Abstract
Objective
To determine real-world trends in antidiabetic drug use, and persistence and adherence, in Japanese patients with type 2 diabetes mellitus (T2DM).
Design
Retrospective evaluation of administrative claims data (2011–2015) using the Japan Medical Data Center (JMDC) and Medical Data Vision (MDV) databases.
Setting
Analysis of two administrative claims databases for Japanese patients with T2DM.
Participants
Adults (aged ≥18 years) with an International Classification of Diseases, 10th Revision code of T2DM and at least one antidiabetic drug prescription.
Main outcome measures
Treatment patterns in untreated (UT) or previously treated (PT) patients receiving antidiabetic therapy; persistence with treatment at 12 months; adherence to treatment at 12 months.
Results
40 908 and 90 421 patients were included from the JMDC and MDV databases, respectively. The most frequently prescribed therapy at the index (first prescription) date was dipeptidyl peptidase-4 inhibitor (DPP-4i) in UT patients (JMDC: 44.0%, MDV: 54.8%) and combination therapy in PT patients (74.6%, 81.1%). Most common combinations were DPP-4i plus: biguanide (BG; 11.4%, 10.9%), sulfonylurea (SU; 8.4%, 11.0%) or BG+SU (7.8%, 9.1%). In UT or PT patients from either database whose index prescription was for any antidiabetic drug class(es) other than DPP-4i, the most frequent add-on or switch was to DPP-4i. 12-month persistence with index monotherapy was highest with DPP-4i and BG. Adherence was high (≥80%) for all monotherapy schedules, except insulin and glucagon-like peptide-1 agonist, and for the five most frequent two-drug and three-drug combinations. Persistence was greater in elderly UT patients and in those receiving ≤5 medications, but comparatively worse in UT patients with ≥3 index antidiabetic drug classes.
Conclusions
The findings indicate that DPP-4i is the most commonly used antidiabetic drug class in Japanese patients with T2DM, and persistence and adherence to this antidiabetic drug class are high.
Keywords: adherence, administrative claims-based study, antidiabetic drug therapy, dipeptidyl peptidase-4 inhibitors, persistence, type 2 diabetes
Strengths and limitations of this study.
This retrospective evaluation of administrative claims data (2011–2015) using the Japan Medical Data Center (JMDC) and Medical Data Vision (MDV) databases was conducted to determine real-world trends in antidiabetic drug use, and persistence and adherence, in Japanese patients with type 2 diabetes mellitus (T2DM); 40 908 and 90 421 patients were included from the JMDC and MDV databases, respectively.
The main strengths of the study are that it provides robust real-world evidence from two large administrative claims databases for patterns of antidiabetic drug use in Japanese patients with T2DM, highlighting widespread use of dipeptidyl peptidase-4 inhibitor (DPP-4i) schedules (as monotherapy, add-on therapy, switch therapy or in combination regimens) and marked persistence and adherence with DPP-4i therapy.
The study was limited to some extent by the strict inclusion criteria which restricted the number of patients eligible for analysis, and by the use of prescription events rather than patient-derived data to estimate outcomes.
Database-specific limitations were the relative scarcity of data for patients aged ≥65 years (JMDC), the absence of information as to whether patients received care in other medical facilities (MDV), and the inability to examine reasons for treatment discontinuation and potential health benefits resulting from increased persistence (JMDC and MDV).
Uptake of sodium-glucose cotransporter-2 inhibitor use may not have been accurately captured given the timing of their introduction in Japan.
Introduction
The prevalence of diabetes mellitus continues to increase globally. In 2015, approximately 415 million people worldwide had diabetes, and this figure is projected to reach almost 650 million by 2040.1 As about 20% of men and 10% of women in Japan are considered to have or are highly likely to have diabetes, the public health implications are enormous.2
Disease characteristics in Asian individuals with type 2 diabetes mellitus (T2DM) differ from those in Caucasian patients; Japanese patients with T2DM principally have pancreatic β-cell dysfunction, with less insulin resistance and adiposity than Caucasians.1 Nevertheless, even in patients with mild metabolic dysfunction, T2DM has serious long-term consequences (ie, nephropathy, neuropathy and retinopathy) and is an important risk factor for atherosclerotic cardiovascular diseases.3 4
The benefits of early and effective intervention in T2DM are extensively acknowledged. Enhanced glycaemic control can markedly reduce microangiopathic and macroangiopathic development and progression.4 An intensified intervention to achieve stricter treatment targets was shown to be significantly superior to conventional therapy for prevention of cerebrovascular events in patients with T2DM.5 The Japan Diabetes Society (JDS) has developed evidence-based guidelines for the management of diabetes.6 In patients who fail to achieve adequate glycaemic control with diet, exercise and lifestyle improvement alone, treatment options include biguanides (BGs), thiazolidinediones (TZDs), sulfonylureas (SUs), glinides, dipeptidyl peptidase-4 inhibitor (DPP-4i), α-glucosidase inhibitors (α-GIs) and sodium-glucose cotransporter-2 inhibitors (SGLT2i), with treatment selection to be based on the underlying causes of T2DM.6
Despite widespread availability of the JDS guidelines and highly favourable conditions for access to healthcare in Japan, a 2-year longitudinal study using claims data identified that the quality of care for patients with T2DM is often suboptimal.7 Notably, screening for diabetic renal and ocular disease was less frequent than recommended in the guidelines and less than half of patients with diabetes were achieving the glycaemic goal (glycosylated haemoglobin (HbA1c) <7%) recommended by JDS for their circumstances.
Allied to these factors is the potential for suboptimal adherence to, and poor persistence with, treatment. Adherence is typically lower among patients with chronic conditions compared with those with acute conditions, and treatment persistence for chronic conditions is particularly low, tending to decline most dramatically within the first 6 months of treatment.8 The reasons for poor adherence and persistence are complex and multifactorial, involving patient-related and physician-related factors as well as treatment regimen factors such as pill burden, regimen complexity and dosing schedule.9
In Japan, it has been estimated that approximately 60% of patients with diabetes forget to take their medication at some stage.10 Non-adherence to antidiabetic medications is associated with increased healthcare expenditure and higher rates of hospitalisation and death.11 12 It has been suggested that use of a once-weekly DPP-4i or a fixed-dose combination (FDC) therapy may improve adherence in patients with T2DM.13 A 10% increase in adherence has been linked with a 0.1% decrease in HbA1c.11 14 Recent studies suggest that dual-therapy schedules containing a DPP-4i may improve persistence relative to DPP-4i monotherapy,15 or SU-containing schedules.16
Contemporary meta-analyses of studies involving incretin-based treatments (ie, DPP-4i or glucagon-like peptide-1 (GLP-1) receptor agonists) in patients with T2DM have shown that these agents are more effective in Asian than in non-Asian populations, possibly due to greater attenuation of β-cell dysfunction.1 17–19 Moreover, the HbA1c-reducing activity of DPP-4i has been linked with fish intake, suggesting that dietary factors may also contribute to their greater efficacy in Asian patients with T2DM.1 20 21
Despite widespread recognition of the deleterious long-term consequences of poorly managed T2DM, and the proven efficacy of incretin-based therapies in Asian populations with diabetes, surprisingly little is known about actual antidiabetic drug utilisation trends, and persistence and adherence patterns with antidiabetic drug therapy, in patients with T2DM in Japan. Under Japan’s compulsory insurance system, all residents are legally obligated to be covered by a form of public health insurance, and claims-related data are captured and stored in propriety databases. In the current study, data from two large administrative claims databases were used to determine real-world trends in antidiabetic drug use, and treatment persistence and adherence rates, in patients with T2DM in Japan.
Methods
Overview
This was a real-world, retrospective evaluation of data from two administrative claims databases in Japan: the Japan Medical Data Center (JMDC) database (Japan Medical Data Center; Tokyo, Japan) and the Medical Data Vision (MDV) database (Medical Data Vision; Tokyo, Japan). The JMDC database contains monthly claims submitted to health insurance societies from medical institutions since January 2005 and, as at July 2017, covered approximately 4 million beneficiaries (employees and their dependants). MDV is a nationwide hospital-based claims database covering nearly 19 million cumulative patients since April 2008 who, as at July 2017, had been treated as inpatients or outpatients at the approximately 300 hospitals in Japan (20% of total number of hospitals) that participate in the diagnosis procedure combination/per-diem payment system. Both databases hold anonymised information about diagnoses, patient characteristics, drug prescriptions, medical procedures, features of medical facilities and reimbursement costs. All patient data are encrypted before entry.
Study population
Eligible patients were adults (≥18 years) with a diagnosis of T2DM (International Classification of Diseases (ICD)-10 code: E11 or E14) who had been issued at least one prescription for an antidiabetic drug during the target selection period of January 2011 to December 2015. All patients were starting new antidiabetic drug therapy.
The first prescription date for an antidiabetic drug class initiated during the selection period was the index date, and the antidiabetic drug class prescribed was designated as the index antidiabetic drug class. Only patients with a new prescription during the selection period were included for analysis. The minimum 12-month preindex (‘look back’) period allowed time to observe patients’ baseline characteristics and ascertain that the first prescription of a given antidiabetic drug class corresponded to initiation of that drug class. The minimum 12-month postindex observational period allowed time to evaluate treatment-related outcomes of interest.
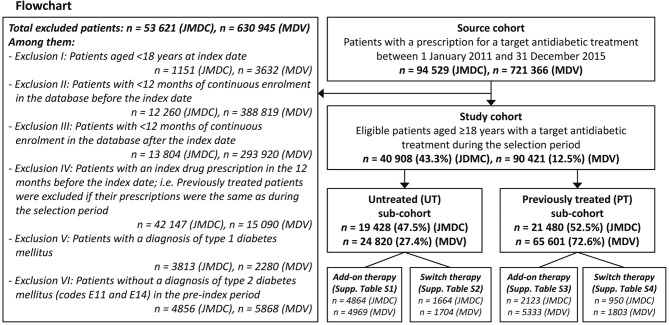
Patients were excluded for the following reasons: age <18 years at the index date; <12 months of continuous enrolment in the database before or after the index date; index prescription received in the 12 months before the index date; no T2DM diagnosis (ICD code E11 or E14) in the preindex period (figure 1).
Figure 1.
Patient disposition. JMDC, Japan Medical Data Center; MDV, Medical Data Vision.
The patient population was divided into two subgroups: (1) untreated (UT) patients, that is, patients without a prescription for any antidiabetic drug class of interest during the preindex period; and (2) previously treated (PT) patients, that is, patients with a prescription for at least one nonindex antidiabetic drug class during the preindex period.
Antidiabetic drug classes of interest
Target antidiabetic drug classes of interest were DPP-4i, BG, SU, α-GI, TZD, glinides, SGLT2i, insulin and GLP-1 receptor agonists and, in PT patients, were the five most common combinations of these same drug classes. Data for insulin and GLP-1 receptor agonists were excluded from persistence and adherence analyses mainly because of inconsistent database information regarding the duration of therapy for these injectable drug classes.
Objectives
The primary objectives of the study were to describe patterns of antidiabetic drug use and persistence with and adherence to antidiabetic drug classes in patients with T2DM, overall and by patient subgroup (UT and PT), in the JMDC and MDV database populations.
Outcomes
A treatment line was defined as the period during which a patient took a specific antidiabetic drug class or a combination of antidiabetic drug classes continuously, that is, without the addition of new class(es) or withdrawal/discontinuation of existing drug class(es). A treatment line-related event was defined as: an ‘add-on’ when a new antidiabetic drug class was prescribed in addition to an existing drug class(es) for more than 21 days (eg, DPP-4i >> add-on event >> DPP-4i+metformin); as a ‘switch’ when at least one new antidiabetic drug class was prescribed in place of an existing drug class(es) within the grace period which was 1.5 times the median prescription duration for a given drug class (eg, DPP-4i >> switch event >> metformin).
Treatment persistence was defined as the time from the index date until discontinuation of at least one index antidiabetic drug class. The median time to discontinuation and the proportion of patients persistent with treatment at 12 months were reported. The date of discontinuation was defined as the date of the last prescription of the first discontinued drug in an antidiabetic drug combination, plus the days of supply of that prescription.
Adherence analyses were performed for patients who received at least two prescriptions of the index antidiabetic drug class(es) during the 12-month postindex follow-up period. Adherence to an antidiabetic drug class of interest was defined as the proportion of days covered (PDC) or the period in which patients had the treatment in their possession (ie, from the index date to first discontinuation of index treatment) and was calculated according to the formula:
Total number of prescription days covered for defined drug class of interest /
Total number of days in the follow-up period.
As the JMDC and MDV databases each contain a field corresponding to the number of days’ supply of a medication, these data were used to calculate the number of prescription days.
Patients were considered adherent if a PDC of ≥0.8 (also expressed as an adherence rate of ≥80%) was achieved. The PDC was calculated from the index date to the first discontinuation of index treatment.
Adherence/persistence was calculated according to the number of antidiabetic drug prescription days, without differentiating between inpatient/outpatient prescribing. No information was available about possible pill dumping or stockpiling.
Statistical analyses
Analyses were performed using SAS V.9.3 (SAS) and were conducted on all patients who met the inclusion criteria and were stratified into the two prespecified patient subgroups (UT and PT) on the index date. Patient demographics, clinical characteristics, treatment-related events affecting index therapy (add-on, switch) and adherence were reported descriptively. The median time to discontinuation was calculated by antidiabetic drug class using Kaplan-Meier survival analysis, with differences between patient subgroups (UT and PT) assessed by log-rank test. The first discontinuation of the index antidiabetic drug class was the survival event and patients were censored if they reached the end of follow-up without discontinuation.
The log-rank test was used to compare the Kaplan-Meier estimates between groups. Cox regression analysis was used to estimate the HR of each event, adjusting for baseline characteristics. For all analyses, a p value of less than α=0.05 was considered statistically significant. For the selection of patient characteristics to be included in regression models, a threshold level of α=0.10 was used.
Patient involvement
No patients were involved in setting the research question or outcome measures, and no patients were involved in developing plans for study implementation. Furthermore, no patients were asked for advice about the interpretation or writing up of results. There are no plans to distribute the research findings to study participants or the specific patient community. Individual patient consent was not required for this study, as the trial was based on anonymised administrative claims data.
Results
Patient disposition
Between January 2011 and December 2015, 94 529 patients in the JMDC database and 721 366 patients in the MDV database with at least one prescription for an antidiabetic drug class of interest were identified. Of these, 40 908 patients (43.3%) in the JMDC database and 90 421 patients (8.0%) in the MDV database met the inclusion criteria and were included in the analyses (figure 1). The ratio of UT to PT patients was approximately 1:1 in the JMDC database and 1:3 in the MDV database.
Patient characteristics
Patient demographics and clinical characteristics are presented in table 1.
Table 1.
Patient demographics and clinical characteristics
| Characteristics | UT patients | PT patients | ||
| JMDC database n=19 428 | MDV database n=24 820 |
JMDC database n=21 480 |
MDV database n=65 601 |
|
| Follow-up, days, median (IQR) | 929 (635–1345) | 942 (675–1356) | 980 (671–1446) | 1027 (715–1521) |
| Age at index date, years, mean (SD) | 51.7 (9.9) | 67.6 (11.8) | 54.4 (9.2) | 65.9 (12.0) |
| Gender: male, n (%) | 14 042 (72.3) | 15 093 (60.8) | 15 779 (73.5) | 40 160 (61.2) |
| Multiple medications*, mean (SD) | 2.0 (4.0) | 3.0 (2.2) | 2.3 (3.1) | 3.3 (2.0) |
| Charlson Comorbidity Index, mean (SD) | 2.2 (1.5) | 2.5 (2.3) | 2.5 (1.6) | 2.6 (2.2) |
| Comorbidities | ||||
| Hypertension (% pts) | 47.8 | 70.1 | 58.3 | 71.3 |
| Hyperlipidaemia (% pts) | 39.8 | 70.0 | 50.0 | 67.2 |
| Dementia (% pts) | 0.2 | 1.9 | 0.2 | 2.0 |
| Diabetic nephropathy (% pts) | 3.7 | 18.1 | 6.1 | 15.7 |
*Number of drugs prescribed (by three-digit Anatomical Therapeutic Chemical Classification System).
JMDC, Japan Medical Data Center; MDV, Medical Data Vision; PT, previously treated; pts, patients; UT, untreated.
The median duration of follow-up in UT patients was 929 days in the JMDC database and 942 days in the MDV database. Mean age was 51.7 years and 67.6 years, respectively. There was a higher proportion of males (72.3% vs 60.8%), a lower mean number of concurrent medications (2.0 vs 3.0) and lower incidences of comorbid hypertension (47.8% vs 70.1%), hyperlipidaemia (39.8% vs 70.0%), dementia (0.2% vs 1.9%) and diabetic nephropathy (3.7% vs 18.1%) among UT patients in the JMDC versus MDV database.
Among PT patients, the median duration of follow-up was 980 days in the JMDC database and 1027 days in the MDV database. Mean age was 54.4 years and 66.9 years, respectively. There was a higher proportion of males (73.5% vs 61.2%), a lower mean number of concurrent medications (2.3 vs 3.3) and lower incidences of comorbid hypertension (58.3% vs 71.3%), hyperlipidaemia (50.0% vs 67.2%), dementia (0.2% vs 2.0%) and diabetic nephropathy (6.1% vs 15.7%) among PT patients in the JMDC versus MDV database.
Index date therapy
Treatment patterns for index antidiabetic drug classes were broadly similar for UT patients and PT patients irrespective of the dataset (JMDC or MDV).
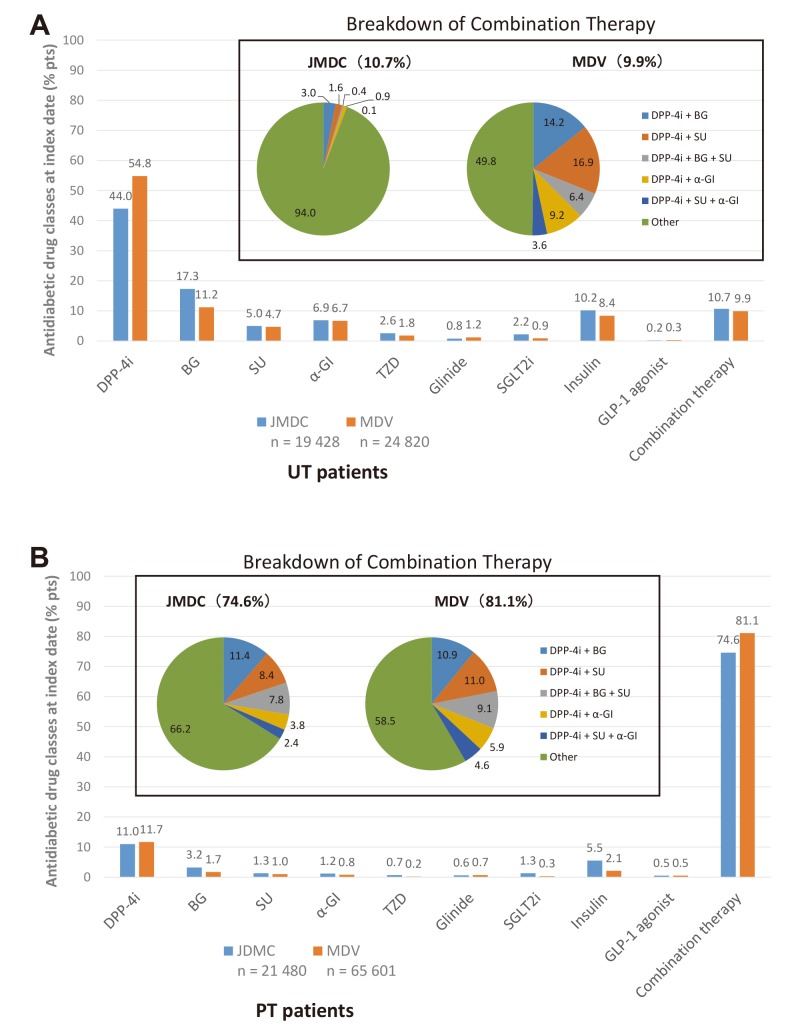
In UT patients (figure 2A), the most common index prescription was for DPP-4i monotherapy (JMDC: 44.0%; MDV: 54.8%), followed by BG, insulin and combination therapy. The composition of combination therapy (ie, combinations of antidiabetic drug classes) was highly varied.
Figure 2.
Antidiabetic drug classes prescribed at the index date in (A) UT patients and (B) PT patients in the JMDC and MDV databases. α-GI, α-glucosidase inhibitor; BG, biguanide; DPP-4i, dipeptidyl peptidase-4 inhibitor; GLP-1, glucagon-like peptide-1; JMDC, Japan Medical Data Center; MDV, Medical Data Vision; PT, previously treated; pts, patients; SGLT2i, sodium-glucose cotransporter-2 inhibitor; SU, sulfonylurea; TZD, thiazolidinedione; UT, untreated.
In PT patients (figure 2B), the most common index prescription was for combination therapy (JMDC: 74.6%; MDV: 81.1%), and the most frequent combinations were a DPP-4i plus a BG or/and a SU. Combinations could consist of single agents in combination, FDC or FDC + single agents in combination. The next most common index therapy in PT patients was DPP-4i monotherapy (JMDC: 11.0%; MDV: 11.7%). Use of other antidiabetic drug classes as monotherapy was low.
Changes to index therapy
In UT patients who had received a DPP-4i as the index prescription, the most frequent add-on was a BG (JMDC: 46.6%; MDV: 36.7%). In UT patients whose index prescription was for any other antidiabetic drug class, the most frequent add-on in all cases (apart from GLP-1 receptor agonists) was a DPP-4i (table 2).
Table 2.
Changes to index therapy: add-on treatment over 12 months according to index antidiabetic drug class in UT patients, n (%)
| JMDC database | |||||||||
| Index treatment | DPP-4i | BG | SU | α-GI | TZD | Glinide | SGLT2i | Insulin | GLP-1 |
| Pts with add-on therapy | n=2839 | n=1102 | n=364 | n=316 | n=102 | n=50 | n=76 | n=7 | n=8 |
| +DPP-4i | NA | 748 (67.9) | 208 (57.1) | 146 (46.2) | 57 (55.9) | 21 (42.0) | 34 (44.7) | 4 (57.1) | 1 (12.5) |
| +BG | 1324 (46.6) | NA | 80 (22.0) | 85 (26.9) | 21 (20.6) | 9 (18.0) | 28 (36.8) | 2 (28.6) | 1 (12.5) |
| +SU | 537 (18.9) | 66 (6.0) | NA | 30 (9.5) | 8 (7.8) | 1 (2.0) | 5 (6.6) | 0 (0.0) | 2 (25.0) |
| +α-GI | 255 (9.0) | 40 (3.6) | 25 (6.9) | NA | 4 (3.9) | 15 (30.0) | 5 (6.6) | 0 (0.0) | 0 (0.0) |
| +TZD | 293 (10.3) | 58 (5.3) | 20 (5.5) | 16 (5.1) | NA | 1 (2.0) | 1 (1.3) | 0 (0.0) | 2 (25.0) |
| +Glinide | 79 (2.8) | 16 (1.5) | 0 (0.0) | 17 (5.4) | 0 (0.0) | NA | 0 (0.0) | 1 (14.3) | 0 (0.0) |
| +SGLT2i | 256 (9.0) | 128 (11.6) | 11 (3.0) | 2 (0.6) | 7 (6.9) | 2 (4.0) | NA | 0 (0.0) | 2 (25.0) |
| +Insulin | 5 (0.2) | 2 (0.2) | 1 (0.3) | 1 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA | 0 (0.0) |
| +GLP-1 | 0 (0.0) | 16 (1.5) | 2 (0.5) | 2 (0.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA |
| MDV database | |||||||||
| Index treatment | DPP-4i | BG | SU | α-GI | TZD | Glinide | SGLT2i | Insulin | GLP-1 |
| Pts with add-on therapy | n=3179 | n=878 | n=342 | n=344 | n=81 | n=74 | n=35 | n=24 | n=12 |
| +DPP-4i | NA | 602 (68.6) | 215 (62.9) | 208 (60.5) | 43 (53.1) | 42 (56.8) | 12 (34.3) | 113 (50.4) | 0 (0.0) |
| +BG | 1168 (36.7) | NA | 51 (14.9) | 36 (10.5) | 14 (17.3) | 7 (9.5) | 12 (34.3) | 26 (11.6) | 4 (33.3) |
| +SU | 736 (23.2) | 44 (5.0) | NA | 36 (10.5) | 3 (3.7) | 1 (1.4) | 4 (11.4) | 10 (4.5) | 4 (33.3) |
| +α-GI | 414 (13.0) | 38 (4.3) | 29 (8.5) | NA | 6 (7.4) | 15 (20.3) | 0 (0.0) | 28 (12.5) | 1 (8.3) |
| +TZD | 168 (5.3) | 29 (3.3) | 13 (3.8) | 4 (1.2) | NA | 4 (5.4) | 0 (0.0) | 1 (0.4) | 0 (0.0) |
| +Glinide | 189 (5.9) | 9 (1.0) | 0 (0.0) | 26 (7.6) | 2 (2.5) | NA | 0 (0.0) | 12 (5.4) | 0 (0.0) |
| +SGLT2i | 190 (6.0) | 94 (10.7) | 7 (2.0) | 5 (1.5) | 8 (9.9) | 0 (0.0) | NA | 2 (0.9) | 3 (25.0) |
| +Insulin | 239 (7.5) | 35 (4.0) | 14 (4.1) | 19 (5.5) | 1 (1.2) | 3 (4.1) | 1 (2.9) | NA | 0 (0.0) |
| +GLP-1 | 2 (0.1) | 10 (1.1) | 4 (1.2) | 1 (0.3) | 0 (0.0) | 1 (1.4) | 4 (11.4) | 0 (0.0) | NA |
‘+’ indicates add-on therapy with new antidiabetic drug class.
α-GI, α-glucosidase inhibitor; BG, biguanide; DPP-4i, dipeptidyl peptidase-4 inhibitor; GLP-1, glucagon-like peptide-1 receptor agonist; JMDC, Japan Medical Data Center; MDV, Medical Data Vision; NA, not applicable; pts, patients; SGLT2i, sodium-glucose cotransporter-2 inhibitor; SU, sulfonylurea; TZD, thiazolidinedione; UT, untreated.
In UT patients who had received a DPP-4i as the index prescription, the most frequent treatment switch to another antidiabetic drug class was to a BG (JMDC: 32.7%; MDV: 26.2%) or insulin (JMDC: 14.3%; MDV: 30.5%). In UT patients whose index prescription was for any other antidiabetic drug class, the most frequent treatment switch was to a DPP-4i (table 3).
Table 3.
Changes to index therapy: switch treatment over 12 months according to index antidiabetic drug class in UT patients, n (%)
| JMDC database | |||||||||
| Index treatment | DPP-4i | BG | SU | α-GI | TZD | Glinide | SGLT2i | Insulin | GLP-1 |
| Pts with switch therapy | n=440 | n=267 | n=224 | n=221 | n=76 | n=44 | n=50 | n=336 | n=6 |
| → DPP-4i | NA | 157 (58.8) | 106 (47.3) | 126 (57.0) | 43 (56.6) | 22 (50.0) | 26 (52.0) | 115 (34.2) | 1 (16.7) |
| → BG | 144 (32.7) | NA | 47 (21.0) | 40 (18.1) | 15 (19.7) | 8 (18.2) | 13 (26.0) | 107 (31.8) | 1 (16.7) |
| → SU | 52 (11.8) | 12 (4.5) | NA | 4 (1.8) | 0 (0.0) | 3 (6.8) | 1 (2.0) | 11 (3.3) | 1 (16.7) |
| → α-GI | 20 (4.5) | 12 (4.5) | 4 (1.8) | NA | 2 (2.6) | 2 (4.5) | 2 (4.0) | 19 (5.7) | 0 (0.0) |
| → TZD | 26 (5.9) | 19 (7.1) | 8 (3.6) | 11 (5.0) | NA | 0 (0.0) | 0 (0.0) | 5 (1.5) | 0 (0.0) |
| → Glinide | 22 (5.0) | 3 (1.1) | 5 (2.2) | 11 (5.0) | 0 (0.0) | NA | 0 (0.0) | 11 (3.3) | 0 (0.0) |
| → SGLT2i | 82 (18.6) | 26 (9.7) | 3 (1.3) | 10 (4.5) | 7 (9.2) | 3 (6.8) | NA | 2 (0.6) | 1 (16.7) |
| → Insulin | 63 (14.3) | 17 (6.4) | 36 (16.1) | 8 (3.6) | 5 (6.6) | 4 (9.1) | 2 (4.0) | NA | 2 (33.3) |
| → GLP-1 | 7 (1.6) | 3 (1.1) | 0 (0.0) | 0 (0.0) | 1 (1.3) | 0 (0.0) | 0 (0.0) | 6 (1.8) | NA |
| MDV database | |||||||||
| Index treatment | DPP-4i | BG | SU | α-GI | TZD | Glinide | SGLT2i | Insulin | GLP-1 |
| Pts with switch therapy | n=446 | n=271 | n=199 | n=224 | n=69 | n=47 | n=20 | n=417 | n=11 |
| → DPP-4i | NA | 206 (76.0) | 144 (72.4) | 155 (69.2) | 41 (59.4) | 24 (51.1) | 6 (30.0) | 224 (53.7) | 5 (45.5) |
| → BG | 117 (26.2) | NA | 15 (7.5) | 21 (9.4) | 14 (20.3) | 4 (8.5) | 6 (30.0) | 34 (8.2) | 2 (18.2) |
| → SU | 51 (11.4) | 12 (4.4) | NA | 15 (6.7) | 4 (5.8) | 4 (8.5) | 2 (10.0) | 25 (6.0) | 0 (0.0) |
| → α-GI | 38 (8.5) | 7 (2.6) | 1 (0.5) | NA | 1 (1.4) | 7 (14.9) | 0 (0.0) | 26 (6.2) | 0 (0.0) |
| → TZD | 18 (4.0) | 9 (3.3) | 0 (0.0) | 4 (1.8) | NA | 0 (0.0) | 0 (0.0) | 7 (1.7) | 0 (0.0) |
| → Glinide | 14 (3.1) | 4 (1.5) | 5 (2.5) | 10 (4.5) | 0 (0.0) | NA | 0 (0.0) | 30 (7.2) | 0 (0.0) |
| → SGLT2i | 52 (11.7) | 14 (5.2) | 2 (1.0) | 1 (0.4) | 3 (4.3) | 0 (0.0) | NA | 2 (0.5) | 2 (18.2) |
| → Insulin | 136 (30.5) | 10 (3.7) | 21 (10.6) | 15 (6.7) | 4 (5.8) | 6 (12.8) | 0 (0.0) | NA | 1 (9.1) |
| → GLP-1 | 11 (2.5) | 4 (1.5) | 1 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (10.0) | 9 (2.2) | NA |
‘→’ indicates treatment switch to new antidiabetic drug class.
α-GI, α-glucosidase inhibitor; BG, biguanide; DPP-4i, dipeptidyl peptidase-4 inhibitor; GLP-1, glucagon-like peptide-1 receptor agonist; JMDC, Japan Medical Data Center; MDV, Medical Data Vision; NA, not applicable; pts, patients; SGLT2i, sodium-glucose cotransporter-2 inhibitor; SU, sulfonylurea; TZD, thiazolidinedione; UT, untreated.
In PT patients who had received a DPP-4i as the index prescription, the most frequent add-on was a BG (JMDC: 30.4%; MDV: 21.6%), SU (JMDC: 24.5%; MDV: 22.8%), or insulin, but only in the MDV population (JMDC: 0.7%; MDV: 24.6%). In PT patients whose index prescription was for any other antidiabetic drug class, the most frequent add-on was a DPP-4i to all drug classes except GLP-1 receptor agonists in the JMDC database and was a DPP-4i to all drug classes except α-GI and GLP-1 receptor agonists in the MDV database (table 4).
Table 4.
Changes to index therapy: add-on treatment over 12 months according to index antidiabetic drug class in PT patients, n (%)
| JMDC database | |||||||||
| Index treatment | DPP-4i | BG | SU | α-GI | TZD | Glinide | SGLT2i | Insulin | GLP-1 |
| Pts with add-on therapy | n=1141 | n=370 | n=163 | n=119 | n=68 | n=71 | n=129 | n=9 | n=53 |
| +DPP-4i | NA | 186 (50.3) | 77 (47.2) | 52 (43.7) | 18 (26.5) | 30 (42.3) | 39 (30.2) | 5 (55.6) | 0 (0.0) |
| +BG | 347 (30.4) | NA | 23 (14.1) | 14 (11.8) | 11 (16.2) | 12 (16.9) | 19 (14.7) | 0 (0.0) | 12 (22.6) |
| +SU | 279 (24.5) | 27 (7.3) | NA | 12 (10.1) | 9 (13.2) | 1 (1.4) | 5 (3.9) | 2 (22.2) | 15 (28.3) |
| +α-GI | 172 (15.1) | 21 (5.7) | 13 (8.0) | NA | 2 (2.9) | 10 (14.1) | 2 (1.6) | 1 (11.1) | 3 (5.7) |
| +TZD | 120 (10.5) | 13 (3.5) | 12 (7.4) | 1 (0.8) | NA | 0 (0.0) | 4 (3.1) | 0 (0.0) | 1 (1.9) |
| +Glinide | 43 (3.8) | 8 (2.2) | 1 (0.6) | 6 (5.0) | 1 (1.5) | NA | 0 (0.0) | 1 (11.1) | 0 (0.0) |
| +SGLT2i | 46 (4.0) | 24 (6.5) | 3 (1.8) | 2 (1.7) | 0 (0.0) | 2 (2.8) | NA | 0 (0.0) | 2 (3.8) |
| +Insulin | 8 (0.7) | 3 (0.8) | 0 (0.0) | 1 (0.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA | 0 (0.0) |
| +GLP-1 | 3 (0.3) | 16 (4.3) | 7 (4.3) | 3 (2.5) | 2 (2.9) | 0 (0.0) | 3 (2.3) | 0 (0.0) | NA |
| MDV database | |||||||||
| Index treatment | DPP-4i | BG | SU | α-GI | TZD | Glinide | SGLT2i | Insulin | GLP-1 |
| Pts with add-on therapy | n=3362 | n=616 | n=322 | n=211 | n=53 | n=180 | n=114 | n=335 | n=140 |
| +DPP-4i | NA | 249 (40.4) | 177 (55.0) | 63 (29.9) | 15 (28.3) | 66 (36.7) | 38 (33.3) | 128 (38.2) | 1 (0.7) |
| +BG | 727 (21.6) | NA | 31 (9.6) | 8 (3.8) | 7 (13.2) | 16 (8.9) | 23 (20.2) | 35 (10.4) | 18 (12.9) |
| +SU | 768 (22.8) | 38 (6.2) | NA | 11 (5.2) | 6 (11.3) | 1 (0.6) | 4 (3.5) | 12 (3.6) | 61 (43.6) |
| +α-GI | 444 (13.2) | 28 (4.5) | 20 (6.2) | NA | 1 (1.9) | 25 (13.9) | 3 (2.6) | 28 (8.4) | 11 (7.9) |
| +TZD | 131 (3.9) | 15 (2.4) | 9 (2.8) | 3 (1.4) | NA | 3 (1.7) | 1 (0.9) | 5 (1.5) | 1 (0.7) |
| +Glinide | 216 (6.4) | 10 (1.6) | 1 (0.3) | 9 (4.3) | 0 (0.0) | NA | 0 (0.0) | 10 (3.0) | 5 (3.6) |
| +SGLT2i | 59 (1.8) | 29 (4.7) | 4 (1.2) | 2 (0.9) | 3 (5.7) | 2 (1.1) | NA | 6 (1.8) | 3 (2.1) |
| +Insulin | 828 (24.6) | 163 (26.5) | 37 (11.5) | 80 (37.9) | 14 (26.4) | 45 (25.0) | 7 (6.1) | NA | 22 (15.7) |
| +GLP-1 | 1 (0.0) | 24 (3.9) | 16 (5.0) | 3 (1.4) | 2 (3.8) | 2 (1.1) | 6 (5.3) | 12 (3.6) | NA |
‘+’ indicates add-on therapy with new antidiabetic drug class.
α-GI, α-glucosidase inhibitor; BG, biguanide; DPP-4i, dipeptidyl peptidase-4 inhibitor; GLP-1, glucagon-like peptide-1 receptor agonist; JMDC, Japan Medical Data Center; MDV, Medical Data Vision; NA, not applicable; PT, previously treated; pts, patients; SGLT2i, sodium-glucose cotransporter-2 inhibitor; SU, sulfonylurea; TZD, thiazolidinedione.
In PT patients whose index treatment was a DPP-4i, the most frequent treatment switch was to insulin (JMDC: 35.0%; MDV: 30.1%). In PT patients whose index prescription was for any other antidiabetic drug class, the most common treatment switch in either dataset was to a DPP-4i or to insulin (table 5).
Table 5.
Changes to index therapy: switch treatment over 12 months according to index antidiabetic drug class in PT patients, n (%)
| JMDC database | |||||||||
| Index treatment | DPP-4i | BG | SU | α-GI | TZD | Glinide | SGLT2i | Insulin | GLP-1 |
| Pts with switch therapy | n=303 | n=92 | n=56 | n=70 | n=46 | n=38 | n=50 | n=268 | n=27 |
| → DPP-4i | NA | 27 (29.3) | 15 (26.8) | 14 (20.0) | 20 (43.5) | 14 (36.8) | 16 (32.0) | 84 (31.3) | 4 (14.8) |
| → BG | 44 (14.5) | NA | 8 (14.3) | 8 (11.4) | 8 (17.4) | 2 (5.3) | 6 (12.0) | 48 (17.9) | 6 (22.2) |
| → SU | 56 (18.5) | 8 (8.7) | NA | 3 (4.3) | 5 (10.9) | 5 (13.2) | 2 (4.0) | 27 (10.1) | 5 (18.5) |
| → α-GI | 15 (5.0) | 2 (2.2) | 1 (1.8) | NA | 1 (2.2) | 3 (7.9) | 1 (2.0) | 17 (6.3) | 2 (7.4) |
| → TZD | 14 (4.6) | 8 (8.7) | 1 (1.8) | 1 (1.4) | NA | 2 (5.3) | 4 (8.0) | 6 (2.2) | 1 (3.7) |
| → Glinide | 12 (4.0) | 2 (2.2) | 4 (7.1) | 1 (1.4) | 0 (0.0) | NA | 1 (2.0) | 7 (2.6) | 0 (0.0) |
| → SGLT2i | 17 (5.6) | 2 (2.2) | 1 (1.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA | 1 (0.4) | 1 (3.7) |
| → Insulin | 106 (35.0) | 37 (40.2) | 18 (32.1) | 37 (52.9) | 6 (13.0) | 6 (15.8) | 9 (18.0) | NA | 7 (25.9) |
| → GLP-1 | 8 (2.6) | 1 (1.1) | 0 (0.0) | 0 (0.0) | 1 (2.2) | 0 (0.0) | 2 (4.0) | 9 (3.4) | NA |
| MDV database | |||||||||
| Index treatment | DPP-4i | BG | SU | α-GI | TZD | Glinide | SGLT2i | Insulin | GLP-1 |
| Pts with switch therapy | n=651 | n=154 | n=135 | n=119 | n=36 | n=119 | n=34 | n=480 | n=75 |
| → DPP-4i | NA | 57 (37.0) | 67 (49.6) | 44 (37.0) | 15 (41.7) | 43 (36.1) | 15 (44.1) | 164 (34.2) | 19 (25.3) |
| → BG | 66 (10.1) | NA | 7 (5.2) | 6 (5.0) | 4 (11.1) | 3 (2.5) | 6 (17.6) | 19 (4.0) | 5 (6.7) |
| → SU | 168 (25.8) | 13 (8.4) | NA | 6 (5.0) | 4 (11.1) | 22 (18.5) | 1 (2.9) | 48 (10.0) | 4 (5.3) |
| → α-GI | 66 (10.1) | 7 (4.5) | 3 (2.2) | NA | 1 (2.8) | 9 (7.6) | 0 (0.0) | 18 (3.8) | 2 (2.7) |
| → TZD | 26 (4.0) | 11 (7.1) | 2 (1.5) | 3 (2.5) | NA | 0 (0.0) | 1 (2.9) | 10 (2.1) | 2 (2.7) |
| → Glinide | 48 (7.4) | 3 (1.9) | 8 (5.9) | 2 (1.7) | 0 (0.0) | NA | 0 (0.0) | 32 (6.7) | 2 (2.7) |
| → SGLT2i | 16 (2.5) | 3 (1.9) | 2 (1.5) | 2 (1.7) | 1 (2.8) | 0 (0.0) | NA | 2 (0.4) | 0 (0.0) |
| → Insulin | 196 (30.1) | 48 (31.2) | 32 (23.7) | 45 (37.8) | 10 (27.8) | 27 (22.7) | 1 (2.9) | NA | 26 (34.7) |
| → GLP-1 | 18 (2.8) | 1 (0.6) | 0 (0.0) | 1 (0.8) | 0 (0.0) | 1 (0.8) | 3 (8.8) | 3 (0.6) | NA |
‘+’ indicates treatment switch to new antidiabetic drug.
α-GI, α-glucosidase inhibitor; BG, biguanide; DPP-4i, dipeptidyl peptidase-4 inhibitor; GLP-1, glucagon-like peptide-1 receptor agonist; JMDC, Japan Medical Data Center; MDV, Medical Data Vision; NA, not applicable; PT, previously treated; pts, patients; SGLT2i, sodium-glucose cotransporter-2 inhibitor; SU, sulfonylurea; TZD, thiazolidinedione.
Persistence and adherence with index monotherapy
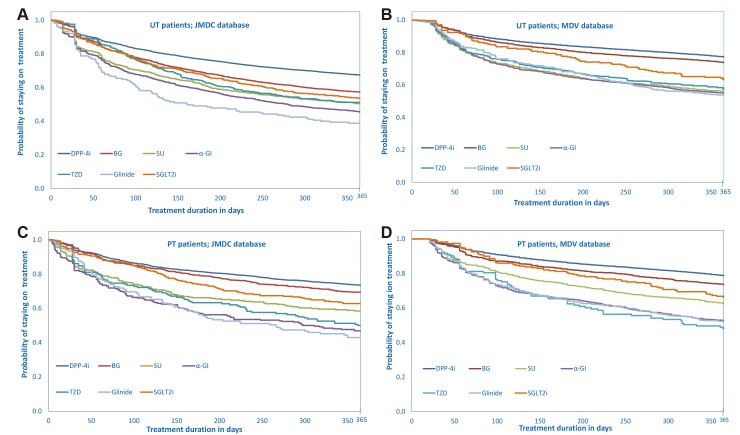
In both patient subgroups across both databases, the probability of remaining on treatment with index monotherapy at 12 months (not including insulin and GLP-1 receptor agonists) was highest with DPP-4i schedules and lowest with glinide schedules (table 6). This is illustrated schematically by Kaplan-Meier survival curves showing the distribution of median time to treatment discontinuation during 12 months’ observation by index antidiabetic drug class for UT and PT patients in each database. Among UT patients, persistence with all antidiabetic drug classes was considerably lower in the JMDC database especially with glinide schedules (figure 3A), than in the MDV database (figure 3B). Among PT patients, persistence with all antidiabetic drug classes tended to be slightly lower in the JMDC database for all antidiabetic drug classes except TZD and especially for glinide schedules (figure 3C) than in the MDV database (figure 3D). Twelve- month persistence rates of approximately 50% or less were recorded for SU, α-GI, TZD and glinides in one or both patient subgroups from one or both datasets (table 6).
Table 6.
Persistence with monotherapy schedules of index antidiabetic drug classes
| UT patients | |||||||
| Index therapy | DPP-4i | BG | SU | α-GI | TZD | Glinide | SGLT2i |
| JMDC database | n=8545 | n=3354 | n=979 | n=1346 | n=504 | n=165 | n=430 |
| Median time to discontinuation (days) | 1138.0 | 582.0 | 384.0 | 280.0 | 400.0 | 161.0 | 471.0 |
| 12-month persistence rate (% pts) | 67.4 | 57.3 | 50.4 | 45.5 | 51.2 | 38.8 | 53.5 |
| MDV database | n=13 598 | n=2777 | n=1174 | n=1666 | n=449 | n=292 | n=224 |
| Median time to discontinuation (days) | 707.0 | 672.0 | 474.5 | 458.0 | 491.0 | 438.5 | 537.5 |
| 12-month persistence rate (% pts) | 77.2 | 73.8 | 56.0 | 54.9 | 57.2 | 53.8 | 63.4 |
| PT patients | |||||||
| Index therapy | DPP-4i | BG | SU | α-GI | TZD | Glinide | SGLT2i |
| JMDC database | n=2354 | n=680 | n=284 | n=256 | n=158 | n=135 | n=285 |
| Median time to discontinuation (days) | 1583.0 | 917.0 | 599.0 | 304.5 | 370.0 | 266.0 | 691.0 |
| 12-month persistence rate (% pts) | 73.5 | 69.3 | 58.1 | 46.9 | 50.0 | 43.0 | 62.8 |
| MDV database | n=7658 | n=1100 | n=633 | n=495 | n=133 | n=446 | n=229 |
| Median time to discontinuation (days) | 764.0 | 666.5 | 532.0 | 422.0 | 333.0 | 396.0 | 553.0 |
| 12-month persistence rate (% pts) | 78.8 | 73.6 | 62.2 | 52.7 | 48.1 | 52.2 | 66.4 |
α-GI, α-glucosidase inhibitor; BG, biguanide; DPP-4i, dipeptidyl peptidase-4 inhibitor; JMDC, Japan Medical Data Center; MDV, Medical Data Vision; PT, previously treated; pts, patients; SGLT2i, sodium-glucose cotransporter-2 inhibitor; SU, sulfonylurea; TZD, thiazolidinedione; UT, untreated.
Figure 3.
Kaplan-Meier survival distribution of median time to treatment discontinuation according to index antidiabetic drug class; (A) UT patients; JMDC database; (B) UT patients, MDV database; (C) PT patients, JMDC database; (D) PT patients, MDV database. α-GI, α-glucosidase inhibitor; BG, biguanide; DPP-4i, dipeptidyl peptidase-4 inhibitor; JMDC, Japan Medical Data Center; MDV, Medical Data Vision; PT, previously treated; SGLT2i, sodium-glucose cotransporter-2 inhibitor; SU, sulfonylurea; TZD, thiazolidinedione; UT, untreated.
Adherence to index antidiabetic drug classes (not including insulin and GLP-1 receptor agonists) was high in both patient subgroups across both databases, with rates ranging from 75.0% to 98.9%. In UT patients (figure 4A) and in PT patients (figure 4B), adherence rates with index antidiabetic drug classes were consistently lower in the JMDC database than in the MDV database. The lowest adherence rates were recorded with SGLT2i in UT patients (75.0%) and PT patients (77.0%) in the JMDC database.
Figure 4.
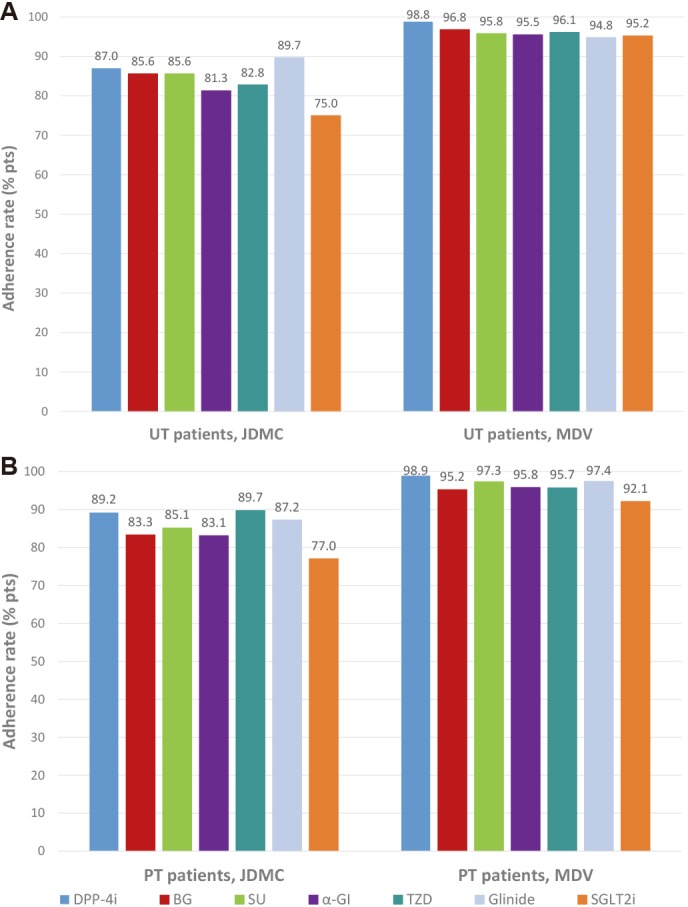
Twelve-month adherence to index antidiabetic drug classes in (A) UT patients and (B) PT patients in the JMDC and MDV databases. α-GI, α-glucosidase inhibitor; BG, biguanide; DPP-4i, dipeptidyl peptidase-4 inhibitor; JMDC, Japan Medical Data Center; MDV, Medical Data Vision; PT, previously treated; pts, patients; SGLT2i, sodium-glucose cotransporter-2 inhibitor; SU, sulfonylurea; TZD, thiazolidinedione; UT, untreated.
Persistence and adherence with index combination therapy
For the five most common antidiabetic drug combinations prescribed to PT patients on the index date (ie, a DPP-4i plus BG, SU, BG+SU, α-GI, or SU+α-GI), 12-month persistence rates were highest for DPP-4i plus BG (JMDC: 53.7%; MDV: 72.0%) and lowest for DPP-4i plus SU+α-GI (JMDC: 30.8%; MDV: 64.2%) (figure 5). Overall, 12-month persistence rates were considerably lower in the JMDC versus MDV database (figure 5).
Figure 5.
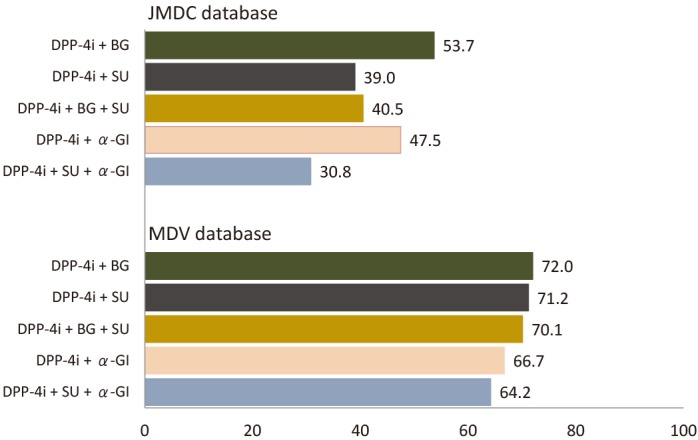
Twelve-month persistence rates with the five most frequent index antidiabetic drug combinations in PT patients in the JMDC and MDV databases. α-GI, α-glucosidase inhibitor; BG, biguanide; DPP-4i, dipeptidyl peptidase-4 inhibitor; JMDC, Japan Medical Data Center; MDV, Medical Data Vision; PT, previously treated; SU, sulfonylurea.
For the five most common antidiabetic drug combinations prescribed to PT patients on the index date (ie, a DPP-4i plus BG, SU, BG+SU, α-GI, or SU+α-GI), adherence rates were ≥80% in both database populations although were slightly lower in the JMDC versus MDV database (figure 6).
Figure 6.
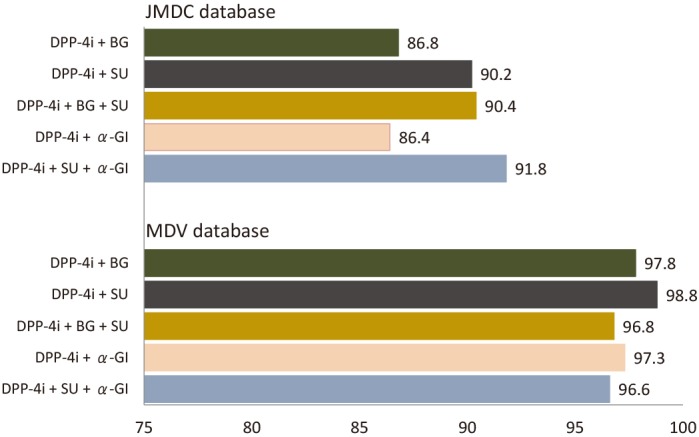
Twelve-month adherence rates to the five most frequent index antidiabetic drug combinations in PT patients in the JMDC and MDV databases. α-GI, α-glucosidase inhibitor; BG, biguanide; DPP-4i, dipeptidyl peptidase-4 inhibitor; JMDC, Japan Medical Data Center; MDV, Medical Data Vision; PT, previously treated; SU, sulfonylurea.
Discussion
Principal findings
This real-world evaluation of data from two administrative claims databases in Japan reveals that the most common index antidiabetic drug class was DPP-4i in UT patients (44%–55%) and combination therapy in PT patients (~75%–80%), with the latter most frequently comprising dual therapy with a DPP-4i plus a BG or SU.
Among patients with a change to their index antidiabetic drug therapy during follow-up: the most common add-on to DPP-4i index therapy was a BG or SU; the most common add-on to BG or SU index therapy was a DPP-4i; the most common switch from DPP-4i index therapy was to a BG or SU; the most common switch from index drug classes other than DPP-4i (except GLP-1 receptor agonists) was to a DPP-4i. Overall patterns for add-on or switch therapy were similar between the JMDC and MDV datasets and between UT and PT patients.
Across all four patient subgroups, 12-month persistence rates were highest with index DPP-4i monotherapy compared with all other index antidiabetic drug classes, although did not exceed 78.8% (with DPP-4i in PT patients in the MDV database) and were around 50% or less with several index antidiabetic drug classes especially in the JMDC database. Mean adherence to antidiabetic monotherapy was high overall, and the proportion of patients with high adherence (≥80%) was higher with index DPP-4i than with all other antidiabetic drug classes. Among drug combinations, 12-month persistence rates were higher for DPP-4i plus BG than for other combinations, although did not exceed 72.0%. Adherence rates were ≥80% for commonly prescribed antidiabetic drug combinations.
We also analysed persistence (≥12 months, <12 months) and drug adherence (<80%, ≥80%) in UT patients according to other patient-related and treatment-related factors. Persistence tended to increase with age (online supplementary table 1). In the JMDC database, the adjusted OR for non-persistence was 3.31 (p<0.05) in the 65–74 years age group compared with the reference group (18–34 years). In addition, persistence with multiple medications tended to be good in patients receiving ≤5 medications, but poorer in patients receiving ≥6 medications. In the MDV database, 29.1% of patients with 4–5 medications were non-persistent, whereas 47.6% of patients with >8 medications were non-persistent. Persistence was good in patients with comorbid hypertension (JMDC: 66.0%; MDV: 71.4%) or hyperlipidaemia (JMDC: 62.3%; MDV: 73.6%). However, persistence was poor in patients treated with multiple antidiabetic drug classes: in both the JMDC and MDV databases, approximately 60%–70% of patients receiving ≥3 index antidiabetic drug classes were non-persistent. Similar findings were evident for adherence (online supplementary table 2). In the MDV database, only 2.0% of patients receiving antidiabetic monotherapy were non-adherent, whereas 6.6%–9.5% of those with ≥3 antidiabetic drugs were non-adherent. All these findings are interesting and suggest that higher rates of persistence and adherence observed in elderly patients treated with multiple medications may reflect greater insight by this group into their disease. Conversely, the relatively low rates of persistence and adherence in patients treated with more index antidiabetic drug classes may have resulted from patient or caregiver difficulties regarding drug management. Therefore, FDC therapy, with its potential to enhance persistence and adherence, may be especially appropriate for patients treated with several index oral antidiabetic drug classes.
bmjopen-2018-025806supp001.pdf (74.7KB, pdf)
bmjopen-2018-025806supp002.pdf (74.7KB, pdf)
Strengths and limitations of the study
The main strengths of the present study are that it provides robust real-world evidence from two large administrative claims databases for patterns of antidiabetic drug use in patients with T2DM in Japan, clearly highlighting the widespread use of DPP-4i schedules (as monotherapy, add-on therapy, switch therapy or in combination regimens), and shows marked persistence and adherence with DPP-4i therapy.
The study was limited to some extent by the strict inclusion criteria, which restricted the proportion of patients from each database eligible for analysis. The analyses did not factor in HbA1c levels at the start of treatment, or the level of HbA1c control achieved during treatment, which may have influenced treatment decisions. Another limitation was the use of prescription events, rather than patient-derived data (eg, patient diaries), to estimate outcomes. A limitation specific to the JMDC database was the relative scarcity of data for patients aged ≥65 years. A limitation specific to the MDV database was the absence of information about whether patients received care in other medical facilities. For example, receipt of a prescription at another medical facility could result in a missing medication history and misclassification of the patient in our analysis. The inability to examine reasons for treatment discontinuation or to analyse any potential health benefits (eg, reduced symptom severity or improved health-related quality of life) resulting from increased persistence, as such data are not collected in administrative claims databases, were limitations that applied to both databases. Lastly, the study may not have accurately captured the uptake of SGLT2i use given the timing of their introduction in Japan. In the first 6 months of their availability (May−October 2015), prescribing of SGLT2i was restricted to 14 days’ therapy for safety reasons, which may have had an impact on usage rates. The restriction applied to this new class of drugs was routine, as directed by the Japanese Pharmaceuticals and Medical Devices Agency. Further analysis of prescribing practices based on updated databases is required to reflect current trends.
Comparison with other studies
A recent update to a position statement from the American Diabetes Association and European Association for the Study of Diabetes regarding management of hyperglycaemia in T2DM stipulates clearly that metformin is the best therapeutic option for monotherapy.22–24 If target HbA1c is not attained after approximately 3 months, progression to double therapy is advocated. If, after a further 3 months, target HbA1c remains unattained, progression to triple therapy is recommended. After a 3-month trial of triple therapy, the introduction of combination injectable therapy with insulin plus a GLP-1 receptor agonist may be indicated.
Conversely, JDS guidelines stipulate that the ‘… choice of glucose-lowering agent should be made based on the disease condition of each particular patient with consideration given to the pharmacological and safety profile of each glucose-lowering agent’.6 In accordance with these recommendations, and in conjunction with appropriate patient education about diet, exercise and lifestyle, treatment of T2DM in Japan may be started with any oral hypoglycaemic agent. As illustrated in the current study, DPP-4i is widely used in Japan, and this concurs with findings from other studies. For example, the ATTAK-J study reported real-world evidence of significant hypoglycaemic activity and favourable safety for DPP-4i therapy in Japanese patients with T2DM.25 The PREFERENCE 4 study documented that treatment-naive Japanese patients preferred (in terms of treatment satisfaction) a DPP-4i to a BG, SU, or α-GI.26 Use of a weekly DPP-4i also improved treatment satisfaction.27 28 However, these are preliminary findings, and additional real-world data from other DPP-4i studies are awaited.
A systematic review and meta-analysis of studies which compared persistence and adherence associated with two or more antidiabetic medications in patients with T2DM found considerable variation among studies in the methods used to define these terms but, nonetheless, was able to ascertain major differences between drug classes.29 Adherence rates were higher with DPP-4i than with TZD, SU and metformin, possibly reflecting the superior tolerability and convenient dosing schedules of these incretin-based agents.
Data about T2DM management in Asian patients indicate that DPP-4i is a viable first-line intervention, in a manner similar to that of metformin in Caucasian patients with T2DM.1 Based on numerous studies involving mainly Japanese or Chinese patients, there is broad recognition that DPP-4i is more effective in East Asian than non-Asian patients1 17–19 30 and, in Japan, >70% of patients treated with antidiabetic drugs receive incretin-based therapies. As approximately 60% of such patients are treatment-naïve, DPP-4i is establishing a definitive role in the first-line treatment of T2DM in Japan.1 31 Although no significant association between DPP-4i and possible pancreatic disorder was observed in several large-scale studies,25 32–34 it is important to remain vigilant for potential safety signals35 since DPP-4i-related pancreatitis is a low but established risk.36
Conclusions and implications
The study indicated that DPP-4i has a prevalent role (as monotherapy, add-on therapy, switch therapy and in combination regimens) in the management of T2DM in Japan. The high persistence and adherence we observed to DPP-4i-containing treatment schedules was a positive finding given the myriad factors contributing to poor adherence,9 but also suggested to us that enhanced diabetes awareness and patient education programmes are needed to improve persistence and adherence rates overall in Japan. For antidiabetic drug therapy in general, research is warranted to quantify the extent to which augmenting persistence and adherence is likely to improve glycaemic control. In the case of DPP-4i, strategies to improve adherence might involve the use of novel once-weekly administration schedules or FDCs.13 37 Frequent prescribing of DPP-4i by Japanese physicians and high patient persistence and adherence with DPP-4i-containing schedules imply satisfaction with treatment. Although there is no current evidence to indicate that DPP-4i provide better glycaemic, microvascular or macrovascular outcomes compared with metformin or other oral antidiabetic agents in Japanese patients, they may be a good treatment option where adherence is an issue.
Supplementary Material
Acknowledgments
The authors thank Kerry Dechant of Content Ed Net for writing and editorial assistance in the preparation of this manuscript, with funding from Takeda Pharmaceutical Company, Tokyo, Japan. The study was presented as a poster (P-039) at the 27th Annual Scientific Meeting of the Japan Epidemiological Association, Yamanashi, Japan, 25–27 January 2017.
Footnotes
Contributors: RN, HK, SH, YO, FG and YS are responsible for the work described in this paper. RN, HK, SH, YO, FG and YS were involved in the conception, design or planning of the study. YO and FG were involved in the analysis of data. RN, HK, KK, AO, SH and YS were involved in the interpretation of results. RN, HK, KK, AO and YO contributed substantially to drafting of the manuscript.
Funding: Funding for this research was provided by Takeda Pharmaceutical Company, Tokyo, Japan.
Disclaimer: The study made use of de-identified data from the JMDC and MDV databases. The opinions, results and conclusions reported are those of the authors. No endorsement by JMDC or MDV or any of its funders or partners is intended or should be inferred.
Competing interests: RN has received speaker honoraria from Astellas Pharma, Nippon Boehringer Ingelheim, Eli Lilly Japan K.K., Kissei Pharmaceutical, Medtronic Japan, MSD, Novartis Pharma KK, Novo Nordisk Pharma, Sanofi KK and Takeda Pharmaceutical and contract research fees for collaborative research with the Japan Diabetes Foundation. HK, KK, AO and YS are employees of Takeda Pharmaceutical. SH was an employee of Takeda Pharmaceutical at the time the study was conducted. FG and YO are employees of Creativ-Ceutical K.K.
Ethics approval: Based on Ethical Guidelines for Epidemiological Research issued by the Japanese Ministry of Health, Labour and Welfare, ethics approval was not applicable to this study.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Given the administrative nature of the data, patients did not provide informed consent for data sharing; however, all data are fully anonymised and the risk of patient identification is low.
Patient consent for publication: Not required.
References
- 1. Seino Y, Kuwata H, Yabe D. Incretin-based drugs for type 2 diabetes: focus on East Asian perspectives. J Diabetes Investig 2016;7:102–9. 10.1111/jdi.12490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fujibayashi K, Hayashi M, Yokokawa H, et al. Changes in antidiabetic prescription patterns and indicators of diabetic control among 200,000 patients over 13 years at a single institution in Japan. Diabetol Metab Syndr 2016;8 10.1186/s13098-016-0187-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Teramoto T, Sasaki J, Ishibashi S, et al. Diabetes mellitus. Executive summary of the Japan Atherosclerosis Society (JAS) guidelines for the diagnosis and prevention of atherosclerotic cardiovascular diseases in Japan--2012 version. J Atheroscler Thromb 2014;21:93–8. [DOI] [PubMed] [Google Scholar]
- 4. Araki E, Haneda M, Kasuga M, et al. New glycemic targets for patients with diabetes from the Japan Diabetes Society. J Diabetes Investig 2017;8:123–5. 10.1111/jdi.12600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ueki K, Sasako T, Okazaki Y, et al. Effect of an intensified multifactorial intervention on cardiovascular outcomes and mortality in type 2 diabetes (J-DOIT3): an open-label, randomised controlled trial. Lancet Diabetes Endocrinol 2017;5:951–64. 10.1016/S2213-8587(17)30327-3 [DOI] [PubMed] [Google Scholar]
- 6. Society JD. Treatment guide for diabetes 2016-2017. Tokyo, Japan: The Japan Diabetes Society. [Google Scholar]
- 7. Tanaka H, Tomio J, Sugiyama T, et al. Process quality of diabetes care under favorable access to healthcare: a 2-year longitudinal study using claims data in Japan. BMJ Open Diabetes Res Care 2016;4:e000291 10.1136/bmjdrc-2016-000291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005;353:487–97. 10.1056/NEJMra050100 [DOI] [PubMed] [Google Scholar]
- 9. Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Proc 2011;86:304–14. 10.4065/mcp.2010.0575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sugita H, Shinohara R, Yokomichi H, et al. Effect of text messages to improve health literacy on medication adherence in patients with type 2 diabetes mellitus: a randomized controlled pilot trial. Nagoya J Med Sci 2017;79:313–21. 10.18999/nagjms.79.3.313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aoki K, Nagakura M, Taguri M, et al. Effect of switching from an anti-diabetic loose dose combination to a fixed dose combination regimen at equivalent dosage for 6 months on glycemic control in Japanese patients with type 2 diabetes: a pilot study. J Clin Med Res 2017;9:719–24. 10.14740/jocmr3067w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fukuda H, Mizobe M. Impact of nonadherence on complication risks and healthcare costs in patients newly-diagnosed with diabetes. Diabetes Res Clin Pract 2017;123:55–62. 10.1016/j.diabres.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 13. Kaku K. First novel once-weekly DPP-4 inhibitor, trelagliptin, for the treatment of type 2 diabetes mellitus. Expert Opin Pharmacother 2015;16:2539–47. 10.1517/14656566.2015.1099630 [DOI] [PubMed] [Google Scholar]
- 14. Rozenfeld Y, Hunt JS, Plauschinat C, et al. Oral antidiabetic medication adherence and glycemic control in managed care. Am J Manag Care 2008;14:71 5. [PubMed] [Google Scholar]
- 15. Kurtyka K, Nishikino R, Ito C, et al. Adherence to dipeptidyl peptidase-4 inhibitor therapy among type 2 diabetes patients with employer-sponsored health insurance in Japan. J Diabetes Investig 2016;7:737 43. 10.1111/jdi.12474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bloomgarden ZT, Tunceli K, Liu J, et al. Adherence, persistence, and treatment discontinuation with sitagliptin compared with sulfonylureas as add-ons to metformin: a retrospective cohort database study. J Diabetes 2017;9:677 88. 10.1111/1753-0407.12461 [DOI] [PubMed] [Google Scholar]
- 17. Kim YG, Hahn S, Oh TJ, et al. Differences in the glucose-lowering efficacy of dipeptidyl peptidase-4 inhibitors between Asians and non-Asians: a systematic review and meta-analysis. Diabetologia 2013;56:696–708. 10.1007/s00125-012-2827-3 [DOI] [PubMed] [Google Scholar]
- 18. Kim YG, Hahn S, Oh TJ, et al. Differences in the HbA1c-lowering efficacy of glucagon-like peptide-1 analogues between Asians and non-Asians: a systematic review and meta-analysis. Diabetes Obes Metab 2014;16:900–9. 10.1111/dom.12293 [DOI] [PubMed] [Google Scholar]
- 19. Davis TME, Mulder H, Lokhnygina Y, et al. Effect of race on the glycaemic response to sitagliptin: Insights from the Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS). Diabetes Obes Metab 2018;20:1427–34. 10.1111/dom.13242 [DOI] [PubMed] [Google Scholar]
- 20. Iwasaki M, Hoshian F, Tsuji T, et al. Predicting efficacy of dipeptidyl peptidase-4 inhibitors in patients with type 2 diabetes: Association of glycated hemoglobin reduction with serum eicosapentaenoic acid and docosahexaenoic acid levels. J Diabetes Investig 2012;3:464–7. 10.1111/j.2040-1124.2012.00214.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Senmaru T, Fukui M, Kobayashi K, et al. Dipeptidyl-peptidase IV inhibitor is effective in patients with type 2 diabetes with high serum eicosapentaenoic acid concentrations. J Diabetes Investig 2012;3:498–502. 10.1111/j.2040-1124.2012.00220.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012;35:1364–79. 10.2337/dc12-0413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012;55:1577–96. 10.1007/s00125-012-2534-0 [DOI] [PubMed] [Google Scholar]
- 24. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015;38:140–9. 10.2337/dc14-2441 [DOI] [PubMed] [Google Scholar]
- 25. Takeda H, Sasai N, Ito S, et al. Efficacy and safety of alogliptin in patients with type 2 diabetes: analysis of the ATTAK-J Study. J Clin Med Res 2016;8:130–40. 10.14740/jocmr2418w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ishii H, Hayashino Y, Akai Y, et al. Dipeptidyl peptidase-4 inhibitors as preferable oral hypoglycemic agents in terms of treatment satisfaction: Results from a multicenter, 12-week, open label, randomized controlled study in Japan (PREFERENCE 4 study). J Diabetes Investig 2018;9:137–45. 10.1111/jdi.12659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oita M, Miyoshi H, Ono K, et al. Satisfaction and efficacy of switching from daily dipeptidyl peptidase-4 inhibitors to weekly trelagliptin in patients with type 2 diabetes--Randomized controlled study. Endocr J 2018;65:141–50. 10.1507/endocrj.EJ17-0303 [DOI] [PubMed] [Google Scholar]
- 28. Tosaki T, Kamiya H, Yamamoto Y, et al. Efficacy and patient satisfaction of the weekly DPP-4 inhibitors trelagliptin and omarigliptin in 80 Japanese patients with type 2 diabetes. Intern Med 2017;56:2563–9. 10.2169/internalmedicine.8184-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McGovern A, Tippu Z, Hinton W, et al. Comparison of medication adherence and persistence in type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab 2018;20:1040–3. 10.1111/dom.13160 [DOI] [PubMed] [Google Scholar]
- 30. Ito Y, Ambe K, Kobayashi M, et al. Ethnic difference in the pharmacodynamics-efficacy relationship of dipeptidyl peptidase-4 inhibitors between Japanese and non-Japanese patients: a systematic review. Clin Pharmacol Ther 2017;102:701–8. 10.1002/cpt.692 [DOI] [PubMed] [Google Scholar]
- 31. Yabe D, Kuwata H, Kaneko M, et al. Use of the Japanese health insurance claims database to assess the risk of acute pancreatitis in patients with diabetes: comparison of DPP-4 inhibitors with other oral antidiabetic drugs. Diabetes Obes Metab 2015;17:430–4. 10.1111/dom.12381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317–26. 10.1056/NEJMoa1307684 [DOI] [PubMed] [Google Scholar]
- 33. White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013;369:1327–35. 10.1056/NEJMoa1305889 [DOI] [PubMed] [Google Scholar]
- 34. Egan AG, Blind E, Dunder K, et al. Pancreatic safety of incretin-based drugs--FDA and EMA assessment. N Engl J Med 2014;370:794–7. 10.1056/NEJMp1314078 [DOI] [PubMed] [Google Scholar]
- 35. Zhang Z, Chen X, Lu P, et al. Incretin-based agents in type 2 diabetic patients at cardiovascular risk: compare the effect of GLP-1 agonists and DPP-4 inhibitors on cardiovascular and pancreatic outcomes. Cardiovasc Diabetol 2017;16:31 10.1186/s12933-017-0512-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. DeVries JH, Rosenstock J. DPP-4 inhibitor-related pancreatitis: rare but real!. Diabetes Care 2017;40:161–3. 10.2337/dci16-0035 [DOI] [PubMed] [Google Scholar]
- 37. Inagaki N, Onouchi H, Maezawa H, et al. Once-weekly trelagliptin versus daily alogliptin in Japanese patients with type 2 diabetes: a randomised, double-blind, phase 3, non-inferiority study. Lancet Diabetes Endocrinol 2015;3:191–7. 10.1016/S2213-8587(14)70251-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-025806supp001.pdf (74.7KB, pdf)
bmjopen-2018-025806supp002.pdf (74.7KB, pdf)