Abstract
A gold-catalyzed oxidative coupling of alkynes was developed as an efficient approach for the synthesis of challenging cyclic conjugated diynes (CCD). Compared to the classical copper-promoted oxidative coupling reaction of alkynes, this gold-catalyzed process exhibits a faster reaction rate due to the rapid reductive elimination from the Au(III) intermediate. This unique reactivity thus allowed a challenging diyne macrocyclization to take place in high efficiency. Condition screening revealed a [(n-Bu)4N]+[Cl-Au-Cl]− salt as the optimal pre-catalyst. Macrocycles with ring size between 13 to 28 atoms were prepared in moderate to good yields, which highlighted the broad substrate scope of this new strategy. Furthermore, the synthetic utilities of the cyclic conjugated diynes for copper-free click chemistry have been demonstrated, which showcased the potential application of this strategy in biological systems.
Keywords: Gold catalysis, cyclic conjugated diynes, macrocyclization, oxidative coupling
eTOC Blurb
A Gold-catalyzed oxidative coupling of alkynes was developed as an efficient approach for the synthesis of challenging cyclic conjugated diyne. Compared to copper promoted oxidative coupling, this protocol allowed macrocyclization under dilute conditions with good overall reactivity and high functional group tolerance. The success to achieve copper-free “click chemistry” on cyclic conjugated diyne highlighted its potential application in biological and medicinal research.
INTRODUCTION
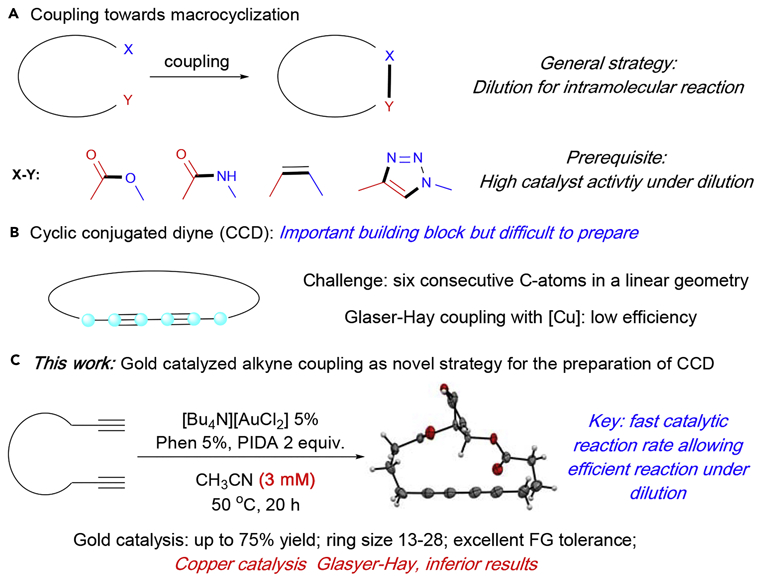
Macrocycles are a group of important and fascinating compounds that exhibits broad utilities in chemical, material and biological research.1–5 Successful macrocyclization strategies often rely on the reaction rate differences between intramolecular cyclization and (problematic) intermolecular oligomerization or polymerization.6–9 Thus, the two general approaches for selective macrocyclization are A) pre-organization of reactant conformation to favor the cyclization and B) reducing intermolecular reaction rate through significant dilution. One major concern for achieving macrocyclization under highly diluted conditions is the efficiency of the catalyst. Some representative macrocyclization strategies are shown in Scheme 1A, including Nozaki-Hiyama-Kishi macrocyclization,10–14 ring closing metathesis15–18 and intramolecular alkyne-azide cycloaddition (CuAAc).19–22
Scheme 1.
Challenges in the synthesis of cyclic conjugated diyne (CCD)
The intrinsic reactivity of C-C triple bonds allows alkynes to occupy a privileged position in organic synthesis. Among alkyne derivatives, conjugated diynes have shown interesting properties in chemical, medicinal and material research.23–27 A typical conjugated diyne is unique in that six consecutive atoms are arranged in a linear geometry (Scheme 1B). Thus, macrocycles containing conjugated diynes must possess a flexible backbone and fairly large ring size so as to minimize the strong ring strain. A significant breakthrough of CCD synthesis was the recent work reported by Collins and coworkers using a phase-transfer system.28–30 In their work, a mixture of MeOH and PEG-400 (4:1) was used to generate a heterogeneous bi-phasic reaction environment. Although the exact mechanism remains uncertain, they proposed that the bi-phase environment allowed the formation of a metal-acetylide at the solvent interface and prevented intermolecular polymerization. This seminal work was the best condition reported so far for the practical synthesis of cyclic conjugated diynes (CCD) with flexible linkers. One major concern of that method was the bi-phase system require very complex conditions for optimal performance (25%−100% CuCl2, 25%−100% Ni(NO3)2 6H2O, 3 equiv Et3N, 5 equiv. pyridine, 60 °C, O2, 1–2 days) and some substrates (such as phenol ester, vide infra) are not tolerated under these conditions. Thus, novel approaches for this extremely challenging transformation is highly desirable, especially with a complementary substrate scope and better functional group tolerability.31–35 In this work, we report the gold-catalyzed oxidative coupling of terminal alkynes as an alternative approach to construct cyclic conjugated diynes. The key to this success was the realization of a faster Csp-Csp reductive elimination on gold(III) complexes (comparing with copper-catalyzed Glaser-Hay conditions),36 which allowed more efficient alkyne coupling under low concentration. Cyclic diynes with size between 13 to 28 atoms were prepared in moderate to good yields, while copper catalysts provided inferior results due to the slow reaction rate under diluted conditions. (Scheme 1C)
RESULTS and DISCUSSION
Our interest in developing new strategies for the synthesis of cyclic conjugated diynes was initiated from our recent success in gold-catalyzed cross coupling of terminal alkynes.37 In that study, we discovered that oxidative coupling of terminal alkynes could be achieved using a gold catalyst under suitable conditions (Phen as ligand and bisacetoxyiodobenzene(PIDA) as oxidant). When comparing with the copper-promoted Glaser-Hay conditions,38–41 gold catalysts gave excellent cross-coupling selectivity between aromatic alkynes and aliphatic alkynes. Besides the excellent selectivity, we also observed a much faster reaction rate with gold-catalyzed condition over the copper.42–45 To further validate this key observation, we conducted kinetic study under three different conditions as shown in Figure 1.
Figure 1.
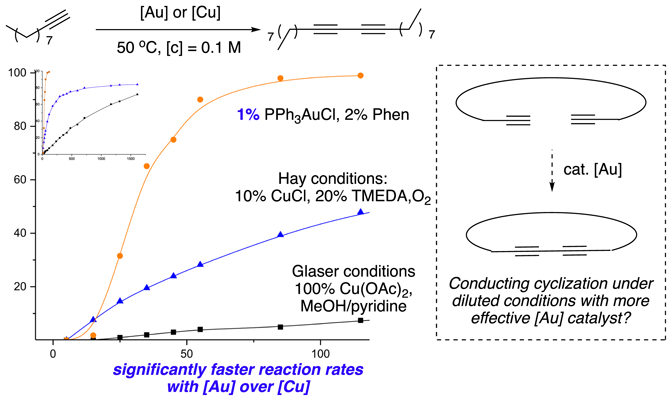
Significantly faster reaction rate of gold-catalyzed coupling
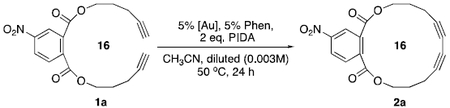
Under identical reaction temperature and concentration (50 °C, 0.1M), 1% PPh3AuCl gave significantly faster reaction rate (t1/2=45 minutes) compared to either Hay or Glaser condition, which required a much higher catalyst loading (20% or 100%). Encouraged by this result, we explored the cyclization of terminal alkyne 1a (Table 1). Interestingly, under previously reported optimal conditions, (5% PPh3AuCl, 10% Phen and 2 eq PIDA), the desired cyclization product 2a (ring size = 16) was only obtained in less than 10% yield, although complete consumption of 1a was achieved within 12 hours even at very low concentration with [c] = 0.003M (entry 1). Slow addition of 1a over time (24 hours) with syringe pump only improved the yield slightly to 12% (entry 2). In both cases, the complete conversion of alkyne 1a indicated the gold catalyst could successfully promote the alkyne oxidative coupling even at low concentration. However, polymerization product was obtained as the dominant side product. These results clearly illustrated the great challenge associated with this challenging macrocyclization. To further optimize this reaction, we screened various gold catalysts. The results are summarized in Table 1.
Table 1.
| Entry | Reaction conditionsa,b | 1a convn. | 2a |
|---|---|---|---|
| 1 | 5% PPh3AuCl, 5% Phen, 2 eq. PIDA, 50 °C | 100% | <10% |
| 2 | Entry 1, syringe pump addition of 1a | 100% | 12% |
| 3 | L[AuCl]2 (10%): L= dppm, dppp, BINAP | 100% | <10% |
| 4 | 10% Xantphos[Au2Cl2], cat-1 | 100% | 15% |
| 5 | 10% t-BuXantphos[Au2Cl2], cat-2 | 100% | 75% |
| 6 | Entry 1, [Au]=5% [t-BuXantphosAu]+[BF4]−, cat-3 | <5% | n.d. |
| 7 | Entry 1, [Au]=5% [(n-Bu)4N]+[Cl-Au-Cl]−; cat-4 | 100% | 75% |
| 8 | Entry 7 without PIDA | <5% | n.d. |
| 9 | Entry 7 without Phen | <5% | n.d. |
| 10 | Glaser cond.: 100% Cu(OAc)2, MeOH/pyridine, 50 °C, 24h | 50% | 12% |
| 11 | Hay cond.: 20% CuCl, 40% TMEDA, iPrOH, O2, 50 °C, 24 h | 20% | <10% |
| 12 | Collins’ bi-phase condition | 100% | 60% |
| 13 | Other metal catalysts: Rh, Ag, Pt, Ru, Fe, Ni, Pd. | <25% | <5% |
Reaction conditions: 5 mol% catalyst and 5% Phen was added to a MeCN solution (30 mL) of 1a (0.1 mmol) and PIDA (0.2 mmol), and reaction was kept under Ar at 50 °C for 24 h.
Conversion and yield were determined by 1H NMR spectroscopy using 1,3,5-trimethoxybenzene as internal standard.
To improve the selectivity towards desired intramolecular cyclization over intermolecular polymerization, we wondered whether bis-gold complexes could improve the reaction performance through a faster transmetalation between two gold atoms in one catalyst.46 Several bis-gold catalysts were tested. Interestingly, while dppm, dppp and BINAP-bound gold complexes gave poor results similar to PPh3AuCl (entry 3), slightly improved yield (15%) of 2a was obtained using Xantphos[AuCl]2 cat-1 (entry 4). Surprisingly, simply switching the catalyst to “t-BuXantphos[AuCl]2” cat-2, CCD 2a was obtained in 75% isolated yield (entry 4), significantly higher than previous cases. It was not clear to us why these two catalysts showed such a dramatic difference until we successfully obtained the crystal structures of both catalysts as shown in Figure 2.
Figure 2.
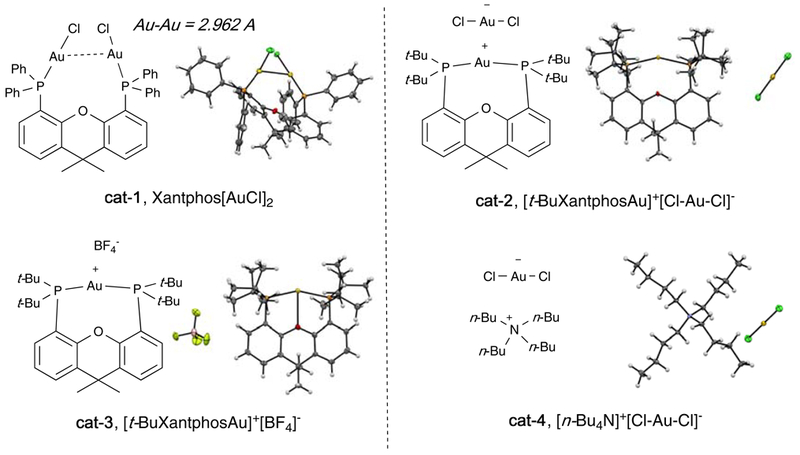
X-ray crystal structures of “Xantphos-Au” complexes.
For Xantphos-Au complex cat-1, a Au-Au distance of 2.962 Å was observed in L-Au-Cl complex. Interestingly, for t-BuXantphos, L-Au-Cl type complex was not formed as revealed by the X-ray. Instead, the complex is a salt with [t-BuXantphosAu]+ as cation and [Cl-Au-Cl]− as anion. The P-Au-P complex was formed presumably due to the strong steric hinderance caused by the t-Bu group.47–49 Clearly, the outstanding catalytic activity of the gold catalyst cat-2 toward cyclic diyne formation must be associated with its unique structure. This discovery is crucial since it revealed a potential new type of gold catalyst (other than L-Au-Cl) that might provide superior reactivity and efficiency towards the preparation of challenging cyclic conjugated diynes. The question is which one of the two species in cat-2, [P-Au-P]+ or [Cl-Au-Cl]− (or both), is the key component for the observed excellent reactivity. To explore this crucial mechanistic question, two gold complexes were prepared: [t-BuXantphosAu]+[BF4]− (cat-3) and [(n-Bu)4N]+[Cl-Au-Cl]− (cat-4). Both complexes are characterized by X-ray (Figure 2). Under identical conditions, [t-BuXantphosAu]+[BF4]− (cat-3) gave almost no reaction (entry 6). In contrast, high yield of cyclization product 2a was obtained using [(n-Bu)4N]+[Cl-Au-Cl]− (cat-4) as the catalyst (entry 7). The active gold species in the catalytic cycle is still unclear at this moment, it is likely that cat-4 only serves as a pre-catalyst, which is oxidized by PIDA to form a Au(III) salt or complex that is the real catalyst in this system. These results not only confirmed that [Cl-Au-Cl]− was a superior pre-catalyst for alkyne macrocyclization over traditional L-Au-Cl catalyst, but also suggested [(n-Bu)4N]+[Cl-Au-Cl]− (cat-4) as the optimal pre-catalyst (cheaper and more efficient) for the challenging CCD synthesis. Although [(n-Bu)4N]+[Cl-Au-Cl]− salt has been known for a long time since 1973 and is commercially available (CAS: 50480–99-4),50 this is the first time to unveil the catalytically reactivity of [(n-Bu)4N]+[Cl-Au-Cl]− salt as a pre-catalyst towards gold redox chemistry. Furthermore, Phen ligand is crucial to stabilize the Au(III) intermediate and presumably has a significant influence on the rate of reductive elimination as suggested in our previous work (entry 9).37 Notably, under typical copper-promoted Glaser or Hay conditions, less than 15% yield of product was obtained with low conversion. Under the bi-phase conditions reported by Collins’ group, a lower yield was obtained. All other metal catalysts tested (Rh, Ag, Pt, Ru, Fe, Ni, Pd) failed to promote this transformation, which greatly highlighted the unique reactivity of this [Cl-Au-Cl]− type of pre-catalyst for the CCD synthesis. With the optimal conditions revealed, we explored the scope of this macrocyclization method. The results are summarized in Figure 3.
Figure 3.
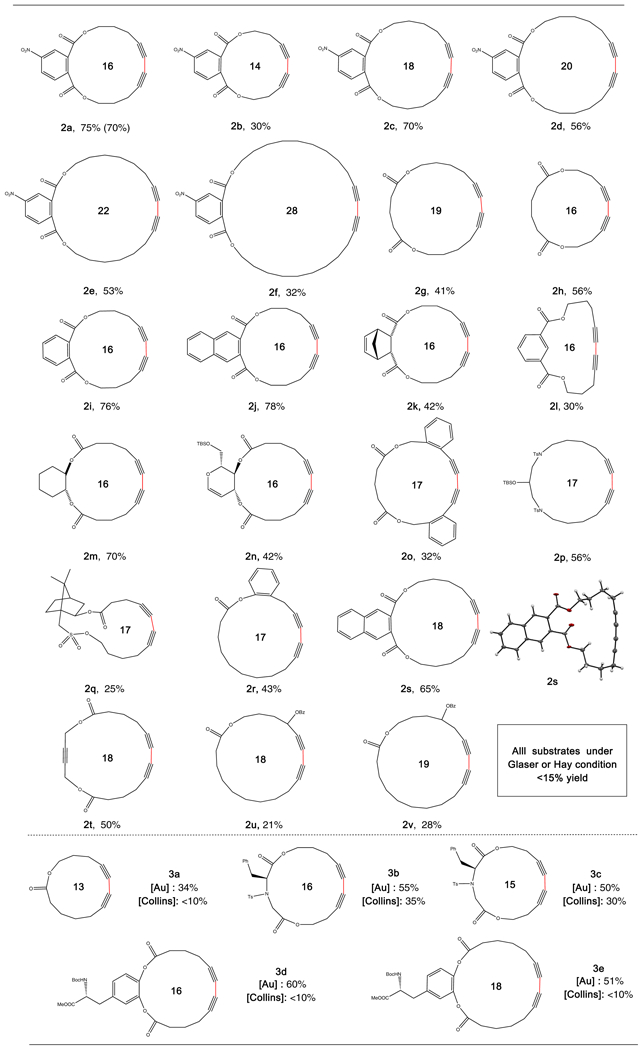
Substrate Scope for Macrocyclizationa,b
aReaction conditions: 5 % catalyst and 5% Phen was added to a MeCN solution (30 mL) of 1a (0.1 mmol) and PIDA (0.2 mmol), and reaction was kept under Ar at 50 °C for 24 h.b Isolated yield.c 0.5 mmol scale.
First, diynes containing different length of alkyl linkers to a 4-nitrophthalic ester backbone (2a-2f) were synthesized and subjected to the optimized reaction conditions. To our delight, macrocycles with ring size ranging from 14–28 were obtained in moderate to good yields. Gram-scale synthesis of 2a was also successfully performed without dramatic erosion in product yield. Notably, despite of significantly increased ring strain, 14-member ring could be effectively achieved with modest yield, which is remarkable for CCD synthesis. Attempts to form 12-member ring gave mainly dimerization products along with polymerization. When the targeted ring size reached 28, the yield of desired products decreased due to the increased polymerization by-products. Next, substrates with different backbones were investigated. Substrates with flexible aliphatic backbone were suitable for this reaction, providing desired products in moderate yields (2g, 2h). Other aromatic ester such as phthalic ester (2i) and naphthalic ester (2j) were also afforded desired products in good yields. Remarkably, substrate with an alkene backbone (2i) were also successful, with no decomposition of the product observed under the oxidative conditions. Substrate containing an alkyne backbone (2t) was also tolerated for this reaction with no hydration product observed. Both aryl alkynes (2o) and benzyl alkynes (2r) were suitable. Alkynes with a labile benzoyl group at the propargyl and homopropargyl position (2u and 2v) also proved successful. The D-Glucal derivative 2n and Camphor derivative 2q were successfully prepared, further demonstrating the exceptional functional group tolerability of this gold catalytic method. The structures of the conjugated dienes were undoubtedly confirmed by the X-ray crystal structure of 2s and 2n. Although Collins’ phase transfer method is a benchmark standard for CCD synthesis, one limitation of Collins’ method was the requirement of a hydrophobic flexible chain to adopt the bi-phase conditions. As a result, it is ineffective towards strained 13-member ring with short alkyne chain (3a). Also, some challenge substrates containing polar amino acid backbones such as 3b-3e gave very low yields. Remarkably, the gold-catalyzed conditions provided significantly better results for these substrates, forming the desired CCD products in moderate yields. It’s worth noting that in all cases, Glaser or Hay conditions only provided desired macrocyclization in less than 15% yield. Overall, all these results clearly demonstrated the great potential of this new method for CCD synthesis, especially as a complementary approach for pervious reported state-of-art Collins’ method.
After the successful realization of this gold-catalyzed oxidative macrocyclization, we envisioned that this protocol could also be employed into the intermolecular alkyne coupling. As demonstrated in Figure 4, homo-coupling of various of aromatic and aliphatic alkynes were achieved in excellent yields. Unlike Corma’s condition using selectfluor as oxidant,42,51 this method successfully promoted the homo-coupling of aliphatic alkynes with long chain (5j, 5k) in excellent yield. Various functional groups were tolerated, such as pyridine (5g), thiophene (5h), propargyl alcohol (5i), even amino acid (5m). Cross-coupling between aryl and aliphatic alkynes were also explored. When the ratio of aryl and aliphatic is 1:3, the selectivity of cross- vs homo- coupling can reach to 7:1 (5n), with 78% isolated yield of cross coupling product. Similar selectivity and yield was observed for the cross-coupling between different aromatic and aliphatic alkynes. Although this result is not superior compared with our previously reported dppm(AuBr)2 system, it offered an alternative option with cheap and readily available [(n-Bu)4N]+[Cl-Au-Cl]− salt. Overall, we demonstrated the capability of [(n-Bu)4N]+[Cl-Au-Cl]− salt in promoting intermolecular alkyne coupling.
Figure 4.
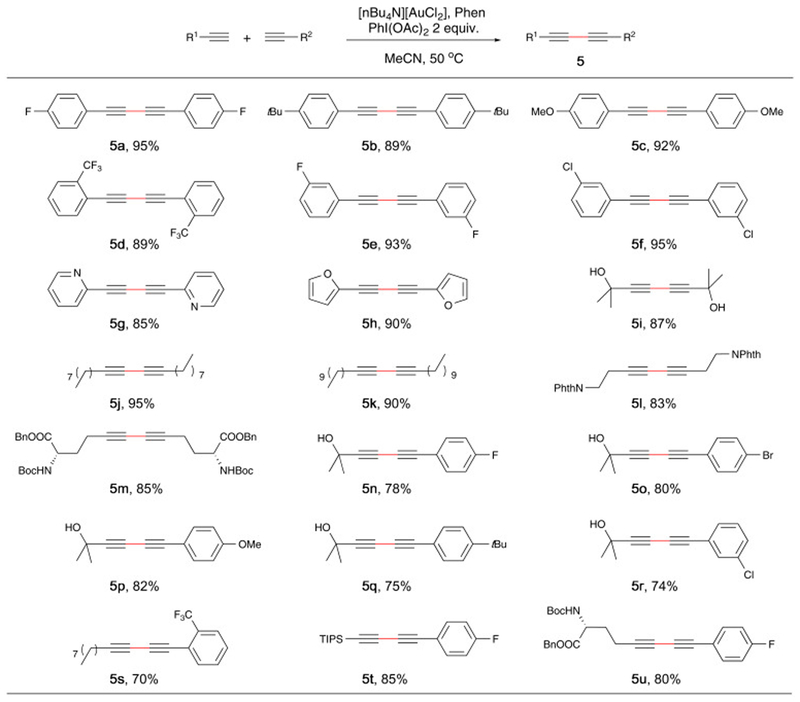
Substrate Scope for intermolecular homo/cross coupling.a,b
aReaction conditions for homo-coupling: 1% catalyst and 2% Phen was added to a MeCN solution (5 mL) of alkyne (1 mmol) and PIDA (1 mmol), and reaction was running at 50 °C. Reaction conditions for cross-coupling: 5 mol% catalyst and 10% Phen was added to a MeCN solution (800 uL) of aryl alkyne (0.2 mmol), aliphatic alkyne (0.6 mmol), and PIDA (0.4 mmol), and reaction was running at 50 °Cb Isolated yield.
Furthermore, the resulting cyclic conjugated diynes are valuable synthons that can be easily converted into other useful compounds. The transformation of diynes into furan 6 was carried out under simple gold-catalyzed conditions as shown in Scheme 2. Conversions to other heterocycles, such as thiophene and pyridine, can be readily achieved based on similar known methods.52–58
Scheme 2.
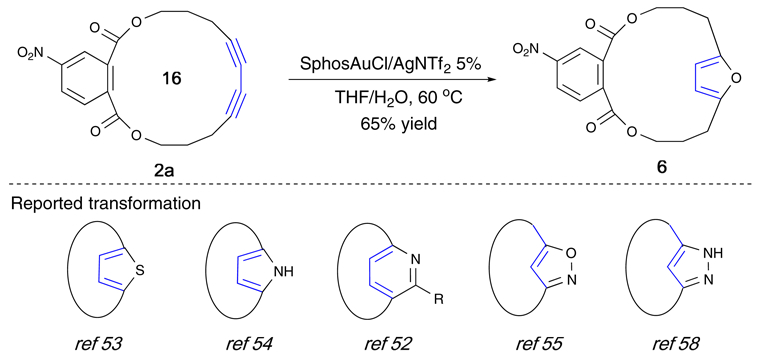
Synthesis of heterocycles from cyclic conjugated diyne (CCD)
One very important application of cycloalkyne is the copper-free azide-alkyne cycloaddition, which has received tremendous attentions in recent years as a bio-compatible labelling strategy under mild conditions.59–65 The success of this strategy relies on the ring strain of the cycloalkyne. Currently, difluoro-modified Cyclooctynes are used as the benchmark cycloalkynes for the metal-free click reaction. However, the preparation of these compounds was not straight forward (multiple steps with overall low yields) and often with poor functional group diversity. Therefore, a new strategy for metal-free click chemistry is highly desirable. With easy access to mid-size cyclic diynes, we postulated that the cyclic conjugated diynes could be another type of coupling partner towards azides, achieving metal-free click chemistry under mild conditions. We envisioned the 14-membered cyclic diynes could be ideal for this purpose with good stability and enough ring strain. To test our hypothesis, cyclic diyne 7 was prepared and charged with BnN3 in MeCN. We are pleased to find out that the desired triazole 8 was obtained in good yield (80% at 60 °C). Notably, single regio-isomer was obtained and its structure was unambiguously conformed by X-ray crystallography (Scheme 3). To the best of our knowledge, this is the first example to achieve the copper-free cycloaddition with a cyclic conjugated diyne. Our group is currently working on evaluation of this new methods in regard to CCD ring size, functional group tolerability and optimal conditions. Those results will be reported in due course. The success of CCD-click chemistry greatly highlighted the potential applications of this gold-catalyzed macrocyclization method in biological and material research.
Scheme 3.
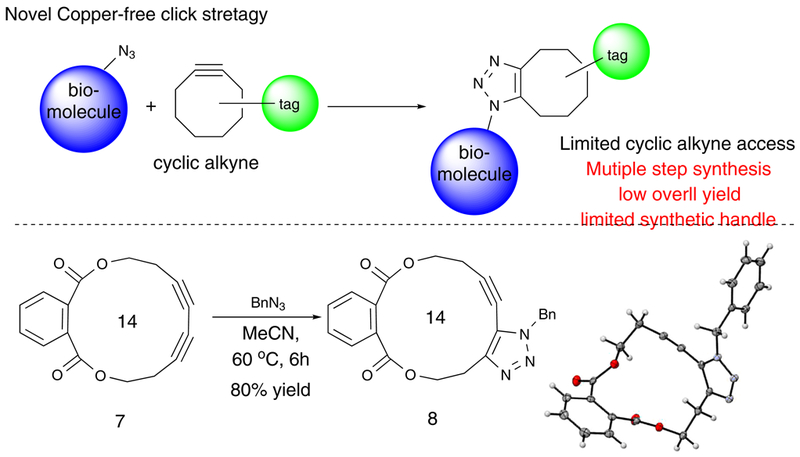
Metal-free click chemistry using cyclic conjugated diyne (CCD)
In summary, we report herein for the first time the synthesis of challenging cyclic conjugated diynes under gold-catalyzed macrocyclization conditions. Gold catalyst [(n-Bu)4N]+[Cl-Au-Cl]− was developed to promote this transformation with broad substrate scope and excellent functional group tolerance. This method is straightforward and efficient, which represents a complementary strategy comparing with current state-of-art methods. Synthetic utility of cyclic diynes was demonstrated by converting them to various heterocycles. The facile copper-free azide-alkyne cyclization with 14-member cyclic conjugated diynes further emphasized the promising future of this new methodology in biological and material research.
EXPERIMENTAL PROCEDURES
Full experimental procedures are provided in the Supplemental Information.
DATA AND SOFTWARE AVAILABILITY
The structure of cat1-cat4, 2n, 2s and 8 reported in this article has been deposited in the Cambridge Crystallographic Data Centre.
Supplemental Information including Supplemental Experimental Procedures can be found with this article online at
Supplementary Material
The Bigger Picture.
Macrocycles are important structural moieties in medicinal and biological research, and efficient methods for macrocyclization are always in high demand. With the unique conformation having six carbon atoms in a linear geometry, the cyclic conjugated diynes (CCD) present greater synthetic challenges and have been much less explored. Therefore, application of these unique macrocycles in biological studies is largely hammered. Herein, we described the discovery of a gold-catalyzed Glaser-Hay type oxidative coupling of terminal alkynes to achieve CCD under diluted conditions with broad substrate scope and great functional group compatibility. Taking advantage of the 14-member cyclic diyne, a copper-free “click chemistry” was achieved, which provided an effective alternative strategy for the traditional cyclooctynes-based azide-alkyne cycloaddition, suggesting a promising future of this method in tackling challenging problems in related biological and medicinal research.
Highlights.
First synthesis of challenging cyclic conjugated diynes via gold catalysis
[(n-Bu)4N]+[Cl-Au-Cl]− salt as a pre-catalyst towards gold redox chemistry
Facile access to functionalized cyclic conjugated diynes with 13–28 member rings
The Copper-free azide-alkyne cycloaddition for potential biological research
ACKNOWLEDGMENTS
We are grateful to the NSF (CHE-1619590), NIH (1R01GM120240–01) and NSFC (21629201) for financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES AND NOTES
- 1.Yudin AK (2015). Macrocycles: lessons from the distant past, recent developments, and future directions. Chem Sci 6, 30–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qi ZH, and Schalley CA (2014). Exploring Macrocycles in Functional Supramolecular Gels: From Stimuli Responsiveness to Systems Chemistry. Acc Chem Res 47, 2222–2233. [DOI] [PubMed] [Google Scholar]
- 3.Marsault E, and Peterson ML (2011). Macrocycles Are Great Cycles: Applications, Opportunities, and Challenges of Synthetic Macrocycles in Drug Discovery. J Med Chem 54, 1961–2004. [DOI] [PubMed] [Google Scholar]
- 4.Iyoda M, Yamakawa J, and Rahman MJ (2011). Conjugated Macrocycles: Concepts and Applications. Angew Chem Int Ed 50, 10522–10553. [DOI] [PubMed] [Google Scholar]
- 5.Driggers EM, Hale SP, Lee J, and Terrett NK (2008). The exploration of macrocycles for drug discovery - an underexploited structural class. Nat Rev Drug Discovery 7, 608–624. [DOI] [PubMed] [Google Scholar]
- 6.Marti-Centelles V, Pandey MD, Burguete MI, and Luis SV (2015). Macrocyclization Reactions: The Importance of Conformational, Configurational, and Template-Induced Preorganization. Chem Rev 115, 8736–8834. [DOI] [PubMed] [Google Scholar]
- 7.Yu XF, and Sun DQ (2013). Macrocyclic Drugs and Synthetic Methodologies toward Macrocycles. Molecules. 18, 6230–6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White CJ, and Yudin AK (2011). Contemporary strategies for peptide macrocyclization. Nature Chem 3, 509–524. [DOI] [PubMed] [Google Scholar]
- 9.Blankenstein J, and Zhu JP (2005). Conformation-directed macrocyclization reactions. Eur J Org Chem, 1949–1964. [Google Scholar]
- 10.Bolte B, Basutto JA, Bryan CS, Garson MJ, Banwell MG, and Ward JS (2015). Modular Total Syntheses of the Marine-Derived Resorcylic Acid Lactones Cochliomycins A and B Using a Late-Stage Nozaki-Hiyama-Kishi Macrocyclization Reaction. J Org Chem 80, 460–470. [DOI] [PubMed] [Google Scholar]
- 11.Pospisil J, Muller C, and Furstner A (2009). Total Synthesis of the Aspercyclides. Chem Eur J 15, 5956–5968. [DOI] [PubMed] [Google Scholar]
- 12.Mi BY, and Maleczka RE (2001). A Nozaki-Hiyama-Kishi Ni(II)/Cr(II) coupling approach to the phomactins. Org Lett 3, 1491–1494. [DOI] [PubMed] [Google Scholar]
- 13.Furstner A (1999). Carbon-carbon bond formations involving organochromium(III) reagents. Chem Rev 99, 991–1045. [DOI] [PubMed] [Google Scholar]
- 14.Wessjohann LA, and Scheid G (1999). Recent advances in chromium(II)- and chromium(III)-mediated organic synthesis. Synthesis, 1–36. [Google Scholar]
- 15.Gradillas A, and Perez-Castells J (2006). Macrocyclization by ring-closing metathesis in the total synthesis of natural products: Reaction conditions and limitations. Angew Chem Int Ed 45, 6086–6101. [DOI] [PubMed] [Google Scholar]
- 16.Bielawski CW, Benitez D, and Grubbs RH (2002). An “endless” route to cyclic polymers. Science. 297, 2041–2044. [DOI] [PubMed] [Google Scholar]
- 17.Blackwell HE, Sadowsky JD, Howard RJ, Sampson JN, Chao JA, Steinmetz WE, O’Leary DJ, and Grubbs RH (2001). Ring-closing metathesis of olefinic peptides: Design, synthesis, and structural characterization of macrocyclic helical peptides. J Org Chem 66, 5291–5302. [DOI] [PubMed] [Google Scholar]
- 18.Furstner A, and Langemann K (1997). Macrocycles by ring-closing metathesis. Synthesis, 792–803. [Google Scholar]
- 19.Chouhan G, and James K (2011). CuAAC Macrocyclization: High Intramolecular Selectivity through the Use of Copper-Tris(triazole) Ligand Complexes. Org Lett 13, 2754–2757. [DOI] [PubMed] [Google Scholar]
- 20.Holub JM, and Kirshenbaum K (2010). Tricks with clicks: modification of peptidomimetic oligomers via copper-catalyzed azide-alkyne 3+2 cycloaddition. Chem Soc Rev 39, 1325–1337. [DOI] [PubMed] [Google Scholar]
- 21.Aprahamian I, Miljanic OS, Dichtel WR, Isoda K, Yasuda T, Kato T, and Stoddart JF (2007). Clicked interlocked molecules. Bull Chem Soc Jpn 80, 1856–1869. [Google Scholar]
- 22.Turner RA, Oliver AG, and Lokey RS (2007). Click chemistry as a macrocyclization tool in the solid-phase synthesis of small cyclic peptides. Org Lett 9, 5011–5014. [DOI] [PubMed] [Google Scholar]
- 23.Ma KQ, Miao YH, Li X, Zhou YZ, Gao XX, Zhang X, Chao JB, and Qin XM (2017). Discovery of 1,3-diyne compounds as novel and potent antidepressant agents: synthesis, cell-based assay and behavioral studies. Rsc. Adv 7, 16005–16014. [Google Scholar]
- 24.Brauer MCN, Neves RAW, Westermann B, Heinke R, and Wessjohann LA (2015). Synthesis of antibacterial 1,3-diyne-linked peptoids from an Ugi-4CR/Glaser coupling approach. Beilstein J Org Chem 11, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi W, and Lei A (2014). 1,3-Diyne chemistry: synthesis and derivations. Tetrahedron Lett 55, 2763–2772. [Google Scholar]
- 26.Yu DG, de Azambuja F, Gensch T, Daniliuc CG, and Glorius F (2014). The C-H Activation/1,3-Diyne Strategy: Highly Selective Direct Synthesis of Diverse Bisheterocycles by Rh-III Catalysis. Angew Chem Int Ed 53, 9650–9654. [DOI] [PubMed] [Google Scholar]
- 27.Yamazaki S (2011). Rearrangements of alkyne and 1,3-diyne in transition metal center forming small ring complexes. Inorg Chim Acta 366, 1–18. [Google Scholar]
- 28.Godin E, Bedard AC, Raymond M, and Collins SK (2017). Phase Separation Macrocyclization in a Complex Pharmaceutical Setting: Application toward the Synthesis of Vaniprevir. J Org Chem 82, 7576–7582. [DOI] [PubMed] [Google Scholar]
- 29.Bedard AC, and Collins SK (2012). Microwave accelerated Glaser-Hay macrocyclizations at high concentrations. Chem Commun 48, 6420–6422. [DOI] [PubMed] [Google Scholar]
- 30.Bedard AC, and Collins SK (2011). Phase Separation As a Strategy Toward Controlling Dilution Effects in Macrocyclic Glaser-Hay Couplings. J Am Chem Soc 133, 19976–19981. [DOI] [PubMed] [Google Scholar]
- 31.Nie F, Kunciw DL, Wilcke D, Stokes JE, Galloway WRJD, Bartlett S, Sore HF, and Spring DR (2016). A Multidimensional Diversity-Oriented Synthesis Strategy for Structurally Diverse and Complex Macrocycles. Angew Chem Int Ed 55, 11139–11143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ungeheuer F, and Fürstner A (2015). Concise Total Synthesis of Ivorenolide B. Chem. Eur. J 21, 11387–11392. [DOI] [PubMed] [Google Scholar]
- 33.Verlinden S, Geudens N, Martins JC, Tourwe D, Ballet S, and Verniest G (2015). Oxidative a,w-diyne coupling as an approach towards novel peptidic macrocycles. Org Biomol Chem 13, 9398–9404. [DOI] [PubMed] [Google Scholar]
- 34.Naveen, Babu SA, Kaur G, Aslam NA, and Karanam M (2014). Glaser-Eglinton-Hay sp-sp coupling and macrocyclization: construction of a new class of polyether macrocycles having a 1,3-diyne unit. RSC Adv 4, 18904–18916. [Google Scholar]
- 35.Lysenko S, Volbeda J, Jones PG, and Tamm M (2012). Catalytic Metathesis of Conjugated Diynes. Angew Chem Int Ed 51, 6757–6761. [DOI] [PubMed] [Google Scholar]
- 36.Wolf WJ, Winston MS, and Toste FD (2014). Exceptionally fast carbon-carbon bond reductive elimination from gold(III). Nature Chem 6, 159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng H, Xi Y, Ronaghi N, Dong B, Akhmedov NG, and Shi X (2014). Gold-Catalyzed Oxidative Cross-Coupling of Terminal Alkynes: Selective Synthesis of Unsymmetrical 1,3-Diynes. J Am Chem Soc 136, 13174–13177. [DOI] [PubMed] [Google Scholar]
- 38.Su L, Dong J, Liu L, Sun M, Qiu R, Zhou Y, and Yin S-F (2016). Copper Catalysis for Selective Heterocoupling of Terminal Alkynes. J Am Chem Soc 138, 12348–12351. [DOI] [PubMed] [Google Scholar]
- 39.Yin W, He C, Chen M, Zhang H, and Lei A (2009). Nickel-Catalyzed Oxidative Coupling Reactions of Two Different Terminal Alkynes Using O2 as the Oxidant at Room Temperature: Facile Syntheses of Unsymmetric 1,3-Diynes. Org Lett 11, 709–712. [DOI] [PubMed] [Google Scholar]
- 40.Bai R, Zhang G, Yi H, Huang Z, Qi X, Liu C, Miller JT, Kropf AJ, Bunel EE, Lan Y, et al. (2014). Cu(II)–Cu(I) Synergistic Cooperation to Lead the Alkyne C–H Activation. J Am Chem Soc 136, 16760–16763. [DOI] [PubMed] [Google Scholar]
- 41.Liu C, Yuan J, Gao M, Tang S, Li W, Shi R, and Lei A (2015). Oxidative Coupling between Two Hydrocarbons: An Update of Recent C–H Functionalizations. Chem Rev 115, 12138–12204. [DOI] [PubMed] [Google Scholar]
- 42.Leyva-Perez A, Domenech A, Al-Resayes SI, and Corma A (2012). Gold Redox Catalytic Cycles for the Oxidative Coupling of Alkynes. ACS. Cata 2, 121–126. [Google Scholar]
- 43.Zhu M, Ning M, Fu WJ, Xu C, and Zou GL (2012). Gold-Catalyzed Homocoupling Reaction of Terminal Alkynes to 1,3-Diynes. Bull Korean Chem Soc 33, 1325–1328. [Google Scholar]
- 44.Banerjee S, and Patil NT (2017). Exploiting the dual role of ethynylbenziodoxolones in gold-catalyzed C(sp)-C(sp) cross-coupling reactions. Chem Commun 53, 7937–7940. [DOI] [PubMed] [Google Scholar]
- 45.Li XD, Xie X, Sun N, and Liu YH (2017). Gold-Catalyzed Cadiot-Chodkiewicz-type Cross-Coupling of Terminal Alkynes with Alkynyl Hypervalent Iodine Reagents: Highly Selective Synthesis of Unsymmetrical 1,3-Diynes. Angew Chem Int Ed 56, 6994–6998. [DOI] [PubMed] [Google Scholar]
- 46.Levin MD, and Toste FD (2014). Gold-Catalyzed Allylation of Aryl Boronic Acids: Accessing Cross-Coupling Reactivity with Gold. Angew Chem Int Ed 53, 6211–6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Partyka DV, Updegraff Iii JB, Zeller M, Hunter AD, and Gray TG (2010). Gold(i) halide complexes of bis(diphenylphosphine)diphenyl ether ligands: a balance of ligand strain and non-covalent interactions. Dalton Trans 39, 5388–5397. [DOI] [PubMed] [Google Scholar]
- 48.Ito H, Saito T, Miyahara T, Zhong CM, and Sawamura M (2009). Gold(I) Hydride Intermediate in Catalysis: Dehydrogenative Alcohol Silylation Catalyzed by Gold(I) Complex. Organometallics. 28, 4829–4840. [Google Scholar]
- 49.Hu J-Y, Zhang J, Wang G-X, Sun H-L, and Zhang J-L (2016). Constructing a Catalytic Cycle for C–F to C–X (X = O, S, N) Bond Transformation Based on Gold-Mediated Ligand Nucleophilic Attack. Inorg Chem 55, 2274–2283. [DOI] [PubMed] [Google Scholar]
- 50.Braunstein P, and Clark RJH (1973). The preparation, properties, and vibrational spectra of complexes containing the AuCl2-, AuBr2-, and AuI2- ions. J Chem Soc, Dalton Trans, 1845–1848. [Google Scholar]
- 51.Leyva-Pérez A, Doménech-Carbó A, and Corma A (2015). Unique distal size selectivity with a digold catalyst during alkyne homocoupling. Nature Commun. 6, 6703. [DOI] [PubMed] [Google Scholar]
- 52.Zhou G, Zhao XM, and Dan WY (2017). Synthesis of 2,3,6-trisubstituted pyridines by transition-metal free cyclization of 1,3-diynes with amino acids. Tetrahedron Lett 58, 3085–3088. [Google Scholar]
- 53.Verlinden S, Ballet S, and Verniest G (2016). Synthesis of Heterocycle-Bridged Peptidic Macrocycles through 1,3-Diyne Transformations. Eur J Org Chem 2016, 5807–5812. [Google Scholar]
- 54.Wang LG, Yu XQ, Feng XJ, and Bao M (2013). Synthesis of 3,5-Disubstituted Pyrazoles via Cope-Type Hydroamination of 1,3-Dialkynes. J Org Chem 78, 1693–1698. [DOI] [PubMed] [Google Scholar]
- 55.Wang LG, Yu XQ, Feng XJ, and Bao M (2012). Synthesis of 3,5-Disubstituted Isoxazoles via Cope-Type Hydroamination of 1,3-Dialkynes. Org Lett 14, 2418–2421. [DOI] [PubMed] [Google Scholar]
- 56.Jiang HF, Zeng W, Li YB, Wu WQ, Huang LB, and Fu W (2012). Copper(I)-Catalyzed Synthesis of 2,5-Disubstituted Furans and Thiophenes from Haloalkynes or 1,3-Diynes. J Org Chem 77, 5179–5183. [DOI] [PubMed] [Google Scholar]
- 57.Kramer S, Madsen JLH, Rottlander M, and Skrydstrup T (2010). Access to 2,5-Diamidopyrroles and 2,5-Diamidofurans by Au(I)-Catalyzed Double Hydroamination or Hydration of 1,3-Diynes. Org Lett 12, 2758–2761. [DOI] [PubMed] [Google Scholar]
- 58.Duan HF, Sengupta S, Petersen JL, Akhmedov NG, and Shi XD (2009). Triazole-Au(I) Complexes: A New Class of Catalysts with Improved Thermal Stability and Reactivity for Intermolecular Alkyne Hydroamination. J Am Chem Soc 131, 12100–12102. [DOI] [PubMed] [Google Scholar]
- 59.Sletten EM, and Bertozzi CR (2011). From Mechanism to Mouse: A Tale of Two Bioorthogonal Reactions. Acc Chem Res 44, 666–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Debets MF, Van Berkel SS, Dommerholt J, Dirks AJ, Rutjes F, and Van Delft FL (2011). Bioconjugation with Strained Alkenes and Alkynes. Acc Chem Res 44, 805–815. [DOI] [PubMed] [Google Scholar]
- 61.Jewett JC, and Bertozzi CR (2010). Cu-free click cycloaddition reactions in chemical biology. Chem Soc Rev 39, 1272–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sumerlin BS, and Vogt AP (2010). Macromolecular Engineering through Click Chemistry and Other Efficient Transformations. Macromolecules. 43, 1–13. [Google Scholar]
- 63.Becer CR, Hoogenboom R, and Schubert US (2009). Click Chemistry beyond Metal-Catalyzed Cycloaddition. Angew Chem Int Ed. 48, 4900–4908. [DOI] [PubMed] [Google Scholar]
- 64.van Dijk M, Rijkers DTS, Liskamp RMJ, van Nostrum CF, and Hennink WE (2009). Synthesis and Applications of Biomedical and Pharmaceutical Polymers via Click Chemistry Methodologies. Bioconjugate Chem 20, 2001–2016. [DOI] [PubMed] [Google Scholar]
- 65.Debets MF, van der Doelen CWJ, Rutjes F, and van Delft FL (2010). Azide: A Unique Dipole for Metal-Free Bioorthogonal Ligations. Chembiochem 11, 1168–1184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The structure of cat1-cat4, 2n, 2s and 8 reported in this article has been deposited in the Cambridge Crystallographic Data Centre.
Supplemental Information including Supplemental Experimental Procedures can be found with this article online at