adenosine A2a receptor involves activation of both N- and P-type calcium channels by different G proteins and protein kinases in the same striatal nerve terminals. J Neurochem 67:374–381 [DOI] [PubMed] [Google Scholar]
- 19.Han Y, Moreira IS, Urizar E, Weinstein H, Javitch JA, 2009. Allosteric communication between protomers of dopamine class A GPCR dimers modulates activation. Nat Chem Biol 5:688–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hillion J, Canals M, Torvinen M, Casado V, Scott R, Terasmaa A, Hansson A, Watson S, Olah ME, Canela EI, Zoli M, Agnati LF, Ibanez CF, Lluis C, Franco R, Ferre S, Fuxe K, 2002. Coaggregation, cointernalization, and codesensitization of adenosine A2A receptors and dopamine D2 receptors. J Biol Chem 277:18091–18097 [DOI] [PubMed] [Google Scholar]
- 21.Kemp JM, Powell TP, 1971. The structure of caudate nucleus of the cat: light and electron microscopy. Philos Trans R Sot Lond B Biol Sci 262:383–401. [DOI] [PubMed] [Google Scholar]
- 22.Kirk IP and Richardson PJ, 1994. Adenosine A2a receptor-mediated modulation of striatal [3H]GABA and [3H]acetylcholine release. J Neurochem 62:960–966 [DOI] [PubMed] [Google Scholar]
- 23.Kirk IP, Richardson PJ, 1995. Inhibition of striatal GABA release by the adenosine A2A receptor is not mediated by increase in cyclic AMP. J Neurochem 64:2801–2808 [DOI] [PubMed] [Google Scholar]
- 24.Márquez-Gómez R, Gutierrez-Rodelo C, Robins MT, Escamilla-Sánchez J, Olivares-Reyes J-A, van Rijn R, Arias-Montaño J-A, 2016. On the existence of a histamine H3–adenosine A2A receptor heteromer. Inflamm Res 65 (Suppl. 1), S29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marquez-Gomez R, Gutierrez-Rodelo C, Robins MT, Arias J-M, Olivares-Reyes J-A, van Rijn RM, Arias-Montaño J-A, 2017. Functional histamine H3 and adenosine A2A receptor heteromers in recombinant cells and rat striatum. bioRxiv 171736; doi: 10.1101/171736 [DOI] [PMC free article] [PubMed]
- 26.Morales-Figueroa GE, Márquez-Gómez R, González-Pantoja R, Escamilla-Sanchez J, Arias-Montaño JA, 2015. Histamine H3 receptor activation counteracts adenosine A2A receptor-mediated enhancement of depolarization-evoked [3H]-GABA release from rat globus pallidus synaptosomes. ACS Chem Neurosci 20:637–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moreno E, Moreno-Delgado D, Navarro G, Hoffmann HM, Fuentes S, Rosell-Vilar S, Gasperini P, Rodriguez-Ruiz M, Medrano M, Mallol J, Cortés A, Casadó V, Lluis C, Ferré S, Ortiz J, Canela E, McCormick PJ, 2014. Cocaine disrupts histamine H3 receptor modulation of dopamine D1 receptor signaling: σ1-D1-H3 receptor complexes as key targets for reducing cocaine’s effects. J Neurosci 34:3545–3558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naaijen J, Zwier MP, Amiri H, Williams SCR, Durston S, Oranje B, Brandeis D, Boecker-Schlier R, Ruf M, Wolf I, Banaschewski T, Glennon JC, Franke B, Buitelaar JK, Lythgoe DJ, 2017. Fronto-striatal glutamate in autism spectrum disorders and obsessive compulsive disorder. Neuropsychopharmacology 42:2456–2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nieto-Alamilla G, Márquez-Gómez R, García-Gálvez A-M, Morales-Figueroa G-E, Arias-Montaño J-A, 2016. The histamine H3 receptor: structure, pharmacology and function. Mol Pharmacol 90:649–673 [DOI] [PubMed] [Google Scholar]
- 30.Panula P, Nuutinen S, 2013. The histaminergic network in the brain: basic organization and role in disease. Nat Rev Neurosci 14:472–487 [DOI] [PubMed] [Google Scholar]
- 31.Panula P, Chazot PL, Cowart M, Gutzmer R, Leurs R, Liu WLS, Stark H, Thurmond RL, Haas HL, Ohlstein EH, 2015. International union of basic and clinical pharmacology. XCVIII. Histamine receptors. Pharmacol Rev 67:601–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Passani MB, Blandina P, 2011. Histamine receptors in the CNS as target for therapeutic intervention. Trends Pharmacol Sci 32:242–249 [DOI] [PubMed] [Google Scholar]
- 33.Pillot C, Heron A, Cochois V, Tardivel-Lacombe J, Ligneau X, Schwartz J-C, Arrang J-M, 2002. A detailed mapping of the histamine H3 receptor and its gene transcripts in rat brain. Neuroscience 114:173–193 [DOI] [PubMed] [Google Scholar]
- 34.Popoli P, Betto P, Reggio R, Ricciarello G, 1995. Adenosine A2A receptor stimulation enhances striatal extracellular glutamate levels in rats. Eur J Pharmacol 287:215–217 [DOI] [PubMed] [Google Scholar]
- 35.Prast H, Tran MH, Fischer H, Kraus M, Lamberti C, Grass K, Philippu A, 1999. Histaminergic neurons modulate acetylcholine release in the ventral striatum: role of H3 histamine receptors. Naunyn Schmiedebergs Arch Pharmacol 360:558–564 [DOI] [PubMed] [Google Scholar]
- 36.Quiroz C, Luján R, Chigashima M, Simoes AP, Lerner TN, Borycz J, Kachroo A, Canas PM, Orru M, Schwarzschild MA, Rosin DL, Kreitzer AC, Cunha RA, Watanabe M, Ferré S, 2009. Key modulatory role of presynaptic adenosine A2A receptors in cortical neurotransmission to the striatal direct pathway. Scientific World Journal 18:1321–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riddy DM, Cook AE, Diepenhorst NA, Bosnyak S, Brady R, Mannoury la Cour C, Mocaer E, Summers RJ, Charman WN, Sexton PM, Christopoulos A, Langmead CJ, 2016. Isoform-specific biased agonism of histamine H3 receptor agonists. Mol Pharmacol 91:87–99 [DOI] [PubMed] [Google Scholar]
- 38.Rivera-Ramírez N, Montejo-López W, López-Méndez MC, Guerrero-Hernández A, Molina-Hernández A, García-Hernández U, Arias-Montaño JA, 2016. Histamine H3 receptor activation stimulates calcium mobilization in a subpopulation of rat striatal neurons in primary culture, but not in synaptosomes. Neurochem Int 101:38–47 [DOI] [PubMed] [Google Scholar]
- 39.Rodríguez-Ruiz M, Moreno E, Moreno-Delgado D, Navarro G, Mallol J, Cortés A, Lluís C, Canela EI, Casadó V, McCormick PJ, Franco R, 2017. Heteroreceptor complexes formed by dopamine D1, histamine H3, and N-Methyl-D-Aspartate glutamate receptors as targets to prevent neuronal death in Alzheimer’s disease. Mol Neurobiol 54:4537–4550 [DOI] [PubMed] [Google Scholar]
- 40.Schiffmann SN, Jacobs O, Vanderhaeghen JJ, 1991. Striatal restricted adenosine A2 receptor (RDC8) is expressed by enkephalin but not by substance P neurons: an in situ hybridization histochemistry study. J Neurochem 57:1062–1067 [DOI] [PubMed] [Google Scholar]
- 41.Schliker E, Fink K, Detzner M, Gothert M, 1993. Histamine inhibits dopamine release in the mouse striatum via presynaptic H3 receptors. J Neural Transm Gen Sect 93:1–10 [DOI] [PubMed] [Google Scholar]
- 42.Schwarzschild MA, Agnati L, Fuxe K, Chen JF, Morelli M, 2006. Targeting adenosine A2A receptor in Parkinson’s disease. Trends Neurosci 29:647–654 [DOI] [PubMed] [Google Scholar]
- 43.Tapper JM, Kóos T, Wilson CJ, 2004. GABAergic microcircuits in the neostriatum. Trends Neurosci 27:662–669. [DOI] [PubMed] [Google Scholar]
- 44.Thomas P, Smart TG, 2005. HEK293 cells line: A vehicle for the expression of recombinant proteins. J Pharmacol and Toxicol Methods 51:187–200 [DOI] [PubMed] [Google Scholar]
- 45.van Rijn RM, Harvey JH, Brissett DI, DeFriel JN, Whistler JL, 2013. Novel screening assay for the selective detection of G-protein-coupled receptor heteromer signaling. J Pharmacol Exp Ther 344:179–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Welch JM, Lu J, Rodriguez RM, Trotta NC, Peca J, Ding J-D, Feliciano C, Chen M, Adams JP, Luo J, Dudek SM, Weinberg RJ, Calakos N, Wetsel WC, Feng G, 2008. Cortico-striatal synaptic defects and OCD-like behaviors in SPAP3 mutant mice. Nature 448:894–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.